Abstract
Background and Objectives
Essential tremor (ET) is one of the most prevalent movement disorders. Because ET is so common, individuals with other neurologic disorders may also have ET. There is evidence, however, that the cooccurrence of ET with Parkinson disease (PD) and/or dystonia is not merely a chance cooccurrence. We have observed combinations of these 3 movement disorders within individuals and across individuals within families containing multiple individuals with ET. This observation has a number of implications. Our objective is to present 4 ET families in whom motor phenomenology was heterogeneous and discuss the implications of this finding.
Methods
ET cases and their relatives were enrolled in the Family Study of Essential Tremor (2015–present). Phenotyping was performed by a senior movement disorders neurologist based on neurologic examination.
Results
We present 4 families, including 14 affected individuals, among whom assigned diagnoses were ET, PD, ET + PD, and ET + dystonia. In those with ET and another movement disorder, the predominant and earliest phenotype was ET.
Discussion
There are assortments of these 3 involuntary motor disorders, ET, dystonia, and PD, both within individuals and in different individuals within ET families. This observation has mechanistic implications. Furthermore, we believe that the concept of the mixed motor disorder should enter into and inform the clinical dialogue. In assigning diagnoses, clinicians are swayed by family history information, and they should be prepared to observe a mix of different motor disorders to manifest within particular families.
Essential tremor (ET), a highly prevalent movement disorder, is commonplace across populations.1 Given its high prevalence, it is entirely conceivable that patients with other movement disorders (e.g., Parkinson disease [PD] or dystonia) might on occasion already have a history of ET. As such, these individuals would have a combination or mix of these conditions. Along similar lines, in families containing multiple individuals with dystonia or families with multiple individuals with PD, it is conceivable that family members would on occasion manifest a highly prevalent condition such as ET, making the family one in which there is a mix of motor disorders. These are examples of what is likely to be chance cooccurrence within individuals and within families.2
Yet there is also evidence that the cooccurrence of ET with other movement disorders, specifically PD and dystonia, is not entirely due to chance. Cross-sectional studies have shown significant associations between ET and PD,3,4 and in a longitudinal, prospective population-based study, the risk of incident PD was 4 times higher in patients with ET than in similarly aged controls.5 Family studies also show associations between ET and PD that are not due to chance.3,6-8 There is growing recognition that patients with ET may develop dystonia on examination.9-11 Furthermore, within a limited number of published ET kindreds, one may find examples of family members with combinations of tremor, dystonia, or both.12-15
Irrespective of the interpretation, evidence suggests that these 3 motor disorders, ET, dystonia, and PD, cooccur within individuals and in different individuals within the same families. Yet this has not been the focus of concentrated scholarly attention, and such families have not been presented with detailed tremor examinations or individual-level phenotypic data. The purpose of this study was to present meticulous case-by-case phenotypic details (i.e., deep phenotyping) of 4 such families with mixed motor phenomenology and to discuss the possible mechanisms.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by Columbia University and Yale University Institutional Review Boards; participants signed written informed consent. The current analyses were approved by Columbia University, Yale University, and the University of Texas Southwestern Institutional Review Boards (respective protocol numbers: AAAQ3307, 1412015096, and STU-2020-0566).
As described,16,17 ET cases (i.e., probands) and their relatives were enrolled in a genetic study of ET, the Family Study of Essential Tremor (phase 2, September 2015–present), which enrolled participants from throughout the United States. Inclusion criteria for probands were as follows: (1) diagnosis of ET assigned by a doctor, (2) age of tremor onset ≤40 years (later changed to ≤50 to be more inclusive), and (3) at least 2 living relatives in the United States who were reported to have ET diagnosed by a doctor.16 The exclusion criterion for probands was a reported diagnosis of dystonia or PD.16 As described,16 during telephone interviews with probands, relatives were identified and reported by the proband to be affected or unaffected.
As detailed elsewhere,16 trained personnel conducted in-person in-home evaluations; the evaluation included clinical questionnaires, including the Mini-Mental State Examination (0 [maximally impaired]–30),18 the Lawton Instrumental Activities of Daily Living Scale (0 [maximally impaired]–8),19 and a standardized videotaped neurologic examination. The latter included a detailed assessment of postural tremor, 5 tests for kinetic tremor, the motor portion of the Unified Parkinson Disease Rating Scale20 excluding an assessment of rigidity, and a comprehensive assessment of dystonia. The assessment of dystonia was performed through review of views of the face, neck, trunk, and extremities: while seated, standing, and walking (including turning); with posture (arms extended in front of body and in “wing-beating” position); and with multiple tests of action (finger taps, hand-opening/closure, finger-to-nose, pouring/drinking/lifting water with a spoon, and alternating toe-heel taps). Handwriting was videotaped and reviewed.21 Audio recordings of sustained phonation and speech were also reviewed to assess for spasmodic dysphonia.21
A senior level movement disorders neurologist (E.D.L.) reviewed all videotaped examinations, rating the severity of postural and kinetic arm tremors on 12 examination items using a reliable scale.22 As reviewed elsewhere,16 ratings were 0 (absent), 0.5 (very low amplitude and almost never present), 1.0 (mild = low amplitude or intermittent tremor), 1.5 (mild-to-moderate [tremor sometimes more than mild]), 2 (moderate = clearly oscillatory and > mild amplitude), 3 (severe), and 4 (extremely severe) and resulted in a total tremor score (range = 0–46 [maximum]).
