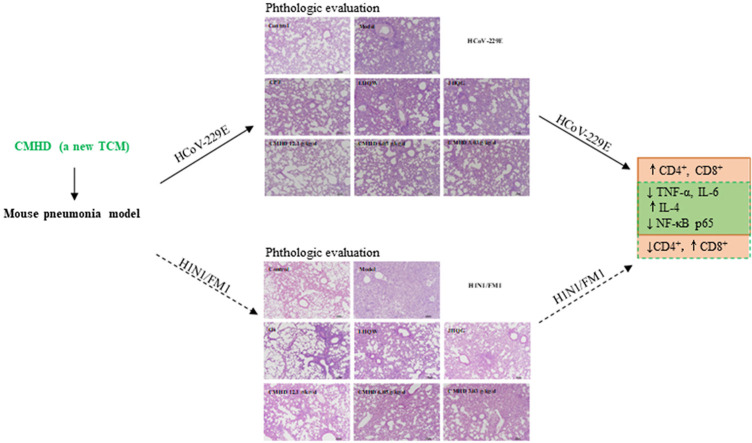
Abstract
Ethnopharmacological relevance
Coronavirus and influenza virus infection seriously threaten human health. Cangma Huadu Granules (CMHD) is an in-hospital preparation composed of eight traditional Chinese medicines (TCM), which has been clinically used against COVID-19 in China and may be a promising candidate for the treatment of influenza. However, the role of its treatment urgently needs to be studied.
Aim of the study: To evaluate the therapeutic effects of CMHD on pneumonia induced by coronavirus (HCoV-229E) and influenza A virus (H1N1/FM1) in mice and explore its mechanism of anti-infection.
Materials and methods
Mice were infected with HCoV-229E or H1N1/FM1 virus through the nasal cavity. CMHD (12.1, 6.05 and 3.03 g/kg/d) or the positive control drugs were administered intragastrically. The lung index and histopathological changes were used to evaluate the therapeutic effect of CMHD. The expression of TNF-α, IL-1β, IL-6 and IL-4 in Serum and the proportion of CD4+ and CD8+ T lymphocytes in peripheral blood were detected to evaluate the anti-inflammatory and immune regulation effects of CMHD, respectively. Furthermore, the levels of p–NF–κBp65/ NF-κB p65, which was the key targets of the NF-κB pathway was analyzed.
Results
In HCoV-229E-induced pneumonia, the lung index was markedly reduced, and lung pathology was improved in mice that treated with CMHD (12.1, 6.05 g/kg/d). Meanwhile, the expression of TNF-α, IL-6 were obviously inhibited, but the expression of IL-4 was significantly increased in CMHD groups. Compared with the model group, CMHD could also markedly upregulate the level of CD4+ and CD8+. Furthermore, CMHD has a markedly effect on inhibit the expression of p–NF–κB p65/NF-κB p65 in the lung. In H1N1-induced pneumonia, the lung index of mice in the CMHD (12.1 g/kg/d) treatment group was lower than that in the model group, and less inflammatory infiltration could be seen in the lung pathological. Moreover, CMHD could also obviously decrease the expression of TNF-α, IL-1β, IL-6, but significantly increase the expression of IL-4. Except for that, CMHD could also markedly downregulate the level of CD4+ and upregulate the level of CD8+ compared with the model group. In addition, CMHD has a markedly effect on inhibit the expression of p–NF–κB p65/NF-κB p65 in the lung.
Conclusion
CMHD can significantly combats viral infections caused by HCoV-229E and H1N1, and the mechanism may be related to its multiple functions of anti-inflammatory, immunity regulating and inhibiting NF-κB signal transduction pathway.
Keywords: HCoV-229E, H1N1/FM1, Pneumonia, Cangma Huadu, Immunomodulation, Inflammation
Abbreviations: CMHD, Cangma Huadu granules; TCM, Traditional Chinese Medicine; COVID-19, new coronavirus disease; BFDA, Beijing Food and Drug Administration; NF-κB, nuclear factor kappa B; CPT, chloroquine phosphate tablets; LHQW, Lianhua Qingwen Granules; JHQG, Jinhua Qinggan Granules; PBS, phosphate buffered saline
Graphical abstract
1. Introduction
Influenza and coronavirus infections are serious threats to human health (GBD 2017 Influenza Collaborators, 2019 V'kovski Philip et al., 2021). Since the beginning of the 21st century, there have been three severe coronavirus infection epidemics, including severe acute respiratory syndrome (caused by SARS-CoV infection) in 2003, Middle East Respiratory Syndrome (caused by MERS-CoV infection) in 2012, and COVID-19 which caused by a newly discovered coronavirus SARS-CoV-2 in 2019 (Al-Tawfiq Jaffar A et al., 2014 and Philip et al., 2021). According to the WHO statistical, as of July 19, 2021, more than 190.0 million confirmed COVID-19 cases and 4.0 million deaths have been reported in 220 countries and regions around the world, and the SARS-CoV-2 is still spreading (WHO organization. 2021). As to influenza, there are also three pandemics which respectively caused by H2N2, H3N2, H1N1 since 2000 (Petersen et al., 2020). In addition, seasonal influenza breaks out every year led to 3 million to 5 million severe cases and 300,00 to 500,000 deaths globally (Paules Catharine I et al., 2018 and Wang et al., 2020). These viruses are easily mutated and difficult to be eliminated, which increases the difficulty of infection treatment.
During the outbreak of viral infections, there are often no specific treatments or preventive vaccines, and considering the high morbidity and mortality rates of these virus as well as their potential to cause epidemics, its highlight the need to find promising effective drugs and increase the stock of antiviral infection drugs reserve. (Petersen et al., 2020; Eccleston-Turner Mark et al., 2019; Mitjà et al., 2021). Traditional Chinese medicine has a long history of preventing and treating emerging infectious diseases. The combination of a variety of herbal medicines endows Chinese herbal formulas with unique medicinal effects, play an anti-infection effect through various ways such as anti-virus, anti-inflammatory, and immune enhancement (Xi et al., 2020). Many classical prescriptions, such as Maxing Shigan Decoction, are used to treat influenza and have good effects (C. Wang et al., 2011). Especially in the process of defending against the COVID-19, 91.50% of the total cases treated by combined conventional and TCM approaches with promising results in all infection stages, including significant symptom management, lower rates of deterioration and mortality, faster recovery as well as disease prevention as of March 23, 2020 (State Administration of Traditional Chinese Medicine, China. 2020; Tian et al., 2020). Many Chinese medicine prescriptions and proprietary Chinese medicines are also recommended in the new coronavirus pneumonia diagnosis and treatment plan jointly issued by the National Health Commission of the People's Republic of China and the State Administration of Traditional Chinese Medicine (Li et al., 2020). Taken together, TCM prescriptions are important resources for the development of antiviral drugs.
