Abstract
Background
Triple negative breast cancer (TNBC) is an aggressive subtype of breast cancer (BC) with ill-defined therapeutic targets. Androgen receptor (AR) and tumour-infiltrating lymphocytes (TILs) had a prognostic and predictive value in TNBC. The relationship between AR, TILs and clinical behaviour is still not fully understood.
Methods
Thirty-six TNBC patients were evaluated for AR (positive if ≥1% expression), CD3, CD4, CD8 and CD20 by immunohistochemistry. Stromal TILs were quantified following TILs Working Group recommendations. Lymphocyte-predominant breast cancer (LPBC) was defined as stromal TILs ≥ 50%, whereas lymphocyte-deficient breast cancer (LDBC) was defined as <50%.
Results
The mean age was 52.5 years and 27.8% were ≥60 years. Seven patients (21.2%) were AR+. All AR+ cases were postmenopausal (≥50 years old). LPBC was 32.2% of the whole cohort. Median TILs were 37.5% and 10% (p = 0.1) and median CD20 was 20% and 7.5% (p = 0.008) in AR− and AR+, respectively. Mean CD3 was 80.7% and 93.3% (p = 0.007) and CD8 was 75% and 80.8% (p= 0.41) in AR− and AR+, respectively. All patients who were ≥60 years old expressed CD20. LDBC was found to be significantly higher in N+ versus N− patients (p = 0.03) with median TILs of 20% versus 50% in N+ versus N−, respectively (p = 0.03). LDBC was associated with higher risk of lymph node (LN) involvement (odds ratio = 6; 95% CI = 1.05–34.21; p = 0.04).
Conclusions
AR expression was evident in older age (≥50 years). Median CD20 was higher in AR− TNBC, while mean CD3 was higher in AR+ tumours. LDBC was associated with higher risk of LN involvement. Larger studies are needed to focus on the clinical impact of the relation between AR and TILs in TNBC.
Keywords: triple negative breast cancer, TNBC, androgen receptor, AR, tumour infiltrating lymphocytes, TILs
Background
Triple negative breast cancer (TNBC) is a challenging heterogeneous disease with distinct molecular subtypes that does not have receptors for oestrogen, progesterone hormones and the human epidermal growth factor receptor 2 (HER2) protein. TNBC was grouped into six molecular subtypes: basal-like (BL) 1, BL2, mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM) and luminal androgen receptor (LAR) [1]. But thereafter, Lehmann et al [2] found that transcripts in the previously defined IM and MSL subtypes came from tumour-infiltrating lymphocytes (TILs) and tumour-associated stromal cells, respectively, and they reduced the number of TNBC molecular subtypes to four (BL1, BL2, M and LAR).
TILs play an essential role in predicting response to chemotherapy and improving clinical outcomes in breast cancer (BC). Moreover, as the immunotherapy landscape continues to evolve, there is interest in whether the immune system could be playing a more substantial role in TNBC specifically. The association between TNBC subtypes and the impact of TILs is still not fully understood. However, accumulating evidence from several studies indicates that intra-tumoural levels of TILs in TNBC are: a) predictive for response to neo-adjuvant chemotherapy and b) prognostic in patients treated with adjuvant chemotherapy, being correlated with improved overall survival (OS) and disease free survival (DFS) [3].
Besides the immune cell markers, the androgen receptor (AR), which controls the transcription of different genes including the immune response genes, has been recognised as a valuable biomarker in TNBC [4]. The AR expression was correlated with better survival outcomes in TNBC [5], albeit its clinical utility and immunological impact remain unclear. However, many opened questions still need to be answered such as what is the prevalence of AR positivity in TNBC and whether AR expression correlates with the mean TILs or with CD3, CD4, CD8, CD20 expression. Also, it remains not fully clear whether there is any relationship between the predominance of TILs and the age or stage. Here, we addressed these questions and explored the correlation between the AR expression and the total and differential TILs in TNBC.
Methods
In this cross-sectional, pilot study, patients’ records were reviewed retrospectively to select patients with TNBC. TNBC was defined based on the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) recommendations (2010) [6], as tumours with negative (<1% of nuclear staining) oestrogen receptor (ER), progesterone receptor (PR) and lack HER2 receptor overexpression or oncogene amplification. From a cohort of 800 BC patients who were diagnosed in 2012, at the clinical oncology department, Ain Shams University; 10% (80 patients) were diagnosed as TNBC in this year; of whom 36 patients had available tumour paraffin tissue and medical records. The clinico-pathological data and survival outcomes were collected. Tumour (T), nodes (N) and metastases (M) (TNM) staging was done according to the seventh edition of the American Joint Committee on Cancer (AJCC). The study protocol was approved by the Research Ethics committee, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Pathological evaluation was performed by a dedicated pathologist (TH), who was blinded for the clinical data. Haematoxylin and eosin-stained sections were revised for the negativity of ER, PR and Her2 and assessed for the histologic type and grade of the tumour. Then, the sections were examined to quantify the stromal TILs according to the 2014 TILs International Working Group [7], where it was defined as the percentage of lymphocytes in direct contact with tumour cells. Lymphocyte-predominant breast cancer (LPBC) was defined as TILs ≥ 50%, while lymphocytic deficient breast cancer (LDBC) was defined as TILs < 50%.
Formalin-fixed, paraffin-embedded tissue specimens were available for the evaluation of both of AR and TILs in 28 patients, AR alone in 5 patients and TILs alone in 3 patients. The AR expression (Code 200M-18) was evaluated by immunohistochemistry (IHC), and considered positive if ≥1% nuclear staining of the tumour cells [4]. Also, immunostaining was performed for T cell markers CD3 (Code 00000 51564), CD4 (Code 104R-28), CD8 (Code 108M-98) and B cell marker CD20 (Code 00000 27500). All antibodies were ready to use, from Cell Marque, California, USA. CD3, CD4, CD8 and CD20 immunostaining results were evaluated as mean per centage of the stained lymphocytes in relation to the total lymphocytes in the whole tissue section. Then the mean (for CD3 and CD8) and median (for CD4 and CD20) were calculated. The primary aim of our study was to describe the expression of AR and immune cells (CD3, CD4, CD8 and CD20) in TNBC, and the percentage of TILs as well. The secondary aim was to correlate the clinico-pathological parameters with these biomarkers.
