Abstract
Background.
SEER-reported cervical cancer incidence rates reflect the total female population including women no longer at risk due to hysterectomy. Hysterectomy rates have been declining in the United States as alternative treatments have become available, which could result in an apparent increase in SEER-reported cervical cancer rates. We aimed to obtain nationally representative historical data on hysterectomy rates in USA, use trends analysis to project rates back to 1935 and forward to 2035, and then predict the impact of changing hysterectomy rates on SEER-reported cervical cancer rates.
Methods.
We performed a systematic search of Medline, Embase, Premedline, Cochrane Central databases and extracted nationally-representative hysterectomy incidence data from 1965 to 2009, including data on the number of cervix-preserving (subtotal) procedures. We then projected rates back to 1935, and forward to 2035 based on trends from joinpoint regression. These rates were then used to estimate hysterectomy prevalence out to 2035, and then to predict the impact of changing hysterectomy rates on SEER-reported cervical cancer rates to 2035. We examined alternative assumptions regarding projected hysterectomy incidence rates out to 2035, including a scenario in which rates decline no further from 2009 rates, and a scenario where rates decline at twice the baseline rate.
Results.
Estimated age-standardized hysterectomy incidence increased from 2.4 to 10.6 per 1000 women between 1935 and 1975. Thereafter, rates are predicted to fall to 3.9 per 1000 by 2035. Subtotal hysterectomy procedures declined from being the predominant method in 1935 to less than 12% of procedures from 1970 onwards. Consequently, holding all else constant, an increase in SEER-reported age-standardized cervical cancer incidence rates (ages 0–85+) of 9% is expected from 2009 to 2035. The predictions were minimally impacted by alternative scenarios for future hysterectomy rates.
Conclusions.
Declining hysterectomy rates have implications for the interpretation of SEER-reported cervical cancer rates. A background increase in cervical cancer rates due to decreasing population hysterectomy exposure may partially offset expected decreases from recent cervical screening changes recommended by the US Preventive Services Task Force. Evaluations of new cervical cancer prevention opportunities should consider the background impact of historical and projected hysterectomy rates.
Keywords: Hysterectomy, Cervical cancer, Trends, USA
1. Introduction
Hysterectomy is one of the most common procedures performed in women in the United States, totalling around 600,000 procedures per year, with approximately 10% of procedures being subtotal (cervix-preserving) [1]. As laparoscopic assisted and robotic hysterectomies have become more common over the past few decades [2–4], the proportion of hysterectomies performed in an outpatient setting has increased [5,6]. Many hysterectomy procedures are performed for benign conditions such as leiomyomas, abnormal uterine bleeding and endometriosis, with only ~10% performed as treatment for cancer [5]. Over recent decades, alternative treatments for benign conditions have become more available. For instance, artery embolization is increasingly used for treatment of leiomyomas [7] and intra-uterine devices have been shown to be a successful alternative treatment for abnormal uterine bleeding [5]. More conservative surgical procedures have also become available for gynaecological cancers, allowing for the preservation of fertility in younger women [8]. Despite the recent decline in hysterectomy rates in the United States [5,6], hysterectomy procedures are still considered to be over-utilised; approximately 60% of hysterectomies are performed for conditions for which alternative therapies exist [9]. Of these, 38% have no documentation of alternative therapies tried before hysterectomy, and 18% had poorly documented indications for performing the hysterectomy [9].
Changes in population-level hysterectomy rates over time have important implications for the interpretation of incidence rates for cervical cancer, because women who have had hysterectomy with removal of the cervix are no longer at risk of the disease and cervical screening is not recommended for women without a cervix [10]. National reporting of cancer incidence in the Surveillance, Epidemiology and End Results (SEER) Program does not correct for the proportion of women who have undergone hysterectomy (which increases with age and is higher in black women versus white [11]), and therefore underestimates the underlying risk of these cancers, particularly in older women. Previous estimates of hysterectomy-adjusted rates of cervical cancer in older women have found that the adjusted incidence of cervical cancer in women aged 45 and over is significantly higher than rates reported by SEER [11]. Black women have higher rates of cervical cancer than white women, and this difference is even greater when considering rates in women without a cervix-removing hysterectomy, implying that the extent of disparity in outcomes by ethnicity has been underestimated [11,12]. Other studies have also shown that SEER-reported rates of endometrioid endometrial cancer have been increasing in the United States over the period 1992–2010 for women aged 20–49 years [13], but this trend was no longer significant after correction for hysterectomy, highlighting the importance of understanding the impact of changing hysterectomy on apparent trends in disease rates.
In addition to influencing the interpretation of current and past SEER-reported cancer rates, underlying assumptions about present and future hysterectomy rates have an important impact on modelled evaluations of future cervical cancer prevention policies, including HPV vaccination and cervical screening. In order to accurately predict the impact of new prevention strategies, models should incorporate estimates of hysterectomy incidence in the population over time [14,15]. Here, we aimed to i) estimate historical incidence rates of hysterectomy via a systematic search of the literature, taking into account subtotal hysterectomy procedures and outpatient procedures, and validate the implied prevalence over time against data from the Behavioral Risk Factor Surveillance System (BRFSS) survey; ii) project hysterectomy incidence rates (overall hysterectomies and subtotal hysterectomies) out to 2035 using Joinpoint regression to identify future trends under a range of assumptions; (iii) use projected estimates to evaluate year-on-year (cross-sectional) hysterectomy prevalence over the course of the century from 1935 to 2035, and (iv) use results from (iii) to estimate the direct impact of cervix-removing hysterectomy prevalence on the SEER-reported cervical cancer incidence rates to 2035.
2. Methods
2.1. Determining historical hysterectomy rates
2.1.1. Systematic literature search
Medline, Embase, Premedline, and Cochrane Central databases were searched to 30th September 2016 using terms for incidence, prevalence and trends, combined with hysterectomy and United States (Appendix Table 1). Titles and abstracts were scanned and potentially relevant articles retrieved. Data sources and references in retrieved articles were searched for further relevant data. Articles, or referenced data sources, were considered potentially relevant if they included incidence or prevalence of hysterectomy, by year and age-group, in the USA or part thereof. National data were preferred, then state-based, but articles examining other large databases, such as insurance data, were also retrieved, in particular if subgroups were examined (e.g., inpatient versus outpatient or total versus subtotal hysterectomy). Data sources which considered hysterectomy procedures in subsets of women only (e.g., hysterectomy procedures in women with fibroids only) were excluded. Data extracted and assessed included the source of data or name of the database coverage of the population of women included (national, state, other), availability of data by calendar year, whether incidence or prevalence was reported, availability of data by single year or grouped years of age of the women, indication for the hysterectomy, the proportion of procedures that were subtotal, and if surgery was performed as an inpatient or outpatient procedure.
2.1.2. Obtaining hysterectomy rates by age and year
Nationally-representative data on all inpatient hysterectomy procedures were collected, either from the literature or from databases, as summarized in Appendix Table 3. Data was available for most years from 1965 to 2009. For years in which data was missing, we used linear interpolation using data from nearby years. Data was available in 5-year age-groups for years 1965–1984 and 1998–2009, and wider age-group strata for intervening years. We generated 5-year age-group estimates for these intervening years by assuming that the age distribution within each 5-year age-group was equivalent to that observed for more detailed data points nearest to the missing years. As nationally-representative hysterectomy were based on inpatient data, we further adjusted these rates for years 2000 onwards to account for the unreported outpatient proportion, based on recent proportional estimates for the relevant year as reported by Doll et al. [6]. The hysterectomy data sources, time periods, years available and age groupings that we identified are summarized in Appendix Table 3 (a) for all hysterectomies.
