Abstract
A pathway sensitive to rapamycin, a selective inhibitor of mammalian target of rapamycin (mTOR), down-regulates effects of insulin such as activation of Akt (protein kinase B) via proteasomal degradation of insulin receptor substrate 1 (IRS-1). We report here that the pathway also plays an important role in insulin-induced subcellular redistribution of IRS-1 from the low-density microsomes (LDM) to the cytosol. After prolonged insulin stimulation, inhibition of the redistribution of IRS-1 by rapamycin resulted in increased levels of IRS-1 and the associated phosphatidylinositol (PI) 3-kinase in both the LDM and cytosol, whereas the proteasome inhibitor lactacystin increased the levels only in the cytosol. Since rapamycin but not lactacystin enhances insulin-stimulated 2-deoxyglucose (2-DOG) uptake, IRS-1-associated PI 3-kinase localized at the LDM was suggested to be important in the regulation of glucose transport. The amino acid deprivation attenuated and the amino acid excess enhanced insulin-induced Ser/Thr phosphorylation and subcellular redistribution and degradation of IRS-1 in parallel with the effects on phosphorylation of p70 S6 kinase and 4E-BP1. Accordingly, the amino acid deprivation increased and the amino acid excess decreased insulin-stimulated activation of Akt and 2-DOG uptake. Furthermore, 2-DOG uptake was affected by amino acid availability even when the degradation of IRS-1 was inhibited by lactacystin. We propose that subcellular redistribution of IRS-1, regulated by the mTOR-dependent pathway, facilitates proteasomal degradation of IRS-1, thereby down-regulating Akt, and that the pathway also negatively regulates insulin-stimulated glucose transport, probably through the redistribution of IRS-1. This work identifies a novel function of mTOR that integrates nutritional signals and metabolic signals of insulin.
Insulin stimulation initiates intracellular signaling by activation of insulin receptor tyrosine kinase, which phosphorylates tyrosine residues of endogenous substrates such as insulin receptor substrate 1 (IRS-1) and IRS-2 (5, 8, 18, 31). Signaling molecules containing a Src homology 2 (SH2) domain, including the p85 subunit of phosphatidylinositol (PI) 3-kinase, Grb2, SHP2, and others, are recruited to the tyrosine-phosphorylated substrate proteins and transmit a cascade of signals, which consists of two major elements, i.e., ras/MAP (mitogen-activated protein) kinase and PI 3-kinase pathways (5, 8, 18, 31).
The PI 3-kinase pathway mediates most of the metabolic actions of insulin, including glucose transport, glycogen synthesis, antilipolysis, and protein synthesis (8, 18, 36). PI 3-kinase phosphorylates the 3′-OH position of the inositol ring in inositol phospholipids, generating 3′-phosphoinositides, such as PI 3,4-bisphosphate [PI(3,4)P2] and PI 3,4,5-trisphosphate [PI(3,4,5)P3] [PI(28, 42). Production of 3′ phosphoinositides by the activation of PI 3-kinase results in recruitment of downstream signaling molecules including Ser/Thr protein kinase Akt (also known as protein kinase B [PKB]) to membranes, which facilitates phosphorylation of regulatory sites of the kinase by upstream regulators including the Ser kinase, 3′-phosphoinositide-dependent kinase 1 (1, 2, 7, 11). Activation of Akt has been shown to mediate many of the cellular effects of insulin (11, 18, 42).
Although there is considerable evidence that PI 3-kinase plays a critical role in insulin-stimulated glucose transport, which is achieved by translocation of GLUT4 from the intracellular pool to the plasma membrane (PM) in insulin target cells, the precise molecular mechanism of insulin-stimulated glucose transport remains unknown. For example, activation of Akt has been reported to be necessary and sufficient to elicit GLUT4 translocation (24, 25, 44), whereas other studies indicated that atypical protein kinase C isoforms ζ and λ are the targets of PI 3-kinase, which mediate GLUT4 translocation (23, 26, 37). Moreover, recent studies suggest that other pathway(s) that may be independent of PI 3-kinase or IRS-1 might have a major role in GLUT4 translocation (3, 20, 35). Another confounding observation is that other growth factors such as platelet-derived growth factor (PDGF) that are equally effective in activating PI 3-kinase do not significantly stimulate glucose transport. Since the majority of insulin-stimulated IRS-associated PI 3-kinase activity resides in the low-density microsomes (LDM), whereas PDGF activates PI 3-kinase recruited to the PDGF receptors in the PM, it has been proposed that IRS-associated PI 3-kinase targeted to a particular intracellular membrane compartment may be important for eliciting GLUT4 translocation (30, 33, 45).
Mammalian target of rapamycin (mTOR) (also known as FRAP, RAFT, and RAPT) is the mammalian counterpart of Saccharomyces cerevisiae TOR1 and TOR2 and is a member of the PI kinase-related kinase family, which includes MEC1, TEL1, RAD3, MEI-41, DNA-PK, ATM, ATR, and TRRAP (9, 41). Although these proteins contain a C-terminal PI kinase homology domain, none of these proteins has been demonstrated to have lipid kinase activity, and both yeast and mammalian TOR are Ser/Thr protein kinases. As part of a complex with cellular protein FKBP, a macrolide immunosuppressant, rapamycin, specifically inhibits TOR function by interacting with the FKBP-rapamycin binding domain in TOR, adjacent to the catalytic kinase domain (9, 41). TORs regulate diverse cellular effects, such as translation, transcription, and autophagy, thereby controlling cell growth and ultimately cell proliferation (9, 41). mTOR controls the mammalian translation machinery via activation of p70 S6 kinase and via inhibition of the eIF-4E inhibitor, 4E-BP1 (also known as PHAS-I) (9). Activation of p70 S6 kinase increases translation of 5′-terminal oligopyrimidine tract mRNAs, which largely encode ribosomal proteins and components of the translational apparatus. Phosphorylated 4E-BP1 dissociates from eIF-4E, thereby allowing cap-dependent translation of mRNAs containing a highly structured 5′ untranslated region. mTOR appears to be regulated downstream of PI 3-kinase/Akt upon stimulation with growth factors including insulin, although the direct link between Akt and mTOR remains to be proven (9, 40). Recent evidence indicates that p70 S6 kinase and 4E-BP1 phosphorylation are dependent on the availability of amino acids, in addition to a growth factor. Thus, mTOR acts as a sensor for amino acids, balancing the availability of nutrients and cell growth (9, 15, 19).
Insulin stimulation induces Ser/Thr phosphorylation of IRS-1, which is seen as a decrease in electrophoretic mobility on sodium dodecyl sulfate (SDS)-polyacrylamide gels (39). In addition, prolonged insulin stimulation causes gradual loss of IRS-1, which we and others have recently reported to be due to degradation by the proteasome (16, 38). We have further shown that a pathway sensitive to rapamycin regulates insulin-induced Ser/Thr phosphorylation and proteasomal degradation of IRS-1 (16). The insulin-induced degradation of IRS-1 plays a major role in down-regulation of insulin signaling, because either inhibition of mTOR with rapamycin or inhibition of the proteasome with the highly specific inhibitor lactacystin (14) enhances insulin-stimulated activation of Akt during prolonged insulin stimulation in parallel with the blockade of insulin-induced degradation of IRS-1 (16). Interestingly, however, rapamycin but not lactacystin enhanced insulin-stimulated 2-deoxyglucose (2-DOG) uptake (16), raising the possibility that glucose transport is negatively regulated after insulin stimulation by a mechanism different from that which down-regulates Akt activation. Insulin stimulation also induces subcellular redistribution of IRS-1 and IRS-2 from the LDM to the cytosol (17, 21). Ser/Thr phosphorylation of IRS proteins (17) or of other component(s) in the intracellular membranes (21) has been suggested to be involved in this phenomenon. However, the molecular mechanism and the functional role of the subcellular redistribution of IRS proteins are not well understood.
In this study, we show that the mTOR-dependent pathway that regulates insulin-induced Ser/Thr phosphorylation and degradation of IRS-1 also plays an important role in insulin-induced subcellular redistribution of IRS proteins. We further provide evidence for distinct mechanisms that are involved in negative regulation of insulin-stimulated glucose transport and Akt activation. We also identify mTOR as an important mediator for cross talk between nutritional signals and metabolic signals of insulin.
MATERIALS AND METHODS
Antibodies.
Anti-IRS-1 and anti-IRS-2 antibodies were purchased from Upstate Biotechnology (Lake Placid, N.Y.). Horseradish peroxidase (HRP)-conjugated monoclonal antiphosphotyrosine (PY20H) and anti-p85 antibodies were obtained from Transduction Laboratories (Lexington, Ky.). Phosphospecific and nonphosphospecific antibodies against Akt, p70 S6 kinase, and p44/42 MAP kinase were from New England Biolabs (Beverly, Mass.). Anti-4E-BP1 and anti-mTOR antibodies were from Zymed Laboratories (San Francisco, Calif.). HRP-conjugated anti-mouse and rabbit immunoglobulin G secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Reagents.
Rapamycin, wortmannin, and LY294002 were purchased from Sigma (St. Louis, Mo.). Lactacystin was obtained from Calbiochem (La Jolla, Calif.). PD98059 was obtained from New England Biolabs. Porcine insulin was kindly provided by the Lilly Research Laboratories (Indianapolis, Ind.). Dulbecco modified Eagle medium (DMEM), minimal essential medium (MEM), MEM Select-amine kit, and fetal calf serum (FCS) were purchased from GibcoBRL Life Technologies (Gaithersburg, Md.). Electrophoresis reagents were from Bio-Rad Laboratories (Hercules, Calif.). [1,2-3H]2-DOG was from NEN Life Science Products (Boston, Mass.). All other reagents and chemicals were from standard suppliers.
