Abstract
p21-activated protein kinases (PAKs) are involved in signal transduction processes initiating a variety of biological responses. They become activated by interaction with Rho-type small GTP-binding proteins Rac and Cdc42 in the GTP-bound conformation, thereby relieving the inhibition of the regulatory domain (RD) on the catalytic domain (CD). Here we report on the mechanism of activation and show that proteolytic digestion of PAK produces a heterodimeric RD-CD complex consisting of a regulatory fragment (residues 57 to 200) and a catalytic fragment (residues 201 to 491), which is active in the absence of Cdc42. Cdc42-GppNHp binds with low affinity (Kd 0.6 μM) to intact kinase, whereas the affinity to the isolated regulatory fragment is much higher (Kd 18 nM), suggesting that the difference in binding energy is used for the conformational change leading to activation. The full-length kinase, the isolated RD, and surprisingly also their complexes with Cdc42 behave as dimers on a gel filtration column. Cdc42-GppNHp interaction with the RD-CD complex is also of low affinity and does not dissociate the RD from the CD. After autophosphorylation of the kinase domain, Cdc42 binds with high (14 nM) affinity and dissociates the RD-CD complex. Assuming that the RD-CD complex mimics the interaction in native PAK, this indicates that the small G protein may not simply release the RD from the CD. It acts in a more subtle allosteric control mechanism to induce autophosphorylation, which in turn induces the release of the RD and thus full activation.
GTP-binding proteins of the Ras superfamily are molecular switches which cycle between the GDP-bound off and GTP-bound on states. In the on state, they interact with effectors which are defined as molecules interacting more tightly with the GTP-bound form than with the GDP-bound form. By interacting with effectors, they mediate downstream biological effects. The switch returns to the GDP-bound off state by the GTPase reaction which is catalyzed by GTPase-activating proteins (4, 5).
Recently, it has become clear that many of the GTP-binding proteins interact with an array of different effectors and thus are involved in more than one signal transduction pathway (26). Many of these effectors are protein kinases that become activated in the course of this interaction. A prominent example are the different Raf kinases (three isoforms) which interact with activated Ras in the course of growth regulation and become activated via a mechanism that involves translocation to the plasma membrane and most likely further allosteric regulatory events which are still incompletely understood.
In the case of the Rho subfamily members Rho, Rac, and Cdc42, a number of Ser/Thr-specific protein kinases have been identified, such as protein kinase N and the Rho kinases for Rho. The first kinases to be identified as downstream targets were the Tyr-specific ACK (activated Cdc42-associated kinase) (23) and the Ser/Thr-specific PAK (p21-activated kinase) (24). PAK constitutes a large family of related protein kinases. The most prominent members are mammalian PAK1 to -4, the PAK homologues Ste20 and Cla4 from Saccharomyces cerevisiae, and the myotonic dystrophy-related kinases, which are involved in cell cycle control, dynamics of the cytoskeleton, apoptosis, and transcription (for reviews, see references 2 and 9).
PAK1 to -3 are 60- to 65-kDa proteins that contain an N-terminal regulatory domain (RD) and a C-terminal kinase domain, the former of which is inhibitory to the latter. The RD contains a conserved CRIB (CDC42/Rac interactive binding region) or GBD (for GTPase-binding domain), and polypeptides encompassing this region are sufficient to bind to the GTP-bound form of Rac and Cdc42 (7, 39). The binding allosterically induces activation of the kinase activity, which in contrast to the Ras-Raf interaction (38) can be demonstrated convincingly with purified components in the test tube (21, 25). Using point mutations in the regulatory region which relieve its inhibitory action (6, 51), it was postulated that the RD directly interacts with the kinase domain. Such a direct interaction has been shown using recombinant fragments (49) and yeast two-hybrid analysis (43). The binding of Cdc42 has been postulated to induce a conformational change, which relieves the inhibitory effect on the kinase domain and opens the kinase structure. The structures of an autoinhibited αPAK fragment (20) and of complexes between Cdc42 and a CRIB-containing fragment (27, 28) seemed to support the model (Fig. 1). In this and other such models, it is postulated that the regulatory and kinase domains dissociate from each other, allowing the latter to become activated by transphosphorylation (9).
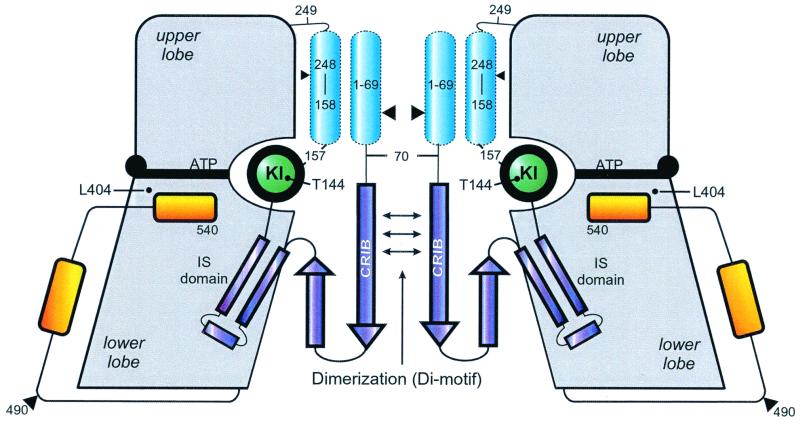
FIG. 1.
Structural model of αPAK, using as a guideline the X-ray structure of a complex between a regulatory fragment from residues 70 to 175 and the complete CD (residues 249 to 545) from human αPAK (18). The regulatory part of the molecule is in dark blue structural elements not included in the three-dimensional structure are in light blue. Approximate locations of residues relevant to the results presented in this report are indicated. It was previously assumed that inhibition by the inhibitory switch (IS) domain and dimerization via the Di motif is relieved by binding of Cdc42 to the CRIB region. KI, kinase inhibitory linker.
Since the conformational changes during kinase activation by GTP-binding proteins are poorly understood, and since PAK activation could serve as an apt structural model, we have investigated the biochemical and structural implications of the model. The results are discussed in the context of the structure of inhibited αPAK (PAK1) (20).
MATERIALS AND METHODS
Protein expression and purification.
The expression clone for rat αPAKL404S in the pGEX-2T vector was a gift of Ed Manser. The glutathione S-transferase (GST) fusion protein was expressed in Escherichia coli BL21 from a slightly modified vector pGEX2T, where, to improve the cleavage with thrombin, a linker sequence (an immunoglobulin A protease cleavage site [33]) was inserted immediately downstream of the thrombin cleavage site. Cells grown to optical density at 600 nm of 0.8 in Luria-Bertani medium with 100 μg of ampicilin per ml were induced at 20°C with 0.1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) and cultivated for 7 to 10 h. After homogenization of the cells with a fluidizer (Microfluidics, Newton, Mass.), the suspension was centrifuged at 30,000 × g for 30 min. GST fusion proteins of PAK, PAKK298A, PAK fragments, and Cdc42 (the G12V mutant was always used) were purified on glutathione (GSH)-Sepharose (Pharmacia). After application, the column was washed extensively with buffer A (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 5 mM dithioerythritol [DTE]), with buffer B (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 5 mM DTE), and again with buffer A. After cleavage with thrombin overnight on the column, the proteins were eluted with buffer A. Thrombin was removed by chromatography on benzamidine-Sepharose 6B (Pharmacia), and the proteins were further purified by gel filtration chromatography (Superdex 200; Pharmacia).
