Abstract
Post-Covid pulmonary fibrosis is evident following severe COVID-19. There is an urgent need to identify the cellular and pathophysiological characteristics of chronic lung squeals of Covid-19 for the development of future preventive and/or therapeutic interventions. Tissue-resident memory T (TRM) cells can mediate local immune protection against infections and cancer. Less beneficially, lung TRM cells cause chronic airway inflammation and fibrosis by stimulating pathologic inflammation. The effects of Janus kinase (JAK), an inducer pathway of cytokine storm, inhibition on acute Covid-19 cases have been previously evaluated. Here, we propose that Tofacitinib by targeting the CD8+ TRM cells could be a potential candidate for the treatment of chronic lung diseases induced by acute SARS-CoV-2 infection.
Keywords: Pulmonary fibrosis, Coronavirus, SARS-CoV-2, JAK inhibitors, Severe lung injury
Graphical Abstract
1. Introduction
A considerable number of COVID-19 patients following their recovery from the acute phase, continue the symptoms for at least more than 12 weeks from the start of the disease and illness is not explained by an alternative diagnosis [1]. However, the underlying mechanisms and possible therapeutic options for this condition have remained unknown [2]. Tissue-specific memory responses have recently been identified in the respiratory tract following SARS-CoV-2 infection. These over-activated tissue-resided CD8+ T cell responses could propagate chronic lung damage and functional impairment following acute COVID-19 [3].
In Covid-19, pulmonary involvement is pathologically characterized by microvascular thrombosis and diffuse alveolar damage (DAD), resulting in the clinical picture of ARDS (Acute Respiratory Distress Syndrome). Direct damage of SARS-CoV-2 combined with deregulated immuno-coagulative pathways affects the pulmonary system in severe Covid-19 that may be modified by individual factors and disease duration [4]. Another important and deleterious effect of COVID-19 is the possibility of post-infection pulmonary fibrosis [5], [6], [7] and anti-fibrotic agents have been suggested to diminish pulmonary fibrotic remodeling in these cases [8], [9]. The lung tissue autopsy results of COVID-19 patients have been compatible with acute DAD, alongside vascular pathology, and fibroproliferative activities. Such patterns can be impacted by age and weight [4]. Therefore, there is an imperative need to recognize the pathophysiological and cellular features of chronic lung squeals of COVID-19 and the development of future preventive and/or therapeutic interventions in these areas [3].
2. Tissue-resident memory T cells in SARS-COV-2 infection
Tissue-resident memory T (TRM) cells are a subgroup of memory T cells that reside in peripheral tissues and independent of circulating memory T cells, they are responsible for immediate and intense response, secreting rapidly antiviral cytokines such as interferon-gamma (IFN-γ) [10]. The TRM cells can facilitate local immune protection against cancer and infections by their rapid and direct mechanisms of action. Localized TRM can provide rapid and robust local protection against viruses, particularly in the lungs. Meanwhile, by stimulating pathologic local inflammation, they induce chronic airway inflammation and fibrosis [11]. They have been involved in the pathogenesis of autoimmune diseases such as inflammatory bowel disease [12], type 1 diabetes mellitus [13], and multiple sclerosis [14]. Additionally, TRM cells are participated in mediating autoimmune skin diseases such as vitiligo, alopecia areata, and psoriasis [15]. Due to their tissue-injurious capability, dysregulated and/or prolonged activities of CD8+ TRM cells can significantly contribute to the development of lung damage/pathology following respiratory viral infection [16].
Tissue-specific memory responses have been discovered following coronavirus infection in the respiratory tract during which tissue CD8+ T cells exert a local protective function [16]. The deposition of parenchymal CD8+ TRM and airway CD4+ TRM cells in animal models could protect against SARS-CoV-1 infection [17], [18]. Moreover, activated profiles of lung CD8+ and CD4+ TRM cells are reported in COVID-19 patients and these cells are frequently detected even ten months after infection in these patients [19], [20]. Recovered patients acquire TRM cells with Th1 phenotype against COVID-19 [20]. Even though less is known about specific-TRM in SARS-CoV-2 infection, data suggest that respiratory tract TRM might play a protective role against COVID-19. However, in a recent study, a sub-lethal mouse model of COVID-19 was used to assess whether the infection-accelerated pulmonary resident CD4+ and CD8+ T cell responses could generate protection against a secondary viral attack or not. It was shown that although resident T cells are elicited by COVID-19 infection, they do not present sufficient protection against secondary viral infection [17].
