Abstract
Objective:
Although some survivors of childhood cancer report significant psychosocial distress, many also report having derived benefits, or post-traumatic growth (PTG), from their cancer experience. This study examines PTG and its correlates among an ethnically diverse sample of adolescent/young adult (AYA) cancer survivors who have recently completed treatment.
Methods:
Survivors of childhood cancer (n = 94; 47% Hispanic), ages 11–21 and within 6 months of completing cancer therapy, were recruited from three pediatric cancer centers. Participants completed a structured interview that assessed demographics, PTG, post-traumatic stress symptoms, health-related quality of life, optimism, and depressive symptoms. Diagnosis/treatment information was collected from each patient’s medical record. Multiple regression analyses were used to identify significant correlates of PTG.
Results:
The majority of survivors reported positive growth. PTG was positively associated with psychosocial functioning and post-traumatic stress symptoms and inversely associated with physical functioning and depressive symptoms. PTG was significantly lower among survivors of bone tumors (vs. survivors of other cancers) and Hispanic survivors who primarily spoke English at home (vs. Hispanics who primarily spoke Spanish at home and non-Hispanics). PTG was not significantly related to age, sex, optimism, cancer treatment modality, duration of treatment, or treatment intensity.
Conclusions:
The AYA survivors commonly reported PTG in the immediate aftermath of cancer treatment. Findings regarding PTG among more acculturated Hispanic and bone tumor AYA survivors may help to inform risk-adapted clinical interventions, among those transitioning from active treatment to post-treatment surveillance, to mitigate negative long-term sequelae and enhance positive psychosocial adaptation from the cancer diagnosis and treatment.
Background
For children and adolescents, a cancer diagnosis and its treatment can disrupt normative developmental trajectories and result in significant psychosocial distress and/or adverse physical sequelae [1,2]. Despite these disruptions and challenges, many young survivors report psychological growth and positive change [3–5]. Such experiential change has been termed post-traumatic growth (PTG). PTG is characterized as a stronger sense of self and values, increased psychological maturity and empathy, improved interpersonal relationships, more engagement in activities, a greater sense of purpose, and greater planning for the future [3,6–8].
Post-traumatic growth occurs when the awareness of vulnerability is accompanied by an augmented sense of becoming more capable and self-reliant [8] and when individuals are able to find new meanings in life and social relationships. These new found meanings in response to cancer may be psychologically protective against depressive symptoms or declining quality of life (QOL) [4,5]. In addition, an underlying positive mindset, such as having optimism, may contribute protective effects against psychosocial decline and potentially foster PTG [9–13].
Although research has typically sought to prevent psychosocial decline and improve QOL among cancer survivors, the presence of psychosocial distress is not entirely avoidable. Both positive and negative outcomes occur in the adjustment to cancer and related treatments. For instance, a positive relationship has been documented between PTG and post-traumatic stress symptoms (PTSS) among childhood cancer survivors [3]. Other research suggests that a curvilinear relationship exists where PTSS may be an important stage in the transition to PTG [14] or the two may occur simultaneously as a natural response to trauma [3,15,16]. Efforts to further clarify the coexistence of positive and negative changes among pediatric cancer patients are needed to better characterize the cancer experience and design tailored psychoeducational interventions for this population [17].
In addition to intrapersonal factors, adaptation can be influenced by ethnic and cultural context. For example, Hispanics tend to report more positive adjustment to cancer [18]. Hispanics generally tend to place greater emphasis on family support and religious coping strategies; two health protective factors that may explain why Hispanics report higher levels of PTG compared with non-Hispanics [18–20]. Furthermore, Hispanics who are more highly acculturated to dominant culture in the United States tend to report lower levels of PTG compared with their less acculturated counterparts [19]. In pediatric oncology, the current understanding of ethnic differences in patterns of adjustment, resiliency, and psychosocial outcomes is limited because of the low diversity of patients recruited for psychosocial studies [21].
The primary aims of this study were to (i) explore PTG among adolescent/young adults (AYAs) who were transitioning from active therapy to surveillance monitoring and survivorship care, (ii) examine differences in PTG by ethnic groups and acculturation, focusing on Hispanics compared with non-Hispanic participants, and (iii) investigate PTG and correlational relationships with constructs of optimism, depressive symptoms, post-traumatic stress, QOL, and disease-related factors.
Methods
Participants and procedures
Patients diagnosed with cancer before age 18 years were recruited between May 2005 and February 2007 from three pediatric cancer treatment centers: Children’s Hospital Los Angeles (Los Angeles, CA), Miller Children’s Hospital (Long Beach, CA), and C.S. Mott Children’s Hospital (Ann Arbor, MI). Patients became eligible for the study as soon as they completed cancer treatment. Oncology nurse care managers at each site identified potential participants according to eligibility criteria that required that participants be 11–21 years of age, disease-free, English speaking, and cognitively able to complete study questionnaires. After receiving the attending physician’s approval to approach the patient, the patient was provided with information about the study during a regularly scheduled clinic visit. Parents and/ or medical staff attested to the patient’s cognitive ability to participate in the study and provide valid self-report data. For patients younger than 18 years of age, signed assent from the patient and signed consent from the parent were both obtained. Patients 18 years and older provided consent on their own behalf.
Within the first 6 months of completing cancer treatment, patients participated in a 35-min structured in-person or telephone interview conducted by trained research assistants. In the structured interview, information was collected on demographics, PTG, PTSS, optimism, depressive symptoms, and QOL. Disease and treatment information were abstracted from each participant’s medical record. Prior to enrolling patients, study procedures were approved by the Institutional Review Board of each treatment center.