Digestion of αPAK.
αPAK was digested with either trypsin, chymotrypsin, papain, or V8 protease at an αPAK/protease ratio of 1,000:1 (wt/wt). The cleavage reaction was carried out on ice in buffer A. Reactions were stopped after various incubation times by adding 1 μl of protease inhibitor cocktail solution (Complete; Boehringer GmbH, Mannheim, Germany). The reaction products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electrospray mass spectrometry, and N-terminal sequencing.
Protein kinase assay.
Kinase activity was measured by assaying the phosphorylation of myelin basic protein (MBP). The reaction was carried out by incubating αPAK (0.1 mg/ml), GST-Cdc42-GppNHp (0.2 mg/ml), and MBP (1 mg/ml) in buffer C (40 mM HEPES-NaOH [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 5 mM DTE) with 3.3 μM [γ-32P]ATP (1 nCi/ml) at 30°C for 15 min (21). The reactions were stopped by addition of SDS sample buffer. Reaction products were run on SDS-PAGE, and radioactivity on the dried gels was quantified using a phosphorimager.
Fluorescence studies.
Fluorescence measurements were done on a Fluoromax spectrofluorimeter (SPEX Instruments S.A., Inc., Edison, N.J.). Fluorescence of 3′-methylanthraniloyl nucleotides was followed at 25°C with λex = 366 nm and λem = 435 nm in buffer C. To determine the affinity of Cdc42 for intact αPAK, the isolated recombinant regulatory fragment, or the RD-catalytic domain (CD) complex, Cdc42 (the G12V GTPase-negative mutant) bound to the appropriate fluorescent 3′-methylanthraniloyl nucleotide was titrated with increasing concentrations of the kinase proteins in buffer C. The resulting fluorescence intensity was recorded, and the data were fitted to a quadratic equation describing a bimolecular association model assuming a 1:1 stoichiometry. To determine the affinity of the autophosphorylated RD-CD fragment, fluorescence intensities were recorded in the presence of 1 mM ATP, giving sufficient time for a kinase reaction (10 min).
The affinities between RD (αPAK residues 57 to 200) or the full-length (FL) αPAK and Cdc42-GppNHp were also determined by stopped-flow measurements. The association rate constants were measured with 0.5 μM Cdc42- mGppNHp and increasing (2.5 to 25.0 μM) concentrations of effector in a stopped-flow apparatus (Applied Photophysics, Leatherhead, United Kingdom) in buffer C at 25°C, with an emission cutoff filter of 408 nm. Dissociation of the labeled Cdc42-mGppNHp complexes was measured by rapid mixing with 100-fold excess (0.2 mM) unlabeled Cdc42-GppNHp and following the fluorescence transient in real time. All kinetic data were fitted to single exponentials in the case of the RD and double exponentials (see below) in the case of FL αPAK, using the program Grafit (Erithacus Software, Staines, United Kingdom). With FL αPAK, the association rate contains a second component whose rate and amplitude are independent of the concentration. It was neglected, as was a second, faster component of the dissociation rate. We have no explanation for the second component, but since the ratio of rates for the first components gave an equilibrium dissociation constant consistent with the one obtained by direct titration, we did not consider it significant.
Pull-down assay.
GST-Cdc42-GppNHp (30 μg) was bound to GSH-Sepharose beads equilibrated in buffer C. Then 20 μg of the RD-CD complex were added; the reaction mixture was incubated for 30 min on ice, either with no nucleotide or with 1 mM ATP or AppNHp together with 5 mM MgCl2, and washed twice with buffer C to remove unbound proteins. The wash eluates together with the contents of the beads (obtained by boiling in SDS sample buffer) were analyzed by SDS-PAGE and stained with Coomassie blue.
RESULTS
Low-affinity binding of PAK to Cdc42.
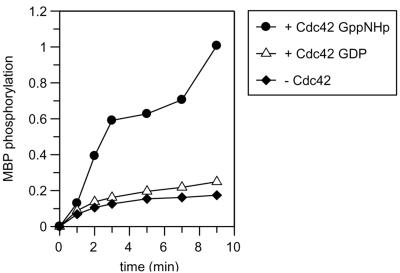
It has been shown that expression of FL native αPAK (PAK1) is lethal to E. coli, whereas an αPAK construct with the point mutation L404S can conveniently be kept in bacteria and used to express large amounts of protein. αPAK with the L404S mutation has a lower basal activity but can still be stimulated by the Rho-type GTP-binding protein Cdc42, albeit not to a similar degree (21, 22). In the three-dimensional structure of autoinhibited human PAK1, the corresponding Leu405 is situated in the β8 strand immediately preceding the activation loop, which according to the structure interacts with the kinase inhibitory segment from the regulatory region (20) (Fig. 1). The side chain points into a hydrophobic pocket but is close to the His387 side chain (Nɛ-to-Cγ distance of 3.7 Å). The L405S mutation could thus possibly bridge these two residues and thereby impede rearrangement of the autophosphorylation loop and positioning of the catalytic machinery containing the essential catalytic Asp389. We confirm that the kinase activity of purified protein, measured either as an autokinase activity or as the phosphorylation of MBP, is absolutely dependent on the presence of activated Cdc42 (Fig. 2). This is further documented in Fig. 5C (see below). The kinetic analysis of the reaction shows a rapid phosphorylation of MBP in the presence of Cdc42-GppNHp, whereas in the absence of Cdc42 (or the presence of Cdc42-GDP), the reaction reaches a much lower level of phosphorylation.
FIG. 2.
Intrinsic and Cdc42-stimulated protein kinase activity of αPAK. αPAK (1.7 μM) was incubated in kinase buffer in the presence of 3.3 μM [γ-32P]ATP and 54 μM MBP, in the absence and presence of Cdc42, in the GDP- or GppNHp-bound conformation. The reaction mixture was incubated at 37°C, and samples from the indicated time points were analyzed for phosphorylation in arbitrary units as described in Materials and Methods.
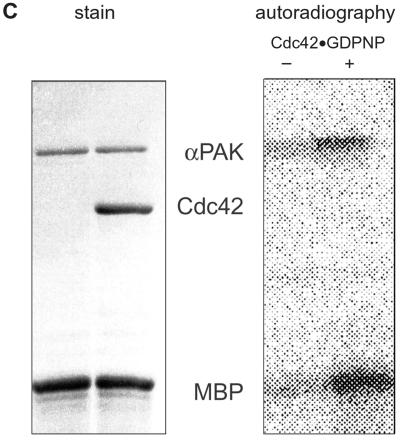
FIG. 5.
