Abstract
Harmine is a β-carboline alkaloid isolated from Banisteria caapi and Peganum harmala L with various pharmacological activities, including antioxidant, anti-inflammatory, antitumor, anti-depressant, and anti-leishmanial capabilities. Nevertheless, the pharmacological effect of harmine on cardiomyocytes and heart muscle has not been reported. Here we found a protective effect of harmine on cardiac hypertrophy in spontaneously hypertensive rats in vivo. Further, harmine could inhibit the phenotypes of norepinephrine-induced hypertrophy in human embryonic stem cell-derived cardiomyocytes in vitro. It reduced the enlarged cell surface area, reversed the increased calcium handling and contractility, and downregulated expression of hypertrophy-related genes in norepinephrine-induced hypertrophy of human cardiomyocytes derived from embryonic stem cells. We further showed that one of the potential underlying mechanism by which harmine alleviates cardiac hypertrophy relied on inhibition of NF-κB phosphorylation and the stimulated inflammatory cytokines in pathological ventricular remodeling. Our data suggest that harmine is a promising therapeutic agent for cardiac hypertrophy independent of blood pressure modulation and could be a promising addition of current medications for cardiac hypertrophy.
Keywords: cardiac hypertrophy, harmine, NE, hESCs-derived cardiomyocytes, inflammatory, SHR
Introduction
Cardiac hypertrophy is an adaptive response to the overload of heart work and also a common pathological process in many cardiovascular diseases such as hypertension, aortic stenosis, and ischemic injury [1, 2]. Through hypertrophic growth, the heart reduces stress and oxygen consumption of ventricular wall and improves contractile function [3]. Cardiac hypertrophy is characterized by cellular hypertrophy, increased protein synthesis, and high arrangement of sarcomeres in cardiomyocytes [4]. Initially, the symptoms often come with hypertrophy of the left ventricle, increase of ventricular wall thickness, and shrinkage of the ventricular chamber. With progress of the disease, it is prone to develop chronic heart failure, fatal arrhythmia, and even sudden cardiac death [5, 6].
Current treatments for cardiac hypertrophy commonly target the symptoms by prescribing medications to alleviate symptoms so that cardiac systolic and diastolic function can be partially improved. These include β-blockers, calcium antagonists, angiotensin-converting enzyme (ACE) inhibitors, and other medicines [7]. However, these drugs often do not play a radical role and only bring about 10%–20% regression of left ventricular mass in patients [8, 9]. If pharmacological treatment fails in patients with severe outflow tract stenosis, surgery is needed to remove the hypertrophic interventricular septum [10]. Cardiac hypertrophy is closely related to various forms of heart failure and has become a risk factor in cardiovascular disease [11]. Therefore, there is an urgent need for developing new drugs and treatment strategies for cardiac hypertrophy.
Many studies have focused on the involvement of inflammation which is a prominent hallmark in the development of cardiac hypertrophy [12, 13]. When the heart is subjected to long-term/chronic stress, the balance between pro-inflammatory and anti-inflammatory response is disturbed, with a shift to the activation of pro-inflammatory cytokines [14–16]. Pro-inflammatory cytokines may cause cardiac hypertrophy through activating the downstream of signal transduction mediators (such as p38 and ERK) and transcription factors (such as nuclear factor kappa B (NF-κB)). Both cardiac hypertrophy and inflammatory signaling cascades share a common downstream transcription target of NF-κB and cAMP response element [17]. Therefore, regulation of immune response to the anti-inflammatory signaling cascade may provide a potential therapeutic target for cardiac hypertrophy [18].
Harmine is a β-carboline alkaloid widely existing in natural plants and can be extracted from Banisteria caapi and Peganum harmala L [19, 20]. As a precious herbal medicine in Chinese Traditional Medicine (TCM), harmine shows a wide range of pharmacological activities, including anti-inflammatory [21], antitumor [22, 23], anti-depressant [24], antioxidant properties [25], as well as anti-leishmanial [26], antibacterial [27], and antiviral bioactivities [28, 29]. In cardiovascular research, previous studies reported that harmine reduced systemic arterial blood pressure and peripheral vascular resistance [30]. Harmine also showed a vasorelaxant effect by inhibiting the L-type voltage-dependent Ca2+ channels in endothelium-intact aortic rings in a dose-dependent manner [31]. Karaki et al. found that harmine inhibited contraction of vascular and intestinal muscles by inhibiting calcium channels [32]. He et al. reported that harmine could improve myocardial infarction (MI)-induced heart injury by upregulating the Dyrk1a-ASF-CAMKIIδ pathway [33]. These studies indicate that harmine has a potential pharmacological effect on the cardiovascular system. However, whether harmine has a therapeutic effect on cardiac hypertrophy has not been reported yet.
Here, we firstly explored the protective effect of harmine on cardiac hypertrophy in spontaneously hypertensive rats (SHRs). We found that harmine significantly alleviated cardiac hypertrophy in a blood pressure-independent manner in vivo. We further established a human cardiac hypertrophy cell model by using human embryonic stem cell (hESC)-derived cardiomyocytes induced by norepinephrine (NE). Our data validated that harmine also attenuated the agonist-induced hypertrophic response of cardiomyocytes in vitro. And the protective effect of harmine on reducing pathological hypertrophy may partially rely on inhibiting NF-κB phosphorylation and thereby suppressing inflammatory response. Our study showed that harmine effectively improved the phenotypes of hypertrophy both in SHRs and in NE-induced hypertrophy of hESCs-derived cardiomyocytes, indicating that harmine could be a promising therapeutic agent for cardiac hypertrophy.
Materials and methods
Animals and treatment
SHR and aged-matched Wistar Kyoto rats (WKYs) at 5 weeks of age (weighing 95–105 g) were obtained from Vital River Co., Ltd. (Beijing, China). All rats were kept under 12/12 h light/dark cycle and given free access to rodent chow diet and water ad libitum. The powder of harmine (Apex Bio, Cas: 442-51-3) and captopril (Targetmol, Cas: 62571-86-2) were mixed well into rat regular food respectively at the ratios of 0.05% and 0.1%. The animals were randomly divided into four groups after 1-week adaptive feed, WKYs (n = 6), SHRs (n = 6), SHRs + Captopril (n = 5) and SHRs + Harmine (n = 7). Following the treatment, the body weight gain was examined every 2 weeks, while daily food intake was examied for 2 weeks. Following 12 weeks treatment, animals were sacrificed, and their hearts were rapidly excised and cut into two parts. The large portion of the myocardial samples was fixed with formalin for histological examination and the small portion of heart tissue from the left ventricular apex were frozen in liquid nitrogen and stored at −80 °C for the following experiments.
