Abstract
Enforced Bcl-2 expression inhibits Myc-induced apoptosis and cooperates with Myc in transformation. Here we report that the synergy between Bcl-2 and Myc in transforming hematopoietic cells in fact reflects a Myc-induced pathway that selectively suppresses the expression of the Bcl-XL or Bcl-2 antiapoptotic protein. Myc activation suppresses Bcl-XL RNA and protein levels in cultures of primary myeloid and lymphoid progenitors, and Bcl-XL and Bcl-2 expression is inhibited by Myc in precancerous B cells from Eμ-myc transgenic mice. The suppression of bcl-X RNA levels by Myc requires de novo protein synthesis, indicating that repression is indirect. Importantly, the suppression of Bcl-2 or Bcl-XL by Myc is corrupted during Myc-induced tumorigenesis, as Bcl-2 and/or Bcl-XL levels are markedly elevated in over one-half of all lymphomas arising in Eμ-myc transgenic mice. Bcl-2 and/or Bcl-XL overexpression did not correlate with loss of ARF or p53 function in tumor cells, indicating that these two apoptotic pathways are inactivated independently. Therefore, the suppression of Bcl-XL or Bcl-2 expression represents a physiological Myc-induced apoptotic pathway that is frequently bypassed during lymphomagenesis.
Many cancers harbor alterations that directly or indirectly lead to constitutive overexpression of the c-Myc oncoprotein (reviewed in reference 3). In most cell types, c-Myc enforces S phase entry (10, 37, 54), although activation of c-Myc also triggers the apoptotic program (reviewed in reference 47). In vivo, activation of apoptosis by c-Myc is evident in the B cells of Eμ-myc transgenic mice, which have intrinsically high proliferative and apoptotic rates (26). Ultimately, secondary genetic changes make these B cells refractory to the Myc apoptotic response, resulting in the outgrowth of clonal pre-B- and B-cell lymphomas (1).
c-Myc activates the ARF-Mdm2-p53 tumor suppressor pathway, which is frequently disabled in human cancers (reviewed in reference 56). c-Myc activation leads to the rapid accumulation of p19ARF (64), a nucleolar protein encoded by an alternative reading frame of the Ink4a/ARF locus (49). In turn, ARF activates p53 both through nucleolar sequestration of p53's inhibitor Mdm2 (59, 62) and by interference with Mdm2 E3 ubiquitin ligase activity (22). Mdm2 is a transcriptional target of p53 that inhibits p53-dependent transactivation (43) and induces p53 ubiquitination (21) and its shuttling to the cytoplasm for destruction by the 26S proteasome (52). Thus, in the presence of oncoproteins such as c-Myc, high ARF levels inhibit Mdm2, allowing a robust p53 transcriptional response that triggers apoptosis (64).
In the majority of lymphomas that arise in Eμ-myc transgenic mice, c-Myc overexpression selects for loss of ARF and/or p53 function (11, 55). Moreover, loss of ARF or p53 markedly accelerates Myc-induced tumor development (11, 23, 25, 55). Although these cooperative effects are associated with a decreased apoptotic rate, even rapidly arising tumors from ARF-null Eμ-myc transgenic mice are clonal (C. M. Eischen and J. L. Cleveland, unpublished data), indicating that additional alterations are required during Myc-induced lymphomagenesis. Furthermore, in primary fibroblasts and pre-B cells the loss of ARF or p53 impairs, but does not fully abolish, c-Myc-induced apoptosis (11, 64). Thus other targets must contribute to the c-Myc apoptotic response.
Bcl-2 or Bcl-XL overexpression blocks many cell death pathways, including those induced by c-Myc (6, 13). Apoptosis induced by c-Myc in hematopoietic cells is effectively suppressed by cytokines, yet high levels of c-Myc override the protective effects of these survival factors (12, 47). Therefore, Myc-induced cell death also likely hinges on proteins regulated by hemopoietins. Potential targets include the Bcl-2 family of proteins, which can either suppress (e.g., Bcl-2, Bcl-XL, and Mcl-1) or augment (e.g., Bax, Bad, and Bak) the apoptotic program (reviewed in reference 17). Although these proteins are regulated by posttranslational modifications and changes in their subcellular localization (reviewed in reference 33), alterations in their steady-state levels also play a pivotal role in hematopoietic cell survival. First, in myeloid progenitors cytokines selectively regulate Bcl-XL expression and apoptosis by a Jak2 kinase-dependent pathway (48). Second, loss of bcl-X in mice results in high levels of apoptosis in embryonic hematopoietic cells (44), whereas bcl-2-deficient mice display profound apoptosis of mature lymphocytes, which disappear by 4 to 6 weeks of age (45, 61). Finally, Bcl-2 transgenes effectively block the severe defects in T-cell lymphopoiesis seen in mice lacking either the interleukin-7 (IL-7) receptor or the common γ chain (2, 32) and enable macrophage production in mice lacking macrophage colony-stimulating factor (CSF-1) (34). Therefore, the appropriate expression of antiapoptotic Bcl-2 family proteins is critical for hematopoietic cell survival.
In vivo, the programmed expression of Bcl-2 in B cells blocks the intrinsically high rates of apoptosis of Eμ-myc transgenic B cells and cooperates with Myc to induce rapid primitive lymphoid tumors (57). We now report that this cooperation reflects an apoptotic pathway induced by c-Myc that selectively suppresses the expression of Bcl-XL or Bcl-2 in hematopoietic cells. Furthermore, Myc-induced suppression of Bcl-2 or Bcl-XL is disabled in over half of the lymphomas arising in Eμ-myc transgenic mice and occurs independently of ARF/p53 status.
MATERIALS AND METHODS
Primary cells.
Myeloid progenitors from the fetal livers of embryonic day 15 (E15) to E17 embryos or from the bone marrow of 6- to 8-week-old bcl-2−/−, p53−/−, ARF−/−, p53 ARF double-null, and wild-type mice were cultured in RPMI 1640 medium supplemented with IL-3 (20 U/ml), IL-6 (10 ng/ml; R&D Systems), and stem cell factor (SCF) (10 ng/ml; R&D Systems) (48). The phenotypes of the cells by fluorescence-activated cell sorting (FACS) were uniformly CD34+, c-Kit+, and Sca-I+. All antibodies used for phenotyping were from Southern Biotechnology (Birmingham, Ala.) or PharMingen (San Diego, Calif.).
