Abstract
Purpose
Create a new definition of near-occlusion with full collapse to predicting recurrent stroke.
Methods
Pooled analysis of two studies. Patients with symptomatic ≥ 50% carotid stenoses were included. Outcome was preoperative recurrent ipsilateral ischemic stroke or retinal artery occlusion within 28 days of presenting event. We analyzed several artery diameters on computed tomography angiography and stenosis velocity on ultrasound.
Results
A total of 430 patients with symptomatic ≥ 50% carotid stenosis were included, 27% had near-occlusion. By traditional definition, 27% with full collapse and 11% without full collapse reached the outcome (p = 0.047). Distal internal carotid artery (ICA) diameter, ICA ratio, and ICA-to-external carotid artery ratio were associated with the outcome. Best new definition of full collapse was distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42. With this new definition, 36% with full collapse and 4% without full collapse reached the outcome (p < 0.001).
Conclusions
Defining near-occlusion with full collapse as distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42 seems to yield better prognostic discrimination than the traditional appearance-based definition. This novel definition can be used in prognostic and treatment studies of near-occlusion with full collapse.
Keywords: Stroke, Carotid stenosis, Carotid near-occlusion, CT-angiography, Ultrasound
Introduction
Carotid near-occlusion is a severe carotid stenosis where the internal carotid artery (ICA) distal to the stenosis is reduced in size (“collapsed”) [1–3]. The distal ICA size reduction is often subtle, resulting in normal-appearing but small distal ICA (near-occlusion without full collapse, Fig. 1a). The distal ICA size reduction can be severe, resulting in a threadlike distal ICA (near-occlusion with full collapse, Fig. 1b). Conventional carotid stenoses do not cause distal ICA size reduction [1].
Fig. 1.
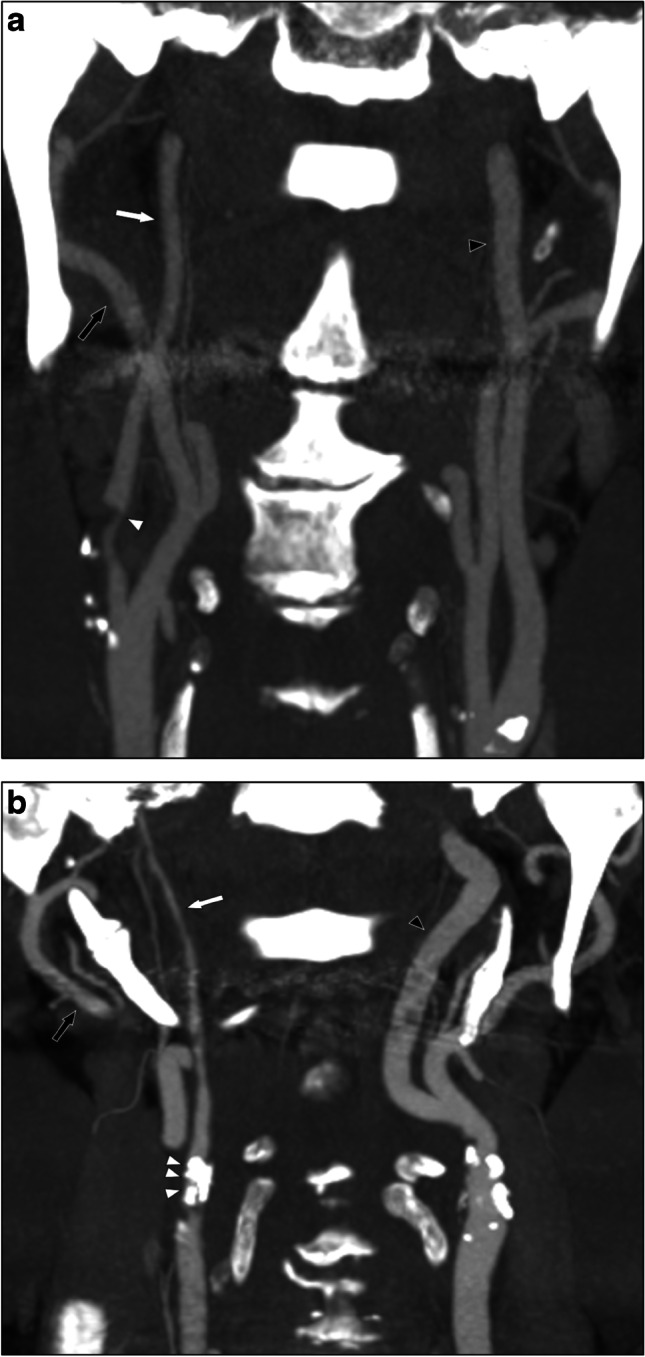
Carotid near-occlusions, coronal views. a Right-sided near-occlusion without full collapse. Beyond a severe stenosis (white arrowhead), the distal ICA is normal-appearing (white arrow, diameter 2.8 mm). However, the distal ICA is smaller than contralateral ICA (black arrowhead, ICA ratio 0.72) and similar to ipsilateral ECA (black arrow, ECA ratio 0.97). It was classified as without full collapse with all presented definitions. b Right-sided near-occlusion with full collapse. Beyond a severe and calcified stenosis (white arrowheads), the distal ICA has a threadlike appearance (white arrow, diameter 1.2 mm), clearly smaller than both contralateral ICA (black arrowhead, ICA ratio 0.20) and ipsilateral ECA (black arrow, ECA ratio 0.40). It was classified as with full collapse with all presented definitions
