Abstract
The signaling of cells by scaffolds of synthetic molecules that mimic proteins is known to be effective in the regeneration of tissues. We report here on peptide amphiphile supramolecular polymers containing two distinct signals and test them in a mouse model of severe spinal cord injury. One signal activates the transmembrane receptor β-1 integrin and a second one the basic fibroblast growth factor 2 receptor and their respective downstream effectors. By mutating the peptide sequence of the amphiphilic monomers in non-bioactive domains, we intensified the motions of molecules within scaffold fibrils. This resulted in remarkable differences in vascular growth, axonal regeneration, myelination, survival of motor neurons, reduced gliosis, and functional recovery. We hypothesize that signaling of cells by ensembles of molecules could be optimized by tuning their internal motions.
One sentence Summary:
Bioactive scaffolds with intense supramolecular motion can greatly improve recovery from spinal cord injury.
Pharmacological signaling of cells usually proceeds through strong binding of small organic molecules to proteins that activate or inhibit particular responses. An emerging signaling strategy is to use nanostructures that target specific cells to deliver a therapeutic cargo, or materials functioning as bioactive scaffolds in the extracellular space. Cell signaling materials that trigger regeneration of tissues mimic the fibrillar components of natural extracellular matrices (ECMs) (1). Mechanobiology has been an important part of the science behind this idea based on discoveries that stiffness and viscoelasticity of materials can mediate multiple aspects of cell behavior (2).
Less developed aspects of this field is the molecular design of materials bearing signals for receptors and the connections between such signals and the motions of molecules within artificial scaffolds. Bioactive signals have been incorporated into covalent polymers (3), and more recently in supramolecular polymers (4). A commonly investigated signal has been the peptide RGDS, present in extracellular fibrils such as fibronectin that promotes cellular adhesion. Supramolecular polymers, which form by non-covalent association among monomers, have potential advantages for regenerative signaling because of the easy tunability of signal density, their ability to architecturally mimic the high persistence length of natural ECM fibrils, and their rapid biodegradation after they serve their function (5).
We report here on a supramolecular scaffold of nanoscale fibrils that integrates two different orthogonal biological signals, the laminin signal IKVAV known to promote differentiation of neural stem cells into neurons and to extend axons (1), and the fibroblast growth factor-2 (FGF-2) mimetic peptide YRSRKYSSWYVALKR, which activates the receptor FGFR1 to promote cell proliferation and survival (6). The two signals were placed at the termini of two different peptides with alkyl tails, known as peptide amphiphiles (PAs), that copolymerize noncovalently in aqueous media to form supramolecular fibrils. We have shown that the IKVAV signal on PA supramolecular polymers could restore partial function after a mild compression injury in a mouse model of SCI (7). Fibril-forming PA molecules that display biological signals at one terminus contain peptide domains between the bioactive moiety and the alkyl tail that can be modified to tune mechanical properties (8, 9).
We therefore investigated different domains that alter the physical properties of a potential scaffold therapy to restore functional recovery in vivo after hind limb paralysis in a murine model of severe spinal cord injury (SCI). The development of SCI therapies that avoid permanent paralysis in humans after traumatic injuries remains a major challenge given the inability of damaged axons to regenerate in the adult central nervous system (CNS) (10, 11). We found that keeping both biological signals at the same density, but slightly mutating the tetrapeptide sequence of these domains, could dramatically change the biological responses of cells in vitro as well the functional recovery from SCI in mice in vivo.
Supramolecular polymer synthesis and characterization
In order to investigate nanofiber-shaped supramolecular polymers with different physical properties that display the same two signals selected, we synthesized a library of different IKVAV PAs in which the tetrapeptide domain controlling physical behavior has different sequences of the amino acids V, A and G (IKVAV PA1-PA8) (see Fig. 1A, fig. S1, and table S1 for the list of PAs used and their peptide sequences). These amino acids were selected because they affect the propensity of molecules within the fibrils to form β-sheets, which have high intermolecular cohesion as a result of their hydrogen-bond density. These interactions in turn results in suppressed mobility of PA molecules within the fibril. For example, V2A2 (PA1) has a high propensity to form β-sheet structure because of its valine content whereas A2G2 (PA2) is potentially a less ordered segment without secondary structure (see Fig. 1A). The rest of the sequences were selected as potential candidates for an intermediate level of motion. All IKVAV PAs utilized the sequence E4G, which spaces this segment from the bioactive signal and provides high solubility in water (12).
Fig. 1. Library of investigated IKVAV PA molecules.
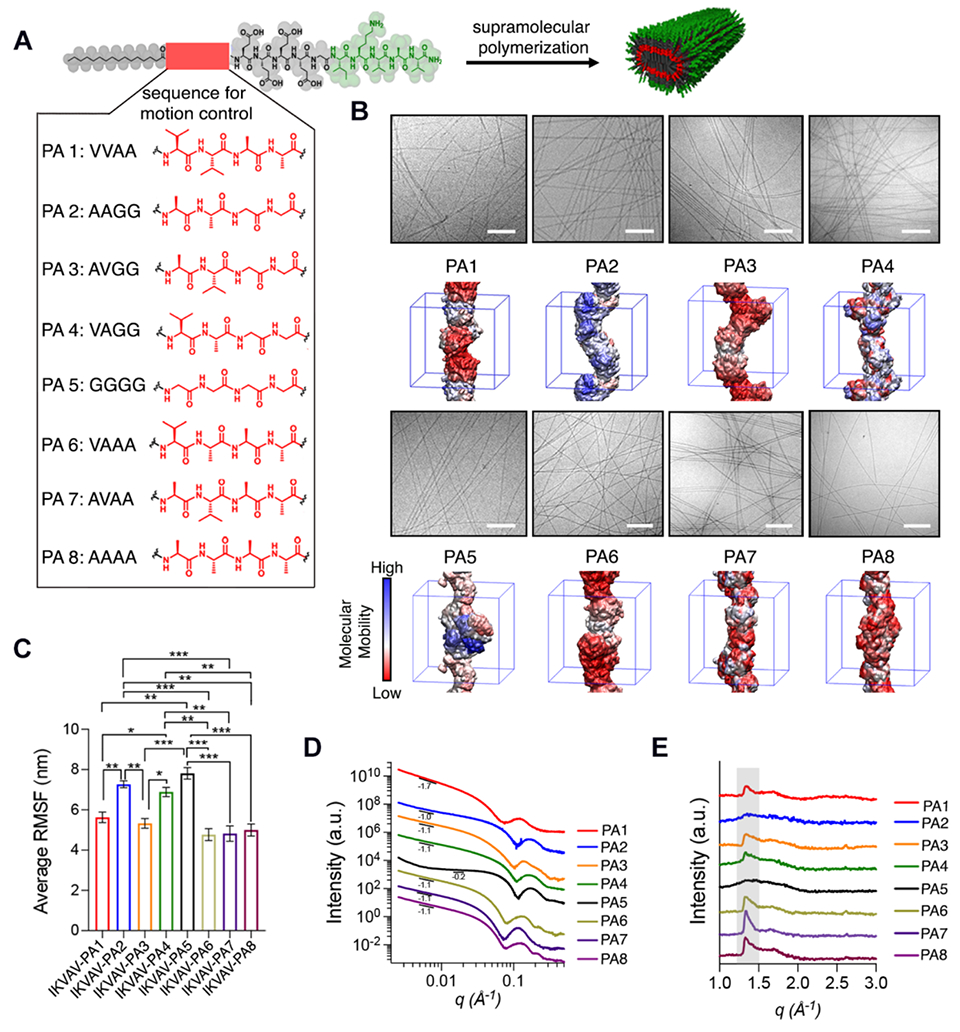
(A) Specific chemical structures of IKVAV PA molecules used and molecular graphics representation of a supramolecular nanofiber displaying the IKVAV bioactive signal. (B) Cryo-TEM micrographs of IKVAV PAs in the library and their corresponding color-coded representation of RMSF values for single IKVAV PA filaments. (C) Bar graphs of the average RMSF values of the different IKVAV PA molecules (error bars correspond to 3 independent simulations; *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA with Bonferroni). (D) SAXS scattering patterns and (E) WAXS profiles of the different IKVAV PA nanofibers (the scattering intensities were offset vertically for clarity; the Bragg peak corresponding to the β-sheet spacing around 1.35 Å is framed in a gray box). Scale bars: 200 nm.
