Abstract
The RNA polymerase II transcription factor TFIID comprises the TATA binding protein (TBP) and a set of TBP-associated factors (TAFIIs). TFIID has been extensively characterized for yeast, Drosophila, and humans, demonstrating a high degree of conservation of both the amino acid sequences of the constituent TAFIIs and overall molecular organization. In recent years, it has been assumed that all the metazoan TAFIIs have been identified, yet no metazoan homologues of yeast TAFII47 (yTAFII47) and yTAFII65 are known. Both of these yTAFIIs contain a histone fold domain (HFD) which selectively heterodimerizes with that of yTAFII25. We have cloned a novel mouse protein, TAFII140, containing an HFD and a plant homeodomain (PHD) finger, which we demonstrated by immunoprecipitation to be a mammalian TFIID component. TAFII140 shows extensive sequence similarity to Drosophila BIP2 (dBIP2) (dTAFII155), which we also show to be a component of Drosophila TFIID. These proteins are metazoan homologues of yTAFII47 as their HFDs selectively heterodimerize with dTAFII24 and human TAFII30, metazoan homologues of yTAFII25. We further show that yTAFII65 shares two domains with the Drosophila Prodos protein, a recently described potential dTAFII. These conserved domains are critical for yTAFII65 function in vivo. Our results therefore identify metazoan homologues of yTAFII47 and yTAFII65.
Transcription factor TFIID is one of the general factors required for accurate and regulated initiation by RNA polymerase II. TFIID comprises the TATA binding protein (TBP) and TBP-associated factors (TAFIIs) (3, 24). A subset of TAFIIs are present not only in TFIID but also in the SAGA, PCAF, STAGA, and TFTC complexes (5, 16, 22, 33, 38, 53).
The function of TAFIIs has been studied using in vitro transcription systems which have provided evidence that they act as specific coactivators by interacting directly with transcriptional activator proteins (48). Such studies also indicated that TAFIIs contribute to promoter recognition and promoter selectivity (8, 10, 49). Genetic evidence obtained from TAFII knockout and depletion experiments and temperature-sensitive (TS) mutants indicates that TAFIIs are involved in the control of gene expression in yeast and mammalian cells and that they act as antiapoptotic factors (11, 23, 37; for reviews, see references 2, 3, and 23).
TFIID has been characterized for Drosophila, humans, and yeast (7, 14, 40, 56). After its initial purification, many subunits were rapidly identified and the corresponding genes were cloned. Sequence comparisons allowed the identification of TAFIIs in each species sharing one or more conserved domains. Many of these domains correspond to structural motifs, such as the histone fold domain (HFD), the β-transducin repeat, and bromodomain, or catalytic domains, such as the histone acetyltransferase domain. These results indicate that the composition and organization of TFIID has been well conserved between yeast and humans (reviewed in reference 16).
In recent years, it has been assumed that all the mammalian TFIID components have been identified. Several observations, however, challenge this assumption. Characterization of highly purified yeast TFIID (yTFIID) has identified two novel TFIID subunits, yTAFII48 (Mpt1) and yTAFII65 (42, 45). While yTAFII48 is the homologue of human TAFII135 (hTAFII135) in yeast (18, 42), no obvious metazoan homologues of yTAFII65 have yet been identified. TAFII65 contains an HFD which selectively heterodimerizes with yTAFII25 (17). Furthermore, a second yTAFII, yTAFII47 (52), contains an HFD and selectively heterodimerizes also with yTAFII25 (17). At present, no homologues of yTAFII47 and yTAFII65 in metazoans have been identified, yet a yTAFII25 homologue has been well characterized for humans (hTAFII30 [27]), and for Drosophila, two homologues have been identified (Drosophila TAFII24 [dTAFII24] and dTAFII16 [19]). Metazoan TFIID must therefore contain a heterodimerization partner(s) for hTAFII30, and dTAFII16, and dTAFII24, which would be considered homologues of yTAFII47 and/or yTAFII65.
In database searches with the yTAFII47 HFD, we previously identified metazoan proteins with HFDs similar to that of yTAFII47 (17). Here, we describe mouse TAFII140 (mTAFII140) and hTAFII140, novel proteins containing an HFD with strong similarity to that of yTAFII47 and a plant homeodomain (PHD) finger. We show that the mouse and human TAFII140 HFDs selectively and directly heterodimerize with that of hTAFII30. Immunoprecipitation experiments confirm that hTAFII140 is a bone fide TAFII which can be immunopurified from HeLa cell extracts under stringent conditions with TBP and other identified hTAFIIs. Mammalian TAFII140 shows high sequence similarity to the Drosophila protein BIP2 (bric-a-brac interacting protein 2), which also contains an HFD and PHD finger. We show that the dBIP2 HFD selectively and directly heterodimerizes with dTAFII24 and that BIP2 is a component of dTFIID.
The Drosophila Prodos (PDS) is a protein essential for cell viability which has been shown to comprise an HFD which selectively heterodimerizes with dTAFII16. Consequently, it has been proposed that PDS may be a dTFIID component (25). Here, we show that PDS shares sequence similarity with yTAFII65 both in the HFD and in a second domain (designated the TAFII65-PDS [TAP] domain [TAPD]) C terminal of the HFD. We show that the yTAFII65 HFD and the TAPD are partially redundant functional domains required for yeast viability. The fact that PDS shares the HF and TAP domains which are required for yTAFII65 function in vivo, along with its observed heterodimerization with dTAFII16, indicates that PDS is the Drosophila homologue of yTAFII65.
MATERIALS AND METHODS
Cloning of mTAFII140 and hTAFII140.
The cDNA encoding mTAFII140 HFD was isolated by reverse transcription (RT)-PCR using mouse F9 embryonal carcinoma cell RNA and oligonucleotide primers based on expressed sequence tag (EST) sequences. To isolate the full-length cDNA, an F9 embryonal carcinoma cDNA library was screened with the 32P-labeled HFD fragment. A clone comprising the 5′ untranslated region (UTR) and amino acids 1 to 340 was isolated. A second overlapping clone encoding amino acids 238 to 747 was isolated by screening the same library with oligonucleotide probes derived from EST sequences. The 3′ end of the cDNA was cloned by RT-PCR using an oligonucleotide primer at amino acid 651 and primers in the 3′ UTR derived from EST sequences. The partial cDNA sequences were cloned in pBSK and sequenced. The three cDNAs were assembled by overlapping PCR into two partially overlapping fragments. In one fragment, a BamHI site was introduced at nucleotide 1286 (GGCTCA-GGATCC) without changing the amino acid sequence. This fragment was cloned into the EcoRI-BamHI sites of expression vector pXJ41 (54). A fragment from nucleotide 1286 to the end of the coding sequence was amplified with primers containing BamHI and XhoI sites, and the resulting fragment was cloned into the pXJ41 vector containing the 5′ fragment, thus reconstituting the full open reading frame. The hTAFII140 cDNA fragment containing the 5′ UTR and genes for amino acids 1 to 727 was cloned by screening a HeLa cell cDNA library with an oligonucleotide corresponding to genes for amino acids 54 to 72 of the HFD based on EST sequences. All constructs were verified by automated DNA sequencing, and further details are available upon request.
Preparation of antibodies and immunoprecipitations.