Echocardiography assessment
Transthoracic echocardiography was performed with a Vevo 2100 Imaging System (VisualSonics) every 2 weeks on SHR rats between 6 weeks and 18 weeks of age. M-mode and B-mode images were obtained both in the long-axis view and short-axis view under general anesthesia with inhalation of 1.5% isoflurane. To evaluate the left ventricular function, ejection fraction (EF), fractional shortening (FS), left ventricular diastolic inner diameter (LVIDd), left ventricular systolic inner diameter (LVIDs), interventricular septum thickness at diastole (IVSd), and left ventricular posterior wall end-diastolic thicknesses (LVPWd) were calculated. The tracings were analyzed by a technician who was blind to group assignment.
Blood pressure measurement
Systolic and diastolic blood pressure of rats were non-invasively measured at the tail artery by the CODA tail-cuff system (Kent Scientific) after 1-month treatment. The resting rat was placed in a holder on a preheated 37 °C platform at least for 10 min to acclimate to the holder of the CODA tail-cuff system. The average of blood pressure was calculated with least 20 consecutive reading cycles.
ELISA
Blood samples were collected from the abdominal aorta of anesthetized SHR rats. The serum was separated from whole blood by centrifuging at 3000 × g and 4 °C for 20 min. Serum levels of NT-ProBNP were measured by enzyme-linked immunosorbent assay kit according to manufacturer’s instructions (Cloud-Clone Corp., Cat#CEA485Ra). Each assay was conducted with four replicates in each group.
Cell culture and cardiac differentiation of hESCs
The H7 hESCs (Wicell) were dissociated with Accutase (Sigma, Cat#A6964) and cultured on Matrigel-coated (Corning, Cat#356231) plates in mTeSR medium (STEMCELL Technologies, Cat#85852). The hESCs were differentiated into cardiomyocytes using a previously described protocol with minor modifications [34]. Briefly, when cells reached 80%–90% confluent, hESCs were treated in RPMI-1640 (Gibco)/B-27 minus insulin (ThermoFisher Scientific, Cat#A1895601) medium containing 6–8 μM CHIR99021 (Selleck, Cat#S1263) for 2 days. At day 3, cells were recovered in fresh RPMI-1640/B-27 minus insulin medium with CHIR99021 removal. At day 4–5, 5 μM IWR-1 (Sigma, Cat#I0161) was added into fresh RPMI-1640/B-27 minus insulin medium. At day 6–7, medium was switched to RPMI-1640/B27-insulin without IWR-1. From day 8 onward, cells were cultured in RPMI-1640/B-27 with insulin for every 2 days. Beating cardiomyocytes were usually observed during day 8–10 after differentiation. Cardiomyocytes of day 30–40 were utilized in this study and maintained in 10% FBS/DMEM at 37 °C with 5% CO2.
Flow-cytometry analyses
Cardiomyocytes were digested with Collagenase I (1 mg/mL, Sigma, Cat#SCR103) for 30 min and 0.05% EDTA/Trypsin (Sigma, Cat#59417 C) for 5–8 min. To detect the differentiation efficiency, cardiomyocytes at differentiation day 30 were fixed with the Fixation/Permeabilization solution (BD Biosciences) at 4 °C for 30 min. Cardiomyocytes were then stained with an anti-cTnT antibody (Thermo Scientific, Cat#MA5-12960) at 4 °C for 1 h and PE-conjugated goat anti-mouse IgG antibody (Invitrogen) at 37 °C for 30 min. DPBS was used to wash the samples before centrifugation (2000 r/min for 2 min). The Annexin V- Alexa Fluor 488/PI Apoptosis Detection Kit (Yeasen) was used to analyze apoptotic cardiomyocytes according to the manufacturer’s instructions. Briefly, 1 × 105 cells were resuspended in 100 μL 1× binding buffer. Five microliters Annexin V-Alexa Fluor 488 and 10 μl PI were added into each tube and incubated at RT for 15 min. Finally, the samples were analyzed with a BD FACSC alibur flow cytometer (BD Biosciences).
Immunofluorescent staining and histological analysis
Single cardiomyocytes generated from beating sheets were plated on 15 mm circular coverslips. After 24 h, cardiomyocytes were treated with 20 μM NE (Sigma, Cat#A7257) or vehicle as a control for 3 days. The harmine/NE group was pre-treated with 10 μM harmine for 3 h and then co-treated with NE. After 72 h, cardiomyocytes were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X for 5 min. After blocking with goat serum for 30 min, cells were stained with the primary antibody against cTnT (1:100, Thermo Scientific, Cat#MA5-12960) at 4 °C overnight and AlexaFluor488 conjugated secondary antibodies (1:300, Thermo Scientific, Cat#A-11001) at RT for 1 h. DAPI (1:1000, Sigma, Cat#D9542) staining for 15 min was carried out at the same time for nuclei visualization.
For histopathological study, hearts were quickly excised and fixed with 4% paraformaldehyde/PBS. Heart tissues were then handled with Masson’s trichrome staining according to standard procedures. After dehydration, 6-μm-thick paraffin-embedded sections were stained with wheat germ agglutinin (WGA) (Invitrogen, Cat#W11261). Images were processed using a Leica IM50 microscope (Leica) and the ImageJ software.
For LPS and tumor necrosis factor-alpha (TNF-α)-induced hypertrophic cardiomyocytes model, cardiomyocytes were treated with 10 μg/mL LPS (Sigma, Cat# L4516), 25 ng/mL, or 100 ng/mL TNF-α (Sigma, Cat#T6674) or vehicle as a control up to 12 h. In harmine-treated group, hESC-derived cardiomyocytes were pre-treated with harmine (25 μM) for 0.5 h before LPS or TNF-α addition.
Calcium imaging
Cardiomyocytes at day 30 of differentiation were dissociated into single cells and reseeded on 20 mm cover glass-bottom dishes (NEST). The beating cardiomyocytes were treated with harmine for 3 h before 24-h NE treatment. And then cardiomyocytes were treated with 5 μM Cal-520®AM and 0.02% Pluronic F-127 (AAT Bioquest) in 10% FBS Tyrode’s solution at 37 °C for 15 min. Calcium signaling was recorded at 37 °C using the line-scan mode (10 ms) of a ZeissLSM-710 laser scanning confocal microscope (Carl Zeiss) and analyzed using the MATLAB software (MathWorks).
