Abstract
Background
Despite the extraordinary speed of mass vaccination efforts, an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant in a vaccinee with coronavirus disease 2019 (COVID-19) mRNA vaccine was identified in an adult day service center (ADSC) of Jeju, South Korea. The primary objective of this study was to investigate the epidemiologic features in infection-vulnerable facilities with a high vaccination rate of BNT162b2 mRNA COVID-19 vaccine. The second was to estimate the secondary transmission prevention effect of the vaccine in the household members by vaccination status.
Methods
We included all ADSC participants, staff and their household members. All COVID-19 infected cases were confirmed by reverse transcriptase polymerase chain reaction. We calculated attack rate in ADSC and the secondary attack rate (SAR) in household members by vaccination status.
Results
Among a total of 42 participants and 16 staff, of which 96.6% were fully vaccinated with BNT162b2 mRNA COVID-19 vaccine, 12 symptomatic cases and 13 asymptomatic confirmed cases of COVID-19 were found. The attack rate was 43.1%, with 13 isolates identified as SARS-CoV-2 virus, delta variant. The SAR in unvaccinated and partially vaccinated household members were 27.8% (5/18) and 25.0% (5/20), respectively, while the SAR in fully vaccinated household members was 12.5% (1/8).
Conclusion
We describe a SARS-CoV-2 delta variant outbreak in ADSC with high vaccine coverage rate, characterized by high infection rate, high transmissibility, and low clinical severity. The outbreak proceeded to unvaccinated or partially vaccinated household members, emphasizing the need for immunizing close contacts of high-risk groups.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Vaccine, Effectiveness
Graphical Abstract
Introduction
Since the availability of coronavirus disease 2019 (COVID-19) vaccines, reductions in COVID-19 infections and hospitalizations, particularly among high-risk populations, have been reported.1,2 Despite this, evidence of ongoing circulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the form of breakthrough infections leading to community outbreaks, have occurred.3,4 More importantly, the newly emerged B.1.617.2 (delta) variant of SARS-CoV-2, has shown to cause high viral loads which then effectively transmits via breakthrough infection.5,6,7
The COVID-19 vaccination in South Korea was introduced since February 2021, prioritizing the high-risk groups and the healthcare workers during the first roll-out.8 The BNT162b2 mRNA COVID-19 vaccines were provided to the participants and staff who attend adult day service centers (ADSCs), reaching high vaccination coverage of 90% as of July 2021, which then have resulted a reduction of cases and mortality among vaccinated population.9 However, unprecedented emergence of delta variant SARS-CoV-2 virus has affected the country, becoming the predominant strain in the country, as of early August.
On August 10th, 2021, an outbreak of SARS-CoV-2 delta variant was identified among participants and staff members of an ADSC in Jeju, South Korea. The high BNT162b2 mRNA COVID-19 vaccine coverage rate among in the ADSC participants and staff provided us opportunity to characterize the effectiveness of COVID-19 vaccines on SARS-CoV-2 delta variant. The primary objective of this study is to investigate the epidemiologic features and the effect of vaccination in infection-vulnerable facilities with a high inoculation rate of BNT162b2 mRNA COVID-19 vaccine. The second purpose is to estimate the secondary transmission prevention effect of the vaccine in the household members by vaccination status.
Methods
The Korea Disease Control and Prevention Agency (KDCA) and Jeju Special Self-Governing Provincial Government conducted a joint-epidemiologic investigation at the ADSC. The study population included all participants and staff who attended the ADSC for at least one day from August 3 until its closure on August 10, and their household contacts. A confirmed COVID-19 case was defined as laboratory confirmation of SARS-CoV-2 via reverse transcriptase polymerase chain reaction (RT-PCR) testing. Participants and staff were interviewed to assess symptoms of COVID-19 in the preceding 14 days. Cases treated with high flow oxygen therapy, mechanical ventilator, Extracorporeal Membrane Oxygenation, or Continuous Renal Replacement Therapy were classified as severe/critical cases. Household contacts were monitored daily for symptoms; and both symptomatic and asymptomatic contacts were tested. Vaccination data (vaccination date, vaccine type, vaccination place) were retrieved from the National Immunization Registry. Cases were defined to be fully vaccinated following 14 days after final vaccine-series dose receipt.