It has been reported that CD103− TRM cells present pathogenic potential in the influenza virus infection model. Persistent T cell receptor (TCR) signaling in the lung of these animals could drive the preservation of CD103− CD69+ T cells [11]. Consistent with this result, it is found that lung CD103− CD69+ T cells of older patients with COVID-19 produce elevated levels of inflammatory molecules that are associated with worse lung pathology and reduced lung function [3]. The transcript profiling of CD103− T cells is related to the TCR signaling pathway, signifying that these cells could be sustained by antigenic or tonic signaling within the damaged lung [3]. It is logical to speculate that after clearance of infectious virus, SARS-CoV-2 antigen might be persistent for a time; thus, stimulating and/or sustaining CD103− TRM cells to cause tissue damage. On the other hand, in those with autoantibodies against type I IFNs that are at higher risk of COVID-19 development, self-antigen may stimulate a portion of CD103− CD69+ T cells [18]. Moreover, CD103− CD69+ T cells in the circulation that have potential lung homing [19] may participate in the pathology of the lung during acute and chronic viral-induced lung injury. It has been found that respiratory CD103- CD69+ T cells exacerbate lung pathology and reduce lung function [3].
It has been also proposed that the expanded TRM17 cells (TRM-like Th17 cells) even continue after the lung's viral clearance and these TRM17 cells could further interact with cytotoxic CD8+ T cells and local macrophages; consequently, they exacerbate the lung injuries and disease severity [20]. The TRM17 cells and their attributed cytokines including GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL17A are the potential orchestrators of the extreme inflammation in severe COVID-19 infection [20]. Following the resolution of acute COVID-19 infection, deregulated pulmonary immune responses, mainly exuberant respiratory CD8+ T cells’ responses, can contribute to the progress of chronic lung injury.
The CD4+ T cells promote T-dependent B cells activation and antibody production, while cytotoxic CD8+ T cells can destroy virus-infected cells [21]. The pulmonary interstitial CD8+ T cells, encompass the majority of infiltrated inflammatory cells that are crucial for virus clearance. In rhesus macaques, low titers of antibody is adequate for protection against SARS-CoV-2 and CD8+ T cells play a role in restricting viral replication when the antibody titers are suboptimal [22]. Depletion of CD4+ T cell is connected with the attenuation of the neutralizing antibody production, recruitment of the lymphocytes to the lung tissue, and cytokine release, which subsequently, postpones the clearance of the virus [21]. However, CD8+ depletion does not influence viral replication. CD8 depletion experiments have proposed its critical role to counteract the rechallenges of SARS-COV-2 infection, indicating the possible restricting role of CD8+ T cells on viral replication [22]. These results indicate the role of CD+8 T cells in virological control which also needs further studies to yield the proof-of-concept of the current findings. It should be noted that the proliferation, differentiation, and survival of CD4+ T and CD8+ T cells are regulated through STAT3 [23].
3. JAK inhibitors
The JAK (Janus kinase)/STAT (signal transducers and activators of transcription) are members of a cascade involved in the COVID-19-related cytokine storm, which is associated with the cytokine receptor signaling in the classical pathway [24]. Cytokine receptor stimulation results in the activation of the SH2 domain of the STAT proteins [25]. Consequently, STAT proteins are dimerized via the JAK-induced phosphorylation leading to their translocation into the nucleus and further stimulation of diverse pathways including immune regulation, apoptosis, cell cycle, and transcription of distinct genes. Importantly, the function of the immune system is extensively affected by the JAK/STAT signaling and the differentiation of Th1, Th2, Th9, and Th17 cells is mediated by JAK/STAT signaling pathway [25].
Several therapeutic strategies have been developed to target the TRM cells. The IFN-γ- producing Th1 cells in the skin initiate the cytotoxic CD8+ T cells infiltration to the perifollicular area, resulting in hair cycle arrest and the development of alopecia areata (AA) [26]. The JAK signaling pathway inhibitors that inhibit type I/II cytokine receptors have the potential efficiency in the treatment of AA [27]. Nezulcitinib, tofacitinib, ruxolitinib, and baricitinib can prevent hyperinflammatory state by modulating cytokine production. These JAK inhibitors have been applied effectively in the clinical treatment of inflammatory (e.g. hemophagocytic lymphohistiocytosis) and rheumatologic (e.g. rheumatoid arthritis) diseases. Tofacitinib is the FD-approved oral JAK2/1/3 inhibitor, declining the release of Th1 and Th17 cytokines and IL-6 that are involved in the pathogenesis of the ARDS.
