Abstract
Condensation of the chromatin fiber and transcriptional inhibition during mitosis is associated with the redistribution of many DNA- and chromatin-binding proteins, including members of the high-mobility-group N (HMGN) family. Here we study the mechanism governing the organization of HMGN proteins in mitosis. Using site-specific antibodies and quantitative gel analysis with proteins extracted from synchronized HeLa cells, we demonstrate that, during mitosis, the conserved serine residues in the nucleosomal binding domain (NBD) of this protein family are highly and specifically phosphorylated. Nucleosome mobility shift assays with both in vitro-phosphorylated proteins and with point mutants bearing negative charges in the NBD demonstrate that the negative charge abolishes the ability of the proteins to bind to nucleosomes. Fluorescence loss of photobleaching demonstrates that, in living cells, the negative charge in the NBD increases the intranuclear mobility of the protein and significantly decreases the relative time that it is bound to chromatin. Expression of wild-type and mutant proteins in HmgN1−/− cells indicates that the negatively charged protein is not bound to chromosomes. We conclude that during mitosis the NBD of HMGN proteins is highly phosphorylated and that this modification regulates the interaction of the proteins with chromatin.
During mitosis the chromatin fiber is fully condensed and most of the transcriptional activity is inhibited (24, 34). These processes are associated with modifications in the tail of the core histones (11, 18, 23, 62) and with changes in the location of DNA and chromatin binding proteins (31, 41). Components of the transcriptional apparatus, transcription factors (35, 50, 51), and chromatin remodeling complexes (43, 54), are displaced from mitotic chromatin, while proteins that promote condensation seem to be enriched in this chromatin (14, 26, 32, 58).
Mitotic chromosomes are also depleted of high-mobility group B (HMGB) (HMG1 and -2) and HMGN (HMG14 and -17) (7, 21, 27, 29, 53) groups of proteins that serve as architectural elements and affect the structure and function of chromatin (5, 10). (Note that the nomenclature of the high-mobility-group proteins has been recently revised; HMGN1 was HMG-14, and HMGN2 was HMG-17. For a full description see the HMG Chromosomal Proteins Nomenclature Home Page http://www.informatics.jax.org/mgihome/nomen/genefamilies /hmgfamily.shtml.) HMGN proteins are the only nuclear proteins that specifically recognize the generic structure of the 147-bp nucleosome core particle (8). The proteins bind cooperatively to nucleosomes and form complexes containing two molecules of either HMGN1 or HMGN2 (49). Several types of experiments suggest that HMGN1 and HMGN2 proteins decompact chromatin and enhance transcription from chromatin, but not DNA, templates (reviewed in references 5 and 10). The proteins decompact the chromatin fiber by targeting two main elements known to compact chromatin: histone H1 (1, 19) and the amino terminus of histone H3 (60).
The intracellular organization of HMGN1 and -N2 proteins is dynamic (46) and related to both transcriptional activity (28) and cell cycle events (27). In cells that are highly active in transcription, the proteins are finely dispersed throughout the entire nucleus and colocalize with sites of active transcription. Upon inhibition of transcription, the proteins are found in large aggregates. The proteins colocalize with DNA in interphase and prophase but not in metaphase or early anaphase. At the end of mitosis, concomitantly with the appearance of the nuclear envelope, the proteins are actively imported into the nucleus and associate with the DNA (27).
The mechanisms regulating the intracellular localization of HMGN proteins are not known. Both HMGN1 and HMGN2 are acetylated (3, 25, 56, 57) and phosphorylated (2, 13, 39, 55; see also references 20 and 30), modifications which are known to affect their target interactions and cellular localization (3, 25, 39, 40, 44, 55).
We address here the mechanisms whereby HMGN1 and -N2 proteins are prevented from binding to mitotic chromosomes. We demonstrate that the nucleosomal binding domain (NBD) of HMGN1 and -N2 proteins is highly and specifically phosphorylated only during mitosis and that this phosphorylation abolishes the ability of the proteins to bind to chromatin. We use point mutants to demonstrate that both in vivo and in vitro, the negative charge in the NBD of HMGN proteins is directly responsible for their inability to bind to chromatin. We demonstrate that, in living cells, the chromatin residence time of HMGN1 is affected by the charge of the NBD, thereby providing the first experimental evidence that this protein domain is functional in vivo. We suggest that the specific phosphorylation of the NBD of HMGN proteins serves to prevent the interaction of these proteins with their chromatin targets during mitosis.
MATERIALS AND METHODS
Generation and characterization of site-specific antibodies.
The phosphopeptide PKRKVS(P)SAEGA was used to elicit anti-S6P, an antibody against HMGN1 phosphorylated at serine 6. The phosphopeptide PKRRS(P)ARLS(P)AKP was used to elicit anti-NBD2P, an antibody specific for the phosphorylated nucleosome binding domains of HMGN1 and -N2. The antibodies were affinity purified by sequential purifications on columns containing either nonphosphorylated or phosphorylated peptide (Quality Controlled Biochemicals, Inc.). The affinity-purified antibodies were characterized by enzyme-linked immunosorbent assay and Western blotting analysis using recombinant HMG-14 and -17 phosphorylated in vitro at specific sites.
HMGN1 and -N2 proteins.
Recombinant wild-type and mutant HMGN1 and -N2 were generated and purified as previously described (6). HMGN1 and -N2 point mutants were generated from the cDNA constructs by site-directed mutagenesis using the Quickchange Site-Directed Mutagenesis Kit (Stratagene). The mutant constructs were subsequently subcloned into either the pet30A expression vector for bacterial expression or the pCI-neo (Promega) or pEGFP-N (Clontech) vectors for mammalian expression.
In vitro phosphorylation of the recombinant HMGN1 and -N2 proteins.
Recombinant HMGN1 and -N2 and recombinant mutated HMGN1S6A were phosphorylated with Rsk2 as recommended by the manufacturer (Upstate Biotechnology), with protein kinase A (PKA) in buffer A (45 mM Tris-Cl, 45 mM boric acid, 10 mM MgCl2, 100 μM ATP, 2 mM CaCl2, 0.5 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 10% glycerol) and with protein kinase C (PKC; Upstate Biotechnology) in buffer A supplemented with a lipid coactivator solution at a final concentration of 50 μg of phosphatidyl serine and 50 μg of diacylglycerol per ml.
Electrophoresis and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was done according to the Laemmli protocol, and acid-urea electrophoresis was performed as previously described with small modifications (37). After electrophoresis, gels were stained with Coomassie blue for densitometric analysis or proteins were transferred using a semidry system onto nitrocellulose membrane for Western blot detection performed with the ECL Plus Kit (Amersham).
Core particle mobility shift assays.
Mobility shift assays to test for the binding of HMG proteins to core particles were done in 2× Tris-buffered saline buffer exactly as described previously (47).
Preparation of HMGN1 and -N2 proteins from the synchronized HeLa cell cultures.
HeLa S3 cells (American Type Culture Collection) were grown in suspension in Eagle essential medium with the Joklik modification (Sigma), supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 1% of penicillin-streptomycin. Logarithmically growing cells (5 × 105 to 6 × 105 cells/ml) were synchronized in S phase by growing the cells in medium supplemented with 5 mM thymidine for 24 h and then allowing them to recover in medium without thymidine for 4 h. G2-phase cells were generated by treatment with thymidine as in the S-phase cells, except that 8 h was allowed for the recovery. HeLa cells were enriched for M phase by an 18.5-h treatment with 0.4 μg of nocodazole per ml (33). The fraction of mitotic cells was determined microscopically using Giemsa (LabChem, Inc.)-stained metaphase spreads. Flow cytometry was used to determine the degree of cell synchronization. Cell cultures enriched for each phase of the cell cycle were harvested by centrifugation, and the HMGN1 and -N2 proteins were extracted and purified as previously described (3).