Proteolyzed PAK is catalytically active. (A) Autoradiogram showing the results of protein kinase assay of the RD-CD complex without Cdc42, with either Cdc42-GDP or Cdc42-GppNHP, using conditions as described in the legend to Fig. 2 and Materials and Methods. Lane 1, kinase assay with FL αPAK in the presence of Cdc42-GppNHp; lane 2, the same reaction in the absence of αPAK. Note that the CD is phosphorylated, whereas RD phosphorylation cannot be detected in the background on MBP. (B) Time course of MBP phosphorylation in (arbitrary units) by the RD-CD complex in the presence or absence of Cdc42-GppNHp. (C) Control incubation with FL αPAK. MBP phosphorylation and autophosphorylation are observed only in the presence of Cdc42-GppNHp.
The interaction of Rac and Cdc42 with effector fragments has been analyzed under equilibrium conditions in solution using fluorescence, scintillation proximity assay, and isothermal calorimetry, with CRIB-containing fragments of various lengths from the regulatory region of Wiscott-Aldrich syndrome protein (WASP), ACK, and PAK (30, 36, 42). Fluorescence intensity decrease (35, 42) as well as increase (30) have been observed to occur, and equilibrium dissociation constants in the nanomolar region for PAK fragments have been measured, also with other techniques (37, 50). Here we measured the interaction of FL αPAK with Cdc42-GppNHp under equilibrium conditions. The fluorescence emission intensity of Cdc42-bound mGppNHp decreases about 50% on addition of FL αPAK (Fig. 3A), in accordance with the results obtained with WASP (35) and PAK (42) fragments. Under these conditions (0.2 μM), Cdc42-mGDP does not show a fluorescence decrease (not shown), confirming the specificity of the interaction and indicating that the affinity to Cdc42-GDP is much lower, as suggested previously by overlay and pull-down techniques (24, 25). Titration of Cdc42-mGppNHp with increasing amounts of αPAK and fitting the resulting fluorescence intensity decrease to a binding equation assuming 1:1 (or 2:2 [see below]) stoichiometry (Fig. 3B) gives an equilibrium dissociation constant of 0.61 μM. This is much higher than what is usually found for CRIB or GBD fragments of PAK, WASP, and ACK (30, 31, 36, 42).
FIG. 3.
Equilibrium measurement of the PAK-Cdc42 interaction. The fluorescence emission spectrum of 0.2 μM Cdc42-mGppNHp in buffer C (upper trace) decreases upon treatment with (from top to bottom) 0.5, 1.0, 1.5, 2.0, and 2.5 μM αPAK. (B) Cdc42-mGppNHp (0.2 μM) was treated with increasing amounts of αPAK as indicated, and the decrease in fluorescence was fitted to a binding equation with 1:1 stoichiometry.
Activation of αPAK by proteolysis and formation of an RD-CD complex.
It has been shown before that limited proteolysis of γPAK (PAK2) induces activation of the kinase activity, as it has been found as an autokinase activity in pig brain and liver (46, 47) or in rabbit reticulocytes (40). Furthermore, it has been shown that γPAK, but not α or βPAK, is cleaved by the cysteine protease caspase 3 in response to apoptotic stimuli (18, 34). γPAK has also been isolated as an inactive precursor kinase which is activated in vitro by mild trypsin digestion (3). In those studies, similarly sized fragments containing the regulatory and the catalytic parts of the kinase have been detected. The question of whether or not the proteolysis products separate from each other has not been addressed. However, judging from the available literature, it has tacitly been assumed that they do. To see if αPAK can also be activated similarly and to investigate the structure-function relationship of the cleavage products, we digested αPAK with proteases such as elastase, trypsin, chymotrypsin, and V8. Although all of these enzymes cleave the protein into similar fragments, most of the enzymes eventually degrade the protein during prolonged incubation. In the case of chymotrypsin, however, two proteolysis-resistant, stable fragments are obtained (Fig. 4). By mass spectrometric analysis and N-terminal sequencing, these fragments can be identified as the N-terminal residues 57 to 200 and the C-terminal residues 201 to 491 from αPAK, which we call the RD and CD, respectively (Fig. 1). It should be mentioned that in the presence of Cdc24-GppNHp, proteolysis with chymotrypsin is much faster (not shown), indicating a conformational change of the kinase, as postulated from the structural and biochemical studies.
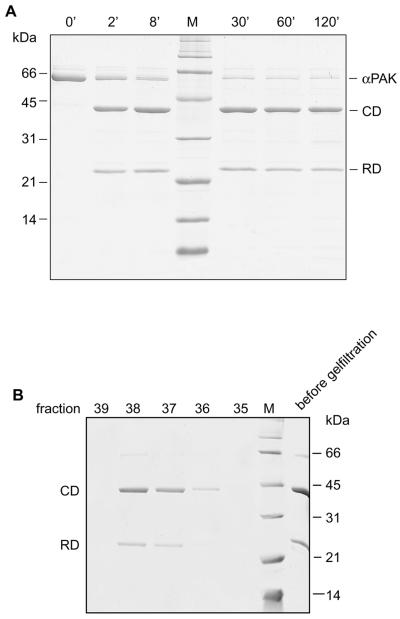
FIG. 4.
Proteolytic digestion of αPAK. (A) αPAK (66 μM) was digested with chymotrypsin (1,000:1; wt/wt). At the indicated time intervals, aliquots of the reaction mixture were treated with protease inhibitors and analyzed by SDS-PAGE as described in Materials and Methods. (B) The reaction mixture from chymotrypsin digestion was applied to a Superdex 75 (10/30) gel filtration column equilibrated in buffer B, and the eluted fractions were analyzed by SDS-PAGE.
