Abstract
Background and aims:
Psoriasis is an immune-mediated inflammatory disease with increased risk of myocardial infarction. Preclinical studies in psoriasis models show an association between chronic inflammation and immune cell proliferation in the spleen and bone marrow (BM). We sought to test the hypothesis that splenic and BM 18F-fluorodeoxyglucose (18F-FDG) uptake is heightened in psoriasis and that higher uptake associates with systemic inflammation and subclinical atherosclerotic disease measures in this cohort.
Methods:
Multimodality imaging and biomarker assays were performed in 240 participants (210 with psoriasis and 30 healthy). Splenic and BM uptake was obtained using 18F-FDG positron emission tomography/computed tomography (PET/CT). Coronary artery plaque characteristics including non-calcified burden (NCB) and lipid rich necrotic core (LRNC) were quantified using a dedicated software for CT angiography. All analyses were performed with StataIC 16 (Stata Corp., College Station, TX, USA).
Results:
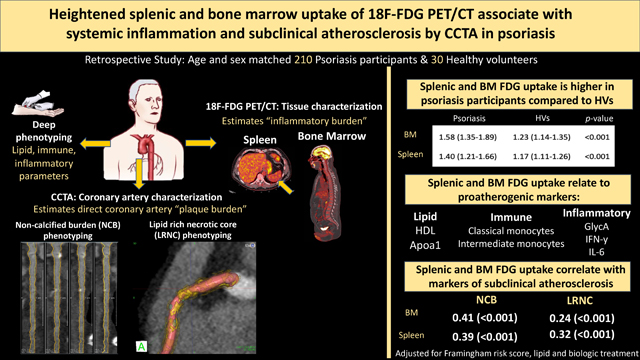
Splenic and BM 18F-FDG uptake was increased in psoriasis (vs. healthy volunteers) and significantly associated with proatherogenic lipids, immune cells and systemic inflammation. Higher splenic 18F-FDG uptake associated with higher total coronary burden (β=0.37; p<0.001), NCB (β=0.39; p<0.001), and LRNC (β=0.32; p<0.001) in fully adjusted models. Similar associations were seen for BM 18F-FDG uptake in adjusted models (β=0.38; β=0.41; β=0.24; respectively, all p<0.001).
Conclusions:
Heightened splenic and BM uptake of 18F-FDG is associated with proatherogenic lipids, immune cells, inflammatory markers and coronary artery disease. These findings provide insights into atherogenic mechanisms in psoriasis and suggest that immune cell proliferation in the spleen and BM is associated with subclinical atherosclerosis.
Keywords: Inflammation, Coronary artery disease, Atherosclerosis, Computerized Tomography (CT), Nuclear cardiology and PET
Graphical Abstract
1. Introduction:
Atherosclerosis is a multifactorial disease that is being recognized as an inflammatory process. Infiltration of leukocyte subsets into the arterial wall is a crucial component of atherogenesis.[1–3] Recent studies indicate that, in conditions of inflammation or metabolic stress, inflammatory mediators mobilize hematopoietic stem cells (HSCs) from the bone marrow (BM) to seed and proliferate at extramedullary sites, such as the spleen.[4, 5] In patients without chronic inflammatory disease or active disease, splenic but not BM metabolic activity independently associates with risk of subsequent major adverse cardiovascular events (MACE). [6] Studies in humans investigating these relationships in states of chronic inflammation and the effects of hematopoietic tissue activation on coronary artery disease (CAD) prior to MACE are limited.
Inflammation may play an active role in hematopoietic proliferation. Increased cytokine receptor expression on HSCs, proinflammatory remodeling of leukocytes, and inflammation-driven monocyte splenic egress demonstrate the regulatory role of inflammation in the spleen and BM niche.[4, 7] Psoriasis is a hyperproliferative cutaneous skin disorder associated with increased cardiovascular risk and serves as a reliable model to study inflammatory atherogenesis.[8] Increasing evidence suggests immune dysfunction is central to both psoriasis and the development of cardiometabolic diseases such as atherosclerosis.[9] The associations between splenic and BM 18F-fluorodeoxyglucose (18F-FDG) uptake with vascular inflammation in psoriasis[10] suggest a role of these tissues in exacerbating the systemic inflammatory load and, thereby, promoting subclinical atherosclerosis.