CMHD is a new Chinese Medicine and approved by BFDA (Z20200008000) in China, which is suitable for the treatment of mild COVID-19 patients. This prescription is derived from the empirical prescriptions summarized by Professor Qingquan Liu in China's epidemic prevention work. During the outbreak of COVID-19, Professor Qingquan Liu, as one of the first batch TCM experts to arrive in Wuhan, went deep into the clinical frontline. In the Jiangxia Temporary Hospital where he is in charge, none of the COVID-19 patients has become serious. Through the diagnosis and treatment of the patients, Professor Qingquan Liu concluded that COVID-19 belongs to the category of “epidemic disease” and “wet fever” in TCM and “wet poison” is the core of the pathogenesis of COVID-19. In this case, CMHD was created to play the role of dampening spleen, clearing heat and detoxifying. CMHD are mainly composed of Atractylodes lancea (Thunb.) DC., Ephedra sinica Stapf, Pogostemon cablin (Blanco) Benth. and other TCM, and these herbs in CMHD have anti-inflammatory, anti-allergic, anti-bacterial and other pharmacological effects (Xu et al., 2020; Liang et al., 2018; Zhao et al., 2020). However, its pharmacodynamic level and mechanism need to be further studied. In addition, the traditional Chinese medicine treatment of influenza often uses heat-clearing and detoxifying drugs, just in the functional scope of CMHD. In view of the multi-targeted and multi-functional of Chinese medicine prescriptions, CMHD may also be an effective drug for the treatment of influenza. Herein, its efficacy needs to be confirmed.
HCoV-229E is one of seven known coronaviruses that affect humans. Patients with infection of HCoV-229E typically develop mild to moderate upper respiratory infections, like the common cold, and sometimes it may also cause simple and complicated lower respiratory diseases associated with series of respiratory symptoms, such as pneumonia and bronchiolitis (Monto Arnold S et al., 2020; Cimolai Nevio, 2020). The mouse pneumonia model infected with HCoV-229E has pathophysiological changes consistent with human mild coronavirus pneumonia, forming evaluation criteria from multiple aspects of lung inflammation, inflammatory factors, immune cells, and pathological changes of important organs. The model has been identified by the Experimental Animal Model Identification and Evaluation Working Committee of the Chinese Association for Laboratory Animals Sciences and is used for the selection of anti-coronavirus infection drugs with potential clinical value and the study of TCM in the prevention and treatment of COVID-19 (Xia Lu et al. 2021). H1N1/FM1 is a commonly used influenza strain in the laboratory for drug screening, which can cause pneumonia in mice (Shi et al., 2020; Yan Yu-Qi et al., 2018.). Coronaviruses and Influenza can cause a strong and continuous pro-inflammatory response, enhance the host's lung immunopathology, and cause systemic immune function abnormalities. In this study, HCoV-229E and H1N1/FM1 infection mouse models were used to evaluate the efficacy of CMHD. At the same time, two western medicine (Oseltamivir and Chloroquine Phosphate Tablets) and two Chinese patent medicines (Lianhua Qingwen Granules and Jinhua Qinggan Granules) widely used in clinical were selected to evaluate the efficacy level of CMHD, respectively. In addition, the anti-inflammatory and immune-regulating effects of CMHD on two types of infections were analyzed. Our research confirms a new drug with effect against coronavirus and influenza infection, and it can help to study the mechanism of anti-coronavirus infection.
2. Materials and methods
2.1. Reagents
CMHD granules (lot: 20200301) used in this study was provided by Beijing Hospital of Traditional Chinese Medicine, Capital Medical University (Beijing, China). Chloroquine Phosphate Tablets (CPT) was purchased from Shanghai Xinyi Tianping Pharmaceutical Co., Ltd. (Shanghai, China). Oseltamivir Phosphate Capsules (Tamiflu) was purchased from Roche Co. Ltd. (Subpackage in Shanghai, China). Lianhua Qingwen granules were bought from Yiling Pharmaceutical Co. Ltd. (Beijing, China). Jinhua Qinggan granules were purchased from Juxie Chang (Beijing) Pharmaceutical Co. Ltd. (Beijing, China). The mouse IL-6, TNF-α, IL-4 and IL-1β enzyme-linked immunosorbent assay (ELISA) assay kits were provided by Shanghai meilian Biotechnology Co., Ltd. (Shanghai, China). Anti-Mouse CD4 PerCP-Cyanine5.5 (lot: C0042091318653), Anti-mouse CD8a APC (lot: C0081101018203) antibodies, and RBC Lysis (lot: B43000830019) were purchased from Tonbo (Biosciences, USA). NF-κB p65 rabbit monoclonal (lot: 8242), and p–NF–κB p65 S536 rabbit monoclonal (lot: 3033) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
2.2. Animals and viruses
Male and female SPF ICR mice (4 weeks, 13–15 g, for H1N1/FM1 infection) and BALB/c mice (4 weeks, 13–15 g, for HCoV-229E infection) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd (SCXK2016-0006, Beijing, China). All mice were kept at a controlled room (25 ± 2, 45–60% humidity) with 12-hr light/dark cycle. Mice were allowed free access to food and water. All procedures involving animals throughout the experiments were carried out according to the Ethics Committee of the Beijing Institute of Traditional Chinese Medicine (Beijing, China) and the AAALAC and the IACUC guidelines.