Transfection into HmgN1−/− fibroblasts and HeLa cells.
HmgN1−/− fibroblasts were generated from 13-day embryos of mice bearing a targeted disruption of the HmgN1 gene. Northern, Western, and immunocytochemical analyses demonstrated that the mice and cells derived from these mice do not express HMG-14 mRNA and do not contain HMG-14 protein (Y. Birger, unpublished results). Plasmids expressing either native or mutant human HMGN1 were transfected into the fibroblasts by the Lipofectamine 2000 method (Gibco-BRL). Cells expressing the proteins were detected by confocal immunofluorescence microscopy. Plasmid expressing either HMGN1-green fluorescence protein (GFP) fusion protein or HMGN1S20,24E-GFP were transfected into HeLa cells also by the Lipofectamine 2000 method.
Fluorescence loss in photobleaching (FLIP).
HeLa cells expressing either HMGN1-GFP wild-type protein or HMGN1S20,24E-GFP were used for the experiment. A spot ∼1 μm in diameter was repeatedly bleached at intervals of 5 s with a 250-ms laser pulse using the 488-nm laser line of an Ar laser of 20-mW nominal output at a 100% intensity. The fluorescence intensity in areas distant from the bleached spot was measured after each bleach pulse. As a control, the fluorescence intensity in a neighboring, unbleached nucleus was measured. Values represent averages from at least seven cells ± the standard deviation.
Confocal microscopy.
Cells grown on coverslips in Dulbecco modified Eagle medium (Life Technologies, Inc.) supplemented with 10% FBS (Gibco-BRL) at 37°C in 5% CO2 incubator were washed with phosphate-buffered saline (PBS), fixed with 4% formaldehyde in PBS for 10 min at room temperature, washed with PBS, and incubated for 20 min in PBS containing 0.1% Triton X-100–1% FBS–0.1% NaN3 (TNBS buffer). The primary antibody (at about 1 μg/ml in TNBS) treatment was done overnight in room temperature in a moist chamber. The coverslips were washed with PBS and incubated with secondary antibody labeled with either Texas red or fluorescein for 2 h at room temperature in a moist chamber. DNA was stained with TO-PRO3 (Molecular Probes). Following the final wash steps, coverslips were inverted onto glass slides using the ProLong anti-fade reagent (Molecular Probes) as the mounting medium.
Fluorescent cells were examined with an epifluorescence microscope (Optiphot; Nikon) equipped with a confocal system (MRC-1024; Bio-Rad Laboratories, Hercules, Calif.). Sequential excitation at 568, 488, and 647 nm was provided by a 15-mW krypton-argon laser (American Laser, Salt Lake City, Utah), and sequential images were collected using LaserSharp software (Bio-Rad).
RESULTS
The NBD of HMGN1 and -N2 proteins is highly phosphorylated during mitosis.
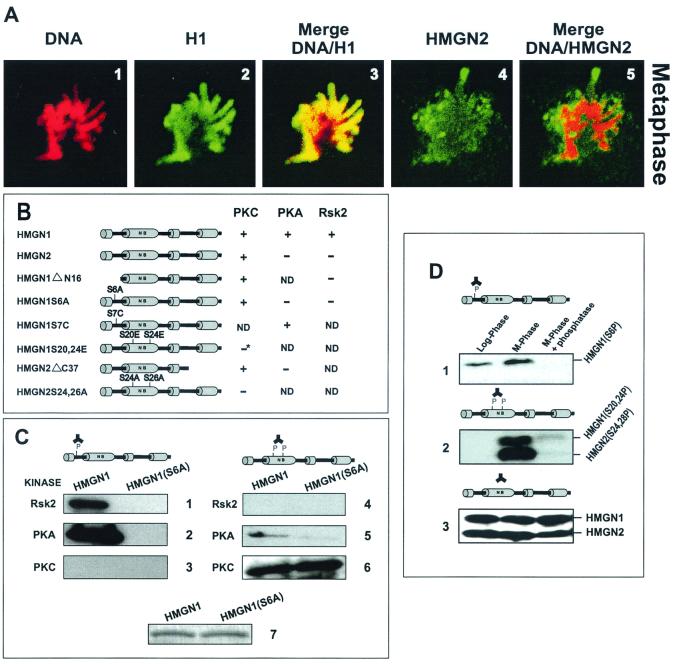
We previously noted that Hep2 mitotic chromosomes are devoid, or highly depleted of HMGN1 and -N2 (HMG-14 and -17) proteins (27). To better understand the molecular mechanism involved in the displacement of HMGN1 and -N2 from mitotic chromatin, we first studied the mitotic location of the proteins in HeLa cells, which have a high content of HMGN1 and -N2 proteins (9). Confocal immunofluorescent microscopy with these cells indicates that, while during interphase the proteins are dispersed throughout the entire nucleus and colocalize with DNA (not shown), HeLa mitotic chromosomes are devoid of HMGN2 (and HMGN1; not shown). In metaphase the proteins are dispersed throughout the entire cell (Fig. 1A4), and their location is clearly distinct from that of the mitotic DNA (Fig. 1A5). In contrast, the linker histone H1, whose nucleosomal location overlaps with that of HMGN1 and -N2 (1), colocalizes with the DNA throughout the entire cell cycle and is present in mitotic chromosomes (Fig. 1A1 to A3). Taken together with previous results (27), these data indicate that the intracellular organization of HMGN1 and -N2 changes during the cell cycle and that the proteins are not present in mitotic chromosomes.
Since numerous proteins are phosphorylated during mitosis, we next examined whether HMGN1 and -N2 are similarly modified and whether this modification affects their binding to chromatin. HMGN1 contains 10 serine residues located at positions 6, 7, 20, 24, 44, 45, 85, 88, and 98, while HMGN2 contains only two serines located at positions 24 and 28. Earlier studies on the location of phosphorylated residues in HMGN1 and -N2 gave conflicting results; however, recent studies suggest that the major phosphorylation sites of this family are serine 6 of HMGN1 and the two serine residues in the conserved NBD, located at positions 20 and 24 in HMGN1 and at positions 24 and 28 in HMGN2 (2, 39, 55). We generated antibodies against these sites: anti-S6P specific for human HMGN1 phosphorylated at serine 6 and anti-NBD2P specific for HMGN1 and -N2 phosphorylated in the NBD.
(i) Characterization of the antibodies to phosphorylated HMGN1 and -N2 proteins.