4. JAK inhibitors reduce the risk of mortality in patients with acute COVID-19
The growth of viral selective CD8+ T cells has been reported to be in close association with the components of the JAK/STAT [28]. SARS-COV-2 infection-related hyper-inflammatory conditions can lead to ARDS and death following cytokine storm [29]. Therefore, the inhibition of the JAK/STAT pathway might prevent the formation of the cytokine storm. However, it is at the expense of a compromised immune response [30]. Recent studies have highlighted the promising role of the JAK/STAT pathway as a valuable marker of a robust immune response to COVID-19 infection [31]. In another interesting study, it was demonstrated that JAK/STAT pathway inhibition plummets the hyperinflammatory status but does not affect the viral clearance [32].
Mounting evidence demonstrated that the overactivation of the JAK/STAT pathway is associated with aggravation of the clinical course of SARS-COV-2 infection [33]. Thus, judicious use of JAK inhibitors in the treatment of severe COVID-19 could be useful as the effect of JAK inhibition has previously been evaluated on acute COVID-19 cases [34], [35]. A meta-analysis on 1190 patients with COVID-19 indicated that the inhibitors of the JAK signaling are meaningfully associated with clinical improvement and a decreased risk of mortality in hospitalized patients [36]. A pooled data from four RCTs (1338 subjects) with COVID-19 infection demonstrated that treatment with JAK inhibitor could decrease the risk for mechanical ventilation and COVID-19 death compared to controls [37].
The effects of JAK inhibition in the treatment of COVID-19 were also evaluated in a combination with remdesivir [38]. Inhibitors of the JAK signaling pathway are promising in the treatment of hospitalized COVID-19 patients by diminishing the risk of respiratory failure or mortality compared to placebo [38]. Phase II trials are currently undergoing for evaluating the efficacy and safety of tofacitinib on COVID-19 patients (NCT04415151 and NCT04469114). Overall, JAK inhibitors are safe drugs in the treatment of acute COVID-19 and result in an improved clinical outcome of hospitalized cases [39].
5. Tofacitinib can be a potential therapy against post- COVID-19 lung injury
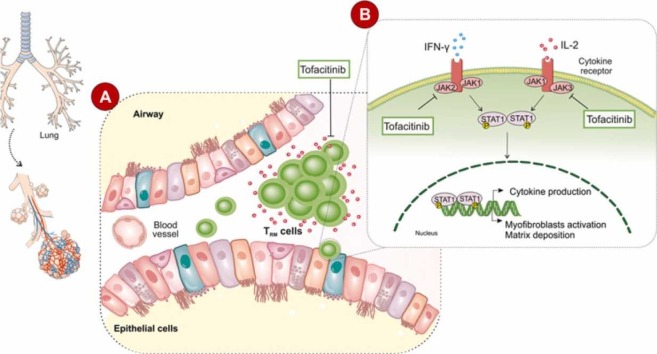
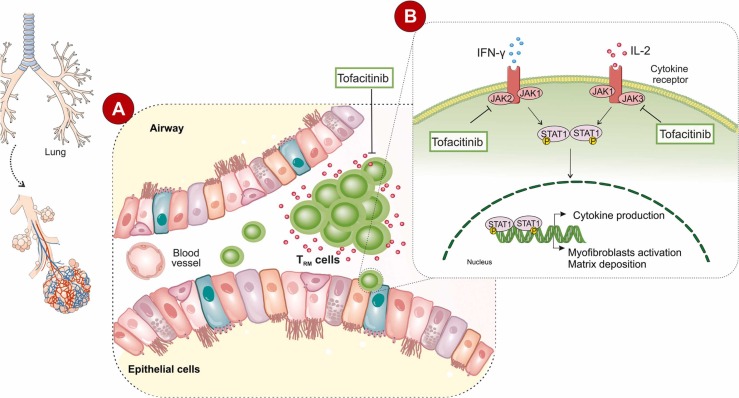
Tofacitinib as a pan JAK inhibitor mainly blocks JAK1 and JAK3 and to a lesser extent the JAK2 and tyrosine kinase 2 (TYK2). Tofacitinib is supposed to be a promising therapeutic agent against COVID-19 lung damage as it inhibits different inflammatory pathways [40]. Although side effects such as abdominal pain, acne vulgaris, diarrhea, nausea, vomiting, headache, and hepatotoxicity, have been reported, Pfizer mainly envisioned how safe and effective Tofacitinib was in COVID-19 patients [41]. Compared with the more general aforementioned side effects of Tofacitinib, the use of other JKA inhibitors such as bariticitinib has been associated with severe complications such as thrombotic events [42]. Therefore, based on the proposed pathophysiological characteristics of dysregulated and/or prolonged activities of CD8+ T cell that is contributed significantly to lung injury following acute COVID-19, there is a need to develop therapeutic interventions for these dysregulated respiratory CD8+ T cell responses. We present the hypothesis that Tofacitinib through targeting the tissue memory CD8+ T cells may be an effective therapy against chronic lung diseases promoted by acute SARS-CoV-2 infection ( Fig. 1). We believe that it is worth conducting clinical trials for evaluating the effects of Tofacitinib administration on patients with post-COVID-19 lung injury. Moreover, the use of Tofacitinib in the treatment of COVID-19 shows extra advantages including its effect on macrophages. SARS-COV-2 infected macrophages release elevated amounts of IL-6, as one of the most important markers of infection connected with JAK/STAT activation [43], [44]. It has been shown that the cellular characteristics of bronchoalveolar lavage fluid from severely ill patients are mainly composed of macrophages [45]. Meanwhile, Tofacitinib not only acts against CD8+ cells but also could be considered as an anti-IL-6R antibody therapy [46].
