Abstract
Objective
To determine whether varenicline is effective for the balance in Parkinson disease (PD).
Methods
This was an investigator-initiated, double-blind, placebo-controlled study. Participants with a clinical diagnosis of PD were randomized to receive varenicline or placebo for 8 weeks. After dose escalation, participants took 1 mg of drug twice daily until the end of the study. Patients with severe tremor were excluded. Primary outcome was a change on the Berg Balance Scale (BBS) from baseline to 8 weeks. The BBS is a 14-item measure consisting of basic balance tasks. The study had a secondary, exploratory outcome of a change in cognition, measured with the Frontal Assessment Battery (FAB) and the Mini-Mental State Exam (MMSE) from baseline to 8 weeks. The FAB is a 6-item measure of executive functioning.
Results
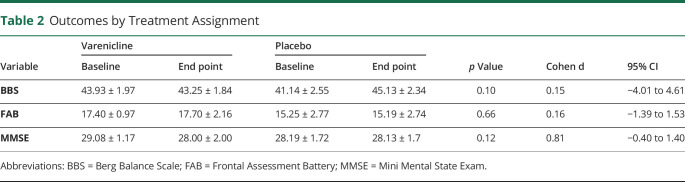
Thirty-six participants were randomized (82% men, 100% White). Average age was 71.0 years (± 8.1). Average baseline motor Movement Disorder Society Unified Parkinson's Disease Rating Scale was 34.7 (± 11.6). There were no differences between treatment groups on the BBS (F[1,28] = 2.85, p = 0.10) or FAB (d = 0.16, 95% confidence interval [CI] = [−1.39 to 1.53]) or MMSE (d = 0.81, 95% CI = [−0.40 to 1.40]).
Conclusion
The results did not suggest that varenicline had an effect on balance in patients with PD. Furthermore, varenicline did not seem to affect cognition. Perhaps, if an objective measure of balance had been used in place of the BBS, the analysis would show a difference between the groups. However, the authors do not recommend further study.
Classification of Evidence
This study provides Class III evidence that in patients with PD with Hoehn and Yahr stages 2, 3, or 4, varenicline does not improve balance as assessed by the BBS.
Roughly 1 million Americans suffer from Parkinson disease (PD),1 with an incidence of falls estimated at 68% for patients who subsequently are at increased risk of injury.2–6 Falling remains an unmet need in PD maintenance. Treatments focus on dopamine replacement that effectively treats some PD motor symptoms but has little effect on instability leading to falls.1,7
Increasingly, disruption of the cholinergic system is implicated in gait dysfunction in PD.8–11 Major centers of cholinergic projections include the basal forebrain complex, pedunculopontine nucleus, and cholinergic interneurons that dominate neurotransmission in the striatum.8,12 These centers and their cholinergic projections play key roles in motor function, and research associates their degeneration with imbalance and falling in PD.10 The relationship between cholinergic degeneration and PD motor symptoms presents an opportunity for alternative therapies targeting these neural pathways. Nicotinic cholinergic receptor (nAChR) agonists are one candidate, with previous studies and preclinical data highlighting a role of the nicotinic cholinergic system in the regulation of movement.13,14
Varenicline is a partial α4β2 nAChR agonist and full α7 nAChR agonist developed as an aid in smoking cessation.15,16 In previous studies, it also improved imbalance in spinocerebellar ataxia (SCA) types 3 and 14,17,18 instability in fragile X-associated-tremor-ataxia-syndrome,19 and Friedriech ataxia.20 Of note, α4β2 is the highest expressed nAChR in the brain. These findings raise the possibility that varenicline may reduce falling by compensating for cholinergic degeneration.9,13,14 The present study sought to determine the efficacy of varenicline for imbalance in PD, using a similar dosing schedule to previous SCA studies.
Methods
Study Design and Participants
This was an investigator-initiated, double-blind, placebo-controlled, parallel-designed study testing varenicline for postural stability in PD. Secondary outcomes were change in cognitive functioning and responsiveness to treatment by PD subtype.