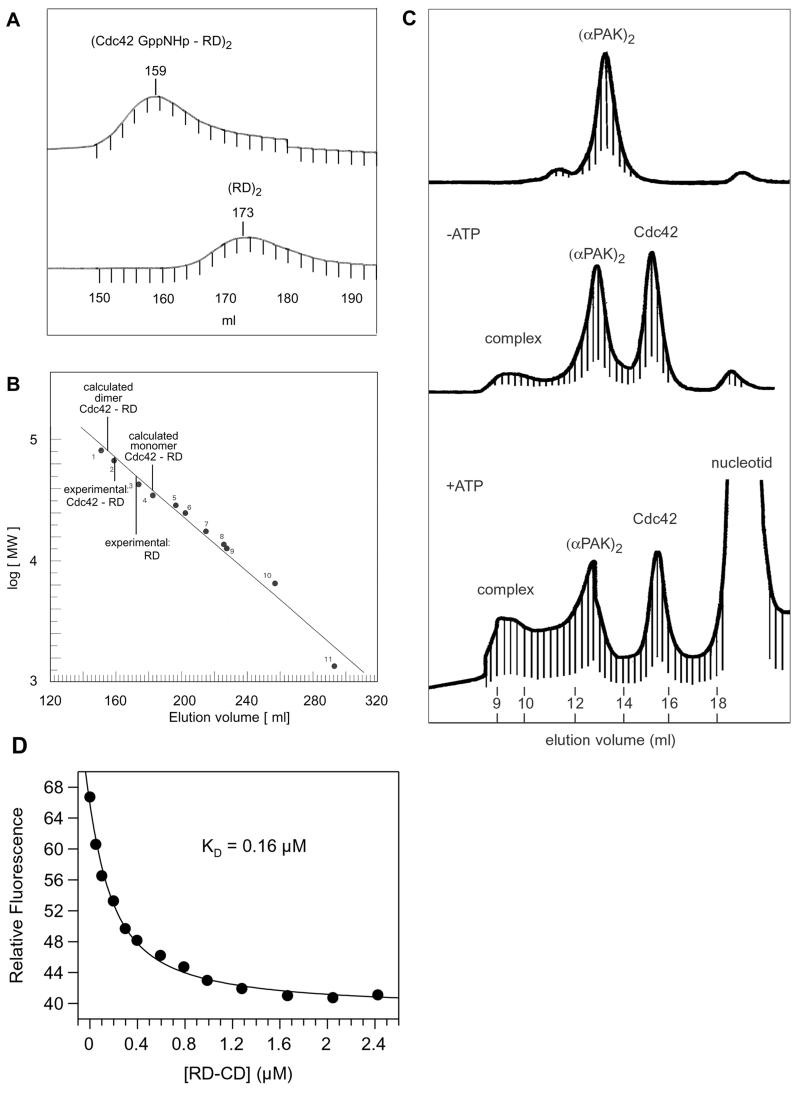
Since the N- and C-terminal fragments of native PAK have been shown to bind to each other (43, 49), we wanted to see if this property is retained with the proteolyzed product. The RD and CD fragments indeed coelute on a calibrated size exclusion chromatography column (Fig. 4B). The elution volume corresponds to an apparent molecular mass of 77 kDa that is intermediate between the masses of a monomer and a dimer (calculated mass of 48.5 kDa). This indicates that the complex may retain the tendency to dimerize, as shown previously for native αPAK and PAK(70-545) (20). The experimental value may also indicate rapid equilibrium between monomer and dimer (see also below).
The RD-CD complex is postulated to be catalytically inactive, as the binding of the regulatory subunit to the catalytic subunit has been shown to inhibit the kinase activity (43, 49). However, contrary to expectation, the complex is catalytically active, as evidenced both by autophosphorylation of the kinase domain and phosphorylation of MBP (Fig. 5A). The kinetics of MBP phosphorylation appear to be similar to those of FL native αPAK in the presence of Cdc42-GppNHp (compare Fig. 2 and 5B), but in contrast to native PAK, activation is uncoupled from the presence of Cdc42 (Fig. 5). This might indicate that activated forms of γPAK obtained by caspase or trypsin digestion (3, 18, 34), just like the activated kinases observed in cell extracts (40, 46, 47), may in fact be similar RD-CD complexes. Mass spectrometry analysis of the CD domain after in gel tryptic digestion (data not shown) indicates that the fragment encompassing Thr422 cannot be found anymore after treatment with ATP, suggesting that this residue in the autophosphorylation loop is the target of modification, as shown by other authors before (8, 49).
The RD (residues 57 to 200).
To show that the RD-CD complex can also be formed from the two isolated subunits, we tried to express the two domains independently in E. coli as recombinant proteins. Whereas the RD comprising residues 57 to 200 could be expressed and purified as a stable protein, we were not able to obtain the CD (comprising residues 201 to 491) by similar protocols. This is somewhat surprising considering that other, albeit different catalytic fragments have been isolated (20, 49). The dissociation constant of 3.7 nM (not shown) determined for the binding of the RD to Cdc42-mGppNHp is somewhat low to be accurately determined by the equilibrium method (see below) but is clearly much lower than that for full-length PAK and also comparable to those for other CRIB domain fragments from either ACK, PAK, or WASP (36, 42).
Recently the structure of a complex between the regulatory fragment (residues 70 to 149) and the complete CD (residues 249 to 545) of αPAK has been solved by X-ray crystallography (20). The N-terminal fragment was shown to contain a motif which mediates dimerization (Di motif) and an inhibitory switch domain which abuts the large lobe of the kinase domain. It also contains a short kinase inhibitory linker that sterically prevents access to the active site and directly interacts with catalytic residues (Fig. 1). The RD-CD complex described here contains all of these regulatory elements plus the PIX (p21-interacting exchange factor) binding motif also involved in the regulation of PAK. The activation model deduced from the crystal and nuclear magnetic resonance (NMR) structures (12, 20, 27) would predict that the regulatory fragment unfolds and forms a monomer due to the interaction with Cdc42 and that its inhibitory interaction with the CD is released. Using size exclusion chromatography, we find that the regulatory fragment (residues 57 to 200) elutes with an apparent higher molecular mass, arguing that the fragment may still form a possibly rapidly interconverting dimer via the Di motif (Fig. 6A and B). The elution profile of the RD-Cdc42 complex also suggests an apparent molecular mass higher than expected for a monomer, not in line with the proposed activation model and with NMR data showing smaller CRIB fragments in complex with Cdc42 to be monomers (12, 27). Since the fragments used for the NMR studies were rather small, they might not contain all of the motifs necessary for dimerization.
FIG. 6.
Dimerization of PAK and the RD-CD complex in the presence of Cdc42 and its affinity to Cdc42. (A) Elution profile of the isolated RD fragment and its complex with Cdc42-GppNHp on a gel filtration (Superdex 75 (26/60)) column with buffer C, which elute with the indicated volumes, plotted against the log of the molecular mass on a calibration curve (B) obtained from marker proteins. The column was equilibrated with a standard where reference proteins 1 to 11 have molecular masses of 81, 67, 43, 35, 29, 25, 17.6, 13.7, 12, 7 and 6.5 kDa, respectively. Calculated elution volume for a Cdc42-RD monomer or dimer complex is indicated. (C) Elution profiles with buffer C on a Superdex 200 (10/30) column of αPAK alone and complexed with Cdc42-GppNHp before and after incubation with 5 mM ATP. The affinity of the RD-CD complex (D) is determined by fluorescence titration with Cdc42-mGppNHp as shown in Fig. 3, giving the indicated affinity of 0.16 μM for the RD-CD complex.
The PAK-Cdc42 complex is a tetramer.
