Abstract
Purpose of Review
Dopa-responsive dystonia (DRD) encompasses a group of phenotypically and genetically heterogeneous neurochemical disorders. Classic GTP cyclohydrolase 1 (GCH-1)–associated DRD consists of early-onset lower limb asymmetrical dystonia, with sleep benefit, diurnal variation, and excellent and sustained response to low l-dopa doses.
Recent Findings
Unlike the classic phenotype, GCH-1–associated DRD may include features inconsistent with the original phenotype. We describe a GCH-1–associated late-onset DRD case with a family history of parkinsonism and cervical dystonia whose response to levodopa was poor and complicated with dyskinesia, blepharospasm, and severe nonmotor symptoms. We use this case as a springboard to review the spectrum of atypical DRD, DRD-plus, and DRD mimics.
Summary
GCH-1–related dystonia may exhibit wide intrafamilial phenotypic variability, no diurnal fluctuation, poor response to l-dopa, and such complications as dyskinesia, epilepsy, sleep disorders, autonomic dysfunction, oculogyric crisis, myoclonus, or tics. More recently, rare GCH-1 variants have been found to be associated with Parkinson disease. Clinicians should be aware of atypical DRD, DRD-plus, and DRD mimics.

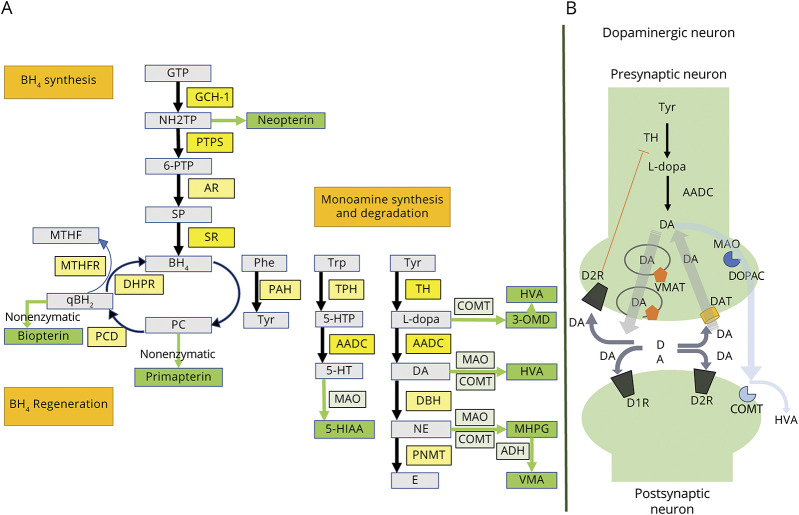
In 1976, Segawa described “hereditary progressive dystonia with marked diurnal fluctuation,”1 followed in 1988 by Nygaard and Duvoisin report of a robust response to l-dopa, for the first time coining the label, dopa-responsive dystonia (DRD).2 DRD is most commonly of childhood or adolescent onset, inherited in an autosomal dominant manner with incomplete penetrance.3,4 Less frequently, there is later onset and autosomal recessive inheritance.2,5 Likely underdiagnosed,3 its prevalence is estimated to be about 0.5 cases per million in the general population, representing 5%–10% of primary dystonias in childhood and adolescence.6 Symptoms emerge as a consequence of a nigrostriatal dopamine deficiency due to enzymatic abnormalities in the catecholaminergic biosynthesis pathway.7 The most frequent enzymatic defect is determined by more than 250 pathologic variants in the GTP cyclohydrolase 1 (GCH-1) gene at locus 14q 22.1–22.2, which encodes the GCH-1 protein.8 This protein catalyzes the synthesis of tetrahydrobiopterin (BH4), a tyrosine hydroxylase (TH) cofactor, whose deficiency limits the synthesis of dopamine, particularly in the ventral portion of the striatum, rich in D1 receptors.1,4 Furthermore, as BH4 is the cofactor for tryptophan hydroxylase and phenylalanine hydroxylase, serotonin and phenylalanine production is also compromised9 (Figure 1).
Figure 1. Biosynthetic Pathways.
Biosynthesis and regeneration of biopterin and monoamines (A). Dopaminergic synapse (B). 3-OMD = 3-ortho-methyldopa; 5-HIAA = 5-hydroxyindoleacetic acid; 5-HT = serotonin; 5-HTP = 5-hydroxytryptophan; 6-PTP = 6-pyruvoyltetrahydropterin; AADC = aromatic l-amino acid decarboxylase; ADH = alcohol dehydrogenase; AR = aldose reductase; BH4 = tetrahydrobiopterin; COMT = catechol-O-methyltransferase; D1R = dopamine receptor type 1; D2R = dopamine receptor type 2; DA = dopamine; DAT = dopamine active transporter; DBH = dopamine beta-hydroxylase; DHPR = dihydropteridine reductase; DOPAC = 3,4-dihydroxyphenylacetic acid; E = epinephrine; GCH-1 = GTP cyclohydrolase 1; GTP = guanosine triphosphate; HVA = homovanillic acid; l-dopa = levodopa; MAO = monoamine oxidase; MHPG = 3-methoxy-4-hydroxyphenylglycol; MTHF = methyltetrahydrofolate; MTHFR = methylene tetrahydrofolate reductase; NE = norepinephrine; NH2TP = d-erythro-7,8-dihydroneopterin triphosphate; PAH = phenylalanine hydroxylase; PC = pterin-4-alpha-carbinolamine; PCD = pterin-4-alpha-carbinolamine dehydratase; Phe = phenylalanine; PNMT = phenylethanolamine N-methyltransferase; PTPS = pyruvoyl-tetrahydropterin synthase; qBH2 = quinonoid-dihydrobiopterin; SP = sepiapterin; SR = sepiapterin reductase; TH = tyrosine hydroxylase; TPH = tryptophan hydroxylase; Trp = tryptophan; Tyr = tyrosine; VMA = vanillylmandelic acid; VMAT = vesicular monoamine transporter.