To test the specificity of the antibodies, we treated recombinant native and mutant proteins with specific kinases. It was previously noted that PKA preferentially phosphorylates serine 6 in human HMGN1 (61), that RSK2 specifically phosphorylates serine 6 of mouse HMGN1 (59), and that PKC phosphorylate both HMGN1 and HMGN2 with similar efficiency (45). As schematically outlined in Fig. 1B, we found that PKA and RSK2 also efficiently phosphorylate recombinant human HMGN1, as measured by 32P incorporation after labeling with [γ-32P]ATP. RSK2 did not incorporate 32P into an HMGN1 point mutant in which serine 6 was mutated to alanine, into a deletion mutant of HMGN1 lacking the first 16 amino acids, or into HMGN2 protein. PKA efficiently phosphorylated a point mutant of HMGN1 in which serine 7 was mutated to cysteine. Thus, RSK2 and PKA phosphorylate only serine 6 of HMGN1. PKC incorporated 32P into wild-type HMGN1, wild-type HMGN2, a deletion mutant of HMGN1 lacking the first 16 amino acids, and an HMGN2 mutant lacking the C-terminal 37-amino-acid residues. In contrast, PKC did not phosphorylate an HMGN2S24,26A, a double mutant in which the only serines present in the protein, located in the NBD, were mutated to alanine. Likewise, the amount of 32P incorporated into the HMGN1S20,24E mutant, in which the two serines located in the NBD were mutated to glutamic acid, was less than 20% of that incorporated into the wild-type protein. We assume that the small amount of phosphate was incorporated into the seven serines present in the C-terminal region of the protein. Thus, PKC specifically phosphorylates the serine residues in the conserved NBDs of HMGN1 and -N2 (Fig. 1B).
FIG. 1.
(A) HMGN1 and -N2 proteins do not colocalize with DNA during metaphase. Confocal laser scanning immunofluorescence of a HeLa metaphase was done, with the antibodies indicated at the top of the panels. The images were recolorized to facilitate comparison of the mitotic localization of the proteins. (B) Schematic diagram of the phosphorylation pattern of recombinant proteins by various kinases. Phosphorylation was determined by incorporation of 32P into the various proteins. HMGN1ΔN16, deletion mutant lacking the first 16 amino acids; HMGN1S6A, a point mutant in which the serine at position 6 was mutated to alanine; HMGN1S7C, a point mutant in which the serine at position 7 was mutated to cysteine; HMGN1S20,24E, a double mutant in which the serines at position 20 and 24 were mutated to glutamic acid (the result marked by an asterisk was due to the presence of serines in the C-terminal region of HMGN1; this mutant incorporated >20% of radioactivity compared to the wild type); HMGN2ΔC37, deletion mutant lacking the 37 C-terminal amino acids; HMGN2S24,26A, a double mutant in which the serines at positions 24 and 26 were mutated to alanine. The plus and minus signs indicate whether the kinases indicated at the top incorporated radioactivity into the proteins. ND, not determined. (C) Characterization of antibodies to specific phosphorylation sites in HMGN1 and -N2 proteins. Western analysis of either wild-type HMGN1 or the HMGN1 Ser6Ala mutant was done as follows: panels 1 to 3, anti-Ser6P (anti-HMGN1 phosphorylated at serine 6); panels 4 to 6, anti-NBD2P (elicited against HMGN1 and -N2 phosphorylated in the two conserved serines present in the NBD); or 7, the corresponding Coomassie blue-stained gel demonstrates equal loading. The kinases used to phosphorylate the proteins are indicated to the left of each panel. The phosphorylation sites in the HMG proteins are diagrammed above the panels. (D) Western analysis of proteins extracted from either logarithmically growing or mitotic cells with the following: 1, anti-Ser6P; 2, anti-NBD2P; or 3, a mix of anti-HMGN1 and anti-HMGN2.
Anti-S6P antibodies reacted with RSK2 and PKA modified native HMGN1 but not with the HMGN1 S6A point mutant or with recombinant HMGN2 treated with the same enzymes. The antibody also did not bind to PKC-treated proteins (Fig. 1C). Thus, the anti-S6P we elicited specifically recognizes human HMGN1 protein phosphorylated at serine 6. Anti-NBD2P reacted only with PKC-modified HMGN1 and HMGN2 (not shown) and not with RSK2-modified proteins (Fig. 1C). PKA phosphorylated the NBD with a very low efficiency and gave a slight background with the anti-NBD2P. Thus, the antibodies reacted with phosphorylated, but not with unphosphorylated HMGN1 and -N2 proteins, in a site-specific manner (Fig. 1 and 2). These highly characterized antibodies are convenient reagents for various studies on the site-specific phosphorylation of HMGN1 and -N2 proteins.
FIG. 2.
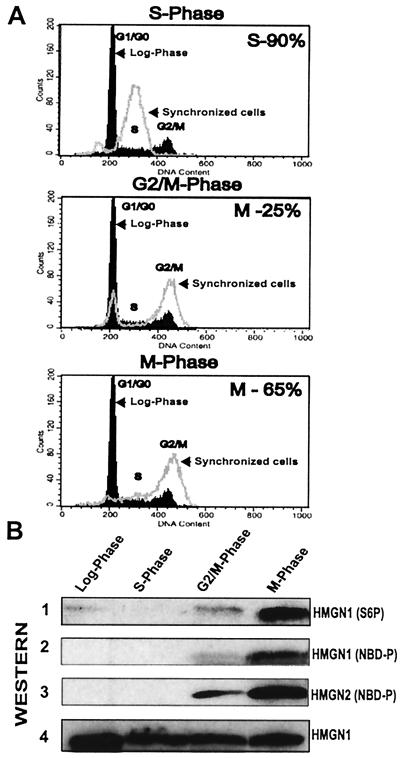
Cell cycle-dependent phosphorylation of HMGN1 and -N2. (A) Flow cytometry analyses. Dark black tracing, logarithmically growing cells; gray tracing, synchronized cells. (B) Western analyses with proteins extracted from the stages indicated at the top with: 1, anti-Ser6P; 2 and 3, anti-NBD2P; 4, anti-HMGN1 (demonstrates equal loading).
(ii) Mitotic phosphorylation of HMGN1 and -N2 proteins.
To test the phosphorylation levels of HMGN1 and -N2 proteins during mitosis, we performed immunoblot analysis with proteins obtained from either nocodazole-arrested or logarithmically growing HeLa cells. Anti-NBD2P produced a strong signal with both HMGN1 and HMGN2 proteins extracted from mitotic cells but not with the HMGN proteins prepared from either phosphatase-treated mitotic extracts or logarithmically growing cells (Fig. 1D). Thus, the NBDs of both HMGN1 and HMGN2 are highly phosphorylated during mitosis. Likewise, the phosphorylation level of serine 6 in HMGN1 is significantly elevated during mitosis (Fig. 1D1).
Western analysis of HMGN protein extracted from synchronized HeLa cell cultures (Fig. 2) provides additional evidence that the NBDs of the HMGN proteins are highly phosphorylated only during mitosis. The site-specific anti-NBD2P antibodies reacted strongly only with HMGN1 and -N2 proteins extracted from mitotic cells, when 65% of the cells were in metaphase as determined by microscopic examination (Fig. 2B). In the G2/M stage of the cycle, when only about 25% of the cells were in metaphase, the antibody signal was significantly lower and can be attributed to the fraction of the mitotic cells in the overall cell population. A similar, cell cycle-related pattern of phosphorylation was observed with antibodies to serine 6 in HMGN1, except that low level of phosphorylation in serine 6 could be detected in logarithmically growing cells (Fig. 2B1). This phosphorylation may be related to specific gene expression (2). Neither antibody reacted with proteins extracted from a culture in which 90% of the cells were in the S phase of the cell cycle.