Statistical analysis
Recorded data were analysed using the Statistical Package for Social Sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Qualitative data were expressed as frequency and percentage. Independent-samples t-test of significance and Mann–Whitney (z) test were used to compare two means and non-parametric data, respectively. Analysis of Variance (ANOVA) test and Kruskal–Wallis test were used to compare more than two means and multiple-group comparisons in non-parametric data, respectively. Chi-square (x2) test was used in order to compare proportions between qualitative parameters. As multivariate analysis is not suitable for small sets of data, estimates are represented according to univariate analysis. Spearman’s correlation coefficient (r) test was used to assess the degree of association between two sets of variables. The p-value was considered significant if ≤0.05.
Results
Patient characteristics
Thirty-six TNBC patients with available enough tumour material were identified for analysis. The patients’ characteristics are shown in Supplementary Material, Table S1. The mean age at diagnosis was 52.5 years (range: 30–75 years), and 27.8% of cases were ≥60 years old. Most of the tumours (58.3%) were of invasive duct carcinoma (IDC) type, while medullary carcinoma and invasive lobular carcinoma (ILC) accounted for 22% and 11%, respectively. Grade II and III tumours were 30.6% and 52.8%, respectively. Stages I, II, III and IV represented 5.6%, 30.5%, 52.7% and 8.3%, respectively. Lymph nodes (LNs) were positive in 77.8% (28 patients). After a median follow-up of 39 months, nine patients had developed a disease progression and the 3-year OS was reached in 44.4% of the patients.
Table S1. Patients’ characteristics of the whole cohort (36 patients).
| Patients’ characteristics | No = 36 | % | |
|---|---|---|---|
| Age category | Age < 60 years Age ≥ 60 years |
26 10 |
72.2 27.8 |
| Menopausal status | Pre-menopausal | 11 | 30.5 |
| Post-menopausal | 25 | 69.5 | |
| Laterality | Bilateral | 2 | 5.6 |
| Right | 17 | 47.2 | |
| Left | 16 | 44.4 | |
| Unknown | 1 | 2.8 | |
| Pathology | IDC Medullary carcinoma ILC Adenoid cystic Unknown |
21 8 5 1 1 |
58.3 22.2 13.9 2.8 2.8 |
| Grade | Grade II | 11 | 30.6 |
| Grade III | 19 | 52.8 | |
| Unknown | 6 | 16.6 | |
| Neo-adjuvant chemotherapy | Negative | 31 | 86.1 |
| Positive | 3 | 8.3 | |
| Unknown | 2 | 5.6 | |
| Type of surgery | MRM | 30 | 83.3 |
| BCS | 6 | 16.7 | |
| Adjuvant chemotherapy | Negative | 3 | 8.3 |
| Positive | 30 | 83.4 | |
| Unknown | 3 | 8.3 | |
| Adjuvant radiotherapy | Negative | 7 | 19.4 |
| Positive | 23 | 63.9 | |
| Unknown | 6 | 16.7 | |
| TNM staging | I II III IV Unknown |
2 11 19 3 1 |
5.6 30.5 52.8 8.3 2.8 |
| T stage | T1 T2 T3 T4 Unknown |
5 18 7 5 1 |
13.9 50.0 19.4 13.9 2.8 |
| N stage | N0 N1 N2 N3 Unknown |
8 9 13 5 1 |
22.2 25.0 36.1 13.9 2.8 |
| LN category | N0 N+ |
8 28 |
22.2 77.8 |
| Relapse/progression | Negative | 27 | 75.0 |
| Positive | 9 | 25.0 | |
| Unknown | 0 | 0.0 | |
| Type of relapse (=9) | Systemic | 6 | 66.7 |
| Regional | 1 | 11.1 | |
| Unknown | 2 | 22.2 | |
| Systemic relapse (=6) | Liver Brain Lung, bone Unknown |
1 1 2 2 |
16.6 16.6 33.4 33.4 |
| 3-year OS | |||
| <3 years ≥3 years |
20 16 |
55.6 44.4 |
|
IDC, Invasive ductal carcinoma; ILC, Invasive lobular carcinoma; MRM, Modified radical mastectomy; BCS, Breast conservative surgery; LN, Lymph node; OS, Overall survival
AR expression and its relation with the clinico-pathological and survival parameters
AR was tested in 33 patients and it was expressed in 21.2% (7 patients). All AR+ cases (100%) were postmenopausal (≥50 years old). Although patients with AR+ tumours were older than those who were AR− (mean age: 55 versus 51.6 years), there was no statistically significant difference in age between the two groups (p = 0.47). LNs were involved in 77% and 85.7% in AR− and AR+, respectively, (p = 0.61). No statistical difference was found in median OS between AR− and AR+ groups (31.5 versus 25 months, p = 0.77). The clinico-pathological parameters according to AR expression are shown in Table 1.
Table 1. The clinico-pathological parameters according to AR expression.
