Abstract
The development of selective targeting of drug molecules towards the mitochondria is an important issue related to therapy efficacy. In this study, we report that gallic acid (GA)-mitochondria targeting sequence (MTS)-H3R9 exhibits a dual role as a mitochondria-targeting vehicle with antioxidant activity for disease therapy. In viability assays, GA-MTS-H3R9 showed a better rescue action compared to that of MTS-H3R9. GA-MTS-H3R9 dramatically exhibited cell penetration and intercellular uptake compared to MTS and fit escape from lysosome release to the cytosol. We demonstrated the useful targeting of GA-MTS-H3R9 towards mitochondria in AC16 cells. Also, we observed that the antioxidant properties of mitochondrial-accrued GA-MTS-H3R9 alleviated cell damage by reactive oxygen species production and disrupted mitochondrial membrane potential. GA-MTS-H3R9 showed a very increased cytoprotective effect against anticancer activity compared to that of MTS-H3R9. We showed that GA-MTS-H3R9 can act as a vehicle for mitochondria-targeting and as a reagent for therapeutic applications intended for cardiovascular disease treatment.
Keywords: Antioxidant activity, Gallic acid, Mitochondria
INTRODUCTION
Cardiovascular disease, including myocardial infarction, myocardial ischemia, and heart failure are a major cause of morbidity and mortality worldwide [1]. These diseases include risk factors such as ageing, hypertension, and obesity, which lead to heart failure presenting as a terminal clinical signal by left ventricular dysfunction due to progressive pathological remodeling characterized by endothelial dysfunction, cardiac hypertrophy, and interstitial fibrosis [2]. However, current treatments such as pharmacologic agents and invasive surgical therapy only delay the progression towards heart failure and the only cure for these diseases is cardiac transplantation [3,4]. Consequently, strategies with better outcomes are needed for these diseases. Nanotechnology has provided important information for therapeutics in various fields including medicine and biology. Nanomaterials are typically in the range of 1–100 nm to specifically target delivery of peptides, protein, drug, and genes to desired therapy sites [5,6]. The commonly used nanomaterials are liposomes, nanoshells, polymeric micelles, dendrimers, graphene, and bacterial nanoparticles [7].
Efficient nanomaterials as drug carriers are stable, biodegradable has easy surface modification and can be used in organelle-specific delivery such as towards the mitochondria and nucleic acid for various diseases [8]. Mitochondria are very complex organelles, which function as powerhouses for cellular energy and are related to energy metabolism, redox regulation, apoptosis, and reactive oxygen species (ROS). Dysfunctional mitochondria caused by mitochondrial oxidative damage due to impaired mitochondrial ATP synthesis are related to many human disorders including neurodegenerative disease, cancers, diabetes, and ischemia-reperfusion injury [9,10]. Until now, a few mitochondrial delivery vehicles have been used for high selective delivery into mitochondria such as triphenylphosphonium, dequalinium, protein transduction domain peptides, and mitochondria targeting sequence (MTS) peptides. However, an important issue regarding safe and efficient functionalization of vehicles to organelle-specific targeting properties remains [11,12]. Among the various mitochondrial targeting vehicles, the MTS which is composed of 15–50 amino acids with amphipathic helical structural feature that can deliver drugs into mitochondria by mitochondrial transport machinery has been studied. It has very low cellular uptake despite having benefits such as simple production and tunable modification for delivery of bioactive cargos to mitochondria [13,14]. According to a report, MTS-H3R9 could be a potential mitochondrial delivery vehicle with improved mitochondria selective targeting efficiency as well as a therapeutic agent, which assists cellular penetrating moieties across the plasma membrane by positively charged conjugation of histidine and arginine resides [15-17].
We characterized an MTS-based vehicle system, GA-MTS-H3R9 to demonstrate that such organ specific delivery vehicles can act as an effective drug. We used MTS-H3R9 with gallic acid (GA, 3,4,5-trihydroxy benzoic acid) as a model drug (GA-MTS-H3R9). GA, a polyphenyl natural product, is found in grapes, black teas, berries, and olive oil. It was known to have anticancer, anti-inflammatory, antioxidant, antibacterial activities and therefore has attracted attention in drug, food, cosmetic, and biomedical fields [18,19]. We aimed to develop a valid mitochondrial carrier and establish the mechanism of cellular function by conjugating GA with MTS peptide-H3R9, denoted as GA-MTS-H3R9 in AC16 cells, derived from human ventricular heart tissues. We demonstrated that MTS-H3R9 showed enhanced mitochondria-targeting efficiency and cellular uptake with or without GA compared to that of unmodified MTS. Here, we showed that GA-MTS-H3R9 exhibits low cytotoxicity, rescued ROS generation, and decreased apoptotic activity via mitochondrial related pathway compared with that of MTS-H3R9 in AC16 human cardiomyocyte cell line. We suggest that the dual effects of GA-MTS-H3R9 as a drug delivery carrier and a therapeutic agent warrant further studies eventually aiming at developing drugs for mitochondria-functional regulation to be used against cardiovascular disease.
METHODS
Synthesis of peptides
All peptides (K [gallic acid], MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, GA-MTS-H3R9, FITC-K [gallic acid], FITC-MTS, FITC-MTS-H3R6, FITC-MTS-H3R9, FITC-GA-MTS-H3R6, and FITC-GA-MTS-H3R9) were provided by Anygen (Gwangju, Korea). The characterization of all the synthesized peptides is shown in Supplementary Table 1.
Cell toxicity assay
AC16 human cardiomyocyte (1.2 × 104) cell line was seeded onto 96-well plates and incubated in Dulbecco’s Modified Eagle’s medium medium containing 10% fetal bovine serum for 16 h at 37°C. The cells were treated with different concentrations of GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 and further cultured for 24 h. The cell viability using all drugs was conducted using the EZ-Cytox assay according to a protocol described previously [20].