Guidelines recommend conservative treatment of symptomatic near-occlusion based on trials where 94% of near-occlusion were without full collapse [1–5]. Two studies have shown that near-occlusion with full collapse has a high risk of recurrent ipsilateral ischemic stroke during the first days after the presenting event, but the risk for near-occlusion without full collapse was modest [6, 7]. One study reported recurrent stroke in 18% of near-occlusion with full collapse, but with unclear timing and no control group [8]. However, one registry study found a low early risk for all near-occlusions [9]. As a high, possibly treatable, risk of stroke seems to exist for near-occlusion with full collapse, the definition of full collapse becomes relevant to establish. The traditional definition of full collapse, a threadlike distal ICA appearance, might not be optimal for prognosis. No other definition has been compared with the risk of recurrent stroke. A measurement-based definition, either anatomical (artery size) or physiological (flow velocity), might not only have better prognostic discrimination, but will likely be more reliable between observers than the traditional definition.
Aim
The aim of this study was to create a new definition of near-occlusion with full collapse based on angiographic and/or ultrasound measurements, aimed at predicting preoperative recurrent ipsilateral ischemic stroke.
Methods
This was a pooled individual patient data analysis of two previously reported studies [6, 7]. The design of the two studies is summarized in Table 1. Both included patients with symptomatic ≥ 50% (NASCET) carotid stenosis that were eligible for revascularization. Symptomatic stenosis was defined as < 6 months since last ipsilateral event (a vast majority was within days). Eligible for revascularization was defined as either undergoing revascularization or were reasonable candidates for revascularization. Reasonable candidate were cases with issues such as recurrent severe stroke, patient refusal, technical considerations, and when risks outweighed benefit after careful consideration. However, cases with very advanced age, severe co-morbidity, and severe index stroke were excluded. In this analysis, only those that were examined with computed tomography angiography (CTA) of sufficient quality within 6 months of the presenting event were included. Ultrasound, which was otherwise used alone, has poor sensitivity for near-occlusion [10, 11]. A total of 430 patients with symptomatic ≥ 50% carotid stenosis were included of which 116 (27%) had near-occlusion, 47 with and 69 without full collapse. Baseline characteristics are presented in Table 2. The included studies had ethical approval as presented in their original articles [6, 7].
Table 1.
Design summary of the two included studies
| Johansson et al. [6] | Gu et al. [7] | |
|---|---|---|
| Years on inclusion | 2007–2009 | 2010–2014 |
| Site | Umeå, Sweden | Umeå, Sweden |
| Main purpose of original study | Assess risk of recurrent stroke in all ≥ 50% stenosis | Assess risk of recurrent stroke in near-occlusion |
| Design | Prospective | Retrospective |
| Case detection approach | Diagnosis of ≥ 50% stenosis in clinic routine | Re-assessment of 4440 consecutive CTAs |
| Total cases with symptomatic ≥ 50% stenosis | 230 | 514 |
| Cases excluded due to no relevant CTA (% of total) | 164 (71) | 149 (29) |
| Included cases (% of total) | 66 (29) | 365 (71) |
| Conventional ≥ 50% stenosis n (%) | 49 (74) | 266 (73) |
| Near-occlusion without full collapsea n (%) | 12 (18) | 57 (16) |
| Near-occlusion with full collapsea n (%) | 5 (8) | 42 (12) |
CTA computed tomography angiography
aDivision by traditional definition
Table 2.