In its prototypical presentation, DRD presents with lower limb action dystonia, expressed as a unilateral or asymmetric equinovarus posture during (and ultimately affecting) walking, accompanied by marked diurnal fluctuation (i.e., sleep benefit and evening worsening). Besides GCH-1 deficiency, mutations in other genes may manifest this phenotype with some clinical variability according to the affected enzyme.2,10 Such is the case of sepiapterin reductase (SR) and TH,2 collectively classified within the clinically heterogeneous group of disorders defined as DRD-plus.7,11
We present here an atypical presentation of GCH-1–related DRD using the opportunity to review the relevant literature.
Case
This 34-year-old woman with no perinatal history nor exposure to dopamine receptor–blocking drugs or other medications manifested a slowly progressive equinovarus position of the right foot when walking, beginning at the age of 20 years. There was no diurnal fluctuation. She endorsed a sensation of rigidity and pain in her lower limbs when walking, greater on the right, and experienced partial relief when at rest. The lateral part of her shoes was excessively worn and could not use high-heel shoes.
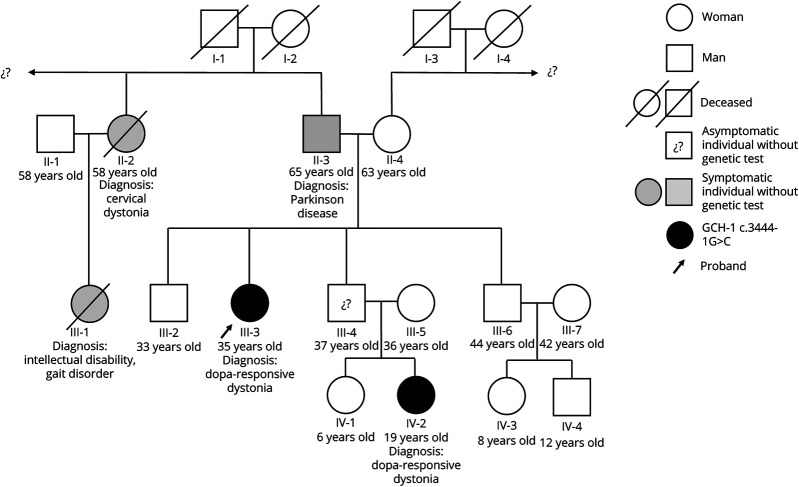
Her father (II-3) developed Parkinson disease (PD) at age 58 years, with REM sleep behavior disorder and constipation as prodromal manifestations. He was treated with l-dopa at low doses (300 mg/d) with sustained response and no complications after 5 years of treatment. Her niece (IV-2) was diagnosed with DRD at age 13 years, caused by the variant GCH-1 NM_000161.3(GCH1):c.344-1G>C, which is classified as pathogenic. This variant is located in a canonical splicing site, and it has previously been reported in clinvar (#RCV000540760.2) as causing a condition similar to that of our report. She exhibited a typical walking-induced foot dystonia with an optimal response to l-dopa. Finally, her paternal aunt (II-2) had adult-onset cervical dystonia, treated with botulinum toxin chemodenervation, apparently never treated with levodopa (Figure 2).
Figure 2. Genogram in Case Study.
Only the 2 youngest members of the family have had genetic confirmation of a GCH-1 c.3444-1G>C mutation. GCH-1 = GTP cyclohydrolase 1.
Examination at the age of 28 years showed bilateral foot dystonia. At that time, genetic evaluation confirmed the same mutation documented in her niece in a heterozygous form. Treatment with l-dopa 100 mg daily, with gradual adjustment up to 300 mg/d, modestly improved her walking, with substantial residual dystonia in the right foot.
Between the ages of 30 and 34 years, she was diagnosed with blepharospasm, insomnia, and early arousals with diurnal hypersomnia, anxiety, and depression. There was increasing bilateral lower limb pain along with persistent right-foot inversion when walking and new onset of jerky postural tremor of the upper limbs (Video 1). Motor symptoms had a partial response to increasing doses of l-dopa/benserazide, reaching a maximum dose of 600 mg/d divided into 3 doses. Her blepharospasm disappeared with l-dopa, but dyskinesia expressed as chorea of the upper limbs and right torticollis prompted a reduction in the dose to 400 mg/d divided into 4 doses.