Fig. 1.
Tofacitinib can be used for post-COVID-19 lung injury by targeting tissue-resident memory T cells.
CRediT authorship contribution statement
MRA developed the idea and SZV prepared the manuscript, SMHK depicted the figure. All the authors read and approved submitted version.
Conflict of interest
There is no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the Kidney Research Center of Tabriz University of Medical Sciences, Tabriz, Iran.
Financial support
No financial support was provided.
References
- 1.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheon I.S., Li C., Son Y.M., Goplen N.P., Wu Y., Cassmann T., Wang Z., Wei X., Tang J., Li Y., Marlow H., Hughes S., Hammel L., Cox T.M., Goddery E., Ayasoufi K., Weiskopf D., Boonyaratanakornkit J., Dong H., Li H., Chakraborty R., Johnson A.J., Edell E., Taylor J.J., Kaplan M.H., Sette A., Bartholmai B.J., Kern R., Vassallo R., Sun J. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci. Immunol. 2021;6(65):eabk1741. doi: 10.1126/sciimmunol.abk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauad T., Duarte-Neto A.N., da Silva L.F.F., de Oliveira E.P., de Brito J.M., do Nascimento E.C.T., de Almeida Monteiro R.A., Ferreira J.C., de Carvalho C.R.R., do Nascimento Saldiva P.H., Dolhnikoff M. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir. Res. 2021;22(1):32. doi: 10.1186/s12931-021-01628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barisione E., Grillo F., Ball L., Bianchi R., Grosso M., Morbini P., Pelosi P., Patroniti N.A., De Lucia A., Orengo G., Gratarola A., Verda M., Cittadini G., Mastracci L., Fiocca R. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2021;478(3):471–485. doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W., Wu Q., Chen Z., Xiong Z., Wang K., Tian J., Zhang S. The potential indicators for pulmonary fibrosis in survivors of severe COVID-19. J. Infect. 2021;82(2):e5–e7. doi: 10.1016/j.jinf.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng L., Khan A., Zhou W., Dai Y., Md E., Chen R., Cheng G. Follow-up study of clinical and chest CT scans in confirmed COVID-19 patients. Radiol. Infect. Dis. 2020;7(3):106–113. doi: 10.1016/j.jrid.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Wu J., Wang S., Li X., Zhou J., Huang B., Luo D., Cao Q., Chen Y., Chen S., Ma L., Peng L., Pan H., Travis W.D., Nie X. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2021;78(4):542–555. doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitiello A., Pelliccia C., Ferrara F. COVID-19 patients with pulmonary fibrotic tissue: clinical pharmacological rational of antifibrotic therapy. SN Compr. Clin. Med. 2020:1–4. doi: 10.1007/s42399-020-00487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin H. Formation and function of tissue-resident memory T cells during viral infection. Curr. Opin. Virol. 2018;28:61–67. doi: 10.1016/j.coviro.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z., Wang S., Goplen N.P., Li C., Cheon I.S., Dai Q., Huang S., Shan J., Ma C., Ye Z., Xiang M., Limper A.H., Porquera E.C., Kohlmeier J.E., Kaplan M.H., Zhang N., Johnson A.J., Vassallo R., Sun J. PD-1(hi) CD8(+) resident memory T cells balance immunity and fibrotic sequelae. Sci. Immunol. 2019;4(36) doi: 10.1126/sciimmunol.aaw1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zundler S., Becker E., Spocinska M., Slawik M., Parga-Vidal L., Stark R., Wiendl M., Atreya R., Rath T., Leppkes M., Hildner K., López-Posadas R., Lukassen S., Ekici A.B., Neufert C., Atreya I., van Gisbergen K., Neurath M.F. Hobit- and Blimp-1-driven CD4(+) tissue-resident memory T cells control chronic intestinal inflammation. Nat. Immunol. 2019;20(3):288–300. doi: 10.1038/s41590-018-0298-5. [DOI] [PubMed] [Google Scholar]