Cell culture.
3T3-L1 cells were obtained from the American Type Culture Collection and were cultured, maintained, and differentiated essentially as previously described (16). Briefly, cells were plated and grown for 2 days postconfluence in DMEM containing 25 mM glucoase supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% FCS in a 10% CO2 environment. Differentiation was then induced by changing the medium to the same medium but with 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 1 μM insulin for 3 days and the medium containing 0.8 μM insulin for another 3 days. The medium was then changed every 3 days. 3T3-L1 adipocytes were used for experiments between 13 and 16 days after initiation of differentiation, when more than 95% of the cells exhibited an adipocyte-like phenotype.
Human embryonic kidney 293 cells obtained from the American Type Culture Collection were cultured in DMEM containing 10% FCS in an atmosphere of 5% CO2.
Infection of recombinant adenovirus vectors.
The recombinant adenoviruses, adenovirus type 5 (Ad5)-p110CAAX containing bovine p110α cDNA with the CAAX motif at the COOH terminus and Ad5-CT that has no insert (12), were amplified in 293 cells, and viral stock solutions with a viral titer of >108 PFU/ml were prepared. 3T3-L1 adipocytes were infected with the vectors by incubating the cells at a multiplicity of infection of 50 PFU/cell, as described previously (16).
Amino acid treatment.
Amino acid-free MEM (0×) and MEM containing fourfold concentration of amino acids (4×) were prepared using MEM Select-amine kit according to the manufacturer's protocol (GibcoBRL Life Technologies). The amino acids and their concentrations in the standard MEM (1×) are as follows: arginine, 600 μM; cystine, 100 μM; glutamine, 2 mM; histidine, 200 μM; isoleucine, 400 μM; leucine, 400 μM; lysine, 397 μM; methionine, 101 μM; phenylalanine, 194 μM; threonine, 403 μM; tryptophan, 49 μM; tyrosine, 199 μM; and valine, 393 μM.
Subcellular fractionation.
3T3-L1 adipocytes were rinsed twice with phosphate-buffered saline and once with HES buffer (255 mM sucrose, 20 mM HEPES [pH 7.4], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 2 μg of aprotinin per ml, 50 ng of okadaic acid per ml) and immediately homogenized by 20 strokes with a motor-driven homogenizer in HES buffer at 4°C. The homogenates (two 10-cm-diameter dishes per condition) were subjected to subcellular fractionation as described previously (17, 21) to isolate PM, high-density microsomes (HDM), LDM, and cytosol with some modifications. Briefly, the homogenate was centrifuged at 19,000 × g for 20 min. The resulting supernatant was centrifuged at 41,000 × g for 20 min, yielding a pellet of HDM. The supernatant from this spin was centrifuged at 250,000 × g for 90 min, yielding a pellet of LDM. Remaining supernatant was concentrated by Centricon-30 (Amicon Inc., Beverly, Mass.) and used as cytosol. The pellet obtained from the initial spin was resuspended in HES buffer, layered onto a 1.12 M sucrose cushion, and centrifuged at 100,000 × g in a swing rotor for 60 min. A white fluffy band at the interface was collected and resuspended in HES buffer and centrifuged at 40,000 × g for 20 min, yielding a pellet of PM. All fractions were adjusted to a final protein concentration of 1 to 3 mg/ml and stored at −80°C. The protein concentrations of these fractions were measured by the Bradford method.
Immunoprecipitation and immunoblotting.
3T3-L1 adipocytes were solubilized in cell lysis buffer containing 20 mM Tris (pH 7.5), 140 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 2 mM Na3VO4, 50 mM sodium fluoride, 10 μg of aprotinin per ml, and 1 mM phenylmethylsulfonyl fluoride on ice. In some experiments, each subcellular fraction was solubilized in homogenization buffer supplemented with 1% Nonidet P-40. Immunoprecipitation with anti-IRS-1 antibody was performed by incubating the lysates with the antibody at 4°C overnight. The immune complexes were collected on protein G-Sepharose for 2 h at 4°C, washed three times with the lysis buffer, and boiled in Laemmli buffer. Samples were electrophoresed on SDS-7.5 or 12% polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with TBS-T (10 mM Tris [pH 7.6], 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat dry milk for immunoblotting with an anti-IRS-1, anti-IRS-2, or anti-mTOR antibody or with TBS-T containing 4% BSA for immunoblotting with anti-4E-BP1 antibody, incubated with the indicated antibodies for 1 h at room temperature, and then incubated with HRP-conjugated secondary antibody. For antiphosphotyrosine immunoblotting, the membrane was blocked in TBS-T containing 4% BSA overnight at 4°C and incubated with HRP-conjugated antiphosphotyrosine antibody (PY20H) for 1 h at room temperature. For detection of phosphorylated or nonphosphorylated Akt, p70 S6 kinase or p44/42 MAP kinase, the membrane was blocked with TBS-T containing 5% nonfat dry milk, incubated with the indicated antibody at 4°C overnight, and then incubated with HRP-conjugated secondary antibody. The proteins were visualized with enhanced chemiluminescence reagents according to the manufacturer's protocol (Amersham Pharmacia Biotech). In some experiments, the intensities of blots were quantitated with a scanning densitometer.
2-DOG uptake.
After serum starvation for 3 h, 3T3-L1 adipocytes were stimulated with 20 nM insulin for the periods of time indicated in the figures and figure legends. Unlabeled and labeled 2-DOG (0.1 mM, 0.74 kBq/well) were added to the cells in the KRP-HEPES buffer (10 mM HEPES [pH 7.4], 131.2 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 2.5 mM NaH2PO4) with 1% BSA at 37°C and incubated for 4 min. The reaction was stopped by adding 10 μM cytochalasin B and washing the cells with ice-cold phosphate-buffered saline three times. The cells were solubilized in 1 ml of a solution of 0.2 N NaOH and 0.2% SDS. The radioactivity was quantitated in a liquid scintillation counter. The nonspecific uptake and absorption values were determined by [3H]2DOG uptake in the presence of 10 μM cytochalasin B, and the results were corrected for these values. Nonspecific uptake and absorption were always less than 10% of total uptake.
Statistical analysis.
Data were analyzed by Student's t test. P values of <0.05 were considered statistically significant.
RESULTS
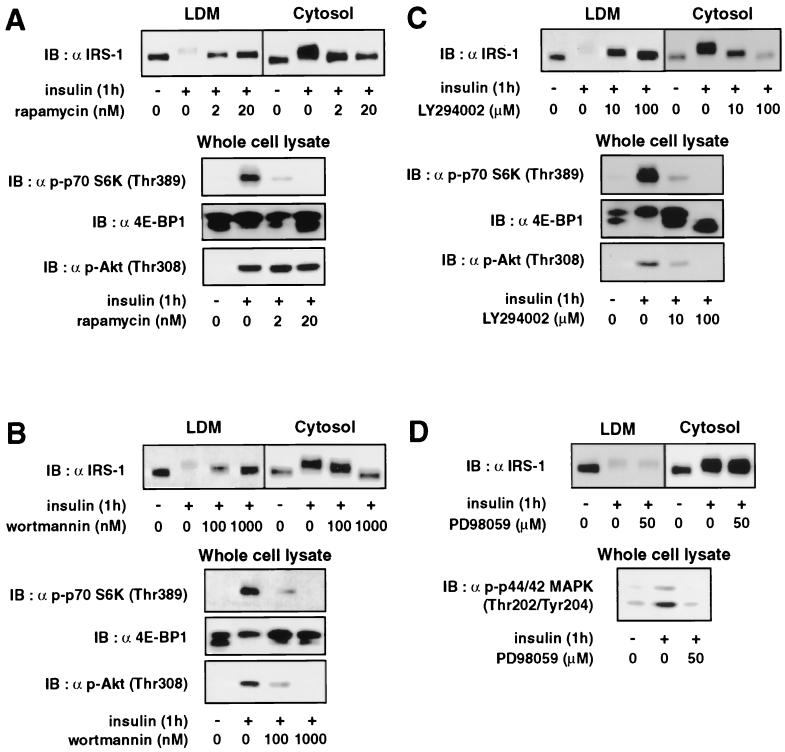
Insulin-induced subcellular redistribution of IRS-1 is inhibited by rapamycin and the PI 3-kinase inhibitors.
Our previous finding that prolonged insulin stimulation results in degradation of IRS-1 by the proteasome by a mechanism sensitive to rapamycin (16), together with the fact that insulin stimulation induces subcellular redistribution of IRS-1 from the LDM to the cytosol, led us to postulate that the insulin-induced redistribution of IRS-1 may be regulated by the mTOR pathway and facilitate the degradation of IRS-1 by the proteasome. To test this possibility, we first examined the role of mTOR pathway in the subcellular redistribution of IRS-1. Fully differentiated 3T3-L1 adipocytes were pretreated with the indicated concentrations of rapamycin, stimulated with insulin for 1 h, and then subjected to subcellular fractionation and immunoblotting with anti-IRS-1 antibody (Fig. 1A). As reported previously (6, 17, 21), IRS-1 was distributed in the LDM and cytosol, and insulin stimulation for 1 h resulted in a decrease in IRS-1 in the LDM and an increase in IRS-1 in the cytosol, as a result of insulin-induced redistribution of IRS-1 from the LDM to the cytosol (Fig. 1). IRS-1 was not detected in the PM or HDM either in the basal or insulin-stimulated state (data not shown). Rapamycin inhibited the insulin-induced redistribution of IRS-1 in a dose-dependent manner, reducing both the insulin-induced decrease in IRS-1 in the LDM and the increase in IRS-1 in the cytosol (Fig. 1A). To monitor the effectiveness of the inhibitor, the phosphorylation status of p70 S6 kinase and 4E-BP1, which are well-documented downstream elements of the mTOR signaling pathway, as well as that of Akt, which is immediately downstream of PI 3-kinase, were determined (Fig. 1). Insulin stimulation increased phosphorylation of p70 S6 kinase, as assessed by immunoblotting with anti-phospho-Thr389 p70 S6 kinase antibody (antibody against p70 S6 kinase phosphorylated at Thr389) and phosphorylation of 4E-BP1, which was shown by increased intensities of the upper bands and decreased intensities of the lower bands among the multiple bands detected by anti-4E-BP1 antibody immunoblotting (Fig. 1). Insulin stimulation also increased phosphorylation of Akt, as shown by immunoblotting with anti-phospho-Thr308 Akt antibody (Fig. 1). As expected, rapamycin inhibited the insulin-stimulated phosphorylation of p70 S6 kinase and 4E-BP1, but not Akt, in a dose-dependent manner (Fig. 1A). Thus, rapamycin inhibited insulin-stimulated redistribution of IRS-1 in parallel with the inhibition of insulin-stimulated activation of the mTOR pathway.
