Abstract
Practical relevance:
The feline cardiomyopathies are the most prevalent type of heart disease in adult domestic cats. Several forms have been identified (see Parts 2 and 3), with hypertrophic cardiomyopathy (HCM) being the most common. Clinically the cardiomyopathies are often indistinguishable. Cats with subclinical cardiomyopathy may or may not have characteristic physical examination findings (eg, heart murmur, gallop sound), or radiographic cardiomegaly. Cats with severe disease may develop signs of heart failure (eg, dyspnea, tachypnea) or systemic arterial thromboembolism (ATE; eg, pain and paralysis). Sudden death is possible. Treatment usually does not alter the progression from subclinical to clinical disease and often the treatment approach, once clinical signs are apparent, is the same regardless of the type of cardiomyopathy. However, differentiating cardiomyopathy from normal variation may be important prognostically.
Patient group:
Domestic cats of any age from 3 months upward, of either sex and of any breed, can be affected. Mixed-breed cats are most commonly affected but certain breeds are disproportionately prone to developing HCM.
Diagnostics:
Subclinical feline cardiomyopathies may be suspected based on physical examination findings, thoracic radiographs and cardiac biomarker results but often the disease is clinically silent. The definitive clinical confirmatory test is echocardiography. Left heart failure (pulmonary edema and/or pleural effusion) is most commonly diagnosed radiographically, but point-of-care ultrasound and amino terminal pro-B-type natriuretic peptide (NT-proBNP) biomarker testing can also be useful, especially when the stress of taking radiographs is best avoided.
Key findings:
Knowledge of pathophysiological mechanisms helps the practitioner identify the feline cardiomyopathies and understand how these diseases progress and how they manifest clinically (heart failure, ATE). Existing diagnostic tests have strengths and limitations, and being aware of these can help a practitioner deliver optimal recommendations regarding referral.
Conclusions:
Several types of feline cardiomyopathies exist in both subclinical (mild to severe disease) and clinical (severe disease) phases. Heart failure and ATE are the most common clinical manifestations of severe cardiomyopathy and are therapeutic targets regardless of the type of cardiomyopathy. The long-term prognosis is often guarded or poor once overt clinical manifestations are present.
Areas of uncertainty:
Some cats with presumed cardiomyopathy do not have echocardiographic features that fit the classic cardiomyopathies (cardiomyopathy – nonspecific phenotype). Although no definitive treatment is usually available, understanding how cardiomyopathies evolve remains worthy of investigation.
Keywords: Cardiomyopathies, myocardial diseases, echocardiography, heart failure, arterial thromboembolism

Classification
A cardiomyopathy is classically defined as a primary disease of the heart muscle (myocardium).1,2 In general, the myocardium becomes weak, thick, stiff or a combination of these due to a disease inherent to the myocardium. ‘Primary’ means the disease is an intrinsic condition of the myocardium, which in humans is usually heritable/genetic, and is not caused by (secondary to) any disease process that is not inherent to the myocardium. Thus, for example, left ventricular (LV) hypertrophy secondary to aortic stenosis, hyperthyroidism, systemic hypertension or acromegaly is not a cardio myopathy.3,4 However, the lines between what is primary and what is secondary can be blurred. For example, taurine deficiency is not a problem inherent to or limited to the myocardium, yet it causes what is termed dilated cardio myopathy. While a term such as ‘taurine-deficient myocardial failure’ is more descriptive, it is also longer and flouts convention and so ‘dilated cardiomyopathy (due to taurine deficiency)’ is generally used. 5 As another example, doxorubicin causes myocardial failure (a decrease in contractility) and is commonly termed ‘doxorubicin cardiomyopathy’ or ‘doxorubicin-induced cardiomyopathy’. 6 Consequently, some clinician scientists use the terms primary and secondary cardiomyopathy. 7
Categorization in humans differs slightly depending on the organization describing it (eg, World Health Organization, American Heart Association, European Society of Cardiology), but, in general, consists of dilated cardiomyopathy (DCM; weak myocardium), hypertrophic cardiomyopathy (HCM; thick, stiff myocardium), restrictive cardiomyopathy (RCM; stiff myocardium) and arrhythmogenic right ventricular cardiomyopathy (ARVC; myocardial deterioration and replacement).8,9 Subcategories and synonyms of these categories exist in both human and veterinary medicine. While it is possible that atrial myopathies exist in cats (eg, persistent atrial standstill), all the classic feline cardiomyopathies refer to ventricular (left or right) cardiomyopathies.10,11 In cats, left heart failure is much more common. Right heart failure primarily occurs with ARVC.
In addition to the common forms of cardiomyopathy, in humans there are several uncommon forms, including left ventricular noncompaction (LVNC). 12 At times, in humans, these have been placed in a category called unclassified cardiomyopathy. This term has also been used differently and inconsistently in veterinary medicine, mainly as a catch-all category to describe a cat with an abnormal echocardiographic pattern that does not fit the structural and functional features (phenotypes) of the common cardiomyopathies or congenital malformations. It was originally used to describe cats that have a large left atrium (LA) and a normal-appearing left ventricle (LV) without demonstrably abnormal diastolic function, but it has been used more broadly since then.13,14 The most recent recommendation has been to abandon this category of ‘unclassified’ because it includes cats with so many different echocardiographic changes that no one knows what each cat’s heart might look like if only that term is provided. The current recommendation is to say that such a cat has cardiomyopathy – nonspecific phenotype (NCM) and then to describe exactly what is seen echocardiographically.2,15
Up until 1987, when taurine deficiency was identified as a cause of DCM in cats, and 1990, when the first mutation that causes HCM in humans was discovered, the etiology of most cardiomyopathies in all species was unknown.5,16 Since then, in humans, thousands of mutations in dozens of genes have been identified that cause cardiomyopathy.9,17,18 These primarily consist of mutations in genes that code for myocardial proteins, including sarcomeric (contractile apparatus), cytoskeletal (cellular scaffold) and mitochondrial proteins. Mutations in the same gene can cause different phenotypes (eg, mutations in the myosin heavy chain gene can cause HCM or DCM), and mutations in different genes can cause the same cardiomyopathy (eg, mutations in the myosin heavy chain gene and in the myosin binding protein C gene both cause HCM). 19 This diversity of genes and their mutations means that a cardiomyopathy like HCM probably represents a final common pathway for many distinct disease subtypes, each of which has its own pathophysiology, rate of progression and prognosis. 20 Mutations in genes encoding components of the sarcomere, Z-band, nuclear membrane, desmosome, mitochondria and calcium-handling proteins have all been found in humans with cardiomyopathy.21,22 In domestic cats the causes of almost all cardio-myopathies remain unsolved. The exceptions are taurine deficiency causing DCM and two breed-specific myosin binding protein C mutations that cause HCM in Maine Coon and Ragdoll cats.5,23
The cardiomyopathies classically cause changes in cardiac anatomical structure and mechanical function. In humans, numerous gene mutations also cause ion channel abnormalities and, in turn, electrical abnormalities in the myocardium that result in electrocardiographic (ECG) changes, notably arrhythmias. They are often termed channelopathies. 24 They have also more recently been added to the cardiomy-opathy classification scheme in humans. 9 None of these ion channel abnormalities has been identified in cats, although one family of dogs with a heritable channelopathy has been described. 25
Staging
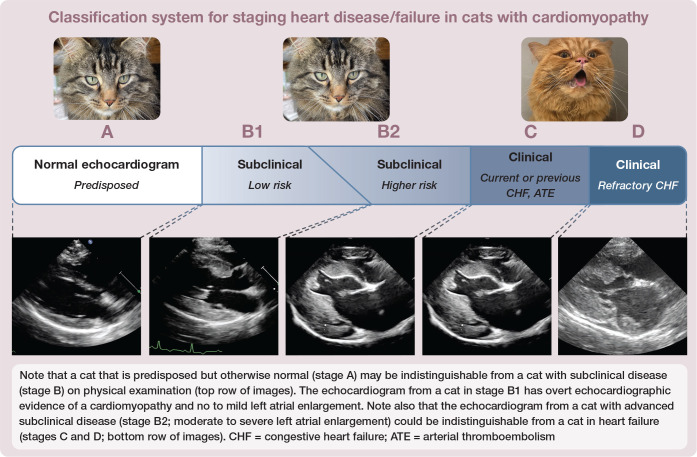
Cats present in different prognostic stages of disease (see box). 2 Some cats present for examination because they belong to a breed known to be predisposed to a particular type of cardiomyopathy, most commonly HCM, but have no identifiable evidence of cardiomyopathy on physical examination. This is stage A.
Most cats with cardiomyopathy have mild, moderate or severe disease detectable via echocardiography but are subclinical, meaning they are showing no overt clinical manifestations of heart failure, thromboembolism or syncope. This is stage B. Cardiomyopathy severity can be judged at both the level of the affected ventricle and at the level of the affected atrium. For example, a cat with HCM can have a severely thickened ventricle but no or only mild LA enlargement. Since it is the degree of atrial enlargement that seems to contribute the most to prognosis, it is atrial enlargement that is used for staging the disease. Stage B is divided into B1 and B2, where cats with no to mild left or right atrial enlargement are in stage B1 and cats with moderate to severe atrial enlargement are in stage B2. In general, cats in stage B1 are not at high risk of developing heart failure or aortic thromboembolism (ATE) in the ensuing months to few years (eg, only 7% of cats with subclinical HCM die of their cardiovascular disease within 1 year of diagnosis; 5- and 10-year cardiac mortality rates are 23% and 28%, respectively), meaning many of these cats stay in a subclinical stage for years, and often for life. 26 Cats in stage B2 are at higher risk and may require medical intervention, such as clopidogrel administration, to try to prevent a thrombus from forming in a severely enlarged left auricle. They should also be monitored for heart failure (eg, the owner may be instructed to monitor the cat’s sleeping respiratory rate [RR] periodically).
Stage C is reserved for cats in left heart failure (pulmonary edema [PE] and/or pleural effusion [PLE]) or right heart failure (ascites and/or PLE), or those that have experienced ATE. By convention, once a cat is in stage C it stays in stage C, rather than moving back to stage B if the heart failure or thromboembolic disease is being successfully treated and clinical signs have resolved. Most, although not all, cats in stage C will eventually die from their cardiomyopathy due to relapsing/progressive clinical signs, and so have a terminal disease. However, their quality of life can be good for months and sometimes a year or two if they are treated appropriately and respond well.
Stage D is reserved for those cats that have become refractory to a loop diuretic (eg, >6 mg/kg/day furosemide). 2 They usually have terminal disease and most live days to months, with occasional cats having longer survival.
