Abstract
The ongoing global pandemic of coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and significantly impacts the world economy and daily life. Symptoms of COVID-19 range from asymptomatic to fever, dyspnoea, acute respiratory distress and multiple organ failure. Critical cases often occur in the elderly and patients with pre-existing conditions. By binding to the angiotensin-converting enzyme 2 receptor, SARS-CoV-2 can enter and replicate in the host cell, exerting a cytotoxic effect and causing local and systemic inflammation. Currently, there is no specific treatment for COVID-19, and immunotherapy has consistently attracted attention because of its essential role in boosting host immunity to the virus and reducing overwhelming inflammation. In this review, we summarise the immunopathogenic features of COVID-19 and highlight recent advances in immunotherapy to illuminate ideas for the development of new potential therapies.
Key words: Cellular exhaustion, COVID-19, cytokine storm, immunopathology, immunotherapy, SARS-CoV-2
Introduction
At the end of 2019, a total of 41 cases of pneumonia of unknown aetiology were first reported and then spread rapidly throughout the world (Ref. 1). Further studies identified this novel zoonotic virus as an enveloped, positive-sense single-stranded RNA coronavirus belonging to the subgenus Sarbecovirus of the genus Betacoronavirus (Ref. 2). It is named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by SARS-CoV-2 was termed corona virus disease 2019 (COVID-19) by the World Health Organization (WHO). On 31 January 2020, the WHO announced that COVID-19 is listed as Public Health Emergency of International Concern. By the end of July 2021, SARS-CoV-2 has spread rapidly and affected more than 200 countries, resulting in more than 230 million identified cases and 4.7 million confirmed deaths (Ref. 3). SARS-CoV-2 is the seventh coronavirus known to infect humans, and the third coronavirus emerged as a public health issue over the past two decades. SARS-CoV-1, MERS-CoV and SARS-CoV-2 can cause serious illness, whereas HKU1, NL63, OC43 and 229E are associated with mild symptoms (Ref. 4). In a previous study of 44 672 patients with COVID-19 in China, 81% of infected patients had a broad spectrum of clinical manifestations ranging from asymptomatic to cough, fever, coagulation dysfunction and metabolic acidosis. A total of 14% had severe manifestations, and 5% had critical manifestations, such as hyper-inflammation, multiple organ dysfunction syndromes and acute respiratory distress syndrome (ARDS) (Ref. 5). Most mortalities happened in elderly patients or patients with multiple comorbidities, including cardiovascular diseases, respiratory diseases, diabetes mellitus, hypertension and immune-compromised patients, such as cancer (Ref. 6).
The pathogenesis of COVID-19 is thought to be determined by two main courses. In the early phase of infection, SARS-CoV-2 identifies host cell angiotensin-converting enzyme 2 (ACE2) receptors and invades the host cell to complete the replication cycle. The cellular damage caused during the replication cycle can lead to respiratory disease, a decrease in lymphocytes and local/systemic inflammation. As the infection progresses, immune pathologies such as cytokine storm and lymphopoenia occur (Ref. 7).
There is currently no specific treatment available. Treatment strategies for COVID-19 infection are early detections, the quarantine of new cases and supportive therapies for the confirmed individual (Ref. 8). Immunotherapy plays an important role in inhibiting viral infection or modifying the overactivated immune response against SARS-CoV-2. In this review, we summarise the immunopathogenic features of COVID-19 and highlight current advances in immunotherapies to combat COVID-19, hoping to enlighten ideas of developing new potential therapies in the future.
Viral entry and replication
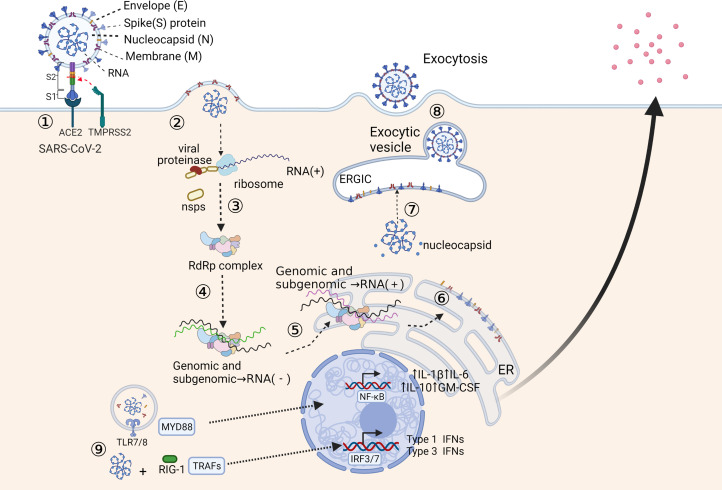
Epidemiological and virological studies suggest that SAR-CoV-2 is transmitted to others mainly by symptomatic and asymptomatic individuals through close contact via respiratory droplets or direct contact with infected individuals, sometimes through contaminated objects and surfaces (Ref. 8). The spike (S) protein of SARS-CoV-2 determines the tropism of receptors in host cells and plays a vital role in the invasion process. Figure 1 summarises the recognition and replication cycle of SARS-CoV-2. The S protein is composed of two subunits: S1 and S2. S1 is responsible for the binding of the viral receptor-binding domain (RBD) to the host cell ACE2, and S2 ensures the fusion of the virus with the host cell membrane (Ref. 9). SARS-CoV-2 employs the cellular transmembrane serine protease 2 (TMPRSS2) for S protein priming (Ref. 10). Most recently, neuropilin 1 has been identified as an important cofactor for entry, particularly in cells with low-level ACE2 expression (Ref. 11). Studies also show that the interaction of the S protein with the CD26 and CD209L could be a possible way of viral entry, but the mechanism is still unclear (Refs 12, 13). During the fusion process, binding of the virus to ACE2 causes stabilisation of the RBD, which triggers conformational changes in the S-complex, leading to the release of the S1 subunit and activation of the fusogenic activity of S2 (Ref. 14). A study demonstrated that strains isolated from COVID-19 patients could use the host cell protease TMPRSS2 and cathepsins B/L to prime the S protein in vitro (Ref. 15). Structural changes in the S protein may prevent the immune system from recognising the virus. For example, the current delta variant has three mutations at the S1 subunits, which increases the affinity of RBD to bind to ACE2 and enhances its ability to escape our immune system (Ref. 16).
Fig. 1.
Recognition and replication cycle of SARS-CoV-2. (1) The SARS-CoV-2 engages the host cell surface through the binding of spike (S) protein to ACE2. TMPRSS2 cleaves the S protein into S1 and S2 subunits, and the S2 subunit ensures the fusion of the virus with the host cell membrane. (2) The host cell ribosome translates the positive-sense RNA genome into polyproteins, which are cleaved by viral proteases to produce viral nonstructural proteins (nsps). (3) The nsps subsequently assembled into RNA-dependent RNA polymerase complex (RdRp). (4) RdRp uses the positive-sense RNA genome as a template to generate the negative-sense sub-genome. (5) Sub-genomes are used as templates for synthesising full-length positive-sense RNA genomes and sub-genomic mRNAs. (6) The sub-genomic mRNAs are translated into the endoplasmic reticulum (ER) to generate structural and accessory proteins. (7) The assembly of newly generated positive-sense RNA genome and viral structural proteins occurs in the endoplasmic-reticulum–Golgi intermediate compartment (ERGIC). (8) The enveloped virions are released through exocytosis. (9) In recognition of the SARS-CoV-2 RNA genome, TLR7/8 and RIG-1 recruit MYD88 and TRAFs. These adaptor molecules translocate transcriptions factors, NF-κB and IRFs into the nucleus leading to the production of and secretion of inflammatory cytokines.
ACE2 is an enzyme that belongs to the renin–angiotensin system and is located on the cell surface of type II alveolar epithelial cells in the lungs and other tissues (Ref. 17). ACE2 plays a critical role in controlling vasoactive effects in the body and is highly co-expressed with genes related to TMPRSS in the upper airways (Refs 18, 19). The affinity of SARS-CoV-2 to ACE2 is 10–20 times higher than that of SARS-CoV, possibly because of the RBD of SARS-CoV-2 having a more compact conformation (Ref. 20). Several residue changes in the RBD of SARS-CoV-2 stabilise two virus-binding hotspots at the RBD–ACE2 interface and increase ACE2 binding affinity (Ref. 21).