Segment 1. Dystonic posture with inversion of the right foot during gait with increased wearing of the shoe sole on the right. Segment 2. Frequent blinking and blepharospasm. Segment 3. Dyskinesia predominantly in the upper limbs.Download Supplementary Video 1 (37.7MB, mp4) via http://dx.doi.org/10.1212/001125_Video_1
Regarding her nonmotor symptoms, there was persistent insomnia, refractory to l-dopa and nonpharmacologic treatments. A polysomnography was performed, which showed a long sleep latency (45 minutes) and long REM latency time (175 minutes), with nocturnal awakenings (48 minutes), poor sleep efficiency, reduced slow-wave sleep (8.8% of the total sleep time) and REM sleep (14.8% of the total sleep time), high index of microarousals, and no respiratory events. Sleep hygiene recommendations and the sequential use of bedtime melatonin 6 mg, mirtazapine 15 mg, and trazodone 100 mg yielded no benefits. Symptomatic response was only achieved with zolpidem CR 10 mg at bedtime. At the time of this report, she was also on treatment with duloxetine 60 mg once daily for anxiety and depression, achieving only partial response. Collectively, her phenotype was considered atypical for DRD.
Considering the low penetrance of the GCH-1 variant found and the atypical features, we also tested ceruloplasmin levels and thyroid, liver, and renal function, which were normal. Her brain MRI was also normal.
Dopa-Responsive Dystonia
Because of the conservation of a normal allele, autosomal dominant DRD (DYT5a) is associated with a less severe enzymatic defect than the autosomal recessive variants.2,7,10 Its penetrance is higher in women (87%) compared with men (35%),12 and accordingly, its incidence is 2.5–4 times higher in women.6,13 The age at presentation shows a bimodal peak with sex differences. Onset typically occurs in childhood or adolescence, with a mean age of 11.6 years,2,4,14 and a younger onset in females. The second onset peak is in adulthood, between the third and sixth decades.2 This last type is more frequent in males,15 with parkinsonism or tremor as the main or only clinical manifestation.12,15,16 Some cases manifest both dystonia and parkinsonism.17
One-third of patients with DRD have a family history of PD.18 It has been hypothesized that mutations in GCH-1 represent a risk factor for PD, even in the absence of a family history of DRD.17-19 Exome sequencing studies showed a 7-fold greater risk of PD development.19 Whether parkinsonian or dystonic, the phenotype may depend on epigenetic and environmental factors.18,19
In young-onset cases, dystonia is initially markedly asymmetric, involving 1 lower or upper limb, and progressing in half of those without treatment, to a segmental or generalized dystonia.1,3 Physical activity exacerbates clinical symptoms,2,7 and diurnal fluctuation is a feature in 80%–94% of the cases4,8,15 but decreases with age.2
On neurologic examination, muscle stretch reflexes may be enhanced, but no other pyramidal signs should be present, as neither should cerebellar, sensory, or cognitive signs.8 There have been reports of cognitive impairment in patients with young-onset DRD without treatment.20 Cardiologic and autonomic systems are generally spared.21,22 In parkinsonian phenotypes, patients can manifest postural tremor23 and retropulsion on the pull test.1 Myoclonus-dystonia, spastic paraplegia, and cerebral palsy have been reported as atypical phenotypes.4,16 Mood disorders, generalized anxiety, agoraphobia, and obsessive-compulsive disorder can be present because of associated serotonin pathway dysfunction.21,23 Major depression has been reported in more than 20% of cases and depressive symptoms in ∼50%, negatively affecting the quality of life. Psychiatric symptoms can appear in the premotor phase.21
Although infrequent in children, sleep disorders are present in about 55% of adults with DRD. Other than sleep benefit in motor symptoms, daytime sleepiness, nonrestorative sleep, and insomnia are common.24 Polysomnographic studies have revealed periodic limb movements, REM sleep behavior disorder, and increased latency to REM sleep. Abnormal movements during sleep have been reported after the onset of levodopa.25
Classic DRD patients show a marked and sustained response to l-dopa, with doses lower than 300 mg per day, in most cases.7,8 Complications such as motor fluctuations, end-of-dose wearing off, and l-dopa–induced dyskinesia are atypical.25 Some authors report the absence of motor fluctuations but chronic dyskinesia.23 However, a systematic review documented a rate of l-dopa–induced dyskinesia in GCH-1–associated DRD of 13.3% in autosomal recessive cases and 5.4% in autosomal dominant cases.26 Dyskinesia has been considered peak-dose and responds to a dose reduction. Anticholinergic agents can attenuate the dystonia and be relied on to reduce the need for l-dopa.27
Structural neuroimaging studies are normal. PET and SPECT with presynaptic dopaminergic markers have most frequently shown a normal dopamine uptake in the striatum of patients with DRD and parkinsonism due to pathogenic variants of GCH-1,28 suggesting that dopaminergic nerve terminals are not impaired.8 However, there are several reports of abnormal dopamine transporter (DAT) SPECT, implying nigrostriatal denervation in GCH-1 carriers manifesting adult-onset parkinsonism or, more rarely, in asymptomatic adult cases.29,30 The progression of nigrostriatal degeneration was demonstrated in a woman carrying a GCH-1 pathogenic variant, manifesting parkinsonism at age 47 years. Serial FP-CIT PET scans showed a normal initial pattern followed by a continuous decline of the putaminal binding ratio at 2, 8, and 11 years from disease onset.31,32 Therefore, an abnormal DAT scan does not rule out GCH-1 cases, and these patients are at high risk of developing a neurodegenerative parkinsonism.