Prevalence
The cardiomyopathies are by far the most common form of heart disease in domestic cats. For example, a prospective study of cardiac biomarkers in 425 cats identified a ratio of myocardial disease to congenital heart malformations of 23:1. 27 An echocardiographic study of 780 cats in rehoming shelters revealed 115 cases of HCM and four congenital cardiovascular malformations (29:1). 28
Of the cardiomyopathies, HCM is the most prevalent in cats referred for an echocardiogram, representing approximately 60% of cases (see Part 2). 29 RCM and NCM phenotypes constitute 20–30% (see Part 3). DCM and ARVC are rare (see Part 3). In apparently healthy cats screened for cardiomyopathy, HCM is by far the most prevalent. 28 Historically, in humans it has been estimated that 1 in 500 people are affected with HCM, although more recent evidence suggests it may be much higher (1 in 200 to 1 in 70).30,31 In cats it appears to be closer to 1 in 7, based on screening for HCM echocardiographically.28,32 The prevalence of HCM severe enough to result in clinical signs and death is largely unknown. In Maine Coons, most cats homozygous for the mutation that causes HCM in that breed will develop severe HCM and so will be at risk for serious sequelae, while those that are heterozygous for the mutation will not. 33 While 30-40% of Maine Coons have the mutation, only around 3–5% of Maine Coon cats are homozygous for that mutation. 34
Presentation
Subclinical cardiomyopathy
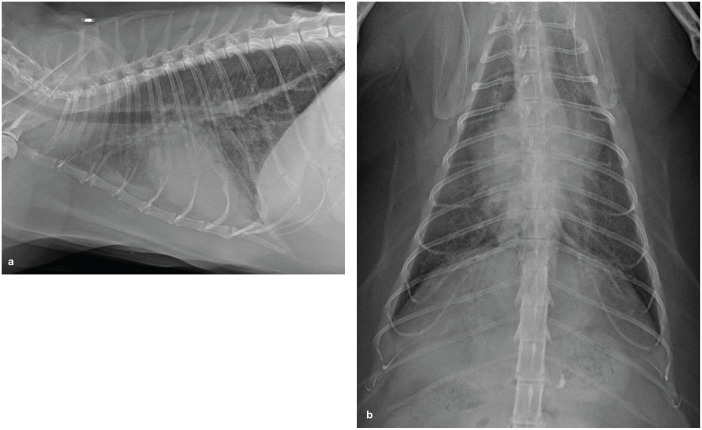
Cats with subclinical cardiomyopathy often go undetected. However, some are diagnosed via echocardiography because they are screened for the disease, most commonly as part of a breeding program, or have an auscultatory abnormality such as a heart murmur, gallop sound or arrhythmia. Some may be identified when screening for a cardiomyopathy using a cardiac biomarker test (eg, amino terminal pro-B-type natriuretic peptide [NT-proBNP] or cardiac troponin I [cTn I]). A few are identified incidentally when thoracic radiographs reveal a left auricular bulge (see Figure 2b later) on a ventrodorsal (VD) or dorsoventral (DV) radiograph (valentine-shaped heart). 35 Cats with severe HCM, RCM and DCM can all have a left auricular bulge when the LA is severely enlarged and cardiomyopathy type is indistinguishable radiographically. 36
Figure 2.
(a) Right lateral radiograph from a cat with PE due to left heart failure from RCM. The densest infiltrate lies between the cardiac silhouette and the diaphragm. (b) Ventrodorsal radiograph from the same cat. There is a left auricular bulge at the 1 o’clock to 2 o’clock position indicating the cat has severe left atrial enlargement
The most common reason a cat with subclinical cardiomyopathy is presented for cardiac diagnostic evaluation is for an incidentally identified soft to moderately loud left parasternal or sternal systolic heart murmur (supplementary files 1 and 2 – see list on page 1023). However, many cats with subclinical cardiomyopathy do not have a heart murmur and, conversely, many cats (~25-33%) that have a left parasternal systolic heart murmur, which is most commonly soft (grade 1–2), do not have cardiomyopathy.28,32,37–39 For example, a cat with a normal heart can have dynamic right ventricular outflow tract obstruction, a benign flow disturbance.40,41 Consequently, a heart murmur in a cat is a much less reliable indicator of structural heart disease than, for example, a heart murmur in a dog with mitral regurgitation.
Supplementary file 1 Audio recording of a left parasternal systolic heart murmur from a cat with hypertrophic cardiomyopathy, played at full speed
Supplementary file 2 Same audio recording as in supplementary file 1 (left parasternal systolic heart murmur from a cat with hypertrophic cardiomyopathy), played at half speed
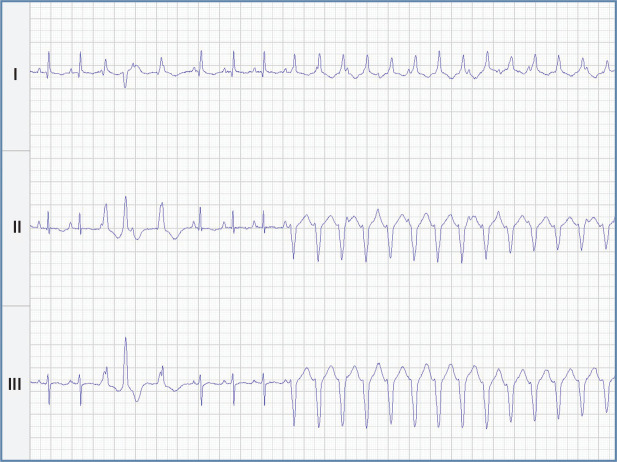
Some cats with subclinical cardiomyopathy present with an arrhythmia, the most common of which is ventricular premature complexes (VPCs; also known as premature ventricular complexes – PVCs). In cats, ventricular hypertrophy makes the myocardium more susceptible to arrhythmias including VPCs and ventricular fibrillation (Figure 1). 42 While only approximately 7% of cats with sub-clinical HCM have VPCs on a resting ECG at the time of diagnosis, almost all cats diagnosed with a ventricular tachyarrhythmia (VPCs, ventricular tachycardia) on a resting ECG have some form of subclinical or clinically apparent cardiomyopathy.26,43 Ventricular tachyarrhythmias are more common in cats with subclinical and clinical HCM than in normal cats when they are examined with a Holter monitor (24-h ambulatory ECG).44,45 Atrial and ventricular tachyarrhythmias are common in cats with ARVC. 46 Cats with cardiomyopathies other than HCM also frequently have VPCs when examined via a Holter monitor. 47 These are most commonly single and uniform. They only rarely show up on a resting ECG. Third degree atrioventricular block has been described in cats with HCM but it also occurs in adult to older cats with no structural heart disease and so may be coincidental.48–50
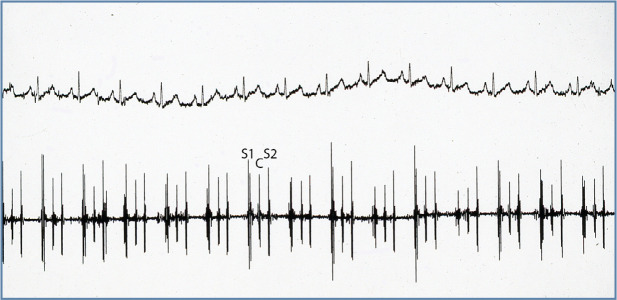
Figure 1.
Electrocardiogram from a cat with HCM. There are multiform ventricular premature complexes (complexes 3, 4 and 5) followed by non-sustained ventricular tachycardia (complexes 9 through 22). Simultaneously recorded leads I, II, III; 50 mm/s
Clinical cardiomyopathy
Most cats with severe, clinically apparent left-sided cardiomyopathy present in left heart failure or with ATE regardless of the type of cardiomyopathy. Left heart failure in cats causes PE and/or PLE, and leads to affected cats presenting with tachypnea (see box), and many with dyspnea (supplementary files 3 and 4).54,55 Hypothermia (due to low cardiac output) is also common.56,57 The exception to left-sided predominance of heart failure in cats with cardiomyopathy is ARVC. These cats present with right heart failure, which manifests as ascites and/or PLE.46,58
Supplementary file 3 Video of a cat with tachypnea (respiratory rate = 60 breaths/min). Note the absence of visible distress and anxiety. This cat had chronic, large-volume cardiogenic pleural effusion
Supplementary file 4 Video of a cat with paradoxical breathing. Note the exaggerated movement of the abdominal walls with respiration

In cats with HCM and respiratory distress (dyspnea), PE is more often responsible for the respiratory signs than is large-volume PLE (86% vs 14%, respectively, in one study 54 ) (Figure 2). In the authors’ experience, the severity of dyspnea is greater in cats with PE than in those with a comparable degree of PLE, especially when the PLE is chronic.
While some cats with heart failure due to a cardiomyopathy have a heart murmur, many others do not. 59 And while a gallop sound is common with severe cardiomyopathy, it is not always present.29,54 Any cat with or without cardiomyopathy can also have a systolic click, which can sound identical to a gallop sound in a cat (Figure 3 and supplementary files 5–7).
Figure 3.
Phonocardiogram from a cat with a systolic click mistaken for a gallop sound (third heart sound). S1 = first heart sound; C = systolic click; S2 = second heart sound
Supplementary file 5 Audio recording of a systolic click in a cat
Supplemental Material
Supplementary file 6 Phonocardiogram of the same audio recording as in supplementary file 5 (systolic click in a cat). The heart rate is 240 beats/min and the interval between S1 and S2 (similar to the QT interval on an ECG) is 120 ms, which is appropriate for that heart rate. The interval between S1 and C is only 80 ms, meaning that C cannot be S2. S1 = first heart sound; C = systolic click; S2 = second heart sound.
Supplementary file 7 Video/audio synchronous display of a systolic click in a cat (different cat to that in supplementary files 5 and 6)
Therefore, cardiac auscultation contributes to the knowledge base around a feline patient, but it cannot be used to rule in or rule out heart failure due to cardiomyopathy in a cat presented with, for example, dyspnea.
Pulmonary auscultation is also limited in its diagnostic value. 60 While some cats with PE have coarse crackles, others do not, and while a lack of, or decrease in, lung sounds in some cats with PLE is obvious, in others it is not. The breathing pattern is also not consistently different for cats with PE vs those with PLE, although prominent abdominal wall movement during respiration (‘paradoxical breathing’) is a clue that suggests PLE may be present (supplementary file 4). In one study, 66% of dyspneic cats with paradoxical breathing had pleural disease, compared with 13% of dyspneic cats without paradoxical breathing. 61 In the authors’ experience, cough is uncommon in cats in heart failure (but is common in cats with asthma). 62 The history of cough in 25% of cats with cardiogenic dyspnea in one study 56 stands counter to general opinion and supports further evaluation of the prevalence of cough in cats with heart disease.