Cryogenic transmission electron microscopy (Cryo-TEM) revealed that all IKVAV PAs formed nanofibers after supramolecular polymerization in water (Fig. 1B). Furthermore, synchrotron solution small-angle x-ray scattering (SAXS) confirmed the formation of filaments revealing a slope in the range −1 to −1.7 in the Guinier region except for PA5, which suggests a mixture of filaments and spherical micelles (slope = −0.2) (Fig. 1D). We also compared the physical behavior of the various assemblies in the library using coarse-grained molecular dynamic (CG-MD) simulations using the MARTINI force field (13) (fig. S2 and supplementary information). These simulations predicted that molecules within the various IKVAV fibers had different degrees of internal dynamics (Fig. 1B). Differences in the ability of the molecules to change positions internally over appreciable distances (on the order of nanometers) were suggested by the simulations, which yielded values of the parameter defined as the root-meansquare fluctuation (RMSF), which is a measure of the average displacement of a PA molecule during the last 5 μs of the simulation (Fig. 1C). These simulations indicate that molecules in PA2 fibers indeed have a high degree of internal motion, as well as PA5 which only contains G residues. Wide-angle X-ray analysis (WAXS) also revealed the presence of internal order (β-sheet Bragg peak with a d-spacing of 4.65 Å) in all the IKVAV PAs except for those with low RMSF values (PA2 and PA5) (Fig. 1E).
In order to probe differences in dynamics among the IKVAV PAs, we performed fluorescence depolarization (FD) measurements by encapsulating 1,6-diphenyl-1,3,5-hexatriene (DPH) within PA nanofibers to measure the microviscosity of the inner hydrophobic core. As expected, PA2 and PA5 had the lowest anisotropy values (0.21 and 0.18, respectively) indicating they formed the most dynamic supramolecular assemblies, PA4 had intermediate dynamics (0.30), and the remaining PAs had less intense supramolecular motion (0.40 to 0.37) (Fig. 2A). We also measured molecular dynamics in the IKVAV epitope using transverse-relaxation nuclear magnetic resonance (T2-NMR) spectroscopy. These experiments obtained the relaxation rate for the methylene protons attached to the ε carbon (Hε) of the K residue in the IKVAV sequence (observed at 2.69 to −2.99 parts per million) (see figs. S3 to S10 and table S1). IKVAV PA1 showed the highest relaxation rate (a low degree of motion), whereas IKVAV PA2 and PA5 had the lowest relaxation rates in the IKVAV PA library (1H-R2= 2.7±0.1 and 2.6±0.003 s−1, respectively, consistent with greater motion (Fig. 2B, figs. S3 to S10, and table S2). Consistent with FD results, IKVAV PA4 reveals an intermediate level of supramolecular motion between PA1 and PA2 (or PA5). Collectively, the simulations as well as FD, WAXS, and T2-NMR measurements are effectively consistent with three levels of supramolecular motion in the library of molecules investigated.
Fig. 2. Effect of supramolecular motion on hNPCs signaling in vitro.

(A) Molecular graphics representation of an IKVAV PA nanofiber indicating the chemical structure and location of DPH used as a probe in fluorescence depolarization measurements (top); bar graph of fluorescence anisotropy of IKVAV PA solutions (error bars correspond to 3 independent experiments; n.s. no significant, ***P<0.0001, one-way ANOVA with Bonferroni). (B) Chemical structure of the IKVAV peptide sequence highlighting the K residue probed by NMR (top); bar graphs of the K relaxation time for the different IKVAV PAs investigated (error bars correspond to 3 runs per condition; ***P<0.0001 vs IKVAV PA1, #P<0.05, ###P<0.0001 vs IKVAV PA2 and +P<0.05, +++P<0.0001 vs IKVAV PA5, one-way ANOVA with Bonferroni). (C) Differentiation conditions used for hNPCs. (D) Representative micrographs of hNPCs treated with IKVAV PA1, PA2, PA4 and PA5; NESTIN-stem cells (red), ITGB1-receptor (green), and DAPI-nuclei (blue). (E) WB results of ITGB1, p-FAK, FAK, ILK, and TUJ-1 in hNPCs treated with laminin and the various IKVAV PAs. (F) Representative confocal micrographs of hNPCs treated with IKVAV PA1, PA2, PA4 and PA5; NESTIN-stem cells (red), SOX-2-stem cells (green), TUJ-1-neurons (white), and DAPI-nuclei (blue). (G, H) Bar graphs of the percentage of SOX-2+ and NESTIN+-stem cells (G) and TUJ-1+ cells (H) treated with the various IKVAV PAs (error bars correspond to 3 independent differentiations; **P<0.01, ***P<0.001 vs IKVAV PA2 and ##P<0.01, ###P<0.001 vs IKVAV PA5, one-way ANOVA with Bonferroni). (I) Fluorescence anisotropy (left) and K residue relaxation times (right) obtained for IKVAV PA2 nanofibers in the absence (No Ca2+) or presence (Ca2+) of calcium ions (***P<0.001, student’s t-test). (J) WB results of ITGB1, p-FAK, FAK, ILK, TUJ-1 in hNPCs treated with IKVAV PA2 in the absence (−) or presence (+) of Ca2+. Scale bars: (D) 10 μm, (F) 100 μm.
Supramolecular motion and in vitro bioactivity
We performed in vitro experiments to determine if the IKVAV signal was equally bioactive in the library of IKVAV PAs. To establish the bioactivity of IKVAV PAs, neural progenitor cells derived from human embryonic stem cells (hNPCs) were treated either with the different IKVAV PA fibers in solution or the recombinant protein laminin (Fig. 2C). PA filaments associate closely with cells and can activate receptors when their surfaces display signals (14).