Antibodies against human and mouse TAFII140 were generated against a synthetic peptide corresponding to amino acids 108 to 127 of hTAFII140 (as indicated in Fig. 2) coupled to ovalbumin. Monoclonal antibodies were generated as previously described (32). Two resulting monoclonal antibodies, 1C7 and 2F5, were used. The anti-BIP2 polyclonal antiserum was collected from rabbits immunized with a bacterially expressed glutathione S-transferase (GST)-fusion protein comprising amino acid residues 719 to 909 of the BIP2 protein. The anti-SAP130 and GCN5 antibodies were as previously described (M. Brand, J. G. Moogs, F. Lejeune, J. Stevenin, M. Oulad-Abdelghani, G. Almouzni, and L. Tora, submitted for publication). The anti-TBP monoclonal antibodies 2C1 and 3G3 and the anti-TAFII monoclonal antibodies were as previously described (6, 13, 27, 32, 35, 36). The polyclonal antisera against dTBP, dTAFII16, dTAFII24, and dTAFII230 were as previously described (19, 28). Immunoprecipitations of HeLa cell nuclear extracts with monoclonal antibody 2C1 and elutions with the epitope peptide were performed as previously described (6, 27, 35, 36). Immunoprecipitations of Drosophila embryo extracts were performed as previously described (19). Western blottings were performed by standard techniques, and the proteins were detected using an ECL kit (Amersham) and autoradiography.
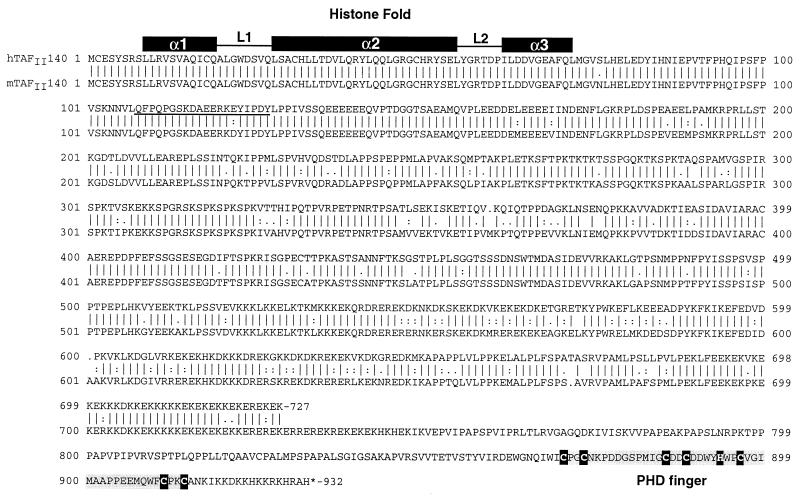
FIG. 2.
The amino acid sequences of mTAFII140 and hTAFII140 are aligned. The positions of the HFD and PHD fingers are shown. In the PHD finger, the critical cysteine and histidine residues are shown in white letters. The amino acid sequences used to generate monoclonal antibodies are underlined. The alignment was generated using the GCG program.
Two-hybrid and coexpression assays.
All yeast and bacterial expression vectors were constructed by PCR using primers with the appropriate restriction sites, and constructs were verified by automated DNA sequencing. Details of constructions are available on request. LexA fusions were constructed in the multicopy vector pBTM116 containing the TRP1 marker and the VP16 fusions were constructed in the multicopy vector pASV3 containing the LEU2 marker (18), and assays were performed with the L40 strain as previously described (17, 50). Quantitative β-galactosidase assays on individual L40 transformants were determined as previously described (18). Reproducible results were obtained in several independent experiments, and the results of a typical experiment are shown in the figures.
Yeast complementation assays.
Complementation assays were performed by the plasmid shuffle technique. Wild-type or mutated yTAFIIs were cloned in the multicopy pAS3 plasmid with a LEU marker. All yeast strains were transformed by the LiAc technique. Yeast strain YSLS58 that was used for plasmid shuffling of TAF65 was derived from YSLS41 (45) by sporulation and tetrad dissection as previously described (17). For complementation assays, the indicated rescue plasmids were transformed and the wild-type TAF (URA3) plasmid was shuffled out by two passages on media containing 5-fluoroorotic acid. In all experiments, cultures were grown in yeast-peptone-dextrose unless selection was necessary, in which case cultures were grown in the appropriate selective synthetic dextrose (SD) medium.
Immunostaining of Drosophila embryos.
The 0- to 2-h and 2- to 24-h embryos were dechorionated, and antibody staining and diaminobenzidine visualization were performed as previously described (44). The anti-BIP2 polyclonal antibody was diluted 1/3,000, and the peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) was diluted 1/1,000.
Immunostaining of Drosophila salivary gland polytene chromosomes.
Squashes of polytene chromosomes and antibody stainings were performed as previously described (57). The anti-BIP2 primary antibody was diluted 1/200. The Cy3-conjugated secondary antibodies were diluted 1/500. DNA was stained with Hoechst 33258.
Nucleotide sequence accession numbers.
The sequences reported here have been assigned the following EMBL database accession numbers: mTAFII140, AJ292189; hTAFII140, AJ292190; dTAFII155, AJ292191.
RESULTS
HFD of mTAFII140 heterodimerizes with hTAFII30.
We previously reported the existence of a putative HFD at the N terminus of yTAFII47 which is necessary and sufficient for yeast viability (17). This HFD shows strong sequence similarity to those of the hTAFII135/ADA1/H2A family and mediates a selective heterodimerization with the yTAFII25 HFD (17). Database searches with the yTAFII47 HFD identified several metazoan proteins with potential HFDs located at their N termini (reference 17 and Fig. 1A).
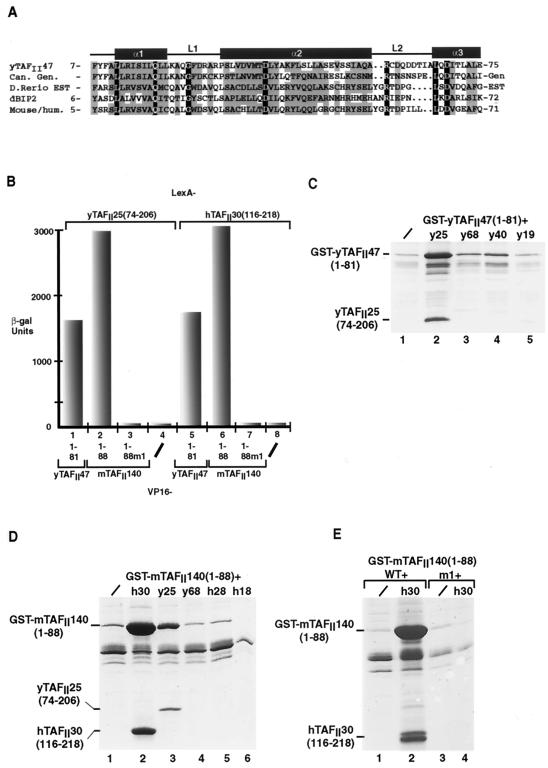
FIG. 1.
Functional analysis of the HFD from a putative mouse and human yTAFII47 homologue. (A) Alignment of the HFD sequence of yTAFII47 with those of its putative Candida albicans (Can), zebrafish (Danio rerio), Drosophila melanogaster (dBIP2), and human and mouse homologues. The positions of the predicted α-helices and loops are indicated above the sequences. Identical amino acids are shown in white letters on a black background. Positions with conserved, mainly hydrophobic amino acids are boxed in gray. Amino acids were classified as follows: small residues, P, A, G, S, T; hydrophobic residues, L, I, V, A, F, M, C, Y, W; polar and acidic residues, D, E, Q, N; basic residues, R, K, H. Threonine residues are occasionally present in otherwise hydrophobic positions. The amino acid sequences shown without numbers are predicted from genomic (gen) or EST sequences. The accession numbers for the indicated sequences are as follows: C. albicans, 396380B03; zebrafish, GenBank accession no. AW343321fi76b06.y1; Drosophila BIP2, Q9XZU7; mouse, GenBank accession no. AA692266ur52c07. (B) Graphical representation of quantitative two-hybrid β-galactosidase assays. The LexA chimeras shown above the graph were tested with the VP16 chimeras shown below each column. −, negative controls with the VP16 domain alone. (C, D, and E) Coexpression of the yTAFII47 and mTAFII140 HFDs with those of other TAFIIs in E. coli cells. Bacteria were transformed to express the proteins shown above each lane, and following extract preparation, the soluble protein retained on glutathione-Sepharose beads was analyzed by SDS-PAGE and staining with Coomassie brilliant blue. /, GST-fusion was expressed alone. The locations of the GST-yTAFII and GST-mTAFII fusions and the retained yTAFII25 and hTAFII30 proteins are indicated.
