Abstract
Axin acts as a negative regulator in Wnt signaling through interaction with various molecules involved in this pathway, including β-catenin, adenomatous polyposis coli, and glycogen synthase kinase 3β. We show here that Axin also regulates the effects of Smad3 on the transforming growth factor β (TGF-β) signaling pathway. In the absence of activated TGF-β receptors. Axin physically interacted with Smad3 through its C-terminal region located between the β-catenin binding site and Dishevelled-homologous domain. An Axin homologue, Axil (also called conductin), also interacted with Smad3. In the absence of ligand stimulation, Axin was colocalized with Smad3 in the cytoplasm in vivo. Upon receptor activation, Smad3 was strongly phosphorylated by TGF-β type I receptor (TβR-I) in the presence of Axin, and dissociated from TβR-I and Axin. Moreover, the transcriptional activity of TGF-β was enhanced by Axin and repressed by an Axin mutant which is able to bind to Smad3. Axin may thus function as an adapter of Smad3, facilitating its activation by TGF-β receptors for efficient TGF-β signaling.
The Wnt signaling pathway plays important roles in the regulation of cellular proliferation, differentiation, motility, and morphogenesis in vertebrates and invertebrates (1, 3, 27, 32, 37). In mammals, Wnt acts on the cell surface receptor Frizzled, which in turn activates the cytoplasmic proteins of the Dishevelled family, including Dvl-1, Dvl-2, and Dvl-3. Dvl antagonizes the effects of glycogen synthase kinase 3β (GSK-3β), leading to the stabilization of β-catenin. β-Catenin then accumulates in the cells, resulting in its nuclear translocation. In the nucleus, β-catenin binds members of the T cell-specific factor (Tcf)/lymphoid enhancer binding factor 1 (Lef1) transcription factor family and regulates transcription of various genes. Axin is the product of the mouse gene Fused (44) and plays a critical role in the regulation of embryonic axis formation by inhibiting Wnt signaling (20). An Axin homologue, Axil (also termed conductin), also functions as a negative regulator of the Wnt signaling pathway (2, 42). Axin interacts with various proteins involved in the Wnt signaling pathway, including β-catenin, adenomatous polyposis coli (APC), and GSK-3β, and regulates the phosphorylation and stability of β-catenin (15, 20, 23). Rat Axin (rAxin) is a protein with 832 amino acids. APC binds to the N-terminal regulator of G-protein signaling (RGS)-homologous domain of Axin and to GSK-3β and β-catenin at two nearby domains in the central part of Axin (2, 9, 14, 22). Although the function of the C-terminal third of Axin has not been fully elucidated, protein phosphatase 2A and Dvl binding sites appear to be located in this region (20).
Members of the transforming growth factor β (TGF-β) superfamily are multifunctional proteins that regulate various cellular functions, including proliferation, differentiation, migration, and apoptosis (26). The TGF-β superfamily includes TGF-βs, activins and inhibins, bone morphogenetic proteins (BMPs), and Müllerian inhibiting substance. Members of the TGF-β superfamily bind to type II and type I serine/threonine kinase receptors and transduce intracellular signals by Smad proteins (12). Type II receptor kinases are constitutively active; upon ligand binding and complex formation with type I receptors, type II receptors transphosphorylate type I receptors, resulting in the activation of Smads by type I receptor kinases. There are three distinct subclasses of Smads. Receptor-regulated Smads (R-Smads) are direct substrates of the type I receptors (6, 29; J. Wrana [http://www.stke.org/cgi/content/full/OC-sigtrans;2000/23/re1]). R-Smads are phosphorylated at the C-terminal SSXS motif by serine/threonine kinase receptors and form heteromeric complexes with the second class of Smads, common-mediator Smads (Co-Smads). The Smad complexes translocate into the nucleus, where they regulate the transcription of various target genes. Smad2 and Smad3 are R-Smads activated by TGF-β and activin receptors, whereas Smad1, -5, and -8 are activated by BMP receptors. Smad4 is the only Co-Smad in mammals. The third class of Smads includes inhibitory Smads (I-Smads), which negatively regulate the signaling activity of R-Smads and Co-Smads. Smad6 and Smad7 are I-Smads in mammals.
R-Smads have a nuclear localization signal at the MH1 domain and tend to translocate into the nucleus through interaction with importin β (31, 39). Under unstimulated conditions, however, R-Smads are retained in the cytoplasm through interaction with membrane-anchoring proteins, including SARA and Hgs (also termed Hrs) (28, 36). SARA is an FYVE domain protein which specifically interacts with Smad2 and Smad3 and facilitates their activation by TGF-β receptors. Hgs is also an FYVE domain protein and cooperates with SARA for the activation of Smad2 and Smad3. Smad2 and -3 have also been reported to bind to microtubules in the cytoplasm by binding to β-tubulin (7).
In addition to their interaction with SARA, Hgs, and β-tubulin, we show here that Smad2 and Smad3 interact and colocalize with Axin in the cytoplasm. Upon activation of TGF-β receptors, Smad3 bound to Axin is efficiently targeted to TGF-β type I receptor (TβR-I) and released from Axin. Since Axin facilitates phosphorylation and transcriptional activity of Smad3, it may function as an adapter of Smad3, enhancing the activation of Smad3 in the TGF-β signaling pathway.
MATERIALS AND METHODS
Plasmids.
cDNAs for Smad1 through -5, a Smad3 mutant (Smad3D407E), and various forms of TβR-I have been described (10, 16, 19). rAxin and Axil, rAxin mutants, and Dvl-1 have been previously reported (14, 42, 43). rAxin mutants were also prepared using a PCR-based method. β-Catenin and Tcf-4 were provided by Tetsuo Noda. SARA was obtained from J. Wrana. Adenovirus vectors containing Axin (Ad-Axin) and β-galactosidase (Ad-LcZ) have been previously described (34).
Cell culture and cDNA transfection.
COS7 cells, Mv1Lu mink lung epithelial cells, 293T cells, and HepG2 human hepatoblastoma cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. For transient transfection, 60 to 80% confluent cells were transfected using FuGENE6 transfection reagent (Roche Molecular Biochemicals).