Contractility assessment
Dissociated single cardiomyocytes were seeded on cover glass-bottom dishes (NEST) and treated with harmine and/or NE for 24 h after 2–3 days culture. Single beating cardiomyocyte was detected by a video-based motion edge-detection system (Nikon). Kinematics trail of cardiomyocytes were recorded with the Video Sarcomere Length software (900B: VSL, Aurora Scientific) and analyzed with the FelixGX software (Photon Technology International). All tests were conducted at 37 °C. Intensity of Y-axis reflected relative contractility, while the number of peaks reflected indirectly the beating frequency.
Quantitative real-time PCR (qPCR) analysis
Total RNA was extracted using Trizol (Invitrogen) and reverse-transcribed into cDNA by the HiScript® II Q RT SuperMix for qPCR (Vazyme). Quantitative RT-RCR was performed with ChamQ Universal SYBR qPCR Master Mix (Vazyme) on a LightCycler 96 PCR System (Roche). Relative mRNA levels were analyzed using the 2ΔΔCt method. β-actin was used as an internal control in cardiomyocytes, while 18S was used as an internal control in heart tissues. All primers were obtained from GENEWIZ (Suzhou, China). Primer sequences in this study are listed in Supplementary Tables 1 and 2.
Cell viability evaluation
Cardiomyocytes at day 30 of differentiation were reseeded at 5000 cells per well in a 96-well plate (Corning). Cells were treated with harmine at 0–300 μM for 3 days. Afterward, cell viability assays were performed using the CellTiter-Glo Viability Assay kit (Promega) on an EnSpire® Multimode Plate Reader (PerkinElmer). Curve fitting and IC50 calculation were conducted with Prism7 (GraphPad).
Western blot analysis
Total protein from heart tissues were extracted with RIPA lysis buffer (Beyotime, Cat#P0013C) containing PMSF (Selleck, Cat#S3025), protease cocktail inhibitor (Bimake, Cat#B14001) and phosphatase inhibitor (Roche, Cat#4906837001). After lysing on ice for 30 min and centrifuging at 12,000 r/min for 15 min, the supernatants were collected and all samples were quantified by BCA Protein Assay Kit (TIANGEN, Cat#PA115-02). Equal amounts of protein (30–50 μg) were subjected to 10% SDS-PAGE and transferred to PVDF membranes (Millipore). Membranes were blocked with 5% skim milk (BD Pharminigen) in TBST (containing 0.1% Tween 20) at room temperature for 1 h and incubated with primary antibodies overnight at 4 °C. After washing with TBST for three times and 10 min per time, the membrane was incubated with HRP-conjugated Affinipure Goat Anti-Mouse/Rabbit antibodies (1:2000, Proteintech, Cat#SA00001-1/SA00001-2) at room temperature for 1 h. Signal was detected using ECL kit (Tanon, Cat#180-5001) on an image system (Tanon 4200). The following primary antibodies were used: anti-phospho NF-κB p65 (1:1000, Cell Signaling Technology, Cat#3033), anti-NFAT3 (1:1000, Cell Signaling Technology, Cat#2183), anti-Pan-Calcineurin (1:1000, Cell Signaling Technology, Cat#2614), anti-NF-κB p65 (1:1000, Proteintech, Cat#10745-1-AP), anti-IκBα (1:1000, Proteintech, Cat#10268-1-AP), anti-β-Actin (1:5000, Proteintech, Cat#66009-1-Ig), anti-BNP (1:1000, Abcam, Cat#19645), anti- Collagen III (1:1000, Abcam, Cat#7778), anti-β-Tubulin (1:10000, Affinity Biosciences, Cat#T0023). Immunoreactive bands were quantified with ImageJ software.
Statistical analysis
All statistical calculations and graphs were performed using Prism7 (GraphPad). Data were presented as mean ± SEM. Statistical differences were evaluated by unpaired two-tailed Student’s t-test between two groups. *P < 0.05 was considered statistically significant.
Results
Harmine alleviates pressure overload-induced cardiac hypertrophy in SHRs
To explore whether harmine has a therapeutic effect on cardiac hypertrophy in vivo, we firstly chose SHR rats as the animal model. Previous studies showed that SHR rats exhibited elevated blood pressure and perivascular resistance from 4–6 weeks after birth, accompanying with gradual cardiovascular symptoms from 7–8 weeks, among which cardiac hypertrophy and fibrosis were most typical [35, 36]. We started harmine administration by oral intake through feedstuff from week 6 after birth when blood pressure of SHRs started to elevate, while the heart did not show obvious hypertrophy. The powder of harmine was added into the regular rat feedstuff at a final ratio of 0.05% in weight (Fig. 1a). Considering Captopril is an active inhibitor of ACE, which is broadly used as anti-hypertensive agent, we used captopril as the positive control and added it into the feedstuff at 0.1% concentration. The standard daily food consumption of ~20 g/day for a rat would approximately yield a total of 50 mg· kg−1· d−1 intake of harmine and 100 mg· kg−1 ·d−1 intake of captopril, respectively. After administration, we also recorded the daily food consumption and body weight of each rat. The results showed that the diet intake of the captopril and the harmine treatment group was not significantly different from the SHR group. This indicated that the addition of captopril and harmine drug powders did not affect the diet of rats, thus ensuring the adequate intake of the drugs (Supplementary Fig, 1a, b). Moreover, there was no significant change in the body weight of all rats after 3 months administration (Supplementary Fig. 1c, d).