In clinical practice, these imaging techniques aid in the distinction of DRD-like diseases, such as homozygous carriers of pathologic variants in the Parkin gene (PARK2) or spinocerebellar ataxia types 2 and 3 (SCA2 and SCA3).2,33
Pathologic studies in carriers of GCH-1 variants developing DRD have shown decreased levels of tetrahydrobiopterin and neopterin, with preserved nigrostriatal dopaminergic terminals. Absence of nigral cell degeneration was shown in the autopsy of a patient with DRD with more than 80 years of disease progression.34 Although we are not aware of pathologic reports of cases of GCH-1–related adult-onset parkinsonism, Lewy body pathology and neuronal loss in the nigral cells and locus coeruleus was reported in a case (heterozygous for c.276delC) who presented with juvenile-onset DRD and parkinsonism complicated by early development of disabling levodopa-induced dyskinesia and death at 39 years.35,36 This is consistent with the recent discovery that rare GCH-1 variants, previously reported as pathogenic of DRD, are associated with PD19 with a phenotype consistent of younger age at onset and milder motor symptoms but more autonomic dysfunction.37
When the clinical manifestations are prototypical of classic DRD, the diagnosis can be straightforward. However, in cases like ours with atypical manifestations (older age at onset, absence of diurnal fluctuation, partial response to l-dopa, peak-dose dyskinesia, sleep, and neuropsychiatric difficulties), a broader spectrum of differential diagnoses needs to be considered.
DRD-Plus
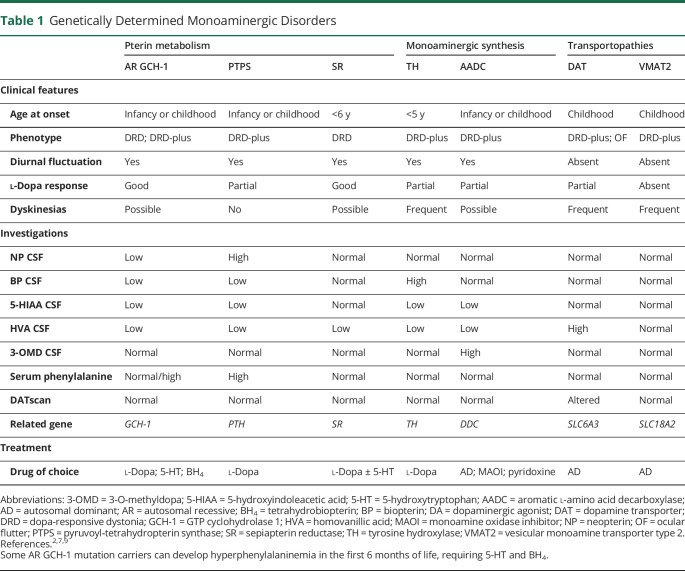
DRD-plus is a term used in reference to young-onset cases in which l-dopa–responsive dystonia includes motor or nonmotor symptoms beyond those expected for classic DRD.5,7 Atypical features include psychomotor retardation, progressive encephalopathy, microcephaly, hypotonia, spasticity, eating disorders, epilepsy, sleep disorders, hyperthermia, ptosis, autonomic dysfunction, ataxia, oculogyric crises, ocular flutter, striatal foot, laryngeal dystonia, myoclonus, tics, different types of focal dystonia, childhood-onset parkinsonism, late-onset parkinsonism, poor response to l-dopa, and l-dopa–induced dyskinesia7,8,12,23,38 (Table 1). These clinical manifestations are more frequent in monoaminergic disorders with an autosomal recessive inheritance pattern, involving pterin metabolism, enzymatic disorders in monoaminergic synthesis, and transport disorders related to DAT and VMAT (Figure 1).
Table 1.