Cats with PLE due to non-cardiac causes, cats with asthma and cats with other primary lung diseases also present with tachypnea and dyspnea. Distinguishing heart failure from respiratory disease can be difficult on initial presentation, especially without further diagnostic testing. A gallop sound makes heart failure much more likely but is frequently not present. 56 Similarly, a rectal temperature <37.5°C makes the diagnosis of heart failure more likely than other causes of dyspnea but this is also an insensitive and non-specific test. Absolute heart rate and RR are not useful for distinguishing heart failure from other causes of dyspnea. 56
Cats with severe dypsnea due to left heart failure usually present acutely, often as an emergency, primarily because owners usually do not notice tachypnea and even dypsnea until it is severe. Dyspneic cats are fragile and subject to dying, if stressed. The initial goals are to: 1) differentiate cats in heart failure from cats with severe respiratory disease; 2) identify if they have PE or PLE; and 3) stabilize them. Differentiating a cat with primary respiratory disease (eg, asthma) from a cat in heart failure can be undertaken by obtaining thoracic radiographs, thoracocentesis if PLE is suspected, thoracic and lung ultrasound, point-of-care ultrasound assessment of LA size and point-of-care cardiac biomarker (NT-proBNP; cTn I) determination.2,63,64 Initial stabilization involves avoiding physical and emotional stress, administering oxygen in a non-stressful manner, possible sedation (eg, with butorphanol 0.2 mg/kg IV), thoracocentesis if large-volume PLE is present, and parenteral loop diuretic administration if PE is present.
Heart failure is the most common cause of PLE in cats, accounting for approximately 40% of cases. 65 Cats with PLE and hypothermia are more likely to be in heart failure. The various types of effusion include: high-protein (‘modified’) transudate; pseudochylous (a milky PLE mimicking chylothorax, associated with increased lipids – cholesterol or lecithin-globulin complexes – potentially seen in any chronic PLE); and chylous (lymphocyte- and triglyceride-rich). 65 The effusion can also be hemorrhagic if the centesis is traumatic (eg, entering a very enlarged atrium if following landmarks that assume normal cardiac dimensions).
Sudden death
Sudden death (presumably most commonly due to ventricular fibrillation but potentially also due to a large thromboembolus in the LV outflow tract or a central nervous system thromboembolus) probably occurs in all forms of cardiomyopathy. It is notable that cardiac disease (primarily HCM) is the most common cause of unexpected death in cats presented for necropsy in studies performed in areas where heartworms are not endemic (eg, the UK).66,67 The prevalence of sudden unexpected death is likely underestimated in cats because such deaths may not be reported to a veterinarian by an owner. A cat with a history of syncope may be at increased risk for sudden death. 68
Diagnosis
The definitive diagnosis of a cardiomyopathy relies on echocardiography. The echocardiographic diagnosis of the specific forms of cardiomyopathy are covered in Parts 2 and 3.
Subclinical cardiomyopathy
Cardiac auscultatory abnormalities
Although many cats with cardiomyopathy are normal on cardiac auscultation, the echocardiographic diagnosis of subclinical feline cardiomyopathy most commonly occurs when an auscultatory abnormality is identified – either a systolic heart murmur or a gallop sound. The systolic heart murmur in a cat with cardiomyopathy is most commonly auscultated over the left apex, either on the sternum (ventral midline) just ventral to the left apex beat or just to the left of the sternum (left parasternal). Localization is best achieved using a pediatric stethoscope. A gallop sound (third ± fourth heart sound) is generated by the LV vibrating. 69 This vibration is low frequency in larger animals (including large dogs) and is best heard with the bell of a stethoscope. Because cats have a smaller heart that vibrates at a higher frequency, gallop sounds in cats are heard best, or at least heard just as well, with the diaphragm of the stethoscope, in the authors’ experience. A systolic click is also heard best with the diaphragm, which contributes to the challenge of identifying the exact nature of third heart sounds (three-heart-sound rhythms) when auscultating cats.
Some third heart sounds in cats are of a nonspecific character that does not allow categorization based on auscultation alone. In the authors’ opinion, a third heart sound that is sometimes present and sometimes absent during a single auscultation in sinus rhythm (eg, the third heart sound is present, then resolves, then recurs, all in 1 minute or less) is unlikely to be an S3 or S4 gallop sound, and more likely to be a systolic click, because the former sounds indicate high ventricular diastolic filling pressures that are not expected to cycle from being audible to inaudible and back again in a short period of just several seconds; also systolic clicks are known to be labile in other species. 70
Clinical cardiomyopathy
Diagnosing a cat with severe PE or PLE due to left heart failure can be accomplished in several ways. The first thing to do is to determine if the cat is tachypneic (see ‘Diagnosing tachypnea’ box on page 1013), as almost all cats in left heart failure with even mild PE or moderate to severe PLE will have an elevated RR (many will also be dyspneic). The next thing to do is to determine if the tachypnea and/or dyspnea is due to severe PLE; if it is, the PLE should be removed promptly.
Diagnosing PLE
Thoracic radiographs are the most common means of diagnosing PLE (Figure 4) but the process of obtaining them can be stressful and the stress can result in death. 74 However, it is not usually necessary to have more than one view to make the diagnosis (a DV or a lateral view often suffices), which is less stressful than obtaining two or three views.
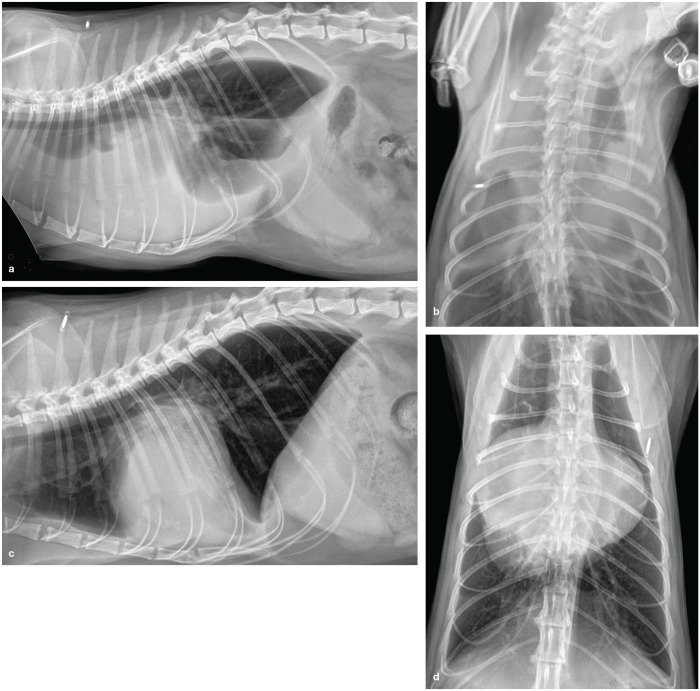
Figure 4.
(a) Right lateral thoracic radiograph from a cat with HCM and severe PLE due to left heart failure. (b) Dorsoventral thoracic radiograph from the same cat, again showing severe PLE. (c) Right lateral thoracic radiograph and (d) ventrodorsal radiograph from the same cat after thoracocentesis and furosemide administration. In (c), the left atrium is so severely enlarged it pushes the trachea dorsally, creates a slight bulge in the caudal aspect of the cardiac silhouette and is more radiopaque than the left ventricle that is ventral to it. In (d) the left auricle is markedly enlarged and bulges out from the cardiac silhouette from the 1 o’clock to 4 o’clock position
If radiographs are deemed too stressful and an ultrasound machine is available, ultrasound is the quickest, least stressful and easiest way to diagnose severe PLE. 64 While PLE is usually readily apparent with ultrasound, in some cases it can be a challenge to differentiate pleural from pericardial effusion. This most commonly happens when the probe is oriented to look at the heart (supplementary file 8). PLE commonly accumulates between the heart and the diaphragm in cats with even mild effusion and has fibrin floating in it, whereas pericardial effusion does not (supplementary file 9). Orienting the probe to examine the caudal thoracic cavity will usually reveal the characteristic PLE, if it is present.
Supplementary file 8 Video showing a thoracic ultrasonographic view of a cat with mild to moderate pleural effusion (echolucent space toward the top). The tip of a lung lobe cat be seen within the fluid (upper right)
Supplementary file 9 Video showing a thoracic ultrasonographic view from the same cat as in supplementary files 5 and 6, with pleural effusion residing between the heart and the diaphragm. Note the mound of fibrin oscillating in the fluid
If it is deemed that radiography is too stressful and ultrasound is not available, and especially if large-volume PLE has been identified in a particular cat in the past, some clinicians advocate thoracocentesis if there is a clinical suspicion of PLE based on physical examination. 75 This procedure, however, is not devoid of risk of complications, including pneumothorax and death. Nevertheless, in a cat with severe dyspnea due to left heart failure and a reasonable clinical suspicion of large-volume PLE, thoracocentesis can be lifesaving. The procedure can be performed in the examination room without sedation and with the cat in sternal recumbency. Only limited skin preparation is needed. The centesis is usually best undertaken with a butterfly catheter. The needle is inserted mid-thorax on either side. If fluid is found, as much of it as possible should be removed, but with the understanding that respiratory effort is probably helped best with the initial part of the centesis, whereas trying to remove as much fluid as possible is aimed at delaying recurrence of dyspnea in the future. Most cats with severe dyspnea due to PLE have 200-350 ml of fluid in their thoracic cavity. If that amount is not removed from one side, the other side may need to be tapped also. In this situation, ultrasound guidance is preferred, but again not necessary. When due to heart failure, the fluid can be a protein-rich (‘modified’) transudate, a pseudochylous effusion or a chylous effusion. 76
Once the fluid is removed, many cats improve quickly and often remarkably. If significant improvement is not seen, PE may also be present or complications (eg, pneumothorax, hemothorax, re-expansion PE) from the thoracocentesis may have occurred. In the authors’ experience, complications are rare when centesis is performed correctly in a cat in heart failure. Thoracocentesis in this emergency situation is very different to when it is performed in a cat with chronic non-cardiogenic PLE. When thoracocentesis is performed in a non-emergent situation, aseptic skin cleaning/preparation should be ensured, and an over-the-needle catheter can be used instead of a butterfly catheter. Caution is always warranted when there is evidence of chronic PLE (eg, rounding of lung lobe margins on radiographs), especially if the effusion is visibly chylous. 77 Such cats have a higher risk of pleural fibrosis causing non-recruitable lung. Iatrogenic pneumothorax can occur, not from contact between the needle and lung, but by visceral pleural rupture. 78 With any evidence of chronicity, a conservative volume of effusion (eg, 120 ml) should be removed, in the authors’ opinion, unless pleural manometry is available. 78
Diagnosing PE
Radiographs can be obtained if the cat is not severely dyspneic and overly stressed by the procedure (see box). The combination of a left auricular bulge (valentine-shaped heart) on a VD or DV radiograph and pulmonary infiltrates is specific for identifying cardio-genic PE (left heart failure).35,79 However, the left auricular bulge is not always apparent, especially if the heart failure has been precipitated by something immediate like acute stress or exogenous fluid overload. 80 In other cases the cardiac silhouette may be obscured by PLE. If the cardiac silhouette is obscured by PLE on a DV view, a VD view can be considered, if tolerated, since the cardiac silhouette is often visible in this view even if it is obscured in the DV view.