FIG. 1.
Effects of rapamycin, the PI 3-kinase inhibitors, and the MEK inhibitor on insulin-induced subcellular redistribution of IRS-1. 3T3-L1 adipocytes were serum starved for 16 h, incubated with the indicated concentration of rapamycin (A), wortmannin (B), LY294002 (C), or PD98059 (D) for 30 min, and then stimulated with 20 nM insulin for 1 h (+). The cells were homogenized and subjected to subcellular fractionation to yield the LDM and cytosol fractions. Proteins in each fraction were separated by SDS-PAGE and immunoblotted (IB) with anti-IRS-1 antibody (α IRS-1) (upper panels). Proteins in the whole-cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho-Thr389-p70 S6 kinase [α p-p70 S6K (Thr389)], anti-4E-BP1 [α 4E-BP1], anti-phospho-Thr308-Akt [α p-Akt (Thr308)], or anti-phospho-Thr202/Thr204-p44/42 MAP kinase antibody [α p-p44/42 MAPK (Thr202/Tyr204)2] (lower panels).
Since insulin-stimulated activation of the mTOR pathway has been reported to be regulated, at least in part, via the PI 3-kinase pathway, we next examined the effects of PI 3-kinase inhibitors wortmannin and LY294002 on the insulin-induced subcellular redistribution of IRS-1. The insulin-induced redistribution of IRS-1 was inhibited by either wortmannin or LY294002 in a dose-dependent manner, with concomitant dose-dependent inhibition of phosphorylation of p70 S6 kinase and 4E-BP1 as well as that of Akt (Fig. 1B and C). The inhibition of the insulin-induced redistribution of IRS-1 by rapamycin and the PI 3-kinase inhibitors was accompanied by inhibition of the insulin-induced mobility shift of IRS-1 (Fig. 1).
The MEK inhibitor PD98059 did not affect the insulin-stimulated redistribution or mobility shift of IRS-1 at the concentration that inhibited insulin-stimulated activation of p44/42 MAP kinase, as assessed by immunoblotting with anti-phospho-Thr202/Tyr204 p44/42 MAP kinase antibody (Fig. 1D). The proteasome inhibitor lactacystin also had no effect on the insulin-induced redistribution of IRS-1 (data not shown). None of these compounds affected the basal distribution of IRS-1 (data not shown). These results indicated that the insulin-induced subcellular redistribution of IRS-1 is regulated by the PI 3-kinase and mTOR-dependent pathway.
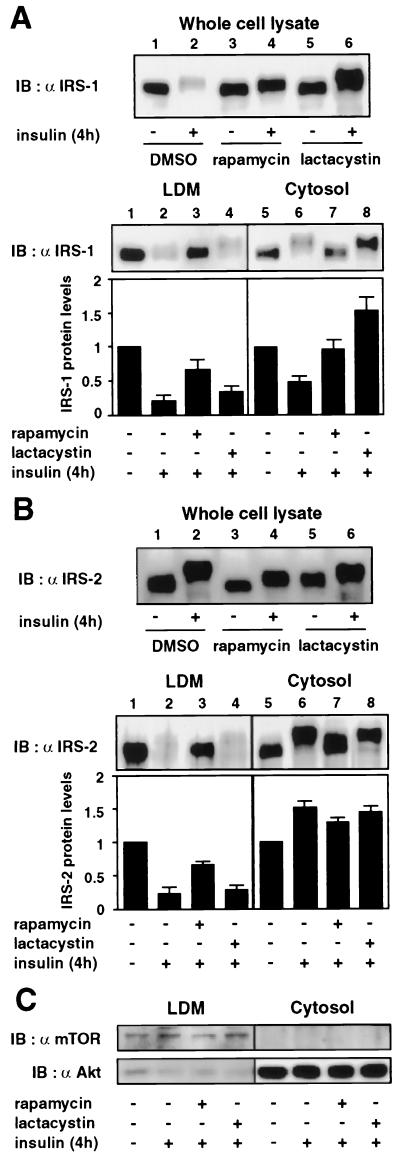
Rapamycin increases IRS-1 in both the LDM and cytosol, whereas lactacystin increases it only in the cytosol after prolonged insulin stimulation.
To evaluate the role of the insulin-induced subcellular redistribution of IRS-1 in its degradation by the proteasome, we next determined the effects of rapamycin and lactacystin on the subcellular distribution of IRS-1 after prolonged insulin stimulation (Fig. 2A). As we have previously reported (16), the total amount of IRS-1 in the whole-cell lysates was markedly decreased after 4 h of insulin stimulation (Fig. 2A, upper panel, lane 2), and rapamycin (Fig. 2A, upper panel, lane 4) and lactacystin (Fig. 2A, upper panel, lane 6) inhibited the insulin-induced decrease of IRS-1. The mobility shift of IRS-1 was increased by lactacystin (Fig. 2A, upper panel, lane 6). Subcellular fractionation of the cells showed that IRS-1 levels were decreased not only in the LDM (Fig. 2A, lower panel, lane 2) but also in the cytosol (Fig. 2A, lower panel, lane 6) from the basal levels of IRS-1 in each fraction, indicating that a large amount of IRS-1 translocated from the LDM to the cytosol was degraded after 4 h of insulin stimulation. The mobility shift of IRS-1 in the cytosol (Fig. 2A, lower panel, lane 6) was greater than that in the LDM (Fig. 2A, lower panel, lane 2). Rapamycin inhibited the mobility shift of IRS-1 and increased the IRS-1 level both in the LDM (Fig. 2A, lower panel, lane 3) and in the cytosol (Fig. 2A, lower panel, lane 7). In contrast, lactacystin did not significantly affect the insulin-induced decrease of IRS-1 in the LDM (Fig. 2A, lower panel, lane 4) but markedly increased the IRS-1 level in the cytosol to a level greater than that prior to insulin stimulation (Fig. 2A, lower panel, lane 8). These results indicated that inhibition of Ser/Thr phosphorylation and subcellular redistribution of IRS-1 by rapamycin results in inhibition of proteasomal degradation of IRS-1 in the cytosol and that lactacystin inhibits proteasomal degradation of IRS-1 in the cytosol without affecting the subcellular redistribution of IRS-1.
FIG. 2.
Effects of rapamycin and lactacystin on subcellular distribution of IRS-1 and IRS-2 after prolonged insulin stimulation. 3T3-L1 adipocytes were serum starved for 16 h, incubated with vehicle (0.1% dimethyl sulfoxide [DMSO]), 20 nM rapamycin, or 10 μM lactacystin for 30 min, and stimulated with 20 nM insulin for 4 h (+). Cells were homogenized and subjected to subcellular fractionation to yield the LDM and cytosol fractions. Proteins in the whole-cell lysates or each fraction were separated by SDS-PAGE and immunoblotted (IB) with anti-IRS-1 (α IRS-1) (A) or IRS-2 (α IRS-2) (B) antibody. Data were analyzed by densitometry and expressed as fold increase compared with the values for each fraction in control cells. Results are means ± standard errors for four independent experiments. Proteins in each fraction were separated by SDS-PAGE and immunoblotted with anti-mTOR (α mTOR) or anti-Akt (α Akt) antibody (C).
Rapamycin inhibits the mobility shift and subcellular redistribution of IRS-2 after prolonged insulin stimulation.
It has been reported that insulin stimulation also induces a mobility shift and subcellular redistribution of IRS-2 but does not significantly reduce its protein level (38). To evaluate the role of mTOR pathway in insulin-induced Ser/Thr phosphorylation and subcellular redistribution of IRS-2, we next determined the effects of rapamycin and lactacystin on the subcellular distribution of IRS-2 after prolonged insulin stimulation (Fig. 2B). Insulin stimulation for 4 h produced a mobility shift of IRS-2, which was greater than that of IRS-1, but did not significantly decrease IRS-2 levels in the cell lysates (Fig. 2B, upper panel, lane 2). However, insulin stimulation for a longer period (16 h) clearly decreased IRS-2 level (data not shown), indicating that the rate of insulin-induced degradation of IRS-2 is much less than that of IRS-1. The mobility shift of IRS-2 was decreased by rapamycin (Fig. 2B, upper panel, lane 4) but was not significantly affected by lactacystin (Fig. 2B, upper panel, lane 6). Neither rapamycin (Fig. 2B, upper panel, lane 4) nor lactacystin (Fig. 2B, upper panel, lane 6) significantly affected IRS-2 levels in the cell lysates. Subcellular fractionation revealed that the IRS-2 levels were decreased in the LDM (Fig. 2B, lower panel, lane 2) and slightly increased in the cytosol (Fig. 2B, lower panel, lane 6) after 4 h of insulin stimulation, indicating that IRS-2 was translocated from the LDM to the cytosol without significant degradation. Rapamycin inhibited the insulin-induced decrease of IRS-2 in the LDM (Fig. 2B, lower panel, lane 3) and did not significantly affect the IRS-2 level in the cytosol (Fig. 2B, lower panel, lane 7). Lactacystin did not affect the distribution of IRS-2 (Fig. 2B, lower panel, lanes 4 and 8). These results indicated that the mTOR-dependent pathway regulates insulin-induced Ser/Thr phosphorylation and subcellular redistribution but causes much less degradation of IRS-2.