To determine whether the HFD, which is identical in the human and murine proteins, would heterodimerize with hTAFII30, two-hybrid experiments were performed in yeast by using LexA fusions of the hTAFII30 HFD and VP16 fusions of the mouse protein (hereafter designated mTAFII140). As previously described (17), strong interactions between the HFDs of yTAFII47 and yTAFII25 or hTAFII30 were observed (Fig. 1B, lanes 1 and 5). Similarly, the mTAFII140 HFD also interacted strongly with both the yTAFII25 and hTAFII30 HFDs (Fig. 1B, lanes 2 and 6). These interactions were abolished by mutation of residue W23 (W23R) in the L1 loop of mTAFII140 HFD [mTAFII140(1-88)m1] (Fig. 1B, lanes 3 and 7).
Direct heterodimerization of the mTAFII140 and hTAFII30 HFDs was tested by coexpression in E. coli (17, 18). When expressed alone, a GST fusion of the yTAFII47 or mTAFII140 HFDs was poorly soluble [Fig. 1C and D, lane 1, GST-yTAFII47(1-81) and GST-mTAFII140(1-88)]. In contrast, when coexpressed with the yTAFII25 HFD, GST-yTAFII47(1-88) was solubilized and formation of a heterodimeric complex was observed (Fig. 1C, lane 2, and reference 17). Similarly, GST-mTAFII140(1-88) was also solubilized when coexpressed with the yTAFII25 and hTAFII30 HFDs, and the formation of a heterodimeric complex was observed in each case (Fig. 1D, lanes 2 and 3, and Fig. 1E, lane 2). Solubilization by the hTAFII30 HFD and complex formation was abolished by the W23R mutation, which eliminated interaction in yeast (Fig. 1E, lane 4). No heterodimerization was seen, however, when the yTAFII47 or mTAFII140 HFDs were coexpressed with the HFDs of other TAFIIs (Fig. 1C, lanes 3 to 5, Fig. 1D, lanes 4 to 6, and data not shown).
Together, these results indicate that the HFD of mTAFII140 selectively and directly heterodimerizes with hTAFII30, indicating that it may be a novel component of mammalian TFIID.
TAFII140 is a HeLa cell TFIID subunit.
The full-length mTAFII140 open reading frame was cloned by a combination of screening an F9 cell cDNA library and RT-PCR using information from overlapping clones in the EST databases (see Materials and Methods). Mouse TAFII140 comprises 932 amino acids with the HFD located between amino acids 9 and 72 (Fig. 2). Interestingly, mTAFII140 also possesses a single PHD finger of the type Cys4-His-Cys3 originally described for the HAT3.1, Polycomb-like, and HRX proteins (1, 47) (sometimes also called the leukemia-associated protein [LAP] domain [43]) at the C terminus of the protein. This PHD finger is preceded by a highly charged region comprising multiple lysine and glutamic acid residues and a region rich in proline and other small residues (Fig. 2).
A partial cDNA encoding the first 727 amino acids of hTAFII140 was also isolated by screening a HeLa cell cDNA library. Comparison with mTAFII140 showed that the HFD was identical in the two proteins which otherwise share strong sequence similarity (Fig. 2). Although we could not isolate a full-length clone for hTAFII140, database searches identified a genomic clone and ESTs encoding a highly related PHD finger followed by a stop codon, as in mTAFII140 (Fig. 4B). This provides strong evidence for the existence of an hTAFII140 protein with an overall structure analogous to that of mTAFII140.
FIG. 4.
(A) Alignment of the mTAFII140 and dBIP2 amino acid sequences. The locations of the histone fold and PHD fingers are indicated. (B) Comparison of the PHD fingers of mTAFII140, hTAFII140, and dBIP2 with some of those previously characterized. Identical amino acids are shown in white letters against a black background. Critical cysteine and histidine residues are indicated (∗).
Monoclonal antibodies were raised against a peptide of hTAFII140 downstream of the HFD (Fig. 2, underlined), which is almost perfectly conserved between humans and mice. This monoclonal antibody recognizes a protein with a molecular mass of around 140 kDa in Cos cells transfected with an mTAFII140 expression vector (Fig. 3A, lane 2). Although no endogenous protein was seen with small amounts of control untransfected Cos cell extract (Fig. 3A, lane 1), hTAFII140 was clearly detected in more-concentrated HeLa cell nuclear extract (lane 3). The antibody also recognized a nuclear protein in HeLa cells in immunofluorescence experiments (data not shown).
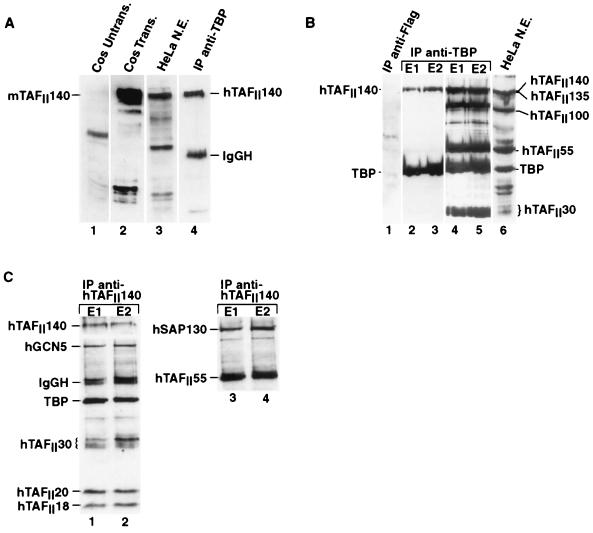
FIG. 3.
Immunoprecipitations. (A) The origin of each extract is shown above each lane. Cos Untrans, 1 μg of untransfected Cos cell extract; Cos Trans, 1 μg of extract from Cos cells transfected with 5 μg of pXJ41-mTAFII140; HeLa N.E., 15 μg of HeLa cell nuclear extract; IP anti-TBP, TFIID immunoprecipitated with monoclonal antibody 2C1 and eluted with the corresponding peptide. The positions of mTAFII140 and hTAFII140 are indicated along with that of residual 2C1 IgGH detected by the secondary conjugated antibody used in ECL. All lanes were probed with anti-TAFII140 antibody 1C7. (B) The layout is the same as that in panel A, with the origin of the protein indicated above each lane. E1 and E2 indicate the first and second elutions of the 2C1 immunoprecipitation with the epitope peptide. The first lane shows a control immunoprecipitation with anti-Flag antibody m2. Lanes 1 to 3 were probed with the anti-TBP antibody 3G3 and the anti-hTAFII140 antibody 2F5. Lanes 4 and 5 show the same blots that are in lanes 2 and 3, probed with additional antibodies; lane 6 shows HeLa cell nuclear extract incubated with all of the antibodies. (C) HeLa cell nuclear extract was precipitated with monoclonal antibody 1C7, and the precipitates eluted with the epitope peptide. The blot was probed with the antibodies used to detect the proteins indicated. In lanes 3 and 4, the blot shown in lanes 1 and 2 was reprobed with the indicated antibodies after inactivation of the first signal by prolonged incubation with ECL.
