Abstract
Objective:
Regional citrate anticoagulation (RCA) is the preferred anticoagulation method for continuous kidney replacement therapy (CKRT) recommended by the KDIGO guidelines. Limited availability of calcium-free solutions often imposes challenges to the implementation of RCA for CKRT (RCA-CKRT). The principal purpose of this study was to characterize the outcomes of RCA-CKRT using calcium-containing solutions.
Study design:
Retrospective cohort study.
Setting & Participants:
We evaluated the safety and efficacy of RCA-CKRT with calcium-containing dialysate and replacement fluid used for 128 patients. A total of 571 filters and 1,227 days of CKRT were analyzed.
Exposures:
Liver disease, sepsis in the absence of liver disease, and sepsis with liver disease.
Outcomes:
Filter life and metabolic complications per 100 CKRT days.
Analytical Approach:
Linear mixed effects model and generalized linear mixed effects models.
Results:
The majority of patients were male 91 (71.1%), 32 (25%) had liver disease, and 29 (22.7%) had sepsis without liver disease. Median filter life was 50.0 (IQR, 22.0, 118.0) hours with a maximum of 322 hours and was significantly lower (33.5 (17.5~ 60.5) hours) in patients with liver disease. Calcium-containing replacement solutions were used in 41.6% of all CRRT hours, and reduced intravenous calcium requirements by 31.7%. Hypocalcemia (ionized calcium lower than 0.85mmol/L) and hypercalcemia (total calcium higher than 10.6mg/dL) were observed in 6.0 and 6.7 per 100 CKRT days respectively. Citrate accumulation was observed in 13.3% of all patients and was associated with metabolic acidosis in 3.9%, which was not different in patients with liver disease (9.3% p=0.165).
Limitations:
Lack of control groups that used calcium-free dialysate and replacement solutions with RCA-CKRT. Possible overestimation of filter life from incomplete data on cause of filter failure.
Conclusions:
Our study suggests that RCA-CKRT with calcium-containing solutions is feasible and safe in critically ill patients including those with sepsis and liver disease.
Keywords: Regional citrate anticoagulation, Continuous renal replacement therapy, Calcium-containing dialysate, Calcium -containing replacement solution
Plain-language summary
Calcium-containing solutions for citrate anticoagulation in continuous dialysis. Regional citrate anticoagulation for continuous dialysis (RCA-CRRT) prevents clotting by binding free calcium in the blood. The procedure utilizes calcium-free dialysate and replacement solutions for fear that calcium-containing solutions would lead to ineffective anticoagulation or increased citrate requirements. We implemented RCA-CRRT using calcium-containing solutions in 128 patients including 32 patients with liver disease and 28 patients with sepsis. In our study, median filter life was 50 hours, comparable to studies using calcium-free solutions. Patients with liver disease and sepsis had shorter median filter life than those without these conditions, however there was no difference in the incidence of complications. This study describes the technique using calcium-containing solutions and practical considerations for patients with liver disease or sepsis.
Introduction
Continuous renal replacement therapy (CRRT) is widely used in the management of critically ill patients and regional citrate anticoagulation (RCA) is the anticoagulation method recommended by KDIGO. 1–6 Traditional CRRT with RCA (RCA-CRRT) protocols have used calcium-free dialysates and replacement solutions for an effective chelation of ionized calcium in the circuit7–9 and calcium is infused intravenously to keep the blood level of ionized calcium sufficient for normal coagulation and hemostasis.7, 8 In our center, we had utilized customized, low sodium, calcium-free bicarbonate-based dialysate and replacement solutions for RCA-CRRT and 10% calcium chloride for replacing calcium for more than 22 years. In 2012, nationwide shortages of bicarbonate solutions, 10% calcium chloride and phosphate were encountered and have continued over the last several years making it difficult to provide traditional RCA-CRRT. 10, 11 Commercially available calcium-containing CRRT solutions have 2.5mEq/L of ionized calcium and a higher concentration of bicarbonate (32 mmol/L) over calcium-free solution (22 mmol/L), thereby providing an external source of calcium and bicarbonate to reduce the need of injectable solutions. However, this approach is challenging because it may affect filter life and metabolic complications associated with RCA-CRRT. Critically ill patients with chronic liver disease or sepsis are the most at risk for these complications since acute on chronic liver failure 12, 13 or multiple organ dysfunction can lead to coagulopathy 14–16 and impair citrate metabolism.
We leveraged the available commercial solutions to develop an alternate approach to RCA-CRRT using calcium-containing dialysate and replacement solutions. In this study, we describe our experience of the efficacy and safety of RCA with calcium-containing solutions for Continuous veno-venous hemodiafiltration (CVVHDF) at our institution.
Methods
We reviewed medical records of patients ≥ 18 years admitted to the University of California, San Diego Health System between January 1 and December 31, 2017, who received CVVHDF. Patients who received CRRT less than 24 hours and those with missing data were excluded. Clinical information, dialysis parameters and laboratory data were extracted from electronic medical records. Hourly recordings of dialysis parameters and anticoagulation variables including citrate flow rate, intravenous calcium replacement flow rate were grouped into 6 hour Periods. Effluent Fluid urea nitrogen (FUN)/ Blood urea nitrogen (BUN) ratio was measured every 12 hours. 1, 17, 18 Comorbidities and hospital admission diagnoses were obtained from chart review. The Sequential Organ Failure Assessment (SOFA) score was calculated at CRRT start. In patients with liver disease, degree of hepatic dysfunction at CRRT start was assessed using the Acute on Chronic Liver Failure (ACLF) score12, 19. Approval to perform anonymous analyses of routinely collected clinical data was obtained with a waiver of informed consent from the Institutional Review Board of the University of California, San Diego.