Measures
Post-traumatic growth
A modified version of Tedeschi and Calhoun’s PTG inventory [22] for assessing change following a traumatic event was used for this study. The modified scale includes 16 items designed to measure survivors’ perceived change in response to their cancer experience. The items were modified such that wording was simplified for younger respondents. For example, ‘my priorities about what is important in life’ was reworded to ‘what I think is truly important’. Scale items assess change in all areas of the original PTGI including the areas of relationships, life goals, personal strengths, appreciation for life, and spirituality. Response choices were provided on a 5-point scale (1: very negative change, 2: somewhat negative change, 3: no change, 4: somewhat positive change, and 5: very positive change) with a higher mean score indicating higher PTG. These items and response format have been used previously among adolescent populations [20], and the response format has been used in separate studies of adults being treated for HIV/AIDS [23] and cancer [24]. For each PTG item, the number of survivors who reported a positive change (some or very positive change), no change, and negative change (some or very negative change) were examined. The mean score across all PTG items was calculated with the scale demonstrating high internal consistency (Cronbach’s alpha = 0.93).
Post-traumatic stress symptoms
To assess PTSS, the University of California at Los Angeles Post-Traumatic Stress Disorder Reaction Index for DSM-IV, Adolescent Version was used [25]. This instrument is a 22-item questionnaire that evaluates adolescent subjective re-experiencing, avoidance, and arousal reactions to traumatic experience and has been used previously in pediatric oncology research [26]. Respondents rate how much of the time during the past month they have experienced the symptom listed (e.g., ‘When something reminds me of cancer, I get very upset, afraid, or sad’). Using a 5-point Likert scale (0 = none to 4 = most of the time), a total post-traumatic stress severity score was calculated by summing 20 of the items. In prior studies, the Post-traumatic Stress Disorder Reaction Index has demonstrated high internal consistency (α = 0.90) and good to excellent test–retest reliability [25]. The Cronbach’s alpha in this sample was 0.99.
Depressive symptoms
The 20-item Center for Epidemiological Studies Depression Scale was used to assess symptoms of depression [27]. Participants indicated how often they had experienced symptoms (e.g., depressed mood, loss of appetite, sleep and psychomotor disruption, feelings of guilt and worthless-ness and/or helplessness, and hopelessness) during the previous week on a 4-point scale (i.e., ‘rarely or none of the time’ (less than 1 day) to ‘most or all of the time’ (5–7 days)). A total score was calculated with higher scores representing more elevated levels of depressive symptoms. The scale yields high internal consistency, adequate test–retest repeatability and good construct validity in both clinical and community samples [28]. The Cronbach’s alpha in this sample was 0.91.
Life Orientation Test—Revised
Optimism was assessed using the Life Orientation Test—Revised (LOT-R) [29]. The LOT-R measures global expectations about positive versus negative outcomes in the future. Participants were asked to rate to what extent they agreed with six coded statements (e.g., ‘If something can go wrong for me, it will’ and ‘Overall, I expect more good things to happen to me than bad’) on a 5-point scale (0 = strongly disagree to 4 = strongly agree). Three items were reverse scored and a total score was calculated for all six items with higher scores indicating a more optimistic outlook. The Cronbach’s alpha was 0.77 in this sample.
The PedsQL™ 4.0 Generic Core Scales-Adolescent and Young Adult Self-Report
The PedsQL™ 4.0 Generic Core Scales (23 items) measure Physical Functioning (8 items), Emotional Functioning (5 items), Social Functioning (5 items), and School Functioning (5 items) [30]. For each item, respondents indicate the extent to which the issue referred to has been a problem (0 = never to 4 = almost always) during the preceding month. Items are reverse scored and linearly transformed to a 0–100 scale with higher scores indicating better QOL. Two summary scores were calculated: Physical Functioning and Psychosocial Functioning (including social, emotional, and school functioning). The PedsQL™ 4.0 Generic Core Scales have been tested with pediatric oncology patients and demonstrated strong internal consistency and test–retest reliability [31]. In this sample, Cronbach’s alphas for the physical and psychosocial summary scales were 0.89 and 0.87, respectively.
Intensity of treatment
The Intensity of Treatment Rating Scale 2.0 was used to determine objective ratings of treatment intensity [32]. Ratings were based on information retrieved from medical chart review, including cancer type, stage at diagnosis, treatment modalities, and relapse history. Two study investigators (KR and KM) served as raters with high kappa for inter-rater reliability (κ = 1.0). Treatment was categorized by four levels of intensity: 1 = least intensive (e.g., surgery only), 2 = moderately intensive (e.g., standard risk leukemia), 3 = very intensive (e.g., high risk leukemia), and 4 = most intensive (e.g., stem cell transplant).
Ethnicity and acculturation
Acculturation was measured by primary language spoken at home (English, Spanish, and other). Although language spoken at home is one aspect of the multidimensional construct of acculturation, it is one of the more robust methods used in health research [33], accounting for a substantial proportion of variance of broader acculturation measures [34,35] and is used in the National Health Interview Survey [36]. We created five categorical variables to capture the survivor’s ethnicity/race and acculturation level: (i) White non-Hispanic: primary language English; (ii) Hispanic: primary language English; (iii) Hispanic: primary language not English; (iv) Other ethnicity/race: primary language English; and (v) Other ethnicity/race: primary language not English.
Data analysis
The primary outcome for this study was PTG. Frequencies for each PTG item as well as a mean score across all PTG items were calculated. Socioeconomic status was calculated using father’s educational level, although when missing, mother’s educational level was substituted (correlation coefficient for mother’s and father’s education: r = 0.64, p < 0.0001). We analyzed race/ethnicity by using the three primary ethnic/racial groups (non-Hispanic white, Hispanic, and other) and the five derived categories that combined race/ethnicity and language spoken in the home. Univariate and multi-variable linear regression procedures were used to identify variables significantly associated with PTG (mean score), including demographics, disease/treatment factors, depressive symptoms, post-traumatic stress disorder, optimism, and QOL (PedsQL psychosocial and physical summary scores). The initial multivariable regression model was constructed by including variables that were related to PTG in univariate analyses (at p ≤ 0.20). To identify correlates of PTG in constructing the final multivariable model, stepwise backward elimination procedures were used (p ≤ 0.05). Data analyses were conducted using SAS statistical software (Version 9.2) (SAS Institute; Cary, NC).