As described,16 all ET diagnoses were assigned by E.D.L. based on review of questionnaires and videotaped neurologic examination using the published diagnostic criteria.23 The criteria include gradations of possible, probable, and definite ET.23 At a minimum, possible ET required moderate or greater amplitude kinetic tremor during 3 or more activities in the absence of another known cause (e.g., medication-induced tremor and tremor from hyperthyroidism) (Table 1).23 These diagnostic criteria for ET were developed for a population-based genetic study, and based on data from approximately 2,000 nondiseased controls, the criteria carefully detail the specific examination maneuvers during which tremor should be present and the severity of tremor that should be evident during these maneuvers to distinguish normal from ET.16 These criteria have been shown to be both reliable22 and valid24 and have been used by tremor investigators in the United States and worldwide.16
Table 1.
Diagnostic Features of the Movement Disorders Observed in Our Families
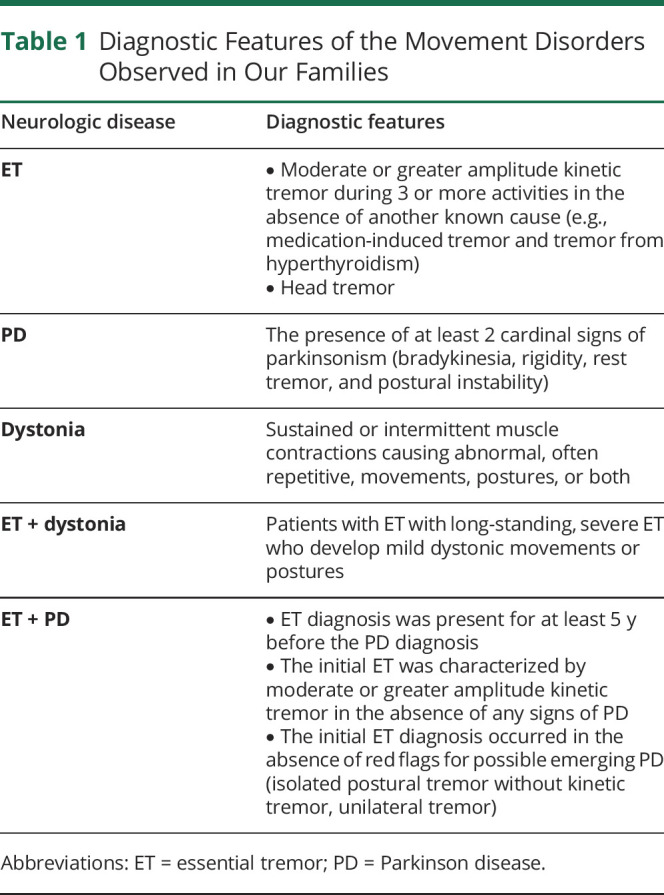
The diagnosis of dystonia was made using the published diagnostic criteria (sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both) (Table 1).21 PD was diagnosed using the published diagnostic criteria, which required the presence of at least 2 cardinal signs (Table 1).25,26 As described,16 some patients with ET may develop mild dystonia; ET and dystonia were the diagnostic categories used for patients with long-standing, severe ET who were developing mild dystonic movements or postures (Table 1). As described,26 the diagnosis of ET-PD required that (1) the ET diagnosis was present for at least 5 years before the PD diagnosis, (2) the initial ET was characterized by moderate or greater amplitude kinetic tremor in the absence of any signs of PD, and (3) the initial ET diagnosis occurred in the absence of red flags for possible emerging PD (isolated postural tremor without kinetic tremor, unilateral tremor) (Table 1).
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
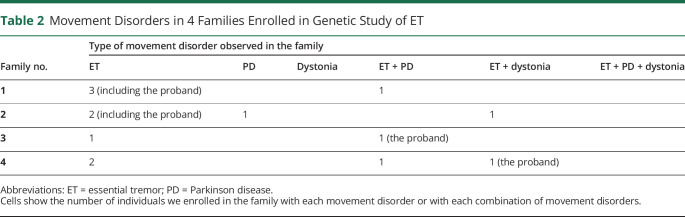
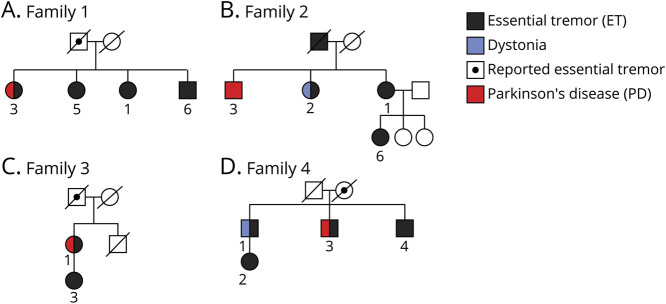
There were 4 families, including 14 affected individuals (Table 2). Pedigrees are shown in Figure 1, A–D.