CRRT technique and RCA anticoagulation
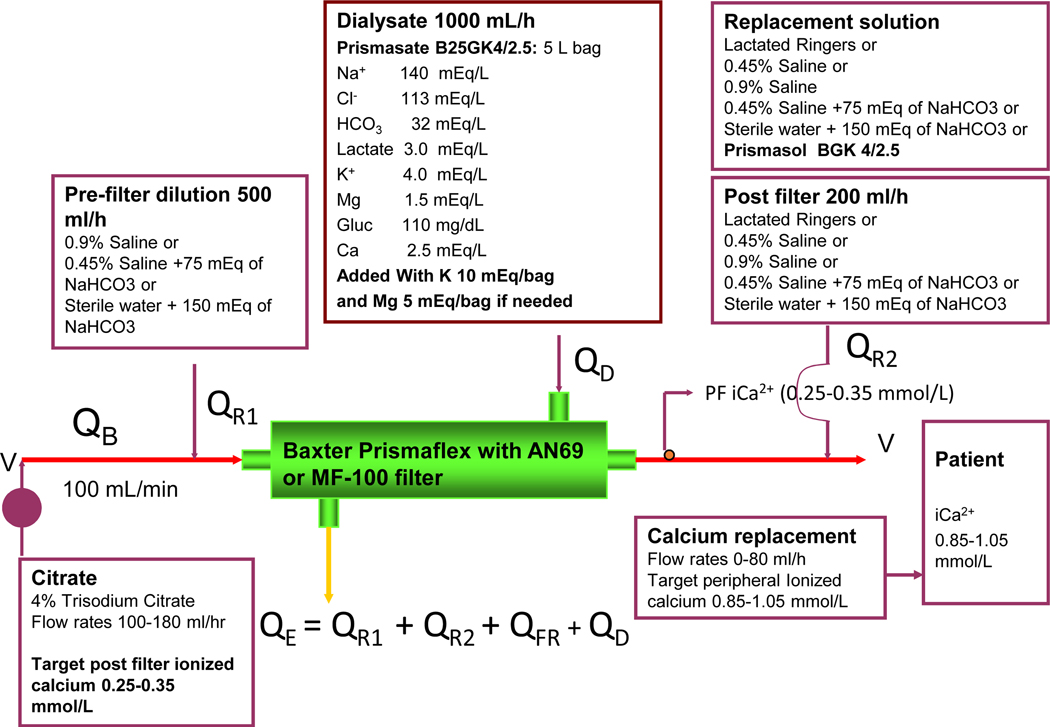
Vascular access was obtained with ultrasound guidance using an uncuffed dual lumen catheter placed in the internal jugular vein or femoral vein. We performed pre- and post-dilution CVVHDF using a Baxter PRISMAFLEX (Lakewood, CO, USA) CRRT machine, with a 0.9 m2 acrylonitrile membrane (AN69, MF-100 filter set for PRISMAFLEX). The operational characteristic and circuit schematic are shown in Figure 1. A 3 - way stopcock was placed between the arterial catheter and the tubingconnecting the filter, and 4% trisodium citrate (140 mmol citrate and 420 mmol sodium/liter, Baxter Corp.) was infused at this site at an initial rate of 140–180 ml/hr (16.8–25.2 mMol citrate per hour) with a range of 100 to 210 ml/ hr for a blood flow rate (Qb) of 100 ml/min. Calcium was replaced via a separate central venous access using a solution of 10% calcium chloride (comprised of 20 ml of 10% calcium chloride added to 250 ml of 0.9% saline; or 10% calcium gluconate (14g of calcium gluconate 10% solution to the 500ml of 0.9% sodium chloride saline) to provide 1 mEq of calcium per l0 ml in cases where calcium chloride solution was not available. The calcium solution was infused at an initial rate of 40 ml/ hour, (4 mEq/hr), with a range of 3 to 5 mEq/hr depending upon the level of ionized calcium and the citrate infusion rate to maintain peripheral blood ionized calcium of 0.85~1.05mmol/L. Ionized calcium levels were sampled from venous blood (pre) and post filter circuit (post) every 6 to 8 hours. Citrate flow rates were adjusted, to maintain post-filter levels ionized calcium values between 0.25 to 0.35mmol/L adjusted to 0.35 to 0.45mmol/L in patients with liver disease. We used commercially available Prismasate (Baxter, Dearborn, Chicago) (4/2.5 solution 2.5 mEq/L calcium) as a dialysate. We added 0~10mEq of KCL and 0~5mEq of MgSO4 to each 5 liter bag based on the UCSD electrolyte protocol (Table S1). Standard dialysate flow rate was 1000mL/hr. We infused 0.9% saline at 500 ml/hr pre-filter to maintain filtration fraction < 30% and an additional 200ml/hr to maintain patency of the aeration chamber as required by the manufacturer. Fluid balance was achieved by adjusting the volume of substitution fluid administered through an IV line outside the circuit to replace the hourly fluid removed adjusting for all intakes and outputs outside the circuit. Hourly fluid balance targets were set by the clinician and adjusted as needed. Fluid composition of the substitution fluid was based on the clinical condition and included Prismasol (32mmol/L bicarbonate, 3mEq/L lactate, 2.5Meq/L ionized calcium) (Baxter, Dearborn, Chicago); or customized bicarbonate solutions composed of sterile water mixed with 150mEq of bicarbonate, sterile water mixed with 75mEq of bicarbonate, and 0.45% saline mixed with 75mEq of bicarbonate, 0.45% saline, or 0.9% saline to achieve metabolic and acid base balance.
Figure 1.
Regional citrate anticoagulation circuit for the PrismaFlex machine with pre and post dilution CVVHDF using calcium-containing solutions. Prismasate and Prismasol are the commercial dialysate and substitutions fluid solutions sourced from Baxter (Baxter, Dearborn Chicago). Abbreviations: QB, blood flow rate; QR1, pre-filter fluid flow rate; QD, dialysate flow rate; QR2, post-filter fluid flow rate; QE, effluent flow rate; QFR, patient fluid removal rate. * Commercial solutions
Efficacy measurement
Filter performance was evaluated by two parameters, filter longevity and filter efficacy. Based on filter life-span filters were assigned into one of 4 groups; ≤ 24 hour, 25~48 hour, 49~96 hour and >96 hour. Reasons for filter change were categorized as: “access”, “clotting”, “low efficacy”, “time” and “unrelated to therapy”. “Access” problems included filters that were changed due to catheter obstruction or kinking. “Low efficacy” was defined as the FUN/BUN ratio below 0.85; and the “time” when filters that were electively changed after more than 96 hours. “Unrelated to therapy” was considered when filters were changed due to CRRT stops related to transfer from the ICU for radiological procedures or room changes, machine problems other than filter, access or line problems; and clinical decisions to stop therapy.
Additionally, we assessed the influence of calcium containing substitution fluids on the amount of calcium replacement required to maintain the peripheral ionized calcium values in target range. For these calculations we considered that the amount of calcium delivered from dialysate was constant because the blood flow rate and dialysate flow rate were maintained at 100ml/min and 1 liter per hour respectively thereby alterations in peripheral ionized calcium were corrected by calcium provided through the calcium gluconate and the calcium delivered through the intravenous substitution fluid. The total amount of calcium delivered was calculated as the sum of the hourly replaced calcium from the calcium gluconate and the intravenous substitution solutions. The contribution was calculated as the proportion of calcium from the substitution fluid among the total amount of calcium delivered.
Safety Assessments
The prevalence of new onset metabolic complications per 100 CRRT days was calculated by evaluating daily worst values of serum electrolyte from the second day of IC U admission. The selected value was categorized as “low”, “within target”, and “high” according to the cutoff values summarized at Table S2(a) and abnormal values were further grouped into mild, moderate, and severe cases (Table S2(b)). C itrate accumulation was defined per patient as the worst calcium ratio during C R R T higher than 2.5.
Data are presented as absolute number and percentages, or medians and interquartile ranges (IQRs), as appropriate. Comparisons of filter life and other filter-level or filter-hour level data were analyzed using a linear mixed effects model that included a patient-specific or patient-filter-specific random effect. The incidence of metabolic complications between groups was compared using a Generalized linear mixed effects model included a patient-specific random effect. Cox-regression analyses were used to define factors associated patient outcome, adjusted with factors that were significant in uni-variable analyses. We considered p < 0.05 as statistically significant. We performed analyses using IBM SPSS statistical (v. 26.0, SPSS Inc., Chicago, IL, USA).
Results
During the study period 226 patients were treated with CRRT and 128 patients were included for this analysis excluding 9 patients who received CRRT less than 24 hours, and 89 patients whose dialysis information for the outcomes included in this analysis was incomplete (Figure S1). Patient baseline characteristics are summarized in Table 1. The mean age was 58.2±14.4 years and 71.1% of the patients were male. A total of 32 out of 128 (25%) had liver disease and 23 (71.9%) had grade 3 ACLF (Table S3). Other co-morbidities included diabetes (40.6%), chronic kidney disease (CKD, 25%) and end stage kidney disease (ESKD, 16.5%). The most common main diagnosis when starting CRRT was sepsis (35.9%) followed by non-septic shock (33.6%). The mean SOFA score at CRRT initiation was 14.9±3.3. The median duration of hospital stay was 27.0 (IQR 12.5–43.5) days. In-hospital mortality rate was 50.8%.
Table 1.