Results
Participants
One hundred and nineteen AYAs were eligible for this study. Of these, 102 (85%) were interviewed. The primary reason for nonparticipation was lack of interest. One patient was deemed ineligible on the basis of the English-speaking eligibility requirement. There were no statistically significant differences between nonparticipants and participants with respect to age, sex, race/ethnicity, type, or duration of treatment. Eight participants with incomplete information were excluded from analyses. The final analytic sample included 94 adolescents: 56 from Children’s Hospital Los Angeles, 27 from C.S. Mott Children’s Hospital (Ann Arbor, MI) and 11 from Miller Children’s Hospital (Long Beach, CA).
Characteristics of the study sites
No significant differences were detected by study site for PTG, PTSS, QOL measures, depressive symptoms, optimism, sex, or age at diagnosis. Statistically significant differences were noted among the three centers for race/ethnicity (p < 0.001), parental education (p = 0.04), cancer type (p = 0.04), and treatment intensity (p = 0.03). Race/ethnicity and parental education were correlated, with a highly significant association between Hispanic ethnicity and lower parental education (p < 0.0001). The majority of Hispanic patients (43/44) were recruited from the two California sites. Differences in the distribution of cancers (and cancer-related treated intensity scores) across sites reflect each institution’s cancer subspecialties and referral patterns.
Participant’s average age at interview was 14.8 years (SD = 2.74). Slightly more than half of the participants were male, and nearly half were Hispanic (Table 1). Eighteen of the 44 Hispanic participants (40%) were from Spanish-speaking homes. Mean age at diagnosis was 12.3 (SD = 3.14). Participants were diagnosed with a variety of cancers, the majority of which were treated with chemotherapy. Treatment was rated as ‘very’ or ‘most’ intensive in 70% of our sample. Length of treatment ranged from 1 to 119 months (mean = 20 months, SD = 18.4), with most patients receiving 1–12 months of treatment.
Table 1.
Sample characteristics and relationship to post-traumatic growth
| n (%) or variable mean (SD) | PTG mean (SD) | Unadjusted univariate test statistic for the relationship with PTG (ANOVA for categorical; Pearson R for continuous) | |
|---|---|---|---|
|
| |||
| Gender | t(1) = 1.99, p = 0.05* | ||
| Male | 49 (52) | 3.89 (0.59)a | |
| Female | 45 (48) | 3.66 (0.54)a | |
| Race/ethnicity | f(2) = 0.65, p = 0.52 | ||
| White non-Hispanic | 40 (44) | 3.85 (0.58) | |
| Hispanic | 44 (47) | 3.71(0.62) | |
| Otherb | 10 (11) | 3.79 (0.32) | |
| Race/ethnicity and language spoken at home | f(4) = 1.80, p = 0.14* | ||
| White non-Hispanic: English is primary language spoken in the home | 40 (44) | 3.85 (0.58)a | |
| Hispanic: English is primary language spoken in the home | 26 (28) | 3.54 (0.51)a | |
| Hispanic: English is not primary language spoken in the home | 18 (19) | 3.95 (0.69) | |
| Other: English is primary language spoken in the home | 5 (5) | 3.85 (0.22) | |
| Other: English is not primary language spoken in the home | 5 (5) | 3.74 (0.43) | |
| Father’s education | r = 0.19, p = 0.08* | ||
| <High school | 20 (24) | 3.60 (0.61)a | |
| High school graduate/GED | 27 (333) | 3.78 (0.59)a | |
| College or technical school | 9 (11) | 3.59 (0.60) | |
| Graduate degree | 27 (33) | 3.94 (0.52) | |
| Diagnosis | f(4) = 2.85, p = 0.03** | ||
| Leukemia | 36 (38) | 3.79 (0.56) | |
| Lymphoma | 23 (24) | 3.72 (0.61) | |
| CNS tumor | 9 (10) | 3.42 (0.50) | |
| Bone tumor | 7 (7) | 3.52 (0.28) | |
| Soft tissue tumor | 19 (20) | 4.09 (0.56) | |
| Age at diagnosis (years) | r = 0.07, p = 0.52 | ||
| <7 | 4 (4) | 3.51 (0.82) | |
| 8–9 | 13 (14) | 3.79 (0.55) | |
| 10–12 | 23 (24) | 3.72 (0.51) | |
| 13–15 | 32 (34) | 3.83 (0.56) | |
| 16–18 | 22 (23) | 3.80 (0.66) | |
| Treatment | |||
| Radiation | t(1) = −1.54, p = 0.13* | ||
| No | 57 (61) | 3.85 (0.58) | |
| Yes | 36 (39) | 3.66 (0.56) | |
| Bone marrow transplant | t(1) = −1.05, p = 0.29 | ||
| No | 88 (95) | 3.79 (0.59) | |
| Yes | 5 (5) | 3.51 (0.28) | |
| Chemotherapy | t(1) = −0.19, p = 0.85 | ||
| No | 4 (4) | 3.83 (0.89) | |
| Yes | 89 (96) | 3.77 (0.57) | |
| Duration of treatment (months) | r = −0.06, p = 0.58 | ||
| 0–12 | 44 (47) | 3.79 (0.63) | |
| 13–24 | 16 (17) | 3.85 (0.58) | |
| 25–36 | 19 (20) | 3.76 (0.52) | |
| 36+ | 15 (16) | 3.69 (0.53) | |
| Treatment intensity (levels 1–4) | 2.86 (0.76) | r = −0.05, p = 0.63 | |
| 1: least intensive | 3 (3) | ||
| 2: moderately intensive | 25 (27) | ||
| 3: very intensive | 49 (51) | ||
| 4: most intensive | 18 (19) | ||
| Depressive symptoms (range 0–38) | 10.9 (9.7) | r = −0.49, p = <0.0001*** | |
| Optimism (range 3–24) | 15.5 (4.3) | r = 0.43, p = <0.0001*** | |
| Post-traumatic stress symptoms (range 0–68) | 17.64 (15.37) | r = −0.23, p = 0.03** | |
| Quality of Life | |||
| PedsQL Psychosocial Summary Scale(range 43–100) | 76.92 (14.53) | r = 0.38, p = 0.0001*** | |
| PedsQL Physical Summary Scale(range 14–100) | 75.87 (21.26) | r = 0.14, p = 0.17* | |
GED, General Education Development Test; CNS, central nervous system; SD, standard deviation; PTG, post-traumatic growth; ANOVA, analysis of variance.