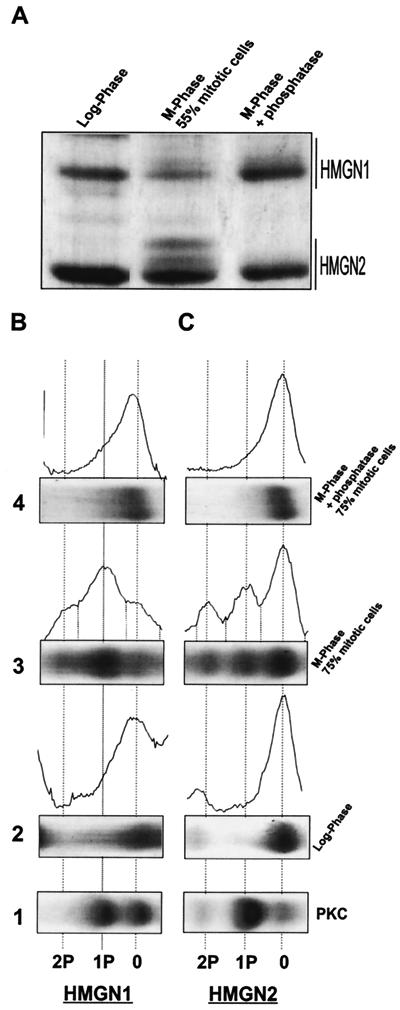
To quantify the relative levels of HMGN phosphorylation, the phosphorylated forms of the proteins were resolved by electrophoresis in long acid urea gels, a fractionation system that separates proteins according to both size and charge (37). HMGN proteins extracted from the mitotic cells cultures contained slow-migrating bands, with mobilities indistinguishable from the PKA- and PKC-modified recombinant proteins. These bands were not observed in HMG preparation obtained from logarithmically growing cells or in proteins isolated from mitotic cells and treated with phosphatase (Fig. 3). Densitometric analysis revealed a high degree of correlation between the level of phosphorylated HMGN1 and -N2 proteins and the percentage of metaphase cells (Table 1). When 55% of the cells were in metaphase (acid urea gel shown in Fig. 3A), 34% of the HMGN1 and 26% of the HMGN2 were monophosphorylated, and 15% of the HMGN1 and 20% of the HMGN2 were diphosphorylated. Part of the HMGN1 phosphorylation is due to the modification at serine 6, which also occurs in the logarithmically growing cells (Fig. 1 and 2). In extracts prepared from cultures in which 75% of the cells were in metaphase, 75% of the HMGN1 and 58% of the HMGN2 were phosphorylated (Table 1). We estimate that the NBDs of at least 80% of the proteins are phosphorylated during mitosis.
FIG. 3.
High level of phosphorylation of the HMGN1 and -N2 proteins during mitosis. (A) Acid urea gel of proteins extracted from either logarithmically growing cells (left panel), HeLa cultures in which 55% of the cells were mitotic (center panel), and phosphatase-treated proteins isolated from 55% mitotic cells (right panel). The migration areas of the HMGN1 and HMGN2 bands are indicated on the right side of the gel. (B) Quantitative analysis of mitotic phosphorylation. High-resolution acid urea gels and corresponding densitometric scans of proteins were isolated either from logarithmically growing cells (panel 2), from 75% mitotic HeLa cells (panel 3), or phosphatase-treated proteins isolated from these mitotic cells (panel 4). The elec- trophoretic profile of PKC phosphorylated recombinant proteins, which served as markers, are shown in panel 1. Dotted lines show the positions of nonmodified mono- and diphosphorylated proteins.
TABLE 1.
Content of phosphorylated HMGN1 and -N2 proteins in mitotic HeLa cellsa
| Expt no. | % cells in mitosis | % HMGN1
|
% HMGN2
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-P | 1-P | 2-P | ΣP | 0-P | 1-P | 2-P | ΣP | ||
| 1 | 55 | 51 | 34 | 15 | 49 | 54 | 26 | 20 | 46 |
| 2 | 70 | 34 | 43 | 23 | 66 | 47 | 30 | 28 | 53 |
| 3 | 75 | 27 | 46 | 27 | 73 | 42 | 37 | 21 | 58 |
| Avg | 67 | 37 | 41 | 22 | 63 | 48 | 31 | 21 | 52 |
Proteins were extracted from nocodasole-treated HeLa cells and resolved on an acid urea gel (see Fig. 3). The relative amounts of each phosphorylated species were quantified by densitometric scanning and ImageQuant (Molecular Dynamics) analysis. The values shown are from three separate experiments. P, phosphate group; ΣP, sum of mono- and diphosphorylated proteins (1-P and 2-P, respectively).
Phosphorylation of the NBD abolishes the binding of HMGN1 and -N2 to nucleosome core particles.
The high level of mitotic phosphorylation of the NBD of HMGN1 and -N2 raises the possibility that this modification prevents the binding of the proteins to mitotic chromatin. The primary binding target of HMGN1 and -N2 proteins in chromatin is the nucleosome core particle. At physiological ionic strengths, each nucleosome core binds two molecules of either HMGN1 or HMGN2 to form a specific complex that can be detected by mobility shift assays (49). The interaction of HMGN with nucleosomes involves specific contact not only with the nucleosomal DNA but also with the amino termini of the core histones (17, 60). We modified the proteins with the site-specific kinases using trace amounts of 32P and used mobility shift assays to assess the effect of phosphorylation on nucleosome binding.
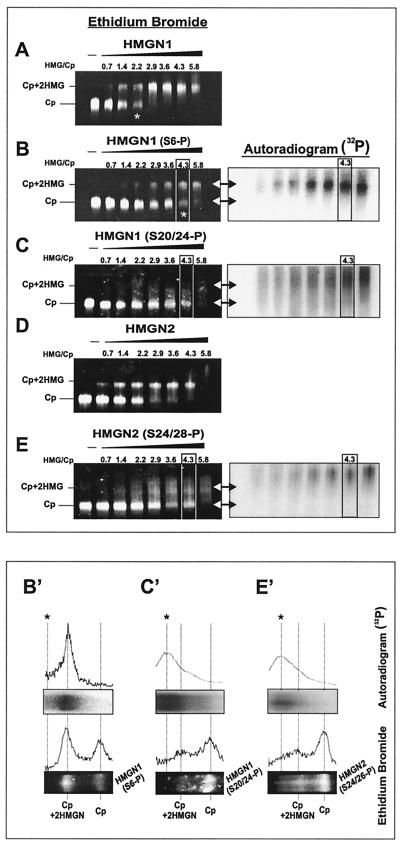
Since the kinases may not fully phosphorylate all of the protein and the reactions may not go to completion, the 32P serves as a specific trace of the labeled protein. Indeed, acid urea gels indicated that about 65% of the protein is phosphorylated (not shown). Thus, the mobility shift reactions contain nucleosome cores visualized by ethidium bromide staining, phosphorylated protein which is visualized by autoradiography, and nonmodified protein. To accurately visualize the location of the various complexes formed, we scanned the lanes in which the original ratio of HMGN to core particle input was 4.3 (boxed lanes in Fig. 4).
FIG. 4.