Patient characteristics (N=128)
| Variable | Value |
|---|---|
| Demographics | |
| Age, y | 58.2 ± 14.4 |
| Male sex | 91 (71.1%) |
| Weight, kg | 84.2 ± 23.2 |
| Height, cm | 169.1 ± 10.1 |
| BMI. kg/m> | 29.6 ± 7.8 |
| Comorbtdites | |
| Hypertension | 67 (52.3%) |
| Diabetes | 52 140.6%) |
| CKD stages 1 –4 | 32 (25.0%) |
| Kidney failure | 22 (16.6%) |
| CHF | 43 (33.6%) |
| Liver disease | 32 (25.0%) |
| Cancer | 18 (14.1%) |
| Main clinical diagnosis at CKRT initiation | |
| Sepsis | 46 (36.9%) |
| Shock other than sepsis | 43 (33.6%) |
| Cardiovascular disease | 38 (29.7%) |
| Urinary calculus | 1 (0.8%) |
| Disease severity | |
| SOFA score at dialysis start | 14.9 ± 3.3 |
| Laboratory data at CKRT initiation | |
| Serum urea nitrogen, mg/dL | 55.5 ± 41.3 |
| Creatinine, mg/dL | 3.6 ± 2.5 |
| pH | 7.3 ± 0.1 |
| Patient outcomes | |
| CKRT duration, d | 5.7 [2.9–12.5] |
| Hospital length of stay, d | 27.0 [12.5–43.5] |
| ICU length of stay, d | 13.0 [8.0–22.5] |
| ICU mortality | 56 (42.9%) |
| In-hospital mortality | 65 (50.8%) |
Values for continuous variables presented as mean ± SD or median [interquartile range]; categorical variables, as counts (percentage). Abbreviations: BMI, body mass index; CHF, congestive heart falure: CKO, chronic kidney disease; CKRT, continuous kidney replacement therapy; ICU, intensve care unit; SOFA, Sequential Organ Failure Assessment.
Median CRRT duration was 5.7 (IQR 2.9–12.5) days and 571 filters, a median of 3 (IQR 1–6) filters per person, were used. During a total of 28,671 filter hours, the median filter life was 32.0 (IQR 16.0–69.0) hours with a minimum duration of 1.0 hours and a maximum duration of 322 hours. Median FUN/BUN ratio was 1.0 (IQR 1.00–1.03) with a minimum of 0.71 and a maximum of 1.39. During the RCA with calcium-containing solutions, median blood flow rate was 100 (IQR 100, 100) mL/min; with the median citrate infusion rate of 150 (IQR134.0–165.0) mL/hr and median post-filter ionized calcium level was 0.32 (IQR 0.30–0.36) mmol/L. Peripheral ionized calcium levels were maintained within the median of 1.05 (IQR 1.00–1.09) mmol/L throughout the CRRT with the median calcium gluconate solution infusion rate of 44.0 (IQR 33.0–55.0)mL/hr (Table 2).
Table 2.
CKRT operational characteristics
| All | LD | SEP | NSLD | P | |
|---|---|---|---|---|---|
| No. of filters | 571 | 104 | 99 | 368 | — |
| Blood flow rate. mL/min | 99.9 ± 3.9 | 99.8 ± 1.5 | 99.8 ± 1.4 | 100.0 ± 4.8 | 0.7 |
| Dialysate flow rate. mL/h | 982.7 ± 224.9 | 984.4 ± 91.7 | 995.1 ± 131.2 | 978.6 ± 270.5 | 0.3 |
| Pre-filter replacement flow rate, mL/h | 482.5 ± 10.7 | 481.3 ± 10.4 | 483.1 ± 9.8 | 482.7 ± 11.1 | 0.5 |
| Postfilter fluid for deaeration chamber, mL/h | 195.5 ± 16.2 | 193.9 ± 8.7 | 198.0 ± 27.7 | 195.2 ± 13.2 | 0.9 |
| IV substitution flow rate, mL/h | 618.9 ± 101.1 | 603.4 ± 104.7 | 621.2 ± 111.2 | 622.9 ± 96.9 | 0.046 |
| Patient fluid removal, mL/h | 908.8 ± 78.2 | 911.1 ± 41.6 | 909.3 ± 72.4 | 907.9 ± 87.8 | 0.8 |
| Effluent flow rate, mL/h | 2,572.1 ± 197.9 | 2,579.2 ± 127.2 | 2.605.1 ± 169.9 | 2.560.4 ± 221.2 | C.07 |
| Citrate drip rate, mL/h | 147.1 ± 20.2 | 140.3 ± 17.7a | 148.5 ± 20.9 | 148.8 ± 20.4 | •-0.001 |
| Postfilter iCa, mmol/L | 0.35 ± 0.06 | 0.38 ± 0.07a | 0.35 ± 0.08 | 0.34 ± 0.05 | <0.001 |
| Peripheral iCa, mmol/L | 1.04 ± 0.10 | 1.01 ± 0.09a | 1.04 ± 0.14 | 1.05 ± 0.08 | 0.004 |
| IV calcium infusion rate, mL/h | 43.19 ± 17.2 | 49.5 ± 20.5a | 47.9 ± 19.1a | 41.1 ±14.8 | <0.001 |
| FUN:SUN ratio | 1.0 [1.0–1.03] | 1.0 (1.0–1.04) | 1.C [0.98–1.03] | 1.0 [1.0–1.03] | 0.08 |
| No. of filters used per person | 3(1–6] | 3 [1–5] | 3 [1–5] | 2 [1–7] | 0.8 |
| Filter life, h | 32.0 [16.0–69.0] | 26.0 [14.0–51.0]a | 24.0 [14.0–46.5]a | 37.0 [17.5–72.0] | 0.01 |
| Excluding filters changed for reason unrelated to therapy | |||||
| No. of filters | 166 | 40 | 35 | 91 | |
| Filter life, h | 50.0 (22.0–118.0) | 33.5 (17.5–60.5]a | 42.0 [23.0–114.0] | 67.0 [21.0–124.0] | 0.008 |
Values for continuous variables are given as mean ± SD or median [interquartile range]. Abbreviations: FUN, fluid urea nitrogen; iCa, ionized calcium: IV, intravenous; LD, liver disease; NSLD, no sepsis or liver disease; SEP, sepsis in the absence of liver disease: SUN, serum urea nitrogen.
P < 0.06 vs NSLD. P value obtained using a linear mixed-effects model that included patient-specific random effect.
In our circuit, the most commonly used pre-filter solutions were 0.9% saline (39.1%), 0.45% saline (48.4%) and post-filter solutions to maintain the patency of the deaeration chamber were 0.9% saline (46.2%) and 0.45% half saline (39.7%). Intravenous substitution fluid utilized to maintain hourly fluid balance included 0.9% saline (48.4%), calcium-containing Prismasol (32mmol/L bicarbonate, 3mEq/L lactate, 2.5Meq/L ionized calcium) (Baxter, Dearborn, Chicago) (41.6%), and individualized bicarbonate solution (7.3%) (Summarized in Table S4).
In 41.6% of the cases with calcium-containing Prismasol, total calcium requirements reduced by 31.7% and a greater amount of calcium was delivered with less infusion of calcium gluconate compared to the cases with calcium-free substitution fluid (Table 3).
Table 3.
Calcium saving effect with calcium containing substitution solution
| Prismasol | Calcium-free substitution fluid | P | |
|---|---|---|---|
| No. filter hours | 5,846 | 22,825 | |
|
| |||
| Calcium gluconate infusion rate, mL/hr | 38.48±16.31 | 47.59±21.71 | <0.001 |
| iCa from calcium gluconate, mmol/hr | 1.93±0.82 | 2.39±1.09 | <0.001 |
| Prismasol infusion rate, mL/hr | 619.15±175.11 | 0 | NA |
| iCa from Prismasol, mmol/hr | 0.77±0.20 | 0 | NA |
| Total iCa delivered, mmol/hr | 2.71 ±0.81 | 2.39±1.09 | <0.001 |
| % of calcium from Prismasol | 31.66±15.51 | 0 | NA |
| Peripheral iCa, mmol/L | 1.06±0.07 | ||
Filter Performance
Of the 571 evaluated filters, median filter life was 32 (IQR 16.0–69) hrs; 39.8% were changed within 24 hours and 25.4% between 24 and 48 hours (Table 2 and 4). Excluding 215 filters where reasons for change were not recorded, the most common cause of filter change was events unrelated to therapy (50.8%) and only 98 out of 356 filters (27.5%) were changed due to clotting or low efficacy. Access problems, clotting and low efficacy were mostly observed in filters with early failure (Table 4). Excluding filters changed unrelated to the therapy, the median filter life was 50.0 (IQR 22.0~118.0) hours (Table 2). In filters that were changed within 24 hours, post-filter ionized calcium was higher, and peripheral ionized calcium was lower than the others despite no difference in citrate flow rate (Figure S2).