Primary pre-B-cell cultures were generated from the bone marrow of wild-type, bcl-2−/−, ARF−/−, and/or p53-null mice as described previously (11). Immunophenotyping established that all cultures were greater than 98% pre-B cells (i.e., CD19+ CD43− CD24+ immunoglobulin M− [IgM−]). B cells (IgM+ CD19+) and B-cell precursors (IgM− CD19+) from bone marrow and spleens of age- and gender-matched wild-type and Eμ-myc transgenic mice (prior to signs of disease) were sorted by FACS after being stained with anti-IgM–fluorescein isothiocyanate and anti-CD19–phycoerythrin.
Virus infection.
Primary myeloid and pre-B cells were infected with the murine stem cell virus (MSCV) Myc-estrogen receptorTM (ERTM)-internal ribosome entry site (IRES)-green fluorescent protein (GFP) virus or with the MSCV-IRES-GFP control virus, as previously described (11). Comparable levels of Myc-ERTM fusion protein in all cultures were established by immunoblotting. The phenotype of the Myc-ERTM virus-infected myeloid progenitors was indistinguishable from that of uninfected or MSCV-GFP virus-infected myeloid cell cultures. The Myc-ERTM chimeric protein was activated by the addition of 1 μM 4-hydroxytamoxifen (4-HT) (Sigma, St. Louis, Mo.). Addition of 1 μM 4-HT to uninfected or MSCV-GFP virus-infected cells had no effect on myeloid or pre-B-cell growth or viability.
Viability and apoptosis assays.
Cell viability was determined at specific intervals by trypan blue dye exclusion following cytokine deprivation or the addition of 1 μM 4-HT to activate Myc-ERTM. Apoptosis was quantitated by measuring fragmented DNA (sub-G1) by flow cytometry following propidium iodide staining.
Transgenic and knockout mice.
The inbred C57BL/6 Eμ-myc transgenic mouse strain was provided by Alan Harris (Walter & Eliza Hall Institute, Melbourne, Australia) and Charles Sidman (University of Cincinnati). We generated ARF+/− Eμ-myc and ARF −/− Eμ-myc transgenic mice as previously described (11). Fifth-generation Teconic 129S6/SvEv backcrossed bcl-2−/− mice were generated, and the control 129S6/SvEv strain was purchased from Taconic Laboratories (Germantown, N.Y.). p53- and ARF-null mice were kindly provided by Gerard Grosveld and Charles Sherr (St. Jude Children's Research Hospital), respectively.
Western blotting.
Whole-cell protein extracts were isolated as previously described (11). Equal amounts of protein (20 to 125 μg/lane) were separated in sodium dodecyl sulfate–7.5 or 10% polyacrylamide gel electrophoresis gels. Proteins were transferred to nitrocellulose (Protran; Schleicher & Schuell, Dassel, Germany) and blotted with antibodies specific for murine c-Myc (06-340) and Bak (both from Upstate Biotechnology, Lake Placid, N.Y.); p19ARF (49); p53 (AB-7; Calbiochem, La Jolla, Calif.); Bcl-2 (15021) and Bax (13686E) (both from PharMingen); Bcl-XL (B2260), Mcl-1 (B54020), and Bad (B36420) (all three from Transduction Labs, San Diego, Calif.); and β-actin (Amersham, Arlington Heights, Ill.). Bound immunocomplexes were detected by enhanced chemiluminescence.
Northern blotting.
Following addition of 1 μM 4-HT, primary pre-B and myeloid cells were harvested at the intervals indicated on the figures and total RNA was isolated using Trizol reagent (Life Technologies, Grand Island, N.Y.). Forty or 20 μg of total RNA from pre-B cells or myeloid cells, respectively, was run into formaldehyde agarose gels and transferred to nitrocellulose (Protran; Schleicher & Schuell). The membranes were probed sequentially with the coding portions of murine bcl-X, bcl-2, and β-actin cDNAs and stripped after each hybridization and autoradiography. For the cycloheximide experiment, primary pre-B cells were pretreated for 30 min at 37°C with 10 μg of cycloheximide/ml or vehicle control prior to the addition of 4-HT.
RESULTS
Loss of ARF and/or p53 protracts, but does not abolish, Myc-induced apoptosis of hematopoietic cells.
In primary mouse embryo fibroblasts (MEFs) and pre-B cells, c-Myc-induced apoptosis involves the activation of the ARF-Mdm2-p53 apoptotic pathway (11, 64). However, MEFs lacking ARF or p53 are not totally resistant to Myc-induced apoptosis (64), indicating that Myc also activates apoptosis independent of ARF or p53. To study such effects in primary hematopoietic cells, we isolated fetal liver- and bone marrow-derived myeloid progenitors and bone marrow-derived pre-B cells from wild-type, ARF-null, p53-null, and/or ARF p53 double-null mice. Primary myeloid progenitors (CD34+ c-Kit+ ScaI+ Lin−) derived from E15 to E17 fetal livers or bone marrow were cultured in IL-3, IL-6, and SCF (48), whereas primary pre-B cells (CD19+ IgM− CD24+ CD43−) derived from bone marrow were grown in medium containing IL-7 (11). FACS analysis demonstrated that loss of ARF and/or p53 had no overt effect on the myeloid or pre-B-cell phenotype (11) (data not shown).