The radiographic pattern of cardiogenic PE in cats is variable. While the common caudodorsal distribution seen in dogs with cardiogenic PE can also occur in cats, most have other patterns.79,81,82 A frequent finding is to see an alveolar infiltrate between the heart and the diaphragm on a lateral view (Figure 5). This pattern is only rarely seen with other lung diseases.
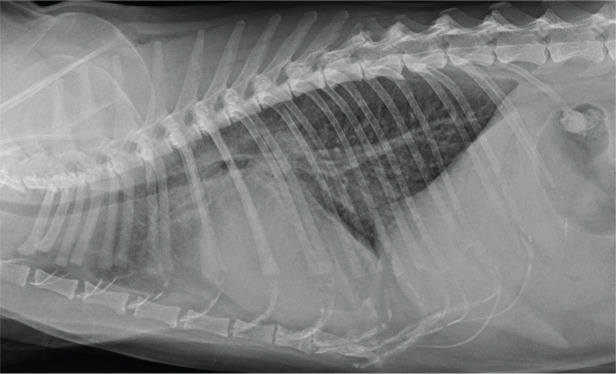
Figure 5.
Right lateral thoracic radiograph from a cat with NCM showing a more diffuse form of PE but still with the heaviest infiltrates between the heart and diaphragm
A cranial and ventral distribution is also common, which is very different from the pattern seen in dogs. Some cats have both PE and (most commonly a small amount of) PLE (Figure 6). 65
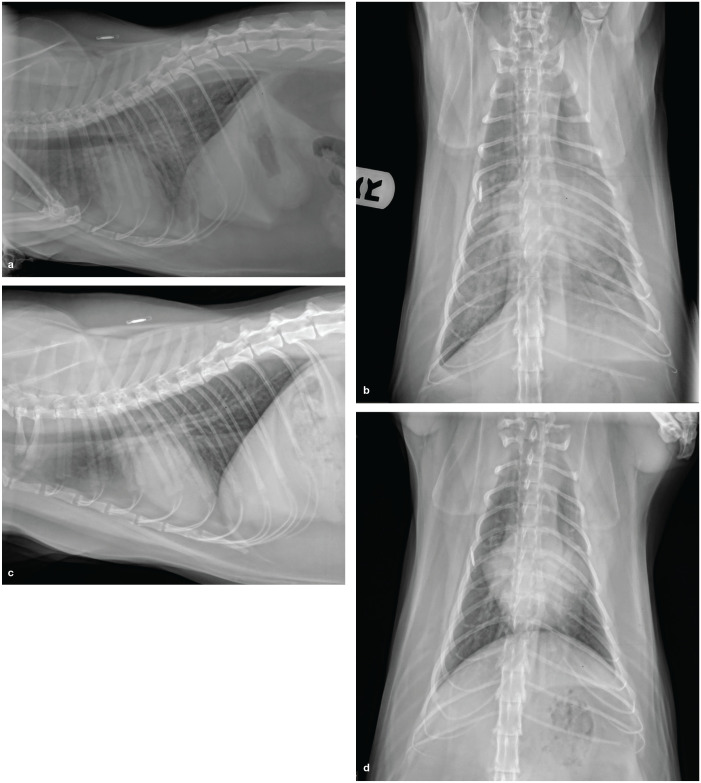
Figure 6.
(a) Right lateral thoracic radiograph from a cat in left heart failure. PE is present, and infiltrates are most prominent between the heart and diaphragm. PLE is also present dorsally at the angle between the spine and the diaphragm. (b) Ventrodorsal thoracic radiograph from the same cat. PE and PLE make it difficult to see the left side of the cardiac silhouette, but the left auricle appears to be enlarged. (c) Right lateral thoracic radiograph and (d) ventrodorsal thoracic radiograph from the same cat after loop diuretic therapy. In (c), the PE and PLE are both improved. In (d), a left auricular bulge at the 2 o’clock to 3 o’clock position is now visible
Diagnosing PE without thoracic radiographs can be problematic. While some cats have pulmonary crackles on auscultation, many do not, and non-cardiac causes of dyspnea may produce crackles in cats. B-lines can be identified using ultrasound but this requires an ultrasound machine and training. 63 Diagnosing PE without radiographs thus often comes down to inference or response to therapy.
Determining whether PE and/or PLE are due to heart failure
One may infer that dyspnea is due to cardiogenic PE by using a biomarker to identify a dyspneic cat as having severe cardiac disease. In the cat presented for severe dyspnea this needs to be undertaken using a point-of-care measure of plasma NT-proBNP or cTn I concentration, not a quantitative test that must be sent to a central laboratory. If available, the point-of-care tests are reasonably accurate (NT-proBNP: 94–100% sensitivity and 72% specificity; cTn I: 65-84% sensitivity and 86–90% specificity), with the result being positive if the dyspnea is due to heart failure and negative if the cat has respiratory disease.64,83–86 Since this type of test is not 100% accurate (ie, not definitive), it should generally be used in combination with other diagnostic tests, if possible.
If an ultrasound machine is available, the best way to infer that dyspnea is due to left heart failure is to do a point-of-care (focused examination) of LA size. If the LA is moderately to severely enlarged then heart failure is almost always the cause of the dyspnea. 64 In most instances the LA will be >18-19 mm in diameter in a right parasternal short-axis view in a cat in left heart failure and the LA:Ao will be >1.8–2.0.51,87
Doppler echocardiography by a skilled examiner can also be used to help predict whether a cat is in left heart failure. 51 Variables that are useful include the ratio of peak velocity of early diastolic transmitral flow to peak velocity of late diastolic transmitral flow (value >1.8), and the ratio of peak velocity of fused early and late transmitral flow velocities to peak velocity of fused early and late diastolic tissue Doppler waveforms (value >15.1).
The accuracy of plasma NT-proBNP concentration determination has also been examined in cats with PLE. 88 In this situation, the quantitative laboratory test is reasonably accurate at differentiating cardiogenic PLE from other causes of PLE when either plasma (sensitivity, 95%; specificity, 82%) or pleural fluid (sensitivity, 100%; specificity, 76%) is used. The point-of-care test (SNAP; IDEXX) is also reasonably accurate for this purpose if plasma is used (sensitivity, 100%; specificity, 79%) or if pleural fluid is used (sensitivity, 100%; specificity 86%). 83
Arterial thromboembolism
ATE is a common and devastating complication of the left-sided cardiomyopathies. The thrombus most commonly forms in a severely enlarged LA, most often in the left auricle (LA appendage). At least two factors are thought to predispose to thrombus formation in a large LA – blood flow stasis and endothelial injury. Endothelial injury is evident as an increase in the amount of von Willebrand factor in the endothelium of enlarged feline left atria and by its integral presence in LA thrombi from cats with severe cardiomyopathy. 89 Severe LA enlargement can occur with HCM, RCM, DCM and NCM and causes blood flow stasis. 90 This predisposes to red cell clumping. This can be visualized with ultrasound as spontaneous echocardiographic contrast (‘smoke’). This presumably predisposes to thrombus formation in cats since it is found in the majority of cats with ATE. 90 It is a negative prognostic sign. 91 When an intracardiac thrombus is identified using echocardiography, it is, as mentioned above, most commonly in the left auricle (supplementary files 10–12). Computed tomographic angiography can also be used to identify LA thrombi, but this is not yet demonstrably superior to echocardiography. 92
Supplementary file 10 Video showing a right parasternal long-axis view of spontaneous echocardiographic contrast in the body of the left atrium (LA) near the mitral valve. There is also a large thrombus in the left auricle at the top. LVFW = left ventricular free wall; IVS = interventricular septum
Supplementary file 11 Video showing spontaneous echocardiographic contrast in the body of the left atrium at the start and then dense contrast in the left auricle and a thrombus occupying the top of the left auricle. Ao = aorta; LA = left atrium. Reproduced, with permission, from Hogan DF. Arterial thromboembolic disease. In: Ettinger SJ, Feldman EC and Côté E (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017, pp 1344–1348
Supplementary file 12 Video showing two-dimensional (left) and three-dimensional (right) echocardiographic views of a thrombus in the left atrium. Courtesy of Seunggon Lee, DVM
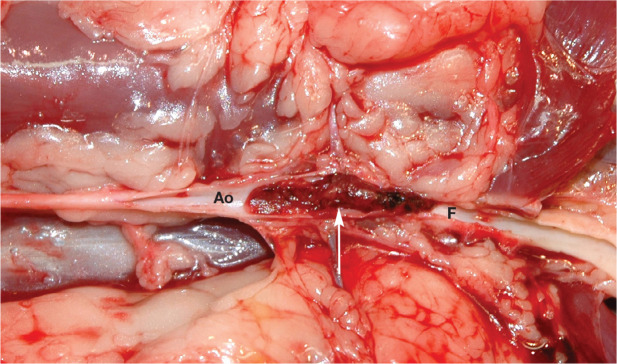
Most left auricular thrombi attain a size larger than any of the entrances to arteries that exit the aorta, and most will dislodge. Because of their size, most are carried to (embolize) the terminal aorta (Figure 7), causing acute occlusion of blood flow to the caudal limbs and resulting in acute pain and caudal limb paresis or paralysis.93,94 Other signs include lack of, or very weak, femoral pulses (pulselessness), cool caudal limb(s) (poikilothermia), pale and/or blue/purple (cyanotic) hindlimb nailbeds (pallor), and firm, painful gastrocnemius muscles. In a small number of cats, the left auricular thrombus is small enough at the time of dislodgement that it can embolize a systemic arterial branch including, but not limited to, subclavian/brachial, coronary and mesenteric arteries, where it can cause forelimb ischemia/lameness, myocardial infarction and intestinal ischemia/infarction, respectively.95,96 Renal infarcts can be detected using ultrasound in some cats with a terminal aortic thromboembolus. 97 Rarely a larger thromboembolus will lodge in the more proximal abdominal aorta where it can occlude renal blood flow causing acute ischemic kidney injury. In theory, a very large thromboembolus can lodge in the LV, completely occlude aortic flow, and so result in sudden death.
Figure 7.
Aortic thromboembolus residing at the aortic trifurcation (arrow). Ao = abdominal aorta; F = femoral artery
A cat with a thromboembolus lodged in the terminal aorta (saddle thromboembolus) initially shows signs of intense pain and paralysis. 93 Vocalization, which is often agonizing, is common. Typically, the pain abates within 12-24 h as the sensory nerves also become non-functional and so may not always be present when the owner finds the cat. Most cats are paralyzed in both caudal limbs, but a few will only have one affected caudal limb. Cats with only one limb affected may be in less pain. Initially the femoral pulses are absent. When the caudal limbs are affected, most cats can move both limbs above the stifles and are paralyzed distal to the stifles. 98 Skin sensation typically is absent below the mid-tibial region. The gastrocnemius muscles are firm. The affected limbs are cool or cold. The cat can usually move its tail.