Table 4.
Circuit outcomes
| Filter Life Group | Total | ||||
|---|---|---|---|---|---|
| ≤24 h | 25–48 h | 49–96 h | >96 h | ||
| No. of fitters | 227 | 145 | 104 | 95 | 571 |
| Filter change reason not recorded | 98 (43.2%) | 68 (46.9%) | 49 (47.1%) | 0 | 215 |
| Filter change reason recorded | 129 (56.8%) | 77 (53.1%) | 55 (52.9%) | 95 (100%) | 356 |
| Unrelated to therapy | 76 (58.9%) | 46 (60%) | 35 (64%) | 24 (25%) | 181 (50.8%) |
| Time | 0 | 0 | 0 | 58 (61%) | 58 (16.3%) |
| Access | 11 (8.5%) | 4 (5%) | 1 (2%) | 3 (3%) | 19 (5.3%) |
| Clotting | 33 (25.6%) | 19 (25%) | 14 (25%) | 5 (5%) | 71 (19.9%) |
| Low efficacy | 9 (7%) | 8 (10%) | 5 (9%) | 5 (5%) | 27 (7.6%) |
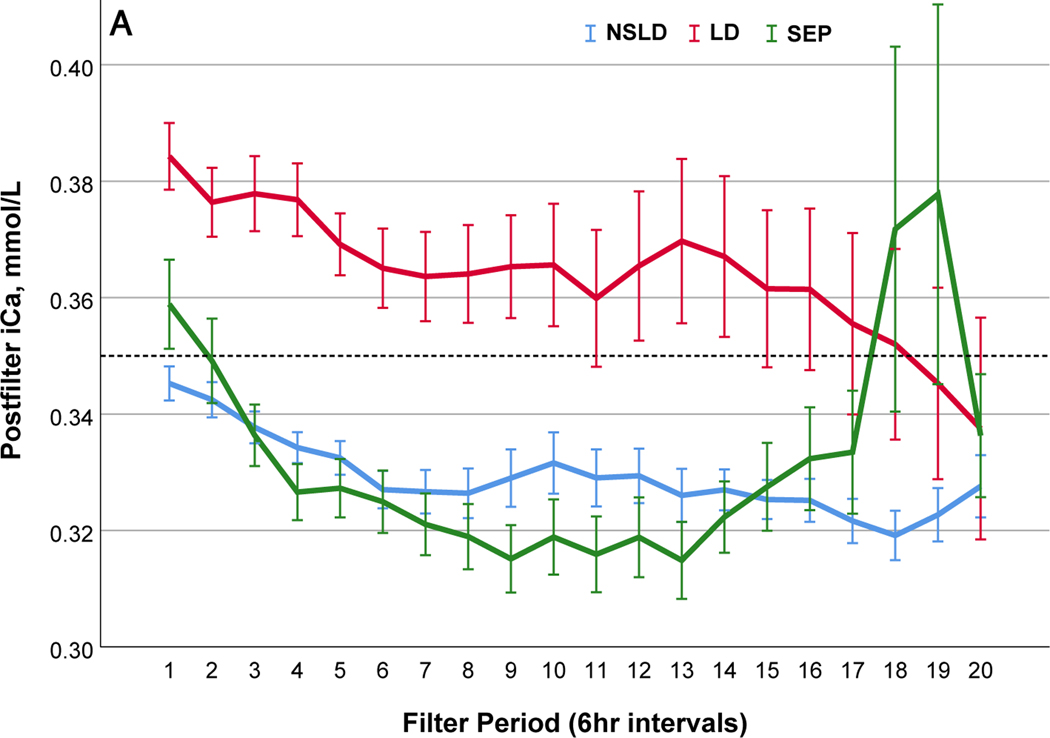
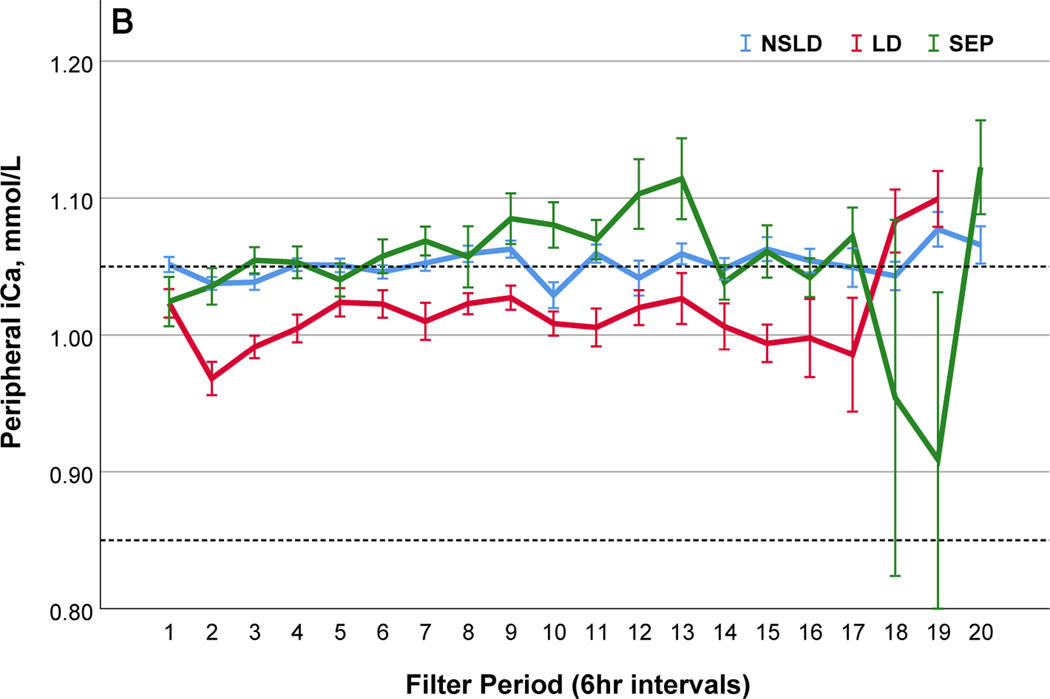
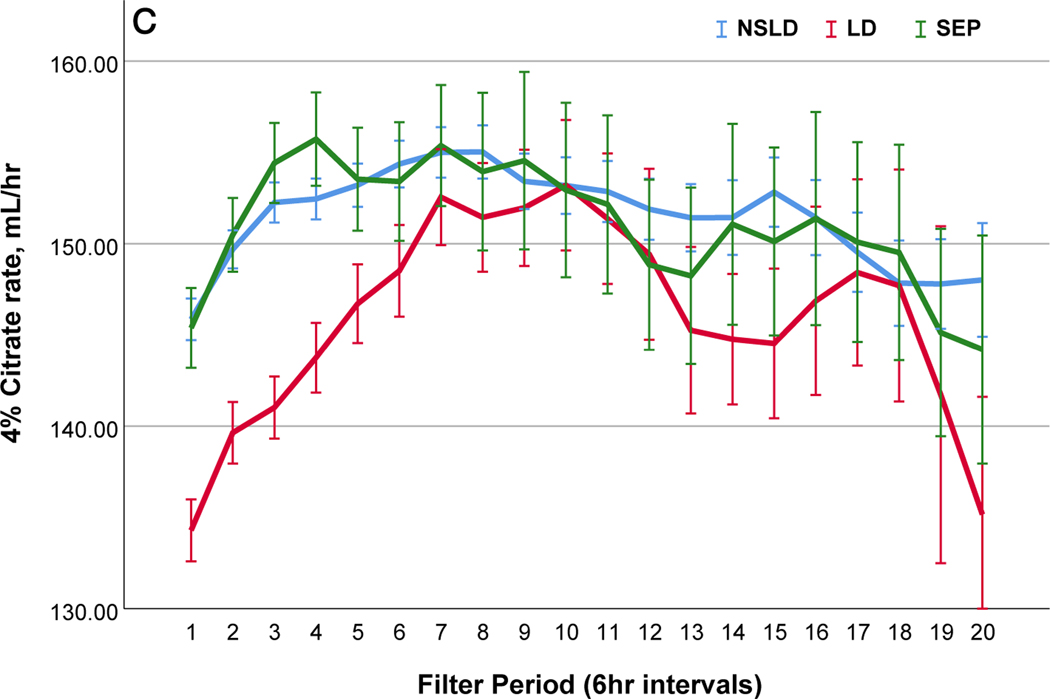
We further compared filter performance in patients with sepsis in the absence of liver disease (SEP) (n=29; 99 filters), those with liver disease (LD) (n=32; 104 filters) and those without sepsis or liver disease (NSLD) (n= 67; 368 filters) (Table 2). The median filter life was significantly lower in patients with SEP (24.0 (IQR 14.0~46.5) hrs) and LD (26.0 (IQR 14.0–51.0) compared to the filters with NSLD 37.0 (IQR 17.5–72.0) hrs; p=0.01). In patients with LD, post-filter ionized calcium levels kept higher and citrate infusion rate was lower, but peripheral ionized calcium levels were lower compared to patients with NSLD (Table 2 and Figure 2). In patients with SEP, peripheral ionized calcium levels were not statistically different from NSLD however a larger amount of calcium replacement was needed (Figure 2). Filter changes due to low efficacy or clotting were more common in patients with LD (48.4%) than in SEP (28.4%) or NSLD (22.4%, p <0.001 (Table S5 a and b)).
Figure 2.
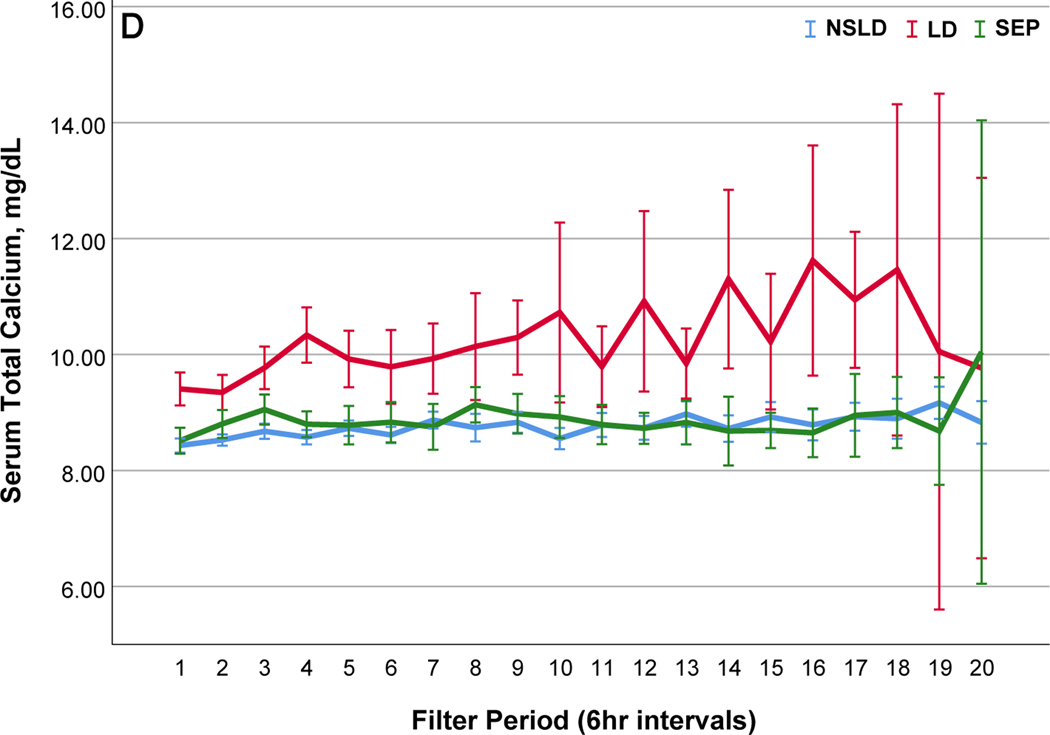