Phosphorylation in the NBD abolishes the binding of HMGN1 and -N2 to core particles. Equal amounts of nonphosphorylated or either HMGN1 or HMGN2 protein trace labeled with [32P]ATP were incubated with 1 μg of the core particles (Cp) at the molar ratios indicated at the top of each lane. (A) Wild-type HMGN1. (B) HMGN1 phosphorylated at serine 6. (C) HMGN1 phosphorylated in the NBD. (D) Wild-type HMGN2. (E) HMGN2 phosphorylated in the NBD. The mobilities of the HMG-core particle complex and of the core particle are indicated at the left of each panel. The asterisk indicates the molar concentration at which 50% of the cores were shifted. The location of the phosphorylated proteins was visualized by autoradiography (right side of panels B, C, and E). The double-headed arrows indicate the mobility of the Cp and the HMG-Cp complex. Panels B′, C′, and E′ are scans and images of the boxed areas (HMG/Cp = 4.3) in the respective panels. Note that the phosphorylation at serine 6 of HMGN1 reduces, but does not abolish, the binding of the protein to nucleosomes. Part of the radioactivity comigrates with the shifted core particles. The HMGN1 and HMGN2 phosphorylated in the NBD failed to generate an HMG-Cp complex. The line with the asterisk indicates the position of the nonspecific aggregates.
HMGN1 and HMGN2 proteins phosphorylated in the NBD by PKC did not bind to nucleosome cores and failed to produce specific mobility shifts even at high protein concentrations at which all of the nucleosomes are fully shifted by the unmodified proteins (compare panels A and C and panels D and E in Fig. 4). Autoradiographic analysis did not detect any radioactivity specifically associated with the HMGN-nucleosome complex. Note that panel E contains a small amount of Cp+2HMGN2 complex formed by the nonphosphorylated protein in the reaction mixture. As expected, this complex does not overlap with the radioactivity, i.e., it does not contain phosphorylated protein. The phosphorylated protein smears throughout the gel and forms a small amount of nonspecific aggregates with a mobility lower than that of the Cp+2HMGN complexes (Fig. 4C and E, right panels, and Fig. 4C′ and E′). In contrast, phosphorylation of HMGN1 at position 6 by RSK2 reduces, but does not abolish, the ability of the protein to interact with nucleosome cores. The RSK2-modified protein produced specific complexes that contained radioactive protein (Fig. 4B and B′). Thus, the inability of the PKC-treated HMGN1 and HMGN2 (Fig. 4C and C′ and Fig. 4E and E′, respectively) to bind to nucleosome cores is due to specific phosphorylation of the proteins in the NBD.
We conclude therefore that phosphorylation of the NBD serves as a mechanism to prevent the interaction of HMGN1 and -N2 with nucleosomes.
In vivo, a negatively charged NBD prevents the binding HMGN1 to chromatin.
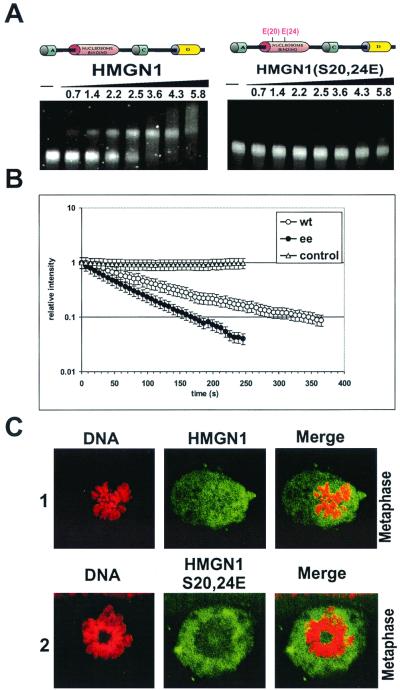
Although it is generally accepted that the HMGN proteins interact with chromatin mainly through their NBDs, this point has never been examined in intact, living cells. To critically examine the hypothesis that the introduction of two negative charges into the NBD is sufficient to abolish the binding of the protein to chromatin in living cells, we generated a double point mutant of HMGN1, S20,24E, in which the two serine residues in the NBD were mutated to glutamic acid. Mobility shift assays with the bacterially expressed proteins revealed that, just like the phosphorylated wild-type proteins, the S20,24E double mutant does not bind to nucleosomes (Fig. 5A [compare with Fig. 4]). Thus, the negative charge in the NBD abolishes the interaction of the HMGN1 and -N2 proteins with nucleosome cores, in spite of the fact that the binding of HMGN protein to nucleosome core requires the presence of the positively charged histone tails (17, 60).
FIG. 5.
A negative charge in the NBD inhibits binding of HMGN1 to chromatin either in vitro or in vivo. (A) Mobility shift assays with wild-type HMGN1 and with the HMGNS20,24E double mutant. The HMG/Cp molar ratios are indicated on the top of the lanes. Note that the double-mutant HMGN1 S20,24E does not form a complex with core particles. (B) FLIP analysis in HeLa cells expressing either HMGN1-GFP or the double-mutant HMGN1S20,24E-GFP. Note that the fluorescence intensity of the cells expressing the mutant proteins was lost faster than in the cells expressing the native proteins, indicating that the mutant is more mobile. As a control, the fluorescence intensity in neighboring, unbleached nucleus was also measured. Values represent averages of at least seven cells with the standard deviations. (C) Confocal laser scanning fluorescence microscopy visualizing the mitotic location of either the human HMGN1 or the human double-mutant HMGN1S20,24E in transfected HmgN1−/− mouse fibroblasts. The proteins are labeled green, and the DNA is labeled red. Both the wild-type protein and the mutated protein do not colocalize with the metaphase chromosomes.
To determine whether the negative charge in the NBD affects the chromatin interaction of the protein in vivo, we transfected HeLa cells with vectors expressing either HMGN1-GFP or HMGN1S20,24E-GFP fusion proteins and measured the intranuclear mobility of the proteins by FLIP. In FLIP experiments, a living cell expressing a fluorescence tagged protein of interest is repeatedly bleached in the same spot using a high laserpower. The loss of fluorescence in an area outside of the bleached region is measured by an imaging scan after each bleach pulse. The rate of loss of fluorescence signal is an indicator of the mobility of the protein. The fluorescence signal from freely mobile molecules decreases rapidly, whereas the fluorescence signal from slowly mobile molecules decreases slowly (46). Note that in this method the movement of unbleached molecules is monitored, excluding the possibility that damage by the bleach pulse affects the mobility of the monitored proteins.
The results presented in Fig. 5B indicate that the loss of fluorescence in the cells expressing HMGN1-GFP was significantly slower that in the cells expressing HMGN1S20,24E-GFP. Thus, in cells expressing the wild-type protein the fluorescence intensity was reduced by 90% after bleaching for a total time of 325 s, whereas in cells expressing the HMGN1S20,24E-GFP double mutant only 155 s was required to reach the same reduction. Note that in nontreated controls the fluorescence intensity was stable during the time of the experiment. Thus, the presence of a negative charge in the NBD of the HMGN proteins disrupts their chromatin interactions not only in vitro but also in vivo, in living cells.
To further confirm that in metaphase the negatively charged HMGN1S20,24E protein indeed does not bind to chromosomes, we expressed either the native or the mutant proteins in mouse fibroblasts lacking endogenous HMGN1 protein, i.e., HmgN1−/− cells. In these experiments the transfected proteins were not GFP fusion constructs; since the cells lacked endogenous HMGN1, the location of the transfected proteins could be visualized by immunofluorescence. The results reveal that both the wild-type and the negatively charged S20,24E double-mutant proteins were not present in metaphase chromosomes (Fig. 5C1 and C2, respectively). Thus, the negatively charged proteins do not bind to either interphase chromatin or to metaphase chromosomes.