Participants were recruited from the Section of Movement Disorders at the Rush University Medical Center (RUMC) between December 28, 2010, and November 2, 2018. Participants were enrolled if they had a diagnosis of PD by the UK Brain Bank criteria,21 a minimum Hoehn and Yahr score of 2, one or more falls or near falls in the 6 months before screening, a stable medication regimen, and safety laboratory test results within normal limits (including serum creatinine kinase, complete metabolic panel, complete blood count, liver function tests, renal function tests, platelets, and an electrocardiogram). All patients had to be 40 or older and were excluded if they had a Hoehn and Yahr score of 5; history of a major psychiatric disorder, cerebral trauma, cardiac arrhythmia, renal insufficiency, or severe renal disease (Blood Urea Nitrogen 50% greater than normal or creatinine clearance <60 mL/min); a cardiovascular procedure within 5 years of baseline or cardiovascular instability; dementia, or other psychiatric illness that prevented the informed consent process; concurrent treatment with trihexyphenidyl, benztropine mesylate, or AChase inhibitors; severe dysphagia; abnormal creatinine kinase or platelet count within 6 months of baseline; used varenicline within 30 days of baseline; allergy to varenicline; were participating in another clinical trial; active substance or tobacco use; moderate-to-severe chronic obstructive pulmonary disease; and, if a woman of childbearing potential, pregnant at the time of screening or were unwilling to take contraception during study participation.
Intervention
The study drug and placebo were manufactured by Pfizer and shipped to the RUMC where they were dispensed to participants with dosing instructions. Participants were randomized to receive 1 mg twice daily of oral varenicline or matched placebo. Randomization was performed using a semirandom algorithm on Statistical Analysis Software (SAS). The participants, investigators, and study staff were masked to the treatment arm of each subject. The therapeutic dose was escalated over the first week of the study, starting at 0.5 mg daily for 3 days and then to 0.5 mg twice daily for 4 days. Thereafter, participants took 1 mg twice daily for 8 weeks until the end of the study. Participants were asked to keep all other medications stable during this period. Outcome measures were performed in person at baseline and the end of the study. Adverse events (AEs) were monitored throughout the study, with a designated phone call at week 5 to assess AEs and drug compliance.
Evaluations
Demographics, including age, sex, and race, were collected. The Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Score was also collected. Efficacy was measured as a change on the Berg Balance Scale (BBS) from baseline to the end of the study after 8 weeks on drug. The BBS is a 14-item measure consisting of basic balance tasks, with a final score indicative of overall balance ability.22,23
The change in cognitive functioning was measured with the Frontal Assessment Battery (FAB) and the Mini-Mental State Exam (MMSE) from baseline to 8 weeks on drug. The FAB measures executive functioning and consists of the following 6 sections: conceptualization, mental flexibility, motor programming, sensitivity to interference, inhibitory control, and environmental autonomy.24,25 Tremor-dominant (TD) and postural-instability-and-gait-disturbance (PIGD) subtype scores were calculated using the MDS-UPDRS classification method.26 The ratio of the mean MDS-UPDRS tremor scores (11 items) and the mean PIGD scores (5 items) defined TD patients (≥1.15), PIGD patients (≤0.90), and indeterminate patients (<1.15 and >0.90).
Statistics
The sample size was estimated using published means and SDs of the BBS in patients with PD.23 The mean of the rating scale is 40.22 with a SD of 8.48. Based on a published effect size of a change of 8 points and using a power of 0.80 and a significance of 0.05 with a 2-sided test, a sample size of 19 participants for each group was estimated, with a target enrollment of 40 to allow for attrition.
Descriptive statistics were used for demographic data. To evaluate efficacy and change in cognition, repeated measures analysis of variance was run on BBS, FAB, and MMSE scores using SAS. The scale score was the dependent measure, time point (baseline or end of study) was the repeated measure, and treatment group (varenicline or placebo) was the independent measure for all analyses; significance was defined as alpha <0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the institutional review board at the RUMC. Each participant signed an Institutional Review Board-approved informed consent form before study participation. The study was registered at ClinicalTrials.gov, identifier: NCT01341080.
Data Availability
All data and statistical analyses are available from the authors on request.