Once the virus enters the host cell cytoplasm, translation begins using viral RNA as a template to generate virus-specific mRNAs. After the translation of virus-specific mRNAs is completed, the production of structural and nonstructural viral proteins is incorporated into the endoplasmic reticulum or Golgi membrane (Ref. 22). Finally, the vesicles containing the virus particles fuse with the plasma membrane to release the virus (Ref. 23). Another possible mechanism for SARS-CoV-2 entry is that the virus binds to its corresponding antibody to form an antigen–antibody complex and then enters the target cell through the Fc receptor (Ref. 24).
Innate and adaptive immunity against SARS-COV-2
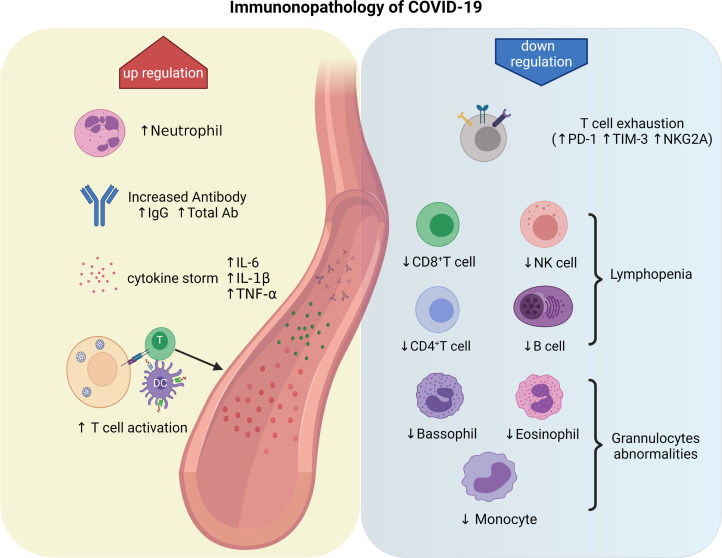
Activated natural killer (NK) cells exert a major histocompatibility complex-independent immune response against SARS-CoV-2 and may limit viral pathogenesis in the early stages of infection (Ref. 25). The respiratory tract is the first front line of the immune system, which contains many immune effector cells such as epithelial cells, type 1 macrophages (M1) and dendritic cells (DCs). After infection, epithelial cells produce immune cytokines and express adhesive molecules, recruiting immune cells to the lung tissue to combat the virus. After virus recognition, activation of interferon regulatory factor 3 (IRF-3) in M1 leads to the production of various immune cytokines such as type I interferon (IFN-I) and phagocytosis (Ref. 26). IFN-I plays an essential role in the immune response against SARS-CoV. One of the mechanisms of anti-viral immunity by IFN-I is activating IFN-induced transmembrane family proteins, which inhibit virus entry into the host cell. DCs can serve as the antigen-presenting cell (APC)-mediated immune response to SARS-CoV-2 and produce IFN-I and interleukin 6 (IL-6) to inhibit viral infection. A study shows that excessive production of IFN-I by DCs leads to severe inflammation and ARDS in severe patients (Ref. 27). The APCs, such as monocytes and DCs, distinguish the viral antigen on infected cells, introduce the antigen to the helper T (Th) cells, and produce cytokines that direct the anti-viral immune response of the T cells. Serum levels of Th1-associated cytokines, including IFN-γ, tumour necrosis factor (TNF)-α and IL-2, are reported to be elevated in COVID-19 patients (Ref. 28). These cytokines activate cytotoxic T cells (CTLs) to attack the virus-infected cells and destroy them by producing perforin and granzymes. In addition, Th2 cells present the viral antigen to B lymphocytes, which subsequently produce neutralising antibodies against the S protein of the virus. The neutralising antibodies inhibit the replication of the virus in the body and generate humoral immunity, which is one of the most important concepts for developing vaccines against SARS-CoV-2 (Ref. 23). Figure 2 briefly summarises the immunopathology of COVID-19.
Fig. 2.
Immunopathology of COVID-19. The neutrophil is significantly increased in severe cases, despite decreased eosinophil, basophil and monocyte counts. Activated B cells produce and secrete a large number of antibodies to neutralise the virus. The antigen-specific T cells are activated, and numerous cytokines, such as IL-6, IL-1β and TNF-α are secreted, leading to cytokine storm. On the other hand, lymphopoenia is an important feature of COVID-19, mainly manifested by a decreased count of CD8+ T cells, CD4+ T cells, NK cells and B cells. The increased expression of programmed cell death protein-1 (PD-1), T cell immunoglobulin domain and mucin domain-3 (TIM3), and killer cell lectin-like receptor subfamily C member 1 (NKG2A) on T cells could accelerate lymphopoenia.
Humoral immunity plays a vital role in the induction of adaptive immunity to coronaviruses. Activation of B cells and plasma cells leads to the production of neutralising antibodies that prevent further infection with the virus. The seroconversion time of Ab and immunoglobulin M (IgM) and IgG antibodies appeared consecutively, with a median seroconversion day of 11, 12 and 14, respectively (Ref. 29). Within 4 weeks of infection, 90–99% of individuals infected with the SARS-CoV-2 virus develop detectable neutralising antibodies (Ref. 30). The RBD domain of the SARS-CoV-2 S protein is the primary target of these viral species-specific neutralising antibodies (Ref. 31). Appropriate anti-viral antibodies can prevent patients from reinfecting the virus. Some cases of reinfection because of insufficient serum antibody levels have been reported, questioning the humoral immunity of SARS-CoV-2 (Ref. 32). Further research is needed to understand the exact mechanism of the immune response to SARS-CoV-2.
Cytokine storm
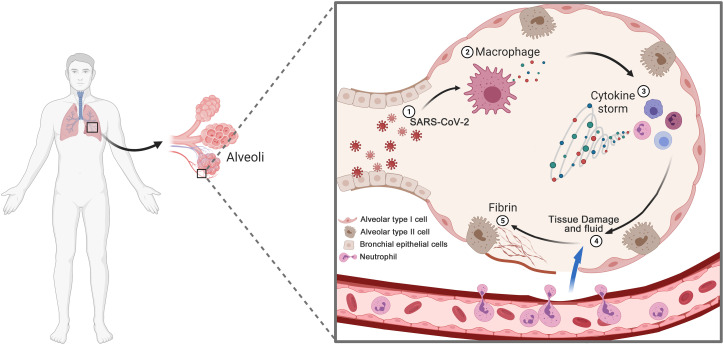
As illustrated in Figure 3, cytokine storm is a state of uncontrolled systemic hyper inflammation caused by an excess of cytokines, leading to multiorgan failure and even death. Rather than direct cytopathic effects of the COVID-19, cytokine storm activates inflammatory immune cells to attack alveolar cells, causing lung tissue damage (Ref. 33). The inflamed lung tissue also stimulates fibroblast cells to form fibrotic tissue in the lungs. In addition, the leakage of fluid into the alveoli and the accumulation of the inflammatory exudate lead to the formation of hyaloid membranes and further ARDS (Ref. 34). Damaged lung cells and M1 produce chemotactic factors that attract other immune cells and trigger an uncontrolled inflammatory response in the lung. Then the inflammatory cells begin an uncontrolled production of pro-inflammatory cytokines and chemokines contribute to the cytokine storm, including IL-1, IL-6 (Ref. 35), IL-8, IL-10, TNF-α, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), CXCL-1, -3, -10 and CCL-2, -3 (Refs 36–38). Increased IL-6 and IL-8 lead to severe inflammation in critical patients (Ref. 26).
Fig. 3.