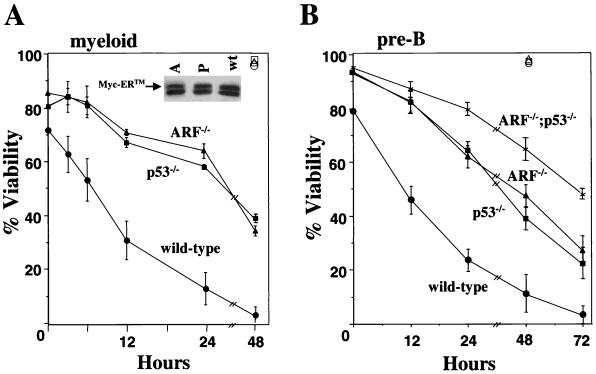
Primary myeloid and pre-B cells were infected with the MSCV-Myc-ERTM-GFP recombinant retrovirus or with the MSCV-GFP control virus. The MSCV-Myc-ERTM-GFP retrovirus encodes a conditionally active form of c-Myc, in which c-Myc is fused to the hormone binding domain of the ER (Myc-ERTM) modified to respond to 4-HT (36). Furthermore, GFP is expressed in cis via an IRES, allowing for direct selection of infected cells. Immunoblotting analysis revealed that comparable levels of Myc-ERTM protein were expressed in cells with the different genotypes (Fig. 1A, inset) (11). When grown in complete medium containing both serum and cytokines, wild-type myeloid and pre-B cells infected with the Myc-ERTM retrovirus exhibited a higher apoptotic index (20 to 30%) than uninfected cells, those infected with control vector, and ARF- and p53-null cells infected with the Myc-ERTM retrovirus (Fig. 1 and data not shown). This basal level of Myc-induced apoptosis in wild-type hematopoietic cells is likely due to the somewhat leaky nature of the Myc-ERTM construct (64). Activation of Myc-ERTM by 4-HT induced rapid cell death of wild-type myeloid (Fig. 1A) and pre-B (Fig. 1B) cells, despite the presence of potent survival factors in the medium. Importantly, although myeloid and pre-B cells lacking ARF and/or p53 were more resistant to 4-HT-induced Myc-ERTM activation, they ultimately underwent apoptosis in the presence of cytokines (Fig. 1). By 48 or 72 h following Myc activation, <40% of the ARF- or p53-null myeloid and pre-B cells, respectively, were alive, and all cells eventually died. Thus, effectors other than ARF and p53 must contribute to c-Myc-induced hematopoietic cell apoptosis.
FIG. 1.
Myc-induces apoptosis in hematopoietic cells lacking ARF and/or p53. 4-HT was added to the culture medium of wild-type, ARF-null, and/or p53-null primary myeloid precursors (A) and pre-B cells (B) infected with a retrovirus encoding Myc-ERTM, and at the indicated intervals the percentages of viable cells were assessed by trypan blue dye exclusion. Data shown for the myeloid cells are the means of five (wild type), four (ARF−/−), and two (p53−/−) independent experiments, and three independent experiments were performed with pre-B cells. Error bars represent one standard deviation. Open symbols, viability of wild-type (triangle), ARF-null (circle), and p53-null (square) cells containing GFP vector controls following 4-HT addition for the indicated intervals. (Inset) Immunoblotting of ARF−/− (A), p53−/− (P), and wild-type (wt) Myc-ERTM retrovirus-infected primary myeloid cells with a Myc-specific antibody. Arrow, location of Myc-ERTM. Myc-ERTM was expressed at similar levels in pre-B cells derived from wild-type, ARF-null, p53-null, and ARF p53 double-null mice (11).
Hematopoietic cell apoptosis induced by cytokine deprivation is independent of ARF and p53 but is augmented by the loss of bcl-2.
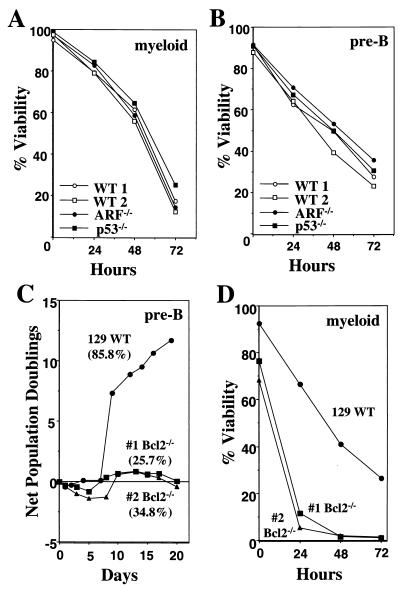
Myc activation renders cells hypersensitive to many apoptotic insults including the withdrawal of survival factors, which suppress c-Myc-induced apoptosis (4, 12, 19). To address the relevance of ARF and p53 to apoptosis that follows the withdrawal of survival factors, we compared the rates of death of wild-type, ARF-null, and p53-null primary hematopoietic cells following removal of their required hemopoietins. Both ARF- and p53-null primary myeloid and pre-B cells grew at accelerated rates (data not shown) (11). However, when deprived of cytokines these myeloid (Fig. 2A) and pre-B (Fig. 2B) cells died at rates essentially identical to those derived from wild-type mice. Thus, ARF and p53 do not regulate hematopoietic cell apoptosis triggered by cytokine deprivation.
FIG. 2.
Apoptosis induced by cytokine withdrawal is independent of ARF or p53 yet is accelerated by bcl-2 loss. Wild-type (WT), ARF-null, or p53-null myeloid progenitors were deprived of IL-3, IL-6, and SCF (A), and wild-type, ARF-null, or p53-null pre-B cells were deprived of IL-7 (B). At the indicated intervals the percentages of viable cells were assessed by trypan blue dye exclusion. Results shown are representative of three independent experiments. (C) Growth curves of bone marrow cells from two bcl-2-deficient mice and one 129/SvEv wild-type mouse in media containing IL-7. The mean percentages of viable cells between days 12 to 17 of culture are in parentheses; standard deviations were less than 5% for all three cultures. (D) Wild-type or bcl-2−/− myeloid progenitors were deprived of IL-3, IL-6, and SCF, and at indicated intervals the percentages of viable cells were assessed by trypan blue dye exclusion. Data shown are representative of two independent experiments. Apoptosis was confirmed by analysis of subdiploid DNA content after propidium iodide staining.