Immunoprecipitation and immunoblotting.
COS7, HepG2, 293T, or Mv1Lu cells were transfected with expression constructs. Infection of Mv1Lu cells with adenoviruses was performed as described previously (34). Twenty-four to 48 h after transfection or infection, cells were solubilized in a buffer containing 20 mM Tris-HCI (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, 1% aprotinin, and 1 mM phenylmethylsulfonyl fluorides. The cell lysates were precipitated by centrifugation, and the supernatants were incubated with anti-FLAG M2 (Eastman Kodak Co.), anti-myc 9E10 (PharMingen), anti-Smad2 and -Smad3 (Transduction Laboratories), or anti-Smad3 (24) (gift of P. ten Dijke) antibodies for 2 h, followed by incubation with protein A- or G-Sepharose beads. The beads were washed four times with the buffer used for cell solubilization. The immune complexes were then eluted by boiling for 3 min in sodium dodecyl sulfate (SDS) sample buffer (100 mM Tris-HCl [pH 8.8], 0.01% bromophenol blue, 36% glycerol, 4% SDS, 10 mM dithiothreitol) and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Aliquots of the cell lysates were directly subjected to SDS-PAGE without immunoprecipitation. Proteins were electrotransferred to ProBlott membranes (Applied Biosystems), immunoblotted with the anti-FLAG M2, anti-myc 9E10, antihemagglutinin 3F10 (Boehringer Mannheim), anti-maltose-binding protein (anti-MBP) (New England BioLabs), anti-Smad2 and -Smad3, anti-phospho-Smad3 (24) (gift of P. ten Dijke), or anti-Axin (our unpublished data) antibodies; and detected using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech). For reblotting, the membranes were stripped according to the manufacturer's protocol.
Interaction of GST-Smad3 with Axin.
Direct interaction between Smad3 and Axin was determined in vitro as described previously (22). Glutathione S-transferase GST–Smad3 Smad3 (500 nM) (41) or GST alone was incubated with 500 nM MBP-Axin for 1 h at 4°C in 50 μl of reaction mixture (20 mM Tris-HCl, pH 7.5, and 1 mM dithiothreitol). GST-Smad3 or GST was precipitated by glutathione-Sepharose 4B, and the precipitates were subjected to SDS-PAGE, followed by immunoblotting with anti-MBP antibody. Aliquots (1:500,000) of MBP-Axin and MBP were directly subjected to SDS-PAGE as controls.
Immunofluorescence labeling.
Immunohistochemical staining of Smad3 and Axin in transfected HepG2 cells was performed using anti-Myc, anti-FLAG, anti-Axin, or anti-Smad3 antibodies followed by incubation with fluorophore-labeled goat anti-mouse or anti-rabbit immunoglobulin G (Alexa Fluor; Molecular Probes) as described previously (8). Intracellular localization was determined by confocal laser-scanning microscopy.
Luciferase assay.
Promoter-reporter constructs, i.e., p3TP-lux, pAR3-lux, and Xtwn-lux, were provided by J. Massagué, J. Wrana, and K. W. C. Cho, respectively. After transient transfection of DNAs (total, 2 μg) into Mv1Lu cells or HepG2 cells in six-well tissue culture plates, cells were incubated for 24 h in the presence and absence of TGF-β (10 pM), and luciferase activity in the cell lysates was determined using a luminometer. Luciferase activities were normalized to sea pansy luciferase activity under the control of the thymidine kinase promoter.
Northern blot analysis.
Total cellular RNA was extracted using Isogen (Nippongene) by following the manufacturer's protocol. Twenty micrograms of RNA was electrophoresed on 1% agarose-formaldehyde gels and transferred to nylon membrane's (Biodyne A; Pall BioSupport Co.). The membranes were hybridized at 42°C overnight with randomly primed DNA probes labeled with [α-32P]dCTP in a hybridization buffer containing 5× SSC (1× SSC is 0.5 M NaCl plus 0.015 M sodium citrate), 50% formamide, 1% SDS, 5× Denhardt's solution, and 0.2 mg of denatured salmon sperm DNA/ml. The membranes were washed to a final stringency of 0.1× SSC and 0.1% SDS at 65°C and were analyzed by autoradiography.
RESULTS
Physical interaction of Axin with Smad3.
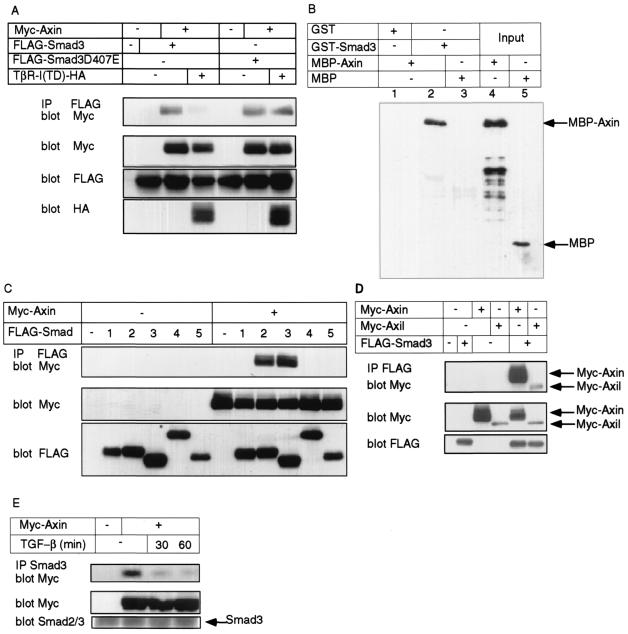
In order to examine cross talk between the signaling pathways of TGF-β and Wnt, we first examined the physical interaction between Smads and various molecules involved in the Wnt signaling pathway. In addition to the interaction between Smads and β-catenin (reference 30 and our unpublished data), we found that Axin coimmunoprecipitated with Smad3 in transfected COS7 cells (Fig. 1A). The interaction between Axin and Smad3 was, however, dramatically decreased in the presence of a constitutively active form of the TβR-I, TβR-I(TD), indicating that Smad3 is bound to Axin under unstimulated conditions and released from it upon receptor activation. This was further confirmed by the use of a Smad3 mutant, Smad3D407E, which binds to TβR-I(TD) but is neither phosphorylated by the receptor nor released from it (reference 10 and our unpublished data). Smad3D407E physically interacted with Axin as the wild-type Smad3, but this interaction was still observed even in the presence of TβR-I(TD) (Fig. 1A). In order to determine whether Axin directly binds to Smad3, a GST pull-down assay was performed using GST- and MBP-fused proteins. As shown in Fig. 1B, MBP-Axin, but not MBP alone, was found to directly bind to GST-Smad3 in vitro.