Schematic diagram of cytokine storm in COVID-19. (1) SARS-CoV-2 infects lung bronchial, alveolar type I and type II epithelial cells. (2) Immune cells, including macrophages, recognise viruses and produce cytokines. (3) Cytokines attract more immune cells, such as neutrophils, T cells, monocyte and DCs, which in turn produce more cytokines, creating a cycle of inflammation that damages the lung cells. (4) Persistent inflammation lead to tissue damage, leucocytes infiltration and fluid leakage from the blood vessel, causing respiratory failure. (5) Excessive deposit of fibrin in the lungs.
Currently, one article introduces the concept of dividing the cytokine storm into two phases: the first phase is a temporary state of immunodeficiency, and the second phase is an overactive state of the immune system to compensate for the failure of target clearance, resulting in a cytokine storm (Ref. 39). Another report described the effects of human coronavirus on cytokines, noting delayed secretion of types I and III IFN in the early phase (within 3 days) of infection and excessive secretion of pro-inflammatory cytokines in the later phase (10–14 days) (Ref. 40). A study highlights a low level of IFN-I activity and downregulation of IFN-stimulated genes in COVID-19 patients and hyper-inflammatory responses represented by IL-6 and TNF-α (Ref. 41). These studies underscore that the cytokine storm results from the failure of the initial response to the type I and III IFNs of SARS-CoV-2, leading to the delayed response of the immune system the inability to clear the virus.
According to the pathogenesis of cytokine storm, appropriate treatments such as IL-1, IL-6 and TNF-α inhibitors can reduce lung tissue damage and lead to a better outcome in COVID-19 patients (Ref. 42). Several strategies, such as injection of immunomodulatory drugs and mesenchymal stem cells (MSCs), have been used to prevent lung injury and multiple organ failure in severe patients with cytokine storm. Targeting inflammatory cytokines could also reduce the severity of cytokine storm.
Lymphopoenia, granulocytes and monocytes disorders
Lymphopoenia, the decreased number of lymphocytes in the peripheral blood, usually accompanied by an increased number of neutrophils, is one of the most important features and common symptoms in severe cases of COVID-19 (Ref. 38). The most common cause for lymphopoenia could be apoptosis/pyroptosis of lymphocytes triggered by SARS-CoV-2 infection (Ref. 43). Other reasons include increased production of glucocorticoids, a storm of cytokines (Ref. 44), weakened vascular cell adhesion (Refs 45, 46) and FAS–FASL interaction (Ref. 47). Lymphopoenia results in decreased numbers of CD8+ T cells, CD4+ T cells, B cells or NK cells and weakens patients' immunity to the virus (Refs 28, 48). A remarkably decreased T cell is almost always observed in severe cases, and patients admitted to the intensive care unit show significantly decreased T cells, especially CD8+ T cells (Ref. 49). Lymphopoenia may serve as a prognostic marker for COVID-19 (Ref. 50). Since the number of neutrophils is increased, the neutrophil-to-lymphocyte ratio can be used as a prognostic criterion for COVID-19 (Ref. 51). In addition to lymphopoenia, abnormalities in granulocytes and monocytes have also been observed in COVID-19 patients (Ref. 51).
Abnormalities in granulocytes and monocytes have also been observed in COVID-19 patients (Fig. 2). The number of neutrophils is significantly higher in severe patients than in non-severe patients (Ref. 52). At the same time, a lower percentage of eosinophils, basophils and monocytes was observed in the serve patients (Ref. 53). The mechanisms are currently unclear, and further research is needed.
Immunotherapy of COVID-19
Currently, there is no proven specific treatment modality for COVID-19. Initial treatments are mainly supportive, including oxygen therapy, ventilator support in patients with respiratory failure, antibiotics to prevent secondary bacterial infections, anti-inflammatories and fluid management (Ref. 54). Since the COVID-19 outbreak, scientists have begun to evaluate the impact of currently available anti-viral drugs on the virus. Because the SARS-CoV-2 is an RNA virus, adenosine analogues such as remdesivir can block the viral RNA synthesis process. Remdesivir has shown clinical improvement and reduced mortality (Ref. 55). Other nucleoside analogues, such as ribavirin, are together with IFN beta-1b, lopinavir–ritonavir through a triple therapy in phase 2 clinical trial of treating COVID-19 (Ref. 56).
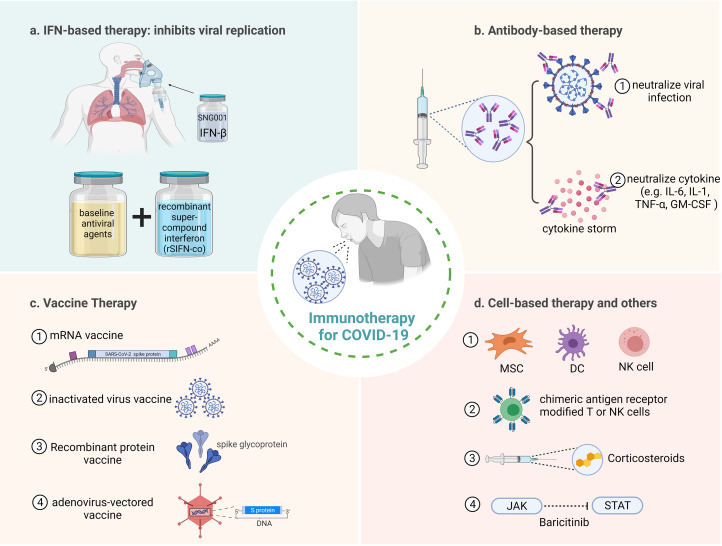
Immunotherapy has achieved remarkable results in the treatment of many diseases such as cancer and viral infections. The use of immune-enhancing agents to boost and strengthen the immune system could have a positive impact on the treatment of COVID-19. Figure 4 summarises the immunotherapy of COVID-19, which will be discussed step by step below. The clinical trials of immunotherapy for COVID-19 are also summarised (Table 1).
Fig. 4.
Immunotherapeutic approaches for COVID-19. (a) IFN is used to inhibit virus replication, the therapy of inhaled nebulised IFN-β1a (SNG001) and baseline anti-viral agents combined with recombinant super-compound interferon (rSIFN-co) have better targeted therapeutic effect. (b) Antibody-based therapy, including (1) neutralise viral infection and (2) neutralise cytokines to prevent damage caused by cytokine storm. (c) Vaccines used to treat COVID-19, including (1) mRNA vaccine, (2) inactivated virus vaccine, (3) recombinant protein vaccine and (4) adenovirus-vectored vaccine. (d) Cell-based therapies and other treatments, including (1) therapies based on MSC, DC and NK cells, (2) CAR-modified T and NK cells have better targeted therapeutic effect, (3) corticosteroids injections act as anti-inflammatory agents and (4) blocking JAK-STAT signalling pathways to inhibit cytokine storms.
Table 1.