Indicates a significant difference in mean PTG (p < .05) between variable category levels.
Because of small numbers, Asian (n = 5), Black (n = 1) and unspecified ethnicity (n = 4) subgroups were consolidated into an ‘Other’ category.
p ≤ 0.20.
p ≤ 0.05.
p ≤ 0.001.
Post-traumatic growth
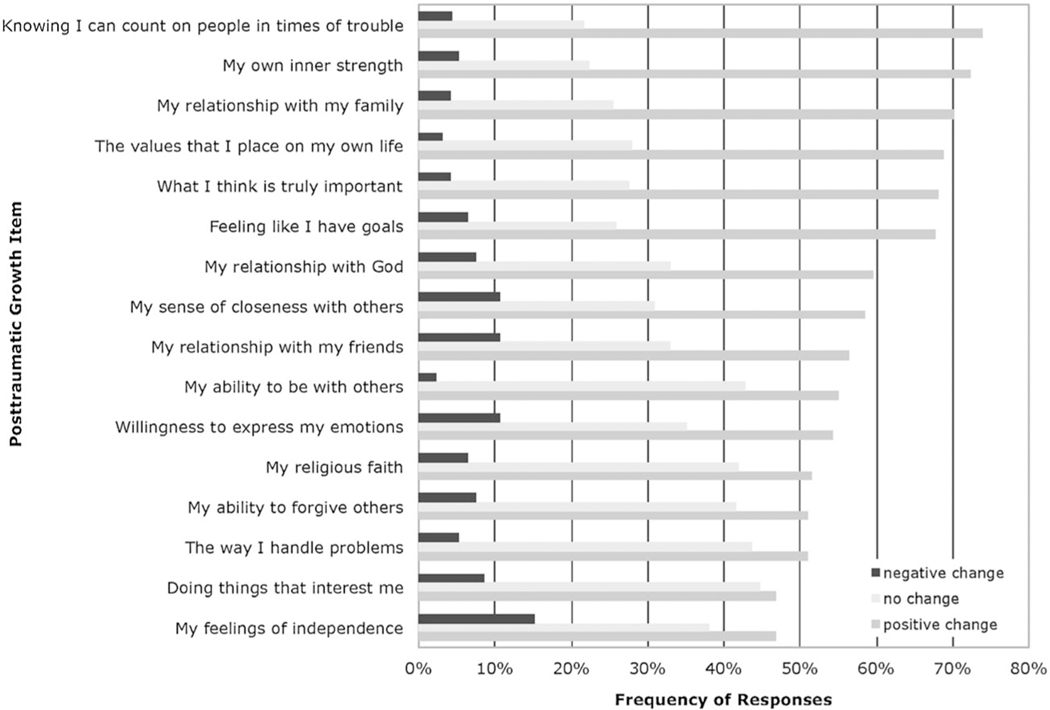
Survivors were more likely to report a positive change than negative or no change across all PTG items (Figure 1). The mean PTG score for the full sample was 3.78 (SD = 0.58), indicating that the majority of respondents reported overall positive change from their cancer experience on the PTG items.
Figure 1.
Responses to post-traumatic growth items: negative, no, and positive change
Correlates of post-traumatic growth
Univariate analyses revealed that men reported significantly higher PTG scores than women (mean difference = 0.23, p = 0.05) (Table 1). PTG scores did not differ significantly among the three primary ethnic/racial groups. However, among the five racial/ethnic groups that included language spoken in the home, PTG scores were lowest for Hispanic survivors who came from English-speaking homes (p = 0.03). There was a positive relationship between PTG and optimism (p < 0.0001) and psychosocial functioning (PedsQL™ psychosocial summary scale) (p < 0.0001) and a negative relationship with depressive symptoms (p < 0.0001) and PTSS (p = 0.03). Survivors with higher PTSS and Center for Epidemiological Studies Depression Scale scores (depressive symptoms) had lower PTG scores. Further, type of cancer diagnosed was significantly related to PTG (p = 0.03). No significant univariate relationships were found between PTG and disease/treatment variables (age at diagnosis, treatment methods, intensity of treatment, or length of therapy) or father’s education.
Consistent with univariate analyses, multivariable analyses of PTG found a positive relationship with psychosocial functioning (p = 0.006) and a negative relationship with depressive symptoms (p = 0.0006) (Table 2). PTG scores were significantly lower for Hispanic survivors from English-speaking homes compared with the referent White non-Hispanic group (p = 0.01). Our final multivariable model revealed that lower physical functioning was associated with higher PTG (p = 0.04). In contrast to our univariate results, our multivariable analyses found a positive relationship rather than a negative relationship between PTG and PTSS (p = 0.02). In this adjusted model, higher levels of PTSS were associated with higher levels of PTG. Also, our multivariable model identified bone tumor patients as the cancer group least likely to endorse PTG (p = 0.02). The final adjusted regression model for PTG also eliminated sex, father’s education, and optimism as significant correlates of PTG.
Table 2.