Baseline comparisons between stenosis groups, using traditional definition of full collapse
| Missing data | Conventional ≥ 50% stenosis (n = 315) | Near-occlusion without full collapse (n = 69) | Near-occlusion with full collapse (n = 47) | pa | ||
|---|---|---|---|---|---|---|
| Age meanb (SD) | 0 | 72 (8) | 70 (9) | 70 (8) | 0.02 | |
| Womenb n (%) | 0 | 96 (30) | 19 (28) | 16 (34) | 0.77 | |
| Myocardial infarctionb n (%) | 1 | 65 (21) | 12 (17) | 6 (13) | 0.40 | |
| Symptomatic peripheral artery diseaseb n (%) | 1 | 25 (8) | 8 (12) | 3 (6) | 0.56 | |
| Current anginab n (%) | 2 | 50 (16) | 7 (10) | 6 (13) | 0.48 | |
| Heart failureb n (%) | 2 | 21 (7) | 3 (4) | 2 (4) | 0.68 | |
| Current smokerb n (%) | 3 | 51 (16) | 16 (23) | 9 (19) | 0.39 | |
| Diabetesb n (%) | 1 | 78 (25) | 18 (26) | 9 (19) | 0.68 | |
| Hypertension† n (%) | 1 | 289 (92) | 56 (81) | 42 (89) | 0.02 | |
| Previous arterial revascularizationb,c n (%) | 1 | 68 (22) | 11 (16) | 9 (19) | 0.54 | |
| Type of presenting event | Afx | 0 | 33 (10) | 13 (20) | 3 (6) | 0.16 |
| RAO | 16 (5) | 2 (3) | 1 (2) | |||
| TIA | 117 (37) | 28 (41) | 15 (32) | |||
| Stroke | 149 (47) | 26 (38) | 28 (60) | |||
| Sought medical attention on day of presenting event n (%) | 0 | 232 (74) | 44 (64) | 34 (72) | 0.26 | |
| Delay presenting event—CTA median (IQR) | 0 | 3 (1–7) | 5 (2–15) | 4 (1–14) | 0.03 | |
| Delay presenting event—ultrasound median (IQR) | 93d | 6 (3–12) | 7 (4–16) | 9 (5–18) | 0.15 | |
| Underwent revascularizatione n (%) | 0 | 209 (66) | 55 (78) | 13 (28) | < 0.001 | |
| Delay presenting event—revascularization median (IQR) | 0 | 10 (7–21) | 13 (7–24) | 18 (9–29) | 0.12 | |
| Stenosis diameter mm mean (SD) | 11f | 1.4 (0.5) | 0.8 (0.3) | 0.7 (0.3) | < 0.001 | |
| Distal ICA diameter mm mean (SD) | 0 | 4.2 (0.6) | 2.9 (0.5) | 1.1 (0.8) | < 0.001 | |
| ICA-ratio mean (SD) | 16 g | 1.00 (0.26) | 0.64 (0.11) | 0.30 (0.28) | < 0.001 | |
| ECA ration mean (SD) | 0 | 1.64 (0.40) | 1.08 (0.47) | 0.40 (0.29) | < 0.001 | |
| Stenosis PSV m/s mean (SD) | 93d | 2.9 (1.4) | 3.7 (1.7) | 2.2 (2.4) | < 0.001 | |
Afx amaurosis fugax, CTA computed tomography angiography, ECA external carotid artery, ICA internal carotid artery, IQR interquartile range, PSV peak systolic velocity, RAO retinal artery occlusion, SD standard deviation, TIA transient ischemic attack
aχ2-test, one-way ANOVA, and Kruskal–Wallis
bUsed as co-variate in the step-wise removal multivariable analysis
cCoronary, carotid, or leg artery interventions
d84 had no ultrasound exam and 9 had echo shadow. 31 were among near-occlusions
eCarotid endarterectomy or angioplasty with stenting
f7 were among near-occlusions, caused by severe calcification
g4 were among near-occlusions, caused by contralateral occlusion
Outcomes
The outcome was defined the same way as in both underlying studies and major trials [3, 6, 7]: recurrent ipsilateral ischemic stroke or ipsilateral retinal artery occlusion (RAO). Stroke was defined by the World Health Organization definition [12], i.e., required symptoms to last > 24 h, whereas transient ischemic attack (TIA, not part of main outcome) was symptoms lasting < 24 h, regardless of imaging evidence of fresh ischemia. RAO was defined as monocular vision loss lasting > 24 h, whereas amaurosis fugax (not part of outcome) lasted < 24 h. In both studies, only anterior circulation events were considered. The risk of the outcome was assessed during the first 28 days after presenting event, until revascularization or death, whichever came first.
Parameters
Both previously published and unpublished data were available for analysis. Available data included baseline factors, recurrent events, CTA assessments, CTA measurements, and velocity data from carotid ultrasound. In Gu et al., CTAs were performed locally or at any of 11 referring hospitals, in Johansson et al., only the referring hospital. In both, various machines and clinical protocols were used for CTA. In both studies, ultrasound was performed at the local hospital. The ultrasound exams were performed by several experienced sonographers using a standardized protocol including long- and short-axis assessment of common, internal, and external carotid arteries, with color doppler, pulsed doppler in duplex mode, and power doppler. Main emphasis was assessment of highest peak systolic velocity (PSV) in the stenosis, with adjustable angle correction ≤ 60°. Various ultrasound machines were used during the studies.