In most cases the diagnosis can be made using history and physical examination findings (including lack of femoral pulse[s]). Other diagnostic aids include Doppler blood pressure measurement of an affected limb and analysis of a blood sample from the affected limb for glucose (will be low) and lactate (will be high), comparing the results with those from a forelimb blood sample. 99 Infrared thermography shows the caudal limbs distal to the stifle and the tail are cooler than the rest of the body. 100 A limitation of blood sampling and of thermography is that the diagnostic value has been shown in studies of cats with unmistakable physical signs of ATE; the performance of such tests when the diagnosis is in question (‘partialocclusion thromboemboli’), which is when additional diagnostic information would be most useful, is not well established. Ultrasound can be used to identify the thromboembolus and aortic blood flow occlusion, as can computed tomographic angiography. 92
Serum creatine kinase (CK) concentration is always elevated in a cat with complete occlusion of the terminal aorta, and usually markedly so. 101 Consequently, a normal serum CK concentration in a paralyzed cat essentially rules out ATE as the cause. The serum concentrations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are also commonly elevated, sometimes markedly so.101,102 Since these enzymes are also elevated in cats with rhabdomyolysis due to a dystrophinopathy and since there is usually no reason for a cat with ATE to have hepatic injury, these elevations are presumably due to skeletal muscle necrosis in cats with ATE, not hepatic disease. 103
Most cats with ATE have severe underlying heart disease (a markedly enlarged LA) or hyperthyroidism and many (40% in one study 101 ) are in overt left heart failure (usually PE) at presentation. However, only around 10% have had a previous diagnosis of heart disease, meaning the onset of signs of ATE is unexpected. A few cats have no underlying heart disease.101,102
The prognosis for a cat with heart failure and ATE causing bilateral caudal limb paralysis is grave, as is the prognosis for cats with ATE that are hypothermic.93,101,104 The prognosis for any cat (even without heart failure) with bilateral caudal limb paralysis due to ATE is poor to grave and many cats are in extreme pain. Consequently, euthanasia is the most common solution. 104 However, if the owner is willing, or if euthanasia is not an option, time (48-72 h) can be allowed to see if the cat can lyse its own thromboembolus via activation of plasmin. 105 It should be noted that in one study of 127 cats presented with ATE, 89 were treated with supportive care and 39 (45%) survived to discharge. 101 But it should also be noted that median survival time for that group of 39 cats was only 117 days. In another study, only around 30% survived to hospital discharge. 106 This represents a broad swath of survival with a generally somber outlook, but it should also be noted that there are recorded single instances of survival of several years. 54
If the cat is not in heart failure and especially if there is no severe underlying heart disease or if only one limb (fore or caudal) is affected, supportive treatment should be attempted, if the owner is willing, giving the cat at least 48 h to break down its own thromboembolus. Up to 80% of cats with one affected limb will survive. 107 Regardless, overall 1-year survival for the entire population of cats with ATE is only around 10%. 104 An additional problem is that cats that survive to discharge can experience another ATE despite antithrombotic therapy. 101
There is no absolute standard of care for treating ATE, but pain control is paramount if signs of pain are present. A potent analgesic needs to be titrated to effect. 93 Intravenous methadone or a constant rate infusion of fentanyl are examples. Unfractionated heparin or a low molecular weight heparin is usually administered soon after presentation. This will not lyse the thromboembolus but aims to prevent a new thrombus from forming on the existing thromboembolus. That this is important is suggested by one study which found that the distal portions of feline aortic thromboemboli are composed primarily of erythrocytes, while the proximal portions also contain remnants of neutrophils (neutrophil extracellular traps) that may propagate further thrombus for-mation. 108 Oral anticoagulation/antiplatelet therapy should also be started with clopidogrel (18.75 mg/cat q24h) or a combination of clopidogrel and aspirin to try to prevent a new left auricular thrombus from developing. A loading dose of clopidogrel might be considered.2,109 Aspirin alone is inadequate. 110 Another approach is to administer clopidogrel (antiplatelet) and rivaroxaban (anticoagulant; 0.5-1 mg/kg PO q24h) together. 111 The primary problem with this approach is the high cost of rivaroxaban.
After initial stabilization it is simply a matter of administering supportive care and waiting to see if the cat’s own thrombolytic system (plasmin) can break down the thromboembolus. The degree to which circulation is re-established is apparent through return of the femoral pulse(s) and limb function and visible/palpable signs of improved perfusion. Serial testing (eg, of local blood lactate concentration) is not known to be helpful in prognostication. Time to reperfusion is highly variable. It can occur within as little time as 1 h. If reperfusion does not occur within the first 48-72 h, the chances of it occurring at all are exceedingly poor.
If perfusion to the affected areas is reestablished, all signs may resolve or signs may only partially resolve. As such, some cats will regain full function in both caudal limbs while others will regain full or partial function of only one limb (supplementary file 13) and so may require amputation of part or all of the other limb. In some, reperfusion is inadequate and results in the development of tissue necrosis (wet or dry gangrene; Figure 8), days to a few weeks following the event, requiring surgery (including debridement and amputation) or justifying euthanasia. 101 Reperfusion syndrome (acute hyperkalemia and metabolic acidosis due to abrupt reperfusion of a limb) only rarely occurs with spontaneous resolution of a thromboembolus. 112
Figure 8.
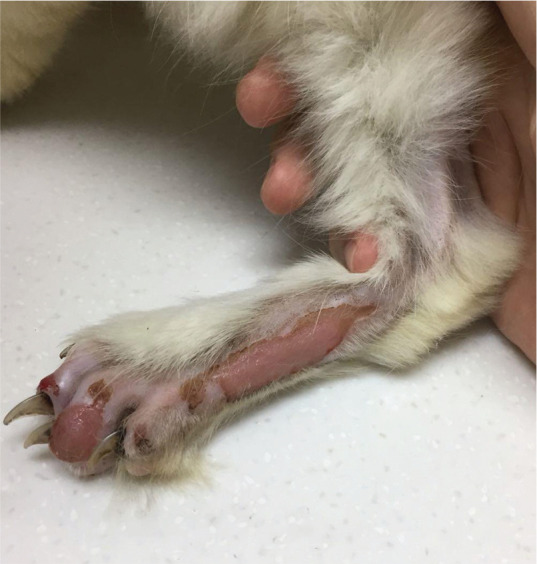
Dry gangrene of the distal caudal limb in a cat that survived an acute bout of ATE
Supplementary file 13 Video of a cat that experienced a bout of acute arterial thromboembolism 2 weeks prior showing residual neurologic deficits in the right caudal limb. Note the lack of signs of pain in this patient that survived the acute stage of arterial thromboembolism. Reproduced, with permission, from Hogan DF. Arterial thromboembolic disease. In: Ettinger SJ, Feldman EC and Côté E (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017, pp 1344–1348
Amputation of one caudal limb is almost always a viable option since cats missing one caudal limb generally do extremely well, barring pre-existing disease of the other caudal limb, assuming a reasonable anesthetic risk and also assuming thromboprophylaxis will prevent ATE recurrence. The use of a thrombolytic agent, such as tissue plasminogen activator or streptokinase, is not indicated since such an approach, while it might shorten the time to reperfusion, does not improve survival and increases the risk of reperfusion syndrome and life-threatening bleeding, such as into the affected limb(s), catheter or venipuncture sites, and/or the gastrointestinal tract.106,113,114 While success with surgical and rheolytic embolectomy has been reported, these techniques are rarely attempted.115,116
Treatment of heart failure
While anatomy and pathophysiology of the left-sided cardiomyopathies (HCM, RCM and DCM) are distinctly different, presentation and treatment are the same or very similar. Consequently, once a cat is diagnosed with a large LA and left heart failure, identifying the type of cardio-myopathy has not yet been proven to influence most treatment approaches. Knowing if a cat has severe outflow tract obstruction due to systolic anterior motion of the mitral valve (see Part 2) may be valuable in certain cases. Knowing that a cat has DCM so that diet history can be obtained, and blood samples submitted for taurine analysis, may be beneficial. By and large, however, administering a loop diuretic during all phases of treatment, supportive therapy (oxygen, warming blanket) during the emergent phase, antiplatelet/anticoagulant medication when there is severe LA enlargement, possibly administering oral pimobendan and an angiotensin-converting enzyme (ACE) inhibitor, and reducing, or preferably eliminating, high-sodium foods and treats to avoid negating the natriuretic purpose of diuretics, will be undertaken regardless of the underlying type of cardiomyopathy. Treatment is discussed further under the specific types of cardiomyopathies in Parts 2 and 3.
Complications of therapy
Azotemia is common in cats in heart failure, primarily those that are being treated with a loop diuretic. While primary renal insufficiency/ failure may be present in some cats, in the authors’ clinical experience the preponderance of the azotemia is most likely due to the administration of a loop diuretic and/or an ACE inhibitor. Therefore, the azotemia is most commonly prerenal. 117 This is often characterized by a greater increase in blood urea nitrogen (BUN) than in serum creatinine concentration. Serum concentration of symmetric dimethyl-arginine (SDMA) is also increased.
In the authors’ experience, prerenal azotemia is not harmful to the kidneys in normotensive patients and is generally reversible. Mild pre-renal azotemia is essentially expected in a cat in severe heart failure being treated with an ACE inhibitor and a moderate to high dosage of a loop diuretic. Even moderate prerenal azotemia (BUN up to 60 mg/dl [21 mmol/l]) is not inherently harmful, especially when temporary (eg, associated with initial high-dose loop diuretic administration) and subclinical (eg, associated with a normal appetite). Severe azotemia, which results in clinical signs of uremia, is expected to be overtly harmful to the cat. While terms such as acute kidney injury and cardiorenal syndrome are often equated with the azotemia seen in cats with heart failure being treated with furosemide and/or an ACE inhibitor, neither agent is nephrotoxic. 118 A loop diuretic causes prerenal azotemia by reducing renal blood flow and so glomerular filtration rate (GFR), while an ACE inhibitor decreases GFR by altering intraglomerular blood flow (ie, selectively dilates the efferent glomerular arterioles by decreasing the circulating concentration of angiotensin II). 119 The effects of both drugs are usually reversible.
Most cats with congestive heart failure that have a less than three-fold elevation above normal in BUN and/or less than two-fold elevation above normal in creatinine have a greater health concern with their cardiac disease than with kidney function. Therefore, while one cannot ignore mild to moderate azotemia in a cat in chronic heart failure receiving a loop diuretic with or without an ACE inhibitor, since the cat will require ongoing monitoring, there is usually no need to address it by reducing the dose of the loop diuretic or by discontinuing the administration of the ACE inhibitor if the cat is eating and feeling well, and certainly no indication to administer parenteral fluids solely to try to reduce the azotemia. While azotemia may be associated with poor outcome in cats in heart failure, in the authors’ opinion most likely this is because cats in severe heart failure have both a poorer prognosis and a need for a higher dose of loop diuretic. 120
That being said, excessive loop diuretic administration should also be avoided, especially since cats that become severely dehydrated tend to stop eating and drinking and so may spiral downward clinically, particularly in the emergent phase (during hospitalization). On occasion, parenteral fluid administration and cessation of loop diuretic administration is required for cats in that situation, particularly in those that are anorexic for a prolonged period and that are receiving high doses of a loop diuretic. This is considered a rescue approach for cats that are profoundly (eg, ≥8%) dehydrated. For cats that show prerenal azotemia but otherwise feel well, administering parenteral fluids and a diuretic simultaneously is discouraged because these treatments simply negate each other.