Potential mediators of apoptosis induced by cytokine deprivation include members of the Bcl-2 family. Gene targeting studies have demonstrated that Bcl-2 and Bcl-X are rate limiting for hematopoietic cell survival (44, 45, 61). However, only bcl-2-deficient mice are amenable to analyses, as bcl-X-deficient mice die at E13.5 (44). We therefore derived primary pre-B cells and myeloid progenitors from the bone marrow of bcl-2−/− mice and assessed their rates of apoptosis following cytokine deprivation. Strikingly, bcl-2−/− pre-B cells had a very high apoptotic index and therefore could not be expanded in tissue culture (Fig. 2C). After 12 to 17 days in culture, pre-B cells lacking bcl-2 were only 25 to 35% viable, whereas cells from wild-type mice were healthy and readily expanded (Fig. 2C). Although bcl-2−/− primary myeloid progenitors only had a slightly higher apoptotic index in complete medium (Fig. 2D), they died at an accelerated rate when deprived of cytokines (Fig. 2D). The majority of the bcl-2-deficient myeloid cells were dead within 24 h after cytokines were removed, whereas only a small fraction of the wild-type progenitors died during this interval (Fig. 2D). Thus, bcl-2 loss potentiates the apoptotic program initiated when hematopoietic cells are deprived of survival factors.
c-Myc suppresses Bcl-XL expression in primary myeloid and pre-B cells.
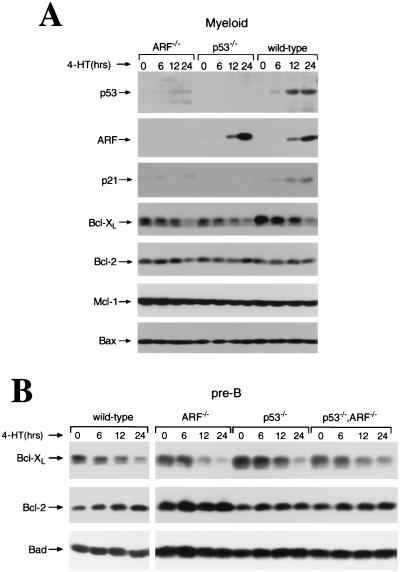
Given the profound effects of bcl-2 loss on hematopoietic cell survival, we assessed the effects of c-Myc on the expression of Bcl-2 family proteins. Activation of Myc-ERTM by 4-HT in wild-type primary myeloid cells revealed, as expected, the induction of ARF and p53 in wild-type myeloid cells, whereas the induction of p53 and p53 transcriptional target p21Cip1 was severely impaired in ARF-null cells (Fig. 3A). As previously reported for MEFs and pre-B cells (11, 64), Myc activation induced ARF in wild-type and p53-null primary myeloid cells. Notably, Myc activation in primary myeloid and pre-B cells led to an obvious and selective reduction in Bcl-XL levels without affecting the expression of Bcl-2 or of proapoptotic proteins Bax, Bad, and Bak (Fig. 3 and data not shown). The levels of the antiapoptotic Mcl-1 protein in the primary myeloid cells were also not altered (Fig. 3A), and Mcl-1 was not detected in any of the pre-B cell cultures (data not shown). One prediction was that Myc-mediated suppression of Bcl-XL was ARF and/or p53 dependent. However, Myc activation in ARF−/−, p53−/−, and ARF p53 double-null myeloid and pre-B cells also resulted in rapid and similar reductions in Bcl-XL levels (Fig. 3). Therefore, Myc activation selectively represses Bcl-XL protein expression in primary hematopoietic cells, and this occurs independent of ARF and/or p53 status.
FIG. 3.
Myc selectively suppresses the expression of Bcl-XL protein in primary hematopoietic cells independent of ARF and p53 status. (A) The expression of p53, ARF, p21Cip1, Bcl-XL, Bcl-2, Mcl-1, and Bax protein was assessed at the indicated intervals by immunoblotting extracts prepared from Myc-ERTM retrovirus-infected primary myeloid precursors following activation of Myc-ERTM by 4-HT. (B) Expression of Bcl-XL, Bcl-2, and Bad protein was assessed at the indicated intervals by immunoblotting extracts prepared from primary pre-B cells harboring Myc-ERTM following activation by 4-HT.
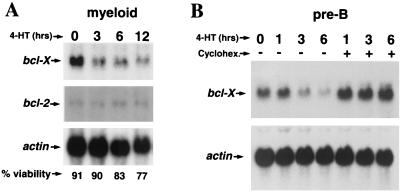
To address whether the decrease in Bcl-XL protein levels following Myc activation was due to changes in bcl-X transcripts, we performed Northern blot analysis of RNA isolated from primary myeloid and pre-B cells infected with the Myc-ERTM retrovirus. The levels of bcl-X RNA decreased rapidly (within 3 h) following Myc activation by 4-HT in both cell types, whereas the levels of bcl-2 transcripts remained unchanged (Fig. 4). The selective suppression of bcl-X transcripts was not simply a secondary effect of cell death, as at 3 h Myc-ERTM-activated cells were as viable as untreated cells (time zero) (Fig. 4A). Notably, new protein synthesis was required for Myc to suppress bcl-X RNA levels (Fig. 4B). Cycloheximide blocked the Myc-induced decrease in bcl-X expression in pre-B cells, indicating that Myc suppresses bcl-X levels by an indirect mechanism. Interestingly, bcl-X transcripts appear to be somewhat induced by cycloheximide, as levels of the transcripts were higher than those in untreated cells (Fig. 4B). Therefore, in primary hematopoietic cells Myc selectively suppresses bcl-X RNA expression and this occurs at either a transcriptional or posttranscriptional level and requires new protein synthesis.
FIG. 4.
New protein synthesis is required for the suppression of bcl-X RNA levels by Myc. The expression of bcl-X and bcl-2 transcripts from primary myeloid cells (A) and bcl-X transcripts from pre-B cells (B) expressing Myc-ERTM was assessed following no pretreatment (A) or a 30-min pretreatment with 10 μg of cycloheximide/ml or vehicle control followed by activation of Myc-ERTM with 4-HT for the indicated intervals. Hybridization with a β-actin probe was used to equalize loading of RNA.
Bcl-2 and Bcl-XL expression is suppressed in precancerous Eμ-myc transgenic B cells.