Table 2.
Movement Disorders in 4 Families Enrolled in Genetic Study of ET
Figure 1. Pedigrees of 4 Families.
Pedigrees are shown, demonstrating the affected individuals in each family.
Family 1
The proband in family 1 (person 1), age 67 years, was from a White family of mixed German-English ancestry. Her deceased father and paternal grandmother were reported to have had ET. Age of tremor onset was 23 years, and a neurologist had diagnosed ET. She was taking primidone for ET. On examination, there was mild postural tremor and kinetic tremor scores (pouring, using a spoon, drinking, finger-nose-finger, and while drawing spirals) were moderate-to-severe (Table 3, Figure 2A). There was mild voice tremor but no head tremor. The total tremor score was 24.5, indicating moderate overall tremor. The diagnosis was probable ET.
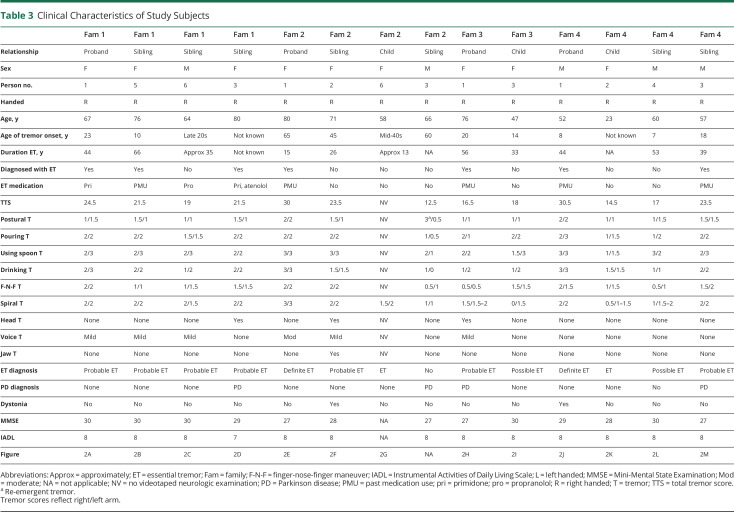
Table 3.
Clinical Characteristics of Study Subjects
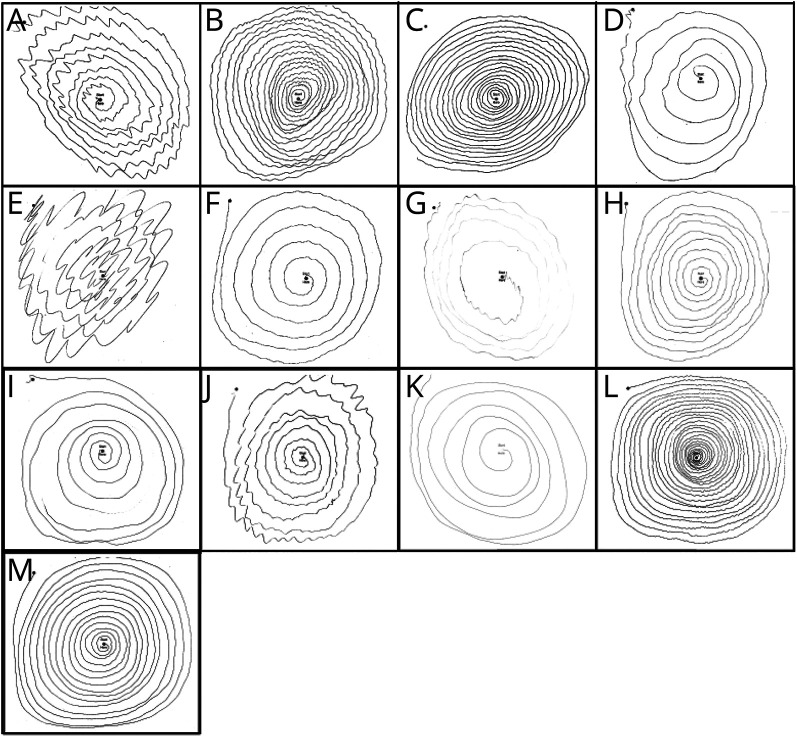
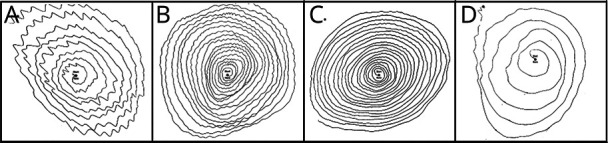
Figure 2. Spirals Demonstrating Kinetic Tremor.