Summary of the clinical trials of immunotherapy for COVID-19 (www.clinicaltrails.com)
| Treatment | Phase | Drug/method | Clinical trial |
|---|---|---|---|
| IFN | 2 | IFN-β1a (SNG001) | NCT04385095, NCT04860518, NCT04330690 |
| 2 | IFN-λ | NCT04354259, NCT04534673 | |
| 2 | IFN-β1b | NCT04465695, NCT04647695, NCT04494399, | |
| 3 | IFN-λ | NCT04967430 | |
| 4 | IFN-β1a | NCT04350671 | |
| NA | Nasal IFN-γ | NCT05054114 | |
| 2 | Inhaled IFN-α and IFN-β | NCT04469491 | |
| Antibody-based therapy | 1 | VIR-7831 (sotrovimab) | NCT04988152 |
| 2 | VIR-7831 (sotrovimab) | NCT04779879 | |
| 2, 3 | Bamlanivimab + etesevimab | NCT04427501 | |
| 3 | VIR-7831 (sotrovimab) | NCT04913675 | |
| 3 | Casirivimab + imdevimab | NCT01152318 | |
| 1, 2 | Convalescent plasma | NCT04521309 | |
| 2 | Convalescent plasma | NCT04347681, NCT04644198 | |
| 2, 3 | Convalescent plasma | NCT04891172 | |
| 2 | PD-1 blocking antibody | NCT04268537, NCT04356508, NCT04413838 | |
| 2 | Narsoplimab (anti-MASP-2) | NCT04488081 | |
| 2 | Anti-CD14 mAb | NCT04391309 | |
| 2 | Leronlimab (anti-CCR5) | NCT04343651, NCT04347239 | |
| 3 | Leronlimab (anti-CCR5) | NCT04901676, NCT04901689 | |
| 2 | Bevacizumab (anti-VEGF) | NCT04344782, NCT04275414 | |
| 3 | Bevacizumab (anti-VEGF) | NCT04822818 | |
| Vaccine therapy | |||
| mRNA vaccines | 1 | BNT162b1 | NCT04523571 |
| 1 | mRNA-1273 | NCT04283461 | |
| 1 | PTX-COVID19-B | NCT04765436 | |
| 2 | CVnCoV | NCT04515147 | |
| 2, 3 | BNT162b2 | NCT04368728 | |
| 2, 3 | mRNA-1273 | NCT04649151 | |
| 2, 3 | SCTV01C | NCT05043311 | |
| 2, 3 | CVnCoV | NCT04652102 | |
| 3 | mRNA-1273 | NCT04470427 | |
| 3 | CVnCoV | NCT04674189 | |
| DNA vaccines | 1 | GX-19N | NCT04915989 |
| 1 | CORVax | NCT04627675 | |
| 1 | INO-4800 | NCT04336410 | |
| 1 | COCIGEN | NCT04742842 | |
| 1, 2 | COVID-eVax | NCT04788459 | |
| 1, 2 | GX-19N | NCT04715997 | |
| 1, 2 | GX-19 | NCT04445389 | |
| 1, 2 | Covigenix VAX-001 | NCT04591184 | |
| 1, 2 | AG0301-COVID19 | NCT04463472 | |
| 1, 2 | AG0302-COVID19 | NCT04527081, NCT04993586 | |
| 1, 2 | GLS-5310 | NCT04673149 | |
| 2, 3 | INO-4800 | NCT04642638 | |
| 2, 3 | AG0302-COVID19 | NCT04655625 | |
| Shingles vaccines | 1 | MVA-SARS-2-S vaccine | NCT04569383 |
| 3 | HZ/su (herpes zoster subunit) | NCT05047770 | |
| Adenovirus | 1 | Ad5-nCoV | NCT04568811 |
| 2 | Ad5-nCoV | NCT05005156 | |
| Treatment | Phase | Drug/method | NCT ID |
| Protein vaccines | 1 | Protein vaccine (V-01) | NCT05050474 |
| 1 | Spike protein vaccine | NCT04982068 | |
| 1 | COVAX19 | NCT04453852 | |
| 1, 2 | EuCorVac-19 | NCT04783311 | |
| 1, 2 | RBD candidate vaccine | NCT05007509 | |
| 2 | Spike protein vaccine | NCT04990544 | |
| 3 | Recombinant protein vaccines | NCT04904549 | |
| Live-attenuated virus vaccines | 1 | COVI-VAC | NCT04619628 |
| 1 | MV-014-212 | NCT04798001 | |
| Cytokine storm management | |||
| IL-17 inhibitors | 1, 2 | Secukinumab | NCT04731116 |
| 3 | Ixekizumab | NCT04724629 | |
| 2 | Gimsilumab | NCT04351243 | |
| 2 | Sargramostim | NCT04707664 | |
| IL-1 receptor inhibitors | 2, 3 | Anakinra | NCT04357366, NCT04643678 |
| 3 | Canakinumab | NCT04348448, NCT04362813 | |
| TNF-α inhibitors | NA | Infliximab | NCT04734678 |
| 2 | Infliximab | NCT04922827, NCT04425538 | |
| 3 | Infliximab | NCT04593940 | |
| IL-6 receptor inhibitors | 2, 3 | Tocilizumab | NCT04332094, NCT04335071, NCT04356937, NCT04377659, NCT04577534 |
| 2, 3 | Sarilumab | NCT04315298, NCT04357808, NCT04357860, NCT04327388 | |
| 3 | Canakinumab | NCT04362813 | |
| IFN-γ inhibitors | 2, 3 | Emapalumab + anakinra | NCT04324021 |
| GM-CSF inhibitors | 2 | Otilimab | NCT04376684 |
| 2 | Gimsilumab | NCT04351243 | |
| 2, 3 | TJ003234 | NCT04341116 | |
| 3 | Lenzilumab | NCT04351152 | |
| C5 inhibitors | 2 | Eculizumab | NCT04288713, NCT04346797 |
| C3 inhibitors | 2 | AMY-101 | NCT04395456 |
| Steroids | Dexamethasone and methylprednisolone | NCT04909918 | |
| JAK inhibitors | 2, 3 | Baricitinib | NCT04321993, NCT04358614 |
| 2, 3 | Ruxolitinib | NCT04362137, NCT04348071 | |
| 2, 3 | Tofacitinib | NCT04750317, NCT04469114 | |
| BTK inhibitors | 2 | Ibrutinib | NCT04375397, NCT04665115, NCT04439006 |
| 2 | Acalabrutinib | NCT04382586, NCT04346199 | |
| Cell therapy | |||
| Mesenchymal stem cells | 1, 2 | Blood-derived MSCs | NCT04565665 |
| 1, 2 | UC-MSC | NCT04288102, NCT04333368, NCT04490486 | |
| 1 | Dental pulp MSCs | NCT04302519 | |
| NK cells | 1 | NK cells | NCT04280224 |
| CAR-NK cells | 1, 2 | NKG2D-ACE2 CAR-NK | NCT04324996 |
| Dendritic cells | 1, 2 | AV-COVID-19 | NCT04690387, NCT05007496, NCT04386252 |
| T cells | 1, 2 | PD-1 and ACE2 Knockout T | NCT04990557 |
| 1, 2 | Allogeneic Hybrid TREG/Th2 | NCT04482699 | |
NA, not available.
IFN therapy
IFN plays an essential role in host defense against SARS-CoV-2 infection. Upon viral infection, the endogenous pattern recognition receptor recognises the genetic material of the virus, which triggers a series of downstream cascade responses such as the JAK-STAT signal and stimulates the production of IFN-stimulating genes (ISGs) and IFN-I and other products (Ref. 57). The downstream molecules controlled by ISGs and IFN-I can directly inhibit viral replication, recruit and activate immune cells to regulate viral infection (Refs 58, 59). It was found that binding of the SARS-CoV-2 protein nonstructural protein 1 (NSP1) to the ribosomal subunits can block the induction of IFN and ISG expression (Ref. 60). TBK1 can induce TLR3–IFN and TMEM173/STING-IFN signalling pathway (Ref. 61), and NSP13 can block the phosphorylation of TBK1 and inhibit signal transduction, which affects the production of IFN (Ref. 62). IFN-I signal transduction can be antagonised by blocking signal transduction or blocking IRF-3 nuclear translocation (Ref. 63).
IFN-I and IFN-III are particularly important for the anti-viral response. They express distinct homologous receptors: IFN-I receptors are expressed in most mammalian tissues, whereas IFN-III receptors are mainly found in the respiratory and gastrointestinal tracts (Refs 64, 65). The delayed IFN response during SARS infection can cause the accumulation of various immune cells and the appearance of cytokine storm, leading to more severe pathological damage in the lung (Ref. 66). Early administration of IFN-I will forcibly eliminate the virus (Ref. 67). The use of inhaled nebulised IFN-β1a (SNG001) can more effectively deliver the drug to the site of SARS-CoV-2 infection for better treatment efficacy (Ref. 68). IFN combination therapy can activate STAT1 to inhibit viral replication (Refs 69, 70). Recombinant super-composite IFN (rSIFN-co) combined with baseline anti-viral therapy was found to have better clinical improvements and fewer side effects than conventional IFN anti-viral therapeutics (Ref. 71). IFN treatment also has side effects, the most common of which are flu-like symptoms, haematological toxicity, elevated transaminases, nausea, fatigue and psychiatric sequelae (Ref. 72).