In all participants, staff members, and their household members, nasopharyngeal swabs were collected, placed in universal transport medium, and sent to the national designated laboratory for confirmation using RT-PCR method. In the setting of increasing proportion of delta variant in Korea, samples were tested for SARS-CoV-2 sequence to detect its genomic variance. The cycle threshold (Ct) value for each specimen was retrieved to estimate the viral load in each case.
This investigation was carried out in accordance with the legal mandate granted by the Infectious Disease Control and Prevention Act (Article 18) as a part of public health intervention, not research, and therefore ethical approval was waived, and written informed consent was exempted. All data were treated confidentially and analyzed without identifiable information in accordance with the Personal Information Protection Act.
Ethics statement
This study was approved by the Institutional Review Board of the Korea Disease Control and Prevention Agency and informed consent was waived (IRB No. 2021-12-02-PE-A).
Results
A total of 42 participants and 16 staff members were included in the investigation, identifying 12 symptomatic case and 13 asymptomatic confirmed cases of COVID-19, with 13 isolates identified as SARS-CoV-2 virus, delta variant (Table 1). The mean age of confirmed COVID-19 cases was 78.9 years (SD 14.3 years) with a range from 34 years to 99 years (Table 1). Eighteen (72.0%) cases of COVID-19 were female. Among all, 96.6% (n = 56) were fully vaccinated against COVID-19 (all had received BNT162b2 mRNA COVID-19 vaccine), while one COVID-19 case, and one non-COVID-19 case had not received the vaccine. The mean interval post-2nd dose vaccination was 140.0 days (range, 80–117 days). One case was defined as a severe/critical case requiring ventilatory support and succumbed to death after 13 days of hospitalization. Other cases have recovered without requiring oxygen supplementation at follow-up, which was up to 14 days after closing of investigation.
Table 1. Demographic characteristics, vaccination status, and outcome of confirmed COVID-19 among participants (n = 42) and staffs (n = 16), SARS-CoV-2 delta variant outbreak in an adult day service center, Jeju, South Korea, 2021.
| Characteristics | COVID-19 case (n = 25) | Non-COVID-19 case (n = 33) | Total (n = 58) | |
|---|---|---|---|---|
| Cases | ||||
| Participant | 22 (88.0) | 20 (60.6) | 42 (72.4) | |
| Staff | 3 (12.0) | 13 (39.4) | 16 (27.6) | |
| Sex | ||||
| Female | 18 (72.0) | 24 (72.7) | 42 (72.4) | |
| Male | 7 (28.0) | 9 (27.3) | 16 (27.6) | |
| Age group, yr | ||||
| ≤ 49 | 1 (4.0) | 2 (6.1) | 3 (5.2) | |
| 50–59 | 2 (8.0) | 10 (30.3) | 12 (20.7) | |
| 60–69 | 0 (0.0) | 3 (9.1) | 3 (5.2) | |
| 70–79 | 6 (24.0) | 4 (12.1) | 10 (17.2) | |
| 80–89 | 12 (48.0) | 8 (24.2) | 20 (34.5) | |
| ≥ 90 | 4 (16.0) | 6 (18.2) | 10 (17.2) | |
| Vaccination status | ||||
| Fully vaccinateda | 24 (96.0) | 32 (97.0) | 56 (96.6) | |
| Unvaccinated | 1 (4.0) | 1 (3.0) | 2 (3.4) | |
| Outcome | ||||
| Asymptomatic infection | 12 (48.0) | - | 12 (20.7) | |
| Symptomatic infection | 12 (48.0) | - | 12 (20.7) | |
| Fatal case | 1 (4.0) | - | 1 (1.7) | |
Values are presented as number (%).
COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
aInterval post 2nd dose: mean 140.0 days (range, 80–117 days).