2.1.3. Subtotal proportion
Subtotal proportions for the period 1935–1960 were estimated from a study performed in Los Angeles [16], which found that the proportion of hysterectomies performed as subtotal procedures decreased from 100% in 1940 to 29% by 1950–1959 (consistent with a historical account of hysterectomies, which described that almost all hysterectomies were subtotal during the early 1900's [17]). NHDS data was used to directly inform the proportion of hysterectomies that are subtotal for the years 1998–2009. Data from the NIS was used to inform the proportion of hysterectomies that are subtotal from 2010 to 2013 and for the period 1990–1993. The data sources, time periods, years available and age groupings available from the literature are summarized in Appendix Table 3 (b) for subtotal proportions.
2.1.4. Projecting rates back to 1935
We estimated the annual percentage change in our generated hysterectomy incident rates for the period 1965–2009 using the online joinpoint regression analysis software provided by the NIH (https://surveillance.cancer.gov/joinpoint/). Joinpoint was used to identify the lines of best fit to the data over the period 1965–2009, with multiple different linear fits connected by joinpoints. We identified three joinpoints with four different line fits over the four time-periods 1965–1974, 1974–1994, 1994–1997 and 1997–2009, as shown in Appendix Figure 1a. To estimate hysterectomy incidence rates back to 1935, we assumed the trend identified for the period 1965–1974, which was 4.1% increase in utilization per year, had predated 1965, consistent with data indicating that hysterectomy rates increased steadily from very low rates in the early 1900s [17,18].
2.2. Projecting hysterectomy rates to 2035
To project hysterectomy incidence rates beyond 2009, we assumed the trends identified from the most recent segment from the joinpoint analysis continues forward in time, which ended up being the period 1997–2009, with a 2.9% decrease per year in this timeframe. In the base case we assumed that hysterectomies could not fall by more than 30% of the rate observed in 2012 based on the estimate of the over-utilization of hysterectomies performed in the United States of 30% in 2012 [9]. We explored alternative trend assumptions going forward to capture uncertainties in future rates, including a ‘no further decline scenario’ whereby hysterectomy rates do not decline from 2009, and a ‘fast decline scenario’ whereby hysterectomy rates decline at twice the rate observed from 1975 to 2009, and have no lower limit by 2035.
2.2.1. Validation of historical estimates of hysterectomy incidence rates against BRFSS prevalence data
Using the derived incident hysterectomy procedures from 1935 onwards, we constructed a table of incident hysterectomy rates from 1935 to 2009 for each year and 5-year age-group, and used these cross-sectional rates to determine the rate of hysterectomy by birth cohort. We then determined the cumulative proportion of women who have ever undergone a hysterectomy by age for each birth cohort (birth cohorts 1900–2000, assuming no hysterectomies in women under ages 35 years for birth cohorts 1900–1935 and considering only data up to year 2009) and used these birth-cohort hysterectomy incidence rates to produce hysterectomy prevalence estimates for each age and birth cohort.
We then tabulated these birth cohort-specific prevalence rates to produce cross-sectional prevalence estimates, and compared these with self-reported prevalence data by 5-year age-group for women aged 15–94 from BRFSS for years 1988–1990, 1998–2000 and 2008–2009. We also obtained self-reported hysterectomy prevalence data for ages 20–84 for the year 2016 through BRFSS, and validated our estimated hysterectomy prevalence for this year.
Due to the substantial increase in outpatient hysterectomy procedures during early 2000's, we were unable to obtain nationally-representative hysterectomy incidence rates after 2009 as many of the existing data sources (such as NHDS) represent inpatient data only. In 2018, the Healthcare Cost and Utilization Project (HCUP) published a report on hysterectomy rates for both inpatient and outpatient settings using data from five states, and estimated rates for these states during 2010–2013 for ages 18–34, 35–54 and 55+ [21]. We assumed that BRFSS data collected for 2016 would not be impacted by the increase in outpatient hysterectomy procedures, as the survey relies on self-reported questions about ever having had a hysterectomy.
2.3. Modeling the impact of changing hysterectomy rates on reported cervical cancer incidence rates
We used the generated hysterectomy incidence rates from 1935 to 2035, along with the proportion of subtotal hysterectomy procedures, to estimate the prevalence of women who have had a cervix-removing hysterectomy out to 2035. Using these prevalence estimates, we predict the impact of changing hysterectomy rates on SEER-reported cervical cancer incidence rates from 2009 to 2035 (excluding the impact of other factors on cervical cancer rates).
3. Results
3.1. Determining historical hysterectomy rates, and projecting rates out to 2035
3.1.1. Systematic literature search
We identified 1852 publications and reviewed 225 in detail; cited articles of potential interest were also examined. Articles with potentially relevant data are summarized in Appendix Table 2. Key databases identified in the search that were accessed to search for further relevant data included the National Hospital Discharge Survey (NHDS) [19] from the Centers for Disease Control and Prevention (CDC), the National Inpatient Sample (known as the Nationwide Inpatient Sample before 2012) (NIS) [1], the State Inpatient Databases SIDs [20] from the Healthcare Cost and Utilization Project, which include inpatient discharges from community hospitals, and the State Ambulatory Surgery and Services Databases (SASDs) [21] from the HCUP which include data outpatient services.
3.1.2. Estimation of hysterectomy rates by age and year, and the proportion that are subtotal
Using the above databases, we extracted nationally representative data on inpatient hysterectomy procedures for most years from 1965 to 2009 (Appendix Table 3 and Fig. 1). After interpolating hysterectomy incidence data for missing years, projecting trends in rates back to 1935 and forward to 2035, we produced a continuous timeline of hysterectomy rates as shown in Fig. 2. We estimated that hysterectomy incidence rates increased from 2.4 per 1000 women in 1935 to reach a peak of 10.6 per 1000 women in 1975, and thereafter declined steadily to 3.9 per 1000 by 2035 (Fig. 2). By 2035, we predicted that hysterectomy incident rates will be ~32% lower than rates from 2012, and therefore we did not further adjust our projections because the reductions did not significantly exceed the upper limit of 30% reduction from 2012 rates. Although we relied on national data up to 2009, hysterectomy incidence rates for some states have been reported for 2010–2013; during the years 2010–2013, we estimated that the rate of incident hysterectomy procedures in 18–34 year-olds ranged from 185 to 200 per 100,000 women (estimated range is the minimum - maximum rate across years 2010, 2011, 2012 and 2013), similar to the rate reported by HCUP in five US states of 195 per 100,000 women [21]. For ages 35–54 we estimated incident hysterectomy rates of 988 to 1029 per 100,000, which are ~10–15% higher than that observed across five states as reported by HCUP (reported at 872 per 100,000).
Fig. 1.