| Clinico-pathological parameters | AR− (26) | AR+ (7) | Chi-square test | p-value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Age | Mean ± SD Range |
51.65 ± 11.76 30–75 |
55.00 ± 4.40 50–63 |
−0.732a | 0.470 | ||
| Age category | <60 years ≥60 years |
18 8 |
69.2 30.8 |
6 1 |
85.7 14.3 |
0.755 | 0.385 |
| Menopausal status | Pre-menopausal | 9 | 34.6 | 0 | 0.0 | 3.332 | 0.068 |
| Post-menopausal | 17 | 65.4 | 7 | 100 | |||
| Laterality | Right Left Bilateral Unknown |
12 12 1 1 |
46.2 46.2 3.8 3.8 |
4 3 0 0 |
57.1 42.9 0.0 0.0 |
0.689 | 0.876 |
| Pathology | IDC Medullary carcinoma ILC Adenoid cystic Unknown |
14 7 3 1 1 |
53.9 26.9 11.6 3.8 3.8 |
5 1 1 0 0 |
71.4 14.3 14.3 0.0 0.0 |
3.476 | 0.627 |
| Grade | Grade II | 9 | 34.6 | 2 | 28.6 | 2.640 | 0.267 |
| Grade III | 11 | 42.3 | 5 | 71.4 | |||
| Unknown | 6 | 23.1 | 0 | 0.0 | |||
| TNM staging | I II III IV Unknown |
2 7 15 1 1 |
7.7 26.9 57.8 3.8 3.8 |
0 3 4 0 0 |
0.0 42.9 57.1 0.0 0.0 |
1.539 | 0.820 |
| T stage | T1 T2 T3 T4 Unknown |
5 10 6 4 1 |
19.2 38.5 23.1 15.4 3.8 |
0 5 1 1 0 |
0.0 71.4 14.3 14.3 0.0 |
3.139 | 0.535 |
| LN category | N0 N+ |
6 20 |
23.1 76.9 |
1 6 |
14.3 85.7 |
0.255 | 0.614 |
| Relapse/progression | Negative Positive Unknown |
19 7 0 |
73.1 26.9 0.0 |
6 1 0 |
85.7 14.3 0.0 |
0.480 | 0.489 |
| Median OS |
Median (IQR) Range |
31.5 (18–44) 1.5–216 |
25 (17–77) 4–86 |
−0.286b | 0.775 | ||
| 3-year OS | <3 years ≥3 years |
14 12 |
53.8 46.2 |
4 3 |
57.1 42.9 |
0.024 | 0.876 |
IDC, Invasive ductal carcinoma; ILC, Invasive lobular carcinoma; LN, Lymph node; IQR, Interquartile range; OS, Overall survival
Independent t-test
Mann–Whitney test
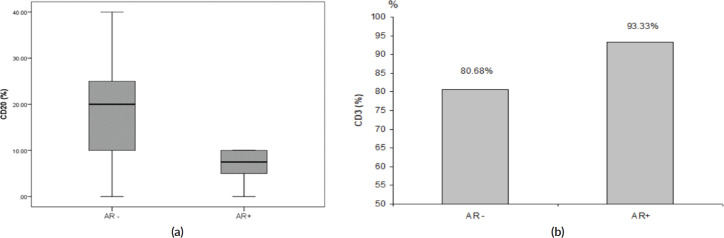
The majority of AR+ tumours (85.7%) was LDBC subtype, with median percentage of TILs was 37.5% and 10% in AR− and AR+ tumours, respectively, (p = 0.10). Median CD20 was significantly higher in AR− versus AR+ (20% versus 7.5%, respectively, p = 0.008), as depicted in Figure 1a, while mean CD3 was significantly lower in AR− versus AR+ (80.7% versus 93.3%, respectively, p = 0.007), as depicted in Figure 1b. On the other side, median CD4 and mean CD8 were not statistically different between AR− and AR+ tumours. Table 2 illustrated the correlation between the AR and total & differential TILs expression.
Figure 1. (a): Relation between AR and median CD20 expression (p = 0.008). (b): Relation between AR and mean CD3 expression (p = 0.007).
Table 2. The correlation between AR and total & differential TILs expression. The p-value was considered significant if ≤0.05.
| Variables | AR− (26) | AR+ (7) | Test value | p-value | |
|---|---|---|---|---|---|
| Median TILs (%) | Median (IQR) | 37.5 (10–50) | 10 (5–20) | −1.607c | 0.108 |
| Range | 1–70 | 3–40 | |||
| LPBC versus LDBC | LDBC | 14 (53.8%) | 6 (85.7%) | 3.082a | 0.214 |
| LPBC | 8 (30.8%) | 0 (0.0%) | |||
| Unknown | 4 (15.4%) | 1 (14.3%) | |||
| Median CD20 (%) | Median (IQR) | 20 (10–25) | 7.5 (5–10) | −2.643c | 0.008 |
| Range | 0–40 | 0–10 | |||
| CD20 expression | Negative | 2 (7.7%) | 1 (14.3%) | 0.290a | 0.865 |
| Positive | 20 (76.9%) | 5 (71.4%) | |||
| Unknown | 4 (15.4%) | 1 (14.3%) | |||
| Mean CD3 (%) | Mean ± SD | 80.7 ± 10.1 | 93.3 ± 4.1 | −2.954b | 0.007 |
| Range | 60–100 | 90–100 | |||
| CD3 expression | Negative | 0 (0.0%) | 0 (0.0%) | 0.005a | 0.943 |
| Positive | 22 (84.6%) | 6 (85.7%) | |||
| Unknown | 4 (15.4%) | 1 (14.3%) | |||
| Median CD4 (%) | Median (IQR) | 0 (0–10) | 12.5 (0–20) | −1.435c | 0.151 |
| Range | 0–20 | 0–30 | |||
| CD4 expression | Negative | 14 (53.8%) | 2 (28.6%) | 1.786a | 0.409 |
| Positive | 8 (30.8%) | 4 (57.1%) | |||
| Unknown | 4 (15.4%) | 1 (14.3%) | |||
| Mean CD8 (%) | Mean ± SD | 75.0 ± 16.3 | 80.8 ± 10.2 | −0.825b | 0.417 |
| Range | 40–100 | 70–95 | |||
| CD8 expression | Negative | 0 (0.0%) | 0 (0.0%) | 0.005a | 0.943 |
| Positive | 22 (84.6%) | 6 (85.7%) | |||
| Unknown | 4 (15.4%) | 1 (14.3%) | |||
IQR, Interquartile range; LPBC, Lymphocytic predominance breast cancer; LDBC, Lymphocytic deficient breast cancer
Chi-square test
Independent t-test
Mann–Whitney test
Total and differential TILs expression and its relation with the clinico-pathological parameters
In the 31 patients, where TILs were evaluated, the median TILs were 30% (range = 1%–70%), while LDBC and LPBC were 67.7% and 32.3%, respectively. CD20 and CD4 were negative in 9.6% and 54.8%, respectively. Table 3 showed descriptive analysis of the total and differential TILs expression. When correlating the lymphocytic predominance with the clinico-pathological parameters (shown in Table 4), LDBC type was found to be significantly higher in N+ versus N− patients (p = 0.03), as depicted in Figure 2. Median TILs were 20% versus 50% in N+ versus N−, respectively, (p = 0.03) as illustrated in Table 5a and b. Total TILs expression < 50% (LDBC) was associated with higher risk of LN involvement (odds ratio (OR) = 6; 95% CI = 1.05–34.21; p = 0.04).