Genetically Determined Monoaminergic Disorders
DRD Mimics
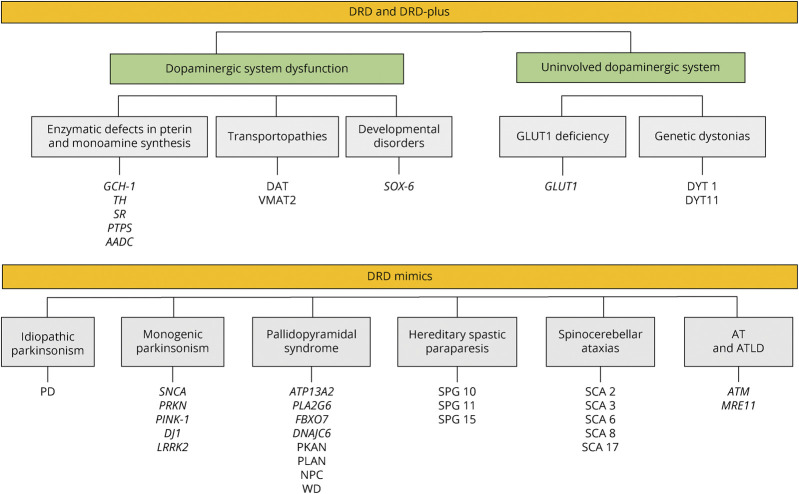
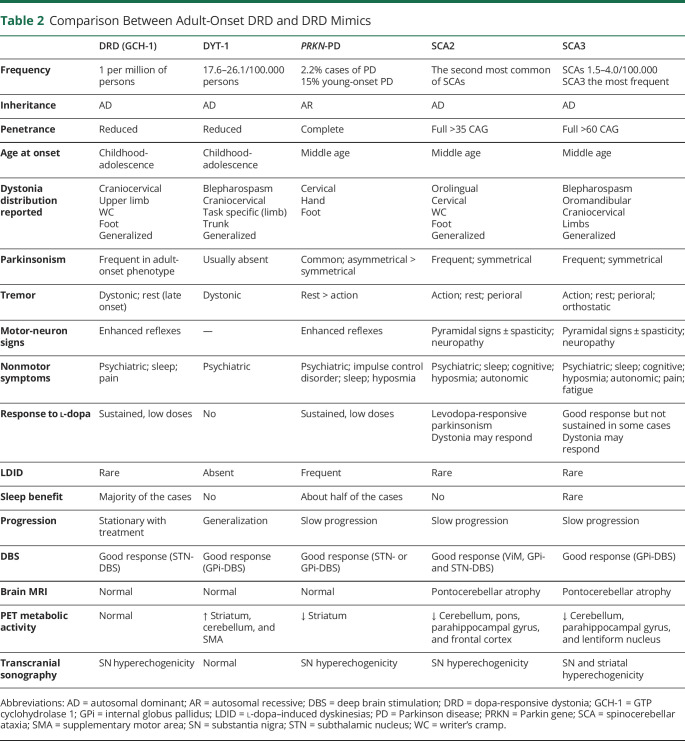
DRD mimics (or “look-alikes”) are a group of neurodegenerative and non-neurodegenerative diseases with or without nigrostriatal dopaminergic impairment, presenting as dystonia and responding to dopaminergic drugs, thus mimicking DRD, but in the absence of pathogenic variants known to express DRD and DRD-plus phenotypes.7 DRD mimics have been reported in cases of DYT1 dystonia,7,39 SPG11-related hereditary spastic paraparesis,1,40 spinocerebellar ataxia (SCA) types 2 and 3,33 juvenile monogenic parkinsonisms, particularly because of PRKN mutations (PARK2),41,42 GLUT1 deficiency, ataxia-telangiectasia,7 and pallidopyramidal syndrome,43 among others (Figure 3). Within this category, Wilson disease and Niemann-Pick disease type C are critical to recognize given the availability of specific disease-modifying treatments. Certain clinical features can help distinguish the most common DRD mimics (Table 2).
Figure 3. Classification and Causes of DRD Syndrome.
AADC = aromatic l-amino acid decarboxylase; AT = ataxia-telangiectasia; ATLD = ataxia telangiectasia–like disorders; DAT = dopamine active transporter; DRD = dopa-responsive dystonia; GCH-1 = GTP cyclohydrolase 1; GLUT-1 = glucose transporter type 1; NPC = Niemann-Pick disease type C; PD = Parkinson disease; PKAN = pantothenate kinase–associated neurodegeneration; PLAN = PLA2G6-associated neurodegeneration; PRKN = Parkin gene; PTPS = pyruvoyl‐tetrahydropterin synthase; SCA = spinocerebellar ataxia; SOX-6 = SRY-Box 6 transcription factor; SPG = spastic paraparesis; SR = sepiapterin reductase; TH = tyrosine hydroxylase; VMAT2 = vesicular monoamine transporter type 2; WD = Wilson disease.
Table 2.