DISCUSSION
We report here that the NBD of the HMGN1 and -N2 protein family is highly and specifically phosphorylated during mitosis and that this phosphorylation has a major functional consequence: it abolishes the interaction of the proteins with its chromatin targets. Moreover, we provide insights into the molecular mechanisms involved in this effect and demonstrate that the abolishment of chromatin binding is accomplished by introducing negative charges in the NBD region of the protein. This finding is relevant to understanding the functional consequences of the mitotic phosphorylation of chromatin binding proteins.
Timing and specificity of HMGN phosphorylation.
Several potential phosphorylation sites in HMGN1 and -N2 have been previously identified (reviewed in references 20 and 30); however, recent studies indicate that serine 6 in HMGN1 and the two conserved serines in the NBD region of the HMGN1 and -N2 protein family are the only in vivo phosphorylation sites (2, 39, 55). Even a detailed analysis of okadaic acid-treated cells failed to reveal additional phosphorylation sites in these proteins (39). Since serine 6 of HMGN1 is associated with the expression of immediate-early genes (2, 13, 59), it can be expected that this modification would be present in logarithmically growing, cycling cells. Indeed, mass spectroscopic analysis also detected a low level of monophosphorylated HMGN1 in cycling K562 (39). In full agreement, our immunological analysis also indicates a low level of phosphorylation in logarithmically growing cells, specifically at serine 6 of HMGN1 (Fig. 2B).
However, the major change in the phosphorylation state occurs during mitosis, when both serine 6 of HMGN1 and the two conserved serine residues in the NBD of both HMGN1 and HMGN2 are highly phosphorylated (Fig. 1 and 2). We estimate that at least 80% of the proteins are phosphorylated in NBD, a significantly higher fraction than previously found in any type of cells, including okadaic acid-treated K562 cells (39). Thus, our findings suggest that HMGN phosphorylation is a highly regulated process that is linked to cell cycle events.
In the immediate-early gene response, serine 6 of HMGN1 is phosphorylated by RSK2 and MSK1 (59). Only a few of the kinases involved in the global mitotic phosphorylation of nuclear proteins have been identified, and those responsible for mitotic phosphorylation of either serine 6 in HMGN1 or the serines in the NBD of HMGN1 and -N2 are not known. We found that the mitotic polo-like kinase1 (22) does not phosphorylate the proteins, and others have reported the same for Cdc2-type kinases (42). Further experiments are needed to identify the mitotic HMGN1 and -N2 kinase and to explore the alternate possibility that the massive phosphorylation is mediated by cytoplasmic kinases that gain access to their targets due to the disaggregation of the nuclear membrane during mitosis.
Phosphorylation prevents the interaction of HMGN proteins with chromatin.
Footprinting (1), cross-linking (4, 16, 60), and site-directed mutagenesis (48) experiments suggest that the interaction of HMGN proteins with nucleosome cores involved multiple precise, but weak, interactions. The interaction of the proteins with nucleosomes is also affected by posttranslational modifications (3, 25, 55). Indeed, we find that phosphorylation of serine 6 in the N-terminal region of HMGN1 weakens the binding of the protein to chromatin, but to a lesser degree than that previously noted by Spaulding et al. (55). In fact, our studies with 32P-labeled proteins clearly demonstrate that even under cooperative binding conditions, the phosphorylated protein binds to nucleosomes (Fig. 3).
The critical region involved in the interaction between core particles and HMGN1 and -N2 proteins is the highly conserved NBD (15, 17). The binding involves interaction with both the DNA and the positively charged amino termini of the core histones (17, 60). Our present studies demonstrate that phosphorylation of the serines residues located in the NBD abolishes the interaction of the proteins with chromatin. The loss of binding to nucleosomes is a direct consequence of the introduction of negative charges in the NBD, since the double point mutant HMGN1 S20,24E also did not bind to nucleosome core (Fig. 5A). Furthermore, FLIP experiments clearly demonstrate that the intranuclear mobility of this negatively charged mutant is significantly higher than that of the native protein, suggesting that the negative charge prevent the HMGN binding to chromatin.
The FLIP experiments are highly significant in that they demonstrate, for the first time, that the NBD is indeed the main chromatin binding site of the proteins in intact, living cells. All of the previous studies on the interaction of HMGN proteins were done in vitro, with purified proteins and isolated chromatin subunits (reviewed in reference 8). It was recently also shown that a peptide corresponding to the NBD of HMGN displaces the proteins from the nuclei of permeabilized cells and inhibits transcription (28). However, the FLIP experiments are the first to validate the finding of the in vitro experiments and to demonstrate that phosphorylation of the NBD has significant biological consequences in living cells.
Biological significance of HMGN mitotic phosphorylation.
Our data, as well as the information previously available, indicate that the phosphorylation of HMGN proteins is associated with specific metabolic events, suggesting that this process serves a role in modulating the activity of HMGN proteins. HMGN1 and -N2 proteins are architectural elements that decondense chromatin and enhance transcription from chromatin templates (5, 10). The proteins colocalize with active sites of transcription and displacement of HMGN from nuclei inhibits transcription (28). In transcriptionally active, logarithmically growing cells the proteins are highly mobile (46), and conceivably their NBD could be readily targeted by kinases. It is therefore possible that in the nucleus the phosphorylation state of these proteins is in a constant state of turnover and that the equilibrium is favoring greatly the dephosphorylated state. However, phosphorylation of the HMGN NBD is clearly incompatible with a nuclear location and, upon okadaic acid treatment, phosphorylated proteins are found only in the cytoplasm (39). We find a very high level of phosphorylation in mitotic cells when the nuclear membrane is disaggregated but not in logarithmically growing, transcriptionally active cells when the nuclear membrane is intact. Thus, all the evidence is consistent with the notion that phosphorylation of the NBD is exclusively a mitotic event.
Mitosis is associated with global phosphorylation of numerous transcription factors, DNA-binding proteins, and histones (24, 35, 43, 50, 51, 54). In the case of histones, this modification may help to recruit factors to mitotic chromatin (11, 12, 38). For chromatin-binding proteins, it has been suggested that this modification displaces the transcriptional machinery and chromatin-modifying enzymes from chromatin and facilitates chromatin condensation. It is highly unlikely that by themselves the HMGN proteins, or any of the phosphorylated regulatory factors, play a major role in the global organization of the chromatin fiber. Most likely, their removal from chromatin is part of the general process necessary for the orderly progression of the cell cycle. We note, however, that about 20% of the HMGN proteins remain unphosphorylated in mitosis. Although we cannot exclude the possibility that all of the phosphatases were fully inhibited and that some phosphate was lost during protein isolation, the data are consistent with the possibility that a fraction of cellular HMGN remains unphosphorylated and can interact with mitotic chromatin.
The NBD typical of the HMGN family is present in other than just the ubiquitous HMGN1 and HMGN2 proteins. This motif was detected in NBP-45, a murine protein with tissue-specific expression (52), in the thyroid hormone interactor protein Trip 7 (36), which was renamed HMGN3a; in a new protein named HMGN4 (Y. Birger et al. unpublished data); and in several additional HMGN-like proteins that are expressed in several tissues (M. Bustin, unpublished data). Just like HMGN1 and HMGN2, these proteins bind cooperatively to nucleosome cores, as shown by mobility shift assay (52; K. J. West, unpublished data). Furthermore, using our specific anti-NBD2P antibodies, we noticed that, just like HMGN1 and HMGN2, all of these proteins could be phosphorylated in their NBD by PKC (M. Prymakowska-Bosak, unpublished data). It is therefore highly likely that all of the proteins containing an NBD functional motif have a very similar pattern of phosphorylation. We suggest that phosphorylation serves to abolish the interaction of these proteins with their chromatin targets until the end of mitosis when the chromatin is patterned for the next round of transcription.