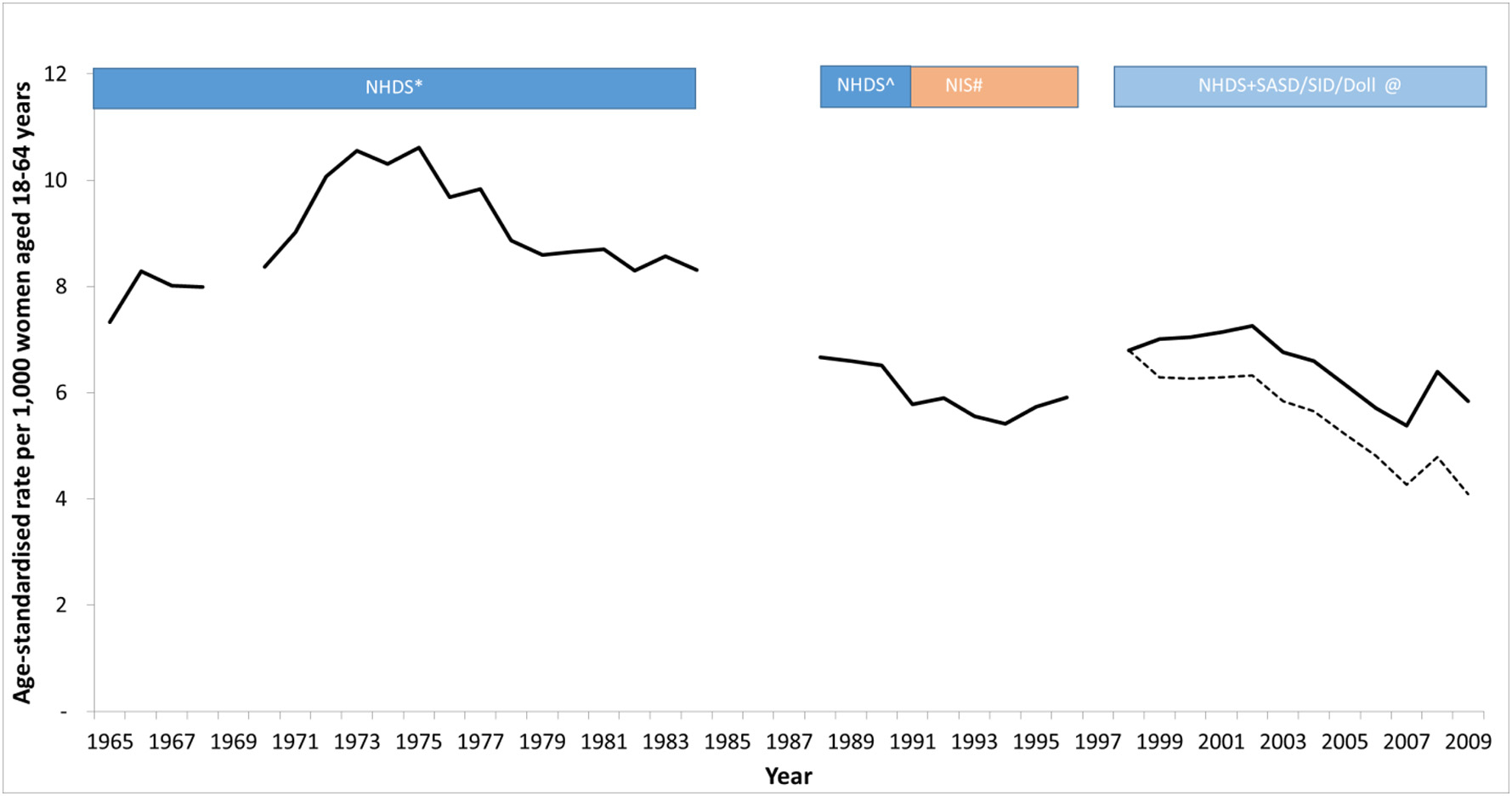
Reported hysterectomy incidence rates (both subtotal and cervix-removing procedures) per 1000 women aged 18–64 years in USA. Data were obtained from analysis of national inpatient datasets from 1965 to 2009. For 2000–2009, the crude reported data from inpatient procedures is shown as the dashed black curve, and the scaled hysterectomy incidence rate after accounting for the under-reported proportion of outpatient procedures is shown as the solid black curve. *Obtained from published rates [22] reported by 5-year age-group and for each individual year from 1965 to 1984 but missing the year 1969. Ôbtained from published rates [23] reported over ages 15+ as one group and across years 1988–1990 as one group. We re-scaled the rates to represent rates for females aged 18–64 (with the assumption that all hysterectomy procedures were performed in this age-range) and assumed that the same number of procedures occurred over the period 1988–1990 each year. #Obtained from published rates [5] reported over ages 15+ as one age-group and for each individual year from 1990 to 1997. We re-scaled the rates to represent rates for females aged 18–64 (with the assumption that all hysterectomy procedures were performed in this age-range). @Obtained from publicly available datasets [27] reported for each year-of-age from 15 to 99 and for each individual year from 1998 to 2009. Raw hysterectomy incidence rates were obtained for inpatient procedures only, and these raw rates are shown as the dashed black curve for 2000–2009. Hysterectomy rates were further adjusted to account for the unreported proportion of hysterectomies that are performed in an outpatient setting, and these corrected rates are shown as the solid black curve.
Fig. 2.
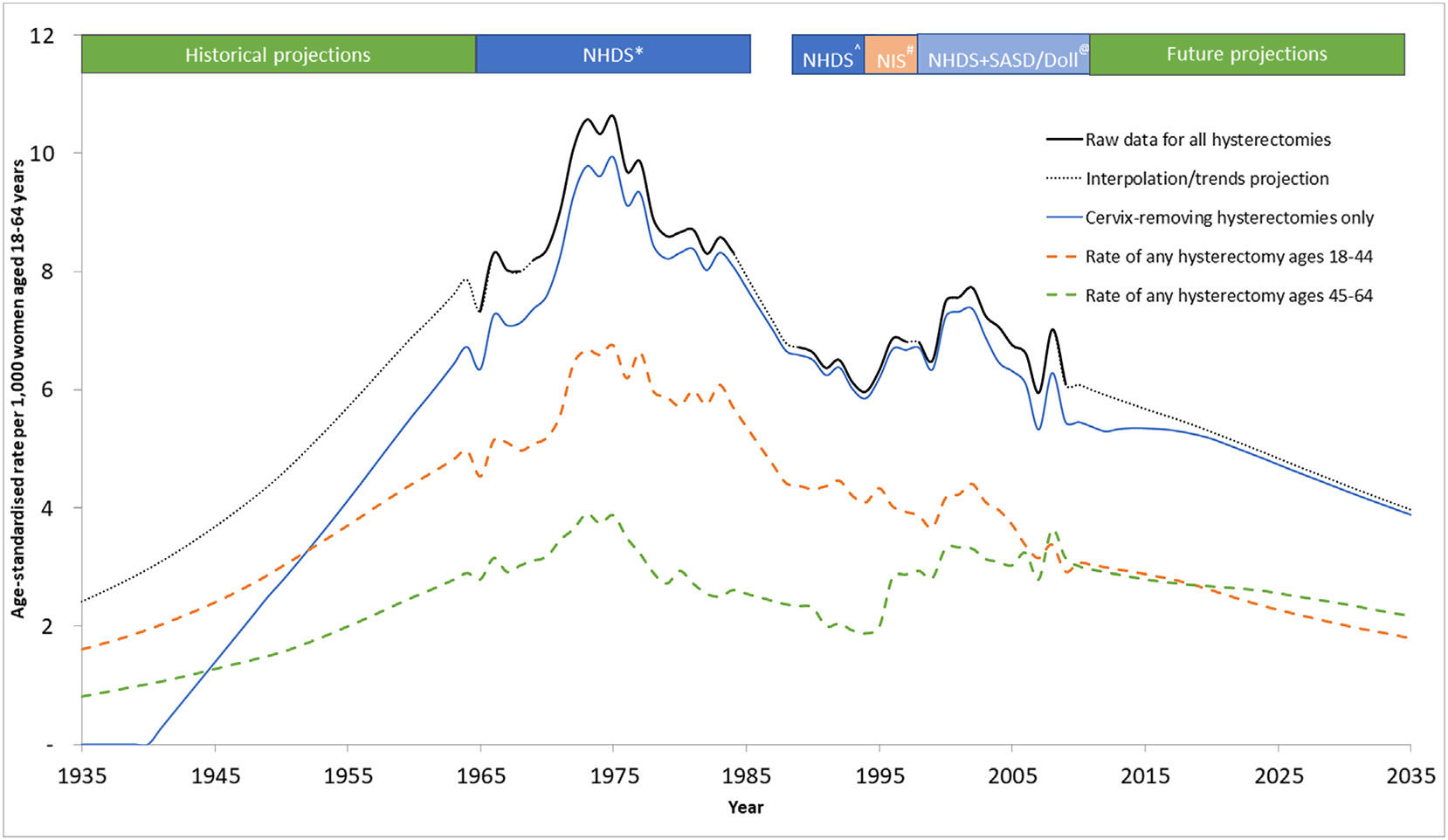
Estimated incidence rate of hysterectomy procedures (both subtotal and cervix-removing procedures) per 1000 women aged 18–64 years in USA from 1935 to 2035^^ ^^ Rates for all hysterectomies are shown as the combined black-solid (real-world informed) and black-dashed (interpolated/trends projection) line. The national datasets used to inform the real-world data segments are listed in the coloured region above the graph. Hysterectomy procedures that are cervix-removing are shown on the graph in the blue solid line. Hysterectomy procedures of any type in females aged 18–44 and 44–64 are shown as the dashed orange and dashed green lines respectively. *Obtained from published rates [28] reported by 5-year age-group and for each individual year from 1965 to 1984, but missing the year 1969. Ôbtained from published rates [29] reported over ages 15+ as one group and across years 1988–1990 as one group. We re-scaled the rates to represent rates for females aged 18–64 (with the assumption that all hysterectomy procedures were performed in this age-range) and assumed that the same number of procedures occurred over the period 1988–1990 each year. #Obtained from published rates [3] reported over ages 15+ as one group and for each individual year from 1990 to 1997. We re-scaled the rates to represent rates for females aged 18–64 (with the assumption that all hysterectomy procedures were performed in this age-range). @Obtained from the publicly available datasets [27] reported for each year-of-age from 15 to 99 and for each individual year from 1998 to 2009. Hysterectomy rates were further adjusted to account for the unreported proportion of hysterectomies that are performed in an outpatient setting.