Hematopoietic tissue activation can be measured in the spleen and BM as increased activity by 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT). 18F-FDG-PET/CT provides a measure of tissue glycolysis, [11] and activity is increased in tissues with rapidly proliferating, highly metabolic cells, including inflammatory cells. [12, 13] Coronary computed tomography angiography (CCTA) is a valuable tool in capturing early atherosclerosis and characterizing coronary burden beyond luminal stenosis. [14, 15] CCTA allows for quantification of non-calcified burden and fibrous and fibrofatty burden, as well as high-risk plaque features such as lipid rich necrotic core (LRNC). These markers of subclinical atherosclerosis associate with traditional cardiovascular risk factors in psoriasis and improve with biologic therapy. [14, 15]
We assessed the effects of splenic and BM 18F-FDG uptake on subclinical atherosclerosis but focused primarily on splenic uptake given that splenic 18F-FDG uptake predicts MACE in a stable population after adjusting for cardiovascular risk factors [6], is associated with vascular inflammation in psoriasis [16], and is implicated in heart failure (a state of chronic inflammation).[17] We aimed to: (1) compare splenic and BM 18F-FDG uptake in psoriasis and healthy volunteers (HVs), (2) characterize patterns of association between splenic and BM 18F-FDG uptake and cardiometabolic risk factors, including proatherogenic lipid, immune, and inflammatory markers, (3) explore differences in those with low and high splenic or BM 18F-FDG uptake, and (4) characterize associations between splenic and BM 18F-FDG uptake subclinical atherosclerotic disease measures and LRNC.
2. Materials and methods:
2.1. Study participants
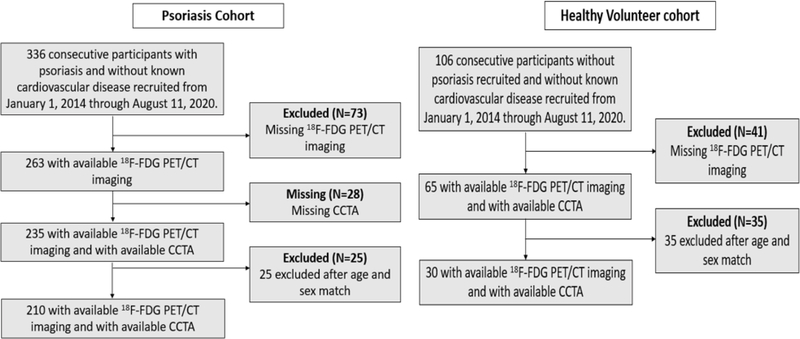
The study included 336 consecutive participants with psoriasis who were 18 years or older and 106 HVs without psoriasis who were recruited from January 1, 2013 through August 11, 2020. The 235 psoriasis participants and 65 HVs had available 18F-FDG-PET/CT imaging and coronary computed tomography angiography (CCTA) results (Figure 1). For detailed inclusion and/or exclusion criteria, as well as clinical and laboratory measurement methods, please see the Supplementary data.
Figure 1: Recruitment scheme for the psoriasis and healthy volunteer cohorts.
18F-FDG PET/CT: 18-fluorine fluorodeoxyglucose positron emission tomography/computed tomography.
2.2. 18F-FDG-PET/CT imaging: Acquisition and analysis
2.2.1. Acquisition
18F-FDG-PET/CT imaging was performed using one Siemens Biograph mCT PET/CT 64-slice scanner (Siemens Medical Solutions USA, Malvern, Pennsylvania) at a single center. After an overnight fast for at least 8 hours, PET/CT images were acquired approximately 60 minutes (mean: 62 ± 1 minutes) [18, 19] after administration of 10 mCi of 18F-FDG. All patients underwent the same PET CT protocol with the same team of technologists with a fixed 18F-FDG dose of 10 mCi. Standard bed positions of 3 min each, scanning cranially to caudally, were obtained for each patient. 1.5-mm axial slices were obtained. Patients with a fasting glucose over 200 mg/dl were excluded.
2.2.2. Image analysis
Splenic and BM 18F-FDG uptake was quantified using previously published methods with a dedicated PET/CT image analysis program (Extended Brilliance Workspace (Philips Electronics, Andover, Massachusetts). [20] Splenic 18F-FDG uptake [6, 21] was measured by placing a single ROI with a volume of 8.0 cm3 within the homogenous-splenic margin and a single maximum standardized uptake value (SUVmax) was taken. Bone marrow 18F-FDG uptake was measured by placing ROI over axial sections within individual vertebrae (T1 to L5) (Figure 2). The average SUVmax of the individual vertebrae was taken. For analysis and results, splenic and BM 18F-FDG uptake was then corrected for mean venous background by dividing the spleen and BM tissue SUVmax by the average supervisor vena cava SUVmean and reported as the target-to-background ratio. Mean and maximum SUVs were generated using a dedicated PET/CT image analysis program (Extended Brilliance Workspace (Philips Electronics, Andover, Massachusetts).