- 13.Kuric E., Seiron P., Krogvold L., Edwin B., Buanes T., Hanssen K.F., Skog O., Dahl-Jørgensen K., Korsgren O. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am. J. Pathol. 2017;187(3):581–588. doi: 10.1016/j.ajpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Machado-Santos J., Saji E., Tröscher A.R., Paunovic M., Liblau R., Gabriely G., Bien C.G., Bauer J., Lassmann H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141(7):2066–2082. doi: 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasson S.C., Gordon C.L., Christo S.N., Klenerman P., Mackay L.K. Local heroes or villains: tissue-resident memory T cells in human health and disease. Cell. Mol. Immunol. 2020;17(2):113–122. doi: 10.1038/s41423-019-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goplen N.P., Cheon I.S., Sun J. Age-related dynamics of lung-resident memory CD8(+) T cells in the age of COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.636118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts L.M., Jessop F., Wehrly T.D., Bosio C.M. Cutting edge: lung-resident T cells elicited by SARS-CoV-2 do not mediate protection against secondary infection. J. Immunol. 2021;207(10):2399–2404. doi: 10.4049/jimmunol.2100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neidleman J., Luo X., George A.F., McGregor M., Yang J., Yun C., Murray V., Gill G., Greene W.C., Vasquez J., Lee S.A., Ghosn E., Lynch K.L., Roan N.R. Distinctive features of SARS-CoV-2-specific T cells predict recovery from severe COVID-19. Cell Rep. 2021;36(3) doi: 10.1016/j.celrep.2021.109414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y., Kilian C., Turner J.E., Bosurgi L., Roedl K., Bartsch P., Gnirck A.C., Cortesi F., Schultheiß C., Hellmig M., Enk L.U.B., Hausmann F., Borchers A., Wong M.N., Paust H.J., Siracusa F., Scheibel N., Herrmann M., Rosati E., Bacher P., Kylies D., Jarczak D., Lütgehetmann M., Pfefferle S., Steurer S., Zur-Wiesch J.S., Puelles V.G., Sperhake J.P., Addo M.M., Lohse A.W., Binder M., Huber S., Huber T.B., Kluge S., Bonn S., Panzer U., Gagliani N., Krebs C.F. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci. Immunol. 2021;6(56) doi: 10.1126/sciimmunol.abf6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal H. The importance of cell-mediated immunity in COVID-19 – an opinion. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., Bondzie E.A., Dagotto G., Gebre M.S., Jacob-Dolan C., Li Z., Nampanya F., Patel S., Pessaint L., Van Ry A., Blade K., Yalley-Ogunro J., Cabus M., Brown R., Cook A., Teow E., Andersen H., Lewis M.G., Lauffenburger D.A., Alter G., Barouch D.H. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchipudi S.V. The complex role of STAT3 in viral infections. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/272359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., Mansouri D. JAK inhibition as a new treatment strategy for patients with COVID-19. Int. Arch. Allergy Immunol. 2020;181(6):467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D.M. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing L., Dai Z., Jabbari A., Cerise J.E., Higgins C.A., Gong W., de Jong A., Harel S., DeStefano G.M., Rothman L., Singh P., Petukhova L., Mackay-Wiggan J., Christiano A.M., Clynes R. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014;20(9):1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim O., Bayart C.B., Hogan S., Piliang M., Bergfeld W.F. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153(6):600–602. doi: 10.1001/jamadermatol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Shea J.J., Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meletiadis J., Tsiodras S., Tsirigotis P. Interleukin-6 blocking vs. JAK-STAT inhibition for prevention of lung injury in patients with COVID-19. Infect. Dis. Ther. 2020;9(4):707–713. doi: 10.1007/s40121-020-00326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satarker S., Tom A.A., Shaji R.A., Alosious A., Luvis M., Nampoothiri M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad. Med. 2021;133(5):489–507. doi: 10.1080/00325481.2020.1855921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas P., Sarmiento M. JAK/STAT pathway inhibition may be a promising therapy for COVID-19-related hyperinflammation in hematologic patients. Acta Haematol. 2021;144(3):314–318. doi: 10.1159/000510179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo W., Li Y.X., Jiang L.J., Chen Q., Wang T., Ye D.W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41(8):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantini F., Niccoli L., Nannini C., Matarrese D., Natale M.E.D., Lotti P., Aquilini D., Landini G., Cimolato B., Pietro M.A.D., Trezzi M., Stobbione P., Frausini G., Navarra A., Nicastri E., Sotgiu G., Goletti D. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology. 2021;60(1):399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijaya I., Andhika R., Huang I., Purwiga A., Budiman K.Y., Bashari M.H., Reniarti L., Roesli R.M.A. The use of Janus Kinase inhibitors in hospitalized patients with COVID-19: systematic review and meta-analysis. Clin. Epidemiol. Glob. Health. 2021;11 doi: 10.1016/j.cegh.2021.100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patoulias D., Doumas M., Papadopoulos C., Karagiannis A. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2021;40(11):4671–4674. doi: 10.1007/s10067-021-05884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M.D., Kim E.S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C.Y., Chen W.C., Hsu C.K., Chao C.M., Lai C.C. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2021;99 doi: 10.1016/j.intimp.2021.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowty M.E., Lin J., Ryder T.F., Wang W., Walker G.S., Vaz A., Chan G.L., Krishnaswami S., Prakash C. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab. Dispos. 2014;42(4):759–773. doi: 10.1124/dmd.113.054940. [DOI] [PubMed] [Google Scholar]
- 41.Guimarães P.O., Quirk D., Furtado R.H., Maia L.N., Saraiva J.F., Antunes M.O., Kalil Filho R., Junior V.M., Soeiro A.M., Tognon A.P., Veiga V.C., Martins P.A., Moia D.D.F., Sampaio B.S., Assis S.R.L., Soares R.V.P., Piano L.P.A., Castilho K., Momesso R., Monfardini F., Guimarães H.P., Ponce de Leon D., Dulcine M., Pinheiro M.R.T., Gunay L.M., Deuring J.J., Rizzo L.V., Koncz T., Berwanger O. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorgensen S.C.J., Tse C.L.Y., Burry L., Dresser L.D. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.A., Merkling S.H., Treluyer J.M., Veyer D., Mouthon L., Blanc C., Tharaux P.L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palasiewicz K., Umar S., Romay B., Zomorrodi R.K., Shahrara S. Tofacitinib therapy intercepts macrophage metabolic reprogramming instigated by SARS-CoV-2 spike protein. Eur. J. Immunol. 2021;51(9):2330–2340. doi: 10.1002/eji.202049159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dentone C., Vena A., Loconte M., Grillo F., Brunetti I., Barisione E., Tedone E., Mora S., Di Biagio A., Orsi A., De Maria A., Nicolini L., Ball L., Giacobbe D.R., Magnasco L., Delfino E., Mastracci L., Mangerini R., Taramasso L., Sepulcri C., Pincino R., Bavastro M., Cerchiaro M., Mikulska M., Bruzzone B., Icardi G., Frisoni P., Gratarola A., Patroniti N., Pelosi P., Bassetti M. Bronchoalveolar lavage fluid characteristics and outcomes of invasively mechanically ventilated patients with COVID-19 pneumonia in Genoa, Italy. BMC Infect. Dis. 2021;21(1):353. doi: 10.1186/s12879-021-06015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antwi-Amoabeng D., Kanji Z., Ford B., Beutler B.D., Riddle M.S., Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review. J. Med. Virol. 2020;92(11):2516–2522. doi: 10.1002/jmv.26038. [DOI] [PMC free article] [PubMed] [Google Scholar]