To determine whether hTAFII140 was a component of immunopurified TFIID, HeLa cell extracts were precipitated with the anti-TBP monoclonal antibody 2C1 (32) and the TFIID was eluted by using the 2C1 epitope peptide. HeLa cell TAFII140 was clearly detected in the eluted TFIID (Fig. 3A, lane 4). HeLa cell TAFII140 was also detected in immunopurified TFIID by using a second independent monoclonal antibody against the same peptide (Fig. 3B, lanes 2 and 3), while neither TBP nor hTAFII140 was detected in control immunoprecipitations using the anti-Flag antibody (lane 1). Reprobing of the immunoblot shows that previously identified TAFIIs were present in the immunopurified TFIID as expected (Fig. 3B, lanes 4 to 6). It is possible that TAFII140 was originally overlooked because it comigrates closely with hTAFII135 (compare lanes 3 and 4).
The anti-hTAFII140 antibodies were also used to immunoprecipitate HeLa cell nuclear extracts. TBP, TAFII30, TAFII20, TAFII18, and TAFII55 as well as other TFIID components (data not shown) were all coprecipitated with TAFII140 (Fig. 3C, lanes 1 to 4). TAFII30 is a component of the TFIID and TFTC complexes (5, 53). To determine whether TAFII140 is also present in TFTC, we asked whether TFTC-specific components could also be immunoprecipitated with the anti-TAFII140 antibody. Incubation of the immunoblots with antibodies against hGCN5 and SAP130 (Brand et al., submitted) indicated that both were coprecipitated (Fig. 3C). Therefore, the anti-TAFII140 antibody precipitates both TFIID-specific subunits (TBP, hTAFII18) and TFTC-specific subunits (SAP130, GCN5), indicating that it is present in both complexes.
BIP2 is a Drosophila TFIID subunit which heterodimerizes specifically with dTAFII24.
Database searches with mTAFII140 show that it shares significant sequence similarity with the Drosophila protein BIP2 (Fig. 4A). BIP2 was first identified (J.-C. Pointud and J.-L. Couderc, unpublished data) in a two-hybrid screen using as bait the BTB (POZ) domain of bric-a-brac 1, a regulatory protein required for several morphogenetic processes in Drosophila development (21, 45). BIP2 contains an HFD in the N terminus and a PHD finger at the C terminus (Fig. 4A). The PHD fingers of mouse and human TAFII140 and BIP2 are more closely related to each other than to PHD fingers from other proteins (Fig. 4B). The significant similarity with hTAFII140 led us to ask whether BIP2 is a component of dTFIID.
We first determined whether the dBIP2 HFD would heterodimerize with those of dTAFII16 or dTAFII24. In yeast two-hybrid assays, the VP16-dBIP2 HFD chimera interacted strongly with the LexA-dTAFII24 fusion, but only a weak interaction with dTAFII16 was observed (Fig. 5A, lanes 1 and 2). In contrast, no significant interaction with the HFDs of yTAFII25 or hTAFII30 was observed (lanes 3 to 5). This indicates that the dBIP2 HFD can discriminate between the HFDs of dTAFII16 and dTAFII24. The selective interaction of the dBIP2 HFD with that of dTAFII24 was also investigated by coexpression in Escherichia coli cells. GST-fusions of the BIP2 HFD were coexpressed with native dTAFII16 and dTAFII24. The GST-BIP2(2-89) fusion was solubilized by coexpression of full-length dTAFII24, for which a complex of the two was retained on the resin, whereas with dTAFII16, no solubilization nor heterodimerization was observed (Fig. 5B, lanes 2 to 3). Similarly, GST-BIP2(2-89) was solubilized by coexpression of the dTAFII24 HFD(57-167), but not by the dTAFII16 HFD(41-146) (lanes 4 and 5). The GST-BIP2-dTAFII24 HFD heterodimerization was almost completely abolished by mutation of residues within the L1 loop [lane 8, G23R, Y24K, GST-BIP2(2-89)m1]. These results indicate a direct and selective dBIP2-dTAFII24 heterodimerization via their HFDs.
FIG. 5.
(A) Graphical representation of quantitative two-hybrid β-galactosidase assays. The VP16-dBIP2 chimera is shown schematically above the graph and interactions with the LexA chimeras shown below each column are indicated. −, negative controls with the LexA domain alone. (B) Direct and selective dBIP2-dTAFII24 heterodimerization. Bacteria were transformed to express the proteins shown above each lane, and following extract preparation, the soluble protein retained on glutathione-Sepharose beads was analyzed by SDS-PAGE and staining with Coomassie brilliant blue. /, the GST-fusion was expressed alone. For lanes 2 and 3, coexpression was performed with the full-length dTAFII16 and dTAFII24 proteins as indicated, while for lanes 4, 5, 7, and 8, coexpression was performed with the HFDs of the indicated proteins. The locations of the GST-BIP2 fusions and the dTAFII24 proteins are indicated. (C and D) Drosophila embryo nuclear extract was precipitated with antisera against TBP (lane 4) or an irrelevant antiserum (raised against GST) (lane 3) as indicated. Lane 2 shows a control immunoprecipitation with the anti-TBP serum in the absence of added nuclear extract. ∗, locations of the precipitated proteins.
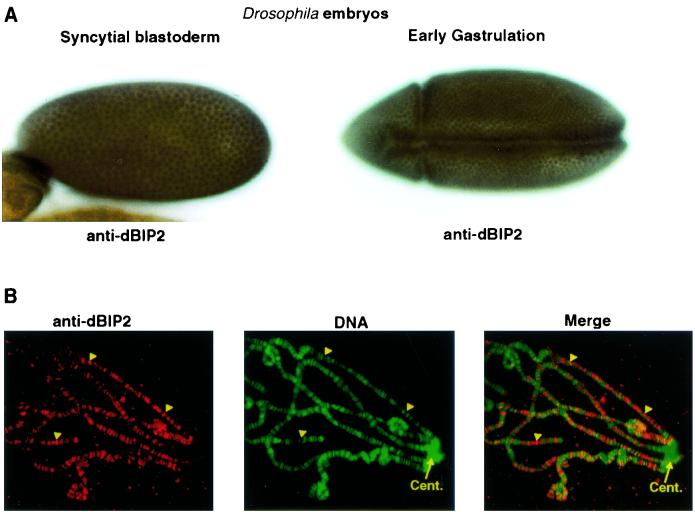
A polyclonal anti-dBIP2 serum was generated (see Materials and Methods). In immunostaining, the anti-dBIP2 serum, but not the preimmune serum, recognized a ubiquitously expressed nuclear protein throughout Drosophila embryogenesis (Fig. 6A and data not shown). This antibody also localized the dBIP2 protein on polytene chromosomes exclusively in decondensed regions, both puffs and interbands (Fig. 6B, arrowheads) which correspond to RNA polymerase II start sites in transcriptionally active chromatin (30, 46). In contrast, it is not found in the transcriptionally inactive heterochromatin around the centromere.
FIG. 6.
(A) Immunodetection of dBIP2 in the nuclei of Drosophila embryos. The relevant stages are indicated. (B) In situ immunodetection of dBIP on salivary gland polytene chromosome spreads. The arrowheads indicate examples of interband regions staining strongly with the anti-dBIP2 serum. Cent., the centromeric region.