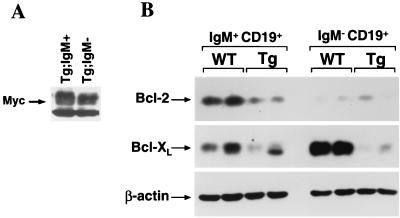
To establish whether Myc also influenced Bcl-XL expression in vivo, we harvested bone marrow from wild-type and Eμ-myc transgenic littermates prior to any detectable disease and sorted B-cell subsets by FACS. B-cell populations were sorted for the pan-B-cell marker CD19 and for the expression of cell surface IgM. The promoter/enhancer used to express the Myc transgene is utilized at the pro- to pre-B-cell stage of B-cell differentiation and stays on throughout B-cell development (1). Therefore, as expected, the levels of Myc in IgM− precursor B cells and the more mature IgM+ B cells were not different (Fig. 5A). Consistent with previous reports (16, 40), we observed high Bcl-XL expression and low Bcl-2 expression in wild-type IgM− B-cell progenitors but low Bcl-XL levels and high Bcl-2 levels in mature IgM+ B cells (Fig. 5B). Comparison of Bcl-XL and Bcl-2 expression revealed that IgM+ B cells from Eμ-myc transgenic mice had markedly reduced levels of Bcl-2 protein relative to those expressed in IgM+ cells from wild-type mice (Fig. 5B). The low levels of Bcl-2 in IgM− (CD19+) B-cell precursors from transgenic and wild-type mice did not differ from each other (Fig. 5B). In contrast, Bcl-XL protein levels were drastically reduced in the IgM− B-cell progenitors from Eμ-myc transgenic mice compared with those in B-cell progenitors from wild-type mice; these differences were less obvious in IgM+ B cells (Fig. 5B). Therefore, c-Myc overexpression selectively suppresses Bcl-XL or Bcl-2 expression in vivo in a cell context-specific fashion.
FIG. 5.
Bcl-2 or Bcl-XL levels are suppressed in B-cell subsets from precancerous Eμ-myc transgenic mice. The levels of Myc (A) and Bcl-2, Bcl-XL, and β-actin (B) proteins in mature (IgM+ CD19+) and precursor (IgM− CD19+) B cells from FACS-sorted bone marrow from two wild-type (WT) and two Eμ-myc transgenic (Tg) mice (age and gender matched) were assessed by immunoblotting with antibodies specific for each protein.
Bcl-2 or Bcl-XL or both are overexpressed in lymphomas arising in Eμ-myc transgenic mice.
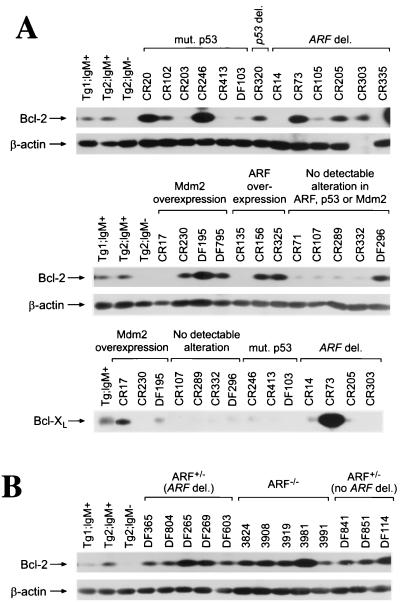
If suppression of Bcl-XL or Bcl-2 expression by c-Myc is relevant to apoptosis in vivo, then this pathway should be disabled in pre-B- and B-cell lymphomas arising in Eμ-myc transgenic mice. Previously we demonstrated that most of the fatal lymphomas (80%) of Eμ-myc transgenic mice have alterations in the ARF-Mdm2-p53 pathway (11). However, the remaining 20% of tumors arising in ARF+/+ Eμ-myc transgenic mice and 8% of the tumors of ARF+/− Eμ-myc transgenic mice lacked alterations in ARF, p53, or Mdm2. We therefore assessed the expression of Bcl-2 and Bcl-XL in this group of lymphomas. We compared their levels to those of Bcl-2 and Bcl-XL present in FACS-sorted B cells derived from precancerous Eμ-myc bone marrow, which expressed reduced levels of Bcl-2 or Bcl-XL relative to levels expressed by wild-type B cells (Fig. 5B). In tumors lacking alterations in ARF, p53, or Mdm2, only two tumors, one from an ARF+/+ Eμ-myc transgenic mouse (DF296) and one from an ARF+/− Eμ-myc transgenic mouse (DF114), overexpressed Bcl-2 and none had elevated levels of Bcl-XL (Fig. 6A and data not shown). However, 56% (14 of 25) of lymphomas from ARF+/+ Eμ-myc transgenic mice expressed much higher levels of Bcl-2 and/or Bcl-XL than those expressed in precancerous B cells (Fig. 6A and data not shown). Bcl-2 was overexpressed in 13 of 25 tumors (CR20, CR102, CR246, CR320, CR73, CR205, CR303, CR230, DF195, DF795, CR156, CR325, and DF296), whereas 2 of 25 tumors (CR73 and CR17) overexpressed Bcl-XL. Only one tumor (CR73) overexpressed both Bcl-2 and Bcl-XL. Therefore, over half of the lymphomas arising in Eμ-myc transgenic mice overexpress Bcl-2 and/or Bcl-XL, indicating that the suppression of Bcl-2 or Bcl-XL expression by Myc in precancerous B cells is bypassed during lymphoma progression.
FIG. 6.
Bcl-2 is overexpressed in over half of the lymphomas arising in Eμ-myc transgenic mice. Levels of Bcl-2, Bcl-XL, and β-actin proteins in tumors from wild-type (A) and from ARF +/− and ARF −/− (B) Eμ-myc transgenic mice were assessed by immunoblotting with antibodies specific for each protein. IgM− B-cell precursors and mature IgM+ B cells were sorted by FACS from bone marrow and spleens of precancerous Eμ-myc transgenic mice and run as controls for Bcl-2 and Bcl-XL expression.