We first investigated the activation of the transmembrane receptor β1-INTEGRIN (ITGB1) known to be expressed in the presence of IKVAV PAs and laminin (15–17) using the active form-specific antibody HUTS4 and also verified activation of the receptor’s intracellular signaling pathway. Fluorescence confocal microscopy and western blot (WB) analysis showed that IKVAV PA2 and PA5 induced substantially higher concentrations of active ITGB1 and the downstream effectors integrin-linked kinase (ILK) and phospho-focal adhesion kinase (p-FAK) relative to the rest of the IKVAV PAs, the IKVAV peptide, and laminin or ornithine coatings as controls (Fig. 2, D and E, and fig. S11). An intermediate level of activation with PA4 revealed correlated with its intermediate level of supramolecular motion relative to the rest of the PAs in the library. As expected, PAs displaying the VVIAK scrambled sequence resulted in minimal cellular activation of ITGB1 (see figs. S12 and S13). Furthermore, pre-treatment with an ITGB1 antibody blocked the attachment of hNPCs on all IKVAV PAs, suggesting that an IKVAV-ITGB1 interaction mediated this process (fig. S14).
Although hNPCs upregulated the neuronal form of β-TUBULIN (TUJ-1+) when treated with IKVAV PAs, this induction (which reflects neuronal differentiation commitment) was higher for IKVAV PA2 and PA5 (20.5±1 % and 20.7±1.2 %, respectively), the two most dynamic supramolecular fibrils (Fig. 2, F to H, and fig. S15). The other IKVAV PAs, with the exception of IKVAV PA4 which showed an intermediate neuronal differentiation commitment (PA4: 14±1.2 %), had a lower percentage of induction of TUJ-1+ neuronal cells (PA1: 8.2±0.7 %, PA3: 7.5±0.6 %, PA6: 7.9±1.3 %, PA7: 7.4±0.6 %, and PA8: 7.5±0.5 %). By using puromycin-based protein synthesis analysis (SUnSET technique), we verified that all of the conditions showed similar protein translation levels, so the observed differences were not linked to a metabolic effect (see fig. S16).
We also performed in vitro experiments in which hNPCs were treated with the most bioactive IKVAV PAs (PA2 and PA5) mixed with 5 mM CaCl2 which is known to electrostatically cross-link negatively charged PA fibers (18, 19). The addition of Ca2+ suppressed supramolecular motion, which was confirmed by FD and T2-NMR experiments (Fig. 2I and fig. S17). When supramolecular motion was decreased by adding Ca2+ ions to the media, the activation of ITGB1 and its downstream intracellular pathway (ILK, p-FAK/FAK) also decreased (Fig. 2J and fig. S18). These results showed a strong positive correlation between dynamics and in vitro bioactivity as mutations were introduced in the tetrapeptide amino acid sequence in the non-bioactive domain of IKVAV PAs.
SCI model: axon regrowth and formation of glial scar
We then proceeded to test the ability of dual signal fibrils to enhance functional recovery after SCI in vivo. Given the low level of in vitro bioactivity observed for IKVAV PA1, PA3, PA4, PA6, PA7 and PA8, we did not to use these PAs in combination with the FGF2 PAs. We also needed nanofibers that display both signals simultaneously, so the binary systems had to be miscible and form hydrogels with similar mechanical properties upon contact with physiological fluids once injected at the site of the injury. Only IKVAV PA2 was both miscible and could form hydrogels with similar mechanical properties when mixed with either FGF2 PA1 or FGF2 PA2, particularly at a molar ratio of 90:10 (Fig. 3, A to C, fig. S19, and table S3). Furthermore, both FGF2 PAs alone formed highly aggregated short fibers that further contributed to immiscibility with other IKVAV PAs such as PA1, PA4 or PA5 (figs. S20 and S21).
Fig. 3. Two chemically different PA scaffolds with two identical bioactive sequences reveal differences in growth of corticospinal axons after SCI.
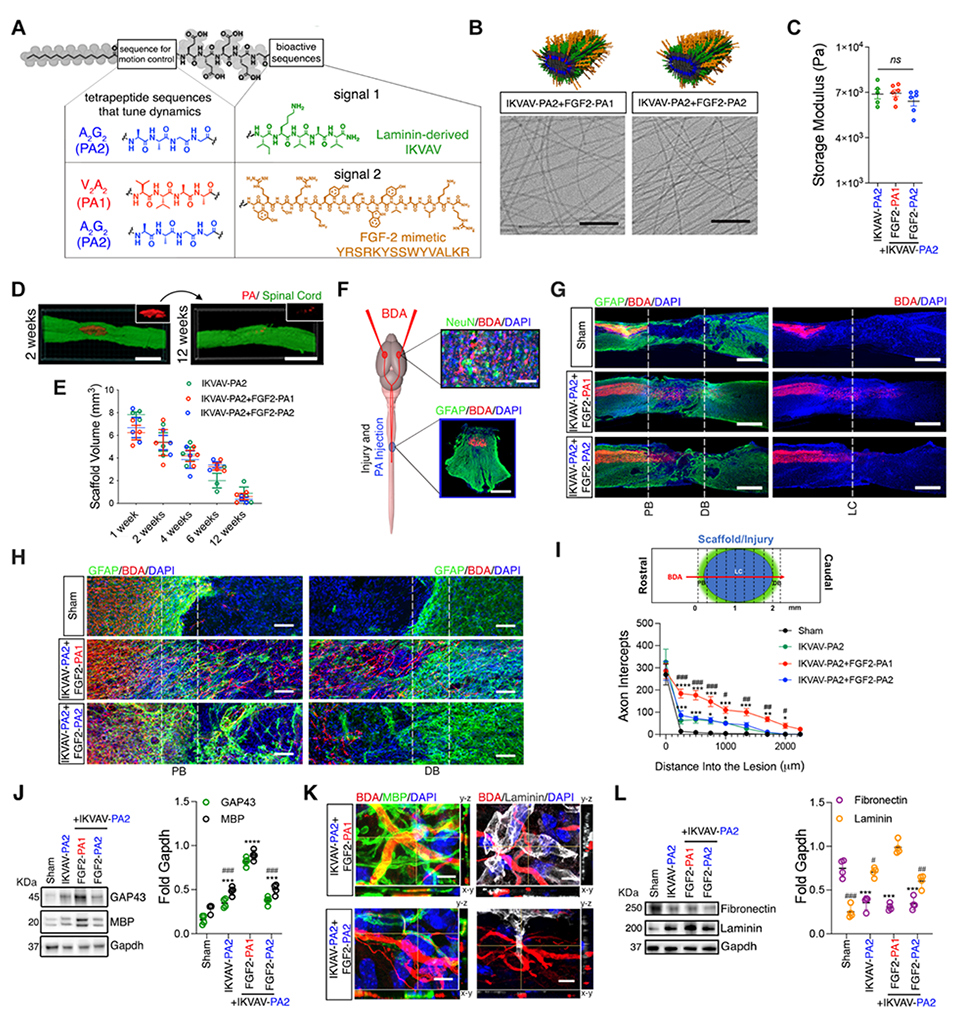
(A) Chemical structures of the two PA molecules used. (B) Molecular graphics representation of a supramolecular nanofiber displaying two bioactive signals (top); cryo-TEM micrographs of IKVAV PA2 co-assembled with FGF2 PAs (FGF2 PA1 and FGF2 PA2) (bottom). (C) Storage modulus of IKVAV PA2 (green) and their respective co-assemblies with FGF2 PAs (FGF2 PA1, red and FGF2 PA2, blue). (D) Fluorescent micrographs of spinal cords (green) injected with IKVAV PA2+FGF2 PA1 (red) covalently labeled with Alexa 647. (E) Plot of PA scaffold volume as a function of time after implantation. (F) Schematic illustration showing the site of BDA and PA injections (left); fluorescent micrographs of the brain cortex (top, right); NeuN-neurons (green), BDA-labelled neurons (red) and DAPI-nuclei (blue) and transverse spinal cord section stained for GFAP-astrocytes (green), BDA-labelled descending axons (red) and DAPI-nuclei (blue) (bottom, right). (G) Fluorescent micrographs of longitudinal spinal cord sections in sham, IKVAV PA2+FGF2 PA1, and IKVAV PA2+FGF2 PA2 groups; GFAP-astrocytes (green), BDA-labelled axons (red) and DAPI-nuclei (blue); vertical white dashed lines indicate the proximal border (PB), the distal border (DB), and the central part of the lesion (LC). (H) Representative magnified images for those in G. (I) Schematic lesion site and vertical lines used to count the number of axons crossing at each location indicated (top); plot of the number of crossing axons (bottom) (error bars correspond to 6 animals per group; *P<0.05, **P<0.01, ***P<0.001 vs sham and #P<0.05, ##P<0.01, ###P<0.001 vs IKVAV PA2 and