Multivariable regression analysis of post-traumatic growth
| Variable | Estimate (SE) | T |
|---|---|---|
|
| ||
| Ethnicity/race | ||
| White non-Hispanic: English is | Reference | |
| primary language spoken in the home Hispanic: English is | −0.33 (0.13) | −2.55** |
| primary language spoken in the home Hispanic: English is not | 0.008 (0.15) | −0.06 |
| primary language spoken in the home Other: English is primary | −0.28 (0.23) | −1.2 |
| language spoken in the home Other: English is not primary language spoken in the home | −0.32 (0.23) | −1.36 |
| Diagnosis | ||
| Leukemia | Reference | |
| Lymphoma | −0.007 (0.13) | −0.05 |
| CNS tumor | −0.003 (0.19) | −1.59 |
| Bone tumor | −0.51 (0.22) | −2.31* |
| Soft tissue tumor | 0.15 (0.15) | 1.06 |
| Depressive symptoms | −0.03 (0.01) | −3.59*** |
| Post-traumatic stress symptoms | 0.01 (0.01) | 2.48* |
| PedsQL Psychosocial Scale | 0.02 (0.006) | 2.83** |
| PedsQL Physical Scale | −0.0061 (0.003) | −2.08* |
| Adjusted R | 0.43 | |
| F | 5.05 | (p < .0001) |
CNS, central nervous system; SE = standard error.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
Discussion
A novel contribution of the present study is that all AYA cancer survivors were evaluated for PTG within 6 months of completing therapy. This represents a unique period in that patients are beginning their transition to being a cancer survivor. Previous research on PTG among survivors of childhood cancer have included participants many years post-treatment or a heterogeneous mix of participants who were on and off therapy [3,16,37–43]. Thus, reports of PTG in this study were less likely to be confounded by subsequent life events (recall bias), as might be the case among childhood cancer survivors who are assessed many years later in the post-treatment trajectory. In addition, although PTG has been studied among survivors of adult cancer, only a few studies have assessed the presence of PTG among AYA survivors of childhood cancer [3,16,37–42,44].
The majority of participants in this study reported positive changes as a result of their cancer experience. Areas of PTG positively endorsed immediately post-treatment were primarily those involving a changed sense of self and perspective on social relationships, with 68%–74% of participants reporting these changes. These areas of positive change are consistent with results from studies among adolescents exposed to other traumatic events [45–47]. Whereas most PTG measures allow only for positive responses [7], the instrument used for this study allowed participants to report negative as well as positive change. A surprisingly small number of patients reported negative changes across PTG items (4%–15%), most frequently in the areas of having more independence and in peer relationships. This makes conceptual sense as a cancer diagnosis and treatment often require adolescent patients to rely more heavily on parental support rather than fostering peer friendships during a time when a normative developmental process for adolescents would be the opposite.
Although preferred language spoken at home does not encompass all aspects of acculturation (i.e., acquiring values, beliefs, customs, and mannerisms of the new country), it has been shown to be a valid indicator and significant component of the process [35,48]. In this study, Hispanic cancer survivors who came from English-speaking homes had significantly lower PTG scores compared with other groups, including Hispanic survivors who came from Spanish-speaking homes. This finding has not been previously reported among childhood cancer survivors; however, it corroborates previous findings among Hispanic women, where those who were more highly immersed in the dominant culture reported lower PTG scores compared with those who were less acculturated [19]. Specifically among Hispanic adult cancer patients, one study has found a strong relationship between higher PTG and coping approaches involving spirituality and religion [18]. To consider spirituality and religion as confounding factors, we performed additional analyses yet found that these factors did not explain the differences in PTG between the two Hispanic groups in this study. Furthermore, several studies have found that particularly among Hispanic adolescents, higher stress from acculturation was associated with a reduction in traditional family values, including parental respect and familism (i.e., protective family factors) [49,50]. Studies are needed that examine whether such alterations in family dynamics and acculturative stress result in a growth-inhibiting dynamic for AYA survivors.
With regard to the third study aim, a positive relationship was found between PTG annd survivors’ psychosocial QOL and mood. This corroborates findings from a study in which survivors of adolescent cancer, diagnosed an average of 14 years prior, demonstrated a positive relationship between PTG and life satisfaction as well as findings of associations between PTG and higher mental QOL and lower depression from several studies conducted among adult cancer survivors [51–54]. Although studies on PTG and its relationship with improved well-being among AYA survivors is limited, prior work with adolescents who have experienced other forms of trauma has demonstrated a protective relationship with health-related outcomes, such as lower levels of substance use, physical inactivity, and depression [15,20,45]. Furthermore, some studies of adult cancer patients suggest that PTG may positively influence physical and psychosocial health by increasing immune functioning [55] and decreasing cortisol levels [56]. These relationships are intriguing and potentially clinically meaningful, suggesting that more longitudinal studies are needed to determine if PTG is a mediator of health, psychosocial well-being, and/or adjustment.
Optimism was positively related to PTG in univariate analyses only. The relationship between these constructs may depend on the type of trauma experienced, time since trauma, and presence of other variables [13,57]. For example, the positive changes derived from the traumatic experience may diminish [58] and thereby attenuate the relationship between PTG and optimism over time. Also, it has been proposed that optimism may be related to PTG in certain circumstances only, such as when an individual perceives more versus less control over the cause of their stress [59]. In this study, other factors such as the lack of depressive symptoms, presence of PTSS, or QOL levels may be more salient to the development of PTG among AYA cancer survivors who recently completed treatment, thus accounting for our finding of no relationship between PTG and optimism in the final model.
The positive relationship between PTG and PTSS in this study supports findings among other samples of young cancer survivors [3,38] and falls in line with theories that explain the development of PTG [60,61]. According to theoretical postulates of PTG, the immediate reactions to traumatic experiences that are considered hall-mark characteristics of post-traumatic stress disorder (i.e., hypervigilance, re-experiencing, perception of life threat, sense of uncontrollability and helplessness, and emotional dissociation) are normal and adaptive behaviors [15]. Through time, if cognitive-emotional processing (i.e., adaptive coping) is facilitated by the appropriate resources (i.e., intrapersonal coping skills, personality characteristics, and supportive social environment), an individual who experiences these reactions to cancer diagnosis and treatment will likely diverge from the path of sustaining PTSS and continue on a path towards finding resolution (e.g., acceptance, meaning in the occurrence, and gaining of a stronger sense of self) and thereby develop PTG [60,62]. Thus, the relationship between PTG and PTSS in this study may be explained by the early timepoint of assessment in survivorship (within 6 months post-treatment), at which AYAs may be experiencing high levels of anxiety upon recently completing treatment and anticipating their next phase ahead, or it may be a celebratory timepoint yet complicated by survivors still having to deal with residual symptoms of treatment. Nevertheless, an expectation is that PTG would increase, whereas PTSS would decrease over time, if assessed at a later timepoint of survivorship. Future research that includes measures of both positive and negative changes at multiple timepoints during the transition period from patient to survivor may help to inform clinicians on the optimal timing for implementing interventions and providing supportive resources aimed at facilitating positive outcomes.