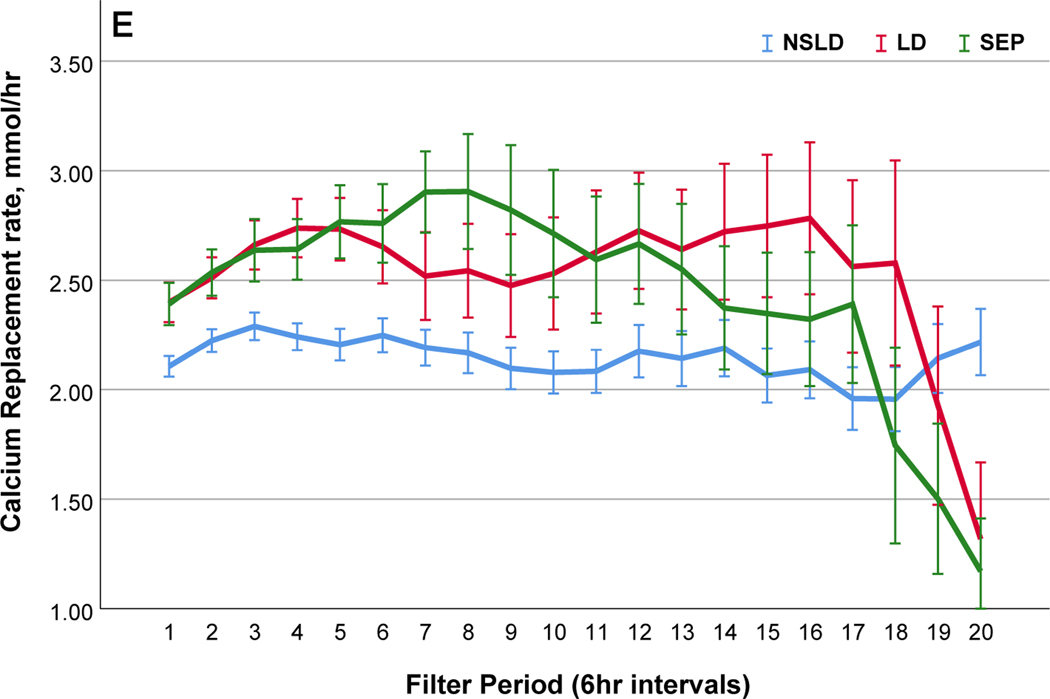
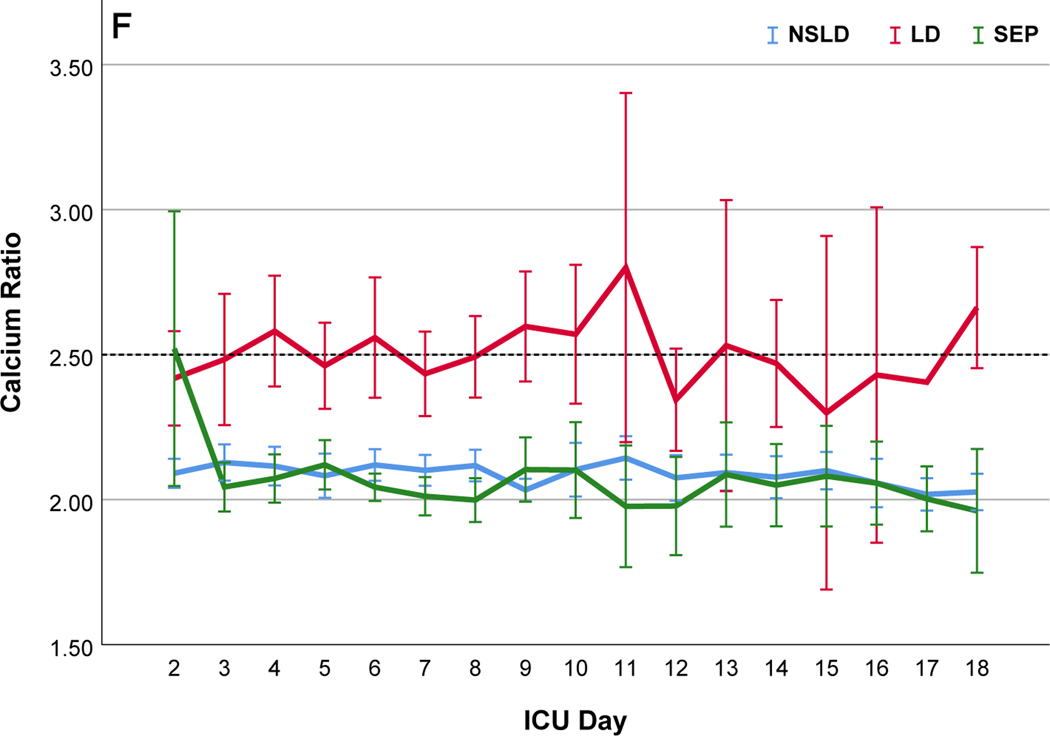
Comparisons of citrate anticoagulation with calcium containing solutions among different disease groups; LD, patients with liver disease (Red line); SEP, patients with sepsis in the absence of liver disease (Green line); NSLD, patients without sepsis or liver disease (Blue line). Filter period is a 6-hour block from the use of each filter to the change and time zero means every new start of CRRT after filter change. (a) Post filter ionized calcium level, dotted line in the middle shows iCa level of 0.35mmol/L : target postfilter iCa level for patients without liver disease, 0.25~0.35 mmol/L; patients with liver disease, 0.35~0.45 mmol/L (b) Peripheral ionized calcium level, two dotted lines show target range of 0.85~ 1.05 mmol/L. (c) 4% Citrate rate, (d) Serum total calcium level, (e) Calcium replacement rate (sum of the replaced calcium from calcium gluconate and Prismasol), (f) Calcium ratio, dotted line in the middle shows calcium ratio of 2.5. Error bars indicate 95% confidence intervals.
Safety
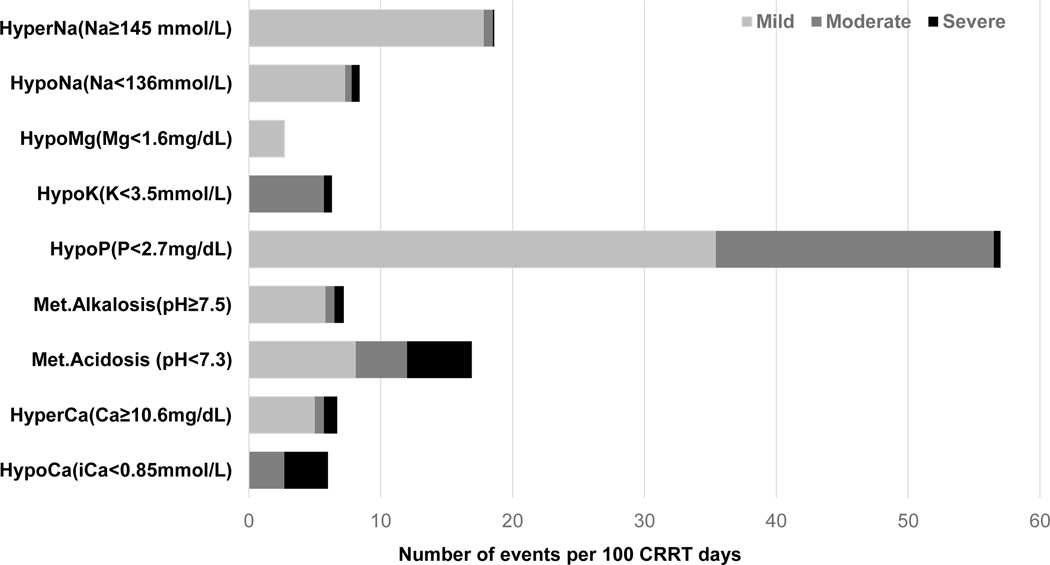
Prevalence of metabolic complications are summarized in the figure 3. Metabolic complications were more frequently observed in cases with LD (Figure 3, Table S6) followed by SEP (Figure S3(a)). Hypocalcemia (ionized calcium < 0.85 mmol/L) was observed in 6.0 days and severe hypocalcemia (ionized calcium < 0.8mmol/L) was observed only in 3.3 days per 100 CRRT days, most frequently in patients with LD (8.4 vs SEP, 6.1 vs NSLD 1.6 days; p=0.004). The frequency of hypocalcemia was not different according to the ACLF grade in LD (Grade1, 5.9 vs Grade 2, 11.1 vs Grade 3, 14.1, p=0.646). Hypercalcemia (total calcium ≥ 10.6 mg/dL) was seen in 6.7 days per 100 CRRT-days and was