IKVAV PA2+FGF2 PA2 groups, repeated measures of two-way ANOVA with Bonferroni). (J) WB results (left) and dot plot of the normalized values for GAP43 and MBP protein in sham, IKVAV PA2, IKVAV PA2+FGF2 PA1, and IKVAV PA2+FGF2 PA2 (right) (**P<0.01, ***P<0.001 vs sham and ###P<0.001 vs IKVAV PA2+FGF2 PA1 group, one-way ANOVA with Bonferroni). (K) Representative 3D fluorescent micrographs of BDA-labelled axon regrowth (red) and myelin basic protein (MBP, green) (left) and laminin (white) (right). (L) WB results (left) and dot plot of the normalized values for laminin and fibronectin expression in conditions described in J (right) (***P<0.001 vs sham and #P<0.05, ##P<0.01, ###P<0.001 vs IKVAV PA2+FGF2 PA1 group, one-way ANOVA with Bonferroni). Data points in E correspond to 3 animals per group and to 4 animals per group in J and L. Scale bars: (D, G) 1500 μm, (F) 25 μm (top) and 200 μm (bottom), (H) 100 μm, and (K) 25 μm.
The miscible and gel-forming binary systems with similar mechanical properties, IKVAV PA2 with either FGF2 PA1 or FGF2 PA2, were taken forward to in vivo experiments (Fig. 3A and fig. S22; for full characterization of these systems see supplementary text). We injected saline solutions of 90:10 molar ratio of IKVAV PA2 co-assembled with either FGF2 PA1 or with FGF2 PA2 into the spinal cord of mice 24 h after a severe contusion in an established murine model of SCI (see supporting information for specific details of the animal model protocol) (20). IKVAV PA2, which was the most bioactive single signal system, was used as a control in all in vivo experiments. All PA solutions gelled in situ when delivered into the spinal cord and localized into the damaged area. To track and quantify the bioactive scaffold’s biodegradation as a function of time, the PA molecules were fluorescently labeled with Alexa 647 dye. We then injected the fluorescent materials into the spinal cord 24 h post-injury and measured their volume at 1, 2, 4, 6, and 12 weeks by fully reconstructing spinal cords using spinning disk confocal microscopy (see Fig. 3D and supplementary information). The soft materials biodegraded gradually within a period of 1 to 12 weeks after implantation, and we did not observe any differences in biodegradation rate among the three experimental materials (see Fig. 3E and fig. S23).
We performed bilateral injections of biotinylated dextran amine (BDA) administered 10 weeks after the injury into the sensorimotor cortex in order to trace the corticospinal tracts (CST), which mediate voluntary motor function (Fig. 3F) (21). We evaluated anterogradely labeled CST axon regrowth 12 weeks after injury in all PA and sham (injection of saline solution only) groups. This process required quantifying the number of labeled axons that regrew to the proximal lesion border and beyond. We also injected IKVAV PA1 and PA fibers lacking any bioactive signals on their surfaces (backbone PA) as controls (see fig. S24 and table S1 for the peptide sequence).
In mice injected with saline solution, we hardly observed any regrown axons within the lesion, whereas we observed some regrowth of axons for IKVAV PA1, in which fibers exhibited low mobility (Fig. 3G and fig. S25; see supplementary text for additional PA controls). On the other hand, in mice injected with IKVAV PA2 alone or co-assembled with FGF2 PA2 (which shares the same A2G2 non-bioactive domain as IKVAV PA2), we only observed a modest, but increased axon regrowth compared to the sham condition. However, injections of IKVAV PA2 co-assembled with FGF2 PA1 (which includes the V2A2 non-bioactive domain instead of A2G2) led to robust corticospinal axon regrowth across the lesion site, even surpassing its distal border (Fig. 3, G and H, and fig. S26). In this group, the total axon regrowth within the lesion was twofold greater than that in the group using the co-assembly of IKVAV PA2 and FGF2 PA2 and 50-fold greater than in the sham group (Fig. 3I). Serotonin axons (5HT), which may also play a role in locomotor function, also regrew within the lesion core with a similar trend as CST (fig. S27).
We hypothesize that the CST and 5HT axon regrowth observed could be in part due to the absence of a significant astrocytic scar which is a strong barrier for axonal regeneration (11). In the sham and backbone PA groups, this barrier was revealed as a dense population of reactive astrocytes expressing high levels of GFAP at the borders of the injury while in all bioactive PA groups, the glial scar was less dense (Fig. 3H and figs. S25 and S26). In agreement with these results, WB analysis showed a higher level of growth-associated protein-43 (GAP-43), which resides in the growth cone of regenerating axons, only in the most bioactive co-assembly (IKVAV PA2+FGF2 PA1) (Fig. 3J).
Finally, we determined whether PA scaffolds could induce remyelination of corticospinal axons 3 months post-injury, and found high levels of myelin basic protein (MBP) within the lesion particularly wrapping the regrown axons in IKVAV PA2+FGF2 PA1 (Fig. 3, J and K). Moreover, in this condition, we observed many growing axons within the lesion to be in contact with high levels of laminin and low levels of fibronectin, indicative of a reduced fibrotic core (Fig. 3, K and L and fig. S26). Our histological and biochemical observations suggested that physical differences between the two supramolecular co-assemblies bearing two bioactive signals could greatly enhance neuro-regenerative outcomes after injury.