(A) Kinetic tremor rating = 2 on drawing a spiral with the left hand (family 1, person 1). (B) Kinetic tremor rating = 2 on drawing a spiral with the right hand (family 1, person 5). (C) Kinetic tremor rating = 2 on drawing a spiral with the right hand (family 1, person 6). (D) Kinetic tremor rating = 2 on drawing a spiral with the left hand (family 1, person 3). (E) Kinetic tremor rating = 3 on drawing a spiral with the right hand (family 2, person 1). (F) Kinetic tremor rating = 2 on drawing a spiral with the right hand (family 2, person 2). (G) Kinetic tremor rating = 2 on drawing a spiral with the left hand (family 2, person 6). (H) Kinetic tremor rating = 1.5–2 on drawing a spiral with the left hand (family 3, person 1). (I) Kinetic tremor rating = 1.5 while drawing a spiral with the left hand (family 3, person 3). (J) Kinetic tremor rating = 2 while drawing a spiral with the right hand (family 4, person 1). (K) Kinetic tremor rating = 1–1.5 while drawing a spiral with the left hand (family 4, person 2). (L) Kinetic tremor rating = 1.5 while drawing a spiral with the left hand (family 4, person 4). (M) Kinetic tremor rating = 2 while drawing a spiral with the right hand. Micrographia is also noticeable (family 4, person 3).
Her sister (person 5), age 76 years, reported an age of tremor onset of 10 years and was diagnosed with ET later in life. In the past, she had taken medication for ET. On examination, there was mild postural tremor and moderate or severe kinetic tremor while pouring, using a spoon, drinking, and drawing spirals (Table 3, Figure 2B). There was mild voice tremor but no head tremor. The total tremor score was 21.5, indicating moderate overall tremor. The diagnosis was probable ET.
A brother (person 6), age 64 years, reported an age of tremor onset in his late 20s. He had not been diagnosed with ET yet reported having taken propranolol. On examination, there was mild postural tremor and mild-to-severe kinetic tremor on various tasks (Table 3, Figure 2C). There was mild voice tremor but no head tremor. The total tremor score was 19, indicating mild-to-moderate overall tremor. The diagnosis was probable ET.
Another sister (person 3), age 80 years, did not recall her age of onset but had seen a neurologist at age 79 years and was diagnosed with ET. Given the severity of her kinetic tremor (Table 3), it is likely that she had had tremor for many years. She was taking primidone and atenolol for tremor. On examination, there were mild postural tremor and moderate kinetic tremor while pouring, using a spoon, drinking from a cup, and drawing spirals (Table 3, Figure 2D). There was a head tremor but no voice tremor. The total tremor score was 21.5, indicating moderate overall tremor. The diagnosis was probable ET. In addition, she had numerous features of early PD: rest tremor while standing (right), mild flexed posturing of her right hand during arm extension, decreased eye blink frequency and pauses, and mild decrement during rapid alternating movements. She was on no medications that could have resulted in these features. Hence, the final diagnosis was ET + PD (Video 1).
Family 1, Person 3. ET is evident when she pours water between cups (moderate kinetic tremor is present bilaterally) and in her spiral drawing (moderate kinetic tremor is seen in Figure 2D). Signs of parkinsonism include a mild flexed posturing of her right hand during arm extension in the wing-beat position, rest tremor in the right hand while standing, hypomimia and reduced eye blink frequency, and pauses and decrement during finger taps.Download Supplementary Video 1 (10.3MB, mov) via http://dx.doi.org/10.1212/001100_Video_1
Family 2
The proband in family 2 (person 1) was age 80 years and was from a White family of mixed European ancestry. Her deceased father was reported to have had ET. The Age of tremor onset was 65 years, and a neurologist had diagnosed ET. She had taken medication for ET in the past. On examination, there was moderate postural tremor. Kinetic tremor was moderate-to-severe while pouring, using a spoon, drinking, finger-nose-finger maneuver, and while drawing spirals (Table 3, Figure 2E). The total tremor score was 30, indicating moderate-to-severe overall tremor. There was moderate voice tremor but no head tremor. Diagnosis was definite ET.
Her sister (person 2), age 71 years, reported that her tremor had started at age 45 years, but she had not been diagnosed with ET or taken medication for ET. On examination, there were mild postural tremor and moderate-to-severe kinetic tremor during multiple tasks (Table 3, Figure 2F). Mild head, jaw, and voice tremors were present. The total tremor score was 23.5, indicating moderate overall tremor. The diagnosis was probable ET. There were also features of mild dystonia: (1) dystonic posturing of the left thumb during arm extension, (2) head tremor, although mild, persisted even while supine (typically a feature of dystonic head tremor rather than ET head tremor),27 and (3) none of her spirals had a clear tremor orientation axis (also a feature aligning them with dystonic tremor rather than ET) (Video 2).28
Family 2, Person 2. ET is evident when she pours water between cups (moderate kinetic tremor is present bilaterally), while using a spoon (severe kinetic tremor is present bilaterally, causing her to spill), in her spiral drawing (moderate kinetic tremor is seen in Figure 2F), during arm extension (postural tremor ratings of 1–1.5), and jaw tremor while using a spoon. The dystonia is subtle—when initially raising her left hand so that her arm is outstretched, the left thumb is abducted (see right thumb for comparison) and during sustained posture holding, the right thumb remains abducted downward and there is a directionality to the tremor, with the thumb tending to be slightly pulled downward rather than oscillating equally around a central plain. The right thumb may be used as a normal comparison point. Furthermore, the spiral has no clear tremor orientation axis (compared with that of Figure 2E, for example, which has a clear tremor orientation axis), a feature that aligns it more with dystonic tremor rather than ET.Download Supplementary Video 2 (19.5MB, mov) via http://dx.doi.org/10.1212/001100_Video_2
The proband's daughter (person 6), age 58 years, had noted the onset of arm tremor in her mid-40s and head tremor at age 58 years. She had not sought medical attention for her tremor. She did not consent to a videotaped examination, but her spirals revealed mild-to-moderate tremor on the right and moderate tremor on the left (Table 3, Figure 2G), consistent with a diagnosis of ET, although a subcategory (possible, probable, and definite) could not be assigned.