Results
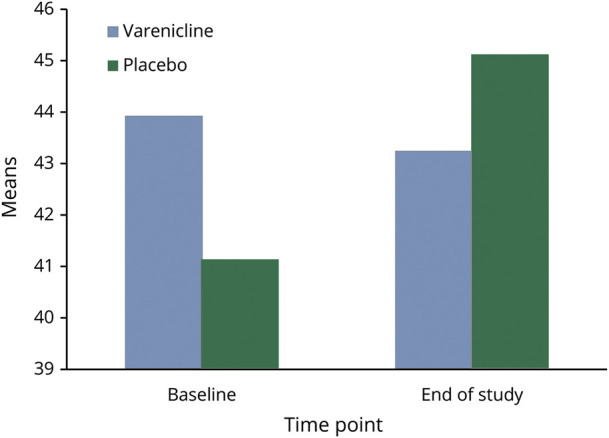
Participants were recruited from the Section of Movement Disorders at the RUMC. Forty participants signed an informed consent form, and 36 were randomized. Six participants terminated participation early, with 2 having sufficient data for last observations carried forward intention-to-treat analyses (Figure 1). The remaining sample of 32 used in the analysis were 82% men and 100% White, with a mean age of 71.0 years (± 8.1), a mean MDS-UPDRS Part III score of 34.7 (± 11.6), and a mean MMSE score of 28.3 (± 1.7) (Table 1). Fifteen participants were randomized to receive varenicline and 17 to placebo. There were no differences between the groups for age, sex, race, motor severity, or cognitive function (Figure 2).
Figure 1. Consort Diagram.
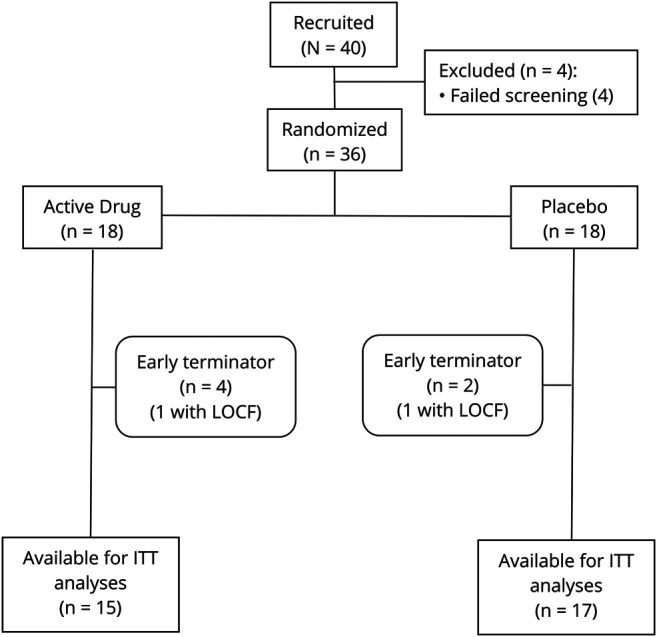
ITT = Intent-to-treat analysis; LOCF = last observations carried forward.
Table 1.
Demographic and Disease Characteristics by Treatment Assignment
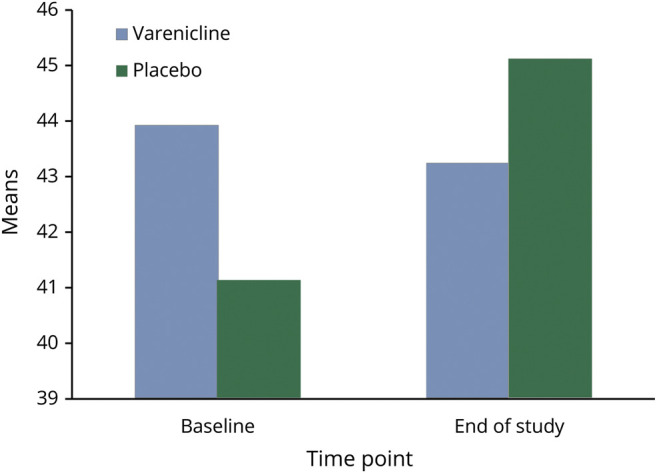
Figure 2. Berg Balance Scale.
Outcomes
There was no need to covary for age, sex, or race on the outcomes because there were no differences in the distributions between treatment groups. On the BBS, there was no effect for time (F[1,28] = 0.11, p = 0.74) or time by treatment interaction (F[1,28] = 2.85, p = 0.10, number needed to treat = 1398, absolute risk reduction = 0.07%), indicating no difference between the groups. Similarly, there was no effect for time (F[1,24] = 0.08, p = 0.77) or time by treatment interaction (F[1,24] = 0.20, p = 0.66) for the FAB (d = 0.16, 95% confidence interval [CI] = [−1.39 to 1.53]) and no effect for time (F[1,26] = 3.35, p = 0.08) or time by treatment interaction (F[1,26] = 2.66, p = 0.12) for the MMSE (d = 0.81, 95% CI = [−0.40 to 1.40]) (Table 2). For the PD subtype (n = 32), 25 participants met the criteria for the PIGD subtype, 5 were tremor dominant, 2 were indeterminate, and 2 had missing values. These groups were not adequately powered to determine any differences between the subtypes.