SCI model: angiogenesis, cell survival and functional recovery
We next explored the impact of both dual signal co-assemblies on angiogenesis at the site of injury, important for a fully anatomical and functional regeneration. Relative to uninjured tissue sections, the transverse spinal cord sections of sham mice revealed a significant degree of tissue degeneration extending rostro-caudally more than 2.0 mm away from the center of the lesion. In this case, a significant decrease in vascular area fraction, vascular length, and branching was observed compared to the uninjured control (Fig. 4, A and B). We assessed the existence of a functional vessel network by transcardially injecting a glucose solution containing 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), a lipophilic carbocyanine dye that incorporates into endothelial cell membranes (Fig. 4A) (22). In groups treated with PA scaffolds, there was high preservation of the ventral tissue structure revealing the maintenance of a functional blood vessel network. However, we again observed that treatment with the most bioactive co-assembly led to an increase in vascular area fraction, vascular length, and branching, especially in the dorsal region. These parameters did not differ significantly between the IKVAV PA2 alone and the less bioactive co-assembly group (IKVAV PA2+FGF2 PA2), implying that the mimetic FGF2 angiogenic signal was not functioning optimally in IKVAV PA2+FGF2 PA2 (Fig. 4, A and B and fig. S28).
Fig. 4. Two chemically different PA scaffolds with two identical bioactive sequences reveal differences in angiogenesis.

(A) Fluorescent micrographs of transverse spinal cord sections in uninjured, IKVAV PA2+FGF2 PA1, IKVAV PA2+FGF2 PA2 and sham groups; GFAP-astrocytes (green), DiI-labelled blood vessels (red), and DAPI-nuclei (blue). (B) Dot plots of the vascular area fraction, perfused vascular length, and number of branches in the transverse sections of groups in A (*P<0.05, ***P<0.0001 vs sham and ##P<0.001, ###P<0.0001 vs IKVAV PA2+FGF2 PA1 group, one-way ANOVA with Bonferroni). (C) Fluorescent images of BrdU+/CD31+ cells in the center of the lesion in animals injected with IKVAV PA2+FGF2 PA1 and IKVAV PA2+FGF2 PA2; CD31-blood vessels (green), BrdU-newly generated cells (red), and DAPI-nuclei (blue). (D) Dot plot of the number of BrdU+/CD31+ cells per mm2 in groups treated with IKVAV PA2 alone, IKVAV PA2+FGF2 PA1, IKVAV PA2+FGF2 PA2, and saline (sham) (*P<0.05, ***P<0.001 vs sham and ###P<0.0001 vs IKVAV PA2+FGF2 PA1 group, one-way ANOVA with Bonferroni). (E) WB results (left) and plot of the normalized values for CD31 protein (right) (**P<0.001, ***P<0.0001 vs sham and ###P<0.001 vs IKVAV PA2+FGF2 PA1 group one-way ANOVA with Bonferroni). Data points in B and D correspond to 6 animals per group and to 4 animals per group in E. Scale bars: (A) 200 μm, (C) 25 μm.
In order to determine the origin of the blood vessels within the lesion, the thymidine analog 5’-bromo-2’-deoxyuridine (BrdU) was intraperitoneally injected during the first week post-injury, and we observed newly formed blood vessels within the lesion of the most bioactive co-assembly group 12 weeks after injury. This was confirmed by a significant increase in the number of BrdU+/CD31+ cells relative to samples for all other groups (Fig. 4, C and D and fig. S29) as well as by WB analysis (Fig. 4E). The IKVAV PA2+FGF2 PA2 co-assembly and IKVAV PA2 alone led to a very modest but yet significantly increased blood vessel formation compared to the sham group.
We also assessed the effect of both dual signal co-assemblies on neuronal survival, maintenance of spinal circuitry and local function. Native FGF-2 has been previously associated with an increase in neuronal viability after SCI (23). Transverse spinal cord sections of the most bioactive co-assembly group showed NeuN+ neurons near the newly generated vessels in the dorsal region similar to the uninjured control group (Fig. 5A). Furthermore, neurons (NeuN+ cells) that were also ChAT+ (motor neurons) were only found in the ventral horn when PAs were utilized, showing a significantly higher number in the most bioactive system relative to other groups (Fig. 5, B and C). The lack of any double BrdU+/NeuN+ neurons within the lesion in any of the groups suggested the absence of local neurogenesis.
Fig. 5. Two chemically different PA scaffolds with two identical bioactive sequences reveal differences in neuronal survival and functional recovery.

(A) Fluorescent micrographs of transverse spinal cord sections corresponding to uninjured, IKVAV PA2+FGF2 PA1, IKVAV PA2+FGF2 PA2 and sham groups; NeuN-neurons (green), DiI-labelled blood vessels (red) and DAPI-nuclei (blue), dashed lines indicate the grey matter (horn). (B) High-magnification images of the ventral horn area for slices in A (left); NeuN-neurons (green), DiI-labelled blood vessels (red), and DAPI-nuclei (blue); ChAT-motor neurons (green), DiI-labelled blood vessels (red), and DAPI-nuclei (blue) (right). (C) Dot plots showing the number of NeuN+ (left) and ChAT+ (right) cells per transverse section (data points correspond to a total of 48 sections; 8 sections per animal and 6 animals per group; **P<0.01, ***P<0.001 vs sham and ###P<0.001 vs IKVAV PA2+FGF2 PA1 group, one-way ANOVA with Bonferroni). (D) Experimental timeline of in vivo experiments (top) and Basso Mouse Scale (BMS) for locomotion (bottom) (error bars correspond to 38 animals per group; **P<0.001, ***P<0.0001 all PA groups vs sham and ###P<0.0001 vs IKVAV PA2+FGF2 PA2 and IKVAV PA2 groups by repeated measures of two-way ANOVA with Bonferroni). Scale bars: (A) 200 μm, (B) 25 μm.
We investigated if the observed axonal regeneration, angiogenesis, and local neuronal cell survival led to behavioral improvement in injured animals. For this purpose, we obtained Basso Mouse Score (BMS) open field locomotor scores and locomotor recovery by footprint analysis in all groups during the 12 weeks post-injury (Fig. 5D and fig. S30). At one-week post-injury and thereafter, all PA groups demonstrated significant and sustained behavioral improvement compared to the sham group. Interestingly, three weeks post-injury, mice treated with the most bioactive co-assembly showed a significant functional recovery (5.9±0.5) compared to mice injected with IKVAV PA2+FGF2 PA2 and IKVAV PA2 alone (4.4±0.5 and 4.3±0.5, respectively) (Fig. 5D). Quantification of footprints revealed significantly larger stride length and width in mice treated with the most bioactive co-assembly relative to other groups (fig. S30). Collectively, these data suggest that neuronal cell survival and functional recovery that we observed in dual signal systems are surprisingly linked to the differences in the chemical composition of their respective non-bioactive tetrapeptides.
In vitro results on human endothelial and neural progenitor cells
Based on results described above, we next investigated the bioactivity of the FGF2 signal in vitro in both co-assemblies using human umbilical vein vascular endothelial cells (HUVECs). As mentioned previously, native FGF-2 enhances endothelial cell proliferation and network formation (24), and we found that within 48 h of culturing HUVECs on the most bioactive co-assembly or FGF2 protein, there was extensive branching and formation of vessel-like capillary networks (Fig. 6, A and B and fig. S31; see also supplementary information for methodology used). We also performed WB analysis to verify whether the observed in vitro bioactivity of the FGF2 PA1 co-assembled with the IKVAV PA2 was linked to the FGF-2 intracellular signaling pathway. HUVECs treated with the most bioactive co-assembly or native FGF-2 revealed high levels of p-FGFR1 and the downstream proteins p-ERK1/2, which activate proliferation and migration of endothelial cells (Fig. 6C) (6, 25). As expected, systems containing the scrambled FGF2 mimetic sequence did not reveal any bioactivity (figs. S31 and S32).