ACKNOWLEDGMENT
We thank Robert Hock for help with the design of the HMGN1-GFP construct.
REFERENCES
- 1.Alfonso P J, Crippa M P, Hayes J J, Bustin M. The footprint of chromosomal proteins HMG-14 and HMG-17 on chromatin subunits. J Mol Biol. 1994;236:189–198. doi: 10.1006/jmbi.1994.1128. [DOI] [PubMed] [Google Scholar]
- 2.Barratt M J, Hazzalin C A, Zhelev N, Mahadevan L C. A mitogen- and anisomycin-stimulated kinase phosphorylates HMG-14 in its basic amino-terminal domain in vivo and on isolated mononucleosomes. EMBO J. 1994;13:4524–4535. doi: 10.1002/j.1460-2075.1994.tb06774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergel M, Herrera J E, Thatcher B J, Prymakowska-Bosak M, Vassilev A, Nakatani Y, Martin B, Bustin M. Acetylation of novel sites in the nucleosomal binding domain of chromosomal protein HMG-14 by p300 alters its interaction with nucleosomes. J Biol Chem. 2000;275:11514–11520. doi: 10.1074/jbc.275.15.11514. [DOI] [PubMed] [Google Scholar]
- 4.Brawley J V, Martinson H G. HMG proteins 14 and 17 become cross-linked to the globular domain of histone H3 near the nucleosome core particle dyad. Biochemistry. 1992;31:364–370. doi: 10.1021/bi00117a008. [DOI] [PubMed] [Google Scholar]
- 5.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustin M, Becerra P S, Crippa M P, Lehn D A, Pash J M, Shiloach J. Recombinant human chromosomal proteins HMG-14 and HMG-17. Nucleic Acids Res. 1991;19:3115–3121. doi: 10.1093/nar/19.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustin M, Neihart N. Antibodies against chromosomal HMG protein stain the cytoplasm of mammalian cells. Cell. 1979;16:181–189. doi: 10.1016/0092-8674(79)90199-5. [DOI] [PubMed] [Google Scholar]
- 8.Bustin M, Reeves R. High mobility group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acid Res Mol Biol. 1996;54:35–100. doi: 10.1016/s0079-6603(08)60360-8. [DOI] [PubMed] [Google Scholar]
- 9.Bustin M, Soares N, Landsman D, Srikantha T, Collins J M. Cell cycle regulated synthesis of an abundant transcript for human chromosomal protein HMG-17. Nucleic Acids Res. 1987;15:3549–3561. doi: 10.1093/nar/15.8.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bustin M, Trieschmann L, Postnikov Y V. The HMG-14/-17 chromosomal protein family: architectural elements that enhance transcription from chromatin templates. Semin Cell Biol. 1995;6:247–255. doi: 10.1006/scel.1995.0033. [DOI] [PubMed] [Google Scholar]
- 11.Cheung P, Allis C D, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 12.Cheung P, Tanner K G, Cheung W L, Sassone-Corsi P, Denu J M, Allis C D. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 13.Clayton A L, Rose S, Barratt M J, Mahadevan L C. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collas P, Le Guellec K, Tasken K. The A-kinase-anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis. J Cell Biol. 1999;147:1167–1180. doi: 10.1083/jcb.147.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook G R, Minch M, Schroth G P, Bradbury E M. Analysis of the binding of high mobility group protein 17 to the nucleosome core particle by 1H NMR spectroscopy. J Biol Chem. 1989;264:1799–1803. [PubMed] [Google Scholar]
- 16.Cook G R, Yau P, Yasuda H, Traut R R, Bradbury E M. High mobility group protein 17 cross-links primarily to histone H2A in the reconstituted HMG 17-nucleosome core particle complex. J Biol Chem. 1986;261:16185–16190. [PubMed] [Google Scholar]
- 17.Crippa M P, Alfonso P J, Bustin M. Nucleosome core binding region of chromosomal protein HMG-17 acts as an independent functional domain. J Mol Biol. 1992;228:442–449. doi: 10.1016/0022-2836(92)90833-6. [DOI] [PubMed] [Google Scholar]
- 18.de la Barre A E, Gerson V, Gout S, Creaven M, Allis C D, Dimitrov S. Core histone N-termini play an essential role in mitotic chromosome condensation. EMBO J. 2000;19:379–391. doi: 10.1093/emboj/19.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding H F, Bustin M, Hansen U. Alleviation of histone H1-mediated transcriptional repression and chromatin compaction by the acidic activation region of chromosomal protein HMG-14. Mol Cell Biol. 1997;17:5843–5855. doi: 10.1128/mcb.17.10.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Einck L, Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Exp Cell Res. 1985;156:295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- 21.Falciola L, Spada F, Calogero S, Langst G, Viot R, Grummt I, Bianchi M. High mobility group 1 protein is not stably associated with the chromosomes of somatic cells. J Cell Biol. 1997;137:19–26. doi: 10.1083/jcb.137.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glover D M, Ohkura H, Tavares A. Polo kinase: the choreographer of the mitotic stage? J Cell Biol. 1996;135:1681–1684. doi: 10.1083/jcb.135.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, Inagaki M. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- 24.Gottesfeld J M, Forbes D J. Mitotic repression of the transcriptional machinery. Trends Biochem Sci. 1997;22:197–202. doi: 10.1016/s0968-0004(97)01045-1. [DOI] [PubMed] [Google Scholar]
- 25.Herrera J, Sakaguchi K, Bergel M, Trieschmann L, Nakatani Y, Bustin M. Specific acetylation of chromosomal protein HMG-17 by P/CAF alters its interaction with nucleosomes. Mol Cell Biol. 1999;19:3466–3473. doi: 10.1128/mcb.19.5.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP- C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- 27.Hock R, Scheer U, Bustin M. Chromosomal proteins HMG-14 and HMG-17 are released from mitotic chromosomes and imported into the nucleus by active transport. J Cell Biol. 1998;143:1427–1436. doi: 10.1083/jcb.143.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hock R, Wilde F, Scheer U, Bustin M. Dynamic relocation of chromosomal protein HMG-17 in the nucleus is dependent on transcriptional activity. EMBO J. 1998;17:6992–7001. doi: 10.1093/emboj/17.23.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isackson P, Bidney J, Reeck G, Neihart N, Bustin M. High mobility group chromosomal protein isolated from nuclei and cytosol of cultured hepatoma cells are similar. Biochemistry. 1980;19:4466–4471. doi: 10.1021/bi00560a013. [DOI] [PubMed] [Google Scholar]
- 30.Johns E W. The HMG chromosomal proteins. London, England: Academic Press; 1982. [Google Scholar]
- 31.Johnson T C, Holland J J. Ribonucleic acid and protein synthesis in mitotic HeLa cells. J Cell Biol. 1965;27:565–574. doi: 10.1083/jcb.27.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura K, Rybenkov V V, Crisona N J, Hirano T, Cozzarelli N R. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 33.Knehr M, Poppe M, Enulescu M, Eickelbaum W, Stoehr M, Schroeter D, Paweletz N. A critical appraisal of synchronization methods applied to achieve maximal enrichment of HeLa cells in specific cell cycle phases. Exp Cell Res. 1995;217:546–553. doi: 10.1006/excr.1995.1121. [DOI] [PubMed] [Google Scholar]