Various techniques have been used to show that FL inactive αPAK as well as the crystallized RD(70-149)-CD(249-545) complex form dimers and tetramers, respectively (20). Figure 6C shows size exclusion chromatography on a calibrated column of FL αPAKL404S which also elutes with a calculated molecular mass of 120 kDa. In the presence of Cdc42, a small amount of complex which runs with a higher molecular mass than the PAK dimer is formed. The small amount of complex formed is most likely due to the only micromolar affinity of FL PAK, but the dynamic nature of complex formation can also be demonstrated from the elution volume of the Cdc42 which is retarded due to complex formation; Cdc42 alone elutes with the expected elution volume (not shown). Higher amounts of complex are formed after incubation of PAK with ATP, in line with a higher affinity expected for an activated kinase. But again the complex shows a higher molecular weight than calculated, suggesting that complex formation with Cdc42 does apparently not dissociate the FL PAK dimer under these conditions. For the affinity measurements between PAK (or the RD-CD complex) and Cdc42, we thus have to assume a 2:2 stoichiometry with two independent binding sites since from the titration curves we have no indication for any other behavior.
Low-affinity binding of Cdc42 to the RD-CD complex.
To see whether binding of Cdc42 to the RD-CD complex resembles the low- or high-affinity interaction of the intact PAK or the isolated CRIB region, we measured the equilibrium dissociation constant. Figure 6D shows that the RD-CD complex forms a low-affinity complex with Cdc42-mGppNHp with an intermediate dissociation constant of 0.16 μM, which is 3.8-fold lower and approximately 10- to 40-fold higher than that of native αPAK and the isolated RD, respectively. This suggests that the RD, or at least the Cdc42-binding site represented by the CRIB region, is not in the high-affinity (4 to 18 nM) conformation found for the isolated RD. This suggests that there are contacts between the CD and RD which modify the interaction of Cdc42 with the latter.
Kinetics of the Cdc42-PAK interaction.
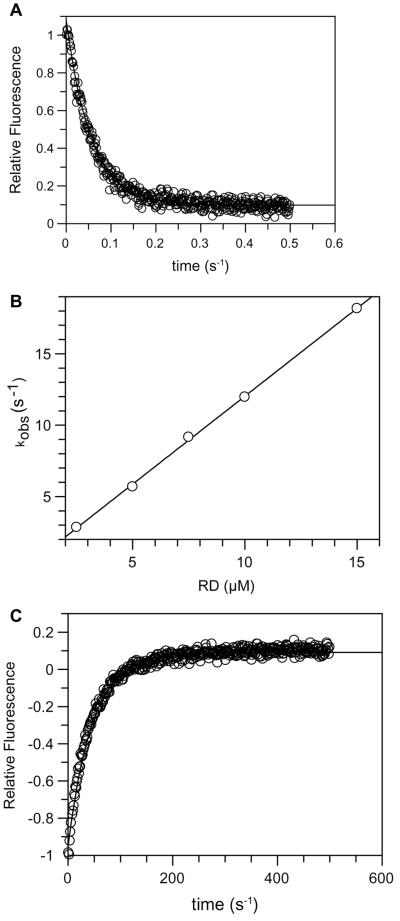
To find the basis for the large difference in affinity between the isolated RD and the full-length protein or the RD-CD complex, we measured the kinetics of interaction between PAK and Cdc42 by stopped-flow analysis. Figure 7A shows that the association between the isolated RD and Cdc42-mGppNHp is fast. Plotting the observed pseudo-first-order rate constants against the concentration of RD (Fig. 7B), an association rate constant (kass) of 1.2 × 106 M−1 s−1 is obtained. Together with the dissociation rate constant (kdiss) of 0.02 s−1 (Fig. 7C) and using the relation Kd = kdiss/kass, an equilibrium dissociation constant of 17.6 nM can be calculated. This is similar to and probably more accurate than what has been found by equilibrium titration due to the high affinity. The association and dissociation rate constants for full-length αPAK are more difficult to interpret because the association and dissociation reactions are not single exponential and contain an extra component (see Materials and Methods). A complex kinetic pattern for the Cdc42-FL PAK interaction may not be too surprising considering the large conformational change of the CRIB domain which has to be postulated considering that in the autoinhibited structure it is not available for binding (20). Neglecting the second step, we get a dissociation rate constant (0.026 s−1) very similar to that of the isolated RD, whereas the association rate constant is only 9.3 × 103 M−1 s−1, which indicates that a process is extremely slow and orders of magnitude slower than a diffusion-limited process. Thus, the much lower association rate between Cdc42-mGppNHp and full-length αPAK (and presumably also the RD-CD complex) may be mostly responsible for the low-affinity binding (>400-fold) compared to the isolated RD fragment.
FIG. 7.
Kinetics of interaction between RD and Cdc42. Increasing concentrations of RD were mixed with Cdc42-mGppNHp in a stopped-flow apparatus, and the fluorescence change due to complex formation was recorded. The primary fluorescence transients, of which panel A is an example, were fitted to a single exponential to give kobs. The latter was plotted against the concentration of RD (B) to give the kass, 1.2 × 106 M−1 s−1. The kdiss of 0.02 s−1 was obtained by mixing a preformed RD-Cdc42-mGppNHp complex with excess unlabeled Cdc42-GppNHp as described in Materials and Methods.
Autophosphorylation but not Cdc42-GppNHp dissociates the RD-CD complex.
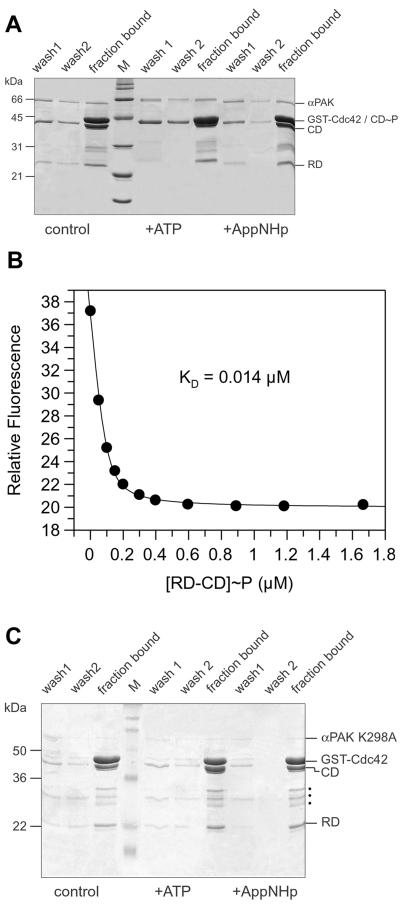
Since the activation model predicts that the binding of the triphosphate form of Cdc42 dissociates the two domains, thereby relieving the inhibition, the chymotryptic digestion product is a valuable tool to test this prediction, as the two domains should separate from each other after binding of Cdc42-GppNHp. We thus coupled GST-Cdc42 in the GppNHp-bound form to GSH-Sepharose beads and incubated them with the RD-CD complex. After the beads were washed, proteins eluted and bound to the GSH-beads were analyzed by SDS-PAGE. The two polypeptide chains are bound to GST-Cdc42 but, due to the low affinity of the RD-CD complex (0.16 μM), are coeluted from the beads in approximately the same 1:1 ratio as was applied to the column (Fig. 8A). Complete elution is mediated by SDS, indicating that the two domains stay bound to each other even after binding to the triphosphate form of Cdc42.