We also determined the protein levels of mTOR and Akt in the subcellular fractions as controls for LDM-associated and cytosolic proteins, respectively (Fig. 2C). It has been reported that mTOR is enriched in cellular membranes (34) and is specifically localized to microsomal membranes in 3T3-L1 adipocytes (46). Consistent with these previous reports, mTOR was found exclusively in the LDM fraction, and the level of mTOR was not significantly affected by insulin or treatment with either rapamycin or lactacystin (Fig. 2C). As previously reported (21), most of the Akt was localized in the cytosol (Fig. 2C). Although insulin stimulation translocates a fraction of Akt to the PM, the level of Akt in the cytosol was not detectably affected by insulin as has been reported previously (21), and treatment with either rapamycin or lactacystin did not affect the level of Akt in the cytosol (Fig. 2C). These data provided evidence for the specificity of subcellular fractions and indicated that the treatment does not affect the level of protein in each fraction other than IRS-1 and IRS-2.
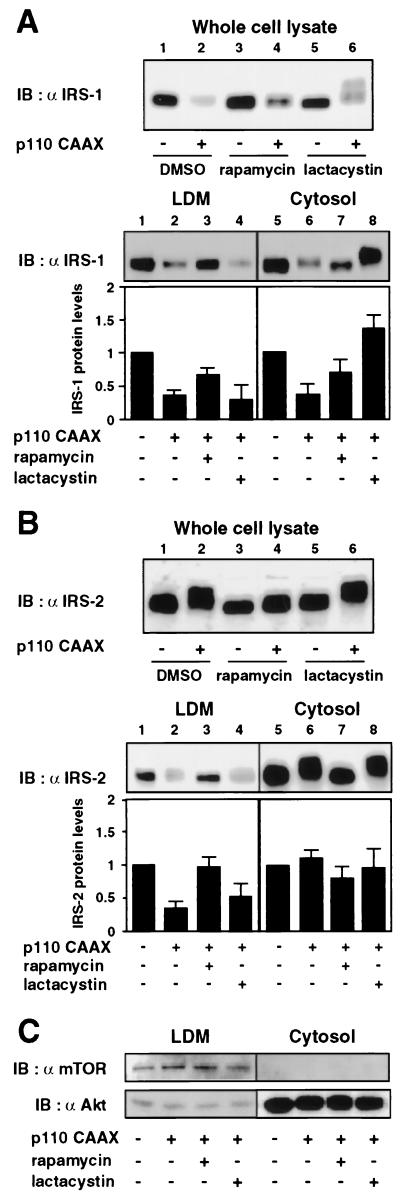
Rapamycin increases IRS-1 in both the LDM and cytosol, whereas lactacystin increases it only in the cytosol in cells expressing p110CAAX.
In a previous study (16), we reported that constitutive activation of the PI 3-kinase pathway by expression of p110CAAX, a membrane-targeted form of the p110 subunit of PI 3-kinase, is sufficient to induce Ser/Thr phosphorylation and degradation of IRS-1 and that these effects are regulated by a mechanism sensitive to rapamycin. To test whether activation of PI 3-kinase pathway is also sufficient to induce subcellular redistribution of IRS-1, we examined the effects of rapamycin and lactacystin on the subcellular distribution of IRS-1 in cells expressing p110CAAX (Fig. 3A). Forty-eight hours after infection of 3T3-L1 adipocytes with Ad5-p110CAAX, cells were treated with either rapamycin or lactacystin for another 16 h and then analyzed by immunoblotting of the total cell lysates and the subcellular fractions. As we have previously reported (16), IRS-1 levels in the cell lysates were lower in cells expressing p110CAAX (Fig. 3A, upper panel, lane 2) than those in cells infected with control adenovirus (Fig. 3A, upper panel, lane 1). Rapamycin partially restored the decrease of IRS-1 (Fig. 3A, upper panel, lane 4), confirming our previous finding (16). Lactacystin also partially restored the total IRS-1 levels and resulted in an appearance of IRS-1 with a marked retardation of the mobility (Fig. 3A, upper panel, lane 6). Subcellular fractionation of the p110CAAX-expressing cells showed that IRS-1 levels were decreased both in the LDM (Fig. 3A, lower panel, lane 2) and in the cytosol (Fig. 3A, lower panel, lane 6) compared to those in cells infected with control adenovirus. The mobility shift of IRS-1 in the cytosol (Fig. 3A, lower panel, lane 6) was greater than that in the LDM (Fig. 3A, lower panel, lane 2). Rapamycin inhibited the mobility shift and partially restored IRS-1 levels both in the LDM (Fig. 3A, lower panel, lane 3) and in the cytosol (Fig, 3A, lower panel, lane 7). In contrast, lactacystin increased the IRS-1 level and its mobility shift in the cytosol (Fig. 3A, lower panel, lane 8) but did not significantly affect them in the LDM (Fig. 3A, lower panel, lane 4). These results are similar to what we have seen for the cells treated with insulin for a prolonged period and indicated that activation of the PI 3-kinase pathway is sufficient to induce subcellular redistribution of IRS-1 and degradation of the IRS-1 by a mechanism sensitive to rapamycin.
FIG. 3.
Effects of rapamycin and lactacystin on subcellular distribution of IRS-1 and IRS-2 in p110CAAX-expressing cells. 3T3-L1 adipocytes were infected with either Ad5-CT or Ad5-p110CAAX at a multiplicity of infection of 50 PFU/cell. At 48 h after infection, cells were incubated in serum-free medium with vehicle (0.1% dimethyl sulfoxide [DMSO]), 20 nM rapamycin, or 10 μM lactacystin for another 16 h. Cells were homogenized and subjected to subcellular fractionation to yield the LDM and cytosol fractions. Proteins in the whole-cell lysates or each fraction were separated by SDS-PAGE and immunoblotted (IB) with anti-IRS-1 (α IRS-1) (A) or IRS-2 (α IRS-2) (B) antibody. Data were analyzed by densitometry and expressed as fold increase compared with the values for each fraction in control cells infected with Ad5-CT. Results are means ± standard errors for three independent experiments. Proteins in each fraction were separated by SDS-PAGE and immunoblotted with anti-mTOR (α mTOR) or anti-Akt (α Akt) antibody (C).
Rapamycin inhibits the mobility shift and subcellular redistribution of IRS-2 in cells expressing p110CAAX.
On the other hand, expression of p110CAAX did not significantly affect IRS-2 levels in the whole-cell lysates, while it induced a mobility shift of IRS-2 (Fig. 3B, upper panel, lane 2) which was greater than that of IRS-1. In some experiments, however, the IRS-2 level was slightly decreased in p110CAAX-expressing cells (data not shown), which was consistent with the previous report (13) and the observation that 16 h of insulin stimulation clearly decreased the IRS-2 level, indicating that expression of p110CAAX induces degradation of IRS-2, the rate of which is much lower than that for IRS-1. Rapamycin inhibited the p110CAAX-induced mobility shift of IRS-2 but did not significantly affect its protein level (Fig. 3B, upper panel, lane 4). Lactacystin did not significantly affect the mobility shift or the protein level of IRS-2 (Fig. 3B, upper panel, lane 6). Subcellular fractionation indicated that expression of p110CAAX decreased the IRS-2 level in the LDM (Fig. 3B, lower panel, lane 2) but did not significantly affect it in the cytosol (Fig. 3B, lower panel, lane 6), indicating that IRS-2 was translocated from the LDM into the cytosol without significant degradation. The mobility shift of IRS-2 in the cytosol (Fig. 3B, lower panel, lane 6) was greater than that in the LDM (Fig. 3B, lower panel, lane 2). Rapamycin inhibited the mobility shift and the decrease of IRS-2 level in the LDM (Fig. 3B, lower panel, lane 3) but did not affect the IRS-2 level in the cytosol (Fig. 3B, lower panel, lane 7). Lactacystin did not affect the distribution and the mobility shift of IRS-2 (Fig. 3B, lower panel, lanes 4 and 8). These results indicated that activation of PI 3-kinase pathway is sufficient to induce Ser/Thr phosphorylation and subcellular redistribution but causes much less degradation of IRS-2 by a mechanism sensitive to rapamycin.
The levels of mTOR and Akt in each fraction were not affected by the expression of p110CAAX or treatment with either rapamycin or lactacystin, confirming the specificity of the subcellular fraction and the specific effect of the treatment on IRS-1 and IRS-2 (Fig. 3C).