It is difficult or next-to-impossible to distinguish prerenal from renal azotemia in a cat on a loop diuretic, primarily because the diuretic renders the urine isosthenuric. Clues that a cat may have renal azotemia include age (older cats are more likely to have chronic kidney disease [CKD]), size of the kidneys (CKD commonly results in smaller kidneys) and hematocrit (CKD often causes a non-regenerative anemia). Cats in heart failure that are azotemic due to renal insufficiency/failure are more susceptible to dehydration and uremia. Systemic hypertension and chronic pyelonephritis are examples of concurrent disorders with renal involvement that can harm a cat with cardiomyopathy, unless they are controlled.
Prognosis
The short-term prognosis for cats with stage B1 cardiomyopathy is good to excellent. Long-term prognosis is fair to guarded and may depend on the severity and type of the disease at the ventricular level. 26 Cats in stage B2 have a guarded short- and long-term prognosis and cats in stages C and D are more likely to die of heart disease than any other disorder (see the earlier ‘Staging’ section).57,91 The unpredictability of individual lifespans means absolute assurances about longevity are never appropriate. Instead, a general outlook (eg, even with a good response to treatment, the cat is more likely to live for months than years) should be provided to assist in the client’s decision-making. One exception to the guarded prognosis for cats with stage C HCM is transient myocardial thickening where the prognosis is excellent if the cat survives the initial phase of the disease (see Part 2). 121 Similarly, in a cat with DCM due to taurine deficiency, taurine supplementation will usually improve cardiac function enough to improve prognosis if the cat survives the initial emergent phase of the disease. 5 In addition, if a cat has HCM exacerbated by hyperthyroidism, controlling the hyperthyroidism can result in regression of LV hypertrophy, a lesser degree of renin-angiotensin system stimulation, and a decrease in metabolic rate, which can make it easier to control the heart failure (see Part 3). 38 In cats with a cardiomyopathy that have experienced acute heart failure precipitated by a stressful event (eg, cat fight), anesthesia/surgery or fluid administration, the prognosis is also better.54,122,123
Several variables can be used to help determine poor long-term prognosis regardless of the type of left-sided cardiomyopathy. Severe LA enlargement is a poor prognostic indicator. 91 Unstable/refractory heart failure and an NT-proBNP >1500 pmol/l also portend an unfavorable outcome. 91
Key Points
- ✜ Cardiomyopathies can be staged clinically according to the American College of Veterinary Internal Medicine’s A–D scale, which reflects the need for treatment and general prognosis:
- – A: Predisposed to developing cardiomyopathy but no identifiable abnormality.
- – B: Positive phenotype (ie, echocardiographically detectable changes such as LV thickening or thinning; atrial enlargement) but no clinical signs.
- – C: Positive phenotype and a decompensated state (most commonly congestive heart failure, but ATE or syncope also possible) at initial presentation or controlled with medication.
- – D: Positive phenotype and a decompensated state that is not fully responsive to treatment
✜ Overall, cardiomyopathies are treated similarly: the onset of heart failure (stage C) warrants treatment with medications that offset fluid retention (eg, a loop diuretic) and any cat with severe LA enlargement should be on antiplatelet/ anticoagulant therapy, most commonly clopidogrel.
Footnotes
Supplementary material: Brief outlines of the supplementary files are provided below; fuller descriptions accompany the files that are available online at: journals.sagepub.com/doi/suppl/10.1177/1098612X211021819.
✜ Files 1 and 2: Audio recordings of a left parasternal systolic heart murmur in a cat with HCM.
✜ File 3: Video of a cat with tachypnea due to chronic, large-volume cardiogenic PLE, with an absence of visible distress and anxiety.
✜ File 4: Video of a cat with paradoxical breathing.
✜ Files 5-7: (File 5) Audio recording, (file 6) phonocardiogram and (file 7) video/audio synchronous display of a systolic click in a cat.
✜ File 8: Video showing a thoracic ultrasonographic view of a cat with mild to moderate PLE.
✜ File 9: Video showing a thoracic ultrasonographic view from the cat in supplementary files 5-7, with PLE residing between the heart and diaphragm and fibrin oscillating in the fluid.
✜ File 10: Video showing a right parasternal long-axis view of spontaneous echocardiographic contrast in the body of the LA near the mitral valve.
✜ File 11: Video showing spontaneous echocardiographic contrast in the body of the LA and then dense contrast in the left auricle and a thrombus occupying the tip of the left auricle. Reproduced, with permission, from Hogan DF (2017). 124
✜ File 12: Video showing two-dimensional (left) and three-dimensional (right) echocardiographic views of a thrombus in the left auricle and LA. Courtesy of Seunggon Lee, DVM.
✜ File 13: Video of a cat that experienced a bout of acute ATE 2 weeks prior to showing residual neurologic deficits in the right caudal limb. Reproduced, with permission, from Hogan DF (2017). 124
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
Contributor Information
Mark D Kittleson, School of Veterinary Medicine, Department of Medicine and Epidemiology, University of California, Davis, and Veterinary Information Network, 777 West Covell Boulevard, Davis, CA 95616, USA.
Etienne Côté, Department of Companion Animals, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada.
References
- 1. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J 1980; 44: 672-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luis Fuentes V, Abbott J, Chetboul V, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med 2020; 34: 1062-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borgeat K, Niessen SJM, Wilkie L, et al. Time spent with cats is never wasted: lessons learned from feline acromegalic cardiomyopathy, a naturally occurring animal model of the human disease. PLoS One 2018; 13: e0194342. DOI: 10.1371/journal.pone.0194342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bond BR, Fox PR, Peterson ME, et al. Echocardiographic findings in 103 cats with hyperthyroidism. J Am Vet Med Assoc 1988; 192: 1546-1549. [PubMed] [Google Scholar]
- 5. Pion PD, Kittleson MD, Rogers QR, et al. Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy. Science 1987; 237: 764-768. [DOI] [PubMed] [Google Scholar]
- 6. Hallman BE, Hauck ML, Williams LE, et al. Incidence and risk factors associated with development of clinical cardiotoxicity in dogs receiving doxorubicin. J Vet Intern Med 2019; 33: 783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brieler J, Breeden MA, Tucker J. Cardiomyopathy: an overview. Am Fam Physician 2017; 96: 640-646. [PubMed] [Google Scholar]
- 8. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J 2008; 29: 270-276. [DOI] [PubMed] [Google Scholar]
- 9. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113: 1807-1816. [DOI] [PubMed] [Google Scholar]
- 10. Gavaghan BJ, Kittleson MD, McAloose D. Persistent atrial standstill in a cat. Aust Vet J 1999; 77: 574-579. [DOI] [PubMed] [Google Scholar]
- 11. Kittleson MD. Case 18. Case studies in small animal cardiovascular medicine. Available at: https://viper.vetmed.ucdavis.edu/public/cardio_kittleson/cases/case18/case18.htm. [Google Scholar]
- 12. Finsterer J, Stöllberger C. Unclassified cardiomyopathies in neuromuscular disorders. Wien Med Wochenschr 2013; 163: 505-513. [DOI] [PubMed] [Google Scholar]
- 13. Gavaghan BJ, Kittleson MD, Fisher KJ, et al. Quantification of left ventricular diastolic wall motion by Doppler tissue imaging in healthy cats and cats with cardiomyopathy. Am J Vet Res 1999; 60: 1478-1486. [PubMed] [Google Scholar]
- 14. Peck CM, Nielsen LK, Quinn RL, et al. Retrospective evaluation of the incidence and prognostic significance of spontaneous echocardiographic contrast in relation to cardiac disease and congestive heart failure in cats: 725 cases (2006-2011). J Vet Emerg Crit Care (San Antonio) 2016; 26: 704-712. [DOI] [PubMed] [Google Scholar]
- 15. Arbustini E, Narula N, Dec GW, et al. The MOGE(S) classification for a phenotype-genotype nomenclature of cardio-myopathy: endorsed by the World Heart Federation. J Am Coll Cardiol 2013; 62: 2046-2072. [DOI] [PubMed] [Google Scholar]
- 16. Geisterfer-Lowrance AA, Kass S, Tanigawa G, et al. A molecular basis for familial hypertrophic cardio-myopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 1990; 62: 999-1006. [DOI] [PubMed] [Google Scholar]
- 17. Jarcho JA, McKenna W, Pare JA, et al. Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N Engl J Med 1989; 321: 1372-1378. [DOI] [PubMed] [Google Scholar]
- 18. Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hyper-trophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020; 76: e159-240. [DOI] [PubMed] [Google Scholar]
- 19. Teekakirikul P, Zhu W, Huang HC, et al. Hypertrophic car-diomyopathy: an overview of genetics and management. Biomolecules 2019; 9: 878. DOI: 10.3390/biom9120878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gannon MP, Link MS. Phenotypic variation and targeted therapy of hypertrophic cardiomyopathy using genetic animal models. Trends Cardiovasc Med 2019; 31: 20-31. [DOI] [PubMed] [Google Scholar]
- 21. Lee TM, Hsu DT, Kantor P, et al. Pediatric cardiomyopathies. Circ Res 2017; 121: 855-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabater-Molina M, Pérez-Sánchez I, Hernández Del Rincón JP, et al. Genetics of hypertrophic cardiomyopathy: a review of current state. Clin Genet 2018; 93: 3-14. [DOI] [PubMed] [Google Scholar]
- 23. Kittleson MD, Meurs KM, Harris SP. The genetic basis of hypertrophic cardiomyopathy in cats and humans. J Vet Cardiol 2015; 17 Suppl 1: S53-S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skinner JR, Winbo A, Abrams D, et al. Channelopathies that lead to sudden cardiac death: clinical and genetic aspects. Heart Lung Circ 2019; 28: 22-30. [DOI] [PubMed] [Google Scholar]