FIG. 8.
Autophosphorylation but not Cdc42-GppNHp interaction dissociates the RD-CD complex. (A) GSH-beads were loaded with 30 μg of GST-Cdc42-GppNHp and incubated with 20 μg of the RD-CD complex. The beads were incubated for 30 min at 4°C with buffer (control) or with 1 mM ATP or AppNHp in the presence of excess Mg2+ as indicated, and washed twice with buffer C (wash 1 and 2), and then washed with SDS sample buffer to remove all bound proteins (fraction bound). The starting materials (lanes 1 to 3) and the contents of the washes were analyzed by SDS-PAGE as indicated, together with a molecular weight marker (M). (B) The RD-CD complex in buffer C was incubated with 1 mM ATP, and the affinity to Cdc42-mGppNHp was determined as in Fig. 3. (C) Pull-down assay as in panel A, but with the RD-CD fragment from kinase inactive (K298A) mutant of αPAK. Dots indicate unidentified impurities.
We have shown above that the RD-CD complex becomes phosphorylated and activated upon incubation with ATP. To test whether phosphorylation changes the affinity of interaction between the two domains, the RD-CD complex was incubated with ATP before application to the GST-Cdc42-GppNHp-loaded beads. In this case, the CD is no longer retained on the beads but is eluted as a single band with a slightly higher molecular weight, presumably due to phosphorylation as shown in Fig. 5A. Upon incubation with the nonhydrolyzable ATP analogue AppNHp, the elution behavior is similar to that of the control, supporting the idea that the release of the CD is indeed due to phosphorylation.
If indeed the autophosphorylation reaction separates the RD from the CD, and since the isolated RD has a high-affinity conformation as shown above (Fig. 6A), treatment of the RD-CD complex (or the mixture of polypeptides) with ATP should induce high-affinity binding. Figure 8B shows this to be the case. The affinity is 14 nM, similar to that of the isolated RD and 11.4-fold higher than that of the unphosphorylated RD-CD complex. To determine whether incubation with ATP influences the affinity of αPAK for Cdc42-GppNHp, the protein was incubated with ATP for 30 min. The affinity is reduced only 3.5-fold, to 0.21 μM, very different from what is found for the RD-CD complex.
To further show that release of the CD from the beads is indeed due to phosphorylation, the experiment was repeated with a kinase-inactive (K298A) (20) version of the RD-CD complex, which could also be prepared from the full-length kinase by chymotrypsin. Here elution from matrix-bound GST-Cdc42-GppNHp is not modified by prior incubation with ATP (Fig. 8C).
DISCUSSION
Reversible protein phosphorylation plays a central role in the regulation of many cellular processes, and the protein kinases necessary for the reaction constitute the second-most-frequent type of proteins in a higher eucaryotic cell. The original estimation of 1,001 protein kinases (14) may well be an underestimation, as 411 full-length proteins and some fragments have been identified in Caenorhabditis elegans alone (32). The large diversity in the number of protein kinases is almost rivaled by the large number of regulatory mechanisms for this kind of protein. Regulation may involve second messengers such as cyclic AMP, cGMP, Ca2+, diacylglycerol, and allosteric control, intrasteric inhibition through pseudo-substrate sequences (17), and positive and negative phosphorylation events (15). One important aspect of kinase regulation involves the interaction with GTP-binding proteins. A large number of bona fide or presumed effectors of the Ras-related proteins are protein kinases, such as c-Raf-1 and Byr2 as effectors of Ras, and Rho kinases and protein kinase N as effectors for Rho proteins (26). PAKs constitute a large family of related kinases, some (but not all) of which interact specifically with the GTP-bound form of Rac and Cdc42. While the mechanism of Raf activation by the interaction with Ras is highly controversial, with the discussion concentrating around Ras being involved in targeting and/or allosteric activation (26), the interaction between Cdc42-GTP and PAK appears to be much more straightforward, as the direct interaction between the purified components leads to phosphorylation of PAK and activation of its kinase activity (2, 9).
Models whereby the binding of the GTP-bound form of Cdc42 or Rac relieves the tight interaction between the RD and CD have been proposed to explain the activation mechanism (2). Here we show that proteolytic digestion of αPAK produces a cleavage of the polypeptide chain between the RD and CD and activates the kinase, even in the absence of Cdc42. Activated forms of PAK have been observed in cell extracts (40, 41, 46, 47), and it has also been shown that trypsin and the apoptotic protease caspase cleave γPAK but not αPAK between the RD and CD and that this leads to activation of the kinase activity (3, 19, 34, 45, 52). Here we show that αPAK is also similarly cleaved and activated by chymotrypsin. We do not understand the nature of the activation process. In light of the recently determined structure of the autoinhibited αPAK consisting of a regulatory fragment from residues 70 to 157 and the complete kinase domain (residues 249 to 545) (20), residue 200 is located away from the active site, and cleavage at this residue is thus unlikely to effect catalytic activity. While the location of the N-terminal residues is not included in the structure, the C-terminal helix ending at residue 540 is rather close to the active site (Fig. 1). The removal of this helix may well be involved in the Cdc42-independent activation mechanism, which is seen with the chymotryptic and previously found proteolytic fragments.
Contrary to what is expected from the conventional activation model for PAKs, the interaction of the chymotryptic regulatory and the catalytic fragments is sufficiently tight even in the presence of Cdc42-GppNHp such that they are coeluted from a GST-Cdc42-GppNHp-bound GSH-Sepharose column. The two fragments separate from each other only after treatment with ATP, which leads to autophosphorylation of the kinase. Figure 5A shows that the kinase domain is phosphorylated, and its mobility on an SDS-gel is retarded (Fig. 8A) after phosphorylation. This is presumably due to a conserved Thr in the activation loop, as demonstrated before for Thr422 of αPAK (8, 49) and Thr402 in γPAK (3, 10, 48), similar to activation of many other protein kinases (15). The RD has been reported to be phosphorylated on residues such as on Ser144 and Ser149 in the autoinhibitory domain of αPAK (8, 22). It is fairly well established by those findings and our results that phosphorylation in both the kinase and regulatory domains is required for the conformational switch and for activation, although it is not yet clear how the mechanism observed for the RD-CD complex investigated relates to that of the intact kinase, just as it is unclear from the crystal structure of another type of RD-CD complex how the missing parts of the regulatory region of αPAK contribute to the regulation (20).