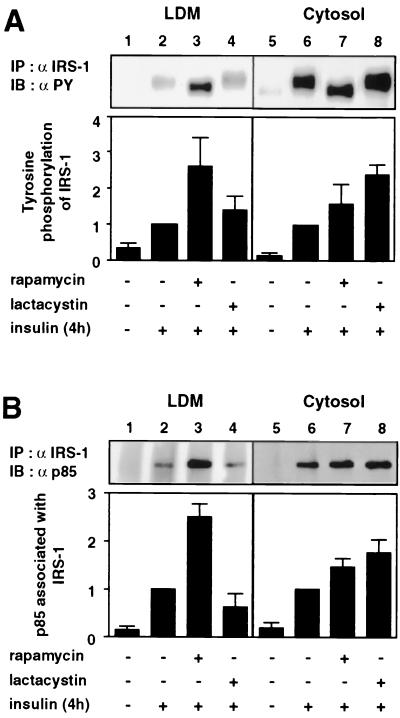
Rapamycin increases tyrosine-phosphorylated IRS-1 and the p85 subunit of PI 3-kinase associated with IRS-1 in both the LDM and cytosol, whereas lactacystin increases them only in the cytosol after prolonged insulin stimulation.
We have previously reported that rapamycin but not lactacystin enhances insulin-stimulated 2-DOG uptake, whereas both rapamycin and lactacystin increase activation of Akt during prolonged insulin stimulation (16). The above result that treatment with either rapamycin or lactacystin during prolonged insulin stimulation resulted in different subcellular localizations of IRS-1 suggested that the differential effects of rapamycin and lactacystin may result from the different localizations of IRS-1-associated signaling components in the cell. To substantiate this hypothesis, we next determined subcellular distribution of tyrosine-phosphorylated IRS-1 and the p85 subunit of PI 3-kinase associated with IRS-1 after treatment with either rapamycin or lactacystin during prolonged insulin stimulation. Proteins in each fraction were immunoprecipitated with anti-IRS-1 antibody and analyzed by immunoblotting with antiphosphotyrosine or anti-p85 antibody (Fig. 4). After 4 h of insulin stimulation, rapamycin increased tyrosine-phosphorylated IRS-1 both in the LDM (Fig. 4A, lane 3) and in the cytosol (Fig. 4A, lane 7), whereas lactacystin increased it mostly in the cytosol (Fig. 4A, lane 8). Similarly, rapamycin increased p85 associated with IRS-1 both in the LDM (Fig. 4B, lane 3) and in the cytosol (Fig. 4B, lane 7), whereas lactacystin increased it only in the cytosol (Fig. 4B, lane 8). Therefore, the levels of tyrosine-phosphorylated IRS-1 and IRS-1-associated PI 3-kinase were affected by rapamycin and lactacystin, apparently in parallel with the level of IRS-1 in each fraction.
FIG. 4.
Effects of rapamycin and lactacystin on subcellular distribution of tyrosine-phosphorylated IRS-1 and IRS-1-associated p85 after prolonged insulin stimulation. 3T3-L1 adipocytes were serum starved for 16 h, incubated with vehicle (0.1% dimethyl sulfoxide [DMSO]), 20 nM rapamycin, or 10 μM lactacystin for 30 min, and stimulated with 20 nM insulin for 4 h (+). The cells were homogenized and subjected to subcellular fractionation to yield the LDM and cytosol fractions. Proteins in each fraction were immunoprecipitated (IP) with anti-IRS-1 antibody (α IRS-1), separated by SDS-PAGE, and immunoblotted (IB) with antiphosphotyrosine antibody (α PY) (A) or anti-p85 antibody (α p85) (B). Data were analyzed by densitometry and expressed as fold increase compared with the values for each fraction in the cells stimulated with insulin in the presence of vehicle only. Results are means ± standard errors for three independent experiments.
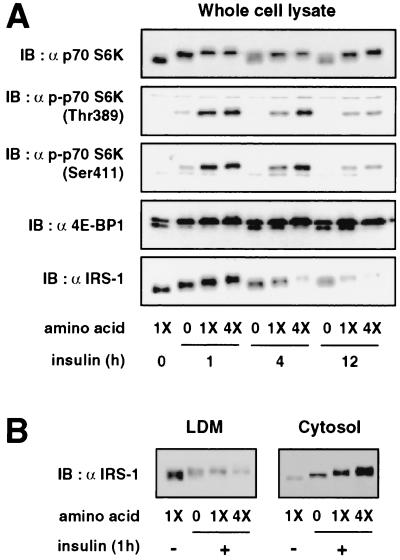
The amino acid deprivation reduces and the amino acid excess enhances insulin-stimulated phosphorylation of p70 S6 kinase and 4E-BP1.
Recent evidence indicates that, in addition to mitogenic signals by various growth factors, amino acid availability regulates the mTOR signaling pathway (9, 15, 19). Therefore, amino acid availability may influence the insulin-stimulated mTOR signaling pathway, thereby modulating insulin-induced Ser/Thr phosphorylation and the subcellular redistribution and degradation of IRS-1, and ultimately control insulin action. To address this possibility, we first tested whether amino acid availability affects insulin-stimulated activation of the mTOR pathway by examining the effects of the deprivation or excess of amino acids on the insulin-stimulated phosphorylation of p70 S6 kinase and 4E-BP1 (Fig. 5A). Activation of p70 S6 kinase, as shown by the retardation of its electrophoretic mobility and by phosphorylation of Thr389 and Ser411, was stimulated by insulin and gradually declined during prolonged insulin stimulation (Fig. 5A). Similarly, phosphorylation of 4E-BP1, as assessed by the relative intensities of the multiple immunoreactive bands, was stimulated by insulin and gradually returned to the basal state during prolonged insulin stimulation (Fig. 5A). The deprivation of amino acids attenuated the insulin-stimulated phosphorylation of p70 S6 kinase and 4E-BP1 (Fig. 5A). Conversely, fourfold excess amino acids augmented them (Fig. 5A). The amino acid excess in the absence of insulin only slightly increased phosphorylation of p70 S6 kinase and 4E-BP1 (data not shown). These results indicated that the amino acid deprivation attenuates and amino acid excess enhances insulin-stimulated activation of the mTOR pathway.
FIG. 5.
Effects of amino acid availability on the insulin-induced phosphorylation of p70 S6 kinase and 4E-BP1 and on electrophoretic mobility shift, proteasomal degradation, and subcellular redistribution of IRS-1. 3T3-L1 adipocytes were serum starved for 16 h, incubated in serum-free MEM without amino acids (0) or with standard concentrations of amino acids (1X) or with fourfold excess (4X) of amino acids for 1 h, and stimulated with 20 nM insulin for the times indicated in the figure. (A) Proteins in the whole-cell lysates were separated by SDS-PAGE and analyzed by immunoblotting (IB) with antibody against nonphosphospecific p70 S6 kinase [α p70 S6K], p70 S6 kinase with Thr389 or Ser411 phosphorylated [α p70 S6K(Thr389) or (Ser411)], 4E-BP1 [α 4E-BP1], or IRS-1 [α IRS-1]. (B) Cells were homogenized and subjected to subcellular fractionation to yield the LDM and cytosol fractions. Proteins in each fraction were separated by SDS-PAGE and immunoblotted with anti-IRS-1 antibody. Representative immunoblots for three independent experiments are shown.
The amino acid deprivation reduces and amino acid excess enhances insulin-induced mobility shift, degradation, and redistribution of IRS-1.
We next examined whether the alterations in the insulin-stimulated activation of the mTOR pathway by the deprivation or excess of amino acids influence insulin-induced Ser/Thr phosphorylation and proteasomal degradation and subcellular redistribution of IRS-1. As shown in Fig. 5A, the insulin-induced mobility shift of IRS-1, maximally induced at 1 h after insulin stimulation (16), was diminished by the amino acid deprivation and augmented by the amino acid excess. Similarly, IRS-1 degradation, clearly observed after 4 and 12 h of insulin stimulation (16), was slowed by the amino acid deprivation and was accelerated by excess amino acids (Fig. 5A). Subcellular fractionation of the cells showed that the insulin-induced redistribution of IRS-1 after 1 h of insulin stimulation, which was seen as the decrease in IRS-1 in the LDM and the increase in IRS-1 in the cytosol, was partially inhibited by the amino acid deprivation and was enhanced by excess amino acids (Fig. 5B).
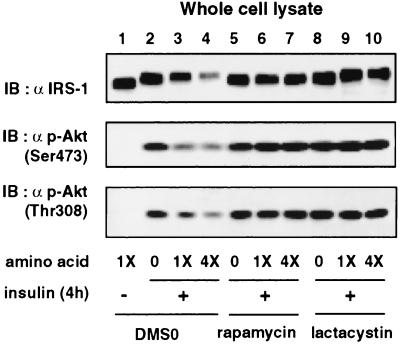
The amino acid deprivation enhances and excess of amino acids attenuates insulin-stimulated activation of Akt after prolonged insulin stimulation.
We have previously shown that degradation of IRS-1 by the proteasome down-regulates activation of Akt during prolonged insulin stimulation (16). Therefore, we next examined whether the alterations of the insulin-induced degradation of IRS-1 by the deprivation or excess of amino acids affect insulin-stimulated activation of Akt (Fig. 6). Activation of Akt, as assessed by phosphorylation of the two regulatory sites of Akt, Ser473 and Thr308, after 4 h of insulin stimulation was increased by amino acid deprivation (Fig. 6, lane 2) and was reduced by excess amino acids (Fig. 6, lane 4), apparently in parallel with the effects on IRS-1 protein levels. The protein levels of Akt were not affected by the chronic insulin treatment or differences in amino acid concentration (data not shown). We also determined the effects of amino acid availability on the phosphorylation of Akt in the presence of either rapamycin or lactacystin (Fig. 6). As we have previously described (16), rapamycin and lactacystin enhanced activation of Akt to comparable extents, in parallel with the comparable inhibition of the insulin-induced degradation of IRS-1 (Fig. 6, lanes 6 and 9). In the presence of either rapamycin or lactacystin, the deprivation or excess of amino acids did not further affect phosphorylation of Akt, and they did not affect protein levels of IRS-1 (Fig. 6), while in the presence of lactacystin, the amino acid deprivation reduced and the amino acid excess augmented the mobility shift of IRS-1 (Fig. 6, lanes 8 and 10). These results indicated that amino acid availability affects insulin-stimulated activation of Akt via the mTOR pathway in parallel with the effects on IRS-1 degradation.