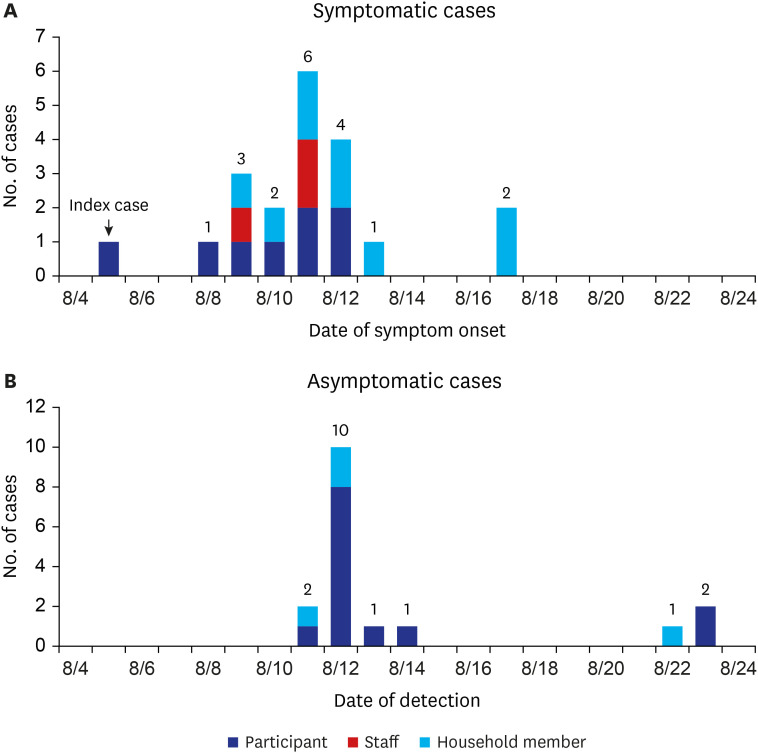
Fig. 1 illustrates the distribution of cases according to presence of symptoms. The index case was identified on August 8, three days after the onset of symptoms. The index case has visited ADSC from August 3 to August 6, and on August 9. A second participant case started symptoms on August 8. The number of symptomatic cases increased from August 9 to August 12, with a peak of six new cases on August 11 (Fig. 1A). Fig. 1-B shows the number of asymptomatic cases by date of detection.
Fig. 1. Epidemic curve of (A) symptomatic cases (n = 20) and (B) asymptomatic cases (n = 17), SARS-CoV-2 delta variant outbreak in an adult day service center and household members, Jeju, South Korea, 2021.
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Overall, the attack rate for the outbreak was 43.1% (25/58, Table 2). Among the 40 participants fully vaccinated against COVID-19, the attack rate was 52.5% (n = 21), while one out of two unvaccinated participants had contracted SARS-CoV-2. All 16 staff were fully vaccinated against COVID-19, resulting in an attack rate of 18.8% (n = 3).
Table 2. COVID-19 cases by vaccination status, SARS-CoV-2 delta variant outbreak in an adult day service center, Jeju, South Korea, 2021.
| Cases | Fully vaccinated (n = 56) | Unvaccinated (n = 2) | Total (n = 58) |
|---|---|---|---|
| Participant | 21/40 (52.5) | 1/2 (50.0) | 22/42 (52.4) |
| Staff | 3/16 (18.8) | - | 3/16 (18.8) |
| Total | 24/56 (42.9) | 1/2 (50.0) | 25/58 (43.1) |
Values are presented as number (%).
COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
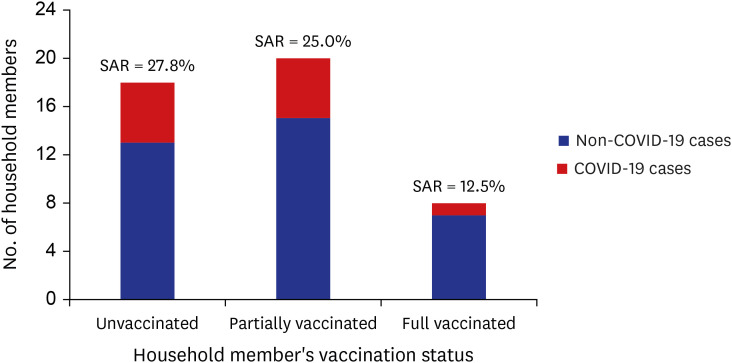
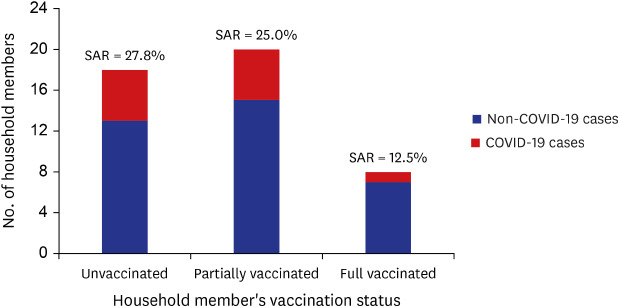
In the 25 COVID-19 confirmed cases of ADSC, 6 patients caused transmission to household members. Forty-six household members were tested to assess secondary transmission from the ADSC outbreak (Fig. 2). Overall, the attack rate of household members for the outbreak was 23.9% (11/46). Among the 6 fully vaccinated index cases, the secondary attack rate (SAR) of unvaccinated and partially vaccinated household members were 27.8% (5/18) and 25.0% (5/20), respectively. The SAR of fully vaccinated household members were 12.5% (1/8).
Fig. 2. SAR to household members by vaccination status, SARS-CoV-2 delta variant outbreak in an adult day service center, Jeju, South Korea, 2021.
SAR = secondary attack rate, COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
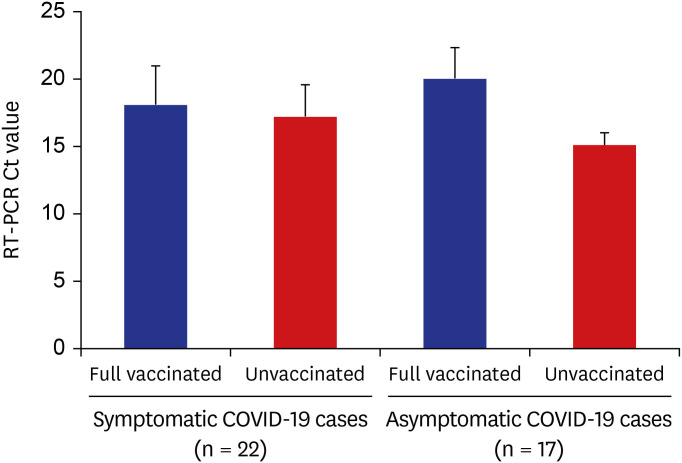
To better characterize the viral load of COVID-19 cases by presence of symptoms and vaccination history, SARS-CoV-2 RT-PCR Ct values were assessed (Fig. 3). The Ct values were comparable among in symptomatic individuals regardless of vaccination status (fully vaccinated vs. unvaccinated, mean 18.1 vs. mean 17.2). Among asymptomatic cases, vaccinated individuals had higher Ct value (mean 20) compared to unvaccinated individuals (mean 15.1).
Fig. 3. SARS-CoV-2 real time RT-PCR Ct values for specimens from COVID-19 cases by vaccination status and symptom, SARS-CoV-2 delta variant outbreak in an adult day service center (n = 25) and household members (n = 12), Jeju, South Korea, 2021.