A graphical representation of the subtotal proportion of hysterectomies is shown in Fig. 3. This figure indicates a small increase in the subtotal proportion during years 2000–2010, which is consistent with the contemporaneous understanding that subtotal procedures preserved sexual and urinary function. However, a subsequent Cochrane review [23] showed that there is little evidence of this effect; furthermore, subtotal laparoscopic hysterectomies have been shown to produce more long term complications when compared to cervix-removing laparoscopic hysterectomies [24]. As a consequence, subtotal proportions declined again in 2010–2013. We assumed that from 2013 onwards, subtotal hysterectomy rates will decline to pre-2000 levels and remain at this rate.
Fig. 3.
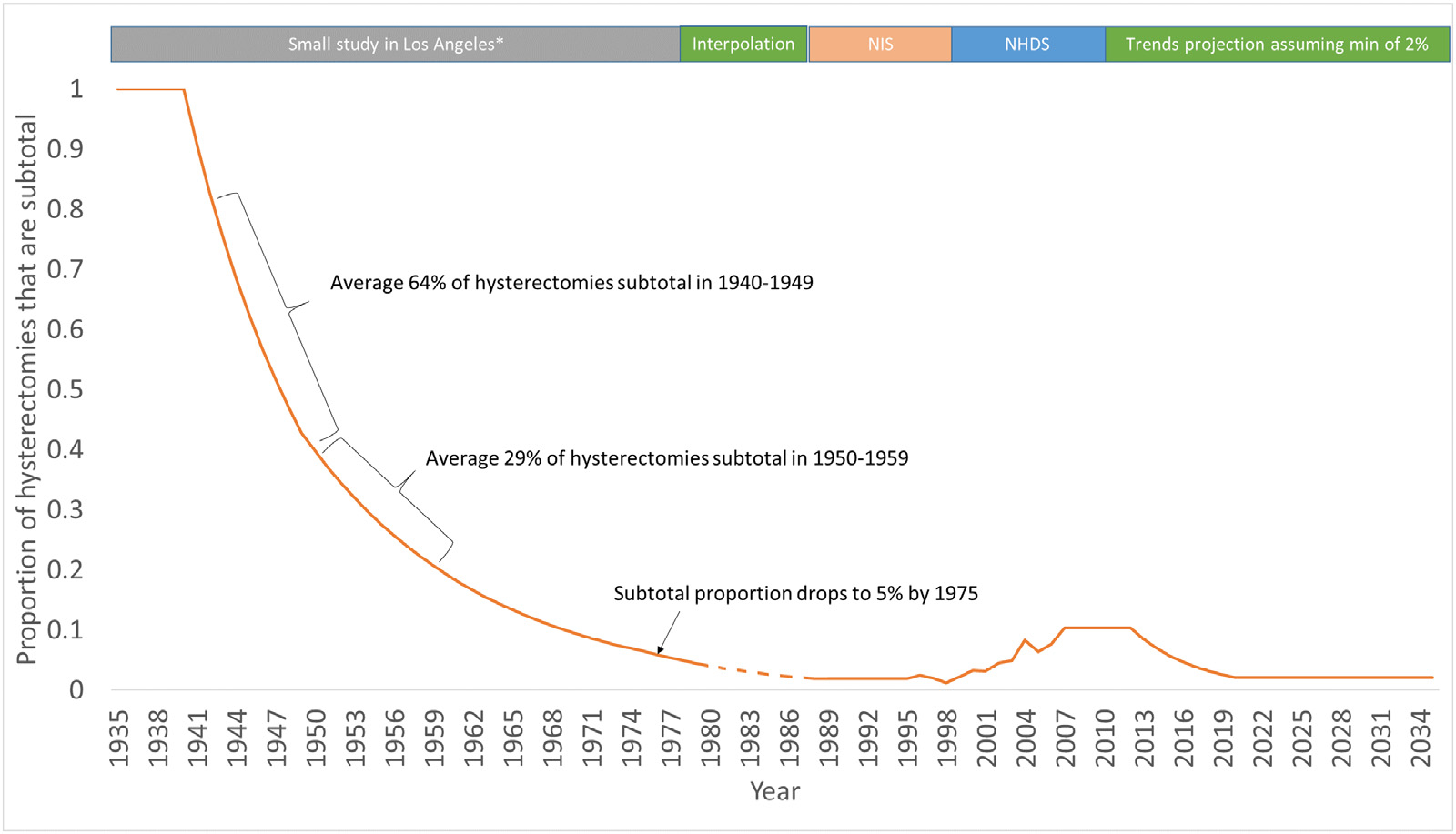
Estimated proportion of hysterectomies that are subtotal. *A small Los-Angeles study [16] reported a declining proportion of subtotal hysterectomies from 100% in 1940, to 64% of procedures between 1940–1949, 39% of procedures over 1950–1959 and dropping to 5% in 1975. @ NIS data was used to inform hysterectomy procedures from 1992–1995 [30]. ** NHDS data was used to directly inform the proportion of hysterectomies that are subtotal for the years 1997–2009 with data obtained from publicly available datasets [27] reported for each year-of-age from 15 to 99 and for each individual year. ^ Data from the NIS was used to inform the proportion of hysterectomies that are subtotal from 2009 to 2013. The proportion of hysterectomies that are subtotal during 1990–1993 was based on NIS data.
3.1.3. Validation of historical hysterectomy incidence rates and projected future rates
The estimated historical incident hysterectomy rates were used to generate age-specific prevalence estimates for the years 1988–1990, 1998–2000 and 2008–2009 for validation against BRFSS data for these years. The estimated hysterectomy prevalence compares well against the more recent BRFSS data reported over the 20-year period (Fig. 4a), although in general, our estimated prevalence is a little lower than BRFSS reported data for ages 65 and over. We also compared our hysterectomy prevalence estimates for the year 2016 against BRFSS reported data on hysterectomy prevalence in this year, which compared well against the data for all ages available (Fig. 4b).
Fig. 4.
