PRACTICAL IMPLICATIONS
Systemic thrombolysis may be considered as treatment in acute spinal cord ischemia.
Acute nontraumatic ischemic myelopathy is a devastating condition with a typically poor outcome occurring in adults, adolescents, and even children.1-3 The acute onset of paraplegia and sensory loss is suggestive of spinal ischemia with little recovery potential.1,4,5 Because of the lack of sufficient data, there are no standardized guidelines regarding the acute treatment of spinal ischemia. Although intravenous thrombolysis is a well-established and effective treatment in cerebral ischemia, its efficacy in spinal cord ischemia has not yet been proven.5 We report the case of a young female patient with acute spinal cord ischemia in whom treatment with systemic thrombolysis led to a complete reversal of paraplegia.
Case
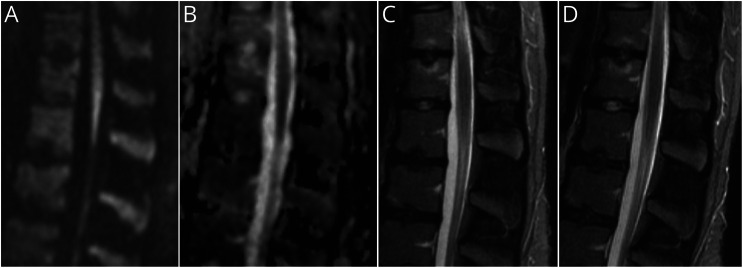
A 17-year-old White woman presented with sudden painless paraplegia and anesthesia from the L4 level downward that had developed 3 hours previously. There were no preexisting medical conditions, especially no history of sickle cell disease, drug abuse or smoking, any medication, or use of oral contraceptives. The deep tendon reflexes were absent in both legs when reflexes of the arms were 1+, and the plantar response was flexor on both sides. Immediate MRI showed a diffusion restriction in the medullary cone with a slightly hyperintense lesion on diffusion-weighted imaging (DWI) and a corresponding signal suppression in the apparent diffusion coefficient (ADC) map (Figure). Apart from marked osteochondritic changes, the MRI revealed no other pathologic changes in the vertebral column or within the spinal canal. With informed consent, the patient received intravenous alteplase 4.5 hours after symptom onset with a dosage of 0.9 mg/kg over 60 minutes and an initial bolus of 10%. Within the following hours, the patient substantially regained motor and sensory function of her legs with improvement of muscle power up to 4/5, 6 hours after treatment. At that time, the follow-up MRI was normal (Figure). As secondary prophylaxis, a long-term treatment with 100 mg aspirin daily was initiated. At the time of discharge 6 days after onset, the patient had a full recovery. Somatosensory and motor evoked potentials yielded no abnormalities. Investigations for cardiovascular risk factors yielded mild hypertension (24 hours mean value 142/74 mm Hg), elevated low-density-lipoprotein-cholesterol (130 mg/dL), lipoprotein (a) (86.2 mg/dL), and homocystein (16.4 µmol/L) were found. Further cardiologic and laboratory investigations (i.e., 24 hours ECG, echocardiography, HbA1c, Fabry disease test, screening for thrombophilia, and vasculitis abnormalities) did not reveal any abnormalities.
Figure. MRI of the Caudal Spinal Cord Recorded Approximately 3.5 Hours After the Onset of the Symptoms.
(A) Sagittal diffusion-weighted imaging (DWI) revealed a hyperintense lesion at the medullary cone. (B) Corresponding signal suppression in the sagittal apparent diffusion coefficient (ADC) map. (C) The sagittal short tau inversion recovery (STIR) sequence ruled out an intervertebral disk prolapse or a myelitic lesion of the spinal cord and medullary cone. Note the cerebrospinal fluid signal between the caudally adjacent spinal roots. (D) At the follow-up assessment 6 hours after treatment, the STIR sequence did not reveal infarct demarcation.
Discussion
The sudden onset of paraplegia and the sensory loss of both legs suggested acute ischemia of the caudal spinal cord.1,2,5 This diagnosis was supported by the diffusion restriction in MRI (Figure, A) in the absence of other structural pathology (Figure, C).1 The simultaneous impairment of protopathic and epicritic sensibility indicated a lack of perfusion in the territories of both anterior and posterior spinal arteries, which suggested a proximal occlusion of the Adamkiewicz spinal artery.6 Notably, spontaneous spinal cord ischemia may occur in any age group, including young adults.1,2 It has a poor prognosis, although good long-term recovery was occasionally observed.3,4 Systemic intravenous thrombolysis, which has been approved for acute cerebral stroke, is also expected to work in spinal cord ischemia, although its use in this condition has not yet been addressed in a clinical trial. In the described patient, the effectiveness of this treatment has probably been enhanced by the good vascular collateralization typical for young age. Importantly, however, thrombolysis probably needs to be initiated within the time frame used in cerebral stroke. Therefore, its therapeutic efficacy and safety profile will be missed if delayed because of lumbar puncture. In contrast to spinal ischemia, polyradiculoneuritis, as in Guillain-Barré syndrome or myelitis, has less limited therapeutic time frames and will not respond to alteplase.
Although the quality of spinal DWI is commonly affected by susceptibility artifacts, it is a promising tool to detect spinal cord ischemia especially in the early stage when T2-weighted images frequently fail to do so.7 Reliable detection of ischemia and differentiation from other spinal pathologies is particularly relevant during this early stage. Thus, we suggest that whenever acute spinal cord ischemia is a valid differential diagnosis, neurologic care should be performed similarly to acute cerebral stroke. Time consuming additional investigations such as lumbar puncture and electrophysiologic measurements bear the danger that the time window for thrombolysis may pass. However, because of the lack of clinical trials, intravenous thrombolysis has to be administered after critical risk-benefit evaluation and with the patient's informed consent.
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76(1):56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maharaj MM, Phan K, Hariswamy S, Rao PJ. Surfer's myelopathy: a rare presentation in a non-surfing setting and review of the literature. J Spine Surg. 2016;2(3):222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76(3-4):95-98. [DOI] [PubMed] [Google Scholar]
- 4.Robertson CE, Brown RD, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012;78(2):114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasr DM, Rabinstein A. Spinal cord infarcts: risk factors, management, and prognosis. Curr Treat Options Neurol. 2017;19(8):28. [DOI] [PubMed] [Google Scholar]
- 6.Vargas MI, Gariani J, Sztajzel R, et al. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR Am J Neuroradiol. 2015;36(5):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006;48(11):795-801. [DOI] [PubMed] [Google Scholar]