Fig. 6. Validating cell signaling differences in vitro between two PA scaffolds exhibiting different supramolecular motion.
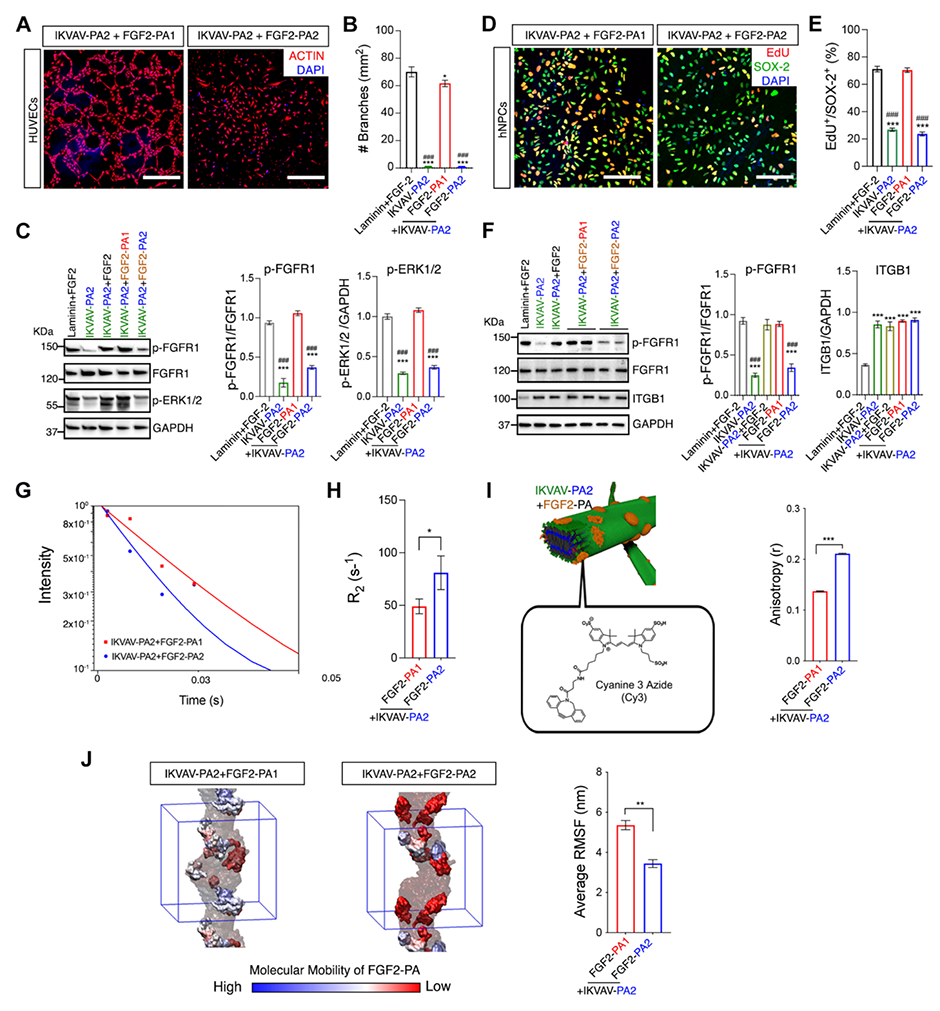
(A) Confocal micrographs of HUVECs treated with IKVAV PA2+FGF2 PA1 and IKVAV PA2+FGF2 PA2; ACTIN-cytoskeleton (red), DAPI-nuclei (blue). (B) Dot plot of the number of branches per mm3 in HUVECs treated with laminin+FGF-2, IKVAV PA2 alone, IKVAV PA2+FGF2 PA1, and IKVAV PA2+FGF2 PA2. (C) WB results (left) and dot plot of the normalized values for active FGFR1 (p-FGFR1) vs total FGFR1 (FGFR1) and active ERK1/2 (p-ERK1/2) using the conditions in B (right). (D) Confocal micrographs of hNPCs on coatings of IKVAV PA2+FGF2 PA1 and IKVAV PA2+FGF2 PA2; EDU-proliferative marker (red), SOX-2-neural stem cell marker (green), and DAPI-nuclei (blue). (E) Dot plot of the percentage of EDU+/SOX-2+ cells on the various coatings. (F) WB results (left) and plot of the normalized values for active FGFR1 (p-FGFR1) vs total FGFR1 (FGFR1) and β1 INTEGRIN (ITGB1) (right). (G) 1H-NMR spin-spin relaxation time of the aromatic protons in Y and W amino acids in the FGF2 mimetic signal at 6.81 ppm (solid lines are single linear best fits). (H) Graph of the aromatic relaxation times measured in G (error bars correspond to 3 runs per condition; *P<0.05 student’s t-test). (I) Fluorescence anisotropy of solutions measured by fluorescent depolarization of FGF2 PAs chemically modified with Cy3 dye (error bars correspond to 3 independent experiments; ***P<0.001 student’s t-test). (J) Color-coded representation of RMSF values in clusters of FGF2 PAs (left) and the corresponding bar graph (right) (IKVAV PA2 molecules are shown in transparent grey, ions and water molecules are removed for clarity, and the simulation box is shown in blue) (error bars correspond to 5 independent simulations; **P<0.01 Student’s T-test). Error bars in B and E correspond to 3 independent experiments and C and F correspond to 4 independent experiments per condition; ***P<0.0001 vs Laminin+FGF2 and ###p<0.0001 vs IKVAV PA2+FGF2 PA1, one-way ANOVA with Bonferroni. Scale bars: (A) 200 μm, (D) 100 μm.
To establish the simultaneous bioactivity of the IKVAV and FGF2 signals in both co-assemblies, we assessed the effects of these molecules on hNPC proliferation in vitro, by quantifying the double positive EdU+/SOX-2+ as well as the induction of ITGB1 and pFGFR-1 (Fig. 6, D to F and fig. S33; see also supplementary text for more information). These experiments suggest that the FGF2 signal in the less bioactive co-assembly is largely non-functional, whereas the IKVAV signal remains operative in both. These results are consistent with our observations in the SCI experiments.
Physical experiments and computer simulations on supramolecular motion
We investigated what might be the physical reasons for the loss of in vitro and in vivo bioactivity when the tetrapeptide that follows the alkyl tail was mutated from V2A2 to A2G2 in the FGF2 PAs. Differences in dynamics between FGF2 PA molecules in the two co-assemblies were studied with T2-NMR spectroscopy and FD (Fig. 6, G to I). We measured the relaxation rates of the aromatic protons in Y and W amino acids, which are only present in the FGF2 mimetic signal (26, 27). The rates were slower in the most bioactive co-assembly, indicating greater supramolecular motion in the signaling peptide (1H-R2= 49.3±11 s−1 vs. 80.9±18.9 s−1 for the less bioactive co-assembly) (Fig. 6, G and H and fig. S34). We also carried out FD experiments on the two co-assemblies using FGF2 PA molecules that were covalently labeled with a Cy3 dye (based on cryo-TEM images the dye did not disrupt the supramolecular assemblies; see fig. S35). A lower anisotropy was found in the most bioactive co-assembly, indicating a higher mobility of the FGF2 signal molecules within the nanofibers (Fig. 6I).