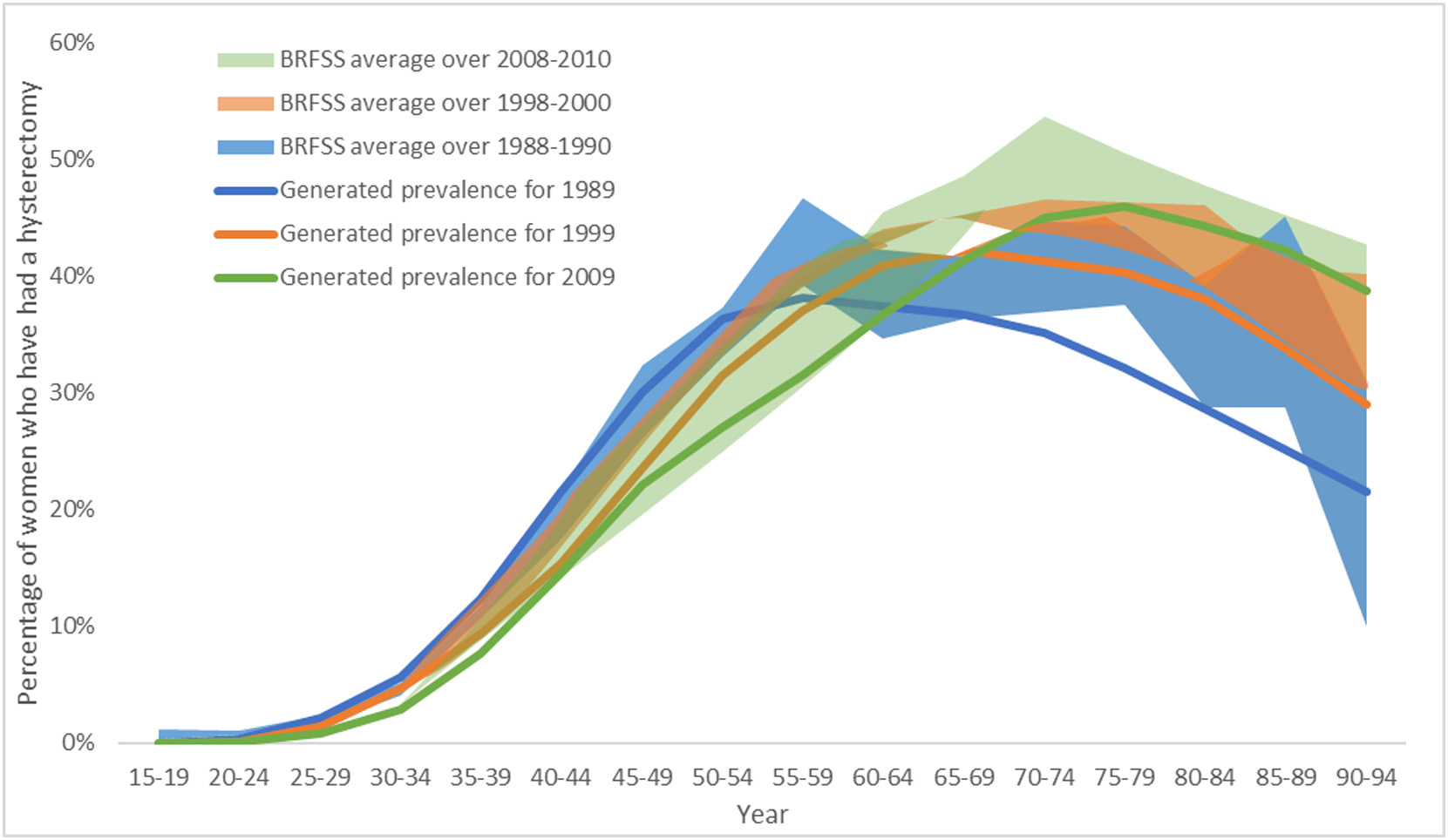
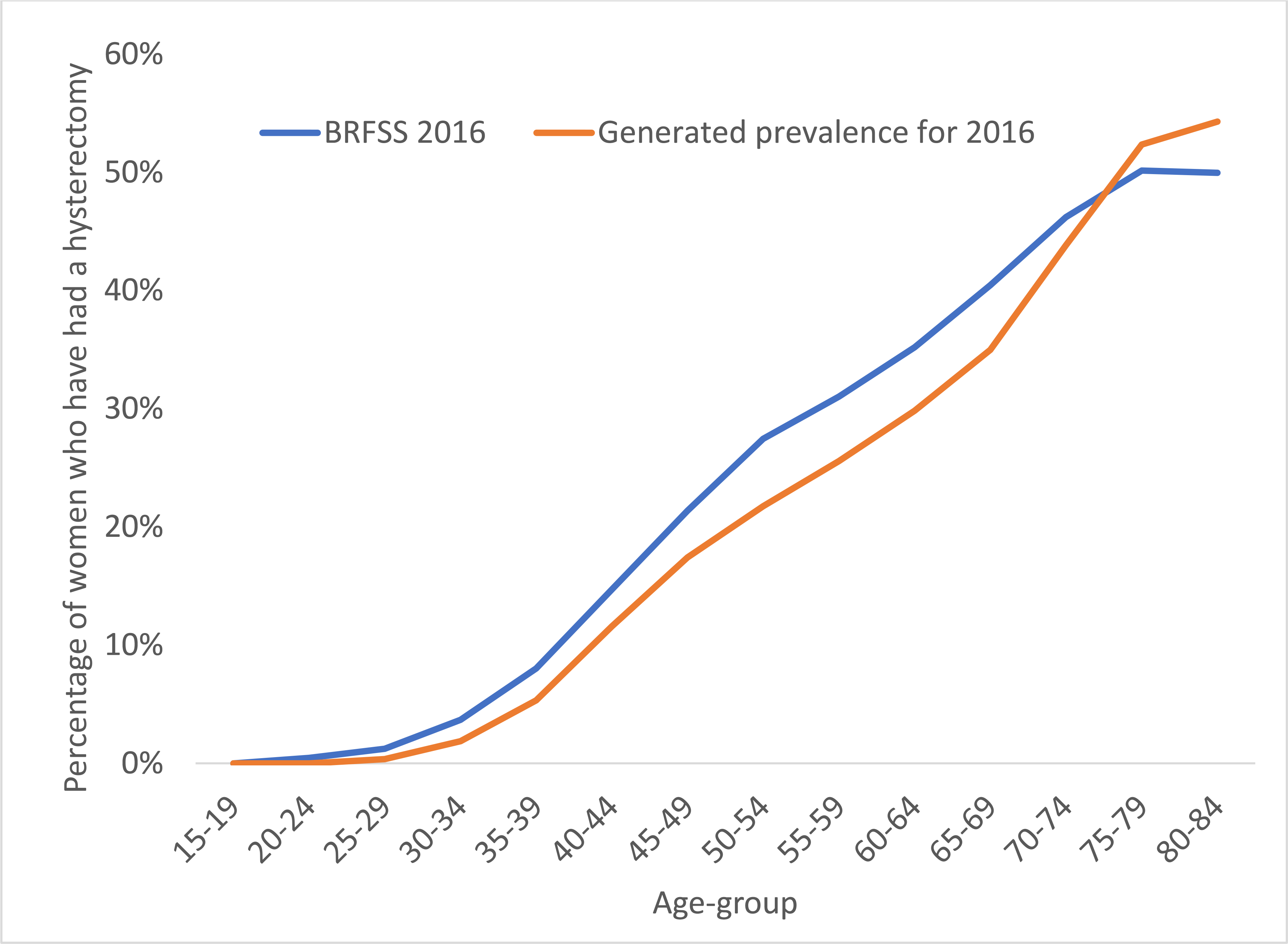
Age-specific hysterectomy prevalence estimates (including subtotal and cervix-removing procedures) as reported by BRFSS (a) for the years shown in shaded regions for 1988–1990 (blue), 1998–2000 (orange) and 2008–2010 (green), and (b) for the year 2016. Estimated historical hysterectomy incidence rates were used to produce cross-sectional prevalence, shown as the solid lines for years 1998 (blue), 1999 (orange) and 2009 (green).