Table 3. Descriptive analysis of the total and differential TILs expression.
| Variables | No. = 31 | |
|---|---|---|
| Median TILs (%) | Median (IQR) | 30 (10–50) |
| Range | 1–70 | |
| LDBC versus LPBC | LDBC | 21 (67.7%) |
| LPBC | 10 (32.3%) | |
| Median CD20 (%) | Median (IQR) | 15 (10–20) |
| Range | 0–40 | |
| CD20 expression | Negative | 3 (9.6%) |
| Positive | 28 (90.4%) | |
| Mean CD3 (%) | Mean ± SD | 80.5 ± 17.7 |
| Range | 2–100 | |
| CD3 expression | Negative | 0 (0.0%) |
| Positive | 31 (100%) | |
| Median CD4 (%) | Median (IQR) | 0 (0–15) |
| Range | 0–30 | |
| CD4 expression | Negative | 17 (54.8%) |
| Positive | 14 (45.2%) | |
| Mean CD8 (%) | Mean ± SD | 73.4 ± 19.7 |
| Range | 2–100 | |
| CD8 expression | Negative | 0 (0.0%) |
| Positive | 31 (100%) | |
IQR, Interquartile range; SD, Standard deviation; LPBC, Lymphocytic predominance breast cancer; LDBC, Lymphocytic deficient breast cancer
Table 4. Relation between lymphocytic predominance and clinico-pathological parameters. The p-value was considered significant if ≤0.05.
| Variables | LDBC (= 21) | LPBC (=10) | Chi-square test | p-value | |||
|---|---|---|---|---|---|---|---|
| Age | Age < 60 | 15 | 71.4% | 7 | 70.0% | 0.007 | 0.935 |
| Age ≥ 60 | 6 | 28.6% | 3 | 30.0% | |||
| Morphology | IDC Medullary ILC Adenoid cystic |
12 5 3 1 |
57.1% 23.8% 14.3% 4.8% |
6 3 1 0 |
60.0% 30.0% 10.0% 0.0% |
0.873 | 0.928 |
| TNM staging | I II III IV |
2 6 11 2 |
9.5% 28.6% 52.4% 9.5% |
0 5 4 1 |
0.0% 50.0% 40.0% 10.0% |
2.045 | 0.563 |
| N stage | N0 | 3 | 14.3% | 5 | 50.0% | 4.762 | 0.190 |
| N1 | 7 | 33.3% | 2 | 20.0% | |||
| N2 | 9 | 42.9% | 2 | 20.0% | |||
| N3 | 2 | 9.5% | 1 | 10.0% | |||
| LN category | N− | 3 | 14.3% | 5 | 50.0% | 4.513 | 0.034 |
| N+ | 18 | 85.7% | 5 | 50.0% | |||
| OS | Median (IQR) | 32 | (18–72) | 25 | (12–39) | −0.993a | 0.320 |
| Range | 2–216 | 9–48 | |||||
| 3-year OS | <3-year OS | 11 | 52.4% | 6 | 60.0% | 0.159 | 0.690 |
| ≥3-year OS | 10 | 47.6% | 4 | 40.0% | |||
IDC, Invasive duct carcinoma; ILC, Invasive lobular carcinoma; LN, Lymph node; IQR, Interquartile range; OS, Overall survival
Mann–Whitney test
Figure 2. Relation between LN involvement and lymphocytic predominance.
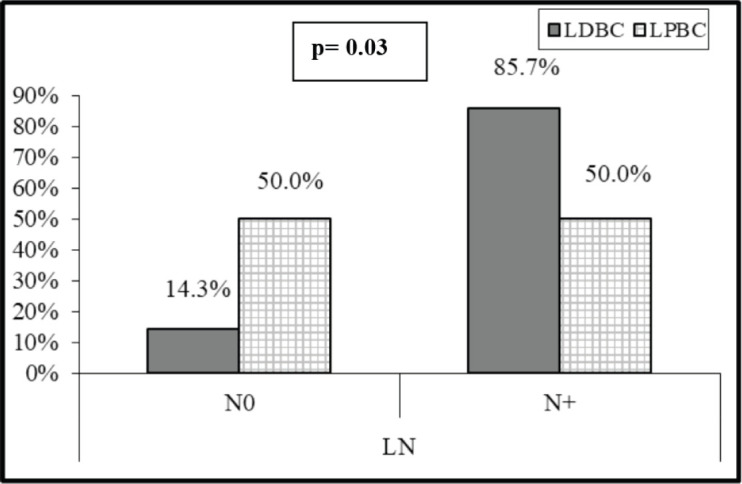
Table 5A. Relation between LN involvement and the total and differential TILs. The p-value was considered significant if ≤0.05.
| Total and differential TILs | LN involvement | Test value | p-value | ||
|---|---|---|---|---|---|
| N− (= 8) | N+ (=23) | ||||
| Total TILs (%) | Median (IQR) | 50 (38–55) | 20 (5–40) | −2.159b | 0.031 |
| Range | 10–60 | 1–70 | |||
| Median CD20 (%) | Median (IQR) | 15 (3–20) | 15 (10–25) | −1.089b | 0.276 |
| Range | 0–20 | 0–40 | |||
| Mean CD3 (%) | Mean ± SD | 85.6 ± 9.8 | 78.8 ± 19.6 | 0.940a | 0.355 |
| Range | 70–100 | 2–100 | |||
| Median CD4 (%) | Median (IQR) | 5 (0–13) | 0 (0–20) | −0.074b | 0.941 |
| Range | 0–20 | 0–30 | |||
| Mean CD8 (%) | Mean ± SD | 74.4 ± 15.0 | 73.1 ± 21.4 | 0.151a | 0.881 |
| Range | 50–100 | 2–100 | |||
LN, Lymph node; IQR, Interquartile range; SD, Standard deviation
Independent t-test
Mann–Whitney test
Table 5B. Relation between LN involvement and the total and differential TILs. The p-value was considered significant if ≤0.05.