Although no relationships were found between PTG and treatment variables (e.g., treatment types, duration, and intensity of treatment), PTG was significantly lower for patients with bone cancers. These patients face a greater risk for treatment-related late effects that manifest as physical and psychosocial problems (e.g., physical deformities/limitations, deficits in education, employment, and marriage) when compared with survivors of other types of cancer and their siblings [63,64]. However, psychosocial problems may develop earlier for bone tumor survivors because they reported less endorsement on PTG domains that other cancer survivors have endorsed more frequently (e.g., relationships with others). Longitudinal studies are needed to examine how psychosocial adjustment for bone tumor survivors changes over time and differs in trajectory compared with AYA survivors of other types of cancers.
This study reports early post-treatment phase findings, and thus, results may not be generalizable to survivors who are further out from diagnosis and treatment. The cross-sectional study design prohibits making causal statements regarding the relationships found. Also, the measurement of acculturation in this study was limited to language spoken in the home, which is one component of acculturation. Although preferred language account for a substantial proportion of the variance of broader acculturation measures [35], in some studies up to 62% [34], future studies may benefit from using more robust measures of the acculturation in examining its relationship with PTG.
This study represents a move away from a deficit-oriented cancer survivorship orientation and towards more competency-based research. Findings demonstrate that young survivors have found meaning in the immediate aftermath of cancer treatment, despite their continuing struggles. Positive changes were associated with higher psychosocial QOL and PTSS and lower depressive symptoms and physical QOL. Positive changes were lowest for the Hispanic subgroup for whom English was the primary language spoken at home and those diagnosed with bone tumors. Thus, on-going assessment of survivors post-therapy may be important to enable AYAs to access supportive services that both integrate the notion of PTG and recognize persisting vulnerabilities when facing cancer-related problems unique to their developmental stage (e.g., gaining a sense of their independence and survivorship identity while conceptualizing their future; moving forward with school, relationships, finding jobs; and navigating a long-term relationship with healthcare). Moreover, findings may help to refine theoretical models (i.e., Adolescent Resilience Model [65]) by describing factors related to PTG, particularly for young survivors who have specific cultural influences. Such models may then be used as a basis for risk-adapted clinical interventions (e.g., distress management and resiliency skills training) planned for implementation during the early transition phase from active treatment to the post-treatment surveillance, to enhance positive psychosocial adaptation from experiences of cancer diagnosis and treatment.
Acknowledgements
Funding for this study was provided by CureSearch National Childhood Cancer Foundation for Adolescent and Young Adult Oncology Research through the generosity of Aflac, Inc. Dr. Meeske is supported by a STOP Cancer Career Development Award. Also, we would like to thank colleagues from C.S. Mott Children’s Hospital in Ann Arbor, MI and Miller’s Children’s Hospital in Long Beach, CA who helped with recruitment and data collection for this study.
Footnotes
Note
1. Source: unpublished qualitative data reported by cancer survivor, May 2008.
‘Cancer has given my life a different perspective. In a way, cancer gave me a gift. A gift the average person cannot comprehend. I saw the best in human nature when I was sick.’
-J. C., cancer survivor1
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- 1.Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med pediatric oncol 2002;38(1):1–10. [DOI] [PubMed] [Google Scholar]
- 2.Felder-Puig R, Formann AK, Mildner A, Bretschneider W, Bucher B, Windhager R, et al. Quality of life and psychosocial adjustment of young patients after treatment of bone cancer. Cancer 1998;83(1):69–75. [DOI] [PubMed] [Google Scholar]
- 3.Barakat LP, Alderfer MA, Kazak AE. Posttraumatic growth in adolescent survivors of cancer and their mothers and fathers. J Pediatr Psychol 2006;31(4):413–419. [DOI] [PubMed] [Google Scholar]
- 4.Jörngården A, Mattsson E, von Essen L. Health-related quality of life, anxiety and depression among adolescents and young adults with cancer: a prospective longitudinal study. Eur J Cancer 2007;43(13):1952–1958. [DOI] [PubMed] [Google Scholar]
- 5.Parry C, Chesler MA. Thematic evidence of psychosocial thriving in childhood cancer survivors. Qual Health Res 2005;15(8):1055–1073. [DOI] [PubMed] [Google Scholar]
- 6.Fritz GK, Williams JR. Issues of adolescent development for survivors of childhood cancer. J Am Acad Child Adolesc Psychiatry 1988;27(6):712–715. [DOI] [PubMed] [Google Scholar]
- 7.Salick EC, Auerbach CF. From devastation to integration: adjusting to and growing from medical trauma. Qual Health Res 2006;16 (8):1021–1037. [DOI] [PubMed] [Google Scholar]
- 8.Tedeschi RG, Calhoun LG. Trauma & Transformation: Growing in the Aftermath of Suffering. Sage Publications, Inc; 1995. [Google Scholar]
- 9.Mannix MM, Feldman JM, Moody K. Optimism and health-related quality of life in adolescents with cancer. Child Care Health Dev 2009;35(4):482–488. [DOI] [PubMed] [Google Scholar]