Bcl-2 levels are higher in IgM+ B cells than in IgM− B-cell precursors (Fig. 5B). We determined by FACS that approximately 73% (27 of 37) of Eμ-myc lymphomas arising in Eμ-myc C57BL/6 transgenic mice are IgM+ or a mixture of IgM+ and IgM− (CD19+), whereas only 27% (10 of 39) are IgM− (CD19+) (C. M. Eischen and J. L. Cleveland, unpublished data). The fact that the majority of our Eμ-myc transgenic mice develop B-cell instead of pre-B-cell lymphomas differs from an early report indicating that only 19% of Eμ-myc transgenic mice develop B-cell lymphomas (20). The genetic backgrounds of our Eμ-myc transgenic mice (congenic C57BL/6) and their Eμ-myc transgenic mice (C57BL/6J Wehi × SJL/J Wehi F1 hybrids) are significantly different, and that is the most likely explanation for these discrepancies. Nevertheless, the expression of Bcl-2 is markedly suppressed by Myc in precancerous IgM+ mature B cells (Fig. 5B). The preponderance of mature B-cell lymphomas and Bcl-2 overexpression in these tumors thus reflects a bypass of this pathway in the more mature B cell and may explain why Bcl-2 is more frequently overexpressed in Eμ-myc lymphomas than Bcl-XL (Fig. 6).
In the lymphomas we found no correlation between Bcl-2 and Bcl-XL expression levels and Mdm2 overexpression, p53 mutation, or ARF deletion (Fig. 6A). Eμ-myc lymphomas with mutated or deleted p53 or deleted ARF were analyzed, and 8 of 13 (62%) expressed high levels of Bcl-2 and/or Bcl-XL. Furthermore, in many lymphomas from ARF+/− Eμ-myc transgenic mice, where 80% harbor deletions of the wild-type ARF allele (11), and in the rapidly arising tumors of ARF nullizygous Eμ-myc transgenic mice, Bcl-2 or Bcl-XL or both were also expressed at abnormally high levels (9 of 13, 69%) (Fig. 6B and data not shown). Thus, disabling the ARF-Mdm2-p53 pathway and loss of Myc-mediated suppression of Bcl-2 or Bcl-XL occur independently during Myc-induced lymphomagenesis.
DISCUSSION
Myc suppresses Bcl-XL and Bcl-2 expression in hematopoietic cells.
Acquiring resistance to Myc-induced apoptosis must occur as cells proceed toward malignancy. The ability of Myc to induce ARF and activate p53 leads to apoptosis, and this inhibits tumor development (11, 55, 64). The ARF-Mdm2-p53 pathway is therefore disabled in most of the lymphomas from Eμ-myc transgenic mice (11, 25, 55). Here we demonstrate that Myc activation in wild-type primary pre-B and myeloid progenitor cells results in a reduction of Bcl-XL levels and that this also occurs in cells lacking ARF and/or p53. In precancerous B-cell subsets of Eμ-myc transgenic mice, Myc suppresses either Bcl-XL or Bcl-2 expression, depending on cell context, whereas over half of the lymphomas arising in these transgenic mice overexpress Bcl-2 or Bcl-XL. Furthermore, the corruption of this second Myc-induced apoptotic pathway occurs independent of ARF, Mdm2, or p53 status in these lymphomas.
Prior to overt lymphoma, B-cell precursors from Eμ-myc transgenic mice have high apoptotic indices in the bone marrow, which are offset by the elevated proliferative rates of premalignant B cells (26). FACS-sorted B-cell subsets from precancerous Eμ-myc transgenic mice had decreased levels of Bcl-2 or Bcl-XL protein (Fig. 5B), whereas the levels of proapoptotic Bcl-2 family members did not change (C. M. Eischen and J. L. Cleveland, unpublished data). This logically should result in apoptosis, as bcl-2- and bcl-X-deficient hematopoietic progenitors are highly prone to apoptosis (Fig. 2) (44). Most Bcl-2 family members appear to control apoptosis by regulating the release of cytochrome c from mitochondria, which activates the caspase-9 regulator Apaf-1 (17). The ratio of pro- and antiapoptotic Bcl-2 family members regulates the susceptibility of cells to apoptosis (33); thus, decreased levels of Bcl-2 or Bcl-XL without changes in proapoptotic proteins in the B cells of Eμ-myc transgenic mice should account for their increased susceptibility to apoptosis. Furthermore, the suppression of Bcl-2 or Bcl-XL expression by Myc independent of ARF or p53 status supports observations by Juin and colleagues that in fibroblasts Myc induces a p53-independent release of cytochrome c from mitochondria, thus facilitating apoptosis (27).
Bcl-2 family members play important roles in programmed cell deaths that occur when cells are deprived of survival factors (48). This is underscored by the high apoptotic index of Bcl-2- and Bcl-XL-deficient hematopoietic progenitors and their accelerated rates of death following deprivation of cytokines (Fig. 2) (44). By contrast, the rates of apoptosis of primary hematopoietic cells lacking p53 or ARF are identical to those of wild-type progenitors when deprived of cytokines. Therefore the suppression of Bcl-2 or Bcl-XL by Myc is more likely to mediate Myc's ability to override the protective effects of survival factors in hematopoietic cells.
Mechanism of Myc-induced Bcl-2 and/or Bcl-XL suppression.
The mechanism by which Myc induces Bcl-2 or Bcl-XL suppression in primary cells is not resolved, yet is most likely transcriptional and indirect. In support of this notion, Myc activation in primary pre-B and myeloid cells results in rapid reductions in bcl-X RNA levels. This Myc-induced decrease in bcl-X RNA requires new protein synthesis, which suggests that Myc controls the expression of a regulator of bcl-X. Whether Myc's transactivation or transrepression functions are required for this response is not resolved. However, it is interesting that cycloheximide induces increases in bcl-X transcripts (Fig. 4B), suggesting that it may remove a labile repressor and that Myc's transrepression functions appear necessary for Myc-induced apoptosis (8). Thus, a model emerges whereby Myc transrepresses a gene or a set of genes that are necessary for maintaining bcl-X expression.