The CRIB/GBD motif whose sequence is conserved in many Cdc42 and Rac effectors such as PAK, ACK, and WASP has been shown to be rather unstructured (1, 12, 16, 26, 28), contrary to what has been found for the Ras-binding domains of Raf kinases and other effectors, which have no sequence homology but show the same stable ubiquitin fold (11, 29, 44). Small fragments containing the CRIB/GBD region of PAK adopt a fixed structure only when bound to the effector region of Cdc42 (12, 27). The structure of an autoinhibited conformation of αPAK (20) showed that the regulatory region forms a dimer which involves part of the CRIB domain and which has to undergo a large conformational change to adopt the conformation found in the Cdc42-CRIB complexes. It was concluded that the interaction with Cdc42 causes dissociation of the dimer and disruption of the contacts between the regulatory and kinase domains, thus leading to an open monomeric active kinase. In their scheme, these and other authors (13) conclude that phosphorylation is a late event in this activation mechanism, which stabilizes the open conformation. We demonstrate here that FL αPAK, the RD-CD complex, as well as the regulatory fragment from residues 57 to 200 alone behave as dimers on a gel filtration column, in agreement with the results of Lei et al. for FL PAK and a different RD-CD complex (20). The regulatory domain in our RD-CD complex is presumably in a conformation similar to that found in the structure of the autoinhibited kinase determined by crystallography, again presumed to be similar to that of intact kinase, which we would biochemically define as binding with low (micromolar) affinity to Cdc42-GppNHp. It was however surprising that the RD-Cdc42 complex apparently does not dissociate into monomers, as judged from size exclusion chromatography. This prompted us to investigate the elution behavior of the PAK-Cdc42 complex. Figure 6C indicates that the complex with FL PAK also elutes with an apparent molecular mass higher than that calculated for a monomer and that complex formation is increased after treatment with ATP and thus autophosphorylation. This is unexpected considering previous activation models and NMR studies which show that (albeit smaller) CRIB region fragments are monomeric when bound to Cdc42.
Furthermore, we show that the phosphorylation but not binding of Cdc42 alone induces the release of the regulatory domain and the activation switch, as monitored by induction of the kinase activity and transition from low- to high-affinity binding of Cdc42. Although quantitative differences might exist between our RD-CD complex and native PAK, we would nevertheless conclude from our experiments that the low-affinity binding of Cdc42 (in the triphosphate conformation) to PAK modifies the closed conformation in a way that the activation loop becomes available for autophosphorylation but the inhibitory interactions between the RD and CD are preserved. This would be in line with the three-dimensional structure where the activation loop is rather flexible and situated on the side of the interface between the RD and CD (only residue Thr423 is determined in the structure) and might become available for transphosphorylation prior to complete release of the regulatory region. Recent studies of αPAK have shown that Ser144 in the regulatory region, which is located directly in the cleft between the two lobes of the kinase domain, has to be phosphorylated for full activation (8), in line with our data. It was discussed there that this phosphorylation is required to allow release of the RD, full activation, and, as our results show, the formation of a high-affinity complex with Cdc42. According to our data, proteolysis of the polypeptide chain thus assumes the role of Cdc42, allowing autophosphorylation by a mechanism—not understood from the present structural data—which then induces the conformational switch leading to high-affinity binding of Cdc42.
ACKNOWLEDGMENTS
We thank Ed Manser for the αPAKL404S clone, members of the structural biology department for helpful discussions, and Rita Schebaum for secretarial assistance.
This work was supported by Deutsche Forschungsgemeinschaft grant SFB 394 (to A.S., H.E.M, K.S., and A.W.)
REFERENCES
- 1.Abdul-Manan N, Aghazadeh B, Liu G A, Majumdar A, Ouerfelli O, Siminovitch K A, Rosen M K. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399:379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 2.Bagrodia S, Cerione R A. PAK to the future. Trends Cell Biol. 1999;9:350–355. doi: 10.1016/s0962-8924(99)01618-9. [DOI] [PubMed] [Google Scholar]
- 3.Benner G E, Dennis P B, Masaracchia R A. Activation of an s6/h4 kinase (Pak 65) from human placenta by intramolecular and intermolecular autophosphorylation. J Biol Chem. 1995;270:21121–21128. doi: 10.1074/jbc.270.36.21121. [DOI] [PubMed] [Google Scholar]
- 4.Bourne H R, Sanders D A, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 5.Bourne H R, Sanders D A, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 6.Brown J L, Stowers L, Baer M, Trejo J, Coughlin S, Chant J. Human STE20 homologue HPAK1 links GTPases to the JNK map kinase pathway. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 7.Burbelo P D, Hall A. 14-3-3 proteins. Hot numbers in signal transduction. Curr Biol. 1995;5:95–96. doi: 10.1016/s0960-9822(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 8.Chong C, Tan L L L, Manser E. The mechanism of PAK activation: autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001;276:17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- 9.Daniels R H, Bokoch G M. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 10.Gatti A, Huang Z D, Tuazon P T, Traugh J A. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J Biol Chem. 1999;274:8022–8028. doi: 10.1074/jbc.274.12.8022. [DOI] [PubMed] [Google Scholar]
- 11.Geyer M, Herrmann C, Wohlgemuth S, Wittinghofer A, Kalbitzer H R. Structure of the Ras-binding domain of RalGEF and implications for Ras binding and signalling. Nat Struct Biol. 1997;4:694–699. doi: 10.1038/nsb0997-694. [DOI] [PubMed] [Google Scholar]
- 12.Gizachew D, Guo W, Chohan K K, Sutcliffe M J, Oswald R E. Structure of the complex of Cdc42Hs with a peptide derived from P-21 activated kinase. Biochemistry. 2000;39:3963–3971. doi: 10.1021/bi992646d. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman G R, Cerione R A. Flipping the switch: the structural basis for signaling through the CRIB motif. Cell. 2000;102:403–406. doi: 10.1016/s0092-8674(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 14.Hunter T. A thousand and one protein kinases. Cell. 1987;50:823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- 15.Hunter T. Signaling—2000 and beyond. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 16.Kim A S, Kakalis L T, Abdul-Manan M, Liu G A, Rosen M K. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 17.Kobe B, Kemp B E. Active site-directed protein regulation. Nature. 1999;402:373–376. doi: 10.1038/46478. [DOI] [PubMed] [Google Scholar]