Intracellular localization
To examine the subcellular localization of each peptide, AC16 (5 × 103) cells were seeded onto an 8-well chamber slide (Ibidi, Munich, Germany). Cells were then incubated for 24 h with 1 µM of FITC-labeled GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9. The cells were treated with 50 nM MitoTracker Red for mitochondria or 100 nM LysoTracker for lysosomes for at least 15 min at 37°C. Cell nuclei were stained with Hoechest dye for 10 min and each sample was analyzed using a confocal microscope (Carl Zeiss, Jena, Germany).
Intracellular uptake and internalization
AC16 (1.5 × 105) cells were seeded onto six well plates. The cells were incubated for 16 h with 3.125 and 6.25 µM of each peptide (with or without FITC-label). To quantitatively analyze the fluorescence intensity, intracellular uptake and internalization of the samples were performed by flow cytometry (BD Bioscience, San Jose, CA, USA).
Analysis of mitochondrial membrane potential
To determine the mitochondrial membrane potential (MMP) of peptides, JC-1 kit (Thermo Fisher Scientific, Grand Island, NY, USA) was used. AC16 (1.5 × 105) cells were seeded onto six well plates. The cells were treated with 6.25 µM of each peptide for 16 h and then incubated with medium containing 10 µg/ml JC-1 solution for 10 min at 37°C. The cells were then analyzed by flow cytometry.
Analysis of intracellular glutathione
Glutathione (GSH) was estimated using a Glutathione Colorimetric Assay Kit (BioVision, Milpitas, CA, USA). AC16 (1 × 106) cells were incubated for 16 h with 6.25 µM of each peptide. GSH levels were measured by following a detailed protocol described previously [20].
Annexin V staining
Apoptosis was evaluated using a FITC Annexin V Apoptosis Detection Kit (BD Bioscience). AC16 (1 × 106) cells were incubated for 24 h with 6.25 µM of each peptide and the assay was performed according to the method previously described by the authors [21].
Cell viability assay
Cellular viability using the peptides was measured using a live/dead assay Kit (Invitrogen). Live cells were stained with green fluorescence by intracellular esterase enzyme activity, while dead cells were stained with red fluorescence in which ethidium homodimer-1 penetrates the plasma membrane thus damaging the cell integrity. AC16 (5 × 103) cells were incubated with 6.25 µM of each peptide for 24 h. The samples were then incubated with 1 µM of calcein acetoxymethyl ester and 4 µM of EthD-1 dissolved in PBS at 37°C for 30 min. The cells were imaged using a confocal microscope (Carl Zeiss).
Mitochondrial superoxide generation
Mitochondria ROS generation in the presence of the peptides were determined using MitoSox Red (Invitrogen). AC16 (5 × 103) cells were seeded onto an 8-well chamber slide (Ibidi). Cells were then incubated with 6.25 µM of each peptide for 16 h. The cells were treated with 5 mM MitoSox Red (Invitrogen) at 37°C for 30 min. The samples were imaged using a confocal laser microscope (Carl Zeiss).
Caspase-3 activity
Caspase-3 activity (BioVision) was determined following the manufacturer’s instructions. AC16 (1 × 106) cells were incubated with 6.25 µM of each peptide for 24 h. Caspase-3 activity was determined as previously described by the authors [21].
Statistical analysis
Data were analyzed using GraphPad Prism software. Statistically significant differences were performed using the unpaired Student’s t-test method.
RESULTS
Characteristics of GA-conjugated MTS-H3R9
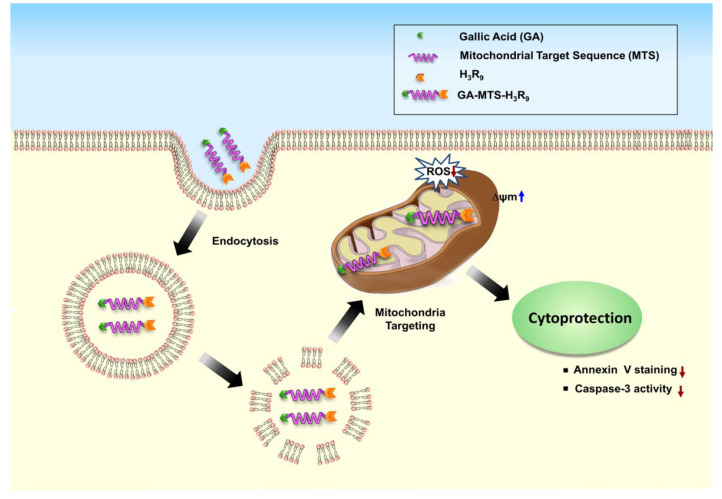
The schematic depiction of the targeting activity of GA-MTS-H3R9 to mitochondria via endo-lysosomal pathway and its continuing activity as a cytoprotective agent is shown in Fig. 1.
Fig. 1. Schematic description for the localization of the GA-MTS-H3R9 inside cells and its cytoprotective effect.