To determine whether dBIP2 is indeed a dTFIID subunit as suggested by its ability to interact with dTAFII24, immunoprecipitation experiments were performed. The anti-dBIP2 serum recognized a protein with a mass of around 155 kDa in Drosophila embryo nuclear extracts (Fig. 5C, lane 1). The embryo extract was precipitated with an antiserum against dTBP, which has previously been shown to precipitate the dTFIID complex (19, 29). BIP2 was precipitated with the anti-dTBP sera (Fig. 5C, lane 4), while no immunoprecipitation was seen using an irrelevant antiserum (lane 3), and the signal did not result from a spurious cross-reaction with the anti-TBP serum (lane 2). As a positive control, dTBP, dTAFII42, dTAFII24, and dTAFII230 were also selectively precipitated by the anti-TBP serum (Fig. 5C and D, lanes 4, and data not shown). These data indicate that dBIP2 (dTAFII155) can be coimmunoprecipitated with dTBP and other dTAFIIs and hence is a subunit of dTFIID.
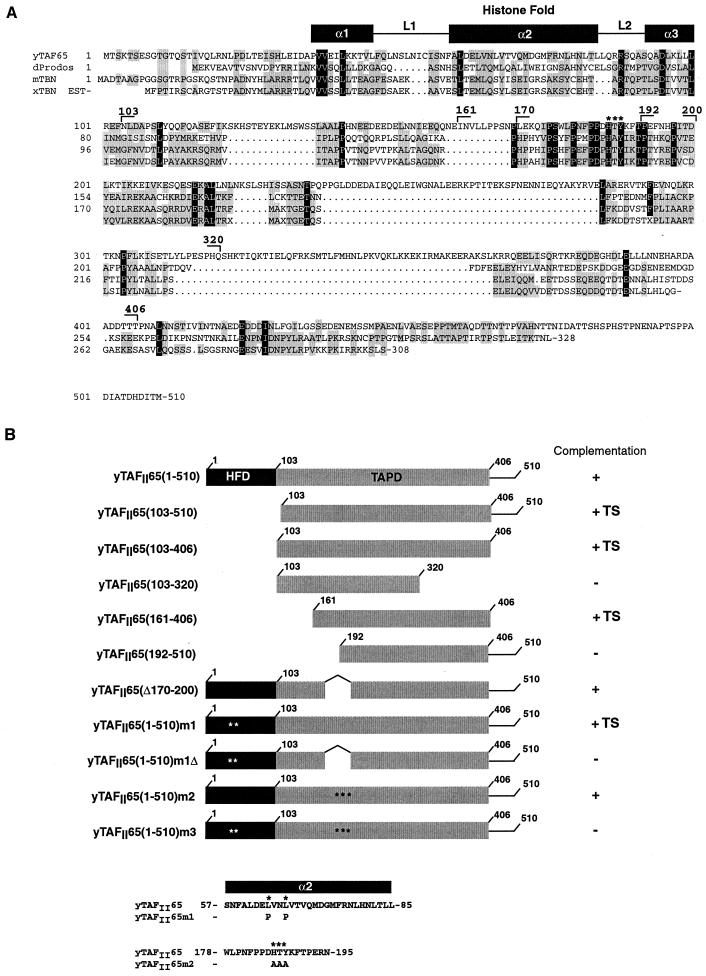
A functional domain of yeast TAFII65 required for viability in yeast is shared with the Drosophila Prodos protein.
The above results indicate that dBIP2 (dTAFII155) is a TFIID component which selectively heterodimerizes with dTAFII24. It has recently been reported that the PDS gene product contains an HFD and interacts specifically with dTAFII16, leading to the idea that PDS is a TFIID subunit (25). Here, we show that dBIP2 (dTAFII155) selectively heterodimerizes with dTAFII24. yTAFII25 can heterodimerize with either yTAFII47 or yTAFII65. Hence, if dTAFII155 and hTAFII140 are homologues of yTAFII47, it is possible that PDS may be a homologue of yTAFII65. We have previously shown that, in addition to the HFD, a second domain of yTAFII65 located between amino acids 103 and 510 was essential for its function in yeast (17). As functional domains in TAFIIs are often evolutionarily conserved, we examined this region for similarities with PDS. Sequence comparisons indicate that yTAFII65 shares significant sequence similarity with PDS not only in the HFD but also in the region C terminal to the HFD (Fig. 7A). Several highly conserved sequence motifs separated by insertions corresponding to potential loop regions in yTAFII65 were observed. We therefore wished to determine whether this conserved domain (designated the TAPD) was essential for yTAFII65 function.
FIG. 7.
(A) Comparison of the amino acid sequences of yTAFII65, PDS, TBN, and a putative Xenopus TBN as described by Voss et al. (51). Identical amino acids are shown as white letters in a black background, and similar amino acids are boxed in gray. The location of the histone fold is indicated. The endpoints of the deletions in yTAFII65 are indicated. The highly conserved HTY amino acids mutated in m2 are indicated (∗). (B) The yTAFII65 deletions are schematized along with their ability to rescue the growth of the yTAFII65 null strain. The presence of mutations is indicated (∗). The mutated amino acid sequences are shown at the bottom. TS, temperature sensitivity.
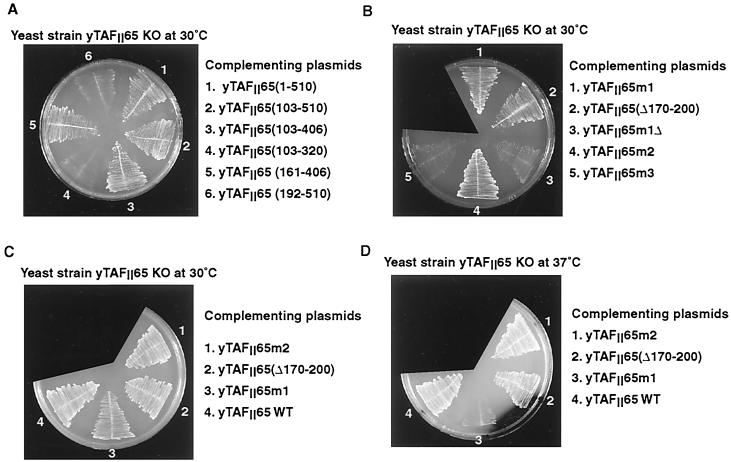
Using the plasmid shuffle technique for yeast, we tested the ability of derivatives of yTAFII65 with a deletion or mutation in the TAPD to complement the growth of a yTAFII65 null strain. Deletion of the yTAFII65 HFD does not abolish growth at 30°C but generates a temperature-sensitive phenotype (17), indicating that the region containing the TAPD contributes to yTAFII65 function. We made further deletions to determine more precisely the location of this functional domain of yTAFII65.
Deletion of amino acids downstream of 406 did not affect the ability of yTAFII65 to rescue the growth of the null strain at 30°C, while a C-terminal truncation at amino acid 320 was unable to rescue growth [Fig. 8A, sections 2 to 4, summarized in Fig. 7B, compare yTAFII65(103-406) and yTAFII65(103-320)]. At the N terminus, a derivative beginning at amino acid 161 was able to complement growth, but a further deletion up to position 192, which deletes a highly conserved region of the TAPD, abolished function [Fig. 8A, sections 5 and 6, summarized in Fig. 7B, compare yTAFII65(161-406) and yTAFII65(192-510)]. As all of the viable constructs lacked the HFD, they showed a temperature-sensitive phenotype, being unable to complement at 37°C (data not shown). Therefore, a minimal TAPD between amino acids 161 and 406 is required for yTAFII65 function.
FIG. 8.
The growth of yeast plated at the indicated temperatures is shown. The complementing plasmids used are next to the plates. WT, wild type.