RT-PCR = reverse transcription-polymerase chain reaction, Ct = cycle threshold, COVID-19 = coronavirus disease 2019, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Discussion
In the setting of SARS-CoV-2 outbreak with delta variant in an ADSC, we found a high breakthrough infection rate of 52.5% among participants who had received BNT162b2 mRNA COVID-19 vaccines. The breakthrough infection rate seems higher than the reports from elsewhere. From one of the earliest reports was from the U.S., in April 2021, among the cohort of 417 persons who had received BNT162b2 or mRNA-1273 vaccines, two cases of breakthrough infections were reported; in which the sequencing revealed variants including E484K, T95I, del142-144, and D614G.7 In Israel in April 2021, among the 1,497 fully vaccinated healthcare workers, 39 SARS-CoV-2 breakthrough infections were documented, predominantly infected by B.1.1.7 (alpha) variant (85%).5 The difference may be largely attributable to the intrinsic property of SARS-CoV-2 variant evading the vaccine-induced immunity.10 Since the emergent of SARS-CoV-2 B.1.617.2 (delta) variant in early 2021, the genotype now predominates among the circulating SARS-CoV-2 strains in most parts of the globe.11,12 In Massachusetts during July 2021, 469 cases of COVID-19 associated with multiple summer events and large public gatherings were identified; while approximately 74% of cases occurred in fully vaccinated persons. Genomic sequencing identified the B.1.617.2 (delta) variant of SARS-CoV-2 in 119 (89%).6 More importantly, the issue may not be limited to certain type of vaccine. An outbreak associated with a gymnastic facility in April-May 2021, caused by delta variant, showed highly transmissible virus affecting those vaccinated with either Moderna or Pfizer-BioNTech or a single dose of Janssen (Johnson & Johnson) vaccines.13 Nevertheless, the high breakthrough infection rate in this case needs to be considered together with the close-contact and densely populated environmental features and demographic and clinical characteristics of residents who were elderly and had chronic underlying disease.
We also found high rates of secondary transmission from vaccinated participants and staff to their household members, suggesting the vaccine's limited effect in preventing secondary transmission, in the setting of an outbreak caused by SARS-CoV-2 delta variant. The finding extends previous reports suggesting the delta variant can pose higher viral load, therefore gaining its efficiency in transmitting the virus to others.14,15 The target priority group for COVID-19 vaccination has been focused on high-risk persons including elderly citizens.16,17
Indeed, low viral load among asymptomatic vaccinated cases suggest virologic effect of the vaccine on potential transmission in regard to the presence of symptoms in affected persons, as in line with previous studies.18,19 Despite this, our study showing the effective transmission of delta variant SARS-CoV-2 from vaccinated person to unvaccinated household members highlights the importance of multi-layered prevention strategy in the ADSC. We also emphasize that vaccination of new participants and staff is completed, symptoms of visitors are monitored, masks are worn indoors, personal hygiene rules are continuously emphasized, and diagnostic tests are required regardless of vaccination history in case of suspicious symptoms. In addition, increased study results that vaccine effectiveness against SARS-CoV-2 infection decreases over time support providing booster vaccination against COVID-19 at the proper time point.20
Even with the vaccine’s limited potency against infection and transmission of the delta variant SARS-CoV-2, the health outcome of this outbreak was reassuring. One case has succumbed to death, resulting in a case fatality rate of 4%; however, all other cases have recovered without complications. More importantly, given that more than 80% of COVID-19 cases were senior citizens aged > 70 years, we find that the BNT162b2 mRNA COVID-19 vaccine was effective in preventing severe disease in older age group, despite the presence of breakthrough infections. It should be noted that although the number was smaller, the breakthrough infection rate was lower among staff (18.8%), who were younger and healthier than the participants.
This investigation has several limitations. Although the sequencing of SARS-CoV-2 was performed to detect the presence of delta variant, the genetic linkage to associate the ADSC outbreak and household infections was not made, therefore is prone to misclassification bias. Furthermore, there is possibility that an asymptomatic case has introduced the SARS-CoV-2 virus to the ADSC outbreak, not the index case, which we could not determine from the investigation.
Despite these, this is the first investigation showing the lowered efficacy of BNT162b2 mRNA COVID-19 vaccine in preventing transmission from breakthrough infection cases to household members. The low clinical severity among vaccinated participants and low viral load in asymptomatic persons emphasizes the need for continued vaccination (booster shot) especially among the high-risk people and their close contacts.
In summary, we describe a SARS-CoV-2 delta variant outbreak in ADSC with high vaccine coverage rate, characterized by high infection rate, high transmissibility, and low clinical severity. The outbreak proceeded to unvaccinated or partially vaccinated household members, emphasizing the need for immunizing close contacts of high-risk groups. Transmission of delta variant SARS-CoV-2 virus from vaccinated persons to others highlight the importance of a multi-layered prevention strategy in high-risk places and continual viral genomic surveillance and assessment of vaccine effectiveness.