significantly higher in patients with LD (31 days) than NSLD (2.0 days, p <0.001). Moderate (total calcium ≥12 mg/dL) or severe hypercalcemia (total calcium ≥ 14mg/dL) was observed only in LD patients with ACLF grade 3 (moderate: 4.2 days, severe: 6.3 days per 100 CRRT days). Hypophosphatemia (phosphate < 2.7 mg/dL) was seen in 57.1 days per 100 CRRT days and 35.4 days were mild (phosphate 2.0 ~ 2.7 mg/dL). Metabolic acidosis (pH <7.3) was observed in 16.9 days (LD 12.7, SEP 23.8, NSLD 16.1, p=0.042) and severe metabolic acidosis (pH < 7.2) was observed in the 4.9 days per 100 CRRT days (LD 4.6, SEP 8.9, NSLD 4.0, p=0.016). The incidence of new onset hypomagnesemia (Mg <1.6 mg/dL) and hypokalemia was not different according to the disease group (Table S6). During the median of 5.7 days of CRRT operation, citrate accumulation was observed in 17 patients (13.3%) (34% in LD, 6.9% Sepsis and 6% NSLD, p <0.001) and 5 (3.9%) of them had combined metabolic acidosis, of whom had ACLF grade 3 liver disease (Table S7). In patients with LD the calcium ratio was significantly higher throughout the filter period with lower peripheral ionized, higher total calcium levels and larger amount of calcium replacement compared to the patients without liver disease (Figure 2).
Figure 3.
Prevalence of new onset metabolic complication after RCA-CRRT with calcium containing solutions (N=1,227 CRRT days); Grading of each metabolic complication is shown in supplement Table S1. Light gray, mild; Dark gray, moderate; Black, severe
Patient outcome
A total of 42.9 % of the patients died in ICU. In the multivariable analysis adjusted with age, sex, BMI, sepsis, liver disease, citrate accumulation, and SOFA score, only SOFA score was a significant factor associated with the ICU mortality (HR 1.233 (1.072~1.419), p=0.003) (Table S8).