Figure 2:
Measurement of tissue activities by 18F-FDG PET/CT and coronary burden by CCTA.
2.3. Coronary artery characterization by CCTA
Participants in the psoriasis study underwent CCTA in the same scanner (320-detector row Aquilion ONE ViSION). Guidelines established by the National Institutes of Health Radiation Exposure Committee were followed. Scans were performed with retrospective gating at 120 kV, tube current of 750–850 mA and a gantry rotation time of ≤420 milliseconds.
2.3.1. CCTA quantification and analysis
Coronary artery characteristics across the main coronary arteries >2 mm diameter were analyzed using QAngio CT (Medis, The Netherlands) with high ICC (>0.95). Clear deviations of the software’s automatic contouring of lumen and outer wall segmentation were edited manually. Total, non-calcified, dense calcified, fibrous, and fibrofatty coronary burden was calculated by dividing the respective coronary artery volume by the corresponding segment length and adjusting for mean lumen intensity. (Figure 2)
2.3.2. Lipid rich necrotic core (LRNC)
Maximum LRNC areas were determined for each major coronary vessel using a commercially available plaque quantification software (vascuCAP, Elucid Bioimaging Inc, Boston, MA) and previously described methods.[14, 22] Segmentations were manually edited only when clear deviations were present. The software then outputted a plaque characterization, which included maximum LRNC area for each coronary artery. Both the intrareader and inter-reader variability was low.[14]
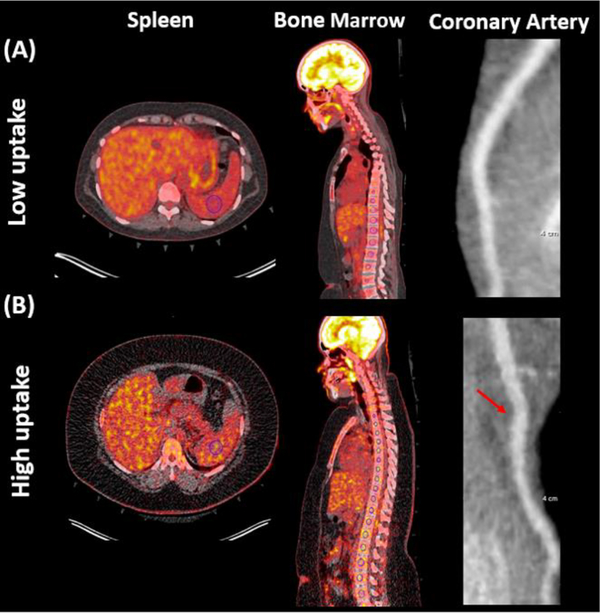
Participant with psoriasis with (A) lower or (B) higher than median splenic and bone marrow 18F-FDG uptake (corrected for mean venous background and reported as target to background ratio). The left anterior descending coronary artery is shown in both participants. Blue circle indicates region of interest. Arrow indicates non-calcified plaque in participants with psoriasis with higher than median splenic and bone marrow 18F-FDG uptake. 18F-FDG-PET/CT: 18-fluorine fluorodeoxyglucose positron emission tomography/computed tomography. CCTA: coronary computed tomography angiography
2.4. Statistical analysis
Values are reported as mean (standard deviation, SD) for parametric variables, median (interquartile range, IQR) for non-parametric variables, and n (%) for categorical variables. Statistical significance was assessed by Student’s t-test for parametric variables, Wilcoxon rank-sum test for nonparametric variables, and Pearson’s χ2 test for categorical variables. For analyses stratified by 18F-FDG uptake, low uptake was defined as less than or equal to median and high uptake was defined as greater than median. Multivariable linear regression analyses were performed to evaluate the associations between splenic or BM 18F-FDG uptake and coronary artery characteristics with adjustment for Framingham risk score, treatment of hyperlipidemia and biologic therapy. Standardized β coefficient values were reported for these analyses with p<0.05 considered significant. All analyses were performed with StataIC 16 (Stata Corp., College Station, TX, USA).
3. Results
3.1. Characteristics of the psoriasis and healthy volunteer study groups
In the cross-sectional study, 210 psoriasis participants were age and sex matched to 30 HVs (Supplemental Table 1). Psoriasis subjects were middle aged 49.2 (±SD 11.9) years, predominantly men (64%), and at low cardiovascular risk with a median Framingham risk score (FRS) of 2.1 (IQR [0.6–6.1]). The psoriasis cohort had significantly higher body mass index (BMI), high sensitivity C-reactive protein (hsCRP), and GlycA than HVs. On 18F-FDG-PET/CT, the psoriasis cohort had significantly higher splenic and BM 18F-FDG uptake compared to HVs (Supplemental Figure 1). Finally, subjects with psoriasis also had higher total coronary artery burden, non-calcified burden (NCB), and dense calcified plaque burden.