Cytotoxicity
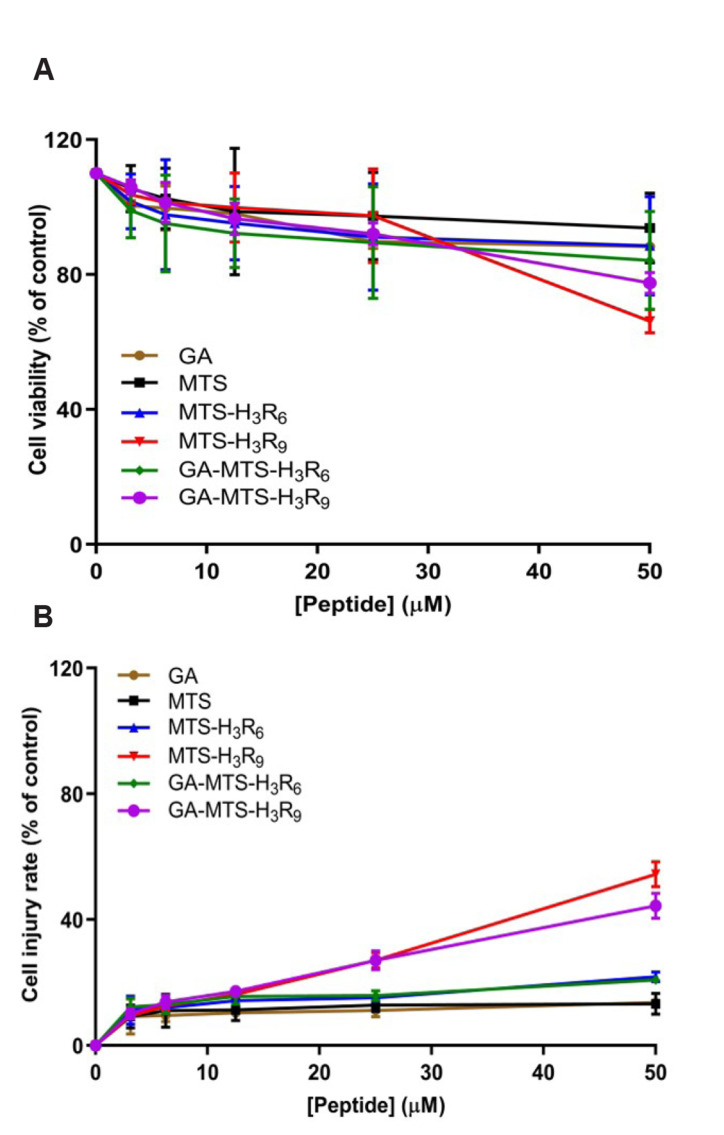
Cytotoxicity can be produced by environment conditions that induces cellular stress by means of intrinsic or extrinsic stimuli [22]. The effect of the MTS peptides on the viability was assessed using WST-1 and lactate dehydrogenase (LDH) cytotoxicity assay [23]. AC16 cells were exposed to GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 at varying doses and exposure time. GA and MTS resulted in higher cell viability even when the peptide concentrations were increased (Fig. 2A). In contrast, MTS-H3R9-exposed cells showed more cytotoxicity than MTS-H3R6-exposed cells. Interestingly, MTS-H3R6 and MTS-H3R9 showed more cytotoxicity than GA-MTS-H3R6 and GA-MTS-H3R9. Also, we performed a LDH release assay. AC16 cells were treated similarly as in the EZ-Cytox cell viability assay. LDH release was higher in AC16 cells exposed to MTS-H3R9 than that with MTS-H3R6 depending on the dosage of peptides (Fig. 2B). Also, GA and MTS showed a low LDH release even when the peptide dosage were increased. However, membrane damage in MTS-H3R9-exposed cells was alleviated by GA-MTS-H3R9 treatment.
Fig. 2. Cell viability assay with GA-MTS-H3R9.
Cell viability was determined using WST-1 and lactate dehydrogenase (LDH) assay. AC16 cells were exposed to different concentrations of GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 up to 50 μM for 24 h. Panel (A) presents the results of the WST-1 assay and Panel (B) presents the results of LDH assay. GA, gallic acid; MTS, mitochondria targeting sequence. Data are indicted as the mean ± SD (n = 3).
Membrane internalization
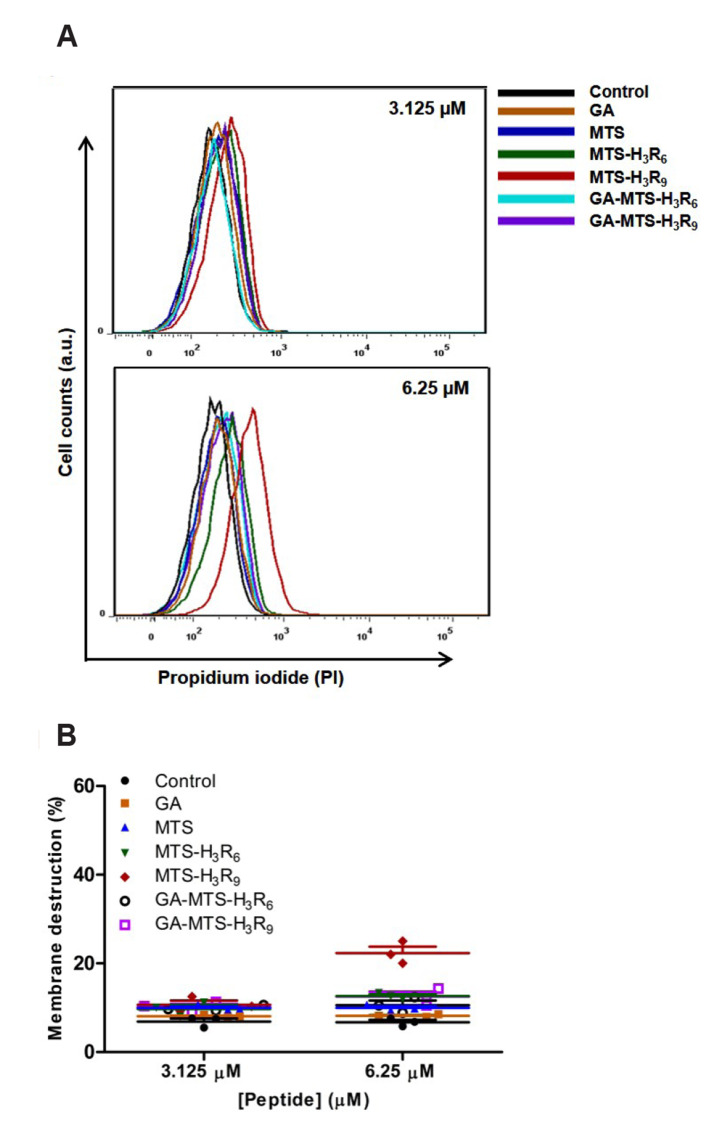
To examine the membrane permeability activity of the peptides, we used propidium iodide (PI), a fluorescent indicator that stains prokaryotic or eukaryotic cells with damaged membranes based on membrane integrity [24]. We exposed AC16 cells with 3.125 and 6.25 μM of GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 and cultured them for 16 h. With increasing doses, MTS-H3R9 exposed cells had increased PI staining compared to the untreated cells (Fig. 3A, B). GA-MTS-H3R9 exposed cells alleviated cellular damage compared to that of the MTS-H3R9 treatment.