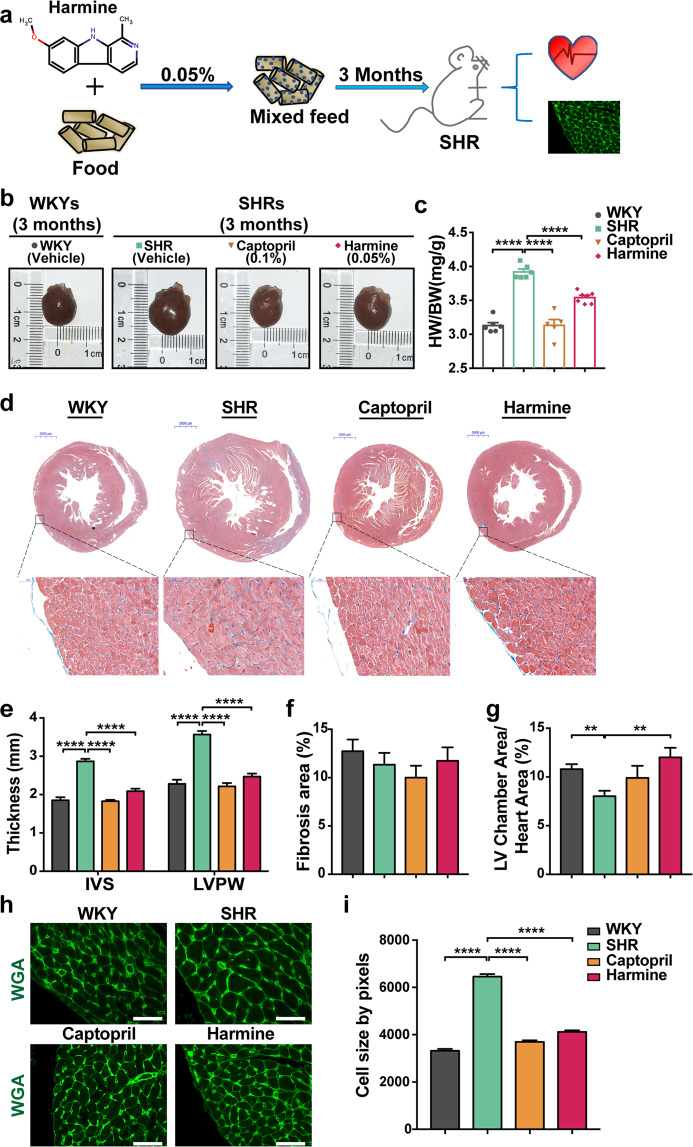
Fig. 1. Harmine alleviates pressure overload-induced cardiac hypertrophy in SHRs.
a Schematic overview of harmine treatments. SHRs at 6 weeks of ages were given the mixed feed with 0.05% harmine or 0.1% captopril for additional 3 months. WKYs and SHRs group as blank control contained no pharmaceutical additives (WKYs, n = 6; SHRs, n = 6; Captopril as positive control, n = 5; Harmine, n = 7). b Macroscopic view of heart size. Scale bar: 2000 μm. c Quantification of heart weight over body weight ratio in each group. d Representative Masson’s trichrome staining images of heart tissues (upper scale bars: 2 mm), and high-magnification views of LV wall (bottom, scale bars: 50 μm). e Statistics of the average thickness of IVS and LVPW in each group. f Quantification the ratio of myocardial fibrosis area. g The ratios of LV chamber area to whole heart area. h Heart tissue slices from each group were stained with wheat germ agglutinin (WGA). Scale bars, 50 μm. i Quantification of CMs cross-sectional area with WGA staining images (n > 400). Data are mean ± SEM. **P < 0.01, ****P < 0.0001 (two-tailed Student’s t test).
After 3 months of harmine intake, the increase of heart size and heart to body weight ratio in SHRs were substantially alleviated (Fig. 1b, c). Harmine-treated hearts exhibited a significant decrease in the thickness of myocardial wall and enlargement of left ventricular chamber compared to those in SHRs without treatment (Fig. 1d–g). Given the fact that an exaggerated accumulation of fibrous tissue is one of the remarkable features of structural cardiac remodeling [37, 38], we also measured the ratio of myocardial fibrosis area and collagen III expression level. However, at the time point we observed (~4.5 months old), there were no significant differences in fibrosis area and collagen III expression between WKY control group and SHR group (Fig. 1f and Supplementary Fig. 2). Considering the increase of cardiomyocyte size is one of the distinctive characteristics in pathological cardiac hypertrophy, we next stained the heart tissue slices with WGA. The WGA-stained sections showed that harmine significantly suppressed the enlargement of myocyte cross-sectional area (Fig. 1h, i). Meanwhile, captopril obviously suppressed cardiac hypertrophy as a validated positive control in parallel test (Fig. 1b–h). Collectively, these data indicated that harmine ameliorated pressure overload-induced cardiac hypertrophy in SHRs.
Previous results reported that harmine showed a vasorelaxant effect in isolated rat aorta [31]. To determine whether the effect of alleviating cardiac hypertrophy by harmine was dependent on lowering the blood pressure, we measured blood pressure of SHRs by tail artery after harmine administration. As shown in Supplementary Fig. 1e, f, SHRs showed significant hypertension, while captopril treatment resulted in an obvious decrease in blood pressure. In contrast, oral intake of harmine did not affect blood pressure of SHRs. These results indicated that the effect of alleviating cardiac hypertrophy by harmine was not dependent on lowering of blood pressure.
Harmine alleviates cardiac remodeling and cardiac dysfunction in SHRs
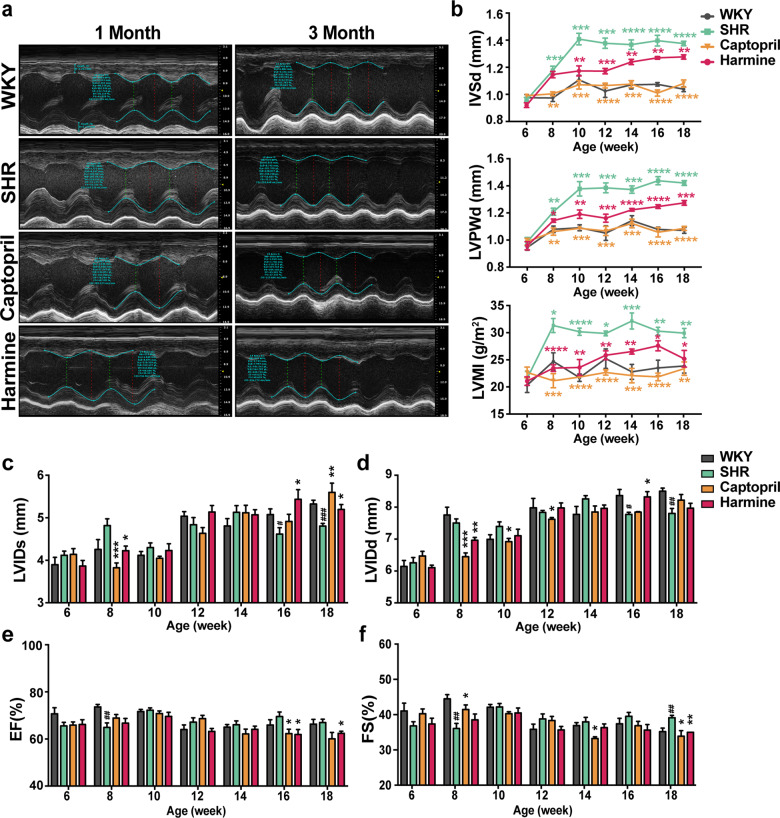
To assess the effect of harmine on cardiac structural and function remodeling in cardiac hypertrophy, echocardiography was conducted every 2 weeks after harmine administration. We observed a significant increase of end-diastolic LVPWd, IVSd, and left ventricular mass index (LVMI) in SHRs without treatment from 8 weeks of ages (Fig. 2a, b). To further evaluate whether harmine treatment demonstrated a long-term therapeutic effect in SHR model, we observed up to 18 weeks. Considering the dramatic lowering effect on blood pressure, captopril treatment achieved a marked improvement in protecting SHR against pressure overload-induced cardiac hypertrophy, which displayed a similar septal, posterior wall thickness and LVMI with aged-matched WKYs. Meanwhile, harmine treatment also obtained a significant reduction of IVSd, LVPWd, and LVMI in SHRs. And we also observed the increased LVIDs, declined EF and FS in harmine-treated SHRs (Fig. 2c–f). Therefore, harmine improved pressure overload-induced cardiac remodeling and dysfunction in SHRs to some extent, which was independently on lowering blood pressure.