- 10.Wenninger K, Helmes A, Bengel J, Lauten M, Volkel S, Niemeyer CM. Coping in long-term survivors of childhood cancer: relations to psychological distress. Psycho-Oncology 2012. [DOI] [PubMed] [Google Scholar]
- 11.Bozo O, Gundogdu E, Buyukasik-Colak C. The moderating role of different sources of perceived social support on the dispositional optimism – posttraumatic growth relationship in postoperative breast cancer patients. J Health Psychol 2009;14 (7):1009–1020. [DOI] [PubMed] [Google Scholar]
- 12.Dunn J, Occhipinti S, Campbell A, Ferguson M, Chambers SK. Benefit finding after cancer: the role of optimism, intrusive thinking and social environment. J Health Psychol 2011;16(1):169–177. [DOI] [PubMed] [Google Scholar]
- 13.Prati G, Pietrantoni L. Optimism, social support, and coping strategies as factors contributing to posttraumatic growth: a meta-analysis. J Loss Trauma 2009;14(5):364–388. [Google Scholar]
- 14.Levine SZ, Laufer A, Hamama-Raz Y, Stein E, Solomon Z. Posttraumatic growth in adolescence: examining its components and relationship with PTSD. J Trauma Stress 2008;21(5):492–496. [DOI] [PubMed] [Google Scholar]
- 15.Linley PA, Joseph S. Positive change following trauma and adversity: a review. J Trauma Stress 2004;17(1):11–21. [DOI] [PubMed] [Google Scholar]
- 16.Kamibeppu K, Sato I, Honda M, Ozono S, Sakamoto N, Iwai T, et al. Mental health among young adult survivors of childhood cancer and their siblings including posttraumatic growth. J Cancer Surviv 2010;4(4):303–312. [DOI] [PubMed] [Google Scholar]
- 17.Jones BL. Promoting healthy development among survivors of adolescent cancer. Fam Community Health 2008;31 Suppl 1:S61–70. [DOI] [PubMed] [Google Scholar]
- 18.Smith BW, Dalen J, Bernard JF, Baumgartner KB. Posttraumatic growth in non-Hispanic White and Hispanic women with cervical cancer. J Psychosoc Oncol 2008;26(4):91–109. [DOI] [PubMed] [Google Scholar]
- 19.Berger R, Weiss T. Posttraumatic growth in U.S. Latinos. Posttraumatic Growth and Culturally Competent Practice: Lessons Learned from Around the Globe John Wiley & Sons, Inc.: Hoboken, New Jersey: 2010;113–127. [Google Scholar]
- 20.Milam JE, Ritt-Olson A, Unger JB. Posttraumatic growth among adolescents. J Adolescent Res 2004;19(2):192–204. [Google Scholar]
- 21.Jones BL, Volker DL, Vinajeras Y, Butros L, Fitchpatrick C, Rossetto K. The meaning of surviving cancer for Latino adolescents and emerging young adults. Cancer Nurs 2010;33(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress 1996;9(3):455–471. [DOI] [PubMed] [Google Scholar]
- 23.Milam J Posttraumatic growth and HIV disease progression. J Consult Clin Psychol 2006;74(5):817–827. [DOI] [PubMed] [Google Scholar]
- 24.Arpawong TE, Richeimer SH, Weinstein F, Elghamrawy A, Milam JE. Posttraumatic growth, quality of life, and treatment symptoms among cancer chemotherapy outpatients. Health Psychol 2012, DOI: 10.1037/a0028223. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg AM, Brymer MJ, Decker KB, Pynoos RS. The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index. Curr Psychiatry Rep 2004;6(2):96–100. [DOI] [PubMed] [Google Scholar]
- 26.Stuber ML, Meeske KA, Leisenring W, Stratton K, Zeltzer LK, Dawson K, et al. Defining medical posttraumatic stress among young adult survivors in the Childhood Cancer Survivor Study. Gen Hosp Psychiatry 2011;33(4):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radloff LS. the CES-D scale. Appl Psychological Meas 1977;1(3):385–401. [Google Scholar]
- 28.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106 (3):203–214. [DOI] [PubMed] [Google Scholar]
- 29.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67(6):1063–1078. [DOI] [PubMed] [Google Scholar]
- 30.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical care 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002;94(7):2090–2106. [DOI] [PubMed] [Google Scholar]
- 32.Werba BE, Hobbie W, Kazak AE, Ittenbach RF, Reilly AF, Meadows AT. Classifying the intensity of pediatric cancer treatment protocols: the Intensity of Treatment Rating Scale 2.0 (ITR-2). Pediatr Blood Cancer 2007;48(7):673–677. [DOI] [PubMed] [Google Scholar]
- 33.Abraido-Lanza AF, Armbrister AN, Florez KR, Aguirre AN. Toward a theory-driven model of acculturation in public health research. Am J Public Health 2006;96(8):1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coronado GD, Thompson B, McLerran D, Schwartz SM, Koepsell TD. A short acculturation scale for Mexican-American populations. Ethn Dis 2005;15(1):53–62. [PubMed] [Google Scholar]
- 35.Epstein AJ, Botvin JG, Dusenbury L, Diaz T, Kerner J. Validation of an acculturation measure for Hispanic adolescents. Psychol Rep 1996;79(3):1075–1079. [DOI] [PubMed] [Google Scholar]
- 36.Corral I, Landrine H. Acculturation and ethnic-minority health behavior: a test of the operant model. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association 2008;27(6):737–745. [DOI] [PubMed] [Google Scholar]
- 37.Currier JM, Hermes S, Phipps S. Brief report: children’s response to serious illness: perceptions of benefit and burden in a pediatric cancer population. J Pediatr Psychol 2009;34(10):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devine KA, Reed-Knight B, Loiselle KA, Fenton N, Blount RL. Posttraumatic growth in young adults who experienced serious childhood illness: a mixed-methods approach. J Clin Psychol Med Settings 2010;17(4):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Love C, Sabiston CM. Exploring the links between physical activity and posttraumatic growth in young adult cancer survivors. Psycho-oncology 2011;20(3):278–286. [DOI] [PubMed] [Google Scholar]