Fig. 3. Membrane internalization by GA-MTS-H3R9.
AC16 cells were treated with 3.125 and 6.25 μM of GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 for 16 h and were subsequently incubated with 5 μl of PI for 20 min. (A) PI internalization using flow cytometry analysis. (B) Quantitative graph showing each peptide from the PI fluorescence data shown in panel (A). GA, gallic acid; MTS, mitochondria targeting sequence; PI, propidium iodide.
Intracellular uptake
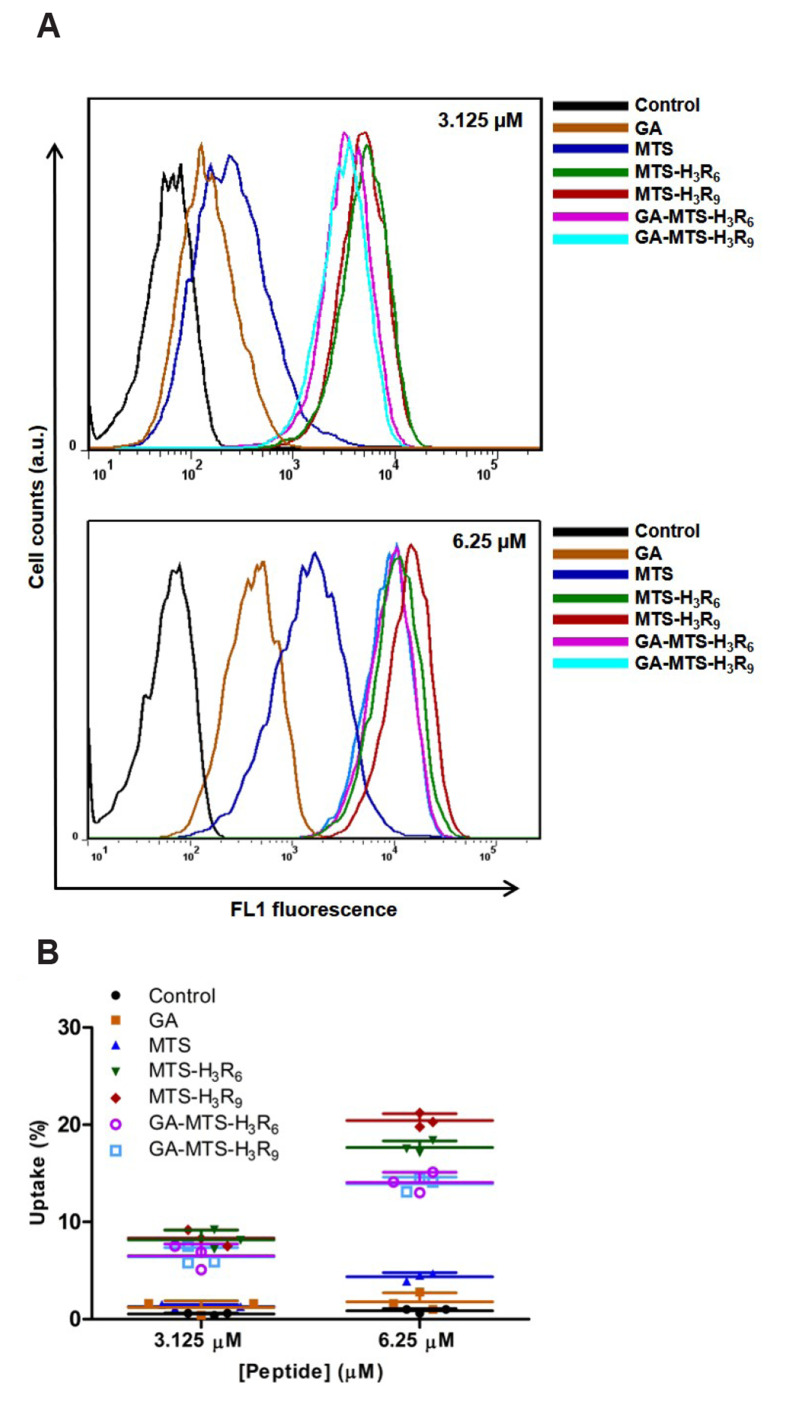
To investigate cellular uptake process of the peptides, AC16 cells were treated with 3.125 and 6.25 µM of FITC-GA, FITC-MTS, FITC-MTS-H3R6, FITC-MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 to assess doses dependent activity of these peptides. Interestingly, when the dose of the peptides was increased, cellular uptake of all the FITC-labelled peptide showed increased activity compared with FITC-MTS except for GA (Fig. 4A, B). According to these results, a dose of 6.25 µM was used for the subsequent experiments.
Fig. 4. Intracellular uptake of GA-MTS-H3R9.
AC16 cells were treated with 3.125 and 6.25 μM of FITC-labeled GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 for 16 h. (A) Cellular uptake of each FITC-labeled peptide using flow cytometry analysis. (B) Quantitative graph showing each peptide from uptake data depicted in panel (A). GA, gallic acid; MTS, mitochondria targeting sequence.