3.2. Association between splenic and BM 18F-FDG uptake and lipid, immune, and inflammatory parameters
3.2.1. Lipid and lipoprotein parameters
In those with psoriasis, splenic 18F-FDG uptake negatively correlated with high density lipoprotein (HDL), HDL particle number, and Apoa1 (Table 1). BM 18F-FDG uptake negatively correlated with HDL, HDL particle number, and Apoa1 but positively correlated with several low density lipoprotein (LDL) phenotypes including LDL level, LDL particle number, and ApoB (Supplemental Table 2). Finally, both splenic and BM 18F-FDG uptake positively correlated with the triglyceride to HDL (TG/HDL) ratio and the triglyceride index (TyG index) in univariable analysis and remained significant for multivariable analysis adjusting for Framingham risk score, lipid treatment, and biologic treatment (Supplemental Table 3).
Table 1:
Baseline associations between splenic 18F-FDG uptake and lipid, immune, and inflammatory parameters in psoriasis cohort
| Splenic 18F-FDG uptake | ||
|---|---|---|
|
| ||
| β | p-value | |
|
| ||
| Lipid and lipoprotein profile | ||
|
| ||
| Total cholesterol | 0.02 | 0.83 |
| HDL | −0.16 | 0.02 |
| LDL | 0.10 | 0.17 |
| Triglycerides | 0.09 | 0.18 |
| LDL particle number | 0.08 | 0.23 |
| HDL particle number | −0.15 | 0.03 |
| Apoa1 | −0.20 | 0.004 |
| ApoB | 0.11 | 0.12 |
|
| ||
| Immune characterization | ||
|
| ||
| White blood cells | 0.07 | 0.32 |
| Absolute lymphocytes | 0.09 | 0.18 |
| Absolute neutrophils | 0.02 | 0.77 |
| Absolute monocytes | 0.47 | <0.001 |
| Classical monocytes | 0.27 | 0.004 |
| Nonclassical monocytes | −0.18 | 0.17 |
| Intermediate monocytes | 0.44 | <0.001 |
|
| ||
| Inflammatory markers | ||
|
| ||
| hsCRP | 0.17 | 0.02 |
| GlycA | 0.24 | 0.001 |
| TNF-α | 0.16 | 0.04 |
| IFN-γ | 0.15 | 0.04 |
| IL-6 | 0.19 | 0.01 |
|
| ||
| 18F-FDG PET/CT TBR | ||
|
| ||
| Bone marrow uptake | 0.81 | <0.001 |
p-value<0.05 deemed significant.
HDL: high density lipoprotein. LDL:low density lipoprotein.
3.2.2. Immune characterization
Splenic 18F-FDG uptake was associated with a proatherogenic immune phenotype including absolute, classical, and intermediate monocyte populations. Splenic 18F-FDG uptake was not associated with nonclassical monocytes (Table 1). BM 18F-FDG uptake correlated with absolute, classical, and intermediate monocyte populations as well as with circulating white blood cells, absolute lymphocytes, and absolute neutrophils. (Supplemental Table 2).
3.2.3. Markers of inflammation
Splenic 18F-FDG uptake significantly correlated with hsCRP and GlycA as well as proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interferon (IFN)- γ, and IL-6. Similar associations with inflammatory parameters were seen with BM 18F-FDG uptake, except it was not significantly associated with TNF-α (Supplemental Table 2).
3.2.4. Tissue activity associations on 18F-FDG-PET/CT
Splenic and BM 18F-FDG uptake were strongly correlated (Table 1).
3.3. Characteristics of the psoriasis study group stratified by splenic and bone marrow 18F-FDG uptake
3.3.1. Splenic 18F-FDG uptake
Those with higher splenic uptake were more likely to be on biologic treatment but had no significant differences in psoriasis area severity index (PASI) score or disease duration. Those with higher uptake also had significantly higher BMI and non-significant trends towards higher hsCRP and GlycA. Both groups had similar total and LDL cholesterol. Those with higher splenic uptake also had higher BM uptake as well as significantly greater prevalence of coronary plaque and high-risk plaque as well as coronary total burden, NCB, and LRNC area (Table 2).