In Gu et al., near-occlusion was diagnosed on CTA by two observers (EJ, 5 years carotid grading experience and AF, > 40 years carotid grading experience) using an approach of systematic interpretation of features, not over interpreting anatomical variance as near-occlusion [7, 13]. Cases without near-occlusion (conventional stenosis) were graded by comparing smallest stenosis lumen diameter with the distal ICA diameter. Per NASCET method, the distal ICA was measured well beyond the bulb as the ICA is usually larger in the bulb region [3]. For this analysis, the same two observers reassessed the CTAs from Johansson et al. with the same approach. In both studies, the two observers were blinded to each other and to the outcome.
Five parameters were candidates for novel definitions of full collapse. Four were CTA-based: stenosis diameter, distal ICA diameter, ipsilateral to contralateral distal ICA diameter ratio (ICA ratio), and distal ICA to ipsilateral external carotid artery diameter ratio (ECA ratio). Stenosis PSV was based on ultrasound. CTA measurements were gathered by one observer (EJ) with a random selection of 49 cases measured twice for reliability analyses. Stenosis PSVs were extracted from reassessment of images by several overlapping observers. All measurement extraction was done blinded to the outcome.
Analyses and statistics
Individual patient data were assessed in a pooled database. The analysis was conducted in four steps. (1) We assessed if any of the five candidate parameters was significantly associated with the outcome among near-occlusions. (2) Possible thresholds of the significant parameters were assessed, balancing high sensitivity (maximum number of outcomes in the full collapse group) with having the smallest full collapse group possible. (3) Combinations of thresholds of the significant parameters were assessed in a similar fashion as in step 2. In cases with one parameter was missing, the other parameter was used alone. (4) The risk of the outcome in the traditional and new near-occlusion groups was compared with conventional ≥ 50% stenosis as reference in both unadjusted and multivariable analyses.
Where appropriate, we used 95% confidence interval (95%CI), mean, median, standard deviation (SD), interquartile range (IQR), 2-sided χ2-test, one-way ANOVA, Mann–Whitney test, and Kruskal–Wallis test. Reliability of CTA measurements was assessed with two-way mixed interclass correlation (ICC) for absolute agreement. The outcome was assessed with Kaplan–Meier (with log rank test) and Cox regression, using revascularization and death as censor. Two multivariable models were assessed: one adjusting for age & sex, the other included baseline parameters and step-wise removing the co-variate with the highest p-value until all variables had p < 0.05. Two risk markers have been suggested in this context: age and type of presenting event [14]. Age was assessed in a dedicated multivariable analysis. Type of presenting event could not be assessed in this fashion as no case with retinal presenting event (amaurosis fugax or RAO) reached the outcome. Thus, type of presenting event is reasonably an important factor, but not assessable in this study. Statistical significance was defined as p < 0.05 and IBM SPSS 26.0 was used in the calculations.