The minimal domain described above comprises the most conserved regions of the TAPD. To further test the significance of the sequence similarity and to evaluate the relative contributions of the HFD and TAPD to yTAFII65 function, we deleted or mutated one of the most conserved blocks between amino acids 170 and 200 in the context of wild-type yTAFII65 and in the context of yTAFII65 m1, where the HFD is mutated. When this region was deleted in the context of a wild-type HFD, complementation was observed [Fig. 8B, section 2, summarized in Fig. 7B, yTAFII65(Δ170-200)]. In contrast, when the deletion was made in the presence of the mutated HFD, which by itself is viable at 30°C (Fig. 8B, section 1), no growth was observed [Fig. 8B, section 3, compare yTAFII65(1-510)m1 with yTAFII65(1-510)m1Δ]. Similarly, mutation of the highly conserved HTY residues within the region had no effect in the presence of the wild-type HFD but led to a loss of function in the context of a mutated HFD [Fig. 8B, sections 4 and 5, compare yTAFII65(1-510)m2 and yTAFII65(1-510)m3, summarized in Fig. 7B]. Hence, when the HFD is functional, the TAPD can be mutated, and likewise, when the TAPD is intact, the HFD is not absolutely required. These results indicate that yTAFII65 contains two partially redundant domains. Nevertheless, the HFD seems to play a more important role since its mutation leads to a temperature-sensitive phenotype while mutation or deletion of the TAPD alone does not [compare yTAFII65m1, yTAFII65m2, and yTAFII65(Δ170-200) in Fig. 8C and D].
DISCUSSION
TAFII140 and BIP2 are novel metazoan TFIID subunits.
We describe here mouse and human TAFII140 and dBIP2 (dTAFII155), novel components of the mammalian and Drosophila TFIID complexes. Both of these proteins contain an N-terminal HFD which selectively heterodimerizes with that of members of the yTAFII25 family. The mouse and human TAFII140 HFD heterodimerizes with those of both yTAFII25 and hTAFII30, whereas the dTAFII155 HFD is much more selective, interacting only with that of dTAFII24 and not with that of dTAFII16. Most other organisms seem to have only one homologue of yTAFII25. The existence of two closely related genes in Drosophila has allowed each one to evolve a more specialized role. For example, as previously reported (19), dTAFII24 is associated with both the TFIID and the GCN5-containing complexes, while dTAFII16 is present only in the TFIID complex. The differential presence of these proteins in the TFIID and GCN5-containing complexes imposes a specialization in partner choice. While yTAFII47 and mouse and human TAFII140 can heterodimerize with yTAFII25 or hTAFII30, the dTAFII155 and PDS HFDs are much less promiscuous in their partners since there is a selective interaction of PDS with dTAFII16 (25) and dTAFII155 with dTAFII24. Consequently, the specialization of the dTAFII16 and dTAFII24 HFDs has forced the dTAFII155 and PDS HFDs to also evolve towards a restricted partner choice. This restriction contributes to the relative subunit compositions of the TFIID and GCN5-containing complexes.
TAFII140 and dTAFII155 also contain a PHD finger at the C terminus of the protein. The PHD finger is found in almost 300 proteins, most of which are nuclear (39). The majority of these proteins are involved in transcription and interactions with chromatin, such as the Polycomb-like family, and more recently the ATP-dependent chromatin remodeling factor ACF (1, 41). Mutations in the PHD fingers of several proteins have been associated with disease (for examples, see references 4 and 20). The structures of the PHD finger of the Williams-Beuren syndrome transcription factor (WSTF) and the KAP-1 corepressor have been recently solved by nuclear magnetic resonance and shown to comprise an interleaved zinc finger which binds two zinc ions (9, 39).
While the function of the PHD finger is unknown, it has been suggested that it is involved in protein-protein interactions. This is the first time a PHD finger has been found in a TFIID subunit, and it may mediate TFIID interactions with chromatin or with chromatin-associated proteins. Its presence in TFIID is particularly intriguing since many of the factors in which it is found are associated with repressor activity, histone deacetylases, and interactions with heterochromatin (for an example, see references 15, 31, and 55). BIP2, however, is not localized on heterochromatin but is associated with transcriptionally active regions of polytene chromosomes. The PHD finger is not the only motif characteristic of chromatin-interacting proteins to be found in TFIID. TAFII250 contains bromodomains, also found in many chromatin-interacting factors, which have recently been shown to mediate interaction with acetyl-lysine groups in the histone tails (12, 26). Hence, both the bromodomains and the PHD finger may be interfaces for directing TFIID to promoters within chromatin.
Immunoprecipitations demonstrate that both hTAFII140 and dTAFII155 are TFIID subunits, since they are coprecipitated under stringent conditions along with TBP and TAFIIs. It has been previously shown that dTAFII24 is coprecipitated by the dTAFII16 antisera and vice versa (19). The simplest interpretation is that both dTAFII24 and dTAFII16 are present in the same dTFIID complex containing one (or more) molecules of each. This TFIID would therefore contain both PDS and dTAFII155. An analogous result was obtained with yeast, where yTAFII47 can be precipitated from strains expressing a tagged version of yTAFII65 (45). These data were supported by immunoelectron microscopy which showed the presence of multiple molecules of yTAFII65, yTAFII47, and yTAFII25 in the same TFIID particle (C. Leurent, S. Sanders, V. Mallouh, P. A. Weil, D. B. Kirschner, L. Tora, and P. Schultz, submitted for publication).
In HeLa cells, hTAFII30 is a component of both TFIID and TFTC. When HeLa cell nuclear extracts were immunoprecipitated with the anti-TAFII140 monoclonal antibody, both TFIID-specific components, such as TAFII18 and TBP, and TFTC-specific components, such as GCN5 and SAP130 (also ADA3 and SPT3; our unpublished data), were detected. The TAFII30-TAFII140 heterodimer therefore appears to be a component of both TFIID and TFTC. At first sight this may seem surprising since, in yeast, yTAFII47 is TFIID specific and is not present in SAGA where the SAGA-specific ySPT7 heterodimerizes with yTAFII25 (17). However, hTAFII135 and hTAFII150 are TFTC components, yet their yeast homologues, yTAFII48 and yTAFII150, are not present in SAGA. In this respect, the findings for yeast and mammalian cells are somewhat different and reflect the fact that TFTC and SAGA have similar, but not identical, compositions. The observation that hTAFII140 is a TFTC component does not, however, rule out the existence of a hSPT7 or dSPT7 homologue, as we previously suggested (17).
The evolutionarily conserved TAPD is required for yTAFII65 function in vivo.
Here, we describe the novel TAPD present in yTAFII65 and PDS. The presence of an HFD and the TAPD in PDS which is known to interact physically and functionally with dTFIID components (25) indicates that PDS is a yTAFII65 homologue. These domains are also present in the mammalian protein TBN, further suggesting that it may also be a TAFII (25, 51).
The TAP domain is located C terminal of the HFD, and in yTAFII65 it is partially redundant with the HFD. In complementation experiments, deletion or mutation of the HFD does not abolish yTAFII65 function when the TAPD is wild type. Similarly, the TAPD can be mutated or deleted without loss of function when the HFD is present. In contrast, simultaneous mutation of both leads to a loss of function. Hence, the HFD and the TAPD play redundant roles in yTAFII65 function.