Table 2.
Outcomes by Treatment Assignment
Discussion
This was one of few clinical trials to test the efficacy of varenicline for imbalance. However, unlike previous studies in ataxia, the results did not suggest a therapeutic effect of varenicline for PD on either the primary or secondary outcomes.
Several limitations of this research may have contributed to its lack of significant findings. However, despite these limitations, the high number needed to treat (N = 1398) and low absolute risk reduction (0.07%) suggest that an unrealistic sample size would be required to detect any differences, if they exist. Regarding the methodology, the primary outcome of efficacy was measured by the BBS. However, the sensitivity of the BBS is poor to moderate, with frequent uncertainty in its scoring.27–29 Note that previous studies in ataxia that showed a beneficial effect of varenicline for ataxia had used the Scale for Assessment and Rating of Ataxia, an 8-item measure of cerebellar ataxia via motor signs that is more sensitive than the BBS.7,18 Future research in imbalance may benefit from replacing qualitative scales such as the BBS with objective posturography tools that directly quantify postural control and are increasingly available.30
The study also faced challenges in recruitment, which was slow and spanned 8 years. This unintentionally large window of enrollment potentially biased the sample and high attrition resulted in a smaller sample size than estimated by power analysis. Although the authors intended to recruit tremor predominant and PIGD patients in equal numbers, the PIGD subtype was overrepresented, prohibiting analysis of efficacy by the PD subtype. This was, in part, the fault of the inclusion criteria. Participants were enrolled if they had a fall history within 6 months of screening and excluded if they had moderate-to-severe tremor because of the reports of varenicline worsening tremor. Thus, the PIGD subtype was unintentionally favored.
Finally, the study was challenged by the general heterogeneity of cholinergic denervation in PD. The rationale for varenicline as an intervention for imbalance in PD was based on the idea that nicotinic agonists compensate for degeneration of cholinergic projections leading to falls. However, the literature shows that this degeneration is neither linear nor uniform across patients.8,31 In a small clinical trial without brain imaging, researchers cannot know whether cholinergic deficits that would be improved on by varenicline were present in participants. Alternatively, if the deficits were present, the findings may suggest that a partial α4β2 nAChR agonist is insufficient to address this degeneration. Full α4β2 agonists should be researched, along with therapies targeting the muscarinic acetylcholine receptors that are also abundant in the brain.
Although limited, the findings suggest that varenicline does not have an effect on balance in PD, which is of note considering previous clinical trials finding the opposite effect in ataxia. This is important because the literature increasingly shows a relationship between the cholinergic system and imbalance in PD and calls for therapies to compensate for its deficit grow. Although the authors do not recommend further study based on their analyses, imbalance and fall prevention remain unmet needs in the management of PD. Given that the mainstay therapies of dopamine replacement do not address this, it is imperative to continue seeking alternative strategies that target other neurotransmitter pathways.
Classification of Evidence
This therapeutic trial provides Class III evidence that 1 mg of varenicline taken twice daily has no effect on the balance in patients with PD (Hoehn and Yahr stages 2, 3, or 4) as assessed by the Berg Balance Scale over 9 months.
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study Funding
Study Funding by Pfizer and registered at ClinicalTrials.gov. Identifier: NCT01341080.
Disclosure
D. Hall received research support from the NIH, Parkinson's Foundation, AbbVie, Biogen, Neurocrine, Biohaven, CHDI, Huntington's Disease Society of America, and Pfizer. S. Kapur received compensation from Teva, Acorda, Acadia, and US World Meds and is a consultant on the science advisory board for Amneal. C. Vaughan, J. Hawkins, and G. Stebbins report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Instability and falling are unmet needs in Parkinson's disease treatment because current therapies focus on dopamine replacement that does not improve balance.
→ Varenicline is a partial α4β2 agonist and full α7 that improved imbalance in previous ataxia studies.
→ Although varenicline did not seem to improve imbalance in PD in the present trial, future research should continue to explore nicotinic agonists that compensate for cholinergic degeneration.