- 40.Phipps S, Long AM, Ogden J. Benefit finding scale for children: preliminary findings from a childhood cancer population. J Pediatr Psychol 2007;32(10):1264–1271. [DOI] [PubMed] [Google Scholar]
- 41.Seitz DCM, Hagmann D, Besier T, Dieluweit U, Debatin K-M, Grabow D, et al. Life satisfaction in adult survivors of cancer during adolescence: what contributes to the latter satisfaction with life? Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation 2011;20(2):225–236. [DOI] [PubMed] [Google Scholar]
- 42.Turner-Sack AM, Menna R, Setchell SR. Posttraumatic growth, coping strategies, and psychological distress in adolescent survivors of cancer. Journal of Pediatric Oncology Nursing: Official Journal of the Association of Pediatric Oncology Nurses 2012;29(2):70–79. [DOI] [PubMed] [Google Scholar]
- 43.Wicks L, Mitchell A. The adolescent cancer experience: Loss of control and benefit finding. Eur J Cancer Care 2010;19(6):778–785. [DOI] [PubMed] [Google Scholar]
- 44.Zebrack BJ, Stuber ML, Meeske KA, Phipps S, Krull KR, Liu Q, et al. Perceived positive impact of cancer among long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Psycho-Oncology 2012;21(6):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milam J, Ritt-Olson A, Tan S, Unger J, Nezami E. The September 11th 2001 terrorist attacks and reports of posttraumatic growth among a multi-ethnic sample of adolescents. Traumatology 2005;11(4):233–246. [Google Scholar]
- 46.Taku K, Calhoun LG, Cann A, Tedeschi RG. The role of rumination in the coexistence of distress and posttraumatic growth among bereaved Japanese university students. Death Stud 2008;32(5):428–444. [DOI] [PubMed] [Google Scholar]
- 47.Harper FWK, Schmidt JF, Beacham AO, Salsman JM, Averill AJ, Graves KD, et al. The role of social cognitive processing theory and optimism in positive psychosocial and physical behavior change after cancer diagnosis and treatment. Psycho-Oncology 2007;16(1):79–91. [DOI] [PubMed] [Google Scholar]
- 48.Orozco S, Thompson B, Kapes J, Montgomery GT. Measuring the acculturation of Mexican Americans: a covariance structure analysis. Measurement and Evaluation in Counseling and Development 1993. [Google Scholar]
- 49.Gil AG, Wagner EF, Vega WA. Acculturation, familism, and alcohol use among Latino adolescent males: Longitudinal relations. J Community Psychol 2000;28(4):443–458. [Google Scholar]
- 50.Casillas J, Kahn KL, Doose M, Landier W, Bhatia S, Hernandez J, et al. Transitioning childhood cancer survivors to adult-centered healthcare: insights from parents, adolescent, and young adult survivors. Psycho-Oncology 2010;19(9):982–990. [DOI] [PubMed] [Google Scholar]
- 51.Seitz DC, Hagmann D, Besier T, Dieluweit U, Debatin KM, Grabow D, et al. Life satisfaction in adult survivors of cancer during adolescence: what contributes to the latter satisfaction with life? Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation 2011;20(2):225–236. [DOI] [PubMed] [Google Scholar]
- 52.Carver CS, Antoni MH. Finding benefit in breast cancer during the year after diagnosis predicts better adjustment 5 to 8 years after diagnosis. Health Psychol 2004;23 (6):595–598. [DOI] [PubMed] [Google Scholar]
- 53.Morrill EF, Brewer NT, O’Neill SC, Lillie SE, Dees EC, Carey LA, et al. The interaction of post-traumatic growth and post-traumatic stress symptoms in predicting depressive symptoms and quality of life. Psycho-Oncology 2008;17(9):948–953. [DOI] [PubMed] [Google Scholar]
- 54.Penedo FJ, Molton I, Dahn JR, Shen B-J, Kinsinger D, Traeger L, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med 2006;31(3):261–270. [DOI] [PubMed] [Google Scholar]
- 55.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res 2004;56(1):1–8. [DOI] [PubMed] [Google Scholar]
- 56.Cruess DG, Antoni MH, McGregor BA, Kilbourn KM, Boyers AE, Alferi SM, et al. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosom Med 2000;62 (3):304–308. [DOI] [PubMed] [Google Scholar]
- 57.Bostock L, Sheikh AI, Barton S. Posttraumatic growth and optimism in health-related trauma: a systematic review. J Clin Psychol Med Settings 2009;16(4):281–296. [DOI] [PubMed] [Google Scholar]
- 58.Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. J Consult Clin Psychol 2006;74(5):797–816. [DOI] [PubMed] [Google Scholar]
- 59.Segerstrom SC. Optimism and immunity: do positive thoughts always lead to positive effects? Brain Behav Immun 2005;19(3):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joseph S, Williams R. Understanding posttraumatic stress: theory, reflections, context and future. Behav Cogn Psychother 2005;33(4):423. [Google Scholar]
- 61.Joseph S, Linley PA. Positive adjustment to threatening events: an organismic valuing theory of growth through adversity. Review of General Psychology 2005;9(3):262. [Google Scholar]
- 62.Christopher M. A broader view of trauma: a biopsychosocial-evolutionary view of the role of the traumatic stress response in the emergence of pathology and/or growth. Clin Psychol Rev 2004;24(1):75–98. [DOI] [PubMed] [Google Scholar]
- 63.Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2009;27 (14):2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang JWY, Friedman DL, Whitton JA, Stovall M, Mertens AC, Robison LL, et al. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2008;50(1):104–110. [DOI] [PubMed] [Google Scholar]
- 65.Haase JE. The adolescent resilience model as a guide to interventions. Journal of Pediatric Oncology Nursing: Official Journal of the Association of Pediatric Oncology Nurses 2004;21(5):289–299; discussion 300–4. [DOI] [PubMed] [Google Scholar]