2.3. Mouse infection and treatment
The HCoV-229E infected mouse model was established as described previously, with slight modification (Lu et al. 2021). Eighty BALB/c mice were randomly divided into eight groups (n = 10 per group, five male, five female): control group (Control), model group (Model), CPT group (0.25 g/kg/d), LHQW group (3.3 g/kg/d), JHQG group (2.75 g/kg/d), CMHD high-dose (12.1 g/kg/d), CMHD middle-dose (6.05 g/kg/d), and CMHD low-dose (3.03 g/kg/d) group. Except for the control group, the mice were intranasally challenged with a 50 μL 100 TCID50 HCoV-229E virus suspension under anesthesia nasally on day 1. On the first day of infection, the mice in the CMHD groups were administered daily with three doses of CMHD orally for four days, 0.2 ml/10 g body weight. The CPT, LHQW and JHQG groups were treated with CPT, LHQW and JHQG, respectively. The mice in the control group were intranasally challenged with 50 μL physiological saline solution and orally administered with distilled water simultaneously. On day 4 after the first time of infection, blood was collected from the orbits and the lungs were dissected for further analysis.
The FM1 infected mouse model was established as described previously, with slight modification (Yu-Qi et al., 2018.). A total number of eighty ICR mice were randomly divided into control group (Control), model group (Model), Oseltamivir group (OS, 0.0275 g/kg/d), LHQW group (LHQW, 3.3 g/kg/d), JHQG group (JHQG, 2.75 g/kg/d), CMHD high-dose (12.1 g/kg/d), CMHD middle-dose (6.05 g/kg/d), and CMHD low-dose (3.03 g/kg/d) group. Then each mouse was respectively administered with FM1 virus solution (35 μL) by the intranasal route after mild anesthesia, except the mice in the control group. On day 1, mice in each CMHD groups were orally administered with three doses of CMHD for 4 consecutive days, once a day and 0.2 ml/10g body weight. The Os, LHQW and JHQG groups were treated with Os, LHQW and JHQG, respectively. The mice in the control group were intranasally challenged with 35 μL physiological saline solution and orally administered with distilled water simultaneously. On day 4 after the first time of infection, blood was collected from the orbits and the lungs were dissected for further analysis.
The Dosage of the drugs (OS, 0.0275 g/kg/d; CPT, 0.25 g/kg/d; LHQW, 3.3 g/kg/d; JHQG, 2.75 g/kg/d; CMHD middle-dose, 6.05 g/kg/d) used in mice is equivalently converted according to the clinical dose of the drug. Meanwhile, 2 times and 1/2 times dose of CMHD middle-dose was set as the high-dose group and the low-dose group, respectively.
2.4. Measurement of lung index
The lung tissues were weighed immediately after excised from the mice body. Lung index was calculated as formula:
| Lung Index =(W1/W0) × 100 |
where W1 is the lung wet weight (g); W0 is the body weight (g).
2.5. Histopathological analysis
The left lung was fixed in 4% poly methyl aldehyde for at least 24 h. After repair block, gradient alcohol dehydration and paraffin embedding, each tissue was cut into 5 μm sections and stained with hematoxylin and eosin.
2.6. Detection of IL-6, TNF-α, IL-4 and IL-1β levels in serum
Blood samples were centrifuged at 3000 rpm and 4 °C for 15 min to obtain the serum. The contents of IL-6, TNF-α, IL-4 and IL-1β in the serum were measured by ELISA. All operations are carried out in strict accordance with the instructions of the kits. The contents of these cytokines were determined by establishing standard curves.
2.7. Flow cytometric detection of T lymphocyte subsets in peripheral blood
Blood samples were centrifuged in phosphate buffered saline (PBS), and after removing the supernatant RBC, lysate was added, and the samples were further treated according to the manufacturer's instructions. The final cell suspension was incubated with the following antibodies according to the instruction of the manufacturer: PerCP-Cy5.5-labeled anti-mouse CD4 and APC-labeled anti-mouse CD8a. Flow cytometric analysis was conducted on an BD FACSVerse™ flow cytometer.
2.8. Western blot analysis
Equal amounts of protein (30 μg/lane) were fractionated by electrophoresis in 12% and 4% polyacrylamide gels and then transferred to Millipore PVDF membranes. The membranes were sequentially incubated with appropriately diluted antibodies targeting β-Actin, NF-κB p65, p–NF–κB p65 at 4 °C overnight, washed with TBST three times, and incubated with HRP-conjugated Affinipure goat anti-mouse IgG diluted 1:5000 in TBST for 1 h at 37 °C. The membranes were then washed with TBST three times, and the protein bands were detected using ECL kit according to the manufacturer's instructions. ImageJ software was used to analyze the target bands of scanned images.
2.9. Data analysis
Data were analyzed using GraphPad Prism v.6 Software. For multiple groups, statistical difference was evaluated by one-way analysis of variance (ANOVA). Data were presented as the mean ± SD. P-value < 0.05 was considered statistically significant.
3. Results
3.1. CMHD inhibit the increase of lung index in mice infected with HCoV-229E and FM1
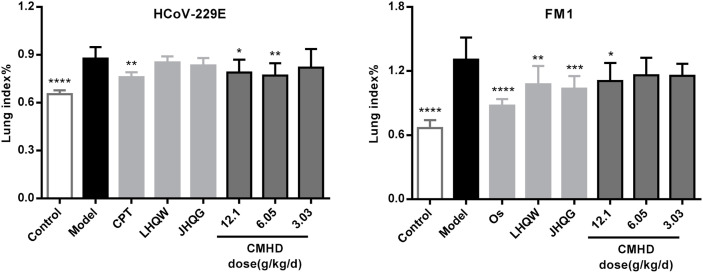
The effect of CMHD against infections caused by HCoV-229E and FM1 viruses were evaluated by inhibiting the increase of lung index (Fig. 1 ). Results showed that after mice infected with HCoV-229E and FM1 virus solution, the lung index of mice increased significantly. CMHD (12.1 g/kg/d and 6.05 g/kg/d) significantly reduced the lung index caused by HCoV-229E infection, but 3.03 g/kg/d CMHD was less effective. Meanwhile, 12.1 g/kg/d CMHD significantly reduced the lung index caused by FM1 infection. To evaluate the efficacy level of CMHD, the trial selected representative drugs in clinical applications as a reference, which include one western medicine and two Chinese patent medicines. The experimental results revealed that the effect of CMHD (12.1 g/kg/d and 6.05 g/kg/d) in reducing lung index caused by HCoV-229E is equivalent to that of CPT. The effect of CMHD (12.1 g/kg/d) in reducing lung index caused by FM1 is equivalent to that of LHQW and JHQG, but weaker than Os.