FIG. 1.
Physical interaction between Axin and Smad3. (A) Interaction of Axin with Smad3 and a Smad3 mutant, Smad3D407E. COS7 cells were transfected with the indicated plasmids. Interaction between Smad3 and Axin in the presence and absence of a constitutively active TβR-I was determined by immunoprecipitation (IP) of Smad3 by anti-FLAG antibody followed by immunoblotting using anti-Myc antibody. The top panel shows the interaction between Smad3 and Axin, and the lower three panels show the expression of each protein. HA, hemagglutinin. (B) Direct interaction of Smad3 with Axin. GST-Smad3 or control GST was mixed with MBP-Axin or MBP. The samples were then incubated with glutathione-Sepharose 4B, and the precipitates were subjected to SDS-PAGE, followed by immunoblotting using anti-MBP antibody (lanes 1 to 3). As a control, MBP-Axin or MBP alone was directly subjected to SDS-PAGE (lanes 4 and 5). (C) Interaction of Smad1 through Smad5 with Axin. Experiments were performed as described for panel A but only in the absence of TβR-I(TD). The top panel shows the interaction between Smads and Axin. (D) Interaction between Axil and Smad3. Interaction of Smad3 with Axin or Axil was determined as described for panel C. (E) Interaction of Axin with Smad3 in HepG2 cells transfected with Axin alone. HepG2 cells were transfected with or without Myc-Axin and stimulated with TGF-β (100 pM) for the indicated periods. Interaction between endogenous Smad3 and Myc-Axin was determined by IP by anti-Smad3 antibody followed by immunoblotting using anti-Myc antibody. The top panel shows the interaction between Smad3 and Axin.
We have also examined the interaction of other Smads, including Smad1 through -5, and an Axin homologue, Axil, in transfected COS7 cells. Similar to Smad3, Smad2 bound to Axin, but the other Smads failed to interact with Axin (Fig. 1C), indicating that interaction between Axin and the Smads occurs specifically for R-Smads involved in the TGF-β and activin signaling pathways. Axil has been shown to function very similarly to Axin, and no functional difference has been observed between these molecules (2, 42). Although the level of expression of Axil in the transfected COS7 cells was lower than that of Axin, Axil was found to interact with Smad3 (Fig. 1D), suggesting that they are functionally redundant in regulation of the TGF-β signaling pathway.
Since antibodies that efficiently recognize endogenous Axin were not available, we were not able to demonstrate interaction between Smad2 or Smad3 and Axin in nontransfected cells. We therefore used HepG2 cells transfected with only Myc-tagged Axin. As shown in Fig. 1E, immunoprecipitation of endogenous Smad3 by a specific Smad3 antibody resulted in coimmunoprecipitation of Myc-Axin. Moreover, Smad3 was dissociated from Axin by the addition of TGF-β.
Domains responsible for the interaction between Axin and Smad3.
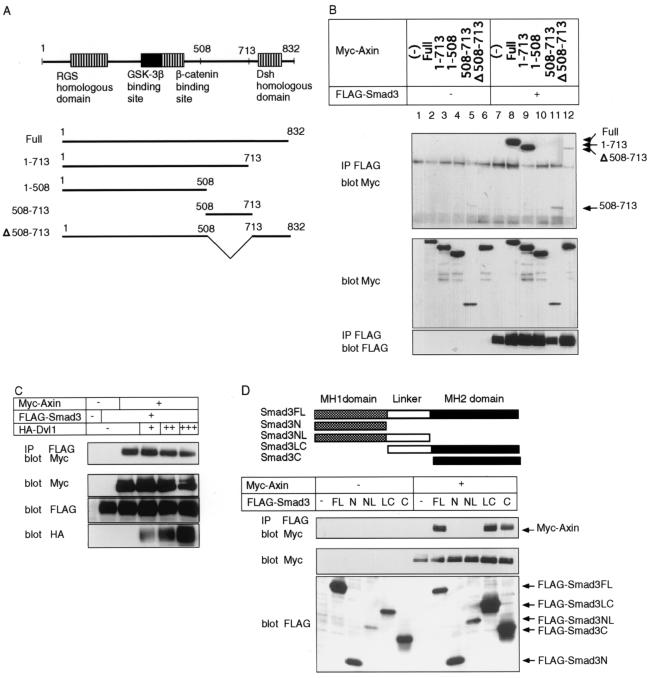
We next determined the domains responsible for the interaction between Axin and Smad3. APC has been reported to bind to the N-terminal RGS-homologous domain, and GSK-3β and β-catenin have been reported to bind to the central part of Axin (Fig. 2A) (2, 9, 14, 22). In contrast, the function of the C-terminal third of Axin has not been fully determined (20).
FIG. 2.
Domains responsible for the interaction between Axin and Smad3. (A) Structure of rAxin and deletion mutants of rAxin used in the present study. The numbers indicate amino acid numbers (14). Dsh, Dishevelled family. (B) Physical interaction between Smad3 and various rAxin mutants. COS7 cells were transfected with the indicated plasmids. Interaction between Smad3 and rAxin mutants in the absence of TβR-I(TD) was determined by immunoprecipitation (IP) of Smad3 by anti-FLAG antibody followed by immunoblotting using anti-Myc antibody. The top panel shows the interaction between Smad3 and rAxin mutants, and the lower two panels show the expression of each protein. (C) Dvl-1 does not affect the interaction between Smad3 and Axin. COS7 cells were transfected with the indicated plasmids. Interaction between Smad3 and Axin in the presence of increasing amounts of Dvl-1 was examined by IP and immunoblotting as described for panel B. The top panel shows the interaction between Smad3 and Axin. Note that the level of expression of Axin decreased in the presence of large amounts of Dvl-1. HA, hemagglutinin. (D) Interaction of Axin with plasmids containing different portions of Smad3. Interaction was determined as described for panel B. Smad3 plasmids used in the present study are shown in the upper panel. FL, full-length; N, N-terminal MH1 domain; NL, N-terminal MH1 domain and linker region; LC, linker region and C-terminal MH2 domain; C, C-terminal MH2 domain.