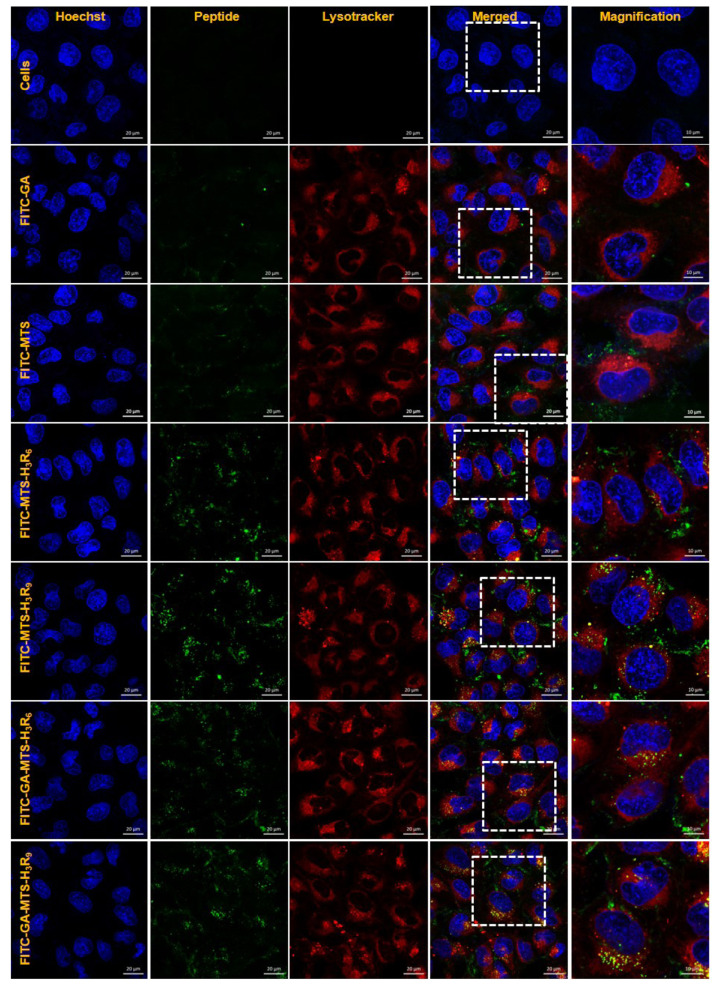
Intracellular distribution
To examine intracellular distribution of the peptides, AC16 cells were treated with 1 µM of FITC-labelled peptides. After 16 h of incubation, the cells were stained with LysoTracker (red) and then visualized by confocal microscopy. Cells incubated with GA and MTS showed a distribution around lysosomes. In contrast, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 displayed colocalization of LysoTracker (red) following endosomal release from the lysosomes into cytosol in the cells due to escape from the endo-lysosome after cellular uptake (Fig. 5).
Fig. 5. Intracellular distribution of GA-MTS-H3R9.
AC16 cells were treated with 1 μM of FITC-labeled GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 for 16 h. The cells were stained with LysoTracker (red) for 20 min and counterstained with Hoechst stain (blue) for 10 min and imaged by confocal microscopy. Scale bar is 10 µm. GA, gallic acid; MTS, mitochondria targeting sequence.
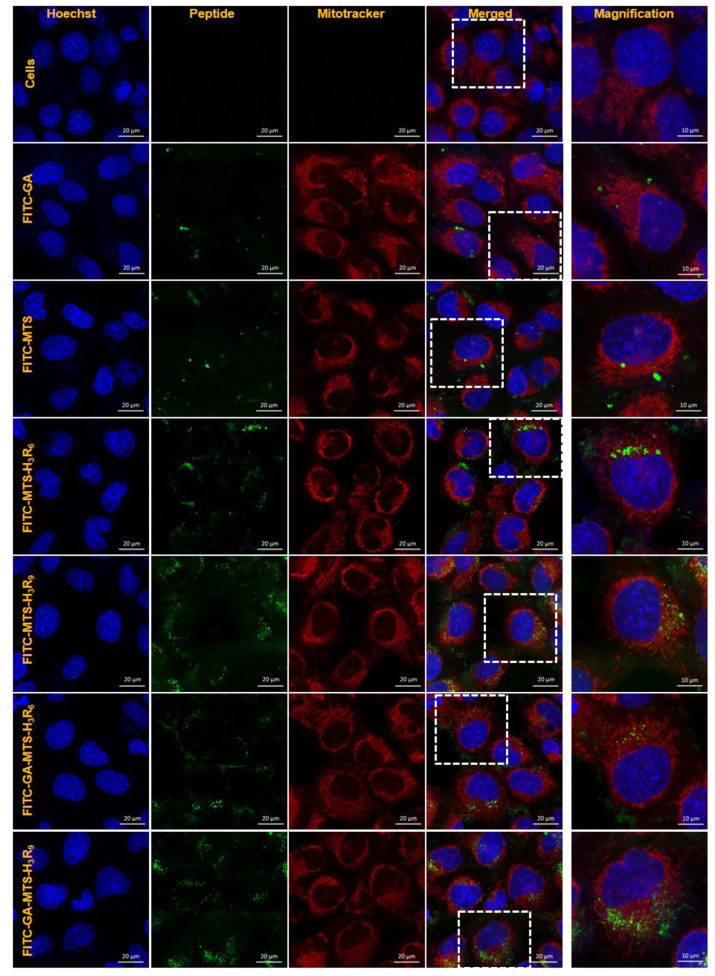
Intracellular mitochondrial targeting
To evaluate mitochondrial-targeting ability of the peptides using confocal microscopy, AC16 cells were treated with 1 µM of FITC-GA, FITC-MTS, FITC-MTS-H3R6, FITC-MTS-H3R9, FITC-GA-MTS-H3R6, and FITC-GA-MTS-H3R9 for 24 h. Cells incubated with FITC-MTS-H3R6 and FITC-MTS-H3R9 with and without GA exhibited colocalization with MitoTracker in the mitochondria of the AC16 cells as compared to MTS treated cells (Fig. 6). Particularly, FITC-MTS-H3R9 and FITC-GA-MTS-H3R9 showed the highest targeting of mitochondria in the cells.
Fig. 6. Intracellular mitochondria localization of GA-MTS-H3R9.