3.2. Modeling the impact of changing hysterectomy rates on cervical cancer incidence rates to 2035
The proportion of women who have ever had a cervix-removing hysterectomy is predicted to decline between 2009 and 2035 for women aged <80 years (Fig. 5a). We predict that hysterectomy prevalence will decline substantially for ages 30–79, ranging from 20 to 45% decline between 2009 and 2035 across these age-groups. Due to changes in hysterectomy prevalence alone (i.e., excluding changes to underlying risk or considering the impact of prevention policies such as HPV vaccination or changes to cervical screening recommendations or practice), age-standardized cervical cancer rates are predicted to increase gradually from an ASR of 7.2 per 100,000 women in 2009 to 7.8 per 100,000 women in 2035 in females aged 0–85+ years (a 9% increase) (Fig. 5b). HPV vaccination commenced in the United States in 2006 and was available to those aged 9–26 years. If we consider the ASR for women who were not age-eligible for HPV vaccination as young females (ages 55+ in 2035), SEER-reported ASR cervical cancer incidence rates are predicted to increase from 9.8 per 100,000 women in 2009 to 11.3 per 100,000 women in 2035 (16% increase).
Fig. 5.
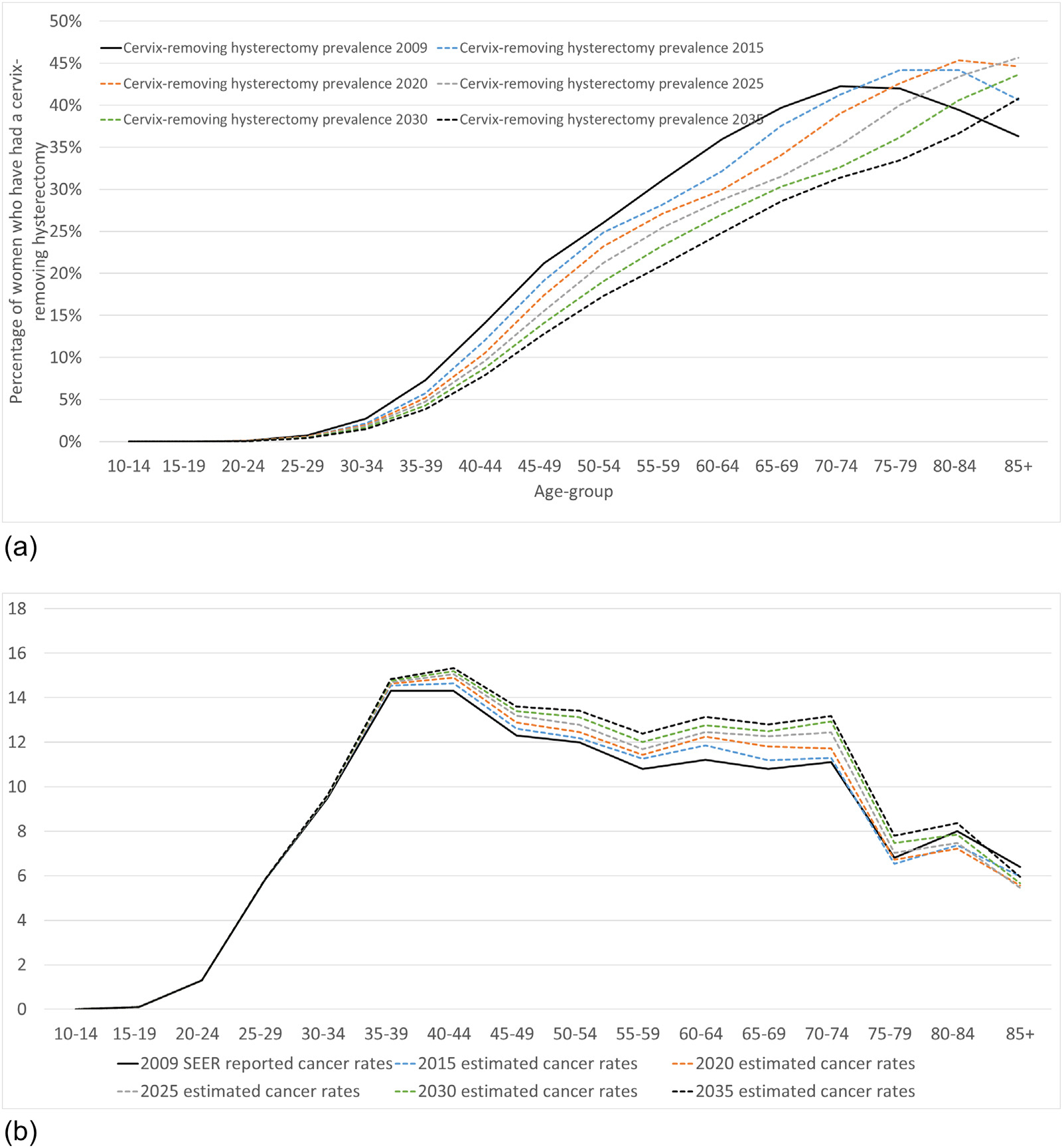
(a) Estimated prevalence of cervix-removing hysterectomies between 2009 and 2035 and (b) projected age-specific rate of cervical cancer incidence from 2009 to 2035.
Varying the assumptions about future trends in hysterectomy, including no further decline in hysterectomy rates from 2009 levels, or double the rate of decline from 2009 levels, had limited impact on cancer rates for women in 2035 (Fig. 6). This is because prevalence estimates for the year 2035 are largely dependent on hysterectomy procedures already performed well before 2035. Therefore, the impact of varying hysterectomy rates between 2009 and 2035 on estimated cervical cancer rates over the next few decades is limited. In addition to investigating the impact of future hysterectomy rates on cervical cancer rates in 2035, we also estimated breakdowns by ethnicity in cervical cancer rates for the year 2035, shown in the Appendix. In this exploratory analysis, we assumed that disparities in cervical cancer risk and hysterectomy incidence rates in black women observed in 2009 persist out to 2035. Under these assumptions, we estimate that SEER-reported ASR (0–85+) of cervical cancer incidence in black women will reach 10 per 100,000 in 2035, compared to 7.7 per 100,000 in white women in 2035 (28% higher rates in black women). For ages 55+, the ASR is predicted to reach 20 per 100,000 in black women in 2035 versus 10.3 per 100,000 in white women (73% higher rates in black women). These findings indicate that SEER-reported cervical cancer rates in black American women will be substantially higher by 2035. These predictions are based on the assumption that disparities in hysterectomy incidence rates and disparities in cervical cancer rates among at-risk women remain unchanged from years 2009–2035.
Fig. 6.