We prepared deletion mutants of rAxin, and examined their interaction with Smad3 in COS7 cells. As shown in Fig. 2B, plasmids encoding amino acids 1 to 713 of rAxin (14), i.e., rAxin(full) and rAxin(1–713), strongly interacted with Smad3. In contrast, rAxin(1–508) failed to do so, suggesting that the region between amino acids 509 and 713 of rAxin is responsible for the interaction with Smad3. Consistent with this finding, rAxin(508–713) bound to Smad3, although it was difficult to obtain a high level of protein expression of rAxin(508–713) in COS7 cells. However, rAxin(Δ508–713), which lacks this region, also weakly interacted with Smad3, suggesting that regions other than amino acids 508 to 713 of rAxin may also be able to bind to Smad3.
Although localization of the Dvl binding domain on Axin has varied among several reports, Dvl appears to bind to the C-terminal region of Axin and inhibit the Axin function to downregulate β-catenin (17, 23, 33). We examined whether Dvl-1 affects the binding between Smad3 and Axin. As shown in Fig. 2C, increasing amounts of Dvl-1 did not significantly affect the interaction between Smad3 and Axin, suggesting that Dvl-1 does not compete with Smad3 for binding to Axin.
The C-terminal MH2 domain of Smad3 is responsible for various functions of Smad3, including association with type I receptors, interaction with SARA, oligomer formation, and transcriptional activation (12, 29). The N-terminal MH1 domain has a nuclear localization signal (31, 39) and is responsible for direct binding to DNA. We examined Smad3 constructs containing different regions of Smad3 for interaction with Axin. A Smad3 construct containing the linker region and MH2 domain and a construct containing only the MH2 domain interacted with Axin, but not the constructs lacking the MH2 domain (Fig. 2D), indicating that Smad3 interacts with Axin through the C-terminal MH2 domain.
Axin is recruited to TβR-I.
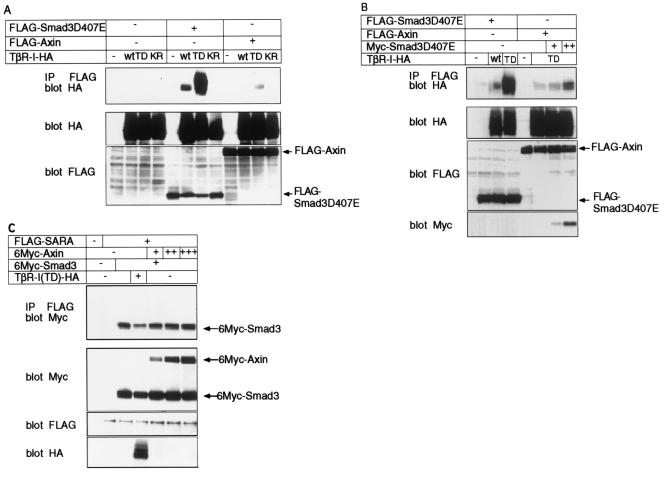
Since Axin interacts with Smad3 only in the absence of activated type I receptors, we next investigated whether Axin can interact with TβR-I. Axin interacted with TβR-I(TD) but interacted only very weakly with wild-type TβR-I and not at all with kinase-inactive TβR-I (Fig. 3A). The interaction between Axin and TβR-I(TD) was weaker than that between Smad3D407E and TβR-I(TD), suggesting that Axin may indirectly associate with TβR-I through Smad3 or that Axin may only transiently associate with TβR-I and immediately dissociate from it. We therefore tested the interaction between Axin and TβR-I in the presence of Smad3D407E, which stably binds to TβR-I(TD). As shown in Fig. 3B, the interaction between Axin and TβR-I(TD) was correlated with the amount of Smad3D407E. These results suggest that Axin interacts with TβR-I as a complex with Smad3 and that upon activation by TβR-I, Smad3 dissociates from TβR-I and Axin.
FIG. 3.
Recruitment of the Axin-Smad3 complex to TβR-I. (A) Interaction of Smad3D407E and Axin with TβR-I. COS7 cells were transfected with the indicated plasmids. Interaction of Smad3D407E or Axin with TβR-I was determined by immunoprecipitation (IP) of Smad3D407E or Axin by anti-FLAG antibody followed by immunoblotting of TβR-I using antihemagglutinin (anti-HA) antibody. The top panel shows the protein interaction, and the lower two panels show the expression of each protein. TβR-I plasmids used were as follows: wt, wild-type TβR-I; TD, TβR-I (TD); and KR, kinase-inactive form of TβR-I. (B) Interaction of Axin with TβR-I in the presence of increasing amounts of Samd3D407E. Interaction was determined as described for panel A. (C) Axin is not involved in the SARA-Smad3 complex. 293T cells were transfected with the indicated plasmids with increasing amounts of 6Myc-Axin. Interaction between SARA and Smad3 in the presence or absence of Axin was determined by IP of SARA by anti-FLAG antibody followed by immunoblotting of Smad3 and Axin using anti-Myc antibody. The top panel shows the interaction of SARA with Smad3 and Axin, and the lower panels show the expression of each protein.
Axin interacts with Smad3 in the absence of activated TβR-I. The mode of interaction of Axin with Smad3 is similar to that of SARA, which anchors Smad3 to the cell membrane. SARA has been shown to interact with Smad2 and Smad3 through the MH2 domain (36, 38). Moreover, both SARA and Axin are distributed in a punctate pattern in the cytoplasm (9, 36) (see Fig. 4A). We therefore examined whether Axin is involved in the interaction of Smad3 with SARA. Smad3 coimmunoprecipitated with SARA, the amount of which was decreased in the presence of TβR-I(TD) (Fig. 3C). Under these conditions, Axin neither was coimmunoprecipitated with SARA nor affected the interaction between SARA and Smad3. This finding suggests that SARA and Axin are independently located in cells.