Fig. 2. Harmine alleviates cardiac hypertrophy and cardiac dysfunction in SHRs.
a Representative images showing the M-mode echocardiograms of rats treated for 1 month and 3 months. b Changes ininterventricular septum thickness at diastole (IVSd), left ventricular posterior wall end-diastolic thicknesses (LVPWd), and left ventricular mass index (LVMI) over time measured by echocardiography. c–f Measurement of left ventricular diastolic inner diameter (LVIDd), left ventricular systolic inner diameter (LVIDs), ejection fraction (EF), and fractional shortening (FS) (WKY group: n = 6; SHR group: n = 6; Captopril group: n = 5; harmine group: n = 7). All data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-tailed Student’s t test compared with SHR group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 by two-tailed Student’s t test compared with WKY group.
Harmine downregulates expression of the cardiac hypertrophy-related markers in vivo
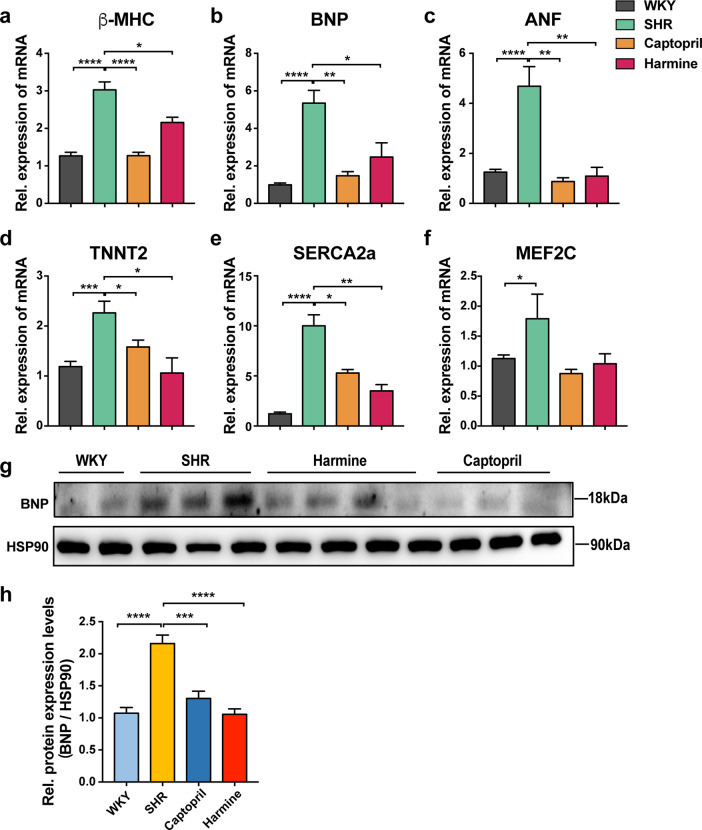
To further confirm whether harmine altered the expression of hypertrophy marker genes in SHRs, we extracted RNA from heart tissues of rats after 3 months treatment. Our data clearly demonstrated that the upregulation of fetal genes β-myosin heavy chain (β-MHC), B-type natriuretic peptide (BNP), and atrial natriuretic factor (ANF), as well as structural and function related proteins, such as NFAT-activated mediators, myocyte-specific enhancer factor 2C (Mef2C) and sarcoplasmic/reticulum Ca2+-ATPase 2A (SERCA2a) in SHRs model were dramatically decreased by captopril or harmine treatment (Fig. 3a–f). Although the mRNA expression level of Mef2C was not significantly different across SHR and treated groups, but there was a tendency for captopril and harmine downregulated Mef2C mRNA abundance. To further confirm the function of these cardiac hypertrophic genes in vivo, we measured the protein level of BNP which exhibited the similar downregulated tendency as mRNA level (Fig. 3g–h). These data indicated that harmine treatment suppressed the excessive expression of pressure overload-induced cardiac hypertrophic markers in vivo.
Fig. 3. Downregulation of cardiac hypertrophy-related genes and proteins in SHRs after treatment of harmine.
a–f qPCR analyses of hypertrophic-related genes in rat cardiac tissues (normalized to 18S expression). g, h Immunoblotting for BNP in isolated cardiac tissues. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (two-tailed Student’s t test).
As NT-proBNP is a discerning marker in cardiac hypertrophy [39, 40], we further measured the level of serum NT-proBNP by ELISA. Both captopril and harmine treatment reduced serum NT-proBNP, which was consistent with the decreased mRNA expression of BNP in the treated groups (Supplementary Fig. 3).
Establishment of the NE-induced hypertrophy model of hESCs-derived cardiomyocytes
Considering the discrepancy between animal models and human, we next established the cardiac hypertrophy model of human cardiomyocytes in vitro. The human cardiomyocytes were derived by differentiating H7 hESCs using a 2D monolayer-based differentiation method as previously described (Fig. 4a). The beating cardiomyocytes were observed at day 8–10 of differentiation. Flow-cytometry analysis of the cardiomyocyte-specific maker cardiac Troponin T (cTnT) revealed that the efficiency of cardiomyocyte differentiation reached more than 90% (Supplementary Fig. 4).