References
- 1.Beitz JM. Parkinson's disease: a review. Front Biosci (Schol Ed) 2014;6:65–74. [DOI] [PubMed] [Google Scholar]
- 2.Sherer TB, Chowdhury S, Peabody K, Brooks DW. Overcoming obstacles in Parkinson's disease. Mov Disord 2012;27:1606–1611. [DOI] [PubMed] [Google Scholar]
- 3.Bloem BR, Van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson's disease. Adv Neurol 2001;87:209–223. [PubMed] [Google Scholar]
- 4.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord 2011;26:1605–1615. [DOI] [PubMed] [Google Scholar]
- 5.Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis 2013;2013:906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson's disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag 2014; 4: 203–221. [DOI] [PubMed] [Google Scholar]
- 7.Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG, Horak FB. Pharmacological treatment in Parkinson's disease: effects on gait. Parkinsonism Relat Disord 2016;31:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res 2011;221:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnen NI, Müller LTM, Dauer WT, Roger LA. Parkinson's disease: what role do pedunculopontine nucleus cholinergic neurons play? Future Neurol 2014;9:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnen NI, Kanel P, Zhou Z, et al. Cholinergic system changes of falls and freezing of gait in Parkinson's disease. Ann Neurol 2019;85:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarter M, Albin RL, Kucinski A, Lusting C. Where attention falls: increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exper Neurol 2014;257:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dautan D, Huerta-Ocampo I, Witten IB, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurol 2014;34:4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quik M, Zhang D, Perez X, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther 2014;144:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quik M, Boyd JT, Bordia T, Perez X. Potential therapeutic application for nicotinic receptor drugs in movement disorders. Nicotine Tob Res 2019;21:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 2007;52:985–994. [DOI] [PubMed] [Google Scholar]
- 16.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci 2007;28:316–325. [DOI] [PubMed] [Google Scholar]
- 17.Zesiewicz TA, Sullivan KL. Treatment of ataxia and imbalance with varenicline (chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14). Clin Neuropharmacol 2008;31:363–365. [DOI] [PubMed] [Google Scholar]
- 18.Zesiewicz TA, Greenstein PE, Sullivan KL, et al. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology 2012;78:545–550. [DOI] [PubMed] [Google Scholar]
- 19.Zesiewicz TA, Sullivan KL, Gooch CL, Lynch DR. Subjective improvement in proprioception in 2 patients with atypical Friedreich ataxia treated with varenicline (Chantix). J Clin Neuromuscul Dis 2009;10:191–193. [DOI] [PubMed] [Google Scholar]
- 20.Zesiewicz TA, Sullivan KL, Freeman A, Juncos JL. Treatment of imbalance with varenicline Chantix(R): report of a patient with fragile X tremor/ataxia syndrome. Acta Neurol Scand 2009;119:135–138. [DOI] [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1993;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992;83:7–11. [PubMed] [Google Scholar]
- 23.Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil 2005;86:789–792. [DOI] [PubMed] [Google Scholar]
- 24.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology 2000;55:1621–1626. [DOI] [PubMed] [Google Scholar]
- 25.Lima CF, Meireles LP, Fonseca R, Castro SL, Garrett C. The frontal assessment battery (FAB) in Parkinson's disease and correlations with formal measures of executive functioning. J Neurol 2008;255:1756–1761. [DOI] [PubMed] [Google Scholar]
- 26.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord 2013;28:668–670. [DOI] [PubMed] [Google Scholar]
- 27.Crouse JJ, Phillips JR, Jahanshahi M, Moustafa AA. Postural instability and falls in Parkinson's disease. Rev Neurosci 2016;27:549–555. [DOI] [PubMed] [Google Scholar]
- 28.McVey MA, Stylianou AP, Luchies CW, et al. Early biomechanical markers of postural instability in Parkinson's disease. Gait Posture 2009;30:538–542. [DOI] [PubMed] [Google Scholar]
- 29.Lima CA, Ricci NA, Nogueira EC, Perracini MR. The berg balance scale as a clinical screening tool to predict fall risk in older adults: a systematic review. Physiotherapy 2018;104:383–394. [DOI] [PubMed] [Google Scholar]
- 30.Chastan N, Debono B, Maltête D, Weber J. Discordance between measured postural instability and absence of clinical symptoms in Parkinson's disease patients in the early stages of the disease. Mov Disord 2008;23:366–372. [DOI] [PubMed] [Google Scholar]
- 31.Bohnen NI, Müller ML, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. J Cereb Blood Flow Metab 2012;32:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and statistical analyses are available from the authors on request.