FIG. 4.
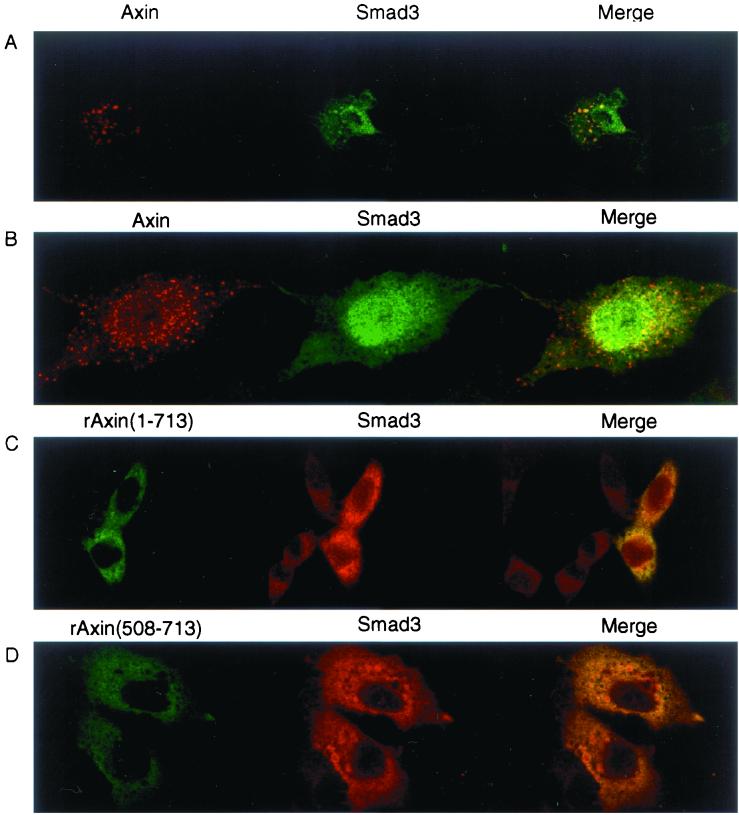
Subcellular localization of Smad3 and Axin. (A) Localization of Smad3 and Axin was examined by confocal microscopy. Anti-FLAG staining for FLAG-Smad3 (green) and anti-Axin antibody staining for Axin (red) followed by Alexa Fluor 488 and 568, respectively, were performed in transfected HepG2 cells. (B) Smad3 was dissociated from Axin upon receptor activation. Immunostaining was performed as described for panel A using transfected HepG2 cells in the presence of TβR-I(TD). (C and D) Localization of rAxin(1–713) (C) or rAxin(508–713) (D) and Smad3 was examined by anti-Myc staining for Axin and anti-Smad3 antibody staining for Smad3 as described for panel A.
Subcellular localization of Axin and Smad3.
We next investigated whether Axin and Smad3 are colocalized in cells in vivo. In various types of cells, Axin was shown to be present in a punctate pattern in the cytoplasm as well as in the plasma membrane (9, 21, 35). We transfected Axin and Smad3 into HepG2 cells and observed their subcellular localization by confocal microscopy. Smad3 tended to spontaneously translocate into the nucleus (40, 45); in the cytoplasm, it was observed as a diffuse pattern with some spots (Fig. 4A). Axin was observed in a punctate pattern as reported previously (9, 21, 35) and was colocalized with Smad3 (Fig. 4A, merge).
Smad3 was dissociated from Axin upon receptor activation when examined by immunoprecipitation and immunoblotting (Fig. 1A and E). In agreement with this observation, Smad3 was translocated into the nucleus in the presence of TβR-I(TD), and was no longer colocalized with Axin (Fig. 4B).
The N-terminal region of Axin is responsible for its characteristic localization in cytoplasmic spots. In addition, the C-terminal region of Axin also induces localization to cytoplasmic spots in Xenopus embryos (9). In HepG2 cells, rAxin(1–713), which lacks the C-terminal tail but has the ability to interact with Smad3, was observed in a diffuse pattern (Fig. 4C). When cotransfected with rAxin(1–713), Smad3 was also observed as a diffuse pattern and partly colocalized with rAxin(1–713). rAxin(508–713), which was able to bind Smad3 (Fig. 2B), exhibited a diffuse pattern with some granules in the cytoplasm and also partly colocalized with Smad3 (Fig. 4D). These results indicate that certain fractions of Smad3 are colocalized even with the deletion mutants of Axin in vivo.
Phosphorylation of Smad3 by TβR-I is facilitated in the presence of Axin.
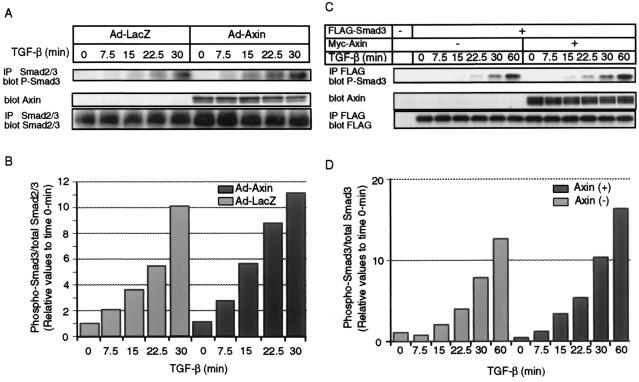
We inquired whether Axin modulates Smad3 activity in the TGF-β signaling pathway. Since infection by adenovirus vectors induces protein expression in more than 90% of cells (data not shown), we used Ad-Axin to infect Mv1Lu mink lung epithelial cells and determined the phosphorylation of endogenous Smad3 in Mv1Lu cells by using anti-phospho-Smad3 (24). Axin enhanced phosphorylation of Smad3 in the Axin-infected cells compared to the control cells, and this result was more prominent at 15 and 22.5 min after the addition of TGF-β than at later time periods (Fig. 5A and B). In order to further confirm the effect of Axin on Smad3 phosphorylation, Smad3 and Axin were cotransfected into Mv1Lu cells, and phosphorylation of transfected Smad3 was determined. As shown in Fig. 5C and D, phosphorylation of Smad3 was observed more strongly in the presence than in the absence of Axin.