Discussion
An effective anticoagulation strategy is essential to maintain CRRT circuits and provide uninterrupted therapy. Regional citrate anticoagulation (RCA) techniques have vastly improved filter performance, however, they have traditionally required calcium free hypotonic solutions to optimize the anticoagulation effect within the circuit.8 Calcium-containing solutions were usually avoided in the RCA-CRRT due to the fear of ineffective anticoagulation or increased citrate accumulation associated with the increased citrate requirement. Shortages of calcium chloride and bicarbonate and calcium free dialysate and replacement solutions prompted us to utilize calcium-containing solutions for RCA in CRRT. Our study suggests that RCA-CRRT10, 11 with calcium-containing solutions is feasible and safe in critically ill patients including those with sepsis and liver disease.
Most studies describing anticoagulation in CRRT focus on filter longevity and circuit loss as parameters for filter performance and fail to recognize that filter efficacy may deteriorate prior to obvious circuit clotting.17, 18, 20, 21 We routinely measure the effluent/plasma fluid to blood urea nitrogen (FUN/BUN) ratios every 12 hours in our CRRT circuits to determine filter efficacy as small molecular clearance should remain intact if the fibers are not clogged with protein or cellular materials.1, 17, 18 We change filters when the FUN/BUN ratio is below 0.85 and when the filter has been in use for more than 96 hours even if it is functioning well. Consequently, we utilized two parameters for filter performance: the filter duration and efficacy and recorded the reasons for filter changes to ascertain the relationship of the operational characteristics to the therapy delivery.
Our median filter life of 32 (16~69) hours and maximum duration of 322 hours was relatively shorter compared to the filter longevity of our previous reports or other groups with calcium-free dialysate. In our previous report of Rolando et al, the median filter life was 75 (IQR 48.5~115.6) with a minimum duration of 1.3 hours and a maximum duration of 183.3 hours.17 Other groups that used RCA with calcium-free dialysate on CVVHDF, the mean or median filter lives were 50 to 67.6 hours.22–25 However, these studies did not measure the filter efficacy and consequently would overestimate filter performance.
Our findings reflect 3 distinct patterns for filter failure, 40% fail early within the first 24 hrs, 44% last between 1–4 days and 16% function over 4 days. Over half of these filter changes are unrelated to the therapy representing the competing events from radiological and other procedures requiring patients to be off therapy. When we re-calculated median filter life after excluding these filters, median filter life was 50 (22.0~118.0) hours with a maximum of 322 hours, comparable to the previous reports.2, 22–25 Clotted circuits account for one quarter of the circuits that fail early and between 1–4 days and only 5% of those that last longer while reduced efficacy accounts for 7.6% and access issues were contributing in 5%. To interpret these findings, it is important to consider the patients underlying condition contributing to the coagulation status and the process of care that is adapted.
At start of CRRT, sepsis and non septic shock accounted for a third each of the patient population, and 25% had liver disease all conditions with changes in coagulation status and citrate metabolism. The early filter failures were associated with clotting in almost half of filters with liver disease and 30% in the sepsis group in the first 48 hours. As we use a standardized protocol for CVVHDF dosing operational characteristics were similar across the filter patterns. However, as liver disease patients are anticipated to have reduced citrate metabolism, citrate flow rates are reduced and have higher target post-filter calcium levels that could potentially influence the higher rate of filter clotting seen in liver disease patients. Low efficacy accounted for filter changes more frequently in both liver disease and sepsis ranging from 16–20% for filters lasting 24–96 hours (Table S5).
The overall complication rate was similar to prior reports with RCA with calcium free solutions.26–28 In RCA-CRRT, a fall in ionized calcium is commonly observed in patients with severe liver disease, shock or sepsis due to the inadequately metabolized citrate which results in an impaired ionized calcium release from the calcium-citrate complex.29, 30 In such cases, increasing intravenous calcium infusion could be a solution for the management of ionized hypocalcemia 31–33 however, contributes to increased total to ionized calcium ratios consistent with citrate accumulation.
Our incidence of citrate accumulation in 13.3% is similar to prior reports of 0~12% in RCA-CRRT with calcium-free dialysate33 and was higher in the patients with liver disease, ranged from 3 to 22%.32 Direct comparison with our data is however difficult because of the heterogeneous mode of CRRT and diverse RCA protocols. Additionally, management strategies for citrate accumulation vary across centers.29,34 We managed citrate accumulation; by a lower blood flow rate, increased post-filter ionized calcium target, increased dialysate flow rate and lowered target systemic ionized calcium level and permitted mild ionized hypocalcemia (0.85 mmol/L≤ ionized calcium< 1.05 mmol/L). Our data shows the intravenous calcium sparing effect of calcium-containing intravenous substitution fluid (Prismasol) used in about 40% of all applications. This reduced calcium gluconate requirement by 30% and compared to the periods when calcium-free substitution fluid was used, a higher amount of calcium was delivered. However, there was no period when calcium was replaced with a substitution fluid alone. Ong et al.,35 reported complete replacement of calcium was possible with commercially used calcium containing replacement solution with replacement flow rate, about 3L per hour. In our protocol, the hourly volume of substitution fluid to maintain fluid balance is limited to approximately 600 ml/hour and would not be sufficient to provide all the calcium necessary to obviate any additional infusion.
Strengths of our study include a detailed assessment of CRRT technique in a large number of patients with heterogeneous conditions. The patient characteristics and process of care elements are well defined to address their influence on filter performance. The delineation of reasons for filter change provides relevant information to guide implementation of this technique in critically ill patients and in those with sepsis and liver disease.
There are some limitations in our study. First this is a single center study with a standardized approach to CRRT technique that may not be applicable elsewhere. Second, we did not perform direct comparisons before and after the use calcium-containing solutions with RCA. Therefore, direct comparison of citrate requirement, filter /patient outcome or metabolic complication was not available. Third, we did not perform a detailed analysis of the severity of liver disease to evaluate its influence as a limiting factor in the use of RCA due to the limited number of patients with liver disease. Finally, reasons for filter loss were not recorded in all the encounters and we may overestimate contributory factors for filter loss because 43.2% of filters changed within 24 hours did not have reasons for failure recorded and could have been due to filter clotting or low efficacy.
In conclusion, calcium containing dialysate and replacement solutions can be safely and effectively used in RCA-CRRT. Further prospective studies will be useful to confirm our findings for wider application.
Supplementary Material
Figure S1. Flow diagrams of included patients
Figure S2. Comparisons of post-filter ionized calcium, citrate flow rate and peripheral ionized calcium levels according to the filter life group
Figure S3. Metabolic complications of RCA with calcium containing solutions in patients with liver disease or sepsis
Table S1: UCSD CRRT electrolyte protocol
Table S2: Cutoff values of electrolyte used in metabolic complication measurement
Table S3. Acute on chronic liver failure grade at CRRT initiation
Table S4: Replacement solutions used in the CRRT circuit
Table S5. Filter changing reasons according to the filter life group in patients with liver disease or sepsis
Table S6. Comparisons of new onset metabolic complications among different disease groups in patients with RCA-CRRT
Table S7. Incidence of citrate accumulation according to patient condition
Table S8. Factors associated with ICU mortality
Acknowledgements:
We thank Ender Sam Kuo for providing us the curated dataset and for his ongoing help in extracting relevant data for analysis.
Support: This work was supported by the National Institutes of Health NIDDK O’Brien Centre for AKI Research Grant DK079337. Funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Financial Disclosure: Dr Mehta reports having a consulting agreement with Baxter Inc. The remaining authors declare that they have no relevant financial interests.