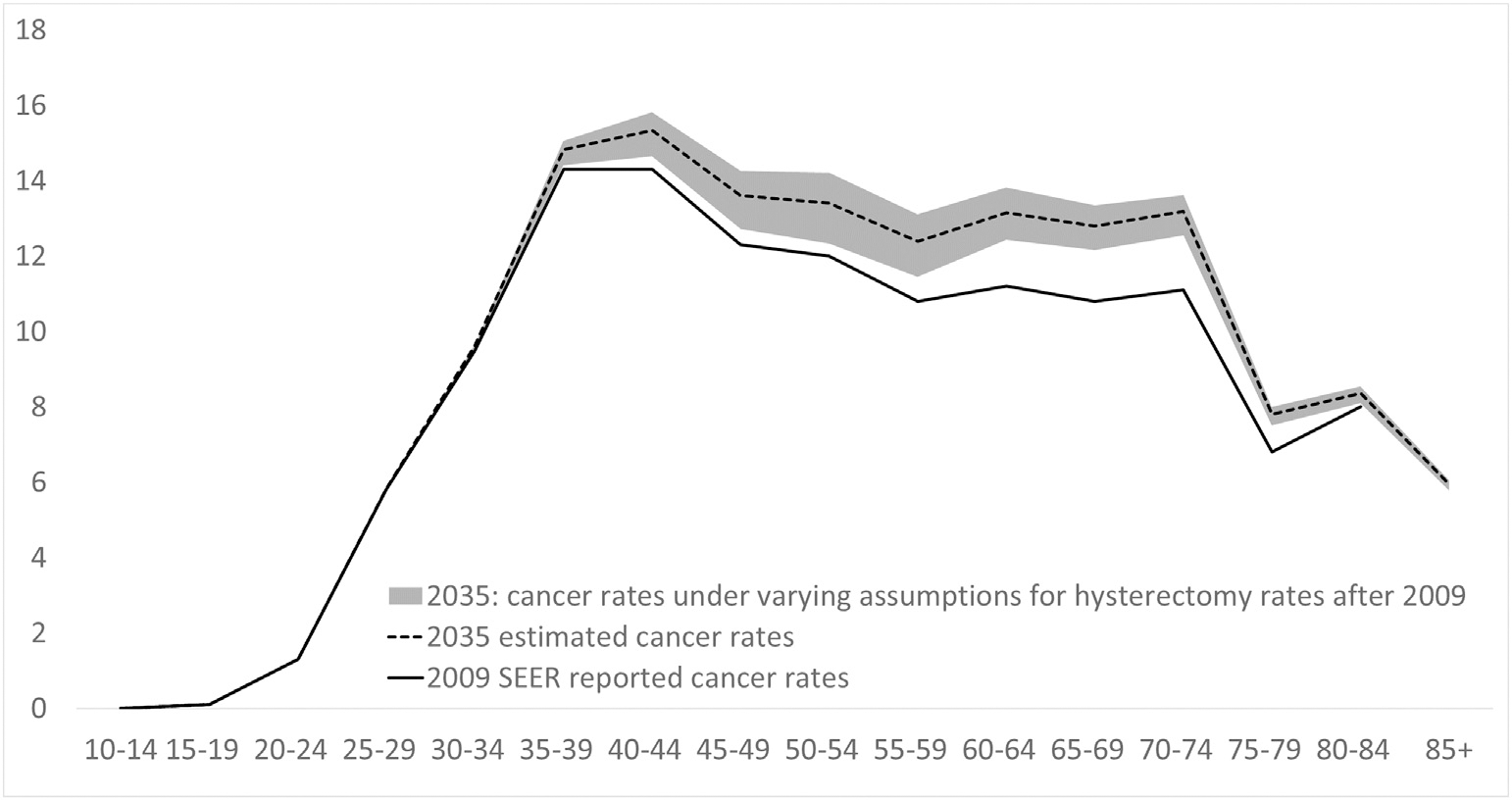
Age-specific cervical cancer incidence rates projected for the year 2035, assuming baseline trends (solid black curve) and taking into account alternative assumptions for projections after 2009 (shaded grey regions) ^The re-estimated cancer incidence rates are based on changing hysterectomy prevalence alone. *shaded grey area represents the potential range of cervical cancer rates under a wide range of trends assumptions (0% change from 2009 versus double the rate of decline in hysterectomy rates from 2009 to 2035 with no lower bound). The dashed black line represents the baseline estimate of cancer rates in 2035. The black solid line represents SEER reported cancer rates for 2009.
4. Discussion
To our knowledge, this is the first analysis to synthesize the data on historical hysterectomy incidence rates to provide future projections of hysterectomy rates and to estimate the impact on future SEER-reported cervical cancer incidence rates. We performed a systematic search of the literature, incorporating data from over 225 articles from the years 1965–2016, as well as data obtained directly from key online data sources, and importantly, we successfully validated our estimated prevalence from these sources against an independent source of information for more recent years, the BRFSS survey. Our estimates suggest that the annual incidence of hysterectomy increased from 2.4 per 1000 women in 1935 to a peak of 10.6 per 1000 by 1975 and based on observed data as available and on information on the most recent trends, we have predicted that thereafter rates will decline steadily, to a predicted 3.9 per 1000 by 2035. Due to these changes in hysterectomy rates alone, we predicted that between 2009 and 2035, SEER-reported cervical cancer incidence rates, which are calculated at the population level and do not correct for women who have had a cervix-removing hysterectomy, will increase by 9% for women aged 10–84 years and 16% for women 55–84 years. As other policy changes with regard to HPV vaccination and cervical screening are likely to impact reported cervical rates in this timeframe, it is important to characterize the background effect of hysterectomy in interpreting future trends in cervical cancer rates.
There are several limitations to our analysis. We found no single database with comprehensive information on national coverage for both inpatient and outpatient procedures, and age-based information on the proportion of subtotal procedures. However, we were able to synthesize age-specific data from a wide range of nationally representative data sources to estimate hysterectomy incidence and prevalence over an entire century. Furthermore, the generated prevalence estimates were successfully validated against self-reported prevalence of hysterectomy in women from the BRFSS survey for more recent years. Although there a major uncertainties in future hysterectomy trends, we did consider the impact of varying future trends assumptions from 2009 to 2035 and found these varying assumptions had only a small impact on the predicted cervical cancer rates by 2035 because the prevalence estimates for future years were largely dependent on hysterectomy procedures already performed prior to 2009. In an initial exploratory analysis, we also provided estimates for cervical cancer incidence rates separately for black women and white women for the year 2035. Previous studies have identified higher hysterectomy rates in black women [11,12], and we found that if disparities in cervical cancer rates and hysterectomy incidence rates in black women persist out to 2035, then ASR of cervical cancer for ages 0–85+ will be 28% higher in black women versus white women in 2035, and the ASR for ages 55+ will be 73% higher for black women in 2035. Finally, in estimating future cervical cancer rates we did not account for changes to cervical screening or HPV vaccination. Guidelines for cervical cancer screening in the United States were revised in 2012 to incorporate 5-yearly cotesting with cytology and HPV in women aged 30–65 [25]. More recently, the U.S. Preventive Services Task Force recommended screening every 3 years with cytology alone in women aged 21–29 years, with or without a switch to every 5-years with high-risk HPV testing alone, or every 5-years with cotesting, in women aged 30–65 years [10]. Although HPV vaccination was introduced in 2007, the first cohorts to be offered this vaccine (age 26 years in 2007) will be <54 years of age in 2035, and so vaccination will unlikely be able to counteract the predicted increase in cervical cancer rates for women aged 55+ in 2035.
Our analysis shows how changing hysterectomy rates can influence reported cancer rates and our predicted background increase in cervical cancer rates from 2009 to 2035 due to the impact of changing hysterectomy rates alone has implications for assessing the success of new prevention strategies. It is anticipated that HPV vaccination and the recent USPSTF recommendations will have a favourable impact on future cervical cancer rates [26]. However, at a population level these reductions may appear to be counteracted in part by declining hysterectomy rates, particularly among older women. Furthermore, current screening guidelines do not recommend continued screening for women over age 65 years who have an adequate history of normal tests, and HPV vaccine impact will not be seen in this age group for many decades. Therefore, it is important that future modelled evaluations of new prevention options explicitly detail their assumptions in relation to hysterectomy uptake, and consider explicitly taking these projections for past and future rates of hysterectomy into account. A range of analyses are currently being performed by the Cancer Intervention and Surveillance Modeling Network (CISNET-Cervical), which involves five teams performing comparative modeling evaluations to identify optimal cancer prevention strategies, and these evaluations will harness the estimates of hysterectomy uptake reported here.
Supplementary Material
HIGHLIGHTS.
Declining rates of hysterectomy have important impacts on SEER-reported cervical cancer rates.