ACKNOWLEDGMENTS
We thank the relevant ministries, including the Ministry of Interior and Safety, Si/Do and Si/Gun/Gu, medical staffs in health centers, and medical facilities for their efforts in responding to COVID-19 outbreak.
Footnotes
Disclaimers: The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Korea Disease Control and Prevention Agency or the institutions with which the authors are affiliated.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yi S, Kim JM, Choe YJ, Park YJ.
- Data curation: Yi S, Kim JM.
- Formal analysis: Yi S.
- Investigation: Hong S, Choi S, Ahn SB, Kim M.
- Methodology: Yi S, Park YJ.
- Supervision: Park YJ.
- Validation: Choe YJ, Kim JM, Yi S.
- Visualization: Kim JM, Choe YJ.
- Writing - original draft: Yi S, Kim JM, Park YJ.
- Writing - review & editing: Yi S, Kim JM, Choe YJ.
References
- 1.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373(1088):n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moline HL, Whitaker M, Deng L, Rhodes JC, Milucky J, Pham H, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥65 years - COVID-NET, 13 states, February-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1088–1093. doi: 10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teran RA, Walblay KA, Shane EL, Xydis S, Gretsch S, Gagner A, et al. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members - Chicago, Illinois, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):632–638. doi: 10.15585/mmwr.mm7017e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi MJ, Choi WS, Seong H, Choi JY, Kim JH, Kim YJ, et al. Developing a framework for pandemic COVID-19 vaccine allocation: a modified Delphi consensus study in Korea. J Korean Med Sci. 2021;36(23):e166. doi: 10.3346/jkms.2021.36.e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control Headquarters. Coronavirus disease-19 updates, Republic of Korea. [Updated 2021]. [Accessed August 29, 2021]. http://ncov.mohw.go.kr/en/
- 10.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 11.Torjesen I. COVID-19: delta variant is now UK's most dominant strain and spreading through schools. BMJ. 2021;373(1445):n1445. doi: 10.1136/bmj.n1445. [DOI] [PubMed] [Google Scholar]
- 12.Vaidyanathan G. Coronavirus variants are spreading in India - what scientists know so far. Nature. 2021;593(7859):321–322. doi: 10.1038/d41586-021-01274-7. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty K, Mannell M, Naqvi O, Matson D, Stone J. SARS-CoV-2 B.1.617.2 (delta) variant COVID-19 outbreak associated with a gymnastics facility - Oklahoma, April-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(28):1004–1007. doi: 10.15585/mmwr.mm7028e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik M, Kunze AC, Bahmer T, Herget-Rosenthal S, Kunze T. SARS-CoV-2: viral loads of exhaled breath and oronasopharyngeal specimens in hospitalized patients with COVID-19. Int J Infect Dis. 2021;110:105–110. doi: 10.1016/j.ijid.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teyssou E, Delagrèverie H, Visseaux B, Lambert-Niclot S, Brichler S, Ferre V, et al. The delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect. 2021;83(4):e1–e3. doi: 10.1016/j.jinf.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi Y, Kim JS, Kim JE, Choi H, Lee CH. Vaccination prioritization strategies for COVID-19 in Korea: a mathematical modeling approach. Int J Environ Res Public Health. 2021;18(8):4240. doi: 10.3390/ijerph18084240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain V, Schwarz L, Lorgelly P. A rapid review of COVID-19 vaccine prioritization in the U.S.: alignment between federal guidance and state practice. Int J Environ Res Public Health. 2021;18(7):3483. doi: 10.3390/ijerph18073483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman KK, Tay DJW, Sen Tan K, Ong SWX, Son TT, Koh MH, et al. Viral load of SARS-CoV-2 in respiratory aerosols emitted by COVID-19 patients while breathing, talking, and singing. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab691. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa R, Bueno F, Albert E, Torres I, Carbonell-Sahuquillo S, Barrés-Fernández A, et al. Upper respiratory tract SARS-CoV-2 RNA loads in symptomatic and asymptomatic children and adults. Clin Microbiol Infect. 2021;27(12):1858.e1–1858.e7. doi: 10.1016/j.cmi.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. SSRN. doi: 10.2139/ssrn.3961378. November 18, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]