FIG. 6.
Effect of amino acid availability on insulin-stimulated phosphorylation of Akt. 3T3-L1 adipocytes were serum starved for 16 h, incubated in serum-free MEM without amino acids (0) or with standard concentrations of amino acids (1X) or with 4-fold excess of amino acids (4X) for 1 h, and stimulated with 20 nM insulin for 4 h (+). Cells were pretreated with vehicle (0.1% dimethyl sulfoxide [DMSO]), 20 nM rapamycin, or 10 μM lactacystin for 30 min prior to the insulin stimulation. Proteins in the whole-cell lysates were separated by SDS-PAGE and analyzed by immunoblotting (IB) with anti-IRS-1 antibody (α IRS-1) or antibody against Akt (Akt phosphorylated at Ser473 or Thr308) [α p-Akt (Ser473) or (Thr308)]. Representative immunoblots for three independent experiments are shown.
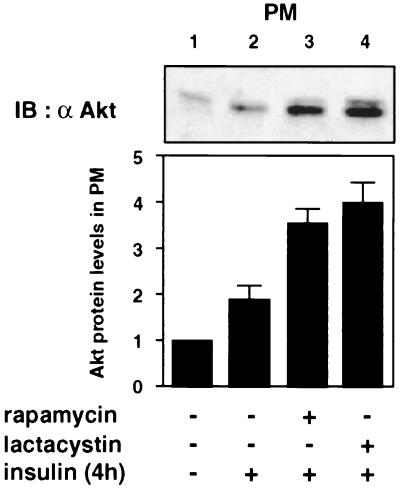
Rapamycin and lactacystin increase the amount of Akt in the PM to comparable extents after prolonged insulin stimulation.
We next examined whether the different subcellular distributions of IRS-1-associated PI 3-kinase after treatment with either rapamycin or lactacystin during prolonged insulin stimulation affect insulin-induced translocation of Akt to the PM, which has been suggested to be one of the necessary processes for activation of Akt (1, 2, 7, 11). In the basal state, Akt was located mostly in the cytosol (Fig. 2C). Insulin stimulation for 4 h increased the amount of Akt in the PM (Fig. 7), indicating that Akt was translocated to the PM. Rapamycin and lactacystin increased the levels of Akt localized in the PM to comparable extents (Fig. 7, lanes 3 and 4).
FIG. 7.
Effects of rapamycin and lactacystin on insulin-induced translocation of Akt to the PM after prolonged insulin stimulation. 3T3-L1 adipocytes were serum starved for 16 h, incubated with vehicle (0.1% dimethyl sulfoxide [DMSO]), 20 nM rapamycin, or 10 μM lactacystin for 30 min, and stimulated with 20 nM insulin for 4 h (+). Cells were homogenized and subjected to subcellular fractionation to yield the PM fraction. Proteins in the fraction were separated by SDS-PAGE and immunoblotted (IB) with anti-Akt antibody (α Akt). Data were analyzed by densitometry and expressed as fold increase compared with the values in control cells. Results are means ± standard errors for three independent experiments.
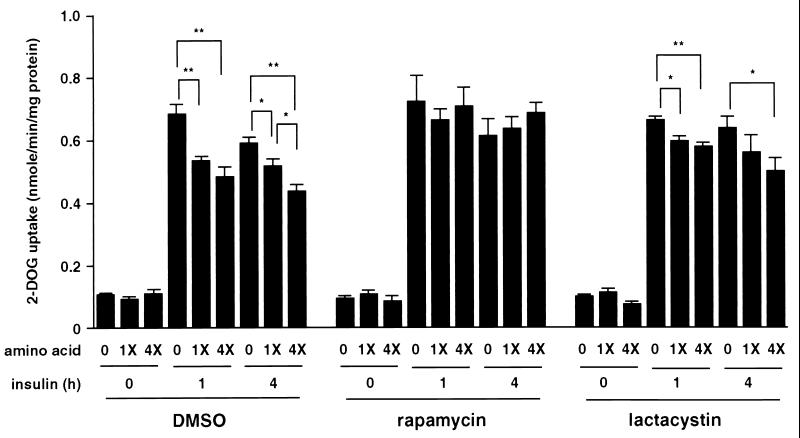
The amino acid deprivation increases and the amino acid excess decreases insulin-stimulated 2-DOG uptake.
Finally, the effect of amino acid availability on the insulin-stimulated 2-DOG uptake in the absence or presence of either rapamycin or lactacystin was determined (Fig. 8). Without treatment with rapamycin or lactacystin, the amino acid deprivation increased and the amino acid excess decreased 2-DOG uptake at 1 or 4 h after insulin stimulation (Fig. 8). As we have previously reported (16), rapamycin but not lactacystin enhanced insulin-stimulated 2-DOG uptake (Fig. 8). In the presence of rapamycin, insulin-stimulated 2-DOG uptake was not further affected by the deprivation or excess of amino acids (Fig. 8). However, in the presence of lactacystin, the levels of insulin-stimulated 2-DOG uptake at 1 or 4 h after insulin stimulation were greater when the cells were deprived of amino acids and were smaller when they were treated with excess amino acids (Fig. 8). The basal levels of 2-DOG uptake were not affected by the deprivation or excess of amino acids either in the absence or presence of rapamycin or lactacystin (Fig. 8). These results indicated that amino acid availability affects insulin-stimulated 2-DOG uptake via the mTOR pathway in parallel with the effects on IRS-1 redistribution rather than its degradation.
FIG. 8.
Effect of amino acid availability on insulin-stimulated 2-DOG uptake. 3T3-L1 adipocytes were incubated in serum-free MEM without amino acids (0) and with standard concentration of amino acids (1X) or with fourfold excess (4X) of amino acids for 3 h and pretreated with vehicle (0.1% dimethyl sulfoxide [DMSO]), 20 nM rapamycin, or 10 μM lactacystin for 30 min, and stimulated with 20 nM insulin for the indicated times. [3H]2-DOG uptake was measured as described in Materials and Methods. Results are shown as means ± standard errors for four independent experiments. (*, P < 0.05; **, P < 0.01)
DISCUSSION
We demonstrated in this study that the mTOR-dependent pathway that regulates insulin-induced Ser/Thr phosphorylation and degradation of IRS-1 (16) also plays an important role in modulating the subcellular distribution of IRS-1 upon insulin stimulation. Thus, rapamycin as well as the two structurally different PI 3-kinase inhibitors inhibited insulin-induced redistribution of IRS-1 from the LDM to the cytosol in parallel with the inhibition of insulin-stimulated phosphorylation of p70 S6 kinase and 4E-BP1, which are well-documented signaling components regulated by mTOR. Additionally, the amino acid deprivation decreased and the amino acid excess increased insulin-induced subcellular redistribution as well as Ser/Thr phosphorylation and degradation of IRS-1, also in parallel with the effects on insulin-stimulated phosphorylation of p70 S6 kinase and 4E-BP1. The mechanism by which amino acid concentration regulates phosphorylation of p70 S6 kinase and 4E-BP1 is poorly defined, although tRNA aminoacylation was suggested to be involved (19). However, a truncation mutant of p70 S6 kinase that is resistant to inhibition by rapamycin is also resistant to inhibition by amino acid withdrawal, indicating that amino acid availability, like rapamycin, may affect p70 S6 kinase and 4E-BP1 via mTOR or an mTOR-controlled downstream element (15). Furthermore, the amino acid-dependent regulation of the mTOR pathway does not seem to involve the FKBP-rapamycin binding domain of mTOR, since amino acid supplementation activates p70 S6 kinase in the presence of rapamycin in cells constitutively expressing a rapamycin-resistant mutant of mTOR, which does not interact with the FKBP-rapamycin complex (19). Therefore, the present data that rapamycin and amino acid treatment, the two different ways to manipulate insulin-stimulated activation of the mTOR pathway, affected insulin-induced subcellular redistribution as well as Ser/Thr phosphorylation and degradation of IRS-1 validate the involvement of the mTOR-dependent pathway in the regulation of these phenomena.
The mTOR pathway involved in insulin-induced redistribution of IRS-1 seems to be, at least in part, regulated via the PI 3-kinase pathway but not via the ras/MAP kinase pathway, because the PI 3-kinase inhibitors inhibited the IRS-1 redistribution with a concomitant inhibition of Akt phosphorylation, which is immediately downstream of PI 3-kinase, and inhibition of p44/42 MAP kinase by the MEK inhibitor had no effect on the IRS-1 redistribution. Furthermore, constitutive activation of the PI 3-kinase pathway by expression of p110CAAX was sufficient to induce IRS-1 redistribution through a mechanism sensitive to rapamycin. However, since relatively high concentrations of the PI 3-kinase inhibitors were required to completely inhibit insulin-induced subcellular redistribution of IRS-1, the inhibitory effects of the PI 3-kinase inhibitors may be due in part to direct inhibition of mTOR, as has been reported previously (4).