Antibody-based therapies
Seow et al. found that the kinetics of the neutralising antibody response against SARS-CoV-2 infection is typical of acute viral infection, with decreasing neutralising antibody titres after an initial peak (average 23.1 days post-onset of symptoms), and that the magnitude of this peak depends on disease severity (Ref. 73). Antibody-based therapies can improve the immune response of COVID-19 patients and inhibit SARS-CoV-2 infection.
Studies have shown that the early use of convalescent plasma therapy can improve the prognosis of patients with moderate to severe COVID-19, but no significant clinical improvement was observed with the late use of convalescent plasma (Ref. 74). The mortality of COVID-19 patients was significantly reduced when convalescent plasma was provided in the early stages of the disease, and the use of plasma with high antibody titres did not increase the risk of death compared with unselected plasma (Refs 75, 76). The use of convalescent plasma not only transiently increased the level of systemic anti-SARS-CoV antibodies but also promoted the specific T-cell response (Ref. 77).
Monoclonal antibodies are used to prevent and treat various diseases, and treatment with monoclonal antibodies against coronavirus is clinically effective (Ref. 78). REGEN-COV, a combination of two monoclonal antibodies, casirivimab and imdevimab, both specifically targeting the spike protein of SARS-CoV-2, has been shown to reduce hospitalisation rates or risk of death in individuals at high risk for COVID-19. Subcutaneous injection of REGEN-COV can prevent SARS-CoV-2 infection and reduce the duration of disease symptoms and high viral load (Ref. 79). Llama-derived single-domain antibodies, also known as nanobodies, are generally more heat stable, accessible and cheaper to produce and can be better used for protein engineering than conventional antibodies. Combining nanobodies with different epitopes or complementary epitopes increases resistance to mutant escape (Ref. 80).
Intravenous immunoglobulin (IVIG) is widely used as an alternative therapy to treat immunodeficiency and reduce inflammatory responses (Ref. 81). The use of high-dose IVIG in hospitalised COVID-19 patients may improve their clinical progress by regulating their immune status. For example, IVIG may reduce the excessive inflammation observed in individuals infected with SARS-CoV-2 by reducing the levels of cytokines, chemokines and some complement factors (C5a) involved in immune activation and cell migration (Ref. 82). A 5% anti-COVID-19 IVIG solution contains 90% immunoglobulins (IgG, IgM and IgA), and the total anti-SARS CoV-2 antibody titre is three times higher than pooled convalescent plasma (Ref. 83).
Vaccine therapy
The first COVID-19 vaccine urgently approved by the U.S. FDA was BNT162b2, an mRNA encoding S protein, which showed 95% efficacy (Ref. 84). The second vaccine, mRNA-1273, had almost the same efficacy as BNT162b2, and it remains to be investigated whether BNT162b2 can elicit a long-term anti-SARS-CoV-2 immune response. The third vaccine, CVnCoV/CV2CoV, has been shown to prevent disease and death caused by the VOCB.1.351 mutant in mouse models, and clinical trials are underway (Ref. 85). The antibody responses to SARS-CoV-2 mRNA vaccination comprise a large proportion of non-neutralising antibodies and are co-dominated by S protein N-terminal domain (NTD) and RBD antibodies. Thus, the NTD portion of the spike represents a vital vaccine target (Ref. 86). DNA vaccine can induce antibodies with robust neutralising ability against SARS-CoV-2 wild-type strains and against some mutants, which could open a new avenue for effective treatment and diagnosis at the point of care (Ref. 87). The new SARS-CoV-2 mutant B.1.526 is spreading at an alarming rate and was identified by a D614G mutation and four novel point mutations in the S protein. The results show that vaccine-induced antibodies are protective against the B.1.526 mutant in the current recovery phase (Ref. 88).
Protein vaccines require adjuvants to enhance the immune response. Novavax's vaccine uses a genetically modified full-length S protein (NVX-CoV2373) that has higher structural stability. When administered to volunteers, the NVX-CoV2373 vaccine has a protective efficacy of 89.7% against SARS-CoV-2 infection and shows effective protection against the B.1.1.7 variant (Ref. 89). Studies have shown that the neutralising antibody response elicited by the Ad5-nCoV vaccine against COVID-19 with two doses of the atomised adenovirus type 5 vector is similar to that elicited by one dose of the intramuscular injection, and nebulisation at 28 days after the first intramuscular injection enhanced the vaccination and induced a strong IgG and neutralising antibody response (Ref. 90). Table 2 lists the COVID-19 vaccines within WHO Emergency Use Listing (EUL) and prequalification (PQ) evaluation process.
Table 2.
COVID-19 vaccines within WHO emergency use listing (EUL) and prequalification (PQ) evaluation process
| Platform | Name of vaccine | Manufacturer/WHO EUL holder | NRA of record |
|---|---|---|---|
| mNRA | BNT162b2/COMIRNATY | Pfizer–BioNTech | USFDA, EMA |
| mRNA-1273/Spikevax | Moderna Biotech | USFDA, EMA | |
| CVnCoV/CV07050101 | CureVac N.V. | EMA | |
| Adenoviral vector | AZD1222/Vaxzevria | Oxford University, AstraZeneca | EMA, MFDS KOREA, Japan, Australia TGA |
| Ad26.COV2.S/Janssen COVID-19 vaccine | Janssen-Cilag International NV | EMA | |
| Gam-COVID-Vac/Sputnik V | Gamaleya Research Institute of Epidemiology and Microbiology | Russian NRA | |
| Covishield | Serum Institute of India Pvt. Ltd. | DCGI | |
| Ad5-nCoV/Convidecia | CanSino Biologics | NMPA | |
| Inactivated | SARS-CoV-2 vaccine (vero cell), inactivated | Sinopharm/Beijing Institute of Biological Products | NMPA |
| COVID-19 vaccine (vero cell), inactivated | Sinovac Life Sciences | NMPA | |
| SARS-CoV-2 vaccine, inactivated (vero cell)/COVAXIN | Bharat Biotech, India | DCGI | |
| Inactivated SARS-CoV-2 vaccine (vero cell) | Sinopharm/WIBP | NMPA | |
| SARS-CoV-2 vaccine, inactivated (vero cell) | IMBCAMS, China | NMPA | |
| Protein | NVX-CoV2373/Covovax | Novavax | EMA |
| CoV2 preS dTM-AS03 vaccine | SANOFI | EMA | |
| SCB-2019 | Clover Biopharmaceuticals | NMPA | |
| Recombinant novel coronavirus vaccine (CHO Cell) | Zhifei Longcom, China | NMPA | |
| Soberana 02 | BioCubaFarma – Cuba | CECMED | |
| Peptide | EpiVacCorona | State Research Center of Virology and Biotechnology | Russian NRA |
NRA, National Regulatory Authority; USFDA, The United States Food and Drug Administration; EMA, European Medicines Agency; MFDS, Ministry of Food and Drug Safety; TGA, Therapeutic Goods Administration; DCGI, Drugs Controller General of India; NMPA, National Medical Products Administration of CHINA; CECMED, Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos, Cuba.
The humoral and cellular immune responses elicited by the different vaccines differed considerably. In particular, inactivated vaccines showed relatively low levels of neutralising antibodies and T-cell responses. Compared with three consecutive doses of inactivated vaccines to enhance the immune response, the use of recombinant subunits, adenoviral vectors or mRNA vaccines after two doses of inactivated vaccines can further boost neutralising antibodies and specific Th1 cell responses (Ref. 91). Modified vaccinia Ankara (MVA) vectors expressing membrane-anchored prefusion-stabilised spike (MVA/S) but not secreted S1 induce strong neutralising antibody responses against SARS-CoV-2 in mice. MVA/S vaccination triggered potent neutralising antibodies and CD8+ T-cell responses in macaques and protected them from SARS-CoV-2 infection, demonstrating that MVA/S is a potential vaccine candidate (Ref. 92).