- 25. Ware WA, Reina-Doreste Y, Stern JA, et al. Sudden death associated with QT interval prolongation and KCNQ1 gene mutation in a family of English Springer Spaniels. J Vet Intern Med 2015; 29: 561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study. J Vet Intern Med 2018; 32: 930-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ettinger S, Yee K, Beardow A, et al. The cat is not a dog -cardiac biomarkers. ACVIM Forum, 2010. https://beta.vin.com/members/cms/project/defaultadv1.aspx?id=4504442&pid=11311&. [Google Scholar]
- 28. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol 2015; 17 Suppl 1: S244-S257. [DOI] [PubMed] [Google Scholar]
- 29. Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994-2001). J Feline Med Surg 2003; 5: 151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Massera D, McClelland RL, Ambale-Venkatesh B, et al. Prevalence of unexplained left ventricular hypertrophy by cardiac magnetic resonance imaging in MESA. J Am Heart Assoc 2019; 8: e012250. DOI: 10.1161/JAHA.119.012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015; 65: 1249-1254. [DOI] [PubMed] [Google Scholar]
- 32. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398-1403. [DOI] [PubMed] [Google Scholar]
- 33. Carlos Sampedrano C, Chetboul V, Mary J, et al. Prospective echocardiographic and tissue Doppler imaging screening of a population of Maine Coon cats tested for the A31P mutation in the myosin-binding protein C gene: a specific analysis of the heterozygous status. J Vet Intern Med 2009; 23: 91-99. [DOI] [PubMed] [Google Scholar]
- 34. Fries R, Heaney AM, Meurs KM. Prevalence of the myosin-binding protein C mutation in Maine Coon cats. J Vet Intern Med 2008; 22: 893-896. [DOI] [PubMed] [Google Scholar]
- 35. Oura TJ, Young AN, Keene BW, et al. A valentine-shaped cardiac silhouette in feline thoracic radiographs is primarily due to left atrial enlargement. Vet Radiol Ultrasound 2015; 56: 245-250. [DOI] [PubMed] [Google Scholar]
- 36. Winter MD, Giglio RF, Berry CR, et al. Associations between ‘valentine’ heart shape, atrial enlargement and cardiomyopathy in cats. J Feline Med Surg 2015; 17: 447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Côté E, Manning AM, Emerson D, et al. Assessment of the prevalence of heart murmurs in overtly healthy cats. J Am Vet Med Assoc 2004; 225: 384-388. [DOI] [PubMed] [Google Scholar]
- 38. Sangster JK, Panciera DL, Abbott JA, et al. Cardiac bio-markers in hyperthyroid cats. J Vet Intern Med 2014; 28: 465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Franchini A, Abbott JA, Lahmers S, et al. Clinical characteristics of cats referred for evaluation of subclinical cardiac murmurs. J Feline Med Surg 2021; 23: 708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rishniw M, Thomas WP. Dynamic right ventricular outflow obstruction: a new cause of systolic murmurs in cats. J Vet Intern Med 2002; 16: 547-552. [DOI] [PubMed] [Google Scholar]
- 41. Ferasin L, Ferasin H, Kilkenny E. Heart murmurs in apparently healthy cats caused by iatrogenic dynamic right ventricular outflow tract obstruction. J Vet Intern Med 2020; 34: 1102-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rials SJ, Wu Y, Ford N, et al. Effect of left ventricular hypertrophy and its regression on ventricular electrophysiology and vulnerability to inducible arrhythmia in the feline heart. Circulation 1995; 91: 426-430. [DOI] [PubMed] [Google Scholar]
- 43. Côté E, Jaeger R. Ventricular tachyarrhythmias in 106 cats: associated structural cardiac disorders. J Vet Intern Med 2008; 22: 1444-1446. [DOI] [PubMed] [Google Scholar]
- 44. Bartoszuk U Keene BW Baron Toaldo M, et al. Holter monitoring demonstrates that ventricular arrhythmias are common in cats with decompensated and compensated hypertrophic cardiomyopathy. Vet J 2019; 243: 21-25. [DOI] [PubMed] [Google Scholar]
- 45. Jackson BL, Lehmkuhl LB, Adin DB. Heart rate and arrhythmia frequency of normal cats compared to cats with asymptomatic hypertrophic cardiomyopathy. J Vet Cardiol 2014; 16: 215-225. [DOI] [PubMed] [Google Scholar]
- 46. Fox PR, Maron BJ, Basso C, et al. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: a new animal model similar to the human disease. Circulation 2000; 102: 1863-1870. [DOI] [PubMed] [Google Scholar]
- 47. Ferasin L, Ferasin H, Borgeat K. Twenty-four-hour ambulatory (Holter) electrocardiographic findings in 13 cats with non-hypertrophic cardiomyopathy. Vet J 2020; 264: 105537. DOI: 10.1016/j.tvjl.2020.105537. [DOI] [PubMed] [Google Scholar]
- 48. Kaneshige T, Machida N, Itoh H, et al. The anatomical basis of complete atrioventricular block in cats with hypertrophic cardiomyopathy. J Comp Pathol 2006; 135: 25-31. [DOI] [PubMed] [Google Scholar]
- 49. Kellum HB, Stepien RL. Third-degree atrioventricular block in 21 cats (1997-2004). J Vet Intern Med 2006; 20: 97-103. [DOI] [PubMed] [Google Scholar]
- 50. Colpitts ME, Fonfara S, Monteith G, et al. Characteristics and outcomes of cats with and without pacemaker placement for high-grade atrioventricular block. J Vet Cardiol 2021; 34: 37-47. [DOI] [PubMed] [Google Scholar]
- 51. Rohrbaugh MN, Schober KE, Rhinehart JD, et al. Detection of congestive heart failure by Doppler echocardiography in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2020; 34: 1091-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Porciello F, Rishniw M, Ljungvall I, et al. Sleeping and resting respiratory rates in dogs and cats with medically-controlled left-sided congestive heart failure. Vet J 2016; 207: 164-168. [DOI] [PubMed] [Google Scholar]
- 53. Ljungvall I, Rishniw M, Porciello F, et al. Sleeping and resting respiratory rates in healthy adult cats and cats with subclin-ical heart disease. J Feline Med Surg 2014; 16: 281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rush JE, Freeman LM, Fenollosa NK, et al. Population and survival characteristics of cats with hypertrophic cardio-myopathy: 260 cases (1990-1999). J Am Vet Med Assoc 2002; 220: 202-207. [DOI] [PubMed] [Google Scholar]
- 55. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009; 11 Suppl 1: S51-S61. [DOI] [PubMed] [Google Scholar]
- 56. Dickson D, Little CJL, Harris J, et al. Rapid assessment with physical examination in dyspnoeic cats: the RAPID CAT study. J Small Anim Pract 2018; 59: 75-84. [DOI] [PubMed] [Google Scholar]
- 57. Locatelli C, Pradelli D, Campo G, et al. Survival and prognostic factors in cats with restrictive cardiomyopathy: a review of 90 cases. J Feline Med Surg 2018; 20: 1138-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fossum TW, Miller MW, Rogers KS, et al. Chylothorax associated with right-sided heart failure in five cats. J Am Vet Med Assoc 1994; 204: 84-89. [PubMed] [Google Scholar]
- 59. Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010; 51: 540-547. [DOI] [PubMed] [Google Scholar]
- 60. Sigrist NE, Adamik KN, Doherr MG, et al. Evaluation of respiratory parameters at presentation as clinical indicators of the respiratory localization in dogs and cats with respiratory distress. J Vet Emerg Crit Care (San Antonio) 2011; 21: 13-23. [DOI] [PubMed] [Google Scholar]
- 61. Le Boedec K, Arnaud C, Chetboul V, et al. Relationship between paradoxical breathing and pleural diseases in dyspneic dogs and cats: 389 cases (2001-2009). J Am Vet Med Assoc 2012; 240: 1095-1099. [DOI] [PubMed] [Google Scholar]
- 62. Corcoran BM, Foster DJ, Fuentes VL. Feline asthma syndrome: a retrospective study of the clinical presentation in 29 cats. J Small Anim Pract 1995; 36: 481-488. [DOI] [PubMed] [Google Scholar]
- 63. Ward JL, Lisciandro GR, Keene BW, et al. Accuracy of point-of-care lung ultrasonography for the diagnosis of cardiogenic pulmonary edema in dogs and cats with acute dyspnea. J Am Vet Med Assoc 2017; 250: 666-675. [DOI] [PubMed] [Google Scholar]
- 64. Ward JL, Lisciandro GR, Ware WA, et al. Evaluation of point-of-care thoracic ultrasound and NT-proBNP for the diagnosis of congestive heart failure in cats with respiratory distress. J Vet Intern Med 2018; 32: 1530-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ruiz MD, Vessières F, Ragetly GR, et al. Characterization of and factors associated with causes of pleural effusion in cats. J Am Vet Med Assoc 2018; 253: 181-187. [DOI] [PubMed] [Google Scholar]
- 66. Wilkie LJ, Smith K, Luis Fuentes V. Cardiac pathology findings in 252 cats presented for necropsy; a comparison of cats with unexpected death versus other deaths. J Vet Cardiol 2015; 17 Suppl 1: S329-S340. [DOI] [PubMed] [Google Scholar]
- 67. Litster A, Atkins C, Atwell R. Acute death in heartworm-infected cats: unraveling the puzzle. Vet Parasitol 2008; 158: 196-203. [DOI] [PubMed] [Google Scholar]
- 68. Payne JR, Borgeat K, Brodbelt DC, et al. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol 2015; 17 Suppl 1: S318-S328. [DOI] [PubMed] [Google Scholar]
- 69. Vancheri F, Gibson D. Relation of third and fourth heart sounds to blood velocity during left ventricular filling. Br Heart J 1989; 61: 144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shah SN, Gangwani MK, Oliver TI. Mitral valve prolapse. In: Treasure Island (FL): StatPearls Publishing. Available at: http://www.ncbi.nlm.nih.gov/books/ NBK470288/. [PubMed] [Google Scholar]
- 71. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N-terminal probrain natriuretic peptide (NT-pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011; 25: 1010-1016. [DOI] [PubMed] [Google Scholar]
- 72. Wess G, Daisenberger P, Mahling M, et al. Utility of measuring plasma N-terminal pro-brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats: NT-proBNP and hypertrophic cardiomyopathy in cats. Vet Clin Pathol 2011; 40: 237-244. [DOI] [PubMed] [Google Scholar]
- 73. Lu T-L, Côté E, Kuo Y-W, et al. Point-of-care N-terminal pro B-type natriuretic peptide assay to screen apparently healthy cats for cardiac disease in general practice. J Vet Intern Med 2021; 35: 1663-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beatty J, Barrs V. Pleural effusion in the cat: a practical approach to determining aetiology. J Feline Med Surg 2010; 12: 693-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schall WD. Thoracentesis. Vet Clin North Am 1974; 4: 395-401. [DOI] [PubMed] [Google Scholar]
- 76. Probo M, Valenti V, Venco L, et al. Pleural lymphocyte-rich transudates in cats. J Feline Med Surg 2018; 20: 767-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fossum TW, Evering WN, Miller MW, et al. Severe bilateral fibrosing pleuritis associated with chronic chylothorax in five cats and two dogs. J Am Vet Med Assoc 1992; 201: 317-324. [PubMed] [Google Scholar]