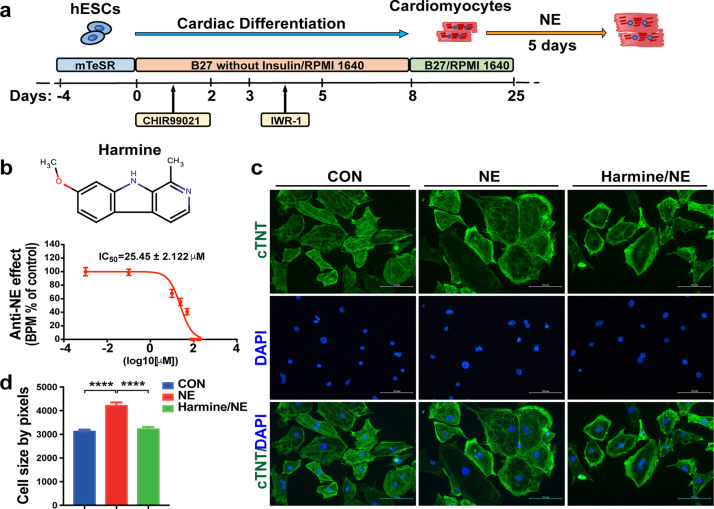
Fig. 4. Harmine reverses the effect of NE on cell size of hESC-derived cardiomyocytes.
a Schematic illustrating the differentiation protocol for H7 hESC line into cardiomyocytes in chemically defined medium. b The dose-dependent effect of harmine on beating rates of NE (20 μM) treated cardiomyocytes for 24 h. IC50 value for harmine was 25.45 ± 2.122 μM (n = 3 for each dose). c Representative immunostaining images of hESC-derived cardiomyocytes stained with cTnT (green) for sarcomere maker and DAPI (blue) for nuclei. Scale bars, 100 μm. d Stimulation with NE (20 μM) for 3 days leads to an increase in cell size, while a reduction after pre-treated with harmine (10 μM) for 3 h and then co-treated with NE for 3 days (n > 400 per group). Data are mean ± SEM. ****P < 0.0001; by two-tailed Student’s t test.
NE is a catecholamine that can induce hypertrophy of neonatal rat cardiomyocytes and adult rat cardiomyocytes [41, 42]. Next, we tested whether hESCs-derived cardiomyocytes could well respond to NE treatment. The results turned out that NE stimulation led to changes of cell size, contractility, beating frequency, calcium activity, as well as upregulated hypertrophic marker genes in hESCs-derived cardiomyocytes shown in the following figures (Figs. 4–6).
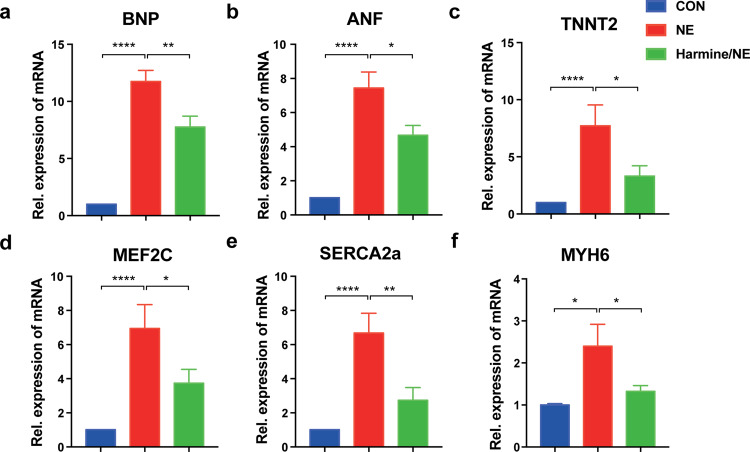
Fig. 6. Downregulation of cardiac hypertrophy-related genes in hESC-derived cardiomyocytes after treatment of harmine.
a–f RT-PCR analyses of relative mRNA expression of hypertrophic-related genes in hESC-derived cardiomyocytes after treated with each compound for 3 days (harmine 25 μM; NE 20 μM). Control group was treated with DMSO. All data were normalized to β-actin expression and expressed as mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001 (n = 3 independent experiments, two-tailed Student’s t test).
Harmine reverses the effect of NE-induced hypertrophy of hESC-derived cardiomyocytes
We further tested whether harmine has an effect of anti-NE-induced hypertrophy on beating hESCs-derived cardiomyocytes. NE-treated hypertrophic cardiomyocytes responded well to harmine in a dose-dependent manner with a 50% inhibitive concentration (IC50) of 25.45 ± 2.122 μM (Fig. 4b). NE treatment led to a significant enlargement of hESC-derived cardiomyocyte size, and this NE-induced hypertrophic growth was obviously suppressed by harmine treatment (Fig. 4c, d). These results indicated that harmine was able to antagonize the NE-induced hypertrophic effect on hESCs-derived cardiomyocytes.
Harmine suppresses the elevated calcium handling and contractility in NE-treated cardiomyocytes
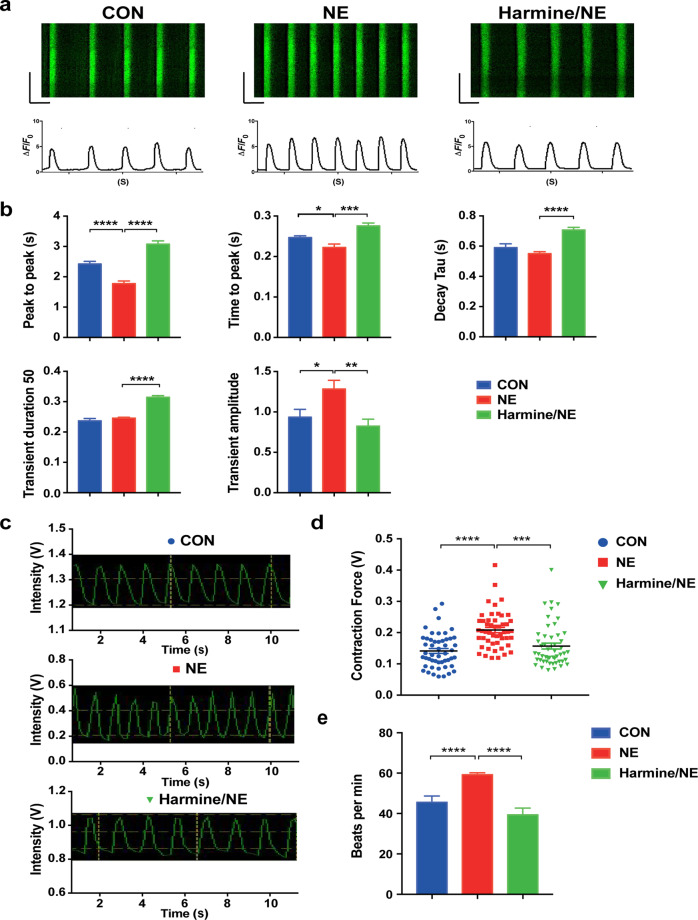
Ca2+ is closely related to the electrophysiological activity and mechanical contraction of cardiomyocytes. Studies have shown that Ca2+ and its related signaling pathways play a critical role in the process of cardiomyocyte hypertrophy, which is often accompanied with an increase of intracellular Ca2+ concentration [43, 44]. To further explore whether harmine affects Ca2+ handling of cardiomyocytes, we examined calcium transients in hESCs-derived cardiomyocytes by Ca2+ imaging after harmine treatment. The results showed that NE treatment exhibited a shorter frequency of calcium transients, slower release time of Ca2+ from sarcoplasmic reticulum, and increased transient amplitudes (Fig. 5a, b). These data indicated that, after NE treatment, more Ca2+was released in a shorter period, correlating with a positive inotropic effect of NE on cardiomyocytes. Harmine treatment resulted in a reduction intransient amplitudes, significantly longer peak to peak time, prolonged time to peak and decay time, as well as prolonged transient duration 50 (Fig. 5a, b). Collectively, these data indicated that harmine treatment inhibited the positive effect of NE on calcium handling of hESCs-derived cardiomyocytes to a certain extent.