The proband's brother (person 3), age 66 years, first noted right arm tremor at age 60 years and had been diagnosed with PD and was taking pramipexole (Table 3). On examination, there was hypomimia with reduced eye blink rate, moderately to severely slowed rapid alternating arm movements, marked tremor at rest in the left arm while seated and while standing, a severe re-emergent tremor in the right arm, no or mild kinetic tremor on numerous tasks, no head tremor, and no voice tremor, consistent with the diagnosis of PD.
Family 3
The proband (person 1), age 76 years, was from a White family of Danish and German ancestry. Her deceased father was also reported to have had ET. Age of tremor onset was 20 years, and a neurologist had diagnosed ET. She had taken medication for ET in the past. On examination, there were mild postural tremor and mild-to-moderate kinetic tremor on a range of tasks (Table 3, Figure 2H) and the total tremor score was 16.5, consistent with mild-to-moderate ET. There were a head tremor and mild voice tremor. The diagnosis was probable ET. There were several features of PD as well: rest tremor in right arm while seated and while standing, which was considered marked given the amount of kinetic tremor; reduced arm swing bilaterally; and rapid alternating movements that were moderately bradykinetic in the left arm and leg. She was on no medications that could have resulted in these features. The final diagnosis was ET + PD.
The proband's daughter (person 3), age 47 years, had noted arm tremor since the age of 14 years but had not been diagnosed with ET or taken medication for it. On examination, there were mild postural tremor and kinetic tremor scores that ranged from none-to-severe (Table 3, Figure 2I). There was no voice or head tremor. The total tremor score = 18, consistent with mild ET, and the diagnosis was possible ET.
Family 4
The proband (person 1), age 52 years, was from a White family of Scandinavian ancestry. His deceased mother was also reported to have had ET. Age of tremor onset was 8 years, and a neurologist had diagnosed ET. He had taken medication for ET in the past. On examination, there was moderate postural tremor bilaterally, although the middle finger on the right was dystonic (flexing involuntarily). There was moderate-to-severe kinetic tremor during multiple tasks (Table 3, Figure 2J) and the total tremor score = 28.5, consistent with severe ET. There was no head or voice tremor. The diagnosis was definite ET with mild dystonia (Video 3).
Family 4, Person 1. ET is evident while drinking water from a cup with each hand and while using a spoon (severe kinetic tremor causes him to spill during each task). There is moderate kinetic tremor while drawing a spiral (Figure 2J). There was moderate postural tremor bilaterally, and the middle finger on the right was dystonic (flexing abnormally and involuntarily at the metacarpophalangeal joint). This dystonic posture was most evident when the arms were stretched outward but was also noted during the wing-beat position in the same finger on the right. The left hand and fingers may be used as a point of normal comparison.Download Supplementary Video 3 (16.6MB, mov) via http://dx.doi.org/10.1212/001100_Video_3
The proband's daughter (person 2), age 23 years, did not endorse tremor, had not been diagnosed with ET, and was taking no medications for ET. On examination, there were mild postural tremor and kinetic tremor ratings that were mild (rating = 1) to mild-to-moderate (rating = 1–1.5) in nearly all tasks (Table 3, Figure 2K). Total tremor score = 14.5. There was no head of voice tremor. Here, although tremor did not reach the threshold severity for possible ET, given her age and family history, it was considered abnormal and a diagnosis of ET was assigned.
The proband's brother (person 4) was 60 years old. He had noticed arm tremor at age 7 years but had not been diagnosed with ET or taken medication to treat tremor. On examination, there were mild postural tremor and kinetic tremor that ranged from mild-to-severe (Table 3, Figure 2L). The total tremor score = 17, consistent with mild ET. There was no voice or head tremor. The diagnosis was possible ET.
The proband's brother (person 3), enrolled at age 57 years, had first noted tremor at age 18 years and had been diagnosed with ET. He had not been diagnosed with PD. He had taken medication for tremor in the past. On examination, there were mild to mild-to-moderate postural tremor and kinetic tremor that ranged from mild-to-moderate to severe (Table 3, Figure 2M). There was no head or voice tremor. The total tremor score = 23.5, consistent with moderate ET. The diagnosis was probable ET. In addition, there were numerous signs of PD: mild hypomimia, decreased eye blink rate, rest tremor in right arm while walking, decreased arm swing, and loss of amplitude and decrement during finger taps on the right. The final diagnosis was ET + PD (Video 4).