Comparison Between Adult-Onset DRD and DRD Mimics
Diagnostic Approach
Basic diagnostic workup depends on the patient's background and symptoms, with l-dopa responsiveness as a major unifying theme. It is generally recommended that every patient with early-onset focal dystonia of unclear nature should have a therapeutic trial with levodopa. Factors in support of the use of levodopa include a family history of dystonia or parkinsonism, atypical age at presentation, dystonia involving the limbs, fluctuations in severity, progression to distant segments, generalization, associated movement disorders, and secondary etiologies. An initial dose of 50 mg levodopa (plus carbidopa or benserazide) 1 to 3 times a day is an acceptable initial dose, which may be slowly increased up to 1000 mg levodopa per day divided into 3 doses for at least 1 month before concluding on its efficacy or lack thereof.2,44
In the presence of the classical DRD phenotype, we must study enzymatic deficiencies related to the GCH-1 gene, explaining about half of the cases, and less frequently TH and SR deficiencies or transport disorders.3 As l-dopa might alter CSF parameters, it is important to obtain dopamine metabolism measures before its use.8
Concentrations of total CSF biopterin, homovanillic acid, 3-O-methyldopa, and total neopterin are reduced in patients with GCH-1 AD deficiency. When a CSF sample is not available, the evaluation of GCH-1 activity in mononuclear blood cells stimulated with phytohemagglutinin or fibroblasts stimulated with cytokines can be useful.8
As a consequence of BH4 deficiency, a bolus of phenylalanine elicits a high serum phenylalanine/tyrosine ratio 1–2 hours after administration in patients with GCH-1 and SR mutations.45,46 This test will be normal in cases because of TH deficiency.47 A high serum level of prolactin can be found in SR and TH deficiencies because of the lack of dopaminergic inhibition; conversely, it can be normal in autosomal dominant GCH-1 cases.2,9,48
In DRD-plus and DRD mimics, PET scans are helpful in recognizing neurodegenerative parkinsonism. MRI can show signal abnormalities in neurodegeneration with brain iron accumulation disorders, SPG11, Wilson disease, and SCAs. From a laboratory standpoint, alpha-fetoprotein, ceruloplasmin levels, acanthocytes, and liver function test should be performed. As we mentioned before, the phenotypic spectrum underlying the term DRD is quite large. Thus, it is desirable to move to a diagnostic approach that incorporates the use of genetic assays such as multigenic panels and/or exome sequencing early in the process, reserving the use of other assays such as structural and functional neuroimaging or biochemical tests to help in the final diagnostic interpretation of a comprehensive genetic evaluation.
Conclusions
DRD encompasses a heterogeneous syndrome of genetic or neurodegenerative diseases, including monoamine and biopterin disorders, with phenotypic pleomorphism compelling their nosologic separation into DRD, DRD-plus, and DRD mimics. GCH-1 deficiency explains about half of the cases exhibiting the classical DRD syndrome originally described by Segawa. Clinicians should be aware of its large clinical variability and the disorders it may mimic. Because of incomplete penetrance, autosomal dominant GCH-1 mutations may seem to “skip generations” and consequently be falsely considered as autosomal recessive. Remarkably, autosomal dominant GCH-1 mutations can be associated with sporadic DRD or PD. Moreover, because of variable expressivity, mutation carriers with atypical phenotypes might be considered affected by alternative diseases. Our case study is illustrative of the wide intrafamilial variability of autosomal dominant GCH-1–related atypical DRD, including late-onset cervical dystonia and parkinsonism, and highlights the absence of diurnal fluctuation, high threshold for the therapeutic benefit of l-dopa, and l-dopa–induced dyskinesia, all of which were originally considered exclusionary for DRD. Other atypical DRD features include epilepsy, sleep or neuropsychiatric disorders, autonomic dysfunction, oculogyric crisis, myoclonus, or tics, challenging the original GCH-1 genotype-phenotype alignment.
Acknowledgment
The authors confirm that patient consent was obtained for this work.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
P. Salles, M. Terán-Jimenez, Á. Vidal-Santoro, and P. Chaná-Cuevas report no disclosures relevant to the manuscript. M. Kauffman is an employee of CONICET Argentina. He has received grant support from the Ministry of Health of Argentina and City of Buenos Aires. A. Espay has received grant support from the NIH and the Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for AbbVie, NeuroDerm, Neurocrine, Amneal, Acadia, Acorda, Kyowa Kirin, Sunovion, Lundbeck, and US WorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from US WorldMeds, Acadia, and Sunovion. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Age at onset of GCH-1 mutation carriers presents a bimodal peak, with “classic” DRD often manifesting in childhood and atypical DRD and parkinsonism in adulthood.
→ Atypical manifestations of GCH-1–DRD include absence of diurnal fluctuation, poor response to l-dopa, and such complications as dyskinesia, epilepsy, sleep disorders, autonomic dysfunction, oculogyric crisis, myoclonus, or tics, incongruent with the original GCH-1 genotype-phenotype observations.
→ Autosomal recessive monoaminergic disorders involving pterin metabolism, enzymatic disorders in monoaminergic synthesis, and transport disorders of DAT and VMAT may express a DRD phenotype with atypical neurologic manifestations.