Fig. 5. The contractility and calcium imaging analyses of hESC-derived cardiomyocytes after treatment of harmine.
a Representative calcium transients recorded in single cardiomyocyte after harmine treatment (25 μM; NE 20 μM). b Quantification of calcium handling parameters (peak to peak, time to peak, decay time, transient duration 50, transient amplitude (ΔF/F0)) for each group. n > 20 cells recorded for each group in every experiment. c Representative traces of contraction movements from single cardiomyocyte recorded by FelixGX detection system. d Quantification of relative contractility in single cardiomyocyte 24 h after harmine (25 μM) or/and NE (20 μM) treatment. n > 50 cells recorded for each group. e Quantification of beating frequency followed harmine and NE treatment (n > 18 for each group). Data are mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-tailed Student’s t test.
Cardiac hypertrophy is often accompanied with augmented contraction force in the compensatory stage [45]. In order to assess whether harmine has an effect on cardiomyocyte contractility, we measured the contraction force of hESCs-derived cardiomyocytes with and without harmine treatment. We found that NE-treated cardiomyocytes exhibited enhanced contractile force and beating frequency, whereas the contractility and beating frequency were decreased after treatment with harmine (Fig. 5c–e). Taken together, these data indicated that harmine inhibited the positive inotropic effect of NE on hESC-derived cardiomyocytes and suggested that harmine could alleviate cardiac hypertrophy by decreasing calcium handling and contraction of cardiomyocytes.
Harmine inhibits NE-induced upregulation of cardiac hypertrophy-related genes in hESCs-derived cardiomyocytes
We next examined the effect of harmine on expression of cardiac hypertrophy-related genes in NE-induced human cardiomyocyte hypertrophy model by qPCR. NE-treated cardiomyocytes exhibited upregulated expression of marker genes for hypertrophic characteristics, including ANF and BNP (Fig. 6a, b). Expression of TNNT2, Mef2C, SERCA2a, and MYH6 were also significantly upregulated (Fig. 6c–f). Upregulation of these hypertrophy-related genes, was significantly suppressed after harmine treatment. Overall, these data indicated that harmine was able to attenuate cardiomyocyte hypertrophy induced by NE.
Harmine has no effect on apoptosis and cell viability of hESCs-derived cardiomyocytes
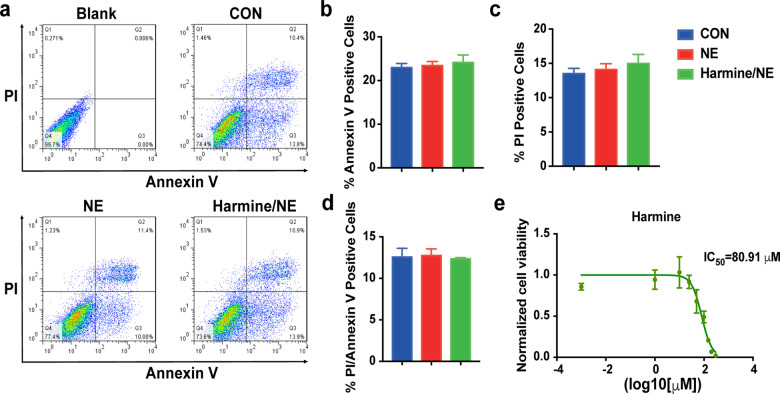
When compounds have toxic effects on cell, it usually impairs cell viability or induces cell apoptosis which could cause cell shrink and impair cardiomyocytes function, thus presenting false-positive results. To test whether harmine has a toxic effect on cardiomyocytes, we then analyzed cell viability and apoptosis rate of hESCs-derived cardiomyocytes after harmine treatment by flowcytometry. Annexin V−/PI− represents living cells, while PI+ indicates necrosis or late apoptosis; annexin V+/PI− indicates early apoptosis and annexin V+/PI+ indicates late apoptosis. To align with the concentration of harmine used in cardiomyocyte function detection, we used the same concentration (25 μM) according to IC50 value measured with beating frequency. As shown, there was no significant difference in the proportion of annexin V+ or / and PI+ cardiomyocytes between control and harmine or NE treatment (Fig. 7a–d). These data indicated that harmine did not promote apoptosis of cardiomyocytes at such concentration. We further performed cell viability assays and found that harmine had little adverse effect on viability of cardiomyocytes with an IC50 of 80.91 μM, which was far higher than the concentration used for cardiomyocyte function detection (Fig. 7e). Taken together, these data indicated that harmine had a low toxicity to cardiomyocytes and the observed improvements of hypertrophic phenotype were not due to the drug cytotoxicity of harmine.
Fig. 7. Harmine has no effect on apoptosis and cell viability of hESC-derived cardiomyocytes.
a Representative flow cytometric analyses of apoptosis in hESC-derived cardiomyocytes following treatment of harmine (25 μM) and NE (20 μM). Cells were double labeled with Annexin V-FITC and propidum iodide after harvesting. The upper-right quadrant represents the annexin V+/PI+ cells; the lower-right quadrant represents the annexin V+/PI− cells; the upper-left quadrant represents the annexin V−/PI+ cells. b–d Quantification of the ratio of annexin V+/PI−cells, annexin V−/PI+ cells and annexin V+/PI+ cells. e Dose–response curves demonstrating the effect of harmine on cell viability of hESC-derived cardiomyocytes using CellTiter-Glo Assay (n = 4 conducted per concentration). Data are mean ± SEM of three independent experiments.