Family 4, Person 3. ET is evident while pouring water between 2 cups (moderate kinetic tremor is present), while using a spoon on the right (moderate kinetic tremor is present), and during spiral drawing (Figure 2M). Several signs of parkinsonism are also evident—there is hypomimia and reduced eye blink frequency, loss of amplitude, and decrement during finger taps on the right, and while walking, there is bilaterally decreased arm swing with transient rest tremor on the right.Download Supplementary Video 4 (9.7MB, mov) via http://dx.doi.org/10.1212/001100_Video_4
Discussion
Ours is not the first study to report the presence of mixed motor disorder in ET families, although there are only a few other reports and none presented detailed tremor examinations with individual-level phenotypic data. A study published in 1997 reported 4 ET kindreds containing 55 persons with ET, numerous individuals with ET + dystonia or ET + PD, and several with ET + dystonia + PD.14 A study published in 2003 reported a family that included 13 members with ET included 3 with ET + PD and 1 with ET and focal hand dystonia.15 A study published in 2006 reported 4 ET kindreds containing 65 individuals diagnosed with ET.13 In that study, several women from Pedigree A had mild cervical dystonia, but their arm tremor was without any dystonic features, and they were classified as ET.13 In addition, there was 1 ET case in Pedigree B with a 30-year history of action tremor; 4 years before death, he also developed PD.13 As noted above, this small number of other reports either did not provide detailed and meticulous data on the phenotypic features of tremor in the ET cases (e.g., description of the physical examination limited to “definite ET”),13,15 restricted their detailed characterization of movement disorders only to the probrand,14 were limited to a single family,15 did not provide visual examples of handwriting samples or spirals,13-15 did not provide videotaped examination material,13-15 or did not include dystonia cases.13
ET is a very common neurologic disease and based on this, it is likely to cooccur within the same individual or within families with other movement disorders.2 This being said, there is abundant evidence in cross-sectional, longitudinal, and family studies of an association between ET and PD3-8 and data indicating that dystonia occurs in the setting of ET,9-11 and these suggest that the cooccurrence is not entirely due to chance. In addition, there is not a comparable literature linking ET to other highly prevalent movement disorders (e.g., tics, restless legs syndrome), arguing that the association is specific and thus less likely to be due to chance. A single gene or genes raising risk of ET could also affect risk of dystonia or PD (pleiotropy), or conversely, multiple disease-specific genes could cooccur within some families. On a pathophysiologic level, both ET and dystonia are linked to cerebellar dysfunction,29-32 and there are patients with ET with Lewy bodies,33 the hallmark feature of PD. Hence, commonalities exist in this regard.
One issue is that PD may in its earliest stages begin as an action tremor, raising the question as to whether the ET + PD cases we report had only PD. This is unlikely because they manifested typical bilateral kinetic predominant action tremor, a feature of ET, rather than the unilateral postural tremor that is typically seen in early cases of PD who present with action tremor.34 Second, among those with clear age of onset data, ET symptoms and diagnoses preceded PD by decades. Third, these cases were embedded in families with a clear stamp of ET.
As seen in Table 2, we note the presence of several movement disorders within a single ET family and within a single individual. In another report of ET kindreds, some family members had isolated dystonia and others had ET + PD + dystonia14 (Table 2, not seen in our 4 families). Hence, the motor phenomenology in these families can involve all combinations of these 3 disorders.
All tested individuals had a Mini-Mental State Examination score of 27 or higher and all of them had Lawton Instrumental Activities of Daily Living Scores of 7 or 8, indicating that these individuals likely did not have dementia or significant cognitive impairment.
We no longer include questions about response to ethanol in our epidemiologic or genetics studies of ET because this question has limited utility. First, many ET cases are elderly, and they do not use ethanol. Studies show that as few of 19%35 to 42.9%36 to 67%37 use ethanol. Second, response to ethanol is not specific to ET, being a feature of many tremor disorders (e.g., primary writing tremor, orthostatic tremor, isolated vocal tremor, and Parkinson disease) and a large number of other movement disorders (e.g., torticollis, spasmodic dysphonia, and generalized dystonia),38,39 both because of its calming effects and likely also because of direct effects. Therefore, whether response to ethanol differs in these families in comparison with families with pure ET could not be assessed, although it is not likely.
Only 8 of these 14 patients had ever used medication for their ET. During the course of the study, we added a question on pharmacologic response phenotype, and this was administered to 2 of the 8, one of whom reported no response to atenolol and the other who did not recall his response to medication. More complete data would have been of interest.