CG-MD simulations supported the T2-NMR and FD results above by yielding higher values of RMSF for FGF2 PA molecules in the most bioactive co-assembly. The simulations also revealed that FGF2 PA molecules form clusters in both co-assemblies (slightly larger in the most bioactive system) with a distribution of mobilities (RMSF values) (Fig. 6J and fig. S36; see also supplementary information). The decreases in bioactivity in one of the systems could be attributed to differences in the extent of co-assembly between the two PA molecules bearing signals. However, 1D 1H-NMR, diffusion ordered spectroscopy (DOSY), and T2-NMR (28, 29) of methylene units in alkyl tails indicate the occurrence of co-assembly in both systems (figs. S37 to S39 and table S4; see supplementary text).
The results obtained on greater degrees of motion in FGF2 PA1 molecules were counterintuitive because the tetrapeptide V2A2 (present in FGF2 PA1) had the least mobility in systems containing only IKVAV PA. The lower mobility in FGF2 PA2 molecules in the co-assembly with IKVAV PA2 was likely the result of greater interactions through hydrogen bonding and side chain contacts among the identical tetrapeptides present in both molecules. In contrast, two dissimilar tetrapeptides are present in the two molecules of the highly bioactive IKVAV PA2+FGF2 PA1 co-assembly, which would not favor a strong interaction between both types of molecules and lead to higher degrees of supramolecular motion.
The evidence for a strong interaction between IKVAV PA2 and FGF2 PA2 and less motion is the essentially invariant CD spectrum when FGF2 PA2 is added to IKVAV PA2. However, the CD spectrum was modified when the less interactive FGF2 PA1 is added to IKVAV PA2, thus suggesting a disruption of secondary structure (fig. S40). Thus, the greater motion detected by NMR for FGF2 PA1 molecules must indicate freer translational motion of its clusters within the fibrils or vertical motion of the signaling clusters in and out of the fibrils. Although we have gathered substantial evidence for the correlation between supramolecular motion and bioactivity of fibrillar scaffolds used here to promote SCI recovery, we could not directly link this physical phenomenon to our in vivo observations with techniques currently available.
Discussion
Our work demonstrates that bioactive scaffolds which physically and computationally reveal greater supramolecular motion lead to greater functional recovery from SCI in the murine model. In one-dimensional scaffolds of non-covalently polymerized bioactive molecules, we expected polyvalency effects to help cluster receptors for effective signaling. We also expected that the internal structure of the supramolecular scaffolds could limit free motion and favorably orient signals toward receptors perpendicular to their fibrillar axis. However, the surprising finding in this work is that the intensity of molecular motions within the bioactive fibrils, as measured on the bench, correlated with enhanced axonal regrowth, neuronal survival, blood vessel regeneration, and functional recovery from SCI. A direct link between the motion and the recovery will require techniques not currently available that could precisely detect supramolecular motion in vivo with high resolution.
However, the computer simulations and experimental data do suggest that translation on the scale of nanometers within or vertically out of the assemblies to reach receptor sites might enhance bioactivity. That is, a highly agile and physically plastic supramolecular scaffold could be more effective at signaling receptors in cell membranes undergoing rapid shape fluctuations. An alternative hypothesis for the cause of the recovery could be broadly more favorable interactions of the molecularly dynamic scaffolds with the protein milieu of the ECM. In the context of our correlative findings between supramolecular motion and bioactivity, it is intriguing to ask why there is such a prevalence of intrinsically disordered proteins in biological systems (30), and one wonders if the added motion of disordered protein domains, in analogy to our bioactive and dynamic supramolecular fibrils, provides greater capacity to signal efficiently in the biological environment. We conclude that our observations suggest great opportunities in the structural design of dynamics to optimize the bioactivity of therapeutic supramolecular polymers.
Supplementary Material
Acknowledgments
The authors are grateful to Mark Karver, Emily Testa and Suvendu Biswas of the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University for their assistance and key insights into the synthesis and purification of the peptide amphiphiles. We also thank the laboratory of Dr. John A. Kessler for initial training of Z.A., A.N.E and F.C. on the SCI model. We thank Mark Seniw for the preparation of graphic illustrations shown in the figures. The authors would also like to thank Dr. Charles Rubert-Perez, Dr. Liam C Palmer, and Dr. Kohei Sato for their initial help with the FGF2 PA materials, especially helpful discussion about materials characterization and CD results.
Funding:
The experimental work and simulations were supported by the Louis A. Simpson and Kimberly K. Querrey Center for Regenerative Nanomedicine (CRN) at the Simpson Querrey Institute for BioNanotechnology (S.I.S.). Work on NMR analysis was supported by the Air Force Research Laboratory under agreement number FA8650-15-2-5518. Part of the biological experiments reported here were supported by the National Institute on Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA) R01NS104219 (E.K.), NIH/NINDS grants R21NS107761 and R21NS107761-01A1 (E.K.), the Les Turner ALS Foundation (E.K.), the New York Stem Cell Foundation (E.K.). We thank the Paralyzed Veterans of America (PVA) Research Foundation PVA17RF0008 (Z.A.), the National Science Foundation (A.N.E. and S.M.C.) and the French Muscular Dystrophy Association (J.A.O.) for graduate and postdoctoral fellowships. We thank the Peptide Synthesis Core and the Analytical Bionanotechnology Equipment Core at the Simpson Querrey Institute for Bionanotechnology for biological and chemical analysis. These facilities have support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS – 1542205). Imaging work was performed at the Center for Advanced Microscopy and CD measurements were performed at the Northwestern University Keck Biophysics facility. Both of these facilities are generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. Spinning disk confocal microscopy was performed on an Andor XDI Revolution microscope, purchased through the support of NCRR 1S10 RR031680-01. Multiphoton microscopy was performed on a Nikon A1R multiphoton microscope, acquired with support from NIH 1S10OD010398-01. Tissue processing was performed at the Pathology Core Facility supported by NCI CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. Electron Microscopy experiments were performed at the Electron Probe Instrumentation Center (EPIC) and the BioCryo facility of Northwestern University’s NUANCE Center which have both received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN. NMR and FTIR characterization in this work made use of IMSERC at Northwestern University, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), the State of Illinois, and IIN. Portions of this work were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, The Dow Chemical Company, and DuPont de Nemours, Inc. This research used resources of the Advanced Photon Source; a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. E.K is a Les Turner ALS Research Center Investigator and a New York Stem Cell Foundation – Robertson Investigator.
Footnotes
Competing interests: A patent pertaining to this work has been filed and is pending: Supramolecular Motion in Bioactive Scaffolds Promotes Recovery from Spinal Cord Injury (inventors: Zaida Alvarez Pinto; Samuel I. Stupp).
Data and materials availability:
All data needed to evaluate the conclusions in the paper are present either in the main text or the supplementary materials.