The finding that the extent of insulin-induced IRS-2 degradation was markedly less than that of IRS-1 is consistent with the finding of a previous report (38) and suggests that the structural difference of IRS-1 and IRS-2 affects the rate of insulin-induced degradation. However, our results indicate that the mechanisms governing Ser/Thr phosphorylation and subcellular redistribution of IRS-1 also apply to IRS-2, because insulin and p110CAAX induced a mobility shift and subcellular redistribution of IRS-2, which were inhibited by rapamycin. The greater mobility shift of IRS-2 compared with that of IRS-1 seems to result from the failure of the Ser/Thr-phosphorylated IRS-2 to be degraded. Thus, after prolonged insulin stimulation or in cells expressing p110CAAX, lactacystin did not further increase the mobility shift of IRS-2, while it increased the mobility shift of IRS-1 as a result of the inhibition of the degradation of Ser/Thr-phosphorylated IRS-1. Therefore, these results indicate that the structural difference of IRS-1 and IRS-2 may affect a process of degradation required after these proteins are Ser/Thr phosphorylated and translocated into the cytosol. Because IRS-1 is the major tyrosine-phosphorylated IRS protein induced by insulin stimulation in 3T3-L1 adipocytes (39), it seems unlikely that subcellular localization of IRS-2 has a significant role in the regulation of insulin signaling in these cells. In support of this, subcellular distribution of pp185 (which includes tyrosine-phosphorylated IRS-1 and IRS-2 in these cells) was similar to that of tyrosine-phosphorylated IRS-1 in cells treated with either rapamycin or lactacystin during prolonged insulin stimulation (data not shown). However, in the cell type in which IRS-2 is dominant, it is possible that the subcellular redistribution of IRS-2 regulated by the mTOR pathway influences insulin signaling.
Since the insulin-induced subcellular redistribution and the mobility shift of IRS proteins are simultaneously affected by rapamycin or amino acid availability, these two phenomena seem to be closely related. The mobility shift of IRS proteins was greater in the cytosol than in the LDM after prolonged insulin stimulation or in cells expressing p110CAAX. One possible explanation for this observation is that the mTOR pathway plays a role in Ser/Thr phosphorylation of IRS proteins in the LDM, which in turn facilitates their redistribution into the cytosol. Alternatively, activation of the mTOR pathway may result in Ser/Thr phosphorylation of a component(s) in the LDM other than IRS proteins, as has been suggested previously (21), which regulates the release of IRS proteins into the cytosol, where they are Ser/Thr phosphorylated. In either case, a Ser/Thr kinase regulated by mTOR or a Ser/Thr phosphatase, which may be negatively regulated by TORs and involved in the effect of rapamycin (10, 22, 29, 32), seems to mediate such phosphorylation events responsible for the redistribution of IRS proteins.
The present data suggest that insulin-induced subcellular redistribution of IRS-1 may play an important role in its degradation by the proteasome, because after prolonged insulin stimulation or in the cells expressing p110CAAX, inhibition of the subcellular redistribution of IRS-1 by rapamycin resulted in inhibition of IRS-1 degradation in the cytosol. One study showed that IRS-1 was ubiquitinated prior to insulin stimulation and that insulin stimulation did not further increase the ubiquitination of IRS-1 (38). If this were the case, the release of the already polyubiquitinated IRS-1 into the cytosol would likely permit interaction with the proteasome in the cytosol and facilitate subsequent degradation. However, another report showed that, in COS7 cells transfected with epitope-tagged IRS-1 and ubiquitin, ubiquitination of IRS-1 was increased by stimulation with IGF-1 (27). Similarly, exposure of prostate epithelial cells to IGF-1 led to an increase in ubiquitin content of IRS-1, which was accompanied by a reduction in IRS-1 levels (47). Therefore, in addition to the insulin-induced redistribution, the increase in ubiquitination of IRS-1, which is likely to be regulated by the mTOR-dependent Ser/Thr phosphorylation of IRS-1, may also be important in the degradation by the proteasome. Based on calculation of the protein contents in the LDM and cytosol fractions, the percentage of the LDM-associated IRS-1 was 50 to 65% of the total IRS-1 under basal conditions, which is consistent with the findings in the previous reports (17, 21). After 4 h of insulin stimulation, the total IRS-1 levels in the whole-cell lysates decreased by approximately 80%, which was greater than the original percentage of IRS-1 in the LDM. This suggests that IRS-1 localized in the cytosol prior to insulin stimulation is also degraded by the proteasome after insulin stimulation and that the originally cytosolic IRS-1 may be degraded by the proteasome, presumably through the mTOR-dependent Ser/Thr phosphorylation and subsequent modification. On the other hand, Ser/Thr phosphorylation and subcellular redistribution may work together to facilitate degradation of the LDM-associated IRS-1 by increasing ubiquitination or some other modification and by allowing the modified IRS-1 to interact with the proteasome, respectively.
Our previous observations that insulin-stimulated 2-DOG uptake is enhanced by rapamycin but not by lactacystin, while Akt phosphorylation is increased by either rapamycin or lactacystin during prolonged insulin stimulation (16) raised the possibility that insulin-stimulated glucose transport may be negatively regulated via the mTOR pathway by a mechanism other than degradation of IRS-1 which down-regulates Akt activation. We have previously shown that, in the presence of lactacystin, the mobility shift of IRS-1 does not change during prolonged insulin stimulation (16), suggesting that such modified form of IRS-1 may not target to the intracellular location appropriate for eliciting GLUT4 translocation. Consistent with this hypothesis, we found in this study that IRS-1 molecules that have been Ser/Thr phosphorylated are accumulated in the cytosol in the presence of lactacystin. On the other hand, rapamycin inhibited Ser/Thr phosphorylation and subcellular redistribution of IRS-1 and, as a consequence, degradation of IRS-1, resulting in increases in IRS-1 levels in both the LDM and cytosol. Accordingly, rapamycin increased tyrosine-phosphorylated IRS-1 and the p85 subunit of PI 3-kinase associated with IRS-1 in both the LDM and cytosol, while lactacystin increased them only in the cytosol. Given the ability of rapamycin but not lactacystin to enhance insulin-stimulated 2-DOG uptake, the enhancement of insulin-stimulated 2-DOG uptake by rapamycin might be explained by the increased level of IRS-1-associated PI 3-kinase in the LDM. This interpretation agrees with the hypothesis that insulin-stimulated GLUT4 translocation and, hence, glucose transport may be dependent on the activation of PI 3-kinase in a particular intracellular membrane compartment (30, 33).
Additionally, we have shown that the amino acid deprivation, which also inhibits insulin-induced IRS-1 redistribution, enhances insulin-induced 2-DOG uptake and, conversely, the amino acid excess, which augments IRS-1 redistribution, decreases it. Furthermore, the amino acid deprivation and excess affected 2-DOG uptake even when IRS-1 degradation was inhibited by lactacystin, while they did not when the IRS-1 redistribution was inhibited by rapamycin. These findings suggest that amino acid availability may regulate insulin-stimulated glucose transport through the effects on IRS-1 redistribution rather than degradation and are consistent with the idea that IRS-1-associated PI 3-kinase localized at the LDM might be important in the regulation of glucose transport. Taken together, our data suggest that the mTOR pathway may negatively regulate insulin-stimulated glucose transport by decreasing the level of IRS-1-associated PI 3-kinase localized at the LDM through subcellular redistribution of IRS-1.
However, although rapamycin completely inhibits IRS-1 redistribution, whereas lactacystin has no effect, the different effects of rapamycin and lactacystin on insulin-stimulated 2-DOG uptake were not so great, and the effects of amino acid availability were small. In this regard, it should be noted that insulin-stimulated 2-DOG uptake does not significantly decrease even after 4 h of insulin stimulation (Fig. 8), despite a marked decrease in the total IRS-1 level due to degradation (Fig. 2A). This suggests that a large fraction of IRS-1 molecules either associated with the LDM or present in the cytosol may not be involved in stimulation of glucose transport and that it may be only a small fraction of LDM-associated IRS-1 that is required for GLUT4 translocation. This fraction may decrease to only a small extent during insulin stimulation, despite a marked decrease in the total LDM-associated IRS-1. Accordingly, inhibition of IRS-1 redistribution by rapamycin or amino acid deprivation increases glucose transport to only a small extent. Another possibility is that insulin-stimulated glucose transport may be regulated by multiple mechanisms, one of which is dependent on IRS-1-associated PI 3-kinase localized at the LDM, but the other may be independent of IRS-1-associated PI 3-kinase, as several recent reports (3, 20) have suggested. It also remains to be tested whether the manipulation of insulin-stimulated activation of the mTOR pathway by rapamycin or amino acid treatment may affect such an IRS-1-independent mechanism of glucose transport.
Unlike 2-DOG uptake, activation of Akt does not seem to be affected by the distribution of IRS-1-associated PI 3-kinase between the LDM and the cytosol but instead is dependent on its total levels in both fractions and, therefore, is down-regulated by the degradation of IRS-1 after insulin stimulation. Thus, when the insulin-induced degradation of IRS-1 was inhibited by either rapamycin or lactacystin, insulin-stimulated phosphorylation of regulatory sites of Akt was increased to a comparable extent despite different subcellular distributions of IRS-1 and the associated PI 3-kinase. Furthermore, the amino acid deprivation increased and the amino acid excess decreased phosphorylation of Akt in parallel with the effects on IRS-1 levels in the cell lysates and amino acid availability did not further influence Akt phosphorylation in the presence of either rapamycin or lactacystin. Following activation of PI 3-kinase, Akt is translocated from the cytosol to the PM and binds 3′-phosphoinositides produced by PI 3-kinase through its PH domain, thereby presenting it to an upstream kinase such as PDK-1 (7, 11). Indeed, the bulk of PI(3,4,5)P3 is synthesized at the PM in response to insulin (43). The present data showed that Akt translocated to the PM after prolonged insulin stimulation was increased by rapamycin and lactacystin to a comparable extent, suggesting that 3′-phosphoinositide production in the PM may occur to comparable levels after treatment of these agents during prolonged insulin stimulation.