Results
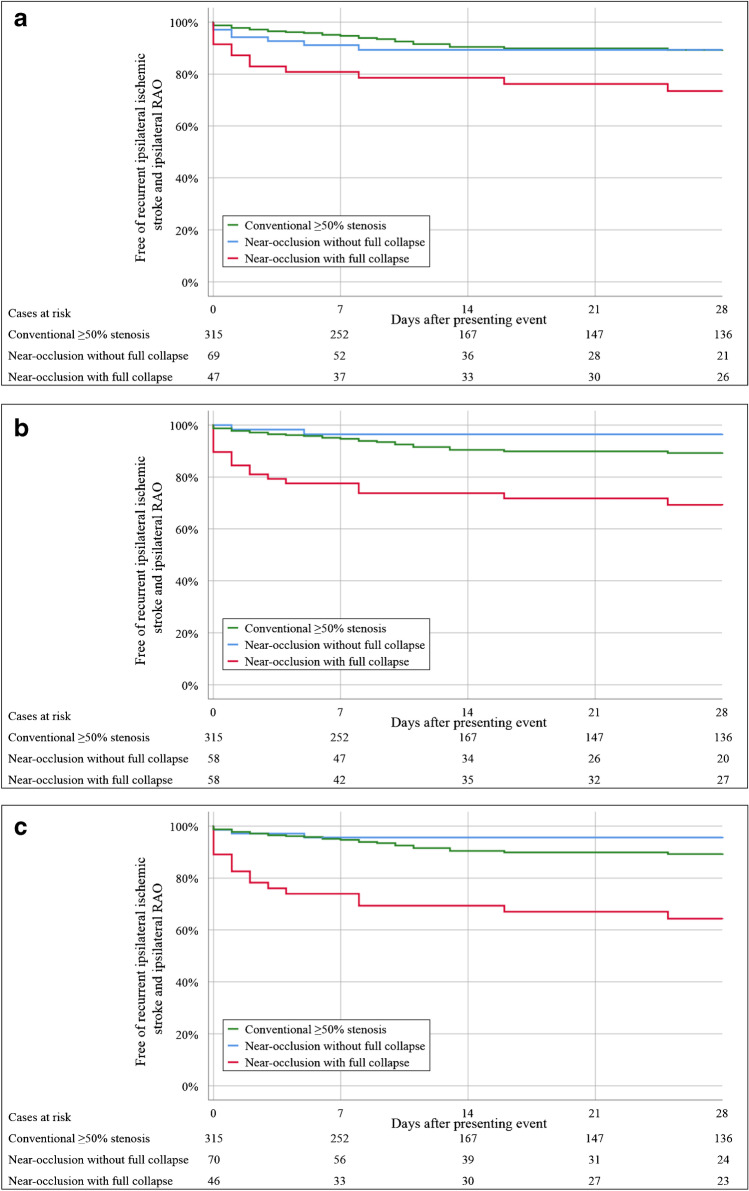
There were 19 outcome events (ipsilateral ischemic stroke or ipsilateral RAO) among the 116 near-occlusions. By traditional definition, the 28-day risk of the outcome was higher in near-occlusion with full collapse (27%) than near-occlusion without full collapse (11%, p = 0.047, Table 3, Fig. 2a). Distal ICA diameter, ICA ratio, and ECA ratio were associated with the outcome among near-occlusions (Table 4). Assessing thresholds < 18 outcomes could reasonably be included in a full collapse group as ≥ 18 outcomes would require ≥ 69% of cases in the full collapse group. Several single parameter thresholds had similar overall performance (Table 3). Combining distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42 was both sensitive and with a small full collapse group (Fig. 2c). Other combinations produced similar results, but only this combination made an improvement in either sensitivity or full collapse group size compared to single parameters (data not shown).
Table 3.
Number of outcomes and Kaplan–Meier curve–based risk of the outcome among near-occlusions at different time intervals after presenting event. Different definitions of full collapse are presented. Conventional ≥ 50% stenosis group presented for general comparison. The outcome was preoperative recurrent ipsilateral ischemic stroke or ipsilateral retinal artery occlusion
| Definition of full collapse | Full collapse | Cases (% of near-occlusions) | 2 days | 7 days | 14 days | 28 days | pa | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % Risk (95%CI) | n | % Risk (95%CI) | n | % Risk (95%CI) | n | % Risk (95%CI) | ||||
| Traditional, by appearance | No | 69 (59) | 4 | 6 (0–11) | 6 | 9 (2–15) | 7 | 11 (3–18) | 7 | 11 (3–18) | 0.047 |
| Yes | 47 (41) | 8 | 17 (6–28) | 9 | 19 (8–30) | 10 | 21 (10–33) | 12 | 27 (14–39) | ||
| Distal ICA diameter ≤ 2.4 mm | No | 58 (50) | 1 | 2 (0–5) | 2 | 4 (0–8) | 2 | 4 (0–8) | 2 | 4 (0–8) | < 0.001 |
| Yes | 58 (50) | 11 | 19 (9–29) | 13 | 22 (12–33) | 15 | 26 (15–38) | 17 | 31 (18–43) | ||
| Distal ICA diameter ≤ 2.0 mm | No | 71 (61) | 3 | 4 (0–9) | 4 | 6 (0–11) | 4 | 6 (0–11) | 4 | 6 (0–11) | < 0.001 |
| Yes | 45 (39) | 9 | 20 (8–32) | 11 | 24 (12–37) | 13 | 29 (16–43) | 15 | 34 (20–48) | ||
| ICA ratio ≤ 0.47 | No | 68 (61) | 2 | 3 (0–7) | 4 | 6 (0–12) | 4 | 6 (0–12) | 4 | 6 (0–12) | < 0.001 |
| Yes | 44 (39) | 10 | 23 (10–35) | 11 | 25 (12–38) | 13 | 30 (16–43) | 15 | 35 (21–50) | ||
| ECA ratio ≤ 0.96 | No | 43 (37) | 2 | 5 (0–11) | 4 | 10 (1–18) | 4 | 10 (1–18) | 4 | 10 (1–18) | < 0.001 |
| Yes | 73 (63) | 10 | 14 (6–22) | 11 | 15 (7–23) | 13 | 18 (9–27) | 15 | 22 (12–32) | ||
| Distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42 | No | 70 (60) | 2 | 3 (0–7) | 3 | 4 (0–9) | 3 | 4 (0–9) | 3 | 4 (0–9) | < 0.001 |
| Yes | 46 (40) | 10 | 22 (10–34) | 12 | 26 (13–39) | 14 | 31 (17–44) | 16 | 36 (22–50) | ||
| Conventional ≥ 50% stenosis | 315 (NA) | 9 | 3 (1–5) | 16 | 5 (3–8) | 25 | 10 (6–13) | 27 | 11 (7–15) | NA | |
95%CI 95% confidence interval, ECA external carotid artery, ICA internal carotid artery, NA not applicable
aLog rank test between the two near-occlusion groups, i.e., conventional ≥ 50% stenosis group not included in these calculations
Fig. 2.