As the function of the yTAFII65 HFD is to mediate heterodimerization with yTAFII25 and hence the integration of yTAFII65 into TFIID, it is likely that the TAPD is also an interface for interactions between yTAFII65 and another TAFII(s). In the absence of the HFD, the TAPD would allow yTAFII65 to associate with TFIID and support growth and vice versa. Obviously, when both are mutated, it is no longer possible for yTAFII65 to interact with the TFIID, and this is lethal for yeast. Note, however, that the HFD seems to be the more critical for yTAFII65 function, since all the viable strains in which it was deleted had a temperature-sensitive phenotype, whereas the strains in which only the TAPD was mutated grow at 37°C. This is consistent with the fact that histone-like heterodimers are extremely stable and suggests that the interactions mediated by the TAPD are less so. Nonetheless, it is worth noting that the TAPD comprises several conserved sequence blocks, suggesting that it may be composite in nature. Thus, even when amino acids 170 to 200 are deleted or mutated, the remainder of the domain may act cooperatively with the HFD to allow growth at 37°C. We have previously reported that the temperature sensitivity resulting from mutation or deletion of the yTAFII65 HFD could be selectively suppressed by overexpression of yTAFII68 (17). This suggests that the TAPD may interact with yTAFII68. Biochemical and structural analyses will be required to determine how the TAPD interacts with yTAFII68 and/or other TFIID components.
In this study, we have proposed that BIP2 (dTAFII155) and hTAFII140 are homologues of yTAFII47 due to the similarity in sequence of the HFDs and their ability to heterodimerize with the yTAFII25 homologues in dTFIID and hTFIID and that PDS is a homologue of yTAFII65 based on the shared HFD and TAPD. These results indicate a remarkable conservation of TFIID subunit composition. One notable exception to this is the presence of a PHD finger in hTAFII140 and its absence in yTAFII47. Nevertheless, in yeast, the bromodomains and kinase activity in hTAFII250 are not present in yTAFII130 (yTAFII145) but are provided by the association of the Bdf1 and Bdf2 proteins with TFIID (34). Database searches with the mTAFII140 and hTAFII140 PHD finger revealed the presence of several yeast proteins with related PHD fingers. It is possible that, by analogy with Bdf1 and Bdf2, one of these PHD finger-containing proteins associates with yTFIID to provide the missing domain.
ACKNOWLEDGMENTS
We thank S. Sanders and P. A. Weil for the yTAFII65 null strain and cDNA, G. Mengus for the Drosophila nuclear extract, A. Ferrus and A. Hernandez for sharing their data prior to publication, Y. Nakatani for the anti-dTBP and -dTAFII230 antisera, S. Hollenberg for the generous gift of yeast strain L40, V. Calco for excellent technical assistance, M. Oulad-Abdelghani and the monoclonal antibody facility, P. Eberling for peptide synthesis, S. Vicaire and D. Stephane for DNA sequencing, the staff of cell culture and oligonucleotide facilities, and B. Boulay for help with illustrations.
Y.-G.G. was supported by a fellowship from the Ligue National Contre le Cancer, S.T. was supported by a fellowship from the Région Alsace, and S.M. was supported by a short-term EMBO fellowship. This work was supported by grants from the CNRS, the INSERM, the Hôpital Universitaire de Strasbourg, the Ministère de la Recherche et de la Technologie, the Association pour la Recherche contre le Cancer, the Ligue Nationale contre le Cancer, and the Human Frontier Science Programme and by grant GREG#59/95 to J.-L.C.
REFERENCES
- 1.Aasland R, Gibson T J, Stewart A F. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 2.Albright S R, Tjian R. TAFs revisited: more data reveal new twists and confirm old ideas. Gene. 2000;242:1–13. doi: 10.1016/s0378-1119(99)00495-3. [DOI] [PubMed] [Google Scholar]
- 3.Bell B, Tora L. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp Cell Res. 1999;246:11–19. doi: 10.1006/excr.1998.4294. [DOI] [PubMed] [Google Scholar]
- 4.Bjorses P, Halonen M, Palvimo J J, Kolmer M, Aaltonen J, Ellonen P, Perheentupa J, Ulmanen I, Peltonen L. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66:378–392. doi: 10.1086/302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand M, Yamamoto K, Staub A, Tora L. Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J Biol Chem. 1999;274:18285–18289. doi: 10.1074/jbc.274.26.18285. [DOI] [PubMed] [Google Scholar]
- 6.Brou C, Chaudhary S, Davidson I, Lutz Y, Wu J, Egly J M, Tora L, Chambon P. Distinct TFIID complexes mediate the effect of different transcriptional activators. EMBO J. 1993;12:489–499. doi: 10.1002/j.1460-2075.1993.tb05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brou C, Wu J, Ali S, Scheer E, Lang C, Davidson I, Chambon P, Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993;21:5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke T W, Kadonaga J T. The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capili A D, Schultz D C, Rauscher I F, Borden K L. Solution structure of the PHD domain from the KAP-1 corepressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J. 2001;20:165–177. doi: 10.1093/emboj/20.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalkley G E, Verrijzer C P. DNA binding site selection by RNA polymerase II TAFs: a TAFII250-TAFII150 complex recognizes the initiator. EMBO J. 1999;18:4835–4845. doi: 10.1093/emboj/18.17.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Manley J L. Robust mRNA transcription in chicken DT40 cells depleted of TAFII31 suggests both functional degeneracy and evolutionary divergence. Mol Cell Biol. 2000;20:5064–5076. doi: 10.1128/mcb.20.14.5064-5076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhalluin C, Carlson J E, Zeng L, He C, Aggarwal A K, Zhou M M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 13.Dubrovskaya V, Lavigne A C, Davidson I, Acker J, Staub A, Tora L. Distinct domains of hTAFII100 are required for functional interaction with transcription factor TFIIF beta (RAP30) and incorporation into the TFIID complex. EMBO J. 1996;15:3702–3712. [PMC free article] [PubMed] [Google Scholar]
- 14.Dynlacht B D, Hoey T, Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991;66:563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 15.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X P, Neilson E G, Rauscher F J. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 16.Gangloff Y G, Romier C, Thuault S, Werten S, Davidson I. The histone fold is a key structural motif of transcription factor TFIID. Trends Biochem Sci. 2001;26:250–257. doi: 10.1016/s0968-0004(00)01741-2. [DOI] [PubMed] [Google Scholar]
- 17.Gangloff Y G, Sanders S L, Romier C, Kirschner D, Weil P A, Tora L, Davidson I. Histone folds mediate selective heterodimerization of yeast TAFII25 with TFIID components yTAFII47 and yTAFII65 and with SAGA component ySPT7. Mol Cell Biol. 2001;21:1841–1853. doi: 10.1128/MCB.21.5.1841-1853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangloff Y G, Werten S, Romier C, Carre L, Poch O, Moras D, Davidson I. The human TFIID components TAFII135 and TAFII20 and the yeast SAGA components ADA1 and TAFII68 heterodimerize to form histone-like pairs. Mol Cell Biol. 2000;20:340–351. doi: 10.1128/mcb.20.1.340-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgieva S, Kirschner D B, Jagla T, Nabirochkina E, Hanke S, Schenkel H, de Lorenzo C, Sinha P, Jagla K, Mechler B, Tora L. Two novel Drosophila TAFIIs have homology with human TAFII30 and are differentially regulated during development. Mol Cell Biol. 2000;20:1639–1648. doi: 10.1128/mcb.20.5.1639-1648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbons R J, Bachoo S, Picketts D J, Aftimos S, Asenbauer B, Bergoffen J, Berry S A, Dahl N, Fryer A, Keppler K, Kurosawa K, Levin M L, Masuno M, Neri G, Pierpont M E, Slaney S F, Higgs D R. Mutations in transcriptional regulator ATRX establish the functional significance of a PHD-like domain. Nat Genet. 1997;17:146–148. doi: 10.1038/ng1097-146. [DOI] [PubMed] [Google Scholar]