There is evidence that, in addition to growth factors, amino acid availability controls activation of the mTOR pathway, which regulates translation of the subsets of mRNAs important for cell growth, via phosphorylation of p70 S6 kinase and 4E-BP1 (9, 15, 19). For example, in Chinese hamster ovary cells overexpressing insulin receptors, amino acid starvation blocks activation of p70 S6 kinase and 4E-BP1 phosphorylation in response to insulin, and supplementation of amino acids restores them (15). This mechanism appears to integrate mitogenic and nutritional signals for a cell to grow (9). We have shown in this study that amino acid availability affects insulin-stimulated activation of the mTOR pathway in differentiated 3T3-L1 adipocytes and consequently controls the mTOR-mediated negative regulation of insulin signaling. Thus, amino acid deprivation inhibits the mTOR-mediated negative regulation of insulin signaling, thereby enhancing insulin-stimulated glucose transport and activation of Akt, which would probably increase other metabolic effects of insulin such as glycogen synthesis, while protein synthesis regulated by the activation of p70 S6 kinase and phosphorylation of 4E-BP1 would be suppressed. Conversely, an excess of amino acids augments the negative regulation, resulting in attenuation of insulin-stimulated glucose transport and other metabolic actions, while increasing translation and promoting cell growth. Therefore, this mechanism identifies a novel function of mTOR that integrates nutritional signals and metabolic signals of insulin and probably plays a role in adaptation of mammalian cells to the environment with different nutrient availabilities.
In conclusion, these results indicate that the mTOR pathway plays an important role in insulin-induced subcellular redistribution of IRS-1, which may facilitate its subsequent degradation by the proteasome. The mTOR pathway negatively regulates insulin-stimulated glucose transport likely through the redistribution of IRS-1, while the subsequent degradation of IRS-1 down-regulates insulin-stimulated activation of Akt. The mTOR-mediated negative regulation of insulin signaling integrates nutritional signals and metabolic signals of insulin.
ACKNOWLEDGMENTS
A. Takano and I. Usui contributed equally to this work.
This study was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (10671060 and 12671103 to T.H.).
We are grateful to Jerrold M. Olefsky for the generous gift of adenovirus constructs and useful comments.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 3.Baumann C A, Ribon V, Kanzaki M, Thurmond D C, Mora S, Shigematsu S, Bickel P E, Pessin J E, Saltiel A R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 4.Brunn G J, Williams J, Sabers C, Wiederrecht G, Lawrence J C, Jr, Abraham R T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 5.Cheatham B, Kahn C R. Insulin action and the insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 6.Clark S F, Martin S, Carozzi A J, Hill M M, James D E. Intracellular localization of phosphatidylinositide 3-kinase and insulin receptor substrate-1 in adipocytes: potential involvement of a membrane skeleton. J Cell Biol. 1998;140:1211–1225. doi: 10.1083/jcb.140.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czech M P, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 9.Dennis P B, Fumagalli S, Thomas G. Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev. 1999;9:49–54. doi: 10.1016/s0959-437x(99)80007-0. [DOI] [PubMed] [Google Scholar]
- 10.Di Como C J, Arndt K T. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- 11.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 12.Egawa K, Sharma P M, Nakashima N, Huang Y, Huver E, Boss G R, Olefsky J M. Membrane-targeted phosphatidylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. J Biol Chem. 1999;274:14306–14314. doi: 10.1074/jbc.274.20.14306. [DOI] [PubMed] [Google Scholar]
- 13.Egawa K, Nakashima N, Sharma P M, Maegawa H, Nagai Y, Kashiwagi A, Kikkawa R, Olefsky J M. Persistent activation of phosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3T3–L1 adipocytes. Endocrinology. 2000;141:1930–1935. doi: 10.1210/endo.141.6.7516. [DOI] [PubMed] [Google Scholar]
- 14.Fenteany G, Schreiber S L. Lactacystin, proteasome function, and cell fate. J Biol Chem. 1998;273:8545–8548. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Yonezawa K, Weng Q P, Kozlowski M T, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 16.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma P M, Olefsky J M, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 17.Heller-Harrison R A, Morin M, Czech M P. Insulin regulation of membrane-associated insulin receptor substrate 1. J Biol Chem. 1995;270:24442–24450. doi: 10.1074/jbc.270.41.24442. [DOI] [PubMed] [Google Scholar]
- 18.Holman G D, Kasuga M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia. 1997;40:991–1003. doi: 10.1007/s001250050780. [DOI] [PubMed] [Google Scholar]
- 19.Iiboshi Y, Papst P J, Kawasome H, Hosoi H, Abraham R T, Houghton P J, Terada N. Amino acid-dependent control of p70(s6k). Involvement of tRNA aminoacylation in the regulation. J Biol Chem. 1999;274:1092–1099. doi: 10.1074/jbc.274.2.1092. [DOI] [PubMed] [Google Scholar]
- 20.Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams J W, Brown J H, Olefsky J M. G Alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3–L1 adipocytes. Mol Cell Biol. 1999;19:6765–6774. doi: 10.1128/mcb.19.10.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue G, Cheatham B, Emkey R, Kahn C R. Dynamics of insulin signaling in 3T3–L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J Biol Chem. 1998;273:11548–11555. doi: 10.1074/jbc.273.19.11548. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Broach J R. Tor proteins and protein phosphatase 2A reciprocally regulate Tap42 in controlling cell growth in yeast. EMBO J. 1999;18:2782–2792. doi: 10.1093/emboj/18.10.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Jr, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 25.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 26.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase C λ for insulin stimulation of glucose uptake but not for Akt activation in 3T3–L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A V, Gooch J L, Oesterreich S, Guler R L, Yee D. Insulin-like growth factor I-induced degradation of insulin receptor substrate 1 is mediated by the 26S proteasome and blocked by phosphatidylinositol 3′-kinase inhibition. Mol Cell Biol. 2000;20:1489–1496. doi: 10.1128/mcb.20.5.1489-1496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leevers S J, Vanhaesebroeck B, Waterfield M D. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 29.Murata K, Wu J, Brautigan D L. B cell receptor-associated protein α4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc Natl Acad Sci USA. 1997;94:10624–10629. doi: 10.1073/pnas.94.20.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nave B T, Haigh R J, Hayward A C, Siddle K, Shepherd P R. Compartment-specific regulation of phosphoinositide 3-kinase by platelet-derived growth factor and insulin in 3T3–L1 adipocytes. Biochem J. 1996;318:55–60. doi: 10.1042/bj3180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pessin J E, Saltiel A R. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Investig. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson R T, Desai B N, Hardwick J S, Schreiber S L. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin-associated protein. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricort J M, Tanti J F, Van Obberghen E, Le Marchand-Brustel Y. Different effects of insulin and platelet-derived growth factor on phosphatidylinositol 3-kinase at the subcellular level in 3T3–L1 adipocytes. A possible explanation for their specific effects on glucose transport. Eur J Biochem. 1996;239:17–22. doi: 10.1111/j.1432-1033.1996.0017u.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabatini D M, Barrow R K, Blackshaw S, Burnett P E, Lai M M, Field M E, Bahr B A, Kirsch J, Betz H, Snyder S H. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P M, Egawa K, Gustafson T A, Martin J L, Olefsky J M. Adenovirus-mediated overexpression of IRS-1 interacting domains abolishes insulin-stimulated mitogenesis without affecting glucose transport in 3T3–L1 adipocytes. Mol Cell Biol. 1997;17:7386–7397. doi: 10.1128/mcb.17.12.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd P R, Withers D J, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Standaert M L, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese R V. Protein kinase C-ζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 38.Sun X J, Goldberg J L, Qiao L Y, Mitchell J J. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359–1364. doi: 10.2337/diabetes.48.7.1359. [DOI] [PubMed] [Google Scholar]
- 39.Sun X J, Miralpeix M, Myers M G, Jr, Glasheen E M, Backer J M, Kahn C R, White M F. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992;267:22662–22672. [PubMed] [Google Scholar]
- 40.Sun X J, Pons S, Wang L M, Zhang Y, Yenush L, Burks D, Myers M G, Jr, Glasheen E, Copeland N G, Jenkins N A, Pierce J H, White M F. The IRS-2 gene on murine chromosome 8 encodes a unique signaling adapter for insulin and cytokine action. Mol Endocrinol. 1997;11:251–262. doi: 10.1210/mend.11.2.9885. [DOI] [PubMed] [Google Scholar]
- 41.Thomas G, Hall M N. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 42.Vanhaesebroeck B, Alessi D R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 43.Venkateswarlu K, Oatey P B, Tavare J M, Cullen P J. Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr Biol. 1998;8:463–466. doi: 10.1016/s0960-9822(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Somwar R, Bilan P J, Liu Z, Jin J, Woodgett J R, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiese R J, Mastick C C, Lazar D F, Saltiel A R. Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3–L1 adipocytes. J Biol Chem. 1995;270:3442–3446. doi: 10.1074/jbc.270.7.3442. [DOI] [PubMed] [Google Scholar]
- 46.Withers D J, Ouwens D M, Nave B T G C, van der Zon M, Alarcon C M, Cardenas M E, Heitman J, Maassen J A, Shepherd P R. Expression, enzyme activity, and subcellular localization of mammalian target of rapamycin in insulin-responsive cells. Biochem Biophys Res Commun. 1997;241:704–709. doi: 10.1006/bbrc.1997.7878. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Hoff H, Sell C. Insulin-like growth factor I-mediated degradation of insulin receptor substrate-1 is inhibited by epidermal growth factor in prostate epithelial cells. J Biol Chem. 2000;275:22558–22562. doi: 10.1074/jbc.M000412200. [DOI] [PubMed] [Google Scholar]