Table 2:
Baseline characteristics in psoriasis cohort stratified by median splenic 18F-FDG uptake
| Splenic 18F-FDG uptake (N=210) | |||
|---|---|---|---|
|
| |||
| Parameter |
Low uptake N=105 | High uptake N=105 | p-value |
| Clinical characteristics | |||
|
| |||
| Age, years | 48.1 (11.7) | 50.3 (12.1) | 0.18 |
| Sex, male | 68 (65) | 67 (64) | 0.89 |
| Body mass index | 28 (25–30) | 30 (26–36) | 0.002 |
| Current smoker, n | 13 (12) | 12 (11) | 0.83 |
| Hypertension, n | 27 (26) | 39 (37) | 0.07 |
| Type 2 diabetes, n | 7 (7) | 10 (10) | 0.45 |
| Hyperlipidemia, n | 40 (38) | 50 (48) | 0.16 |
| Lipid lowering medication, n | 31 (30) | 37 (35) | 0.38 |
| Hypertension treatment, n | 21 (20) | 32 (30) | 0.08 |
| Diabetes treatment, n | 6 (6) | 10 (10) | 0.30 |
|
| |||
| Psoriasis characteristics | |||
|
| |||
| PASI score | 5.8 (2.9–9.8) | 6.0 (3.2–10.6) | 0.51 |
| Disease duration, years | 20.4 (13.6) | 22.3 (14.2) | 0.31 |
| Topical treatment, n | 67 (64) | 62 (59) | 0.44 |
| Phototherapy, n | 18 (17) | 11 (10) | 0.37 |
| Systemic treatment, n | 16 (15) | 11 (10) | 0.30 |
| Biologic treatment, n | 25 (24) | 40 (38) | 0.02 |
|
| |||
| Clinical and lab values | |||
|
| |||
| Systolic blood pressure, mm Hg | 124 (111–132) | 122 (112–131) | 0.96 |
| Diastolic blood pressure, mmHg | 72.5 (66–78) | 72 (66–79) | 0.73 |
| hsC-reactive protein, mg/dL | 1.8 (0.7–3.5) | 2.2 (0.9–4.7) | 0.21 |
| GlyA, umol/L | 391 (361–433) | 413 (356–466) | 0.12 |
| HOMA-IR | 2.5 (1.6–4.2) | 3.5 (1.9–6.1) | 0.008 |
| Framingham risk score | 1.9 (0.5–5.1) | 2.9 (0.7–7.5) | 0.19 |
|
| |||
| Lipid and lipoprotein profile | |||
|
| |||
| Total cholesterol, mg/dL | 176 (153–204) | 184 (165–207) | 0.13 |
| LDL cholesterol, mg/dL | 95 (82–124) | 107 (86–120) | 0.27 |
| HDL cholesterol, mg/dL | 51 (46–67) | 50 (42–62) | 0.27 |
| Triglycerides, mg/dL | 98 (79–128) | 110 (73–187) | 0.15 |
| LDL particle number | 1120 (868–1418) | 1194 (930–1470) | 0.23 |
| HDL particle number | 34 (31–40) | 33 (30–38) | 0.09 |
|
| |||
| 18F-FDG PET/CT TBR uptake | |||
|
| |||
| Bone marrow uptake | 1.4 (1.2–1.5) | 1.9 (1.6–2.1) | <0.001 |
| Splenic uptake | 1.2 (1.1–1.3) | 1.7 (1.5–1.9) | <0.001 |
|
| |||
| Coronary artery characteristics | |||
|
| |||
| Plaque presence | 78 (29.5%) | 90 (39.5%) | 0.02 |
| High risk plaque presence | 45 (17.0%) | 55 (24.3%) | 0.05 |
| Total burden, mm2 | 1.11 (0.36) | 1.37 (0.66) | 0.001 |
| Non-calcified burden, mm2 | 1.06 (0.40) | 1.30 (0.62) | 0.002 |
| Dense-calcified burden, mm2 | 0.08 (0.13) | 0.07 (0.16) | 0.84 |
| Lipid rich necrotic core area, mm2 | 4.43 (2.40) | 6.97 (5.48) | <0.001 |
Less than or equal to median is defined as low uptake, above median defined as high uptake. Values reported as mean (SD) for parametric variables, median (IQR) for non-parametric continuous variables, and n (%) for categorical variables. p-value<0.05 deemed significant.
HDL: high density lipoprotein. LDL: low density lipoprotein. HOMA-IR: homeostatic model assessment for insulin resistance. PASI: psoriasis area severity index.
3.3.2. Bone marrow 18F-FDG uptake
Those with higher BM uptake had a significantly higher PASI score but did not significantly differ in terms of disease duration or biologic treatment. Compared to those with lower uptake, subjects with higher BM uptake were younger and had a higher BMI, hsCRP, and GlycA. Despite having similar total cholesterol, the higher uptake group had lower HDL and higher LDL levels. The group with higher BM uptake also had significantly higher splenic uptake on FDG-PET/CT. Though not significant, the group with higher BM uptake had higher incidence of coronary and high-risk plaque presence. Subjects with higher BM uptake also had significantly higher coronary total burden, NCB, and LRNC area on CCTA (Supplemental Table 4).