| Total and differential TILs | LN involvement | Chi-square test | p-value | ||||
|---|---|---|---|---|---|---|---|
| N− | N+ | ||||||
| No. | % | No. | % | ||||
| LDBC versus LPBC | LDBC | 3 | 37.5 | 18 | 78.3 | 4.513 | 0.034 |
| LPBC | 5 | 62.5 | 5 | 21.7 | |||
| CD20 expression | Negative | 2 | 25.0 | 1 | 4.3 | 2.896 | 0.089 |
| Positive | 6 | 75.0 | 22 | 95.7 | |||
| CD3 expression | Negative | 0 | 0.0 | 0 | 0.0 | NA | NA |
| Positive | 8 | 100 | 23 | 100.0 | |||
| CD4 expression | Negative | 4 | 50.0 | 13 | 56.5 | 0.102 | 0.750 |
| Positive | 4 | 50.0 | 10 | 43.5 | |||
| CD8 expression | Negative | 0 | 0.0 | 0 | 0.0 | NA | NA |
| Positive | 8 | 100 | 23 | 100 | |||
NA, Not applicable
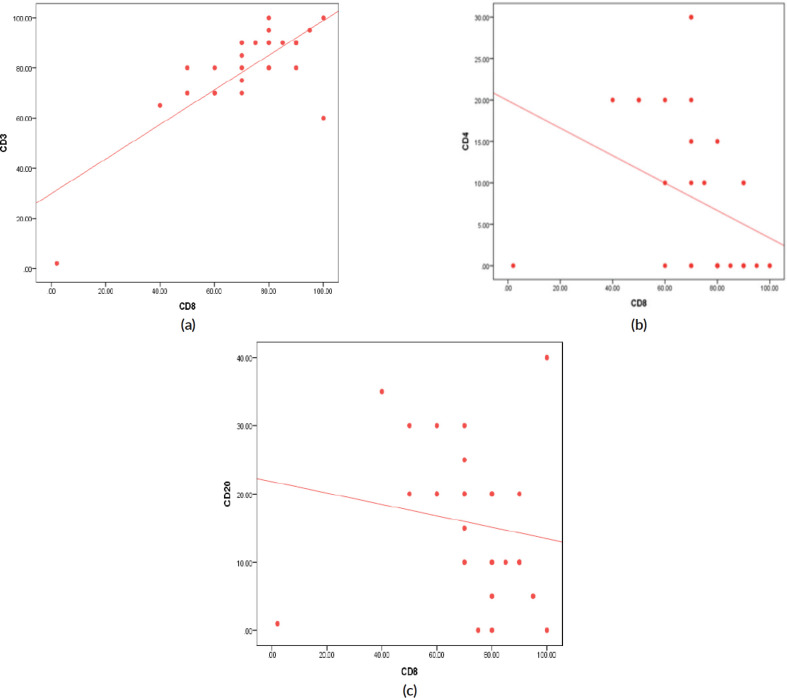
On the other side, when analysing the relationship between the age and TILs (Supplementary Material, Table S2), it was found that median TILs were lower in patients ≥60 years old despite statistically not significant, (median TILs = 10% versus 38% in ≥60 years old versus <60 years old, respectively, p = 0.45). Moreover, all patients who were ≥60 years old expressed B-cell marker (100%) (shown in Supplementary Material, Table S3). Furthermore, a significant positive correlation was present between CD8 and CD3 (correlation coefficient (r) = 0.591, p ˂ 0.001), while significant inverse correlations were present between CD3 and CD20 (r = −0.814, p ˂ 0.001), CD8 and CD20 (r = −0.382, p = 0.03) and CD8 and CD4 (r = −0.52, p = 0.002), as illustrated in Figure 3a–c and Supplementary Material, Table S4.
Table S2. Relation between age and the total and differential TILs.
| Total and differential TILs | Age | Test value | p-value | ||
|---|---|---|---|---|---|
| Age < 60 | Age ≥ 60 | ||||
| Total TILs (%) | Median (IQR) | 38 (10–50) | 10 (3–50) | −0.745b | 0.456 |
| Range | 1–60 | 1–70 | |||
| Median CD20 (%) | Median (IQR) | 18 (10–20) | 10 (10–20) | −0.156b | 0.876 |
| Range | 0–40 | 1–35 | |||
| Mean CD3 (%) | Mean ± SD | 83.64 ± 10.14 | 73.00 ± 28.54 | 1.554a | 0.131 |
| Range | 60–100 | 2–95 | |||
| Median CD4 (%) | Median (IQR) | 5 (0–20) | 0 (0–10) | −0.933b | 0.351 |
| Range | 0–30 | 0–20 | |||
| Mean CD8 (%) | Mean ± SD | 76.36 ± 14.24 | 66.33 ± 29.00 | 1.302a | 0.203 |
| Range | 50–100 | 2–90 | |||
IQR, Interquartile range; SD, Standard deviation
Independent t-test
Mann–Whitney test
Table S3. Relation between age and the total and differential TILs.
| Total and differential TILs | Age | Chi-square test | p-value | ||||
|---|---|---|---|---|---|---|---|
| Age < 60 | Age ≥ 60 | ||||||
| No. | % | No. | % | ||||
| LDBC versus LPBC | LDBC | 15 | 68.2 | 6 | 66.7 | 0.007 | 0.935 |
| LPBC | 7 | 31.8 | 3 | 33.3 | |||
| CD20 expression | Negative | 3 | 13.6 | 0 | 0.0 | 1.359 | 1.000 |
| Positive | 19 | 86.4 | 9 | 100 | |||
| CD3 expression | Negative | 0 | 0.0 | 0 | 0.0 | NA | NA |
| Positive | 22 | 100.0 | 9 | 100 | |||
| CD4 expression | Negative | 11 | 50.0 | 6 | 66.7 | 0.716 | 0.397 |
| Positive | 11 | 50.0 | 3 | 33.3 | |||
| CD8 expression | Negative | 0 | 0.0 | 0 | 0.0 | NA | NA |
| Positive | 22 | 100.0 | 9 | 100 | |||
N, Not applicable
Figure 3. (a): Correlation between CD3 and CD8 expression (positive) (r = 0.591, p ≤ 0.001). (b): Correlation between CD4 and CD8 expression (inverse) (r = −0.527, p = 0.002). (c): Correlation between CD20 and CD8 expression (inverse) (r = −0.382, p = 0.034).