Kaplan–Meier curves of three approaches to define near-occlusion with full collapse. Tests for statistical significance presented in Tables 3 and 5. a Traditional approach. b Distal ICA diameter ≤2.4 mm. c Distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42. The outcome was preoperative recurrent ipsilateral ischemic stroke or ipsilateral retinal artery occlusion
Table 4.
Cox regression of the hazard ratio of the outcome among near-occlusions among the five candidate parameters for novel definitions of full collapse. The outcome was preoperative recurrent ipsilateral ischemic stroke or ipsilateral retinal artery occlusion within 28 days of presenting event
| Near-occlusion parameter | HR (95%CI) | p |
|---|---|---|
| Stenosis diameter (per 1 mm increment) | 0.44 (0.07–2.53) | 0.35 |
| Distal ICA diameter (per 1 mm increment) | 0.51 (0.34–0.77) | 0.001 |
| ICA ratio (per 0.1 increment) | 0.76 (0.64–0.91) | 0.03 |
| ECA ratio (per 0.1 increment) | 0.86 (0.77–0.96) | 0.009 |
| Stenosis PSV (per 1 m/s increment) | 1.11 (0.80–1.56) | 0.53 |
ECA external carotid artery, HR hazard ratio, ICA internal carotid artery, PSV peak systolic velocity
Multivariable analyses were conducted on the traditional and two of the new full collapse definitions (Table 5). With all three definitions, full collapse had a higher risk than conventional ≥ 50% stenosis in both unadjusted and age & sex–adjusted analyses. The two novel approaches tended to have higher HR for full collapse (3.6–4.4) than the traditional approach (2.9–3.0), but with overlapping 95%CIs. Cases without full collapse showed a weak tendency to have a lower risk than conventional ≥ 50% stenosis with the novel definitions, but not with the traditional definition. In the step-wise removal multivariable analyses, no other variable than degree of stenosis remained at p < 0.05 for all three combinations. Age was not associated with the outcome: unadjusted HR 1.1 (95%CI 0.8–1.6, p = 0.57) and adjusted for degree of stenosis HR 1.2 (95%CI 0.8–1.7, p = 0.40) per 10-year increment, when defining full collapse as distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42.
Table 5.
Cox regression of the risk of the outcome in the traditional and two novel definitions of full collapse. Unadjusted and age & sex–adjusted models. Conventional ≥ 50% stenosis was used as reference. The outcome was preoperative recurrent ipsilateral ischemic stroke or ipsilateral retinal artery occlusion within 28 days of presenting event
| Definition of full collapse | Full collapse | Unadjusted HR (95%CI) | p | Age & sex–adjusted HR (95%CI) | p |
|---|---|---|---|---|---|
| Traditional, by appearance | No | 1.2 (0.5–2.8) | 0.66 | 1.3 (0.5–2.9) | 0.59 |
| Yes | 2.9 (1.5–5.7) | 0.002 | 3.0 (1.5–6.0) | 0.002 | |
| Distal ICA diameter ≤ 2.4 mm | No | 0.4 (0.1–1.6) | 0.20 | 0.4 (0.1–1.7) | 0.21 |
| Yes | 3.6 (1.9–6.5) | < 0.001 | 3.7 (2.0–6.9) | < 0.001 | |
| Distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42 | No | 0.5 (0.1–1.6) | 0.24 | 0.5 (0.2–1.7) | 0.27 |
| Yes | 4.2 (2.3–7.8) | < 0.001 | 4.4 (2.3–8.2) | < 0.001 |
HR hazard ratio, ICA internal carotid artery
None of the near-occlusion cases with missing data of stenosis diameter or ICA ratio reached the outcome. However, 58% of near-occlusions with the outcome lacked stenosis PSV data, compared to 21% of near-occlusion without the outcome (p = 0.002). All but one missing PSV data was due to no ultrasound exam, the exception was due to echo shadow. Median time between presenting event and the outcome tended to be longer for those examined with ultrasound (3, IQR 1–14, days) than those not examined with ultrasound (1, IQR 0–3, days), p = 0.15.
Of the 315 conventional ≥ 50% stenoses, 2 (< 1%) had an ECA ratio ≤ 0.96, but none had distal ICA ≤ 2.4 mm or ICA ratio ≤ 0.47.
Intra-rater ICC was excellent for stenosis diameter (0.99; 95%CI 0.98–0.99), distal ICA diameter (0.98; 95%CI 0.97–0.99), and ICA ratio (0.99; 95%CI 0.98–0.99). Inter-rater ICC was very good for ECA ratio (0.93; 95%CI 0.90–0.96).