Our observations have several implications. First, they underscore challenges for studies that attempt to identify candidate genes for ET. Second, they point to what could be common genetic etiologies for these varied movement disorders. An example of this would be DYT24, because of mutations in the anoctamin 3 gene, resulting in a range of movement disorders, including tremor, dystonia, and myoclonus.40 Third, they suggest mechanistic or pathophysiologic commonalities among these disorders, whether it is mutual involvement of the cerebellum or deposition of Lewy bodies. For example, neuroanatomical studies in nonhuman primates demonstrate that cerebellar and basal ganglia outputs converge at the level of the cerebral motor cortex.31 Furthermore, cerebellar and basal ganglia circuits with the cerebral cortex seem to be interconnected at several subcortical levels as well.31 Such evidence is used to support the notion that the cerebellum, a region that plays a central role in the pathophysiology of ET,29 and plays a role in the pathophysiology of dystonia.31 Fourth, we believe the concept of the “mixed motor disorder” should on multiple levels enter into and inform the clinical dialogue and clinical decision making of providers. In assigning diagnoses, clinicians are influenced or swayed by family history information; in doing so, they should be prepared to accept the possibility of a significant mix of motor disorders within the same family rather than feeling constrained to assign a homogeneous diagnosis to every family member. This will serve to lessen the likelihood of diagnostic misclassification (i.e., assigning the incorrect diagnosis to a patient) and reduce the possibility of treating patients for the wrong condition. Furthermore, the discussion with families who seem to have mixed conditions will be informed by understanding that similar families with heterogeneous conditions have been observed and presented in the literature and that their family has a described and documented situation.
In summary, there are “mixes” of these 3 involuntary motor disorders: ET, dystonia, and PD, within individuals and across family members within a family. The recognition of this has implications in both the clinical and research realms and might serve to guide those who are studying disease etiology and pathogenesis.
Appendix. Authors
Study Funding
This work was supported by the NIH National Institute of Neurological Disorders and Stroke R01 NS073872.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534-541. [DOI] [PubMed] [Google Scholar]
- 2.Elble RJ, Dubinsky RM, Ala T. Alzheimer's disease and essential tremor finally meet. Mov Disord. 2007;22(11):1525-1527. [DOI] [PubMed] [Google Scholar]
- 3.LaRoia H, Louis ED. Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology. 2011;37(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan EK, Lee SS, Fook-Chong S, Lum SY. Evidence of increased odds of essential tremor in Parkinson's disease. Mov Disord. 2008;23(7):993-997. [DOI] [PubMed] [Google Scholar]
- 5.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population-based study. J Neurol Neurosurg Psychiatry. 2009;80(4):423-425. [DOI] [PubMed] [Google Scholar]
- 6.Rocca WA, Bower JH, Ahlskog JE, et al. Increased risk of essential tremor in first-degree relatives of patients with Parkinson's disease. Mov Disord. 2007;22(11):1607-1614. [DOI] [PubMed] [Google Scholar]
- 7.Spanaki C, Plaitakis A. Essential tremor in Parkinson's disease kindreds from a population of similar genetic background. Mov Disord. 2009;24(11):1662-1668. [DOI] [PubMed] [Google Scholar]
- 8.Costello S, Bordelon Y, Bronstein J, Ritz B. Familial associations of Alzheimer disease and essential tremor with Parkinson disease. Eur J Neurol. 2010;17(6):871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia KP, Bain P, Bajaj N, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33(1):75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey S, Bhattad S, Hallett M. The problem of questionable dystonia in the diagnosis of ‘essential tremor-plus.’ Tremor Other Hyperkinet Mov. 2020;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey S, Bhattad S. Questionable dystonia in essential tremor plus: a video-based assessment of 19 patients. Mov Disord Clin Pract. 2019;6(8):722-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis ED, Hernandez N, Alcalay RN, Tirri DJ, Ottman R, Clark LN. Prevalence and features of unreported dystonia in a family study of “pure” essential tremor. Parkinsonism Relat Disord. 2013;19(3):359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma S, Davis TL, Blair MA, et al. Familial essential tremor with apparent autosomal dominant inheritance: should we also consider other inheritance modes? Mov Disord. 2006;21(9):1368-1374. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic J, Beach J, Pandolfo M, Patel PI. Familial essential tremor in 4 kindreds. Prospects for genetic mapping. Arch Neurol. 1997;54(3):289-294. [DOI] [PubMed] [Google Scholar]
- 15.Yahr MD, Orosz D, Purohit DP. Co-occurrence of essential tremor and Parkinson's disease: clinical study of a large kindred with autopsy findings. Parkinsonism Relat Disord. 2003;9(4):225-231. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Hernandez N, Sebastian AA, Clark LN, Ottman R. Validity of probands' reports and self-reports of essential tremor: data from a large family study in North America. J Neurol Sci. 2018;393:45-50. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Hernandez N, Ionita-Laza I, Ottman R, Clark LN. Does rate of progression run in essential tremor families? Slower vs. faster progressors. Parkinsonism Relat Disord. 2013;19(3):363-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. [PubMed] [Google Scholar]
- 20.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(15):41-47. [DOI] [PubMed] [Google Scholar]
- 21.Louis ED, Eliasen EH, Kim CY, Ferrer M, Gaini S, Petersen MS. High prevalence of dystonia in the Faroe Islands: a population-based study. Neuroepidemiology. 2019;53(3-4):220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis ED, Ford B, Bismuth B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord. 1998;13(2):287-293. [DOI] [PubMed] [Google Scholar]