Table S4. Correlation between the total and differential TILs expression. The p-value was considered significant if ≤0.05.
| Total TILs (%) | CD20 (%) | CD3 (%) | CD4 (%) | CD8 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Total TILs (%) | - | - | 0.228 | 0.218 | -0.151 | 0.416 | -0.008 | 0.964 | -0.060 | 0.747 |
| CD20 (%) | 0.228 | 0.218 | - | - | −0.814 | <0.001 | 0.200 | 0.282 | −0.382 | 0.034 |
| CD3 (%) | −0.151 | 0.416 | −0.814 | <0.001 | - | - | −0.101 | 0.590 | 0.591 | <0.001 |
| CD4 (%) | −0.008 | 0.964 | 0.200 | 0.282 | −0.101 | 0.590 | - | - | −0.527 | 0.002 |
| CD8 (%) | −0.060 | 0.747 | −0.382 | 0.034 | 0.591 | <0.001 | −0.527 | 0.002 | - | - |
Discussion
It is well established that the expression of AR differs according to molecular subtypes of BC with more frequent expression in ER negative cancers. The prevalence of AR+ expressing tumours is generally ranging from 10% to 41% in TNBC cases [1, 8–14], with rare reports showing rates up to 79% [15, 16]. In accordance with most published reports, our rate of AR expression in TNBC was 21.2%.
Whether clinico-pathologic characteristics of TNBC vary based on AR expression status have been extensively studied [8, 13, 15, 17, 18]. Some studies showed that patients with AR+ tumours were significantly older, exhibited tumours with significantly lower grades (I–II), more frequent nodal involve ment, non-ductal histology and lower Ki67 [14, 15, 17, 18]. Other reports described reduced LN metastases in AR+ TNBCs [8], or just similar clinico-pathologic profile between AR+ and AR− TNBC tumours [13]. Herein, there was no statistically significant difference in the clinico-pathological parameters according to AR expression. However, AR+ cases were older in age and exhibited more regional nodal spread. Despite statistically insignificant, this profile was analogous to the LAR subtype described by Lehmann et al [2].
Available evidence about the prognostic value of AR in TNBC is controversial. Some reports suggested that AR-positivity was associated with good outcomes [8, 13], whereas others concluded that AR status conferred worse prognosis [19] or had no significant impact on disease prognosis [4, 20, 21]. Many factors may explain these discrepant results across studies including the sample size limited cohorts, differences in the ethnic origin, the anti-AR antibodies used for staining, staining/scoring method, as well as variability in the thresholds used to define AR positivity [4]. A meta-analysis published in 2017, demonstrated that AR-positive status was associated with better DFS and OS in TNBC (hazard ratio (HR) = 0.64; 95% CI = 0.51–0.81; p < 0.001 and HR = 0.64; 95% CI = 0.49–0.88; p < 0.001, respectively), in univariate analysis [5]. Of note, no multivariate analysis was provided and this meta-analysis included heterogeneous studies in terms of methods of AR scoring, clinical cohorts’ characteristics, therapies received and length of follow-up. A large multi-institutional study including about 1,407 TNBC tumours issued after this meta-analysis concluded that the AR-positivity was a marker of good prognosis in USA and Nigerian cohorts, whereas it conferred poor prognosis in Norway, Ireland and Indian cohorts, and was neutral in UK cohort [4]. Whereas a more recent meta-analysis (2020) [21] demonstrated that AR expression in TNBC was not associated with DFS (HR = 0.92; 95% CI = 0.67–1.27; p = 0.63), OS (HR = 0.91; 95% CI = 0.67–1.22; p = 0.53), distant-DFS (HR = 1.02; 95% CI = 0.96–1.08; p = 0.48) or recurrence-free survival (HR = 0.95; 95% CI = 0.46–1.98; p = 0.90), regardless of the confounding factors and heterogeneity that existed among included studies. Our study results had matched the latter meta-analysis results, where no statistical difference in median OS (31.5 versus 25 months, p = 0.77) or relapse/progression rate (26.9% versus 14.3%, p = 0.48) was found between AR− and AR+ groups.
Importantly, the presence of more frequent special histological subtypes with poor prognosis as medullary carcinoma, ILC and adenoid cystic carcinoma in the AR− versus the AR+ group (42% versus 28%) in our cohort, may have an impact on survival as pointed to by other studies [22].
Compared to other subtypes, TNBC was shown to exhibit higher levels of TILs [23]. There is heterogeneity of TILs cut-off used in published studies in order to distinguish between LPBC and LDBC. Some studies defined LPBC as showing more than 50% of lymphocyte infiltration [24, 25], whereas others used different cut-offs [26]. In our cohort, median TILs were 30% (range: 1%–70%), with a LPBC prevalence of 32.2%, which is not in full agreement with other reports. Adams et al [24] reported much lower median TILs percentage (10%), and with using the same cut-off of ≥50% TILs, only 4.4% were LPBC, whereas Pruneri et al [25] described a median TILs level of 20%, with LPBC prevalence of 22% of cases.
Little is known about the association between TNBC clinico-pathologic features and lymphocytic predominance. A pooled analysis of nine large studies by Loi et al [26] demonstrated that TILs were significantly lower in older age. Whilst, Adams et al [24] reported no strong associations between TILs scores and age or menopausal status. Despite not statistically significant, we showed lower median TILs in patients ≥60 years versus <60 years old (10% versus 38%, p = 0.45).