Discussion
The main finding of this study was that novel measurement–based definitions of near-occlusion with full collapse seem to provide better prognostic outcomes than the traditional appearance-based approach.
Near-occlusion was defined in NASCET by necessity because the reduction of distal ICA diameter in near-occlusions would lead to stenosis underestimation if the percent comparison was applied [1–3]. Near-occlusion has been, and likely still is, misunderstood by many [2]. Although often described as rare, near-occlusion constitutes 30% of ≥ 50% symptomatic stenosis [15]. Many omit near-occlusion without full collapse [16], even though 94% of near-occlusions in NASCET and ECST were without full collapse [3]. Defining full collapse was initially not done for prognosis, but was rather descriptive and highlighted that not all near-occlusions have a threadlike appearance [1–3]. As symptomatic near-occlusion with full collapse has been shown to have a worse short-term prognosis than those without full collapse [6, 7], a prognostic-based definition is reasonable. Also, “threadlike appearance” is difficult to apply consistently, examples of different applications in the same material exist [3, 17]. By using measurements, better reliability is likely, although that is beyond the scope of this study to assess. The novel definitions only tended to have better prognostic outcome than the traditional definition (not clearly superior), the reliability aspects alone is sufficient to favor the use of a measurement-based approach. As there was no overlap between these new full collapse definitions and conventional ≥ 50% stenosis (except for ECA ratio), the definitions can be applied to all carotid stenosis without risking mistaking a conventional stenosis for near-occlusion with full collapse.
Of the three significant parameters, one was an absolute measurement (distal ICA diameter) and two were relative (ICA ratio, ECA ration), which have different pros and cons: Absolute measurements can vary with study quality, windowing, and handling of the fuzzy edge. While intra-rater reliability was very good, it is not necessarily transferable to raters in routine practice. Relative measurements require a relevant comparison and the comparison can sometimes be misleading, such as bilateral near-occlusion affecting ICA ratio and ECA size vary between patients. Stenosis diameter was not associated with the outcome among near-occlusions, but this could be false-negative due to the use of CTA as stenosis diameter similar to voxel size make assessment difficult. A conventional angiography-based study might yield a positive association. The non-association between stenosis PSV and outcome was possibly due to lacking data. Further study into the prognostic potential of carotid ultrasound in near-occlusion should reasonably include the assessment of distal velocity [18].
Near-occlusion without full collapse had no substantial benefit with CEA in NASCET and ECST due to a relatively low long-term risk of stroke in the medical arm [1–5]. This seems to be applicable to short-term prognosis as well, especially with the new definition. Although our definitions make separation against full collapse easy, a diagnostic challenge remains for separation from conventional stenosis [13, 16]. Further diagnostic work is warranted, which can include both ultrasound and phase contrast approaches [18, 19].
We found that for all symptomatic ≥ 50% stenosis, only degree of stenosis (near-occlusion with full collapse) was associated with the outcome. Higher age and cerebral (not retinal) presenting event have been suggested, but without assessment of near-occlusion [14]. We found no association with age. However, when previously suggested in a study with 377 cases, age was not strongly associated with the outcome (p = 0.02). We showed a strong association for full collapse (p < 0.001) with similar number of cases included (n = 430), suggesting degree of stenosis being more important than age [14].
Our study had several weaknesses. The use of medical treatment could not be assessed as it was gathered with different definitions in the underlying studies [6, 7]. However, dual antiplatelet medication was rarely used [6, 7]. Both of the underlying studies had selection to CTA, albeit it became more standard practice over the 8 years of inclusion [6, 7]. Reliability of ultrasound with test–retest approach was not performed. With the use of adjustable thresholds and limited number of outcomes, some degree of model fitting can be suspected, validation is warranted. Main strengths were the combined data from relevant studies with analyses based on individual data, and reassessment of CTA measurements for a uniform analysis.
In summary, defining near-occlusion with full collapse as distal ICA diameter ≤ 2.0 mm and/or ICA ratio ≤ 0.42 seems to yield better prognostic discrimination than the traditional appearance-based definition. This novel definition can be used in prognostic and treatment studies of near-occlusion with full collapse.
Author contribution
EJ: Concept, design, funding, data gathering, data analysis, and manuscript draft.
TG: Data gathering and manuscript edit.
AJF: Data gathering and manuscript edit.
Funding
Open access funding provided by Umea University. Knut and Alice Wallenberg foundation, Region Västerbotten, the Research fund for Neurological Research at the University hospital of Northern Sweden, Swedish Stroke foundation and the Northern Swedish research fund.
Data availability
Data is available on reasonable request.
Declarations
Conflicts of interest
The authors report no disclosures.