FIG. 5.
Phosphorylation of Smad3 in the presence of Axin. (A and B) Phosphorylation of endogenous Smad3 in Mv1Lu cells was determined in the presence and absence of Axin. Mv1Lu cells were infected with control adenovirus (Ad-LacZ) or Ad-Axin at a multiplicity of infection of 100 and treated with TGF-β (10 pM) for the indicated periods. Cell lysates were then immunoprecipitated (IP) by an anti-Smad2 and -Smad3 antibody, followed by immunoblotting using an anti-phospho-Smad3 antibody (P-Smad3). Expression of Axin and Smad2 and -3 is shown in the lower panels. In the panel demonstrating the expression of Smad2 and -3, the Smad3 bands were not well separated from the Smad2 bands. Intensities of the immunoblotted bands of phospho-Smad3 were therefore quantified compared to the Smad2 and -3 bands, and the values were plotted relative to the 0-min values. (C and D) Phosphorylation of transfected Smad3 by Axin. Mv1Lu cells were transiently transfected with the indicated plasmids and stimulated by TGF-β (10 pM) for the indicated periods. Phosphorylation of Smad3 was examined by FLAG IP followed by anti-phospho-Smad3 immunoblotting. Intensities of the immunoblotted bands of phospho-Smad3 were quantified compared to the Smad3 bands (FLAG IP followed by FLAG immunoblotting), and the values were plotted relative to the 0-min values.
Enhancement of transcriptional activation activity of TGF-β by Axin.
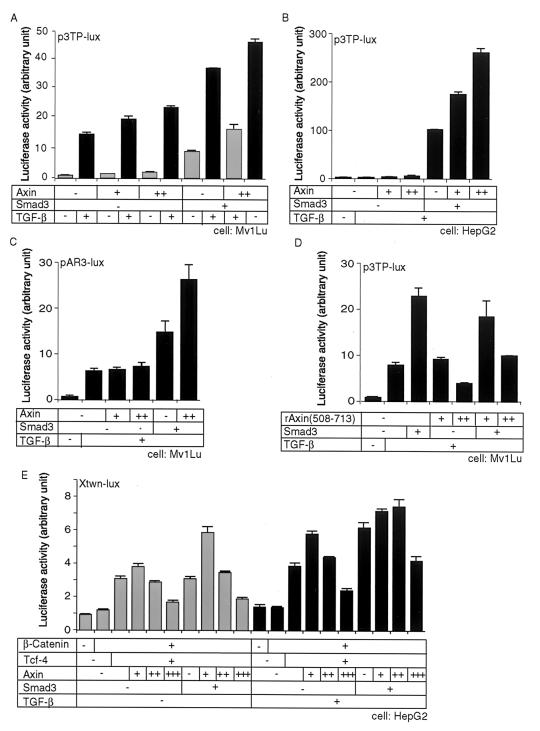
We next examined modulation of the transcriptional activity of TGF-β by Axin using two different promoter-reporter constructs: p3TP-lux, which contains AP-1 binding sequences and a TGF-β-responsive element of PAI-1 (4), and pAR3-lux, which contains an activin-TGF-β-responsive element of the Mix.2 promoter (11). TGF-β induced transcriptional activation of p3TP-lux in both Mv1Lu cells and HepG2 cells (Fig. 6A and B). Smad3 facilitated transcriptional activation by TGF-β, which was further enhanced in the presence of Axin. Similar results were obtained using pAR3-lux in the presence of the transcription factor FoxH3 (originally termed FAST1) (18) (Fig. 6C), although the effect of Axin on pAR3-lux was not significant in the absence of Smad3.
FIG. 6.
Enhancement of TGF-β activity by Axin. (A and B) The effects of Axin on the transcriptional activation of p3TP-lux were determined in the presence of the indicated plasmids with or without TGF-β stimulation (10 pM) in Mv1Lu cells (A) or HepG2 cells (B). For transfection of Axin, + and ++ represent 0.1 and 0.3 μg of rAxin DNA, respectively. Smad3 DNA was used at 0.3 μg. (C) The effect of Axin on pAR3-lux was examined with or without TGF-β (10 pM) in Mv1Lu cells. All cells were transfected with 0.4 μg of FoxH3 DNA. (D) Modulation of transcriptional activation of p3TP-lux by rAxin (508–713) was examined with or without TGF-β (10 pM) in Mv1Lu cells. For transfection of rAxin (508–713), + and ++ represent 0.3 and 1.0 μg of DNAs, respectively, transfected into cells. (E) Effects of Axin on Xtwn promoter activation in the presence of TGF-β and Wnt signaling. HepG2 cells were transfected with the indicated plasmids with or without TGF-β (10 pM). For transfection of the Axin DNA, +, ++, and +++ represent 0.1, 0.3, and 1.0 μg of DNA, respectively. Amounts of other DNAs were as follows: Smad3, 0.3 μg; Tcf-4, 0.01 μg; and β-catenin, 0.5 μg.
rAxin(508–713) was able to bind Smad3 (Fig. 2B) but did not exhibit the characteristic punctate pattern of wild-type Axin (Fig. 4D). rAxin(508–713) did not induce transcriptional activation of p3TP-lux (Fig. 6D); instead, it inhibited the transcriptional activity by TGF-β in both the presence and the absence of Smad3, indicating that rAxin(508–713) exhibits a dominant-negative effect on the transcriptional activity induced by TGF-β.