Fig. 1.
Inhibition effects of CMHD treatment on the lung index levels of HCoV-229E-infected BALB/c mice and FM1-infected ICR mice. Results are represented as mean ± SD (n > 8), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with model group.
3.2. CMHD improve lung pathology
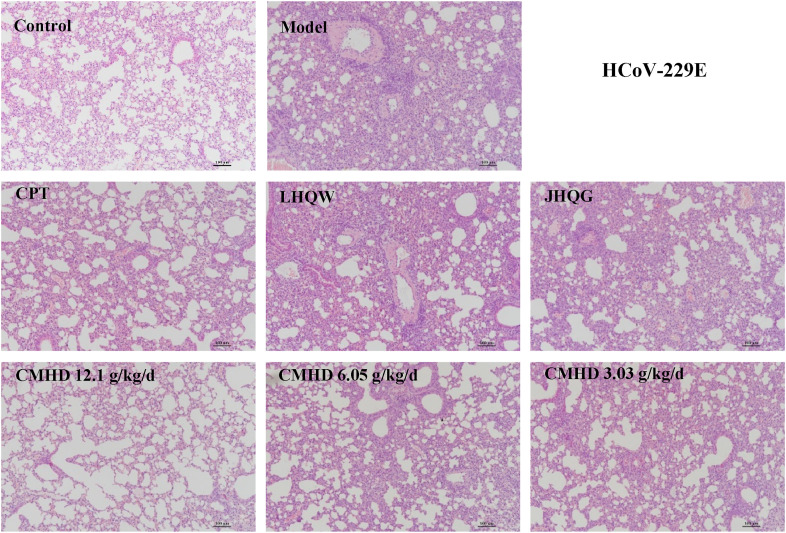
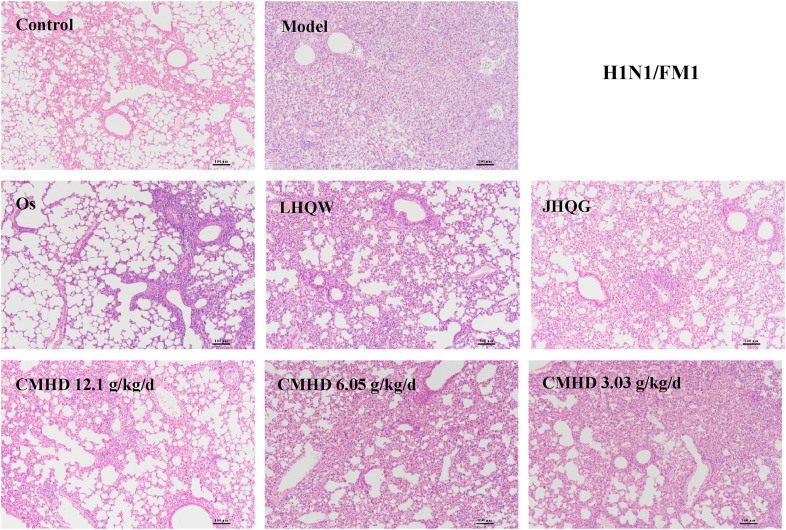
As shown in Fig. 2 and Fig. 3 , whether it is a BALB/c mouse or an ICR mouse, the lung tissue structure of the control group is basically normal, and there is no inflammatory cell infiltration around the bronchus, no necrosis on the wall of the bronchial tube, and no material exudation in the lumen could be observed. In contrast, the main pathological manifestation of the lungs in the HCoV-229E model group was that the alveolar septum significantly widened and accompanied by a large number of inflammatory cell infiltration, which was identified as moderate interstitial pneumonia. In the CMHD (12.1 g/kg/d) group, the lung tissue morphology was significantly improved, mainly manifested as a slight widening of the alveolar septum and a small amount of inflammatory cell infiltration. The 6.05 g/kg/d and 3.03 g/kg/d CMHD group can also reduce the degree of lung tissue pathology, but the effect is weaker than that of the 12.1 g/kg/d group. In the FM1 model group, the lungs of the mice showed different pathological manifestations. Extensive lung injury was observed in the mice, including widening of alveolar septum, bronchial epithelial cell necrosis, and a large number of inflammatory cell infiltration. The 12.1 g/kg/d CMHD can improve the pathology caused by FM1, mainly reflected in reducing the degree of inflammatory cell infiltration, compared with the model group. The experimental results also revealed that the effect of CMHD (12.1 g/kg/d and 6.05 g/kg/d) on improves lung pathology caused by HCoV-229E is better than all the reference drugs. The effect of CMHD (12.1 g/kg/d) on improves lung pathology caused by FM1 is equivalent to that of LHQW and JHQG, but weaker than Os.
Fig. 2.
The treating effects of CMHD on reducing the severity of lung pathology ( × 100) in mice with HCoV-229E pneumonia. The HE method was used in this experiment (n = 6).
Fig. 3.
CMHD reduces the severity of lung pathology ( × 100) in mice with H1N1/FM1 pneumonia. The HE method was used in this experiment (n = 6).