The underlying mechanism(s) by which Bcl-2 or Bcl-XL or both are no longer suppressed by Myc but are rather overexpressed in the lymphomas arising in Eμ-myc transgenic mice is also not resolved but is not a result of gene amplifications or gross rearrangements of the genes by translocations or retrovirus insertions (data not shown). Inactivation of the c-Myb or Pim-1 oncogenes or the induction of the p53 or p16Ink4a tumor suppressors has been shown to down-regulate levels of bcl-2 transcripts (15, 29, 35, 42, 60). We have thus far been unable to implicate any of these proteins in the suppression of Bcl-2 or Bcl-XL by Myc. c-Myc has no effect on c-Myb or Pim-1 expression in myeloid cells (J. L. Cleveland, unpublished data), and the inactivation of p53 or the deletion of the INK4a/ARF locus in lymphomas from Eμ-myc transgenic mice does not necessarily restore Bcl-2 or Bcl-XL levels (Fig. 6). However, our data do support the concept that oncoproteins and tumor suppressors that regulate the apoptotic program do indeed target Bcl-2 or Bcl-XL expression. Notably, overexpression of Bcl-2 cooperates with Myc in accelerating lymphomagenesis in Eμ-bcl-2 Eμ-myc double-transgenic mice (57), and our studies now provide an explanation for this observation. Moreover, these findings may explain why oncogenes that induce Bcl-2, such as v- or c-Myb (15, 60), Pim-1 (35), Ras (30), and BCR-ABL (53), cooperate with Myc in transformation (reviewed in references 24 and 63).
Cooperation of Myc with the ARF-p53 pathway and Bcl-2 and/or Bcl-XL in lymphomagenesis.
Analyses of lymphomas arising in Eμ-myc transgenic mice indicate that Bcl-2 and/or Bcl-XL overexpression and p53 mutation or ARF loss are selected for independently during Myc-induced tumorigenesis. p53 or ARF inactivation occurs in over 70% of human cancers (18), while Bcl-2 or Bcl-XL is overexpressed in many tumor types (51). Genetic studies of mice have demonstrated essential roles for p53 and ARF in inhibiting tumor development (5, 9, 28), whereas the evidence linking the Bcl-2 family of proteins to cancer is less obvious. Although Bcl-2 was cloned as the translocation product in human follicular lymphomas, Bcl-2 overexpression alone is poor at inducing tumors in transgenic mice (38, 58). Nonetheless, inactivating frameshift mutations in proapoptotic Bcl-2 family member Bax are found in adenocarcinomas of the colon (50) and in some human hematopoietic malignancies (39). In addition, Bcl-XL expression is activated by retrovirus insertions in murine myeloid and T-cell leukemias (48).
Myc's induction of the ARF-Mdm2-p53 pathway or the suppression of Bcl-2 or Bcl-XL expression alone results in apoptosis, but when combined both events ensure a complete and rapid cell death response. The ARF-Mdm2-p53 pathway mediates cell cycle arrest and apoptosis, whereas Bcl-2 and Bcl-XL inhibit cell death. Thus, cells that have lost p53 or ARF function and that overexpress Bcl-2 and/or Bcl-XL should resist growth arrest and apoptosis and continue to cycle under growth-limiting conditions, such as those that occur in the tumor microenvironment. Therefore, inactivating both pathways should provide cells with an even greater survival advantage. Forty-four percent of the lymphomas arising in Eμ-myc transgenic mice that are inactivated in the ARF-Mdm2-p53 pathway, 60% of tumors from ARF+/− Eμ-myc transgenic mice bearing deletions of the wild-type allele of ARF, and 80% of tumors from ARF−/− Eμ-myc transgenic mice overexpressed Bcl-2 and/or Bcl-XL. Therefore disabling both Myc-induced pathways appears to provide a selective advantage to Myc-overexpressing B cells. Consistent with this notion, p53 mutation and Bcl-XL overexpression cooperate to favor the accumulation of cells with genetic damage (41).
Myc appears to independently target Bcl-2 and/or Bcl-XL expression and the ARF-Mdm2-p53 pathway, yet the precise mechanisms by which Myc regulates these pathways are unresolved and remain important issues. It will also be interesting to further evaluate Myc-induced tumors that have disabled both pathways, as they should prove more resistant to treatment. Finally, these findings appear to be directly relevant to human cancers such as Burkitt's lymphoma, where p53 mutations, Ink4A/ARF inactivation, and Mdm2 and Bcl-2 overexpression have all been observed (7, 14, 31, 46).
ACKNOWLEDGMENTS
We thank Charles Sherr and Gerard Zambetti for many helpful discussions and for critical review of the manuscript, Robert Hawley and Derek Persons for retrovirus vectors, Alan Harris and Charles Sidman for providing breeders for Eμ-myc mice, and Richard Cross for superb assistance with FACS. We also appreciate the outstanding technical support of Elsie White, Chunying Yang, and Rose Mathew.
This work was supported in part by National Institutes of Health (NIH) grants CA76379 and DK44158 (J.L.C.), CA71907 and CA56819 (M.F.R.), and DK45663 and DK40700 (D.W.); Cancer Center core grant CA-21765; and NIH postdoctoral grant CA81695 (C.M.E.) and by the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children's Research Hospital.