AC16 cells were exposed to 1 μM of FITC-labeled GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 for 24 h. The cells were stained with MitoTracker (red) for 20 min and counterstained with Hoechst stain (blue) and then imaged by confocal laser scanning microscopy. Scale bar is 20 µm. GA, gallic acid; MTS, mitochondria targeting sequence.
In addition, cell cytotoxicity was examined under similar conditions including the mitochondria targeting experiments. AC16 cells were exposed to each peptide for 24 h. For this assay, the cytotoxicity of the peptides was investigated using WST-1 and LDH release assay. Also, we performed a live/dead viability assay. Regardless of the dose of each peptide, all peptides conferred high cell viability (Supplementary Fig. 1).
Reactive oxygen species
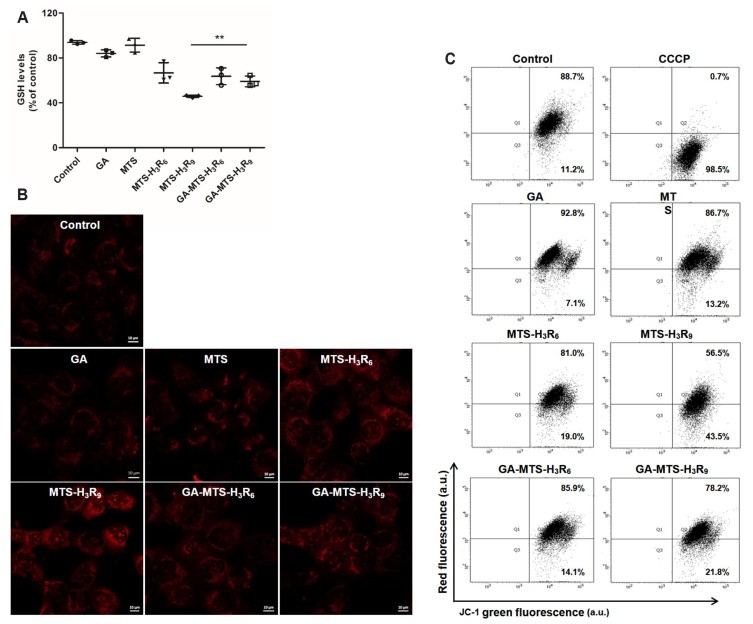
To evaluate the level of ROS generated by cells exposed to these peptides, AC16 cells were treated with 6.25 μM of each peptide for 12 h. We examined the levels of GSH, as hydrophilic antioxidant enzymes act against ROS production for protection of cells [25]. As shown in Fig. 7A, decreased GSH were observed in the cells exposed to MTS-H3R9 in the grafting of GA in MTS-H3R9 compared to MTS treatment.
Fig. 7. ROS levels and mitochondrial membrane potential (MMP) with GA-MTS-H3R9.
(A) AC16 cells were exposed to 6.25 μM of GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 for 16 h. Intracellular ROS levels were assessed by GSH assay. (B) AC16 cells were treated using the same conditions as those panel (A). The cells were treated with 20 μM MitoSox for 16 h. Cells were imaged by confocal microscopy. Scale bar is 10 µm. (C) AC16 cells were treated using the same conditions as those in panel (A). The cells were added to 2 μM JC-1 and then incubated for 20 min. MMP was determined by flow cytometry analysis. Data are indicted as the mean ± SD (n = 3). ROS, reactive oxygen species; GA, gallic acid; GSH, glutathione; MTS, mitochondria targeting sequence; CCCP, carbonyl cyanide 3-chlorophenylhydrazone. **p < 0.01.
To further investigate whether MTS-H3R9 induces ROS production when each peptide was delivered into the mitochondria, we employed Mitosox Red, as an indicator of mitochondria derived ROS production [26]. As shown in Fig. 7B, compared to control cells, GA-MTS-H3R9 showed a weak red fluorescence signal compared to that of MTS-H3R9 via superoxide production. These results suggest that the conjugation of antioxidant GA peptide to the MTS-H3R9 and GA-MTS-H3R9 may rescue cells against oxidative stress by enhancing antioxidant activity in the cells due to the polyphenol compound related to the free hydroxyl groups on the aromatic ring in GA.
Mitochondrial membrane potential
The change in MMP is an indicator of mitochondrial function which representing apoptotic process following cytochrome c release from mitochondria [27]. To investigate the mitochondrial depolarizing effect of GA-MTS-H3R9, we measured the MMP using JC-1 probe, a lipophilic cationic dye that exhibits a J-aggregation form with red fluorescence because of high MMP in healthy cells. However, the monomeric form exhibits green fluorescence in low MMP by depolarization of the mitochondria in apoptotic cells [28]. AC16 cells treated with 6.25 μM of all the peptides were examined using flow cytometry. When compared to untreated cells, treatment of AC16 cells with MTS-H3R6 and MTS-H3R9 induced MMP dissipation (19.0% and 43.5%, respectively). Interestingly, MTS-H3R9-treated cells showed significantly greater MMP disruption compared to that in MTS-H3R6 treated cells. In addition, GA-MTS-H3R9 showed a significant reduction in MMP depolarization compared to that of MTS-H3R9 (43.5% and 21.8%, respectively) (Fig. 7C). Furthermore, ROS generation and MMP experiments were carried out employing the similar conditions as those used in the mitochondria targeting assays. We examined GSH and JC-1 assay and all peptides used in this experiment did not trigger cellular damage and the cells adapted with peptide exposure at a low dose (Supplementary Fig. 2).