- 23.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124-133. [DOI] [PubMed] [Google Scholar]
- 24.Louis ED, Pullman SL. Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Mov Disord. 2001;16(4):668-673. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42(6):1142-1146. [DOI] [PubMed] [Google Scholar]
- 26.Louis ED, Wise A, Alcalay RN, Rao AK, Factor-Litvak P. Essential tremor-Parkinson's disease: a double whammy. J Neurol Sci. 2016;366:47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnew A, Frucht SJ, Louis ED. Supine head tremor: a clinical comparison of essential tremor and spasmodic torticollis patients. J Neurol Neurosurg Psychiatry. 2012;83(2):179-181. [DOI] [PubMed] [Google Scholar]
- 28.Michalec M, Hernandez N, Clark LN, Louis ED. The spiral axis as a clinical tool to distinguish essential tremor from dystonia cases. Parkinsonism Relat Disord. 2014;20(5):541-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis ED, Kerridge CA, Chatterjee D, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol. 2019;138(5):859-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bares M, Filip P. Cerebellum and dystonia: the story continues. Will the patients benefit from new discoveries? Clin Neurophysiol. 2018;129(1):282-283. [DOI] [PubMed] [Google Scholar]
- 31.Shakkottai VG, Batla A, Bhatia K, et al. Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum. 2017;16(2):577-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filip P, Gallea C, Lehericy S, et al. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord. 2017;32(5):757-768. [DOI] [PubMed] [Google Scholar]
- 33.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt12):3297-3307. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long duration asymmetrical postural tremor is likely to predict development of Parkinson's disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005;76(1):115-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis ED, Ford B, Wendt KJ, Cameron G. Clinical characteristics of essential tremor: data from a community-based study. Mov Disord. 1998;13(5):803-808. [DOI] [PubMed] [Google Scholar]
- 36.Dowd H, Zdrodowska MA, Radler KH, et al. Prospective longitudinal study of gait and balance in a cohort of elderly essential tremor patients. Front Neurol. 2020;11:581703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louis ED, Michalec M. Semi-quantitative data on ethanol consumption in 354 ET cases and 370 controls. J Neurol Sci. 2014;347:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajput AH, Jamieson H, Hirsh S, Quraishi A. Relative efficacy of alcohol and propranolol in action tremor. Can J Neurol Sci. 1975;2(1):31-35. [DOI] [PubMed] [Google Scholar]
- 39.Frucht SJ, Riboldi GM. Alcohol-responsive hyperkinetic movement disorders-a mechanistic hypothesis. Tremor Other Hyperkinet Mov. 2020;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamelou M, Charlesworth G, Cordivari C, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord. 2014;29(7):928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Family 1, Person 3. ET is evident when she pours water between cups (moderate kinetic tremor is present bilaterally) and in her spiral drawing (moderate kinetic tremor is seen in Figure 2D). Signs of parkinsonism include a mild flexed posturing of her right hand during arm extension in the wing-beat position, rest tremor in the right hand while standing, hypomimia and reduced eye blink frequency, and pauses and decrement during finger taps.Download Supplementary Video 1 (10.3MB, mov) via http://dx.doi.org/10.1212/001100_Video_1
Family 2, Person 2. ET is evident when she pours water between cups (moderate kinetic tremor is present bilaterally), while using a spoon (severe kinetic tremor is present bilaterally, causing her to spill), in her spiral drawing (moderate kinetic tremor is seen in Figure 2F), during arm extension (postural tremor ratings of 1–1.5), and jaw tremor while using a spoon. The dystonia is subtle—when initially raising her left hand so that her arm is outstretched, the left thumb is abducted (see right thumb for comparison) and during sustained posture holding, the right thumb remains abducted downward and there is a directionality to the tremor, with the thumb tending to be slightly pulled downward rather than oscillating equally around a central plain. The right thumb may be used as a normal comparison point. Furthermore, the spiral has no clear tremor orientation axis (compared with that of Figure 2E, for example, which has a clear tremor orientation axis), a feature that aligns it more with dystonic tremor rather than ET.Download Supplementary Video 2 (19.5MB, mov) via http://dx.doi.org/10.1212/001100_Video_2
Family 4, Person 1. ET is evident while drinking water from a cup with each hand and while using a spoon (severe kinetic tremor causes him to spill during each task). There is moderate kinetic tremor while drawing a spiral (Figure 2J). There was moderate postural tremor bilaterally, and the middle finger on the right was dystonic (flexing abnormally and involuntarily at the metacarpophalangeal joint). This dystonic posture was most evident when the arms were stretched outward but was also noted during the wing-beat position in the same finger on the right. The left hand and fingers may be used as a point of normal comparison.Download Supplementary Video 3 (16.6MB, mov) via http://dx.doi.org/10.1212/001100_Video_3
Family 4, Person 3. ET is evident while pouring water between 2 cups (moderate kinetic tremor is present), while using a spoon on the right (moderate kinetic tremor is present), and during spiral drawing (Figure 2M). Several signs of parkinsonism are also evident—there is hypomimia and reduced eye blink frequency, loss of amplitude, and decrement during finger taps on the right, and while walking, there is bilaterally decreased arm swing with transient rest tremor on the right.Download Supplementary Video 4 (9.7MB, mov) via http://dx.doi.org/10.1212/001100_Video_4
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.