- 18.Lee N, Macdonald H, Reinhard C, Halenbeck R, Roulston A, Shi T, Williams L T. Activation of hpak65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc Natl Acad Sci USA. 1997;94:13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Escalante R, Firtel R A. A Ras GAP is essential for cytokinesis and spatial patterning in dictyostelium. Development. 1997;124:983–996. doi: 10.1242/dev.124.5.983. [DOI] [PubMed] [Google Scholar]
- 20.Lei M, Lu W, Meng W, Parrini M-C, Eck M J, Mayer B J, Harrison S C. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 21.Manser E, Chong C, Zhao Z S, Leung T, Michael G, Hall C, Lim L. Molecular-cloning of a new member of the p21-cdc42/rac- activated kinase (pak) family. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- 22.Manser E, Huang H Y, Loo T H, Chen X Q, Dong J M, Leung T, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature. 1993;363:364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- 24.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 25.Martin G A, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick F, Wittinghofer A. Interactions between Ras proteins and their effectors. Curr Opin Biotechnol. 1996;7:449–456. doi: 10.1016/s0958-1669(96)80123-6. [DOI] [PubMed] [Google Scholar]
- 27.Morreale A, Venkatesan M, Mott H R, Owen D, Nietlispach D, Lowe P N, Laue E D. Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat Struct Biol. 2000;7:384–388. doi: 10.1038/75158. [DOI] [PubMed] [Google Scholar]
- 28.Mott H R, Owen D, Nietlispach D, Lowe P N, Manser E, Lim L, Laue E D. Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature. 1999;399:384–388. doi: 10.1038/20732. [DOI] [PubMed] [Google Scholar]
- 29.Nassar N, Horn G, Herrmann C, Scherer A, McCormick F, Wittinghofer A. The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue. Nature. 1995;375:554–560. doi: 10.1038/375554a0. [DOI] [PubMed] [Google Scholar]
- 30.Nomanbhoy T, Cerione R A. Fluorescence assays of Cdc42 interactions with target/effector proteins. Biochemistry. 1999;38:15878–15884. doi: 10.1021/bi9916832. [DOI] [PubMed] [Google Scholar]
- 31.Owen D, Mott H R, Laue E D, Lowe P N. Residues in Cdc42 that specify binding to individual CRIB effector proteins. Biochemistry. 2000;39:1243–1250. doi: 10.1021/bi991567z. [DOI] [PubMed] [Google Scholar]
- 32.Plowman G D, Sudarsanam S, Bingham J, Whyte D, Hunter T. The protein kinases of Caenorhabditis elegans: a model for signal transduction in multicellular organisms. Proc Nat Acad Sci USA. 1999;96:13603–13610. doi: 10.1073/pnas.96.24.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pohlner J, Klauser T, Kuttler E, Halter R. Sequence-specific cleavage of protein fusions using a recombinant Neisseria type 2 IgA protease. Bio/Technology. 1992;10:799–804. doi: 10.1038/nbt0792-799. [DOI] [PubMed] [Google Scholar]
- 34.Rudel T, Bokoch G M. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of pak2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph M G, Bayer P, Abo A, Kuhlmann J, Vetter I R, Wittinghofer A. The Cdc42/Rac interactive binding region motif of the Wiskott Aldrich syndrome protein (WASP) is necessary but not sufficient for tight binding to Cdc42 and structure formation. J Biol Chem. 1998;273:18067–18076. doi: 10.1074/jbc.273.29.18067. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph M G, Wittinghofer A, Vetter I R. Nucleotide binding to the G12V-mutant of Cdc42 investigated by X-ray diffraction and fluorescence spectroscopy: two different nucleotide states in one crystal. Protein Sci. 1999;8:778–787. doi: 10.1110/ps.8.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens W K, Vranken W, Goudreau N, Xiang H, Xu P, Ni F. Conformation of a Cdc42/Rac interactive binding peptide in complex with Cdc42 and analysis of the binding interface. Biochemistry. 1999;38:5968–5975. doi: 10.1021/bi990426u. [DOI] [PubMed] [Google Scholar]
- 38.Stokoe D, McCormick F. Activation of c-Raf-1 by Ras and SRC through different mechanisms—activation in vivo and in vitro. EMBO J. 1997;16:2384–2396. doi: 10.1093/emboj/16.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symons M, Derry J M J, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the gtpase cdc42hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 40.Tahara S M, Traugh J A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Identification and characterization of a protein kinase activated by proteolysis. J Biol Chem. 1981;256:11558–11564. [PubMed] [Google Scholar]
- 41.Tahara S M, Traugh J A. Differential activation of two protease-activated protein kinases from reticulocytes by a Ca2+-stimulated protease and identification of phosphorylated translational components. Eur J Biochem. 1982;126:395–399. doi: 10.1111/j.1432-1033.1982.tb06793.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson G, Owen D, Chalk P A, Lowe P N. Delineation of the cdc42/rac-binding domain of p21-activated kinase. Biochemistry. 1998;37:7885–7891. doi: 10.1021/bi980140+. [DOI] [PubMed] [Google Scholar]
- 43.Tu H, Wigler M. Genetic evidence for Pak1 autoinhibition and its release by Cdc42. Mol Cell Biol. 1999;19:602–611. doi: 10.1128/mcb.19.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vetter I R, Linnemann T, Wohlgemuth S, Geyer M, Kalbitzer H R, Herrmann C, Wittinghofer A. Structural and biochemical analysis of Ras-effector signaling via RalGDS. FEBS Lett. 1999;451:175–180. doi: 10.1016/s0014-5793(99)00555-4. [DOI] [PubMed] [Google Scholar]
- 45.Walter B N, Huang Z D, Jakobi R, Tuazon P T, Alnemri E S, Litwack G, Traugh J A. Cleavage and activation of pal-activated protein kinase gamma-pak by cpp32 (caspase 3)—effects of autophosphorylation on activity. J Biol Chem. 1998;273:28733–28739. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- 46.Yang S D, Chang S Y, Soderling T R. Characterization of an autophosphorylation-dependent multifunctional protein kinase from liver. J Biol Chem. 1987;262:9421–9427. [PubMed] [Google Scholar]
- 47.Yang S D, Fong Y L, Yu J S, Liu J S. Identification and characterization of a phosphorylation-activated, cyclic AMP and Ca2+-independent protein kinase in the brain. J Biol Chem. 1987;262:7034–7040. [PubMed] [Google Scholar]
- 48.Yu J S, Chen W J, Ni M H, Chan W H, Yang S D. Identification of the regulatory autophosphoylation site of autophosphorylation-dependent protein kinase (auto-kinase)—evidence that auto-kinase belongs to a member of the p21-activated kinase family. Biochem J. 1998;334:121–131. doi: 10.1042/bj3340121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zenke F T, King C C, Bohl B P, Bokoch G M. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B L, Wang Z X, Zheng Y. Characterization of the interactions between the small GTPase Cdc42 and its GTPase-activating proteins and putative effectors—comparison of kinetic properties of Cdc42 binding to the Cdc42-interactive domains. J Biol Chem. 1997;272:21999–22007. doi: 10.1074/jbc.272.35.21999. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Z S, Manser E, Chen X Q, Chong C, Leung T, Lim L. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zumbihl R, Aepfelbacher M, Andor A, Jacobi C A, Ruckdeschel H, Rouot B, Heesemann J. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J Biol Chem. 1999;274:29289–29293. doi: 10.1074/jbc.274.41.29289. [DOI] [PubMed] [Google Scholar]