3.3. CMHD strongly inhibited the expression of proinflammatory cytokines and increase levels of anti-inflammatory cytokines
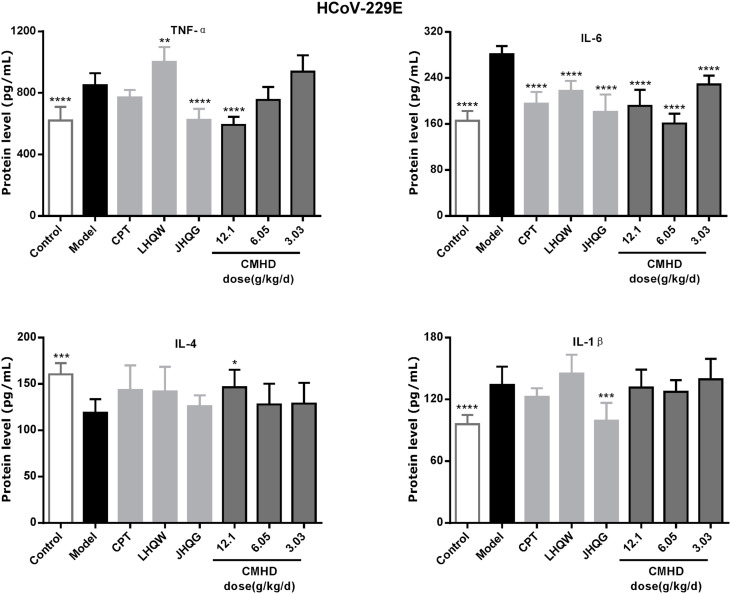
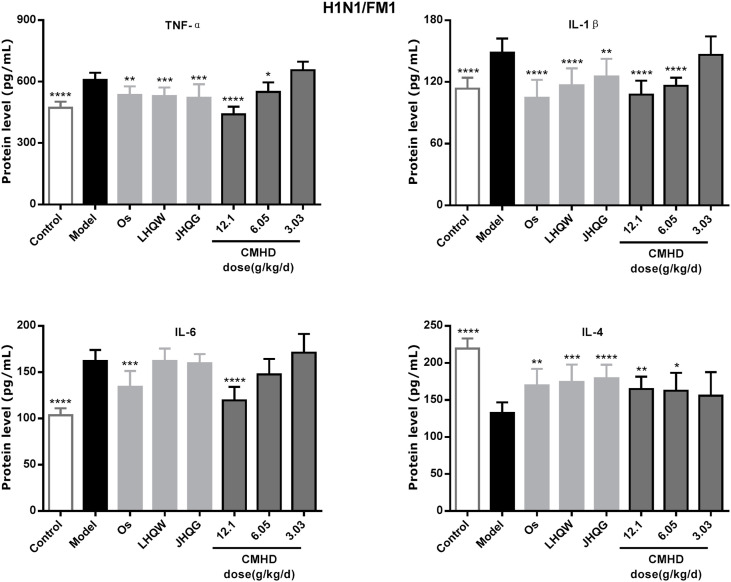
Whether it is a coronavirus or influenza virus infection, it can cause a change in the expression of inflammatory factors, which include the increased expression of pro-inflammatory factors and the decreased expression of anti-inflammatory factors. As shown in Fig. 4 and Fig. 5 , the expression levels of IL-6, TNF-α, IL-1β in the model group were significantly up-regulated and the IL-4 was significantly down-regulated after infected by HCoV-229E and FM1 compared with those in the control group. Obviously, CMHD can significantly reduce the expression level of IL-6, TNF-α and up-regulated the expression level of IL-4 in HCoV-229E infection. CMHD can also significantly reduced the expression levels of IL-6, TNF-α, IL-1β, and increase the expression levels of IL-4 after H1N1/FM1 infection.
Fig. 4.
CMHD can adjust the serum cytokine content of HCoV-229E infected mice. Infect mice as described in Experiment. From the first day of infection, each drug or distilled water was given orally every day. On the 4th day of infection, the concentration of inflammatory cytokines in the serum of mice was detected (n = 8 in each group). The expression levels of TNF-α, IL-1β, IL-6 and IL-4 in mouse serum were detected by ELISA. Results are represented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, compared with model group.
Fig. 5.
CMHD can adjust the serum cytokine content of H1N1/FM1 infected mice. Infect mice as described in Experiment. From the first day of infection, each drug or distilled water was given orally every day. On the 4th day of infection, the concentration of inflammatory cytokines in the serum of mice was detected (n = 10 in each group). The expression levels of TNF-α, IL-1β, IL-6 and IL-4 in mouse serum were detected by ELISA. Results are represented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, compared with model group.
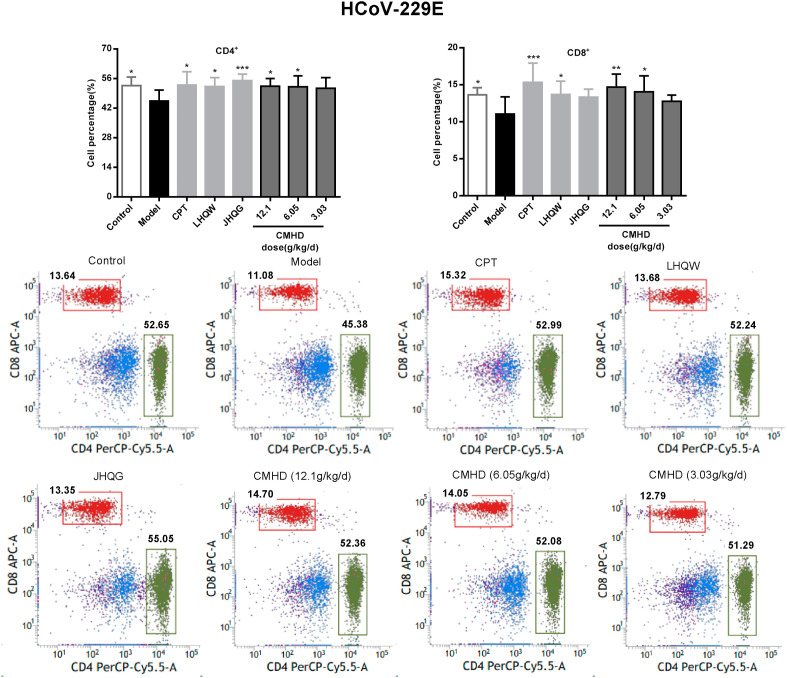
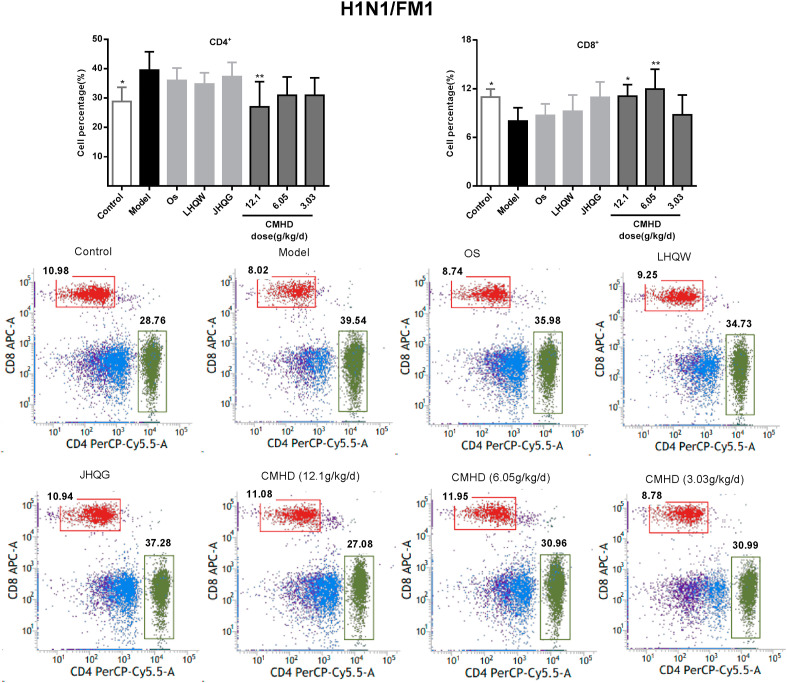
3.4. CMHD regulates the proportion of T lymphocytes in peripheral blood
As shown in Fig. 6 , the proportions of the CD4+ and CD8+ T cell in peripheral blood of the model group mice were obviously decreased after HCoV-229E infection. After administered for 4 days, CMHD increases the proportion of CD4+ and CD8+ T lymphocytes in a dose-dependent manner. While in the H1N1/FM1 model group, the proportions of the CD4+ was obviously increased and the CD8+ were significantly decreased in peripheral blood. CMHD could significantly downregulate the proportions of the CD4+. 12.1 and 6.05 g/kg/d CMHD could upregulate the proportions of the CD8+, obviously. These results were shown in Fig. 7 . The results also revealed that the effects of CMHD regulates the CD4+ and CD8+ T cell in peripheral blood were better than LHQW, JHQG, CPT and Os.