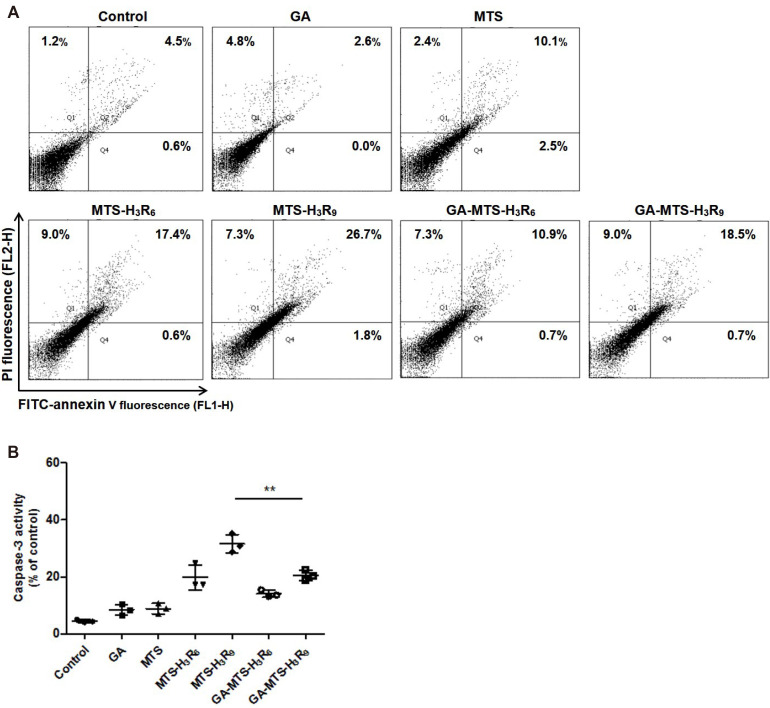
Apoptosis
We evaluated the apoptotic activity of GA-MTS-H3R9 by FACS analysis using an Annexin V/PI double staining. Annexin V binds to a surface exposed to phosphatidyl serine from the plasma membrane during apoptotic processes resulting in the disruption of plasma membrane. In addition, PI passes through the plasma membrane and binds to cellular DNA of necrotic cells [21,24]. AC16 cells were treated with 6.25 μM of each peptide for 24 h. As shown in Fig. 8A, MTS-H3R6 and MTS-H3R9 showed an increase in late apoptosis (17.4% and 26.7%, respectively) compared to that in control group cells (4.5%). Interestingly, AC16 cells exposed to GA-MTS-H3R9 exhibited a decrease in late apoptotic cells (18.5%) than those exposed to MTS-H3R9 (26.7%). Furthermore, experiments related to apoptosis were conducted under the similar conditions similar to the mitochondria targeting assays. We examined annexin V/PI staining. We observed that exposure to all the peptides did not result in cell death in these conditions (Supplementary Fig. 3).
Fig. 8. Induction of cytoprotection by GA-MTS-H3R9.
(A) Anticancer activity of each peptide. AC16 cells were exposed to 6.25 μM of GA, MTS, MTS-H3R6, MTS-H3R9, GA-MTS-H3R6, and GA-MTS-H3R9 for 24 h. Apoptosis levels for each group were measured by flow cytometry analysis after annexin V staining. Q1 indicates necrotic cells. Q2 indicates late apoptotic cells, Q3 indicates intact cells, and Q4 indicates early apoptotic cells. (B) Caspase-3 activity of each peptide. AC16 cells were treated using the same conditions as those in panel (A). Caspase-3 activity was assessed as described in Methods. Data are indicted as the mean ± SD (n = 3). GA, gallic acid; MTS, mitochondria targeting sequence. **p < 0.01.
To further confirm the anti-apoptotic activity of GA-MTS-H3R9, we applied the caspase-3 activity as executioner caspases that was mediated via intrinsic apoptosis pathway due to various cellular stresses [29]. We performed these experiments using similar conditions as those of the annexin V/PI staining. Compared with MTS peptides, GA-MTS-H3R9-exposed AC16 cells displayed decreased caspase-3 activity compared to MTS-H3R9 treated cells (Fig. 8B).
DISCUSSION
In our study, we demonstrated that MTS-H3R9 conjugated to GA (GA-MTS-H3R9) regulated mitochondrial apoptosis by employing dual functional strategies such as by acting as a delivery vehicle and by functioning as a therapeutic agent. Previous reports suggested that cell penetrating peptide (CPP) such as cationic, hydrophobic, and amphipathic peptides comprised of 5–20 amino acids traverse the tissue and cell membranes to biologically deliver active cargo into cells. Among the CPPs, arginine-rich peptides with highly positive charges show characteristics demonstrating penetration of plasma membranes [16]. In our study, we indicated that with or without GA in FITC-MTS-H3R6 and FITC-MTS-H3R9 the cationic peptide-modified MTS may facilitate cell membrane penetration caused by cell penetrating peptides with arginine residues and then subsequently improving cellular uptake, potentially optimizing subcellular targeting of the mitochondria.
Subcellular localization into the cells for cellular targeting via endocytosis is a major factor for successful drug delivery [30]. In our study, we showed that MTS-H3R6 and MTS-H3R9 with or without GA has the potential to surmount endosomal/lysosomal hurdles to achieve mitochondria-targeting via the endocytosis system (Fig. 5).
The results demonstrated that cells exposed to GA-MTS-H3R9 showed high cell viability against mitochondrial-mediated cell death induced by increased ROS generation, disrupted mitochondrial membrane potential, and anticancer activity. However, GA-MTS-H3R9 showed reduced cytotoxicity and mitochondria-triggered cell damage compared with MTS-H3R9 at 6.25 μM dose. Furthermore, GA-MTS-H3R9 alleviated intrinsic cell death in AC16 cells caused by ROS generation, disrupted mitochondria membrane potential, and induced cell death at the 6.25 μM dose compared with that by MTS-H3R9 treatment. Our results indicate that GA-MTS-H3R9 as a targeted mitochondria-selective vehicle displayed cytoprotective effects against cellular damage. It is established that mitochondria-targeted GA-MTS-H3R9 can be used as a bifunctional peptide that provides efficient mitochondrial target carrier as well as function as an effective therapeutic agent. These findings suggest that this peptide vehicle can be employed for therapeutic applications for mitochondria-associated disorders.
FUNDING
This research was supported by the Basic Research Lab Program (2020R1A4A1018943) and the Basic Science Research Program (2018R1A2A3074998, 2019R1I1A1A01061429) through the National Research Foundation of Korea funded by the Ministry of Science and ICT, Korea.