3.4. Association between splenic and bone marrow 18F-FDG uptake and coronary artery plaque characteristics in psoriasis
To evaluate the relationship between splenic or BM 18F-FDG uptake and coronary artery plaque characteristics in psoriasis, associations between tissue 18F-FDG uptake and CCTA-derived plaque parameters were assessed. In models fully adjusted for Framingham risk score, treatment of hyperlipidemia and biologic treatment, splenic 18F-FDG uptake significantly associated with total coronary burden, NCB, fibrous burden, fibrofatty burden, and LRNC (Table 3A). Similar associations were seen between BM 18F-FDG uptake and coronary parameters (Table 3B).
Table 3:
Associations between splenic and BM 18F-FDG uptake and coronary artery plaque characteristics
| Total burden | Non-calcified burden | Dense-calcified burden | Fibrous burden | Fibrofatty burden | Lipid rich necrotic core | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | |
|
| ||||||||||||
| (A) Spleen | ||||||||||||
|
| ||||||||||||
| Unadjusted | 0.40 | <0.001 | 0.41 | <0.001 | −0.03 | 0.73 | 0.26 | <0.001 | 0.31 | <0.001 | 0.35 | <0.001 |
| Adjusted | 0.37 | <0.001 | 0.39 | <0.001 | −0.06 | 0.37 | 0.25 | 0.001 | 0.29 | <0.001 | 0.32 | <0.001 |
|
| ||||||||||||
| (B) Bone marrow | ||||||||||||
| Unadjusted | 0.37 | <0.001 | 0.39 | <0.001 | −0.14 | 0.06 | 0.28 | <0.001 | 0.30 | <0.001 | 0.27 | <0.001 |
| Adjusted | 0.38 | <0.001 | 0.41 | <0.001 | −0.10 | 0.16 | 0.26 | <0.001 | 0.31 | <0.001 | 0.24 | <0.001 |
Coronary burden presented as an average burden measured in the left anterior descending, left circumflex and right coronary arteries. p-value<0.05 deemed significant. Adjusted: Model adjusted for framingham risk score, treatment of hyperlipidemia and biologic therapy.
3.5. Response to biologic therapy in psoriasis
Psoriasis participants that were biologic naïve at baseline, who were started on biologic therapy, and underwent 18F-FDG PET/CT at 1-year follow-up (n=37) were assessed to determine the effect of initiation of biologic therapy on splenic and BM activity. Both splenic and BM 18F-FDG significantly decreased between baseline and 1 year in these participants (Supplemental Table 5).
4. Discussion
In this study, we again show that immune cell proliferation (within the spleen and BM) is elevated in psoriasis[10] and extend these findings by demonstrating that splenic and BM 18F-FDG uptake associates with cardiometabolic risk factors including proatherogenic lipid, immune, and inflammatory markers. Furthermore, we show that splenic and BM 18F-FDG uptake predicts coronary plaque structural features (NCB and LRNC). Taken together, these findings demonstrate that hematopoietic tissue activation, detected by 18F-FDG uptake, associates with pathogenic chronic inflammatory changes, which in turn relate to atherosclerotic disease measures and high-risk features of coronary disease in psoriasis.
The majority of HSCs reside in the BM, remaining in a quiescent state until they undergo activation, proliferation, and differentiation in response to inflammation.[2] For example, during severe systemic bacterial infection, myeloid cell turn over and granulopoiesis rapidly increases even in the absence of peripheral cytopenia triggering interferon expansion. [23, 24] This response may be useful to stimulate the innate immune system against infections through extramedullary hematopoiesis. However, sustained exposure to inflammatory signals can further perpetuate inflammation through positive feedback between HSCs and the ongoing inflammatory state, further propagating downstream disease. [25, 26] In fact, recent studies in humans have shown a relationship between emergency myelopoiesis and states of ischemia in cardiovascular disease.[6, 21]
In support of these findings, not only was splenic and BM 18F-FDG uptake increased in psoriasis compared to matched HVs, but those with elevated splenic and BM 18F-FDG uptake had a more atherogenic coronary profile. This may be in part due to the association of splenic and BM 18F-FDG uptake with inflammatory markers including hsCRP, GlycA, IL-6, and IFN-γ that further propagate leukocyte release. While these findings are consistent with those of Kim et al.[21] and Emami et al. [6], we expand on the prior observations by demonstrating both splenic and BM 18F-FDG uptake correlated with proatherogenic immune cells including classical and intermediate monocytes, highlighting that increased 18F-FDG uptake in the spleen and BM captures immune dysfunction that likely contributes to atherosclerosis. In addition, splenic and BM 18F-FDG uptake correlated with proatherogenic lipids, including the TG/HDL ratio and TyG index. Both the TG/HDL ratio and TyG index have been associated with insulin resistance[27, 28], diabetes incidence[29], and cardiovascular mortality[29, 30], suggesting a role of splenic and BM activation in the pre-diabetic state, lipid dysfunction, and subsequent cardiovascular consequences.