The TGF-β and Wnt signaling pathways cooperate in transcription from the Xenopus twin (Xtwn) gene promoter, but not that from the Topflash promoter, through direct interaction between Smad3 and Lef1 and their binding to the Xtwn promoter (25). Since Axin regulates TGF-β and Wnt signaling in positive and negative fashions, respectively, it is important to examine whether Axin can facilitate the transcription of Xtwn-lux induced by TGF-β signaling in the presence of Wnt signaling. Transcription of Xtwn-lux was induced by β-catenin and Tcf-4, which was inhibited by large amounts of Axin (Fig. 6E). In agreement with the previous report (25), transcription from the Xtwn promoter was enhanced by TGF-β and Smad3. Small amounts of Axin facilitated transcriptional activation of Xtwn-lux in the presence of TGF-β and/or Smad3. When highly expressed, Axin repressed the transcription of Xtwn-lux even in the presence of TGF-β and/or Smad3. These findings thus suggest bimodal modulation of transcription from the Xtwn promoter activity by Axin in the presence of TGF-β and Wnt signals.
Induction of PAI-1 mRNA by TGF-β in the presence of Axin.
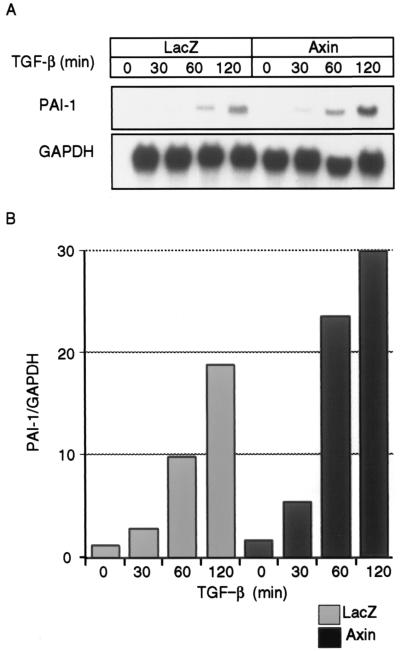
In order to further study the functional role of Axin in the TGF-β signaling pathway, we examined the induction of PAI-1 mRNA by TGF-β in Mv1Lu cells in the absence and presence of Axin. Ad-Axin or control adenovirus (Ad-LacZ) was used to infect Mv1Lu cells, and the expression of PAI-1 mRNA was analyzed by Northern blotting. As shown in Fig. 7, induction of PAI-1 mRNA by TGF-β was facilitated in the Axin-infected cells, compared to induction in the control cells.
FIG. 7.
Enhancement of PAI-1 mRNA induction by TGF-β in the presence of Axin. Mv1Lu cells were infected with control adenovirus (Ad-LacZ) or Ad-Axin as described in the Fig. 5 legend and treated with TGF-β (10 pM) for the indicated periods. Northern blot analysis for PAI-1 was performed. Relative levels of PAI-1 expression were determined by densitometry and normalized to the levels of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control. The values were plotted relative to the 0-min values.
DISCUSSION
Smads play central roles in TGF-β and BMP signal transduction. R-Smads are activated by the serine/threonine kinase receptors, form complexes with Co-Smads, and translocate into the nucleus, where they regulate transcription of target genes (12). I-Smads inhibit the activation of R-Smads by interfering with the activation of R-Smads by their receptors and by preventing complex formation between R-Smads and Co-Smads. Various adapter proteins may regulate the effects of Smads for efficient signaling. SARA has an FYVE domain that interacts with phosphatidylinositol 3-phosphate and anchors SARA to the cell membrane. SARA physically interacts with Smad2 and Smad3 and controls the subcellular localization of Smad2 and -3 for efficient activation by TGF-β receptors (36, 38). Hgs is also an FYVE domain-containing protein, and together with SARA, it facilitates the activation of Smad2 and Smad3 by TGF-β and activin receptors (28). β-Tubulin has been reported to interact with Smad2, -3, and -4 (7). Interaction between β-tubulin and Smads could be observed in the absence of activated receptor and was dissociated upon receptor activation. These proteins may function as adapters for R-Smads, which retain R-Smads in the cytoplasm and target them to activated receptors. In contrast to these molecules interacting with R-Smads, STRAP associates with an I-Smad, Smad7, and stabilizes its interaction with the activated receptor for inhibition of TGF-β signaling (5).
Our results revealed that Axin directly interacts with Smad3 in the absence of receptor activation and facilitates the activation of Smad3 by TGF-β receptors. Thus, the effects of Axin are similar to those of SARA. Subcellular localization studies of Axin revealed that it is present in the cytoplasm in a punctate pattern similar to that of SARA and colocalizes with Smad3. However, Axin did not affect the interaction of Smad3 with SARA, nor did it coimmunoprecipitate with SARA. Thus, Axin and SARA may be independently located in the cytoplasm.
The Axin-Smad3 complex was also able to associate with activated TβR-I, as detected using a Smad3 mutant, Smad3D407E. Axin may thus support the interaction between Smad3 and TβR-I, but after its phosphorylation, Smad3 dissociates from TβR-I as well as from Axin. In agreement with this finding, we found that Smad3 was strongly phosphorylated in the presence of Axin. The released Smad3 then forms a complex with Co-Smad and translocates into the nucleus, where it participates in transcriptional regulation. Consistent with this hypothesis, Axin enhanced the transcriptional activation activity of TGF-β and facilitated the PAI-I mRNA expression induced by TGF-β.
The TGF-β and Wnt/Wingless pathways play pivotal roles in tissue specification and morphogenesis during development. Signaling cross talk between the TGF-β pathway and Wnt pathway through transcription factors Tcf/Lef1 and Smad3 has been reported to occur in regulation of the transcriptional activation of the Xtwn gene (25). Facilitation of Wnt signaling has also been shown to occur through the interaction of Smad4 with β-catenin and Tcf/Lef1, independent of TGF-β receptor activation (30). We have shown here that Axin, a negative regulator involved in the Wnt signaling pathway, also participates in the regulation of TGF-β signaling.
APC, GSK-3β, and β-catenin interact with Axin through the N-terminal and central portions of Axin (9, 20). In contrast, protein phosphatase 2A and Dvl interact with Axin through the C-terminal part of Axin (9, 13, 15, 23), although the functional importance of this region has not been fully determined. Interaction between Axin and Smad3 through the C-terminal part of Axin was observed. We also showed that Dvl-1 does not compete with Smad3 for binding to Axin. The present findings thus suggest that Axin has dual functions in signal transduction: it acts as a negative regulator of the Wnt signaling pathway and as a positive regulator of the TGF-β signaling pathway.