Interestingly, we found that patients with LN involvement were significantly more likely to be LDBC, where a total TILs expression < 50% (LDBC) was associated with higher risk of LN involvement (OR = 6; 95% CI = 1.05–34.21; p = 0.04). This is in agreement with Loi et al [26], but in contrast to a recent meta-analysis which concluded that no significant association between decreased TILs and LN metastasis risk [27].
Our knowledge about the association between TILs and AR is still limited. In a large cohort study about non-metastatic TNBC of LAR subtype, this tumour subset was found to exhibit lower median stromal TILs and to be less likely LPBC (≥50% TILs) compared to non-LAR, although this did not reach statistical significance [28], similarly to our study. However, we did not examine the genetic profiles of our AR+ tumours to classify them into the LAR subtype. Other reports using IHC described significant association between AR expression and lower levels of stromal TILs [11, 17].
Studies about the immune cells subsets composition of TILs according to AR expression are very scarce. In our study, median CD20 was significantly higher in AR− tumours compared to those with AR+ (20% versus 7.5%, respectively, p = 0.008). Whereas, mean CD3 was significantly lower in AR− versus AR+ (80.7% versus 93.3%, respectively, p = 0.007). On the other side, previous publications reported that CD8+ were more frequent in AR+ than AR− tumours [12, 29, 30], in contrast to our study which showed that neither CD8 nor CD4 were statistically different between AR+ and AR− tumours.
Based on two large-scale BC genomics data, evidence from a comprehensive analysis of 26 immune gene-sets including 15 immune cell type and function suggested that TNBC had the strongest tumour immunogenicity. Comparison of the immune infiltrate densities of different immune cell subpopulations demonstrated higher degree of infiltration in TNBC than non-TNBC, including CD3, CD8 and CD20 and others [31].
T-lymphocytes represent the main lymphocyte type in the tumour microenvironment, and the majority of T lymphocytes express a cytotoxic effector phenotype (CD8+). Intra-tumoural and adjacent stromal CD8+ T-cell infiltration have been found to be significantly associated with ER negativity and basal phenotype [32, 33]. Infiltrating CD8+ T-cells have been reported in more than 60% of TNBC cases [33, 34]. In our study, CD8 was expressed in 100% of the cases with the mean of its expression was 73.4%.
The role of tumour-infiltrating B cells (CD20) as components of TILs in BC subtypes is still unclear. A positive correlation between higher numbers of total CD20+ B cells and ER and PR negativity, and basal phenotype has been reported [35]. In our study, CD20 was expressed in 90.3% of the tumours and its median expression was significantly higher in AR− versus AR+ TNBC (20% versus 7.5%, respectively, p = 0.008).
Using a digital pathology computational workflow to quantify the spatial patterns of five immune markers (CD3, CD4, CD8, CD20 and FoxP3) in TNBC, Mi et al [36] demonstrated positive correlations between CD3 and CD8 cells. Similarly, we also showed a significant positive correlation between CD3 and CD8. Data from a study that used multiplexed ion beam imaging to simultaneously quantify in situ expression of 36 proteins in 41 patients with TNBC, suggested that all patients with B cells had also CD4 T cells and CD8 T cells [37]. In contrast, we found in our study significant inverse correlations between CD20 and CD8, as well as CD20 and CD3.
Immune cellular subpopulations in BC representing the innate immunity (natural killer, CD68+ and CD11c+ cells) and adaptive immunity (CD3+ cells (CD8+ or CD4+) and CD20+ cells) [38], worth thorough evaluation in TNBC, with the aim of understanding its clinical implications in BC management. In a recent consensus report for the management of TNBC, the majority of the panellists concluded that more evidence to support the predictive value of TILs and its impact on the clinical decision are warranted [39].
As no prior studies evaluating the exact relation between AR and TILs in this unique disease entity ‘TNBC’, we tended to represent all the statistical analyses and correlations which we evaluated, keeping with the aim of this exploratory study which may help future research. This study had mainly described the expression patterns of AR and TILs in TNBC. Moreover, the correlation between AR and the total and different TILs subpopulations was illustrated. TILs were evaluated by one pathologist who was blinded to the clinical characteristics and according to the International Working Group. However, our findings should be interpreted carefully. The limitations of our study include: i) the retrospective nature, ii) the small sample size (despite this, there were some significant correlations) and iii) the survival data was not mature due to the short follow-up duration (median: 39 months).
Conclusions
This study highlighted the probable relationship between the AR and total and differential TILs expression in TNBC; and the clinico-pathological characteristics as well. Understanding the immune micro-environment in a subset of tumours with poor prognosis and less identified therapeutic targets like TNBC, may pave the way for the advent of immunotherapy in specific group of patients. Moreover, lower TILs density may identify a subpopulation of TNBC who warrants more radical regional LNs management. The prognostic relevance and the potential predictive impact of AR and TILs in TNBCs merit further evaluation in larger scale studies.
Conflicts of interest
All authors declared no conflicts of interest.
Authors’ contributions
Conception and design: HE, JB, TH, AMA, MK, DZA, MO Ahmed, MO Alorabi
Acquisition and interpretation of data: all authors
Drafting the manuscript and revising it critically for important intellectual content: all authors.
Funding
None.