Fig. 6.
The regulating effect of CMHD on the percentage of CD4+ and CD8+T cells in peripheral blood of HCoV-229E infected mice. Results are represented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, compared with model group (n = 8).
Fig. 7.
The regulating effect of CMHD on the percentage of CD4+ and CD8+T cells in peripheral blood of H1N1/FM1 infected mice. Results are represented as mean ± SD, *P < 0.05, **P < 0.01, compared with model group (n = 8).
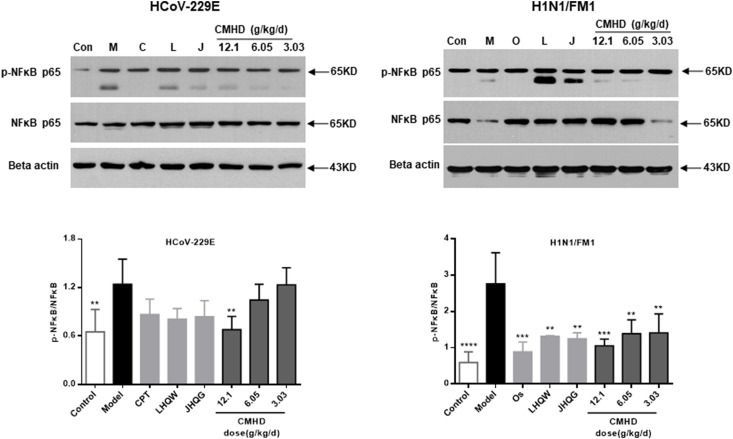
3.5. Relative protein expression analysis of p–NF–κB p65/NF-κB p65
Whether it is a coronavirus or influenza virus infection, compared with those in the control group, the protein expression levels of p–NF–κB p65/NF-κB p65 in the model group were markedly increased as determined by western blot. Compared with those in the model group, the protein expression levels of p–NF–κB p65/NF-κB p65 were downregulated in the CMHD groups. The results were shown in Fig. 8 .
Fig. 8.
CMHD inhibits the inflammation induced by the HCoV-229E and H1N1/FM1 through modulating the NF-κB pathway. Results are represented as mean ± SD, **P < 0.01, ***P < 0.001, ****P < 0.0001, compared with model group (n ≥ 3).
4. Discussion
Both influenza and coronavirus can cause sudden and fatal diseases, such as acute infectious pneumonia, and spread widely and rapidly around the world (GBD 2017 Influenza Collaborators, 2019 Influenza Collaborators. 2019; WHO organization. 2019). During a viral pandemic, there are often no specific antiviral drugs or vaccines available. Therefore, the development of effective and safe antiviral drugs has always been a key issue in scientific research and medicine (Petersen et al., 2020).
CMHD is an anti-epidemic empirical formula in clinical, the main component (Supplementary materials S1) of the medicine has anti-inflammatory, antioxidant, and anti-viral activities. Although clinical evidence shows that CMHD has a certain therapeutic effect on COVID-19, its mechanism was still unclear. Considering that the coronavirus and influenza are both RNA viruses, it is speculated that CMHD may have potential anti-flu effects. In the experiment, the results of lung index and lung pathology were used to determine the therapeutic effect of CMHD on HCoV-229E and H1N1/FM1 infections in vivo. The results show that the increase of lung index caused by HCoV-229E and H1N1/FM1 can significantly inhibited by CMHD. At the same time, the severity of lung pathology was obviously improved, and the lung was protected. The efficacy of the 12.1 and 6.05 g/kg/d CMHD in the treatment of coronavirus pneumonia is like that of CPT. The 12.1 g/kg/d CMHD has the same efficacy in treating influenza pneumonia as LHQW and JHQG. In addition, the possible mechanism of CMHD against viral infection in this experimental were also explored.
Many cytokines are essential for controlling virus replication, pro-inflammatory cytokines exacerbate morbidity and tissue damage in mouse models, while anti-inflammatory cytokines, such as IL-4, have the opposite effect (Shi et al., 2013; Liu et al., 2017). Clinically, the levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, are independent and significant predictors of disease severity and death in the serum of patients with COVID-19 (Del Valle Diane Marie et al., 2020). In addition, these cytokines were also elevated in patients with influenza pneumonia (Novak et al., 2020). By regulating cytokines, it is an effective means to treat infections. In this study, on the 4th day after infection, outbreaks of IL-1β, TNF-α, IL-6 and decreased of IL-4 were observed in mice infected with HCoV-229E and H1N1/FM1 viruses. At the same time, our results show that CMHD significantly inhibit the release of IL-1β, TNF-α, IL-6 and upregulate the expression IL-4 in vivo. The effect of CMHD in regulating the expression of cytokines is consistent with the reduction of lung injury in the treated mice. Strangely, in this test, LHQW significantly increased the expression of TNF-α in HCoV-229E mouse model. We considered that LHQW has no inhibitory effect on this index under these experimental conditions, namely it has no effect on TNF-α. Here, we also found that although HCoV-229E and H1N1/FM1 are two different viruses, the pathophysiological changes caused by them have similarities. As shown in the results of this experiment, the same inflammatory factors have the same changing trend and are consistent with the clinical manifestations of coronavirus and influenza (Del Valle Diane Marie et al., 2020). CMHD can recall a variety of abnormally changed cytokines in the two infection models, demonstrating its potential against multiple respiratory virus infections.