SUPPLEMENTARY MATERIALS
Supplementary data including one table and three figures can be found with this article online at https://doi.org/10.4196/kjpp.2022.26.1.15.
Footnotes
Author contributions: Y.B., G.Y.K., and F.J. performed the experiments. K.S.K. and J.H. supervised and coordinated the study. Y.B. and J.H. wrote the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Wolfram JA, Donahue JK. Gene therapy to treat cardiovascular disease. J Am Heart Assoc. 2013;2:e000119. doi: 10.1161/JAHA.113.000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leo CH, Jelinic M, Ng HH, Parry LJ, Tare M. Recent developments in relaxin mimetics as therapeutics for cardiovascular diseases. Curr Opin Pharmacol. 2019;45:42–48. doi: 10.1016/j.coph.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. 2019;393:1034–1044. doi: 10.1016/S0140-6736(18)31808-7. [DOI] [PubMed] [Google Scholar]
- 4.Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R, Liao R, Kohane DS. Nanoparticles targeting the infarcted heart. Nano Lett. 2011;11:4411–4414. doi: 10.1021/nl2025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan KX, Pan S, Jeevanandam J, Danquah MK. Cardiovascular therapies utilizing targeted delivery of nanomedicines and aptamers. Int J Pharm. 2019;558:413–425. doi: 10.1016/j.ijpharm.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 2014;9:467–483. doi: 10.2147/IJN.S36654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater. 2019;10:4. doi: 10.3390/jfb10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentino K, García-Sáez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181:62–75. doi: 10.1016/j.chemphyslip.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Rin Jean S, Tulumello DV, Wisnovsky SP, Lei EK, Pereira MP, Kelley SO. Molecular vehicles for mitochondrial chemical biology and drug delivery. ACS Chem Biol. 2014;9:323–333. doi: 10.1021/cb400821p. [DOI] [PubMed] [Google Scholar]
- 11.Weissig V, Boddapati SV, Jabr L, D'Souza GG. Mitochondria-specific nanotechnology. Nanomedicine (Lond) 2007;2:275–285. doi: 10.2217/17435889.2.3.275. [DOI] [PubMed] [Google Scholar]
- 12.Lin R, Zhang P, Cheetham AG, Walston J, Abadir P, Cui H. Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting. Bioconjug Chem. 2015;26:71–77. doi: 10.1021/bc500408p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimpel A, Neundorf I. Bifunctional peptide hybrids targeting the matrix of mitochondria. J Control Release. 2018;291:147–156. doi: 10.1016/j.jconrel.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 14.von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986;5:1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu GS, Han J, Ko KS, Choi JS. Cationic oligopeptide-conjugated mitochondria targeting sequence as a novel carrier system for mitochondria. Macromol Res. 2014;22:42–46. doi: 10.1007/s13233-014-2003-3. [DOI] [Google Scholar]
- 16.Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584:1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Bae Y, Joo C, Kim GY, Ko KS, Huh KM, Han J, Choi JS. Cationic oligopeptide-functionalized mitochondria targeting sequence show mitochondria targeting and anticancer activity. Macromol Res. 2019;27:1071–1080. doi: 10.1007/s13233-019-7153-x. [DOI] [Google Scholar]
- 18.Xie M, Hu B, Wang Y, Zeng X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J Agric Food Chem. 2014;62:9128–9136. doi: 10.1021/jf503207s. [DOI] [PubMed] [Google Scholar]
- 19.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 20.Bae Y, Green ES, Kim GY, Song SJ, Mun JY, Lee S, Park JI, Park JS, Ko KS, Han J, Choi JS. Dipeptide-functionalized polyamidoamine dendrimer-mediated apoptin gene delivery facilitates apoptosis of human primary glioma cells. Int J Pharm. 2016;515:186–200. doi: 10.1016/j.ijpharm.2016.09.083. [DOI] [PubMed] [Google Scholar]
- 21.Bae Y, Jung MK, Lee S, Song SJ, Mun JY, Green ES, Han J, Ko KS, Choi JS. Dequalinium-based functional nanosomes show increased mitochondria targeting and anticancer effect. Eur J Pharm Biopharm. 2018;124:104–115. doi: 10.1016/j.ejpb.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 22.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 23.Holder AL, Goth-Goldstein R, Lucas D, Koshland CP. Particle-induced artifacts in the MTT and LDH viability assays. Chem Res Toxicol. 2012;25:1885–1892. doi: 10.1021/tx3001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Xiang Y, Xu M. From red to green: the propidium iodide-permeable membrane of Shewanella decolorationis S12 is repairable. Sci Rep. 2015;5:18583. doi: 10.1038/srep18583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang L, Xie G, Liu C, Zhou J, Chen J, Yu S, Li J, Pang X, Shi H, Liang H. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim Biophys Acta. 2013;1833:2996–3005. doi: 10.1016/j.bbamcr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Soumya RS, Vineetha VP, Salin Raj P, Raghu KG. Beneficial properties of selenium incorporated guar gum nanoparticles against ischemia/reperfusion in cardiomyoblasts (H9c2) Metallomics. 2014;6:2134–2147. doi: 10.1039/C4MT00241E. [DOI] [PubMed] [Google Scholar]
- 27.He H, Li DW, Yang LY, Fu L, Zhu XJ, Wong WK, Jiang FL, Liu Y. A novel bifunctional mitochondria-targeted anticancer agent with high selectivity for cancer cells. Sci Rep. 2015;5:13543. doi: 10.1038/srep13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430. doi: 10.1038/cddis.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancho P, Galeano E, Nieto E, Delgado MD, García-Pérez AI. Dequalinium induces cell death in human leukemia cells by early mitochondrial alterations which enhance ROS production. Leuk Res. 2007;31:969–978. doi: 10.1016/j.leukres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Madani F, Lindberg S, Langel U, Futaki S, Gräslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011;2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.