REFERENCES
- 1.Adams J M, Harris A W, Pinkert C A, Corcoran L M, Alexander W S, Cory S, Palmiter R D, Brinster R L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 2.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo K, Koskinen P, Makela T P, Saksela K, Sistonen L, Winqvist R. myc oncogenes: activation and amplification. Biochim Biophys Acta. 1987;907:1–32. doi: 10.1016/0304-419x(87)90016-3. [DOI] [PubMed] [Google Scholar]
- 4.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 5.Attardi L D, Jacks T. The role of p53 in tumour suppression: lessons from mouse models. Cell Mol Life Sci. 1999;55:48–63. doi: 10.1007/s000180050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissonnette R P, Echeverri F, Mahboubi A, Green D R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- 7.Capoulade C, Bressac-de Paillerets B, Lefrere I, Ronsin M, Feunteun J, Tursz T, Wiels J. Overexpression of MDM2, due to enhanced translation, results in inactivation of wild-type p53 in Burkitt's lymphoma cells. Oncogene. 1998;16:1603–1610. doi: 10.1038/sj.onc.1201702. [DOI] [PubMed] [Google Scholar]
- 8.Conzen S D, Gottlob K, Kandel E S, Khanduri P, Wagner A J, O'Leary M, Hay N. Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis. Mol Cell Biol. 2000;20:6008–6018. doi: 10.1128/mcb.20.16.6008-6018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 10.Eilers M, Schirm S, Bishop J M. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 1991;10:133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eischen C M, Weber J D, Roussel M F, Sherr C J, Cleveland J L. Disruption of the ARF-Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 13.Fanidi A, Harrington E A, Evan G I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 14.Finke J, Fritzen R, Ternes P, Trivedi P, Bross K J, Lange W, Mertelsmann R, Dolken G. Expression of bcl-2 in Burkitt's lymphoma cell lines: induction by latent Epstein-Barr virus genes. Blood. 1992;80:459–469. [PubMed] [Google Scholar]
- 15.Frampton J, Ramqvist T, Graf T. v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 1996;10:2720–2731. doi: 10.1101/gad.10.21.2720. [DOI] [PubMed] [Google Scholar]
- 16.Grillot D A, Merino R, Pena J C, Fanslow W C, Finkelman F D, Thompson C B, Nune G. Bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J Exp Med. 1996;183:381–391. doi: 10.1084/jem.183.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross A, McDonnell J M, Korsmeyer S J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 18.Hainaut P, Soussi T, Shomer B, Hollstein M, Greenblatt M, Hovig E, Harris C C, Montesano R. Database of p53 gene somatic mutations in human tumors and cell lines: updated compilation and future prospects. Nucleic Acids Res. 1997;25:151–157. doi: 10.1093/nar/25.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington E A, Bennett M R, Fanidi A, Evan G I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris A W, Pinkert C A, Crawford M, Langdon W Y, Brinster R L, Adams J M. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167:353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 22.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu B, Marin M C, el-Naggar A K, Stephens L C, Brisbay S, McDonnell T J. Evidence that c-myc mediated apoptosis does not require wild-type p53 during lymphomagenesis. Oncogene. 1995;11:175–179. [PubMed] [Google Scholar]
- 24.Hueber A O, Evan G I. Traps to catch unwary oncogenes. Trends Genet. 1998;14:364–367. doi: 10.1016/s0168-9525(98)01520-0. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs J J, Scheijen B, Voncken J W, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13:2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen K A, Prasad V S, Sidman C L, Osmond D G. Apoptosis and macrophage-mediated deletion of precursor B cells in the bone marrow of E mu-myc transgenic mice. Blood. 1994;84:2784–2794. [PubMed] [Google Scholar]
- 27.Juin P, Hueber A O, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka M, Wiehle S, Spitz F, Schumacher G, Roth J A, Cristiano R J. Down-regulation of bcl-2 is associated with p16INK4-mediated apoptosis in non-small cell lung cancer cells. Oncogene. 2000;19:1589–1595. doi: 10.1038/sj.onc.1203466. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita T, Yokota T, Arai K, Miyajima A. Regulation of Bcl-2 expression by oncogenic Ras protein in hematopoietic cells. Oncogene. 1995;10:2207–2212. [PubMed] [Google Scholar]
- 31.Klangby U, Okan I, Magnusson K P, Wendland M, Lind P, Wiman K G. p16/INK4a and p15/INK4b gene methylation and absence of p16/INK4a mRNA and protein expression in Burkitt's lymphoma. Blood. 1998;91:1680–1687. [PubMed] [Google Scholar]
- 32.Kondo M, Akashi K, Domen J, Sugamura K, Weissman I L. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 33.Korsmeyer S J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(Suppl.):1693s–1700s. [PubMed] [Google Scholar]
- 34.Lagasse E, Weissman I L. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–1031. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- 35.Lilly M, Sandholm J, Cooper J J, Koskinen P J, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999;18:4022–4031. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- 36.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mateyak M K, Obaya A J, Adachi S, Sedivy J M. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 38.McDonnell T J, Korsmeyer S J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 39.Meijerink J P, Mensink E J, Wang K, Sedlak T W, Sloetjes A W, de Witte T, Waksman G, Korsmeyer S J. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- 40.Merino R, Ding L, Veis D J, Korsmeyer S J, Nune G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minn A J, Boise L H, Thompson C B. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 42.Miyashita T, Krajewski S, Krajewska M, Wang H G, Lin H K, Liebermann D A, Hoffman B, Reed J C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 43.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 44.Motoyama N, Wang F, Roth K A, Sawa H, Nakayama K I, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh D Y. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama K, Negishi I, Kuida K, Sawa H, Loh D Y. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newcomb E W. P53 gene mutations in lymphoid diseases and their possible relevance to drug resistance. Leukoc Lymphoma. 1995;17:211–221. doi: 10.3109/10428199509056825. [DOI] [PubMed] [Google Scholar]
- 47.Packham G, Cleveland J L. c-Myc and apoptosis. Biochim Biophys Acta. 1995;1242:11–28. doi: 10.1016/0304-419x(94)00015-t. [DOI] [PubMed] [Google Scholar]
- 48.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nunez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quelle D E, Zindy F, Ashmun R A, Sherr C J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 50.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed J C, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 51.Reed J C, Miyashita T, Takayama S, Wang H G, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez-Garcia I, Grut G. Tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL2. Proc Natl Acad Sci USA. 1995;92:5287–5291. doi: 10.1073/pnas.92.12.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santoni-Rugiu E, Falck J, Mailand N, Bartek J, Lukas J. Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol Cell Biol. 2000;20:3497–3509. doi: 10.1128/mcb.20.10.3497-3509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt C A, McCurrach M E, de Stanchina E, Wallace-Brodeur R R, Lowe S W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherr C J, Weber J D. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 57.Strasser A, Harris A W, Bath M L, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 58.Strasser A, Harris A W, Cory S. E mu-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene. 1993;8:1–9. [PubMed] [Google Scholar]
- 59.Tao W, Levine A J. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor D, Badiani P, Weston K. A dominant interfering Myb mutant causes apoptosis in T cells. Genes Dev. 1996;10:2732–2744. doi: 10.1101/gad.10.21.2732. [DOI] [PubMed] [Google Scholar]
- 61.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 62.Weber J D, Taylor L J, Roussel M F, Sherr C J, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 63.Weston K. Reassessing the role of C-MYB in tumorigenesis. Oncogene. 1999;18:3034–3038. doi: 10.1038/sj.onc.1202728. [DOI] [PubMed] [Google Scholar]
- 64.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]