- 78. Rozanski E. Diseases of the pleural space. In: Ettinger SJ, Feldman EC, Côté E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier Saunders, 2017, p 1136. [Google Scholar]
- 79. Guglielmini C, Diana A. Thoracic radiography in the cat: identification of cardiomegaly and congestive heart failure. J Vet Cardiol 2015; 17: S87-S101. [DOI] [PubMed] [Google Scholar]
- 80. Schober KE, Wetli E, Drost WT. Radiographic and echocardiographic assessment of left atrial size in 100 cats with acute left-sided congestive heart failure. Vet Radiol Ultrasound 2014; 55: 359-367. [DOI] [PubMed] [Google Scholar]
- 81. Benigni L, Morgan N, Lamb CR. Radiographic appearance of cardiogenic pulmonary oedema in 23 cats. J Small Anim Pract 2009; 50: 9-14. [DOI] [PubMed] [Google Scholar]
- 82. Ward JL, Lisciandro GR, DeFrancesco TC. Distribution of alveolar-interstitial syndrome in dogs and cats with respiratory distress as assessed by lung ultrasound versus thoracic radiographs. J Vet Emerg Crit Care (San Antonio) 2018; 28: 415-428. [DOI] [PubMed] [Google Scholar]
- 83. Wurtinger G, Henrich E, Hildebrandt N, et al. Assessment of a bedside test for N-terminal pro B-type natriuretic peptide (NT-proBNP) to differentiate cardiac from non-cardiac causes of pleural effusion in cats. BMC Vet Res 2017; 13: 394. DOI: 10.1186/s12917-017-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wells SM, Shofer FS, Walters PC, et al. Evaluation of blood cardiac troponin I concentrations obtained with a cage-side analyzer to differentiate cats with cardiac and noncardiac causes of dyspnea. J Am Vet Med Assoc 2014; 244: 425-430. [DOI] [PubMed] [Google Scholar]
- 85. Connolly DJ, Soares Magalhaes RJ, Fuentes VL, et al. Assessment of the diagnostic accuracy of circulating natri-uretic peptide concentrations to distinguish between cats with cardiac and non-cardiac causes of respiratory distress. J Vet Cardiol 2009; 11: S41-S50. [DOI] [PubMed] [Google Scholar]
- 86. Hanås S, Holst BS, Höglund K, et al. Effect of feline characteristics on plasma N-terminal-prohormone B-type natriuretic peptide concentration and comparison of a point-of-care test and an ELISA test. J Vet Intern Med 2020; 34: 1187-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duler L, Scollan KF, LeBlanc NL. Left atrial size and volume in cats with primary cardiomyopathy with and without congestive heart failure. J Vet Cardiol 2019; 24: 36-47. [DOI] [PubMed] [Google Scholar]
- 88. Humm K, Hezzell M, Sargent J, et al. Differentiating between feline pleural effusions of cardiac and non-cardiac origin using pleural fluid NT-proBNP concentrations. J Small Anim Pract 2013; 54: 656-661. [DOI] [PubMed] [Google Scholar]
- 89. Cheng W-C, Wilkie L, Kurosawa TA, et al. Immunohistological evaluation of von Willebrand Factor in the left atrial endocardium and atrial thrombi from cats with cardiomyopathy. Animals (Basel) 2021; 11: 1240. DOI: 10.3390/ani11051240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardio-graphic contrast in 89 cats with myocardial disease. J Vet Intern Med 2006; 20: 120-130. [DOI] [PubMed] [Google Scholar]
- 91. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013: 27: 1427-1436. [DOI] [PubMed] [Google Scholar]
- 92. Vititoe KP, Fries RC, Joslyn S, et al. Detection of intra-cardiac thrombi and congestive heart failure in cats using computed tomographic angiography. Vet Radiol Ultrasound 2018; 59: 412-422. [DOI] [PubMed] [Google Scholar]
- 93. Luis Fuentes V. Arterial thromboembolism: risks, realities and a rational first-line approach. J Feline Med Surg 2012; 14: 459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hassan MH, Abu-Seida AM, Torad FA, et al. Feline aortic thromboembolism: presentation, diagnosis, and treatment outcomes of 15 cats. Open Vet J 2020; 10: 340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wallack ST, Hornof WJ, Herrgesell EJ. Ultrasonographic diagnosis – small bowel infarction in a cat. Vet Radiol Ultrasound 2003; 44: 81-85. [DOI] [PubMed] [Google Scholar]
- 96. Lee M, Park N, Kim J, et al. Imaging diagnosis – acute mesenteric ischemia associated with hypertrophic car-diomyopathy in a cat. Vet Radiol Ultrasound 2015; 56: E44-E47. [DOI] [PubMed] [Google Scholar]
- 97. Hickey MC, Jandrey K, Farrell KS, et al. Concurrent diseases and conditions in cats with renal infarcts. J Vet Intern Med 2014; 28: 319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Griffiths IR, Duncan ID. Ischaemic neuromyopathy in cats. Vet Rec 1979; 104: 518-522. [DOI] [PubMed] [Google Scholar]
- 99. Klainbart S, Kelmer E, Vidmayer B, et al. Peripheral and central venous blood glucose concentrations in dogs and cats with acute arterial thromboembolism. J Vet Intern Med 2014; 28: 1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pouzot-Nevoret C, Barthélemy A, Goy-Thollot I, et al. Infrared thermography: a rapid and accurate technique to detect feline aortic thromboembolism. J Feline Med Surg 2018; 20: 780-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Smith SA, Tobias AH, Bruecker KA, et al. Arterial thromboembolism in cats: acute crisis in 127 cases (1992-2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med 2003; 17: 73-83. [DOI] [PubMed] [Google Scholar]
- 102. Laste NJ, Harpster NK. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977-1993. J Am Anim Hosp Assoc 1995; 31: 492-500. [DOI] [PubMed] [Google Scholar]
- 103. Gaschen F, Gaschen L, Seiler G, et al. Lethal peracute rhab-domyolysis associated with stress and general anesthesia in three dystrophin-deficient cats. Vet Pathol 1998; 35: 117-123. [DOI] [PubMed] [Google Scholar]
- 104. Borgeat K, Wright J, Garrod O, et al. Arterial thromboembolism in 250 cats in general practice: 2004-2012. J Vet Intern Med 2014; 28: 102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Welles EG. Antithrombotic | fibrinolytic factors. A review . Vet Clin North Am Small Anim Pract 1996; 26: 1111-1127. [DOI] [PubMed] [Google Scholar]
- 106. Guillaumin J, Gibson RM, Goy-Thollot I, et al. Thrombolysis with tissue plasminogen activator (TPA) in feline acute aortic thromboembolism: a retrospective study of 16 cases. J Feline Med Surg 2019; 21: 340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schoeman JP. Feline distal aortic thromboembolism: a review of 44 cases (1990-1998). J Feline Med Surg 1999; 1: 221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li RHL, Nguyen N, Stern JA, et al. Neutrophil extracellular traps in feline cardiogenic arterial thrombi: a pilot study. J Feline Med Surg. Epub ahead of print 20 September 2021. DOI: 10.1177/1098612X211044986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. deLaforcade A, Bacek L, Blais MC, et al. Consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE): domain 1-defining populations at risk. J Vet Emerg Crit Care (San Antonio) 2019; 29: 37-48. [DOI] [PubMed] [Google Scholar]
- 110. Hogan DF, Fox PR, Jacob K, et al. Secondary prevention of cardiogenic arterial thromboembolism in the cat: the double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J Vet Cardiol 2015; 17 Suppl 1: S306-S317. [DOI] [PubMed] [Google Scholar]
- 111. Lo ST, Walker AL, Georges CJ, et al. Dual therapy with clopidogrel and rivaroxaban in cats with thromboembolic disease. J Feline Med Surg. Epub ahead of print 10 May 2021. DOI: 10.1177/1098612X211013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Blaisdell FW. The reperfusion syndrome. Microcirc Endothelium Lymphatics 1989; 5: 127-141. [PubMed] [Google Scholar]
- 113. Welch KM, Rozanski EA, Freeman LM, et al. Prospective evaluation of tissue plasminogen activator in 11 cats with arterial thromboembolism. J Feline Med Surg 2010; 12: 122-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moore K, Morris N, Dhupa N, et al. Retrospective study of streptokinase administration in 46 cats with arterial throm-boembolism. J Vet Emerg Crit Care 2000; 10: 245-257. [Google Scholar]
- 115. Vezzosi T, Buralli C, Briganti A, et al. Surgical embolectomy in a cat with cardiogenic aortic thromboembolism. J Vet Cardiol 2020; 28: 48-54. [DOI] [PubMed] [Google Scholar]
- 116. Reimer SB, Kittleson MD, Kyles AE. Use of rheolytic thrombectomy in the treatment of feline distal aortic thromboembolism. J Vet Intern Med 2006; 20: 290-296. [DOI] [PubMed] [Google Scholar]
- 117. Macedo E, Mehta R. Prerenal azotemia in congestive heart failure. In: Ronco C, Costanzo MR, Bellomo R, et al. (eds). Contributions to nephrology. Vol 164. Basel: KARGER, 2010, pp 79-87. Available at: https://www.karger.com/Article/FullText/313723. [DOI] [PubMed] [Google Scholar]
- 118. Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Ren Inj Prev 2015; 4: 57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Heller J, Kramer HJ, Horácek V. Comparative effects of the angiotensin II receptor blocker EXP 3174 and of the angiotensin-converting enzyme inhibitor captopril on renal glomerular hemodynamics in the dog. Kidney Blood Press Res 1997; 20: 391-397. [DOI] [PubMed] [Google Scholar]
- 120. Liu M, Köster LS, Fosgate GT, et al. Cardiovascular-renal axis disorder and acute-phase proteins in cats with congestive heart failure caused by primary cardiomyopathy. J Vet Intern Med 2020; 34: 1078-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Novo Matos J, Pereira N, Glaus T, et al. Transient myocardial thickening in cats associated with heart failure. J Vet Intern Med 2018; 32: 48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Smith S, Tobias A, Fine D, et al. Corticosteroid-associated congestive heart failure in 12 cats. Int J Appl Res Vet Med 2004; 2: 159-170. [Google Scholar]
- 123. Block CL, Oyama MA. Echocardiographic and biomarker evidence of plasma volume expansion after short-term steroids administered orally in cats. J Vet Intern Med 2020; 34: 29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hogan DF. Arterial thromboembolic disease. In: Ettinger SJ, Feldman EC, Côté E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017, pp 1344-1348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 6 Phonocardiogram of the same audio recording as in supplementary file 5 (systolic click in a cat). The heart rate is 240 beats/min and the interval between S1 and S2 (similar to the QT interval on an ECG) is 120 ms, which is appropriate for that heart rate. The interval between S1 and C is only 80 ms, meaning that C cannot be S2. S1 = first heart sound; C = systolic click; S2 = second heart sound.