Ethics approval
The underlying studies had ethics approval as presented in the original articles. In both, the ethics review board waived the need for informed consent due to the observational nature of the studies.
Footnotes
Study was presented at the International Stroke Conference 2021.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/23/2021
OA funding note has been added.
References
- 1.Johansson E, Fox AJ. Carotid near-occlusion: a comprehen sive review, part 1—definition, terminology, and diagnosis. Am J Neuroradiol. 2016;37:2–10. doi: 10.3174/ajnr.A4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson E, Fox AJ. Carotid near-occlusion: a comprehen sive review, part 2—prognosis and treatment, pathophysiology, confusions, and areas for improvement. Am J Neuroradiol. 2016;37:200–204. doi: 10.3174/ajnr.A4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox AJ, Eliasziw M, Rothwell PM, et al. Identification, prog nosis, and management of patients with carotid artery near occlu sion. Am J Neuroradiol. 2005;26:2086–2094. [PMC free article] [PubMed] [Google Scholar]
- 4.Naylor AR, Ricco JB, de Borst GJ, et al. Management of ath erosclerotic carotid and vertebral artery disease: 2017 clinical prac tice guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:3–81. doi: 10.1016/j.ejvs.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ ASNR/CNS/SAIP/SCAI/ SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Circulation. 2011;124:e54–e130. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 6.Johansson E, Öhman K, Wester P. Symptomatic carotid near-occlusion with full collapse might cause a very high risk of stroke. J Intern Med. 2015;277:615–623. doi: 10.1111/joim.12318. [DOI] [PubMed] [Google Scholar]
- 7.Gu T, Aviv RI, Fox AJ, Johansson E. Symptomatic carotid near-occlusion causes a high risk of recurrent ipsilateral ischemic stroke. J Neurol. 2020;267:522–530. doi: 10.1007/s00415-019-09605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meershoek AJA, Vonken EPA, Nederkoorn PJ, et al. Carotid endarterectomy in patients with recurrent symptoms associated with an ipsilateral carotid artery near-occlusion with full collapse. J Neurol. 2018;265:1900–1905. doi: 10.1007/s00415-018-8939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Pastor A, Gil-Núñez A, Ramírez-Moreno JM, et al. Early risk of recurrent stroke in patients with symp tomatic carotid near-occlusion: results from CAOS, a multicenter registry study. Int J Stroke. 2017;12:713–719. doi: 10.1177/1747493017714177. [DOI] [PubMed] [Google Scholar]
- 10.Khangure SR, Benhabib H, Machnowska M, et al. Carotid near-occlusion frequently has high peak systolic velocity on Doppler ultrasound. Neuroradiology. 2018;60:17–25. doi: 10.1007/s00234-017-1938-4. [DOI] [PubMed] [Google Scholar]
- 11.Johansson E, Vanoli D, Bråten-Johansson I, et al. Near-occlusion is difficult to diagnose with commonly used carotid ultrasound methods. Neuroradiology. 2011;63:721–730. doi: 10.1007/s00234-021-02687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatano S. Experience from multicenter stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson E, Aviv RI, Fox AJ. Atherosclerotic ICA stenosis coincidental with ICA asymmetry associated with circle of Willis variations can mimic near-occlusion. Neuroradiology. 2020;62:101–104. doi: 10.1007/s00234-019-02309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson E, Cuadrado-Godia E, Hayden D, et al. Recurrent stroke in symptomatic carotid stenosis awaiting revascularization – a pooled analysis. Neurology. 2016;86:498–504. doi: 10.1212/WNL.0000000000002354. [DOI] [PubMed] [Google Scholar]
- 15.Johansson E, Fox AJ (2021) Near-occlusion is a common variant of carotid stenosis: Study and systematic review. Can J Neurol Sci; in press [DOI] [PubMed]
- 16.Johansson E, Gu T, Aviv RI, Fox AJ. Carotid near-occlusion is often overlooked when CT-angiography is assessed in routine practice. Eur Radiol. 2020;30:2543–2551. doi: 10.1007/s00330-019-06636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgenstern LB, Fox AJ, Sharpe BL, et al. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery: North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Neurology. 1997;48:911–915. doi: 10.1212/WNL.48.4.911. [DOI] [PubMed] [Google Scholar]
- 18.Johansson E, Benhabib H, Herod W, et al. Carotid near-occlusion can be identified with ultrasound by low flow velocity distal to the stenosis. Acta Radiol. 2019;60:396–404. doi: 10.1177/0284185118780900. [DOI] [PubMed] [Google Scholar]
- 19.Johansson E, Zarrinkoob L, Wåhlin A, Eklund A, Malm J (2021) Diagnosing carotid near-occlusion with phase contrast MRI. AJNR. 10.3174/ajnr.A7076 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on reasonable request.