We estimate hysterectomy rates increased from 2.4 to 10.6 per 1000 women between 1935 and 1975, dropping to 3.9 by 2035.
Consequently, holding all else constant, a 9% increase in SEER-reported cervical cancer rates are expected from 2009 to 2035.
Declining hysterectomy rates may partially offset expected reductions in cervical cancer after changes in screening.
Acknowledgments
The authors would like to thank Dr Julia Steinberg for her advice on statistical trends analysis.
Funding sources
Research reported in this publication was supported by the U.S. National Cancer Institute of the National Institutes of Health under Award Number U01CA199334 (CISNET-Cervical). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of competing interest
KS receives salary support from the Cancer Institute. MAS receives salary support from the National Health and Medical Research Council (Australia) and the Cancer Institute NSW. KC is co-PI of an unrelated investigator-initiated trial of cytology and primary HPV screening in Australia (‘Compass’), which is conducted and funded by the VCS Foundation, a government-funded health promotion charity. In 2013, the VCS Foundation received equipment and a funding contribution for the Compass trial from Roche Molecular Systems and Ventana Inc. USA. However, neither KC nor her institution (Cancer Council NSW) receives direct funding from industry for this trial or any other project. All other authors declare no conflicts of interest.
Footnotes
Appendix. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.05.030.
References
- [1].Agency for Healthcare Research and Quality R, MD, HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP), https://www.hcupus.ahrq.gov/nisoverview.jsp. (Accessed 9 October 2017).
- [2].Reich H, Decaprio J, McGlynn F, Laparoscopic hysterectomy J Gynecol. Surg. 5 (1989) 213–216. [Google Scholar]
- [3].Farquhar CM, Steiner CA, Hysterectomy rates in the United States 1990–1997, Obstet. Gynecol. 99 (2) (2002) 229–234. [DOI] [PubMed] [Google Scholar]
- [4].Wright JD, Ananth CV, Lewin SN, et al. , Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease, Jama. 309 (7) (2013) 689–698. [DOI] [PubMed] [Google Scholar]
- [5].Wright JD, Herzog TJ, Tsui J, et al. , Nationwide trends in the performance of inpatient hysterectomy in the United States, Obstet. Gynecol. 122 (201) (2013) 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Doll KM, Dusetzina SB, Robinson W, Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000–2014, JAMA Surg. 151 (9) (2016) 876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gupta JK, Sinha A, Lumsden M, Hickey M, Uterine artery embolization for symptomatic uterine fibroids, The Cochrane Library2014. [DOI] [PubMed] [Google Scholar]
- [8].McLaren JF, Bates GW, Fertility preservation in women of reproductive age with cancer, Am. J. Obstet. Gynecol. 207 (6) (2012) 455–462. [DOI] [PubMed] [Google Scholar]
- [9].Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am. J. Obstet. Gynecol..212(3):304.e301–304.e307. [DOI] [PubMed] [Google Scholar]
- [10].Force UPST, Screening for cervical cancer: US preventive services task force recommendation StatementUSPSTF recommendation: screening for cervical CancerUSPSTF recommendation: screening for cervical cancer, JAMA. 320 (7) (2018) 674–686. [DOI] [PubMed] [Google Scholar]
- [11].Rositch AF, Nowak RG, Gravitt PE, Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009, Cancer. 120 (13) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Merrill RM, Impact of hysterectomy and bilateral oophorectomy on race-specific rates of corpus, cervical, and ovarian cancers in the United States, Ann. Epidemiol. 16 (12) (2006) 880–887. [DOI] [PubMed] [Google Scholar]
- [13].Temkin SM, Kohn EC, Penberthy L, et al. , Hysterectomy-corrected rates of endometrial cancer among women younger than age 50 in the United States, Cancer Causes Control 29 (4–5) (2018) 427–433. [DOI] [PubMed] [Google Scholar]
- [14].Simms KT, Smith MA, Lew JB, Kitchener HC, Castle PE, Canfell K, Will cervical screening remain cost-effective in women offered the next generation nonavalent HPV vaccine? Results for four developed countries, Int. J. Cancer 139 (12) (2016) 2771–2780. [DOI] [PubMed] [Google Scholar]
- [15].Kim JJ, Burger EA, Sy S, Campos NG, Optimal cervical cancer screening in women vaccinated against human papillomavirus, J. Natl. Cancer Inst. 109 (2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stern E, Misczynski M, Greenland S, Damus K, Coulson A, “Pap” testing and hysterectomy prevalence: a survey of communities with high and low cervical cancer rates, Am. J. Epidemiol. 106 (4) (1977) 296–305. [DOI] [PubMed] [Google Scholar]
- [17].Sutton C, Past, present, and future of hysterectomy, J. Minim. Invasive Gynecol 17 (4) (2010) 421–435. [DOI] [PubMed] [Google Scholar]
- [18].Morgan DM, Kamdar NS, Swenson CW, Kobernik EK, Sammarco AG, Nallamothu B, Nationwide trends in the utilization of and payments for hysterectomy in the United States among commercially insured women, Am. J. Obstet. Gynecol. 218 (4) (2018) 425.e421–425.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention (CDC), National Hospital Discharge Survey (NHDS), https://www.cdc.gov/nchs/nhds/. (Accessed 9 October 2017).
- [20].Agency for Healthcare Research and Quality R, MD, HCUP State Inpatient Databases (SID). Healthcare Cost and Utilization Project (HCUP), https://www.hcup-us.ahrq.gov/sidoverview.jsp. (Accessed 9 October 2017).
- [21].Agency for Healthcare Research and Quality R, MD, HCUP State Ambulatory Surgery and Services Databases (SASD). Healthcare Cost and Utilization Project (HCUP), https://www.hcup-us.ahrq.gov/sasdoverview.jsp. (Accessed 9 October 2017).
- [22].Moore BJ, Steiner CA, Davis PH, Stocks C, Barrett ML, Trends in hysterectomies and Oophorectomies in hospital inpatient and ambulatory settings, 2005–2013: statistical brief# 214, Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville (MD): Agency for Healthcare Research and Quality, 2006. [PubMed] [Google Scholar]
- [23].Lethaby A, Mukhopadhyay A, Naik R, Total versus subtotal hysterectomy for benign gynaecological conditions, The Cochrane Database of Systematic Reviews, 4, 2012, p. Cd004993. [DOI] [PubMed] [Google Scholar]
- [24].van Evert JS, Smeenk JMJ, Dijkhuizen FPHLJ, de Kruif JH, Kluivers KB, Laparoscopic subtotal hysterectomy versus laparoscopic total hysterectomy: a decade of experience, Gynecol. Surg. 7 (1) (2010) 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saslow D, Solomon D, Lawson HW, et al. , American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer, J Low Genit. Tract Dis. 16 (3) (2012) 175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim JJ, Burger EA, Regan C, Sy S, Screening for cervical cancer in primary care: a decision analysis for the US preventive services task ForceUSPSTF modeling study: screening for cervical cancer in primary CareUSPSTF modeling study: screening for cervical cancer in primary care, JAMA. 320 (7) (2018) 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].National Hospital Dicharge Survey (NHDS), Questioonnaires, datasets and related documentation. 1998–2009, https://www.cdc.gov/nchs/nhds/nhds_questionnaires. (Accessed 11 March 2017).
- [28].Pokras R, Hufnagel VG, Hysterectomies in the United States, Vital Health Stat. 13 (92) (1987) 1–32. [PubMed] [Google Scholar]
- [29].Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB, Hysterectomy in the United States, 1988–1990, Obstet. Gynecol. 83 (4) (1994) 549–555. [DOI] [PubMed] [Google Scholar]
- [30].Merrill RM, Hysterectomy surveillance in the United States, 1997 through 2005, Med. Sci. Monit. 14 (2008) CR24–CR31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