References
- 1.Segawa M. Hereditary progressive dystonia with marked diurnal fluctuation. Brain Dev. 2011;33(22 suppl 1):195-201. [DOI] [PubMed] [Google Scholar]
- 2.Wijemanne S, Jankovic J. Dopa-responsive dystonia—clinical and genetic heterogeneity. Nat Rev Neurol. 2015;11(7):414-424. [DOI] [PubMed] [Google Scholar]
- 3.Malek N, Fletcher N, Newman E. Diagnosing dopamine-responsive dystonias. Pract Neurol. 2015;15(5):340-345. [DOI] [PubMed] [Google Scholar]
- 4.Segawa M, Kimura K, Hoshino K, Terao Y, Hayashi M. Segawa dopa-responsive Dystonia. Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier. 2017:1-7. [Google Scholar]
- 5.Krim E, Aupy J, Clot F, Bonnan M, Burbaud P, Guehl D. Mutation in the GCH1 gene with dopa-responsive dystonia and phenotypic variability. Neurol Genet. 2018;4(2):e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygaard TG. Dopa-responsive dystonia. Curr Opin Neurol. 1995(4):310-313. [DOI] [PubMed] [Google Scholar]
- 7.Lee WW, Jeon B, Kim R. Expanding the spectrum of dopa-responsive dystonia (DRD) and proposal for new definition: DRD, DRD-plus, and DRD look-alike. J Korean Med Sci. 2018;33(28):e184-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furukawa Y. GTP Cyclohydrolase 1-Deficient Dopa-Responsive Dystonia. Genereviews®; 2002:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siu WK. Genetics of monoamine neurotransmitter disorders. Transl Pediatr. 2015;4(2):175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaila EC, McCabe DJ, Delanty N, Costello DJ, Murphy RP. Broadening the phenotype of childhood-onset dopa-responsive dystonia. Arch Neurol. 2006;63(8):1185-1188. [DOI] [PubMed] [Google Scholar]
- 12.Segawa M, Nomura Y, Nishiyama N. Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease). Ann Neurol. 2003(54 suppl 6):S32-S45. [DOI] [PubMed] [Google Scholar]
- 13.Nutt JG, Nygaard TG. Response to levodopa treatment in dopa-responsive dystonia. Arch Neurol. 2001;58(6):905-910. [DOI] [PubMed] [Google Scholar]
- 14.Ichinose H, Ohye T, Takahashi E, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8(3):236-242. [DOI] [PubMed] [Google Scholar]
- 15.Weng YC, Wang CC, Wu YR. Atypical presentation of dopa-responsive dystonia in Taiwan. Brain Behav. 2018;8(2):e00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadic V, Kasten M, Brüggemann N, Stiller S, Hagenah J, Klein C. Dopa-responsive dystonia revisited: diagnostic delay, residual signs, and nonmotor signs. Arch Neurol. 2012;69(12):1558-1562. [DOI] [PubMed] [Google Scholar]
- 17.Mencacci NE, Isaias IU, Reich MM, et al. Parkinson's disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137(pt 9):2480-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshino H, Nishioka K, Li Y, et al. GCH1 mutations in dopa-responsive dystonia and Parkinson's disease. J Neurol. 2018;265(8):1860-1870. [DOI] [PubMed] [Google Scholar]
- 19.Rudakou U, Ouled Amar Bencheikh B, Ruskey JA, et al. Common and rare GCH1 variants are associated with Parkinson's disease. Neurobiol Aging. 2019;73(7):231.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Laso E, Sánchez-Raya A, Moriana JA, et al. Neuropsychiatric symptoms and intelligence quotient in autosomal dominant Segawa disease. J Neurol. 2011;258(12):2155-2162. [DOI] [PubMed] [Google Scholar]
- 21.Antelmi E, Stamelou M, Liguori R, Bhatia KP. Nonmotor symptoms in dopa-responsive dystonia. Mov Disord Clin Pract. 2015;2(4):347-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brüggemann N, Stiller S, Tadic V, et al. Non-motor phenotype of dopa-responsive dystonia and quality of life assessment. Parkinsonism Relat Disord. 2014;20(4):428-431. [DOI] [PubMed] [Google Scholar]
- 23.Trender-Gerhard I, Sweeney MG, Schwingenschuh P, et al. Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J Neurol Neurosurg Psychiatry. 2009;80(8):839-845. [DOI] [PubMed] [Google Scholar]
- 24.Timmers ER, Kuiper A, Smit M, et al. Non-motor symptoms and quality of life in dopa-responsive dystonia patients. Parkinsonism Relat Disord. 2017;45:57-62 [DOI] [PubMed] [Google Scholar]
- 25.Hertenstein E, Tang NK, Bernstein CJ, Nissen C, Underwood MR, Sandhu HK. Sleep in patients with primary dystonia: a systematic review on the state of research and perspectives. Sleep Med Rev. 2016;26:95-107. [DOI] [PubMed] [Google Scholar]
- 26.Bendi VS, Shou J, Joy S, Torres-Russotto D. Motor fluctuations and levodopa-induced dyskinesias in dopa-responsive dystonia. Parkinsonism Relat Disord. 2018;50:126-127. [DOI] [PubMed] [Google Scholar]