Management of cytokine storm
Higher serum IL-6 levels are associated with the severity of COVID-19 (Ref. 93). Therefore, blocking IL-6 could reduce COVID-19 inflammation. Tocilizumab and sarilumab are two FDA-approved IL-6 receptor antibodies investigated in COVID-19 patients in phase III clinical trials (Ref. 94). Tocilizumab and sarilumab improved the survival of COVID-19 patients (Ref. 95). Administration of two doses of tocilizumab (8 mg/kg intravenously) per day reduced C-reactive protein (CRP) and cytokine storm in a man with renal carcinoma who suffered from COVID-19 (Ref. 96). In some cases, adverse effects such as acute pancreatitis, cytopenias, elevated ferritin and lactate dehydrogenase occur with the use of tocilizumab in COVID-19 patients (Ref. 97). IL-1β is a pro-inflammatory cytokine that plays an important role in the progression of airway inflammation in many viral infections (Ref. 98). In response to alveolar infection by SARS-CoV-2, macrophages secrete IL-1β, which induces fever and leads to fibrosis in the lungs (Ref. 35). Inhibition of IL-1β may be a practical approach to reduce cytokine release syndrome and ARDS progression, as higher levels of IL-1β are associated with the severity of COVID-19 infection in critically ill patients (Ref. 99).
TNF-α is an inflammatory cytokine that triggers leucocyte recruitment to the site of infection in response to SARS-CoV-2 (Ref. 99). IFN-γ is a vital biomarker promoting the pathogenesis of ARDS and acute lung injury. The synergistic effect of TNF-α and IFN-γ leads to inflammatory cell death, tissue damage and mortality in SARS-CoV-2 infection (Ref. 100). Therefore, inhibition of TNF-α and IFN-γ is a promising immunotherapy method to inhibit ARDS progression in severe COVID-19 patients. For example, XPro1595, a soluble TNF-α-neutralising protein that inhibits the interaction between soluble TNF-α and its receptor, is currently evaluated in a clinical trial to treat COVID-19 (NCT04370236). Infliximab, an approved mouse/human chimeric monoclonal antibody, is undergoing clinical trials to evaluate its therapeutic effect on COVID-19 (NCT04922827, NCT04425538 and NCT04593940). GM-CSF is an immunoregulatory cytokine that plays a central role in initiating and maintaining inflammatory diseases. Several clinical trials have considered the use of otilimab (NCT04376684), lenzilumab (NCT04351152), gimsilumab (NCT04351243), mavrilimumab (NCT04492514), TJ003234 (NCT04341116) and sargramostim (NCT04707664) to block GM-CSF as potential treatments for COVID-19.
The complement system could cause some pathophysiological aspects of COVID-19 infections, such as thrombotic microangiopathy and acute kidney injury (Ref. 101). The C5 inhibitor eculizumab has be used as a potential therapeutic agent in the clinical treatment of COVID-19 (NCT04288713). Corticosteroids exert their anti-inflammatory effects by inhibiting the expression of pro-inflammatory transcription factors (Ref. 102). Dexamethasone and other oral/intravenous corticosteroids are the first drugs recommended to treat cytokine storm in patients with severe COVID-19. The study shows that the use of dexamethasone resulted in lower 28-day mortality in COVID-19 patients (Ref. 103). JAK pathway inhibitors such as baricitinib, fedratinib and ruxolitinib are effective anti-inflammatory agents against COVID-19-associated cytokine storm (Ref. 104). Using baricitinib in combination with anti-viral drugs such as lopinavir can reduce the inflammatory response and reduce virus recurrence in COVID-19 patients (Ref. 105).
Cell therapy
MSCs can release various cytokines, such as cell growth factor, prostaglandin E2, GM-CSF, IL-6 and IL-13, to promote phagocytosis and activation of alveolar macrophages (Ref. 106). MSC-based therapy releases immunomodulatory factors and suppresses cytokine storm to improve the microenvironment in ARDS caused by SARS-CoV-2 infection and inhibits pulmonary fibrosis and alveolar fluid accumulation (Ref. 107). Human menstrual blood-derived MSCs were used in an exploratory study to treat severe COVID-19 patients, and the results suggested that menstrual blood-derived MSCs could reduce the mortality of COVID-19 patients (Ref. 108). Furthermore, the potential of COVID-19 treatment can be improved by modifying the extracellular vesicles of MSCs (Ref. 109).
Ligands on virus-infected cells induce NK cells to activate and release IFN-γ and TNF-α, thereby lysing virus-infected cells. Ongoing clinical trials NCT04280224 and NCT04324996 in China will investigate the role of NK cells in severe pneumonia or lymphopoenia triggered by COVID-19. Recent studies have shown that chimeric antigen receptor (CAR)-NK cell therapy exhibits effective antitumor activity in haematologic cancers with minimal side effects (Ref. 110). CAR-NK cells use the scFv of S309, a neutralising antibody that targets the highly conserved region of the SARS-CoV-2 S protein can kill target cells expressing SARS-CoV-2 S protein (Ref. 111).
SARS-CoV-2-specific memory CD8+ T cells were detectable in SARS-CoV-2 convalescent individuals (Ref. 112). Furthermore, the peptide-specific memory CD8+ T cells of SARS-CoV-2 could proliferate when the antigen was re-attacked, suggesting that SARS-CoV-2-specific CD8+ T cells are not exhausted but functional (Ref. 113). Overexpression of inhibitory receptors CTLA4 and PD-1/PD-L1 is a typical characteristic of the exhaustion of CD8+ T cells, leading to decreased effector activity and proliferation ability. The total number of NK and CD8+ T cells was significantly decreased, and the function of NK and CD8+ T cells was exhausted in patients with SARS-CoV-2 infection (Ref. 114). Functional blockade of CTLA4 and PD-1/PD-L1 may unleash exhausted NK and CD8+ T cells, enhancing anti-viral immunity against SARS-CoV-2 (Refs 115, 116).
DC is a crucial bridge between innate and adaptive immunity in anti-viral infection. COVID-19 DCs are shown to promote the secretion of cytokines and chemokines, regulate the inflammatory response and induce the differentiation of CD4+ and CD8+ T cells against pathogens (Ref. 117). In addition, plasma cell-like DCs inhibit viral replication by rapidly producing IFN-I (Ref. 118). It may be helpful to inhibit the functions of DC to control the excessive inflammatory process in severe COVID-19 patients.
Other immunotherapies
Fulminant activation of coagulation and consumption of clotting factors occurs in severe cases of COVID-19, leading to disseminated intravascular coagulation and patient death (Ref. 119). On the other hand, coagulation factors can increase inflammation by acting on specific cell receptors. Considering these two aspects, inhibition of blood clotting may decrease the mortality of COVID-19 patients by reducing the risk of small intravascular thrombosis and severe inflammation. In a retrospective study, COVID-19 patients taking anticoagulants at moderate doses (7 mg/kg per 12 h) during hospitalisation were associated with a significantly lower cumulative incidence of in-hospital death compared with patients not receiving anticoagulants (Ref. 120). Low-dose radiotherapy (LDRT, <100 cGy) has been used to treat a variety of chronic inflammatory diseases. LDRT is reported to have an anti-inflammatory effect by downregulating proinflammatory macrophages and upregulating anti-inflammatory macrophages and NK T cells. Thus, LDRT has the potential to counter the immune reaction incited by COVID-19 (Refs 121, 122). The latest research studies suggest that LDRT is feasible in COVID-19 patients and has shown promising results in COVID-19 pneumonia (Refs 123, 124). IL-15 is expressed by myeloid cells to support T-cell responses, activate NK cells and modulate inflammation, and is a key immunomodulatory cytokine with antiviral properties (Ref. 125). Induction of IL-15 can elicit a robust immune response against SARS-CoV-2 (Ref. 126). IL-15 and IL-21 combination therapy for COVID-19 has also been performed (Ref. 127).
Conclusion
In this review, we have reported several aspects of the immunopathogenesis of COVID-19 and analysed the mechanism of the host immune response against this disease. Among immunotherapeutic approaches for blocking viral attachment or entry, monoclonal antibodies are preferred because of their specificity, purity, low risk of contamination with bloodborne pathogens. A monoclonal antibody cocktail or combination of different monoclonal antibodies recognising different epitopes on the viral surface may increase the efficacy of virus neutralisation.