References
- 1.Silva GA et al. , Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Discher DE, Mooney DJ, Zandstra PW, Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandley BK, Schnaar RL, Covalent attachment of an Arg-Gly-Asp sequece peptide to derivatizable polyacrylamide surfaces: Support of fibroblast adhesion and long-term growth. Anal Biochem 172, 270–278 (1988). [DOI] [PubMed] [Google Scholar]
- 4.Hartgerink JD, Beniash E, Stupp SI, Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294, 1684–1688 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Aida T, Meijer EW, Stupp SI, Functional supramolecular polymers. Science 335, 813–817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubert Perez CM, Alvarez Z, Chen F, Aytun T, Stupp SI, Mimicking the Bioactivity of Fibroblast Growth Factor-2 Using Supramolecular Nanoribbons. ACS Biomater Sci Eng 3, 2166–2175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tysseling-Mattiace VM et al. , Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci 28, 3814–3823 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sur S, Newcomb CJ, Webber MJ, Stupp SI, Tuning supramolecular mechanics to guide neuron development. Biomaterials 34, 4749–4757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortony JH et al. , Internal dynamics of a supramolecular nanofibre. Nat Mater 13, 812–816 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahuja CS et al. , Traumatic spinal cord injury. Nat Rev Dis Primers 3, 17018 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Tran AP, Warren PM, Silver J, The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol Rev 98, 881–917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberger JE, Berns EJ, Bitton R, Newcomb CJ, Stupp SI, Electrostatic control of bioactivity. Angew Chem Int Ed Engl 50, 6292–6295 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee O-S, Cho V, Schatz GC, Modeling the Self-Assembly of Peptide Amphiphiles into Fibers Using Coarse-Grained Molecular Dynamics. Nano Lett 12, 4907–4913 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Edelbrock AN et al. , Supramolecular Nanostructure Activates TrkB Receptor Signaling of Neuronal Cells by Mimicking Brain-Derived Neurotrophic Factor. Nano Lett 18, 6237–6247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap L, Tay HG, Nguyen MTX, Tjin MS, Tryggvason K, Laminins in Cellular Differentiation. Trends Cell Biol 29, 987–1000 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Pan L et al. , beta1-Integrin and integrin linked kinase regulate astrocytic differentiation of neural stem cells. PLoS One 9, e104335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei WL et al. , Laminin/beta1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell Res 22, 954–972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stendahl JC, Rao MS, Guler MO, Stupp SI, Intermolecular Forces in the Self-Assembly of Peptide Amphiphile Nanofibers. Adv Funct Mater 16, 499–508 (2006). [Google Scholar]
- 19.Greenfield MA, Hoffman JR, Olvera M S. I de la Cruz. Stupp, Tunable Mechanics of Peptide Nanofiber Gels. Langmuir 26, 3641–3647 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Bhalala OG, Pan L, North H, McGuire T, Kessler JA, Generation of Mouse Spinal Cord Injury. Bio Protoc 3, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson MA et al. , Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y et al. , Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc 3, 1703–1708 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasai M, Jikoh T, Fukumitsu H, Furukawa S, FGF-2-responsive and spinal cord-resident cells improve locomotor function after spinal cord injury. J Neurotrauma 31, 1584–1598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami M, Sakurai T, Role of fibroblast growth factor signaling in vascular formation and maintenance: orchestrating signaling networks as an integrated system. Wiley Interdiscip Rev Syst Biol Med 4, 615–629 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Goetz R, Mohammadi M, Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol 14, 166–180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauto DF et al. , Aromatic Ring Dynamics, Thermal Activation, and Transient Conformations of a 468 kDa Enzyme by Specific 1H–13C Labeling and Fast Magic-Angle Spinning NMR. J Am Chem Soc 141, 11183–11195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyama Y et al. , Dynamic regulation of GDP binding to G proteins revealed by magnetic field-dependent NMR relaxation analyses. Nat Commun 8, 14523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner KH, Kay LE, The use of 2H, 13C, 15N multidimensional NMR to study the structure and dynamics of proteins. Annu Rev Biophys Biomol Struct 27, 357–406 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Kainosho M et al. , Optimal isotope labelling for NMR protein structure determinations. Nature 440, 52–57 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Wright PE, Dyson HJ, Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16, 18–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olbrich KC, Andersen TT, Blumenstock FA, Bizios R, Surfaces modified with covalently-immobilized adhesive peptides affect fibroblast population motility. Biomaterials 17, 759–764 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Wu DH, Chen AD, Johnson CS, An Improved Diffusion-Ordered Spectroscopy Experiment Incorporating Bipolar-Gradient Pulses. J Magn Reson Series A 115, 260–264 (1995). [Google Scholar]
- 33.Price WS, Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part 1. Basic theory. Concept Magn Reson 9, 299–336 (1997). [Google Scholar]
- 34.Palmer AG 3rd, Dynamic properties of proteins from NMR spectroscopy. Curr Opin Biotechnol 4, 385–391 (1993). [DOI] [PubMed] [Google Scholar]
- 35.Hou J, Madsen LA, New insights for accurate chemically specific measurements of slow diffusing molecules. J Chem Phys 138, 054201 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Hanwell MD et al. , Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4, 17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jong DH et al. , Improved Parameters for the Martini Coarse-Grained Protein Force Field. J Chem Theory Comput 9, 687–697 (2013). [DOI] [PubMed] [Google Scholar]
- 38.martinize.py v.2.0 (<http://cgmartini.nl/images/tools/martinize/martinize-2.6/martinize.py>).
- 39.Tang C, Smith AM, Collins RF, Ulijn RV, Saiani A, Fmoc-diphenylalanine self-assembly mechanism induces apparent pKa shifts. Langmuir 25, 9447–9453 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Abraham MJ et al. , GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015). [Google Scholar]
- 41.de Jong DH, Baoukina S, H. I. Ing0lfsson, S. J. Marrink, Martini straight: Boosting performance using a shorter cutoff and GPUs. Comput Phys Commun 199, 1–7 (2016). [Google Scholar]
- 42.Bussi G, Donadio D, Parrinello M, Canonical sampling through velocity rescaling. J Chem Phys 126, 014101 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR, Molecular dynamics with coupling to an external bath. J Chem Phys 81, 3684 (1984). [Google Scholar]
- 44.Topol A, Tran NN, Brennand KJ, A guide to generating and using hiPSC derived NPCs for the study of neurological diseases. J Vis Exp, e52495 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt EK, Clavarino G, Ceppi M, Pierre P, SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6, 275–277 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Hilton BJ, Blanquie O, Tedeschi A, Bradke F, High-resolution 3D imaging and analysis of axon regeneration in unsectioned spinal cord with or without tissue clearing. Nat Protoc 14, 1235–1260 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Rust R et al. , Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc Natl Acad Sci U S A 116, 14270–14279 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi Y et al. , FDISCO: Advanced solvent-based clearing method for imaging whole organs. Sci Adv 5, eaau8355 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fawzi NL, Ying J, Torchia DA, Clore GM, Kinetics of amyloid beta monomer-to-oligomer exchange by NMR relaxation. J Am Chem Soc 132, 9948–9951 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewandowski JR, Halse ME, Blackledge M, Emsley L, Protein dynamics. Direct observation of hierarchical protein dynamics. Science 348, 578–581 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present either in the main text or the supplementary materials.