- 27.Kim R, Jeon B, Lee WW. A systematic review of treatment outcome in patients with dopa-responsive dystonia (DRD) and DRD-plus. Mov Disord Clin Pract. 2016;3(5):435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarman PR, Bandmann O, Marsden CD, Wood NW. GTP cyclohydrolase I mutations in patients with dystonia responsive to anticholinergic drugs. J Neurol Neurosurg Psychiatry. 1997;63(3):304-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L, Ye J, Zhang H, Han ZF, Zheng ZH. Degree of dopaminergic degeneration measured by 99mTc-TRODAT-1 SPECT/CT imaging. Neural Regen Res. 2018;13(7):1281-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukawa Y, Kish SJ. Parkinsonism in GTP cyclohydrolase 1-deficient DOPA-responsive dystonia. Brain. 2015;138(5):e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JJ, Lu CS, Tsai CH. Variability of presynaptic nigrostriatal dopaminergic function and clinical heterogeneity in a dopa-responsive dystonia family with GCH-1 gene mutation. J Neurol. 2018;265(3):478-485. [DOI] [PubMed] [Google Scholar]
- 32.Shin JH, Lee WW, Lee JY, Kim HJ, Jeon B. GCH-1 genetic variant may cause Parkinsonism by unmasking the subclinical nigral pathology. J Neurol. 2020;267(7):1952-1959. [DOI] [PubMed] [Google Scholar]
- 33.Wilder-Smith E, Tan EK, Law HY, Zhao Y, Ng I, Wong MC. Spinocerebellar ataxia type 3 presenting as an L-DOPA responsive dystonia phenotype in a Chinese family. J Neurol Sci. 2003;213(1-2):25-28. [DOI] [PubMed] [Google Scholar]
- 34.Segawa M, Nomura Y, Hayashi M. Dopa-responsive dystonia is caused by particular impairment of nigrostriatal dopamine neurons different from those involved in Parkinson disease: evidence observed in studies on Segawa disease. Neuropediatrics. 2013;44(2):61-66. [DOI] [PubMed] [Google Scholar]
- 35.Segawa M, Nomura Y, Yukishita S, Nishiyama N, Yokochi M. Is phenotypic variation of hereditary progressive dystonia with marked diurnal fluctuation/dopa-responsive dystonia (HPD/DRD) caused by the difference of the locus of mutation on the GTP cyclohydrolase 1 (GCH-1) gene? Adv Neurol. 2004;94:217-223. [PubMed] [Google Scholar]
- 36.Gibb WR, Narabayashi H, Yokochi M, Iizuka R, Lees AJ. New pathologic observations in juvenile onset parkinsonism with dystonia. Neurology. 1991;41(6):820-822. [DOI] [PubMed] [Google Scholar]
- 37.Pan HX, Zhao YW, Mei JP, et al. GCH1 variants contribute to the risk and earlier age-at-onset of Parkinson's disease: a two-cohort case-control study. Transl Neurodegener. 2020;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaltho TC, Jankovic J, Lotze T. The association of Tourette syndrome and dopa-responsive dystonia. Mov Disord. 2011;26(2):359-360. [DOI] [PubMed] [Google Scholar]
- 39.Ozelius L, Lubarr N. DYT1 Early-Onset Isolated Dystonia. Genereviews®; 1993:1-19. [PubMed] [Google Scholar]
- 40.Lee WW, Jeon BS. Clinical spectrum of dopa-responsive dystonia and related disorders. Curr Neurol Neurosci Rep. 2014;14(7):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan NL, Graham E, Critchley P, et al. Parkin disease: a phenotypic study of a large case series. Brain. 2003;126(pt 6):1279-1292. [DOI] [PubMed] [Google Scholar]
- 42.Yamamura Y, Hattori N, Matsumine H, Kuzuhara S, Mizuno Y. Autosomal recessive early-onset parkinsonism with diurnal fluctuation: clinicopathologic characteristics and molecular genetic identification. Brain Dev. 2000(22 suppl 1):S87-S91. [DOI] [PubMed] [Google Scholar]
- 43.Kara E, Hardy J, Houlden H. The pallidopyramidal syndromes: nosology, aetiology and pathogenesis. Curr Opin Neurol. 2013;26(4):381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin. 2015;33(1):77-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Opladen T, Okun JG, Burgard P, Blau N, Hoffmann GF. Phenylalanine loading in pediatric patients with dopa-responsive dystonia: revised test protocol and pediatric cutoff values. J Inherit Metab Dis. 2010;33(6):697-703. [DOI] [PubMed] [Google Scholar]
- 46.Hyland K, Fryburg JS, Wilson WG, et al. Oral phenylalanine loading in dopa-responsive dystonia: a possible diagnostic test. Neurology. 1997;48(5):1290-1297. [DOI] [PubMed] [Google Scholar]
- 47.Bandmann O, Goertz M, Zschocke J, et al. The phenylalanine loading test in the differential diagnosis of dystonia. Neurology. 2003;60(4):700-702. [DOI] [PubMed] [Google Scholar]
- 48.Koht J, Rengmark A, Opladen T, et al. Clinical and genetic studies in a family with a novel mutation in the sepiapterin reductase gene. Acta Neurol Scand Suppl. 2014;129(198):7-12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Segment 1. Dystonic posture with inversion of the right foot during gait with increased wearing of the shoe sole on the right. Segment 2. Frequent blinking and blepharospasm. Segment 3. Dyskinesia predominantly in the upper limbs.Download Supplementary Video 1 (37.7MB, mp4) via http://dx.doi.org/10.1212/001125_Video_1