COVID-19 is an infectious disease that causes an imbalance in the immune system and an inflammatory cytokine storm. Immunosuppressants and anti-inflammatory drugs are potential agents for the treatment of cytokine storm. For example, immunomodulators with IL-6 blockers have the therapeutic potential to specifically inhibit the status of hyperinflammation. Controlling the excessive inflammatory response is crucial to prevent the progression of ARDS in severe cases. On the other hand, enhancing host immunity is crucial to ensure that the host is sufficiently able to fight viral infections. These two aspects are essential concerns of immunotherapy against COVID-19. However, further studies are needed to evaluate the efficacy of immunotherapy in patients infected with COVID-19.
Financial support
This study was supported by the Natural Science Foundation of Guangdong Province, China (XTJ, grant number 2020A1515010981), (XL, 2020A1515110366); the Science and Technology Program of Guangzhou, China (XTJ, grant number 202102080193) and the President Foundation of Nanfang Hospital, Southern Medical University (XL, 2018C013).
Ethical standards
The authors assert that this work does not involve human or animal experimental procedures.
Conflict of interest
None.
References
- 1.Lu H et al. (2020) Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. Journal of Medical Virology 92, 401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C et al. (2020) A novel coronavirus outbreak of global health concern. The Lancet 395, 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization(WHO) Coronavirus Disease (COVID-19) Dashboard. Available at https://covid19.who.int/ (Accessed on October 2021).
- 4.Andersen KG et al. (2020) The proximal origin of SARS-CoV-2. Nature Medicine 26, 450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epidemiology Working Group for Ncip Epidemic Response Chinese Center for Disease Control Prevention (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41, 145–151. [DOI] [PubMed] [Google Scholar]
- 6.Jung SM et al. (2020) Real-time estimation of the risk of death from novel coronavirus (COVID-19) infection: inference using exported cases. Journal of Clinical Medicine 9, 523. doi: 10.3390/jcm9020523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqi HK and Mehra MR (2020) COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. Journal of Heart and Lung Transplantation 39, 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization; COVID-19 clinical management: living guidance. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (Accessed on September 2021).
- 9.Wu A et al. (2020) Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host & Microbe 27, 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantuti-Castelvetri L et al. (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vankadari N and Wilce JA (2020) Emerging Wuhan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerging Microbes & Infections 9, 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amraei R et al. (2021) CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. ACS Central Science 7, 1156–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song W et al. (2018) Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathogens 14, e1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad K et al. (2021) Genomics-guided identification of potential modulators of SARS-CoV-2 entry proteases, TMPRSS2 and cathepsins B/L. PLoS ONE 16, e0256141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khateeb J et al. (2021) Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Critical Care 25, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.South AM et al. (2019) Fetal programming and the angiotensin-(1-7) axis: a review of the experimental and clinical data. Clinical Science 133, 55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H et al. (2020) Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. American Journal of Respiratory and Critical Care Medicine 202, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wruck W and Adjaye J (2020) SARS-CoV-2 receptor ACE2 is co-expressed with genes related to transmembrane serine proteases, viral entry, immunity and cellular stress. Scientific Reports 10, 21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrapp D et al. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang J et al. (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581, 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.V'Kovski P et al. (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nature Reviews Microbiology 19, 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X et al. (2020) Molecular immune pathogenesis and diagnosis of COVID-19. Journal of Pharmaceutical Analysis 10, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karthik K et al. (2020) Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Human Vaccines & Immunotherapeutics 16, 3055–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florindo HF et al. (2020) Immune-mediated approaches against COVID-19. Nature Nanotechnology 15, 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vabret N et al. (2020) Immunology of COVID-19: current state of the science. Immunity 52, 910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiappelli F et al. (2020) COVID-19 immunopathology and immunotherapy. Bioinformation 16, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J et al. (2020) Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55, 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J et al. (2020) Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clinical infectious Diseases 71, 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. COVID-19 natural immunity: scientific brief, 10 May 2021. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Natural_immunity-2021.1 (Accessed on October 2021).
- 31.Ju B et al. (2020) Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584, 115–119. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L et al. (2020) Cause analysis and treatment strategies of ‘recurrence’ with novel coronavirus pneumonia (COVID-19) patients after discharge from hospital. Chinese Journal of Tuberculosis and Respiratory Diseases 43, 281–284. [DOI] [PubMed] [Google Scholar]
- 33.Wiersinga WJ et al. (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 324, 782–793. [DOI] [PubMed] [Google Scholar]
- 34.Wang W et al. (2020) Definition and risks of cytokine release syndrome in 11 critically ill COVID-19 patients with pneumonia: analysis of disease characteristics. Journal of Infectious Diseases 222, 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conti P et al. (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVID-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents 34, 327–331. [DOI] [PubMed] [Google Scholar]
- 36.Chua RL et al. (2020) COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nature Biotechnology 38, 970–979. [DOI] [PubMed] [Google Scholar]
- 37.Costela-Ruiz VJ et al. (2020) SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine & Growth Factor Reviews 54, 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGonagle D et al. (2020) The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmunity Reviews 19, 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanco-Melo D et al. (2020) Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadjadj J et al. (2020) Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye Q et al. (2020) The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. The Journal of Infection 80, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tay MZ et al. (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology 20, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao YC et al. (2002) IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. Journal of Immunology 169, 4288–4297. [DOI] [PubMed] [Google Scholar]
- 45.Chen RF et al. (2006) Role of vascular cell adhesion molecules and leukocyte apoptosis in the lymphopenia and thrombocytopenia of patients with severe acute respiratory syndrome (SARS). Microbes and Infection 8, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao S et al. (2021) Elevated serum levels of progranulin and soluble vascular cell adhesion molecule-1 in patients with COVID-19. Journal of inflammation Research 14, 4785–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellesi S et al. (2020) Increased CD95 (Fas) and PD-1 expression in peripheral blood T lymphocytes in COVID-19 patients. British Journal of Haematology 191, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F et al. (2020) Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. Journal of Infectious Diseases 221, 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavakolpour S et al. (2020) Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunology Letters 225, 31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang G et al. (2020) Prognostic value of leukocytosis and lymphopenia for coronavirus disease severity. Emerging Infectious Diseases 26, 1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang B et al. (2020) Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Frontiers in Molecular Biosciences 7, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lippi G and Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection. Clinical Chemistry and Laboratory Medicine 58, 1131–1134. [DOI] [PubMed] [Google Scholar]
- 53.Qin C et al. (2020) Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious Diseases 71, 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu K et al. (2020) Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang da Xue Xue Bao. Yi Xue Ban 49, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilt TJ et al. (2021) Remdesivir for adults with COVID-19 : a living systematic review for American college of physicians practice points. Annals of Internal Medicine 174, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung IF et al. (2020) Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazewski C et al. (2020) Type I interferon (IFN)-regulated activation of canonical and non-canonical signaling pathways. Frontiers in Immunology 11, 606456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mesev EV et al. (2019) Decoding type I and III interferon signalling during viral infection. 4, 914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ribero MS et al. (2020) Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathogens 16, e1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thoms M et al. (2020) Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 369, 1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liyana A et al. (2019) The emerging role of human TBK1 in virus-induced autophagy. Autophagy 15, 917–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia H et al. (2020) Evasion of type I interferon by SARS-CoV-2. Cell Reports 33, 108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fung SY et al. (2021) SARS-CoV-2 main protease suppresses type I interferon production by preventing nuclear translocation of phosphorylated IRF3. International Journal of Biological Sciences 17, 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mordstein M et al. (2010) Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. Journal of Virology 84, 5670–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazear HM et al. (2019) Shared and distinct functions of type I and type III interferons. Immunity 50, 907–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subbian S and Ramasamy S (2020) Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clinical Microbiology Reviews 34, e00299–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subbian S (2021) The abstruse side of type I interferon immunotherapy for COVID-19 cases with comorbidities. Journal of Respiration 34, 49–59. doi: 10.3390/jor1010005. [DOI] [Google Scholar]