Peer Review: Received Sep 24, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form January 19, 2021.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Macedo E, Claure-Del Granado R, Mehta RL. Effluent volume and dialysis dose in CRRT: time for reappraisal. Nat Rev Nephrol. 2011;8(1): 57–60. [DOI] [PubMed] [Google Scholar]
- 2.Oudemans-van Straaten HM, Kellum JA, Bellomo R. Clinical review: anticoagulation for continuous renal replacement therapy--heparin or citrate? Crit Care. 2011;15(1): 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Laupland KB, Boiteau PJ, Godinez-Luna T. Is regional citrate superior to systemic heparin anticoagulation for continuous renal replacement therapy? A prospective observational study in an adult regional critical care system. J Crit Care. 2005;20(2): 155–161. [DOI] [PubMed] [Google Scholar]
- 4.Honore PM, Spapen HD. Evolution of Vascular Access and Anticoagulation. Contrib Nephrol. 2018;194: 15–24. [DOI] [PubMed] [Google Scholar]
- 5.Claure-Del Granado R, Macedo E, Soroko S, et al. Anticoagulation, delivered dose and outcomes in CRRT: The program to improve care in acute renal disease (PICARD). Hemodial Int. 2014;18(3): 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Section 5: Dialysis Interventions for Treatment of AKI. Kidney Int Suppl (2011). 2012;2(1): 89–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL. Continuous renal replacement therapy in the critically ill patient. Kidney Int. 2005;67(2): 781–795. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, McDonald BR, Aguilar MM, Ward DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int. 1990;38(5): 976–981. [DOI] [PubMed] [Google Scholar]
- 9.Abramson S, Niles JL. Anticoagulation in continuous renal replacement therapy. Curr Opin Nephrol Hypertens. 1999;8(6): 701–707. [DOI] [PubMed] [Google Scholar]
- 10.USFDA. FDA Drug Shortages calcium chloride. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Calcium%20Chloride%20Injection,%20USP&st=c&tab=tabs-1: Accessed July 19, 2020.
- 11.USFDA. FDA Drug Shortages Sodium Bicarbonate Injection, USP. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Sodium%20Bicarbonate%20Injection,%20USP&st=c: Accessed Aug 19, 2020.
- 12.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7): 1426–1437, 1437 e1421–1429. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Mehta G, Jalan R. Acute-on-chronic liver failure. Clin Med (Lond). 2020;20(5): 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premkumar M, Sarin SK. Current Concepts in Coagulation Profile in Cirrhosis and Acute-on-Chronic Liver Failure. Clin Liver Dis (Hoboken). 2020;16(4): 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher C, Patel VC, Stoy SH, et al. Balanced haemostasis with both hypo- and hyper-coagulable features in critically ill patients with acute-on-chronic-liver failure. J Crit Care. 2018;43: 54–60. [DOI] [PubMed] [Google Scholar]
- 16.Blasi A, Calvo A, Prado V, et al. Coagulation Failure in Patients With Acute-on-Chronic Liver Failure and Decompensated Cirrhosis: Beyond the International Normalized Ratio. Hepatology. 2018;68(6): 2325–2337. [DOI] [PubMed] [Google Scholar]
- 17.Claure-Del Granado R, Macedo E, Chertow GM, et al. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol. 2011;6(3): 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claure-Del Granado R, Macedo E, Chertow GM, et al. Toward the optimal dose metric in continuous renal replacement therapy. Int J Artif Organs. 2012;35(6): 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Foundation For The Study Of Chronic Liver Failure. CLIF-C ACLF CALCULATOR. Accessed December 1, 2020. https://www.efclif.com/scientific-activity/score-calculators/clif-c-aclf
- 20.Gattas DJ, Rajbhandari D, Bradford C, Buhr H, Lo S, Bellomo R. A Randomized Controlled Trial of Regional Citrate Versus Regional Heparin Anticoagulation for Continuous Renal Replacement Therapy in Critically Ill Adults. Crit Care Med. 2015;43(8): 1622–1629. [DOI] [PubMed] [Google Scholar]
- 21.Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;59(6): 810–818. [DOI] [PubMed] [Google Scholar]
- 22.Kutsogiannis DJ, Gibney RT, Stollery D, Gao J. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int. 2005;67(6): 2361–2367. [DOI] [PubMed] [Google Scholar]
- 23.Swartz R, Pasko D, O’Toole J, Starmann B. Improving the delivery of continuous renal replacement therapy using regional citrate anticoagulation. Clin Nephrol. 2004;61(2): 134–143. [DOI] [PubMed] [Google Scholar]
- 24.Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70(7): 1312–1317. [DOI] [PubMed] [Google Scholar]
- 25.Oudemans-van Straaten HM, Wester JPJ, de Pont A, Schetz MRC. Anticoagulation strategies in continuous renal replacement therapy: can the choice be evidence based? Intensive Care Med. 2006;32(2): 188–202. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi NA, Altarelli M, Eckert P, Schneider AG. Complications of Regional Citrate Anticoagulation for Continuous Renal Replacement Therapy: An Observational Study. Blood Purif. 2020: 1–9. [DOI] [PubMed] [Google Scholar]
- 27.Klingele M, Stadler T, Fliser D, Speer T, Groesdonk HV, Raddatz A. Long-term continuous renal replacement therapy and anticoagulation with citrate in critically ill patients with severe liver dysfunction. Crit Care. 2017;21(1): 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Peng S, Cen Z, et al. Applying Regional Citrate Anticoagulation in Continuous Renal Replacement Therapy for Acute Kidney Injury Patients with Acute Liver Dysfunction: a Retrospective Observational Study. Kidney Blood Press Res. 2018;43(4): 1065–1074. [DOI] [PubMed] [Google Scholar]
- 29.Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2(6): 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigwalt F, Bouteleux A, Dambricourt F, Asselborn T, Moriceau F, Rimmele T. Clinical Complications of Continuous Renal Replacement Therapy. Contrib Nephrol. 2018;194: 109–117. [DOI] [PubMed] [Google Scholar]
- 31.Slowinski T, Morgera S, Joannidis M, et al. Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: the Liver Citrate Anticoagulation Threshold (L-CAT) observational study. Crit Care. 2015;19: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Bai M, Yu Y, et al. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care. 2019;23(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morabito S, Pistolesi V, Tritapepe L, Fiaccadori E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol. 2014;9(12): 2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier-Kriesche HU, Finkel KW, Gitomer JJ, DuBose TD Jr, Unexpected severe hypocalcemia during continuous venovenous hemodialysis with regional citrate anticoagulation. Am J Kidney Dis. 1999;33(4): e8. [DOI] [PubMed] [Google Scholar]
- 35.Ong SC, Wille KM, Speer R, Tolwani AJ. A continuous veno-venous hemofiltration protocol with anticoagulant citrate dextrose formula A and a calcium-containing replacement fluid. Int J Artif Organs. 2014;37(6): 499–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagrams of included patients
Figure S2. Comparisons of post-filter ionized calcium, citrate flow rate and peripheral ionized calcium levels according to the filter life group
Figure S3. Metabolic complications of RCA with calcium containing solutions in patients with liver disease or sepsis
Table S1: UCSD CRRT electrolyte protocol
Table S2: Cutoff values of electrolyte used in metabolic complication measurement
Table S3. Acute on chronic liver failure grade at CRRT initiation
Table S4: Replacement solutions used in the CRRT circuit
Table S5. Filter changing reasons according to the filter life group in patients with liver disease or sepsis
Table S6. Comparisons of new onset metabolic complications among different disease groups in patients with RCA-CRRT
Table S7. Incidence of citrate accumulation according to patient condition
Table S8. Factors associated with ICU mortality