Furthermore, both splenic and BM 18F-FDG uptake associated strongly with both NCB and LRNC in patients with psoriasis. These associations with high-risk coronary features deviate from a previous study in patients without chronic inflammatory disease or active malignancy that found splenic but not BM 18F-FDG uptake independently predicted MACE.[6] Yet, our findings are supported by existing literature that describes an additional role of the BM in exacerbating the inflammatory state in psoriasis. This is supported by our finding that both splenic and BM 18F-FDG uptake decreases following initiation of biologic treatment in psoriasis participants. For example, case reports demonstrate psoriasis that remits after BM transplant in those with malignancies,[31] and, in our previous cohort, we have shown BM 18F-FDG uptake reduces in response to biologic therapy. [18] In addition, our observation that BM 18F-FDG uptake is associated with neutrophils, white blood cells, and monocytes suggests that BM 18F-FDG uptake may be capturing proliferation of multiple immune cells in psoriasis.
This study uses multimodality imaging to understand the interplay among immune cell activation, various markers of inflammation, metabolic dysfunction and subclinical atherosclerosis. Next steps of this work should include characterizing the differential effect of targeted therapies on splenic and BM 18F-FDG uptake and atherosclerotic endpoints to better understand the role of both tissues in atherosclerosis. In addition, efforts can be focused on identifying potential immune and inflammatory biomarkers, besides traditional lipids, that may be useful in guiding statin and biologic therapy in psoriasis.
Our study has several limitations. First, it is a retrospective study and is subject to the inherent limitations. Further, we aimed to match the psoriasis and HV cohort by age and sex, which led to unequal cohort sizes that may affect the power of our conclusions. We also recognize these groups may have had other differences besides age and sex that introduced bias to the results. In addition, the observed associations between splenic and BM 18F-FDG uptake with inflammatory cells, cytokines, and imaging markers of atherosclerosis do not indicate a causal relationship.
4.2. Conclusion:
In conclusion, splenic and bone marrow 18F-FDG uptake is heightened in psoriasis compared to HVs. Both splenic and BM 18F-FDG uptake is associated with atherogenic serum based proatherogenic parameters, as well as greater subclinical coronary artery plaque burden and LRNC. Collectively, these findings suggest inflammation driven immune proliferation plays an important role in atherosclerosis in psoriasis.
Supplementary Material
Highlights:
Splenic and bone marrow (BM) uptake represent heightened immune cell proliferation
Splenic and BM uptake relate to atherogenic lipid, immune & inflammatory measures
Splenic and BM uptake relate to non-calcified burden & lipid rich necrotic core
Financial support
National Heart, Lung and Blood Institute Intramural Research Program in Bethesda, Maryland (HL006193-07). This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, and the Colgate-Palmolive Company. MTO is supported by the NIH K23HL151909.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
NNM is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc, and Novartis receiving grants and/or research funding and as a principal investigator for the National Institute of Health receiving grants and/or research funding. MTO received consulting fees from Intrinsic Imaging, LLC for unrelated work. AT received institutional grants from Genentech and personal fees from Actelion and Esperion during the conduct of this study for research outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–74. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitroulis I, Kalafati L, Bornhauser M, Hajishengallis G, Chavakis T. Regulation of the Bone Marrow Niche by Inflammation. Front Immunol. 2020;11:1540. doi: 10.3389/fimmu.2020.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8:121–30. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- 8.Harrington CL, Dey AK, Yunus R, Joshi AA, Mehta NN. Psoriasis as a human model of disease to study inflammatory atherogenesis. Am J Physiol Heart Circ Physiol. 2017;312:H867–H73. doi: 10.1152/ajpheart.00774.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frostegard J Atherosclerosis in patients with autoimmune disorders. Arterioscler Thromb Vasc Biol. 2005;25:1776–85. doi: 10.1161/01.ATV.0000174800.78362.ec. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser H, Kvist-Hansen A, Krakauer M, Gørtz PM, Henningsen KMA, Wang X, et al. Association between Vascular Inflammation and Inflammation in Adipose Tissue, Spleen, and Bone Marrow in Patients with Psoriasis. Life. 2021;11:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55:2527–35. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 12.Higashi K, Ueda Y, Yagishita M, Arisaka Y, Sakurai A, Oguchi M, et al. FDG PET measurement of the proliferative potential of non-small cell lung cancer. J Nucl Med. 2000;41:85–92. [PubMed] [Google Scholar]