Supplementary Material
References
- 1.Lehmann B, Bauer J, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehmann B, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLos One. 2016;11(6):e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao G, Wang Z, Qu X, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):179. doi: 10.1186/s12885-020-6668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattarai S, Klimov S, Mittal K, et al. Prognostic role of androgen receptor in triple negative breast cancer: a multi-institutional study. Cancers. 2019;11(7):995. doi: 10.3390/cancers11070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozovic-Spasojevic I, Zardavas D, Brohée S, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: a meta-analysis of clinical and gene expression data. Clin Cancer Res. 2016;23(11):2702–2712. doi: 10.1158/1078-0432.CCR-16-0979. [DOI] [PubMed] [Google Scholar]
- 6.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Peng R, Yuan Z, et al. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol. 2011;29(2):406–410. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
- 9.Astvatsaturyan K, Yue Y, Walts A, et al. Androgen receptor positive triple negative breast cancer: clinicopathologic, prognostic, and predictive features. PLos One. 2018;13(6):e0197827. doi: 10.1371/journal.pone.0197827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gucalp A, Tolaney S, Isakoff S, et al. Phase II trial of bicalutamide in patients with androgen receptor–positive, estrogen receptor–negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leon-Ferre R, Polley M, Liu H, et al. Tumor-infiltrating lymphocytes in androgen receptor-positive, triple-negative breast cancer. J Clin Oncol. 2018;36(5_suppl):5–5. doi: 10.1200/JCO.2018.36.5_suppl.5. [DOI] [Google Scholar]
- 12.Castaneda C, Castillo M, Enciso J, et al. Role of undifferentiation markers and androgen receptor expression in triple‐negative breast cancer. Breast J. 2019;25(6):1316–1319. doi: 10.1111/tbj.13464. [DOI] [PubMed] [Google Scholar]
- 13.Asano Y, Kashiwagi S, Goto W, et al. Expression and clinical significance of androgen receptor in triple-negative breast cancer. Cancers. 2017;9(12):4. doi: 10.3390/cancers9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jongen L, Floris G, Wildiers H, et al. The prognostic role of the androgen receptor in patients with triple-negative early breast cancers and primary surgery. J Clin Oncol. 2019;37(15_suppl):e12042–e12042. doi: 10.1200/JCO.2019.37.15_suppl.e12042. [DOI] [Google Scholar]
- 15.Guiu S, Mollevi C, Charon-Barra C, et al. Prognostic value of androgen receptor and FOXA1 co-expression in non-metastatic triple negative breast cancer and correlation with other biomarkers. Brit J Cancer. 2018;119(1):76–79. doi: 10.1038/s41416-018-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traina T, Miller K, Yardley D, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) J Clin Oncol. 2015;33(15_suppl):1003–1003. doi: 10.1200/jco.2015.33.15_suppl.1003. [DOI] [Google Scholar]
- 17.Dieci M, Tsvetkova V, Griguolo G, et al. Androgen receptor expression and association with distant disease-free survival in triple negative breast cancer: analysis of 263 patients treated with standard therapy for stage I-III disease. Front Oncol. 2019;9:452. doi: 10.3389/fonc.2019.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton L, Cao D, Sarode V, et al. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor–expressing triple-negative breast carcinoma. Am J Clin Pathol. 2012;138(4):511–516. doi: 10.1309/AJCP8AVF8FDPTZLH. [DOI] [PubMed] [Google Scholar]
- 19.Hu R, Dawood S, Holmes M, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17(7):1867–1874. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGhan L, McCullough A, Protheroe C, et al. Androgen receptor-positive triple negative breast cancer: a unique breast cancer subtype. Ann Surg Oncol. 2013;21(2):361–367. doi: 10.1245/s10434-013-3260-7. [DOI] [PubMed] [Google Scholar]
- 21.Xu M, Yuan Y, Yan P, et al. Prognostic significance of androgen receptor expression in triple negative breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2020;20(4):e385–e396. doi: 10.1016/j.clbc.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Balkenhol MCA, Vreuls W, Wauters CAP, et al. Histological subtypes in triple negative breast cancer are associated with specific information on survival. Ann Diagn Pathol. 2020;46:151490. doi: 10.1016/j.anndiagpath.2020.151490. [DOI] [PubMed] [Google Scholar]
- 23.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 24.Adams S, Gray R, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27(2):249–256. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- 26.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, et al. Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): a systematic review and meta-analysis study. Diagnostics. 2020;10(9):704. doi: 10.3390/diagnostics10090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon-Ferre RA, Polley MY, Liu H, et al. Proceedings of the 2018 San Antonio Breast Cancer Symposium. Vol. 79. Philadelphia: AACR; 2019. Characteristics, outcomes and prognostic factors of luminal androgen receptor (LAR) triple-negative breast cancer (TNBC) [abstract] pp. 3–8. (Dec 2018) [Google Scholar]
- 29.Sánchez-Cousido L, López-González A, Honrado Franco E, et al. Relationship between androgen receptor and tumor-infiltrating lymphocytes in triple-negative breast cancer. Ann Oncol. 2018;29:viii80. doi: 10.1093/annonc/mdy270.246. [DOI] [Google Scholar]
- 30.Sánchez-Cousido L, Honrado Franco E, Sanz Guadarrama Ó, et al. 219P Relationship among PDL1, androgen receptor (AR) and tumour infiltrating lymphocytes (TILs) in early triple negative breast cancer. Ann Oncol. 2020;31:S328–S329. doi: 10.1016/j.annonc.2020.08.341. [DOI] [Google Scholar]
- 31.Liu Z, Li M, Jiang Z, et al. A comprehensive immunologic portrait of triple-negative breast cancer. Translat Oncol. 2018;11(2):311–329. doi: 10.1016/j.tranon.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud S, Paish E, Powe D, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Lachapelle J, Leung S, et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanton S, Adams S, Disis M. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes. JAMA Oncol. 2016;2(10):1354. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud S, Lee A, Paish E, et al. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2011;132(2):545–553. doi: 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 36.Mi H, Gong C, Sulam J, et al. Digital pathology analysis quantifies spatial heterogeneity of CD3, CD4, CD8, CD20, and FoxP3 immune markers in triple-negative breast cancer. Front Physiol. 2020;11:583333. doi: 10.3389/fphys.2020.583333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keren L, Bosse M, Marquez D, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. 2018;174(6):1373–1387. doi: 10.1016/j.cell.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goff S, Danforth D. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer. 2021;21(1):e63–e73. doi: 10.1016/j.clbc.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elghazaly H, Rugo HS, Azim HA, et al. Breast-gynaecological & immuno-oncology international cancer conference (BGICC) consensus and recommendations for the management of triple-negative breast cancer. Cancers. 2021;13(9):2262. doi: 10.3390/cancers13092262. [DOI] [PMC free article] [PubMed] [Google Scholar]