The NF-κB signaling pathway is a key factor to control the secretion of inflammatory cytokines and the recruitment of inflammatory cells during viral infection (Lee et al., 2020; Winkler Emma S et al., 2020). NF-кB causes the production of cytokines, and these cytokines can react in turn to produce a positive self-regulating loop and aggravate the inflammatory response (Hu et al., 2016). Therefore, the influence of CMHD on the NF-κB signaling pathway in antiviral immunity were mainly evaluated in this study, and the results may lay the foundation for the control of RNA virus infection. The test results showed that significant activation of the NF-κB signaling pathway was observed on the 4th day after infection, and CMHD could significantly reduce the activation of p–NF–κB p65/NF-κB p65 induced by HCoV-229E and H1N1/FM1. It could be inferred that the underlying mechanism of the antiviral activity of CMHD involves the impairment of virus-induced up-regulation of pro-inflammatory cytokines by inhibiting the activity of the NF-кB signaling pathway. The experimental results further prove that HCoV-229E and H1N1/FM1 infections can cause some of the same pathophysiological changes in the body.
T lymphocytes, which mediate cellular immunity, played an important role in maintained the immune function of the body. It can be divided into CD4+ T cells and CD8+ T cells and each of them has different effects. CD4+ T lymphocytes, also known as helper T lymphocytes, can promote the proliferation and differentiation of B cells and other immune cells. CD8+ T lymphocyte subsets are the main effector cells against viral infections, for its ability to limit viral replication and promote clearance of infected cells. Mice lacking CD8+ T cells showed higher susceptibility and viral replication after infection (Koutsakos Marios et al., 2019). According to the “New Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Eighth Edition)" and related research reports, patients with COVID-19 will experience peripheral blood lymphopenia, and CD4+ T and CD8+ T lymphocyte counts are significantly related to the severity of the disease (Giamarellos-Bourboulis Evangelos J et al., 2020; Zhang et al., 2020). The ratio of CD4+ T and CD8+ T cells in flu patients also changed markedly, and strong and broad CD4+ but not CD8+ T cell responses were observed in the blood, and were higher in those with severe disease (Zhao et al., 2012). In this study, it was found that the proportion of CD4+ T and CD8+ T cells in the peripheral blood of the coronavirus pneumonia model mice was significantly reduced, indicating that the immune function of the HCoV-229E infected mice was reduced and the ability to resist viral infections was inhibited. Compared with the model group, CMHD can significantly increase the proportion of CD4+ T and CD8+ T cells, indicating that drug intervention can effectively maintain the immune function of mice and effectively improve the antiviral ability. However, in influenza virus pneumonia model mice, CD4+ T increased significantly, and the proportion of CD8+ T cells decreased markedly, indicating that the immune function of the mouse was altered after H1N1/FM1 infection. The cellular immune function could be over-activated, and the ability of humoral immunity to directly resist viral infection is reduced. After the intervention of CMHD, the differentially expressed T lymphocytes were called back and the immune function was significantly improved. It can be seen from this experimental study that the immunomodulatory effect of CMHD is better than that of CPT, Os, LHQW and JHQG. Comparing the immune changes caused by HCoV-229E and H1N1/FM1 infections again, it is found that the expression of immune cells is different under different infections, but they are all consistent with the clinical manifestations of coronavirus and influenza (Giamarellos-Bourboulis Evangelos J et al., 2020; Zhao et al., 2012). Although the two viruses cause different changes in immune cells, CMHD has played its role well, that is, it effectively restores abnormally expressed T cells. This is another proof that Chinese medicine can regulate the body's immunity against viral infections.
5. Conclusions
CMHD has obvious therapeutic effects on mice infected with coronavirus and influenza virus. The drug effect is related to the suppression of cytokine burst, reduction of inflammatory cell infiltration in the lung, regulation of immunity and suppression of the activity of NF-кB signaling pathway. This is the first attempt of CMHD to resist viral pneumonia induced by coronavirus and influenza virus. Considering that the pathophysiological changes caused by HCoV-229E and H1N1/FM1 have similarities, such as the expression of TNF-α, IL-6, but also differences, such as the expression of T cells. More detailed studies must be carried out, such as the influence of interferon system, adaptive immune response, and immune escape mechanism, to provide drug applications reference. CMHD may be a very effective tool against coronavirus infection and influenza infection. If its use is combined with anti-viral strategies, it will bring greater benefits to human health.
Author contributions
Qing-quan Liu, Yu-hong Guo and Xu-ran Cui designed the study. Xu-ran Cui performed the experiments, analyzed the data, and wrote the paper. Qing-quan Liu, Yu-hong Guo revised the manuscript. All authors read and approved the final manuscript.
CRediT authorship contribution statement
Xu-ran Cui: Methodology, Data curation, Formal analysis, Validation, Visualization, Writing – original draft, The contributions of authors Qing-quan Liu and Yu-hong Guo are equivalent. Yu-hong Guo: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition, Writing – review & editing. Qing-quan Liu: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare there are no known conflicts of interest associated with this publication.