- 13.Zhou W, Dey A, Manyak G, Teklu M, Patel N, Teague H, et al. The application of molecular imaging to advance translational research in chronic inflammation. J Nucl Cardiol. 2020. doi: 10.1007/s12350-020-02439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi H, Uceda DE, Dey AK, Abdelrahman KM, Aksentijevich M, Rodante JA, et al. Treatment of Psoriasis With Biologic Therapy Is Associated With Improvement of Coronary Artery Plaque Lipid-Rich Necrotic Core: Results From a Prospective, Observational Study. Circ Cardiovasc Imaging. 2020;13:e011199. doi: 10.1161/circimaging.120.011199. [DOI] [PubMed] [Google Scholar]
- 15.Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115:721–8. doi: 10.1093/cvr/cvz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L. Systemic Inflammation and Evidence of a Cardio-splenic Axis in Patients with Psoriasis. Acta Derm Venereol. 2018;98:390–5. doi: 10.2340/00015555-2873. [DOI] [PubMed] [Google Scholar]
- 17.Glezeva N, Voon V, Watson C, Horgan S, McDonald K, Ledwidge M, et al. Exaggerated Inflammation and Monocytosis Associate With Diastolic Dysfunction in Heart Failure With Preserved Ejection Fraction: Evidence of M2 Macrophage Activation in Disease Pathogenesis. Journal of Cardiac Failure. 2015;21:167–77. doi: 10.1016/j.cardfail.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, et al. Chronic Stress-Related Neural Activity Associates With Subclinical Cardiovascular Disease in Psoriasis: A Prospective Cohort Study. JACC Cardiovasc Imaging. 2020;13:465–77. doi: 10.1016/j.jcmg.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35:2667–76. doi:doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, et al. Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis. JAMA Cardiol. 2018;3:949–56. doi: 10.1001/jamacardio.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ Cardiovasc Imaging. 2014;7:454–60. doi: 10.1161/circimaging.113.001093. [DOI] [PubMed] [Google Scholar]
- 22.Sheahan M, Ma X, Paik D, Obuchowski NA, Pierre SS, III WPN, et al. Atherosclerotic Plaque Tissue: Noninvasive Quantitative Assessment of Characteristics with Software-aided Measurements from Conventional CT Angiography. Radiology. 2018;286:622–31. doi: 10.1148/radiol.2017170127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465:793–7. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–92. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trompouki E, Mullen L, Fernandez-Reyes D, Yodoi J, Kim S, Schuettpelz LG. Editorial: Inflammatory Signaling in Bone Marrow Failure and Hematopoietic Malignancy. Frontiers in Immunology. 2017;8. doi: 10.3389/fimmu.2017.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitagawa M, Saito I, Kuwata T, Yoshida S, Yamaguchi S, Takahashi M, et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–54. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- 27.Pantoja-Torres B, Toro-Huamanchumo CJ, Urrunaga-Pastor D, Guarnizo-Poma M, Lazaro-Alcantara H, Paico-Palacios S, et al. High triglycerides to HDL-cholesterol ratio is associated with insulin resistance in normal-weight healthy adults. Diabetes Metab Syndr. 2019;13:382–8. doi: 10.1016/j.dsx.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Kim B, Kim W, Ahn C, Choi HY, Kim JG, et al. Lipid indices as simple and clinically useful surrogate markers for insulin resistance in the U.S. population. Sci Rep. 2021;11:2366. doi: 10.1038/s41598-021-82053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride–to–High-Density-Lipoprotein-Cholesterol Ratio Is an Index of Heart Disease Mortality and of Incidence of Type 2 Diabetes Mellitus in Men. Journal of Investigative Medicine. 2014;62:345–9. doi: 10.2310/jim.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 30.Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The Triglyceride-Glucose Index, an Insulin Resistance Marker, Was Non-linear Associated With All-Cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med. 2020;7:628109. doi: 10.3389/fcvm.2020.628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Li J, Wang L, Niu X, Hou R, Liu R, et al. Transmission of psoriasis by allogeneic bone marrow transplantation and blood transfusion. Blood Cancer J. 2015;5:e288. doi: 10.1038/bcj.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.