- 34.Krebs J, Fry C, Samuels M, Peterson C. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell. 2000;102:587–598. doi: 10.1016/s0092-8674(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn A, Vente A, Doree M, Grummt I. Mitotic phosphorylation of the TBP-containing factor SL1 represses ribosomal gene transcription. J Mol Biol. 1998;284:1–5. doi: 10.1006/jmbi.1998.2164. [DOI] [PubMed] [Google Scholar]
- 36.Lee J W, Choi H S, Gyuris J, Brent R, Moore D D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with thyroid hormone receptor. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 37.Lennox R W, Cohen L H. Analysis of histone subtypes and their modified forms by polyacrylamide gel electrophoresis. Methods Enzymol. 1989;170:532–549. doi: 10.1016/0076-6879(89)70063-x. [DOI] [PubMed] [Google Scholar]
- 38.Lo W S, Trievel R C, Rojas J R, Duggan L, Hsu J Y, Allis C D, Marmorstein R, Berger S L. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gen5-mediated acetylation at lysine 14. Mol Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 39.Louie D F, Gloor K K, Galasinski S C, Resing K A, Ahn N G. Phosphorylation and subcellular redistribution of high mobility group proteins 14 and 17, analyzed by mass spectrometry. Protein Sci. 2000;9:170–179. doi: 10.1110/ps.9.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lund T, Berg K. Metaphase-specific phosphorylations weaken the association between chromosomal proteins HMG 14 and 17, and DNA. FEBS Lett. 1991;289:113–116. doi: 10.1016/0014-5793(91)80921-o. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Balbas M A, Dey A, Rabindran S K, Ozato K, Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83:29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 42.Meijer L, Ostvold A C, Walass S I, Lund T, Laland S G. High-mobility-group proteins P1, I and Y as substrates of the M-phase-specific p34cdc2/cyclincdc 13 kinase. Eur J Biochem. 1991;196:557–567. doi: 10.1111/j.1432-1033.1991.tb15850.x. [DOI] [PubMed] [Google Scholar]
- 43.Muchardt C, Reyes J C, Bourachot B, Leguoy E, Yaniv M. The hbrm and BRG-1 proteins, components of the human SNF/SWI complex, are phosphorylated and excluded from the condensed chromosomes during mitosis. EMBO J. 1996;15:3394–3402. [PMC free article] [PubMed] [Google Scholar]
- 44.Palvimo J, Maenpaa P H. Binding of high-mobility-group proteins HMG 14 and HMG 17 to DNA and histone H1 as influenced by phosphorylation. Biochim Biophys Acta. 1988;952:172–180. doi: 10.1016/0167-4838(88)90113-6. [DOI] [PubMed] [Google Scholar]
- 45.Palvimo J, Mahonen A, Maenpaa P H. Phosphorylation of high-mobility-group chromatin proteins by protein kinase C from rat brain. Biochim Biophys Acta. 1987;931:376–383. doi: 10.1016/0167-4889(87)90229-1. [DOI] [PubMed] [Google Scholar]
- 46.Phair R D, Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- 47.Postnikov Y V, Herrera J E, Hock R, Scheer U, Bustin M. Clusters of nucleosomes containing chromosomal protein HMG-17 in chromatin. J Mol Biol. 1997;274:454–465. doi: 10.1006/jmbi.1997.1391. [DOI] [PubMed] [Google Scholar]
- 48.Postnikov Y V, Lehn D A, Robinson R C, Friedman F K, Shiloach J, Bustin M. The cooperative binding of chromosomal protein HMG-14 to nucleosome cores is reduced by single point mutations in the nucleosomal binding domain. Nucleic Acids Res. 1994;22:4520–4526. doi: 10.1093/nar/22.21.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postnikov Y V, Trieschmann L, Rickers A, Bustin M. Homodimers of chromosomal proteins HMG-14 and HMG-17 in nucleosome cores. J Mol Biol. 1995;252:423–432. doi: 10.1006/jmbi.1995.0508. [DOI] [PubMed] [Google Scholar]
- 50.Segil N, Geurmah M, Hoffman A, Roeder R, Heintz N. Mitotic regulation of TFIID: inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev. 1996;10:2389–2400. doi: 10.1101/gad.10.19.2389. [DOI] [PubMed] [Google Scholar]
- 51.Segil N, Roberts S B, Heintz N. Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science. 1991;254:1814–1816. doi: 10.1126/science.1684878. [DOI] [PubMed] [Google Scholar]
- 52.Shirakawa H, Landsman D, Postnikov Y V, Bustin M. NBP-45, a novel nucleosomal binding protein with a tissue-specific and developmentally regulated expression. J Biol Chem. 2000;275:6368–6374. doi: 10.1074/jbc.275.9.6368. [DOI] [PubMed] [Google Scholar]
- 53.Shirakawa H, Tanigawa T, Sugyama S, Kobayashi M, Terashima T, Yoshida K, Arai T, Yoshida M. Nuclear accumulation of HMG2 is mediated by a basic region interspaced with a long DNA-binding sequence, and retention within the nucleus requires the acidic carboxyl terminus. Biochemistry. 1997;36:5992–5999. doi: 10.1021/bi962487n. [DOI] [PubMed] [Google Scholar]
- 54.Sif S, Stukenberg P T, Kirschner M W, Kingston R E. Mitotic inactivation of a human SWI/SNF chromatin remodeling complex. Genes Dev. 1998;12:2842–2851. doi: 10.1101/gad.12.18.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spaulding S W, Fucile N W, Bofinger D P, Sheflin L G. Cyclic adenosine 3′,5′-monophosphate-dependent phosphorylation of HMG 14 inhibits its interactions with nucleosomes. Mol Endocrinol. 1991;5:42–50. doi: 10.1210/mend-5-1-42. [DOI] [PubMed] [Google Scholar]
- 56.Sterner R, Vidali G, Allfrey V G. Studies on the acetylation and deacetylation of HMG proteins. J Biol Chem. 1979;254:11577–11583. [PubMed] [Google Scholar]
- 57.Sterner R, Vidali G, Allfrey V G. Studies on the acetylation and deacetylation of HMG proteins: identification of the sites of acetylation of HMG-14 and HMG-17. J Biol Chem. 1981;256:8892–8895. [PubMed] [Google Scholar]
- 58.Strunnikov A V, Jessberger R. Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur J Biochem. 1999;263:6–13. doi: 10.1046/j.1432-1327.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 59.Thomson S, Clayton A L, Hazzalin C A, Rose S, Barratt M J, Mahadevan L C. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 1999;18:4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trieschmann L, Martin B, Bustin M. The chromatin unfolding domain of chromosomal protein HMG-14 targets the N-terminal tail of histone H3 in nucleosomes. Proc Natl Acad Sci USA. 1998;95:5468–5473. doi: 10.1073/pnas.95.10.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walton G M, Spiess J, Gill G N. Phosphorylation of high mobility group 14 protein by cyclic nucleotide-dependent protein kinases. J Biol Chem. 1982;257:4661–4668. [PubMed] [Google Scholar]
- 62.Wei Y, Yu L, Bowen J, Gorovsky M A, Allis C D. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]