- 21.Godt D, Couderc J L, Cramton S E, Laski F A. Pattern formation in the limbs of Drosophila: bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119:799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- 22.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates J R, Workman J L. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 23.Green M R. TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes. Trends Biochem Sci. 2000;25:59–63. doi: 10.1016/s0968-0004(99)01527-3. [DOI] [PubMed] [Google Scholar]
- 24.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Hernández A, Ferrús A. Prodos is a conserved transcriptional regulator that interacts with dTAFII16 in Drosophila. Mol Cell Biol. 2001;21:614–623. doi: 10.1128/MCB.21.2.614-623.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson R H, Ladurner A G, King D S, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 27.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 28.Kokubo T, Gong D W, Yamashita S, Takada R, Roeder R G, Horikoshi M, Nakatani Y. Molecular cloning, expression, and characterization of the Drosophila 85-kilodalton TFIID subunit. Mol Cell Biol. 1993;13:7859–7863. doi: 10.1128/mcb.13.12.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokubo T, Takada R, Yamashita S, Gong D W, Roeder R G, Horikoshi M, Nakatani Y. Identification of TFIID components required for transcriptional activation by upstream stimulatory factor. J Biol Chem. 1993;268:17554–17558. [PubMed] [Google Scholar]
- 30.Kramer A, Haars R, Kabisch R, Will H, Bautz F A, Bautz E K. Monoclonal antibody directed against RNA polymerase II of Drosophila melanogaster. Mol Gen Genet. 1980;180:193–199. doi: 10.1007/BF00267369. [DOI] [PubMed] [Google Scholar]
- 31.Le Douarin B, You J, Nielsen A L, Chambon P, Losson R. TIF1alpha: a possible link between KRAB zinc finger proteins and nuclear receptors. J Steroid Biochem Mol Biol. 1998;65:43–50. doi: 10.1016/s0960-0760(97)00175-1. [DOI] [PubMed] [Google Scholar]
- 32.Lescure A, Lutz Y, Eberhard D, Jacq X, Krol A, Grummt I, Davidson I, Chambon P, Tora L. The N-terminal domain of the human TATA-binding protein plays a role in transcription from TATA-containing RNA polymerase II and III promoters. EMBO J. 1994;13:1166–1175. doi: 10.1002/j.1460-2075.1994.tb06366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez E, Kundu T K, Fu J, Roeder R G. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998;273:23781–23785. doi: 10.1074/jbc.273.37.23781. [DOI] [PubMed] [Google Scholar]
- 34.Matangkasombut O, Buratowski R M, Swilling N W, Buratowski S. Bromodomain factor 1 corresponds to a missing piece of yeast TFIID. Genes Dev. 2000;14:951–962. [PMC free article] [PubMed] [Google Scholar]
- 35.Mengus G, May M, Carre L, Chambon P, Davidson I. Human TAFII135 potentiates transcriptional activation by the AF-2s of the retinoic acid, vitamin D3, and thyroid hormone receptors in mammalian cells. Genes Dev. 1997;11:1381–1395. doi: 10.1101/gad.11.11.1381. [DOI] [PubMed] [Google Scholar]
- 36.Mengus G, May M, Jacq X, Staub A, Tora L, Chambon P, Davidson I. Cloning and characterization of hTAFII18, hTAFII20 and hTAFII28: three subunits of the human transcription factor TFIID. EMBO J. 1995;14:1520–1531. doi: 10.1002/j.1460-2075.1995.tb07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger D, Scheer E, Soldatov A, Tora L. Mammalian TAFII30 is required for cell cycle progression and specific cellular differentiation programmes. EMBO J. 1999;18:4823–4834. doi: 10.1093/emboj/18.17.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogryzko V V, Kotani T, Zhang X, Schlitz R L, Howard T, Yang X J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 39.Pascual J, Martinez-Yamout M, Dyson H J, Wright P E. Structure of the PHD zinc finger from human Williams-Beuren syndrome transcription factor. J Mol Biol. 2000;304:723–729. doi: 10.1006/jmbi.2000.4308. [DOI] [PubMed] [Google Scholar]
- 40.Poon D, Weil P A. Immunopurification of yeast TATA-binding protein and associated factors. Presence of transcription factor IIIB transcriptional activity. J Biol Chem. 1993;268:15325–15328. [PubMed] [Google Scholar]
- 41.Poot R A, Dellaire G, Hulsmann B B, Grimaldi M A, Corona D F, Becker P B, Bickmore W A, Varga-Weisz P D. HuCHRAC, a human ISWI chromatin remodelling complex contains hACF1 and two novel histone-fold proteins. EMBO J. 2000;19:3377–3387. doi: 10.1093/emboj/19.13.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese J C, Zhang Z, Kurpad H. Identification of a yeast transcription factor IID subunit, TSG2/TAF48. J Biol Chem. 2000;275:17391–17398. doi: 10.1074/jbc.M001635200. [DOI] [PubMed] [Google Scholar]
- 43.Saha V, Chaplin T, Gregorini A, Ayton P, Young B D. The leukemia-associated-protein (LAP) domain, a cysteine-rich motif, is present in a wide range of proteins, including MLL, AF10, and MLLT6 proteins. Proc Natl Acad Sci USA. 1995;92:9737–9741. doi: 10.1073/pnas.92.21.9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahut-Barnola I, Godt D, Laski F A, Couderc J L. Drosophila ovary morphogenesis: analysis of terminal filament formation and identification of a gene required for this process. Dev Biol. 1995;170:127–135. doi: 10.1006/dbio.1995.1201. [DOI] [PubMed] [Google Scholar]
- 45.Sanders S L, Weil P A. Identification of two novel TAF subunits of the yeast Saccharomyces cerevisiae TFIID complex. J Biol Chem. 2000;275:13895–13900. doi: 10.1074/jbc.275.18.13895. [DOI] [PubMed] [Google Scholar]
- 46.Sass H, Bautz E K. Interbands of polytene chromosomes: binding sites and start points for RNA polymerase B (II) Chromosoma. 1982;86:77–93. doi: 10.1007/BF00330731. [DOI] [PubMed] [Google Scholar]
- 47.Schindler U, Beckmann H, Cashmore A R. HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J. 1993;4:137–50. doi: 10.1046/j.1365-313x.1993.04010137.x. [DOI] [PubMed] [Google Scholar]
- 48.Tjian R. The biochemistry of transcription in eukaryotes: a paradigm for multisubunit regulatory complexes. Philos Trans R Soc Lond B Biol Sci. 1996;351:491–499. doi: 10.1098/rstb.1996.0047. [DOI] [PubMed] [Google Scholar]
- 49.Verrijzer C P, Yokomori K, Chen J L, Tjian R. Drosophila TAFII150: similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science. 1994;264:933–941. doi: 10.1126/science.8178153. [DOI] [PubMed] [Google Scholar]
- 50.vom Baur E, Zechel C, Heery D, Heine M J, Garnier J M, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- 51.Voss A K, Thomas T, Petrou P, Anastassiadis K, Scholer H, Gruss P. Taube nuss is a novel gene essential for the survival of pluripotent cells of early mouse embryos. Development. 2000;127:5449–5461. doi: 10.1242/dev.127.24.5449. [DOI] [PubMed] [Google Scholar]
- 52.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIs. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 53.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAFII-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 54.Xiao J H, Davidson I, Matthes H, Garnier J M, Chambon P. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell. 1991;65:551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, LeRoy G, Seelig H P, Lane W S, Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Q, Lieberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]
- 57.Zink B, Paro R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature. 1989;337:468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]