Acknowledgments
This study was financially supported by the matching project of the North China Regional Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2021.114965.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Al-Tawfiq Jaffar A., Alimuddin Zumla, Philippe Gautret, et al. Surveillance for emerging respiratory viruses. Lancet Infect. Dis. 2014;14:992–1000. doi: 10.1016/S1473-3099(14)70840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diane Marie Del Valle, Kim-Schulze Seunghee, Hsin-Hui Huang, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Influenza Collaborators Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017:an analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis Evangelos J., Netea Mihai G., Nikoletta Rovina, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Zhilin, Song Bin, Xu Lei, et al. Aqueous synthesized quantum dots interfere with the NF-κB pathway and confer anti-tumor, anti-viral and anti-inflammatory effects. Biomaterials. 2016;108:187–196. doi: 10.1016/j.biomaterials.2016.08.047. [DOI] [PubMed] [Google Scholar]
- Lee Wonhwa, June Hong Ahn, Park Hee Ho, et al. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct Target Ther. 2020;5:186. doi: 10.1038/s41392-020-00292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yuxi, Li Juan, Zhong Dongling, et al. Clinical practice guidelines and experts' consensuses of traditional Chinese herbal medicine for novel coronavirus (COVID-19): protocol of a systematic review. Syst. Rev. 2020;9:170. doi: 10.1186/s13643-020-01432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Shanshan, Meng Xianqun, Wang Zhibin, et al. Polysaccharide from Ephedra sinica Stapf inhibits inflammation expression by regulating Factor-β1/Smad2 signaling. Int. J. Biol. Macromol. 2018;106:947–954. doi: 10.1016/j.ijbiomac.2017.08.096. [DOI] [PubMed] [Google Scholar]
- Liu Jian-Xing, Zhang Ying, Hu Qiu-Ping, et al. Anti-inflammatory effects of rosmarinic acid-4-O-β-D-glucoside in reducing acute lung injury in mice infected with influenza virus. Antivir. Res. 2017;144:34–43. doi: 10.1016/j.antiviral.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Lu Xia, Shi Yujing, Su Jie, et al. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19: antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine. 2021;85:153390. doi: 10.1016/j.phymed.2020.153390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marios Koutsakos, Illing Patricia T., Nguyen Thi H.O., et al. Human CD8 T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- Mark Eccleston-Turner, Phelan Alexandra, Katz Rebecca. Preparing for the next pandemic - the WHO's global influenza strategy. N. Engl. J. Med. 2019;381:2192–2194. doi: 10.1056/NEJMp1905224. [DOI] [PubMed] [Google Scholar]
- Mitjà Oriol, Marc Corbacho-Monné, Maria Ubals, et al. A cluster-randomized trial of hydroxychloroquine for prevention of covid-19. N. Engl. J. Med. 2021;384:417–427. doi: 10.1056/NEJMoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto Arnold S., DeJonge Peter M., Callear Amy P., et al. Coronavirus occurrence and transmission over 8 Years in the HIVE cohort of households in Michigan. J. Infect. Dis. 2020;222:9–16. doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevio Cimolai. Complicating infections associated with common endemic human respiratory coronaviruses. Health Secur. 2021;19:195–208. doi: 10.1089/hs.2020.0067. [DOI] [PubMed] [Google Scholar]
- Novak Tanya, Hall Mark W., McDonald Douglas R., et al. RIG-I and TLR4 responses and adverse outcomes in pediatric influenza-related critical illness. J. Allergy Clin. Immunol. 2020;145:1673–1680. doi: 10.1016/j.jaci.2020.01.040. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules Catharine I., Sullivan Sheena G., Kanta Subbarao, et al. Chasing seasonal influenza - the need for a universal influenza vaccine. N. Engl. J. Med. 2018;378:7–9. doi: 10.1056/NEJMp1714916. [DOI] [PubMed] [Google Scholar]
- Petersen Eskild, Marion Koopmans, Go Unyeong, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip V'kovski, Annika Kratzel, Steiner Silvio, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Xunlong, Zhou Wei, Huang Hai, et al. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit. Care. 2013;17:R301. doi: 10.1186/cc13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Yucong, Xu Huachong, Xiao Yike, et al. Gegen qinlian decoction downregulates the TLR7 signalling pathway to control influenza A virus infection. Biomed. Pharmacother. 2020;121:109471. doi: 10.1016/j.biopha.2019.109471. [DOI] [PubMed] [Google Scholar]
- State Administration of Traditional Chinese Medicine . Information Office of the State Council; 2020. https://baijiahao.baidu.com/s?id=1661946820250968958&wfr=spider&for=pc China. Available from: [Google Scholar]
- Tian Jiaxing, Yan Shiyan, Wang Han, et al. Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol. Res. 2020;161:105127. doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Cao B., Li Q.-Q., Zou Z.-Q., Li Z.-A., Gu L., et al. Oseltamivir compared with the Chinese traditional therapy Maxingshigan-Yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann. Intern. Med. 2011;155(4):217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- Wang Xin, You Li, O'Brien Katherine L., et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Global Health. 2020;8:e497–e510. doi: 10.1016/S2214-109X(19)30545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO organization https://covid19.who.int/
- Winkler Emma S., Bailey Adam L., Kafai Natasha M., et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Shengyan, Li Yunhong, Yue Lifeng, et al. Role of traditional Chinese medicine in the management of viral pneumonia. Front. Pharmacol. 2020;11:582322. doi: 10.3389/fphar.2020.582322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Wei, Fang Sijia, Wang Yong, et al. Receptor and signaling pathway involved in bovine lymphocyte activation by Atractylodis macrocephalae polysaccharides. Carbohydr. Polym. 2020;234:115906. doi: 10.1016/j.carbpol.2020.115906. [DOI] [PubMed] [Google Scholar]
- Yu-Qi Yan, Fu Ying-Jie, Wu Sha, et al. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother Res. 2018;32:2560–2567. doi: 10.1002/ptr.6196. [DOI] [PubMed] [Google Scholar]
- Zhang Xiaonan, Tan Yun, Yun Ling, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Zhao Yan, Zhang Yong-Hong, Laura Denney, et al. High levels of virus-specific CD4+ T cells predict severe pandemic influenza A virus infection. Am. J. Respir. Crit. Care Med. 2012;186:1292–1297. doi: 10.1164/rccm.201207-1245OC. [DOI] [PubMed] [Google Scholar]
- Zhao Yuge, Yang Yuting, Zhang Jiaxin, et al. Lactoferrin-mediated macrophage targeting delivery and patchouli alcohol-based therapeutic strategy for inflammatory bowel diseases. Acta Pharm. Sin. B. 2020;10:1966–1976. doi: 10.1016/j.apsb.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.