Mutations of the Axin gene have been found in certain hepatocellular carcinomas (34). Our preliminary results revealed that cells lacking wild-type Axin still respond to TGF-β (our unpublished data), suggesting that the loss of Axin may be compensated for by other Smad-binding proteins, including SARA and Hgs, or the Axin homologue Axil. Further study is required to elucidate the functional roles of Axin and Axil in TGF-β signaling in vivo.
ACKNOWLEDGMENTS
We are grateful to Y. Sasaki for technical help and Tetsuo Noda for discussion.
This study was supported by Grants-in-Aid for Scientific Research and Special Coordination Funds for Promoting Science and Technology of the Ministry of Education, Culture, Sport, Science, and Technology of Japan and by Research for the Future Program, the Japan Society for the Promotion of Science.
REFERENCES
- 1.Akiyama T. Wnt/β-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–282. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 2.Behrens J, Jerchow B A, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 3.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 4.Cárcamo J, Zentella A, Massagué J. Disruption of transforming growth factor β signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol Cell Biol. 1995;15:1573–1581. doi: 10.1128/mcb.15.3.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta P K, Moses H L. STRAP and Smad7 synergize in the inhibition of transforming growth factor β signaling. Mol Cell Biol. 2000;20:3157–3167. doi: 10.1128/mcb.20.9.3157-3167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derynck R, Zhang Y, Feng X-H. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 7.Dong C, Li Z, Alvarez R, Jr, Feng X-H, Goldschmidt-Clermont P J. Microtubule binding to Smads may regulate TGFβ activity. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- 8.Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath T K, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- 9.Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of Axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto D, Yagi K, Inoue H, Iwamoto I, Kawabata M, Miyazono K, Kato M. A single missense mutant of Smad3 inhibits activation of both Smad2 and Smad3, and has a dominant negative effect on TGF-β signals. FEBS Lett. 1998;430:201–204. doi: 10.1016/s0014-5793(98)00658-9. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y Y, Grinnell B W, Richardson M A, Topper J N, Gimbrone M A, Jr, Wrana J L, Falb D. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 12.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 13.Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3β-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by β-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 16.Ishida W, Hamamoto K, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath T K, Kato M, Miyazono K. Smad6 is a Smad1/5-induced Smad inhibitor: characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275:6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- 17.Itoh K, Antipova A, Ratcliffe M J, Sokol S. Interaction of Dishevelled and Xenopus Axin-related protein is required for Wnt signal transduction. Mol Cell Biol. 2000;20:2228–2238. doi: 10.1128/mcb.20.6.2228-2238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaestner K H, Knochel W, Martinez D E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 19.Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikuchi A. Modulation of Wnt signaling by Axin and Axil. Cytokine Growth Factor Rev. 1999;10:255–265. doi: 10.1016/s1359-6101(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 21.Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, Takada R, Takada S, Kikuchi A. Axin prevents Wnt-3a-induced accumulation of β-catenin. Oncogene. 1999;18:979–985. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- 22.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 23.Kishida S, Yamamoto H, Hino S-I, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korchynskyi O, Landstrom M, Stoika R, Funa K, Heldin C-H, ten Dijke P, Souchelnytskyi S. Expression of Smad proteins in human colorectal cancer. Int J Cancer. 1999;82:197–202. doi: 10.1002/(sici)1097-0215(19990719)82:2<197::aid-ijc8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Labbé E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and Wnt pathways. Proc Natl Acad Sci USA. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massagué J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 27.Miller J R, Hocking A M, Brown J D, Moon R T. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 28.Miura S, Takeshita T, Asao H, Kimura Y, Murata K, Sasaki Y, Hanai J-I, Beppu H, Tsukazaki T, Wrana J L, Miyazono K, Sugamura K. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol. 2000;20:9346–9355. doi: 10.1128/mcb.20.24.9346-9355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazono K, ten Dijke P, Heldin C-H. TGF-β signaling by Smad proteins. Adv Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- 30.Nishita M, Hashimoto M K, Ogata S, Laurent M N, Ueno N, Shibuya H, Cho K W Y. Interaction between Wnt and TGF-β signalling pathways during formation of Spemann's organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- 31.Pierreux C E, Nicolás F J, Hill C S. Transforming growth factor β-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol Cell Biol. 2000;20:9041–9054. doi: 10.1128/mcb.20.23.9041-9054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 33.Salic A, Lee E, Mayer L, Kirschner M W. Control of β-catenin stability: reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 34.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 35.Smalley M J, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer L G, Hutchinson L, Fry M J, Dale T C. Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 1999;18:2823–2835. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukazaki T, Chiang T A, Davison A F, Attisano L, Wrana J L. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 37.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Chen Y G, Ozdamar B, Gyuricza C A, Chong P A, Wrana J L, Massagué J, Shi Y. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287:92–97. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- 39.Xiao Z, Liu X, Lodish H F. Importin β mediates nuclear translocation of Smad 3. J Biol Chem. 2000;275:23425–23428. doi: 10.1074/jbc.C000345200. [DOI] [PubMed] [Google Scholar]
- 40.Xu L, Chen Y-G, Massagué J. The nuclear import function of Smad2 is masked by SARA and unmasked by TGFβ-dependent phosphorylation. Nat Cell Biol. 2000;2:559–562. doi: 10.1038/35019649. [DOI] [PubMed] [Google Scholar]
- 41.Yagi K, Goto D, Hamamoto T, Takenoshita S, Kato M, Miyazono K. Alternatively-spliced variant of Smad2 lacking exon 3: comparison with wild-type Smad2 and Smad3. J Biol Chem. 1999;274:703–709. doi: 10.1074/jbc.274.2.703. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol. 1998;18:2867–2875. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 44.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry III W L, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Musci T, Derynck R. The tumor suppressor Smad4/DPC4 as a central mediator of Smad function. Curr Biol. 1997;7:270–276. doi: 10.1016/s0960-9822(06)00123-0. [DOI] [PubMed] [Google Scholar]