- 68.Pdm A et al. (2020) Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Clinical Trials 9, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang XN et al. (2006) Hyper-activated IRF-1 and STAT1 contribute to enhanced interferon stimulated gene (ISG) expression by interferon alpha and gamma co-treatment in human hepatoma cells. Biochimica et Biophysica Acta 1759, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nezhad FS et al. (2020) Therapeutic approaches for COVID-19 based on the dynamics of interferon-mediated immune responses. Preprints, 1–26. doi: 10.20944/preprints202003.0206.v2 [DOI] [Google Scholar]
- 71.Li C et al. (2021) Effect of a genetically engineered interferon-alpha versus traditional interferon-alpha in the treatment of moderate-to-severe COVID-19: a randomised clinical trial. Annals of Medicine 53, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Gool AR et al. (2003) Neuropsychiatric side effects of interferon-alfa therapy. Pharmacy World & Science: PWS 25, 11–20. [DOI] [PubMed] [Google Scholar]
- 73.Seow J et al. (2020) Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiology 5, 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Briggs N et al. (2021) Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. 16, e0254453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Candia PD et al. (2021) Effect of time and titer in convalescent plasma therapy for COVID-19. iScience 24, 102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cross RW et al. (2021) Use of convalescent serum reduces severity of COVID-19 in nonhuman primates. Cell Reports 34, 108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acosta-Ampudia Y et al. (2021) COVID-19 convalescent plasma composition and immunological effects in severe patients. Journal of Autoimmunity 118, 102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blake A et al. (2021) Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infectious Diseases 7, ofab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O'Brien MP et al. (2021) Subcutaneous REGEN-COV antibody combination for COVID-19 prevention. medRxiv. doi: 10.1101/2021.06.14.21258567 [DOI] [Google Scholar]
- 80.Koenig PA et al. (2021) Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science 371, eabe6230. doi: 10.1126/science.abe6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rojas M et al. (2020) Convalescent plasma in COVID-19: possible mechanisms of action. Autoimmunity Reviews 19, 102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Concepción MLRDL et al. (2021) High-dose intravenous immunoglobulins might modulate inflammation in COVID-19 patients. Life Science Alliance 4, e202001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali S et al. (2021) Production of hyperimmune anti-SARS-CoV-2 intravenous immunoglobulin from pooled COVID-19 convalescent plasma. Immunotherapy 13, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chagla Z (2021) The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥7 days after the 2nd dose. Annals of Internal Medicine 174, JC15. [DOI] [PubMed] [Google Scholar]
- 85.Hoffmann D et al. (2021) CVnCoV and CV2CoV protect human ACE2 transgenic mice from ancestral B BavPat1 and emerging B.1.351 SARS-CoV-2. Nature Communications 12, 4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amanat F et al. (2021) SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 184, 3936–3948 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y et al. (2021) Potent RBD-specific neutralizing rabbit monoclonal antibodies recognize emerging SARS-CoV-2 variants elicited by DNA prime-protein boost vaccination. Emerging Microbes & Infections 10, 1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou H et al. (2021) B.1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. bioRxiv, doi: 10.1101/2021.03.24.436620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heath PT et al. (2021) Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. New England Journal of Medicine 385, 1172–1183. doi: 10.1056/NEJMoa2107659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu S et al. (2021) Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. The Lancet. Infectious Diseases 21, 1654–1664. doi: 10.1016/S1473-3099(21)00396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J et al. (2021) Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerging Microbes and Infections 10, 1598–1608. doi: 10.1080/22221751.2021.1957401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Routhu NK et al. (2021) A modified vaccinia Ankara vector-based vaccine protects macaques from SARS-CoV-2 infection, immune pathology, and dysfunction in the lungs. Immunity 54, 542–556 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen L Y C et al. (2021) Assessing the importance of interleukin-6 in COVID-19. The Lancet. Respiratory Medicine 9, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.FDA Approves Phase III Clinical Trial of Tocilizumab for COVID-19 Pneumonia https://www.cancernetwork.com/view/fda-approves-phase-iii-clinical-trial-tocilizumab-covid-19-pneumonia Accessed on September 2021.
- 95.Investigators R-C et al. (2021) Interleukin-6 receptor antagonists in critically ill patients with COVID-19. New England Journal of Medicine 384, 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu B et al. (2020) Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? Journal of Autoimmunity 111, 102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morrison A R et al. (2020) Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. Journal of Medical Virology 92, 1791–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinon F et al. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- 99.Price KN et al. (2020) COVID-19 and immunomodulator/immunosuppressant use in dermatology. Journal of the American Academy of Dermatology 82, e173–ee75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karki R et al. (2021) Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184, 149-168 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahajan R et al. (2020) Eculizumab treatment for renal failure in a pediatric patient with COVID-19. Journal of Nephrology 33, 1373–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramesh G et al. (2015) Anti-inflammatory effects of dexamethasone and meloxicam on Borrelia burgdorferi-induced inflammation in neuronal cultures of dorsal root ganglia and myelinating cells of the peripheral nervous system. Journal of Neuroinflammation 12, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Horby P et al. (2021) Dexamethasone in hospitalized patients with COVID-19. New England Journal of Medicine 384, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richardson P et al. (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 395, e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stebbing J et al. (2020) COVID-19: combining antiviral and anti-inflammatory treatments. The Lancet. Infectious Diseases 20, 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou T et al. (2021) Challenges and advances in clinical applications of mesenchymal stromal cells. Journal of Hematology & Oncology 14, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jamshidi E et al. (2021) Proposed mechanisms of targeting COVID-19 by delivering mesenchymal stem cells and their exosomes to damaged organs. Stem Cell Reviews and Reports 17, 176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu X et al. (2021) Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clinical and Translational Medicine 11, e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Racchetti G and Meldolesi J (2021) Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines 9, 667. doi: 10.3390/biomedicines9060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gang M et al. (2020) CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 136, 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma M et al. (2021) CAR-NK cells effectively target the D614 and G614 SARS-CoV-2-infected cells. bioRxiv. doi: 10.1101/2021.01.14.426742. [Google Scholar]
- 112.Schulien I et al. (2021) Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nature Medicine 27, 78–85. [DOI] [PubMed] [Google Scholar]
- 113.Rha M S et al. (2021) PD-1-expressing SARS-CoV-2-specific CD8(+) T cells are not exhausted, but functional in patients with COVID-19. Immunity 54, 44–52 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng M et al. (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology 17, 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Awadasseid A et al. (2021) Potential protective role of the anti-PD-1 blockade against SARS-CoV-2 infection. Biomedicine & Pharmacotherapy 142, 111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Riva G et al. (2020) COVID-19: room for treating T cell exhaustion? Critical Care (London, England) 24, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alamri A et al. (2021) A missing link: engagements of dendritic cells in the pathogenesis of SARS-CoV-2 infections. International Journal of Molecular Sciences 22, 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cervantes-Barragan L et al. (2007) Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood 109, 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang N et al. (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis: JTH 18, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meizlish ML et al. (2021) Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. American Journal of Hematology 96, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta S et al. (2021) Low dose lung radiotherapy for COVID-19 pneumonia: a potential treatment. Respiratory Medicine 186, 106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodel F et al. (2007) Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. International Journal of Radiation Biology 83, 357–366. [DOI] [PubMed] [Google Scholar]
- 123.Sharma DN et al. (2021) Low-dose radiation therapy for COVID-19 pneumonia: a pilot study. The British Journal of Radiology 94, 20210187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhawan G et al. (2020) Low dose radiation therapy as a potential life saving treatment for COVID-19-induced acute respiratory distress syndrome (ARDS). Radiotherapy & Oncology 147, 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Verbist KC and Klonowski KD (2012) Functions of IL-15 in anti-viral immunity: multiplicity and variety. Cytokine 59, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knudson KM et al. (2019) Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. Journal for Immunotherapy of Cancer 7, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilz SW (2021) A clinical trial of IL-15 and IL-21 combination therapy for COVID-19 is warranted. Cytokine & Growth Factor Reviews 58, 49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]