Harmine alleviated cardiac hypertrophy partially relied on an anti-inflammatory effect via modulating the activity of NF-κB signaling pathway
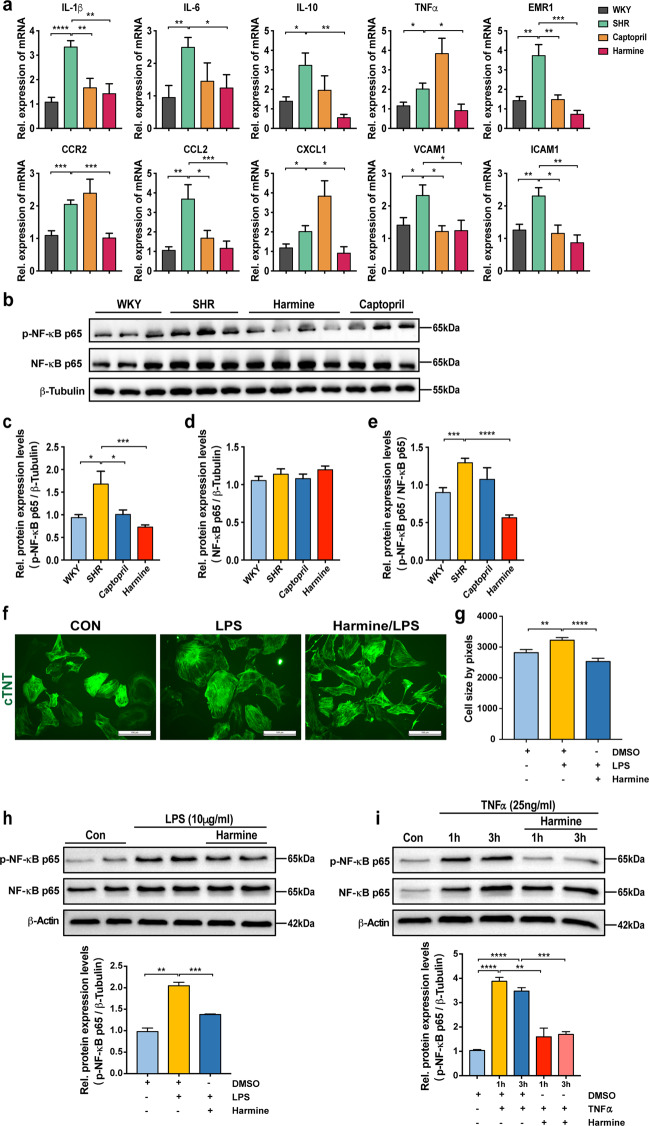
Activation of inflammation response plays a vital role in cardiac hypertrophy development and heart failure [46]. Studies have shown that inflammatory cell infiltration (T-lymphocytes and macrophages), upregulation of crucial pro-inflammatory mediators like interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and TNF-α, as well as activation of NF-κB are the characteristic hallmarks of pathological cardiac hypertrophy [12, 47, 48]. Harmine has a strong anti-inflammatory and antioxidant effect by inhibiting the activation of NF-κB [49–51]. Therefore, we next investigated whether harmine could alleviate inflammation and NF-κB activation in the cardiac hypertrophy model of SHRs. As shown in Fig. 8, harmine effectively downregulated the mRNA level of inflammatory mediators, including IL-1β, IL-6, IL-10, and TNF-α that were upregulated in SHRs. The increase in mRNA levels of macrophage maker (Emr1) and adhesion molecule markers (intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1)) in heart tissues of SHRs were also markedly reduced by harmine. The mRNA levels of chemokines (CCL2, CXCL1) and chemokine receptor (CCR2), chemoattractant for inflammatory cells, were reduced in harmine-treated hearts in SHRs (Fig. 8a). Moreover, western blot showed that the expression of phosphorylated NF-κB (p-NF-κB p65) and the ratio of p-NF-κB p65/NF-κB p65 were markedly lower in harmine-treated SHRs than that in other groups (Fig. 8c–e). There were no significant changes in the protein levels of NF-κB p65 and IκBα (Fig. 8b, Supplementary Fig. 5).
Fig. 8. Harmine attenuates inflammatory response and NF-κB activation both in vivo and vitro.
a qPCR analyses of inflammatory-related genes mRNA levels in rat cardiac tissues (normalized to 18S expression). b Representative Western blot analysis of phosphorylated NF-κB p65 and NF-κB p65 protein expression in the heart tissues of WKYs and SHRs after 3-month treatment. c–e Quantification of phosphorylated NF-κB p65 and NF-κB p65 protein expression normalized to β-Tubulin, and phosphorylated NF-κB p65/NF-κB p65 ratio by densitometric analysis. f Representative images of hESC-derived cardiomyocytes stained with cTnT (green) antibody. The hESC-derived cardiomyocytes were treated with LPS (100 ng/mL) for 3 days or were pre-treated with harmine (10 μM) for 3 h and then co-treated with LPS (100 ng/mL) for 3 days (n > 200 per group). Scale bars, 100 μm. g Quantification of cell size changes in each group. h-i Representative immunoblots of phosphorylated NF-κB p65 (p-NF-κB p65) and NF-κB p65 in hESC-derived cardiomyocytes following treatment of LPS (10 μg/mL) for 3 h, or TNF-α (25;ng/mL) for 1 h and 3 h (upper), and quantification of relative protein expression by densitometry (bottom). In all harmine-treated group, hESC-derived cardiomyocytes were pre-treated with harmine (25 μM) for 0.5 h before LPS or TNF-α addition. β-Actin was used as loading control. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (two-tailed Student’s t test).
To further confirm the contribution of harmine in suppressing inflammation in cardiac hypertrophy via NF-κB pathway, we next explored whether harmine could slowdown pathological hypertrophy progression under LPS and TNF-α stimulation. As shown in Fig. 8f, g, we observed that the cell size of cardiomyocytes were enlarged after LPS treatment. Harmine successfully alleviated this hypertrophic phenotype. The phosphorylated NF-κB level was dramatically increased by LPS induction (Supplementary Fig. 6), which was also reversed by harmine treatment. The similar stimulatory effect was also observed with TNF-α challenge at doses of 25 ng/mL and 100 ng/mL, respectively. These observations were in line with our previous findings in SHR rats in vivo. Taken together, our data suggested that harmine was able to inhibit inflammatory response and NF-κB activation in cardiac hypertrophy both in vitro and in vivo.
It has been reported that harmine may participate in regulating the activity of calcineurin-NFAT signaling pathway. We then examined harmine’s effect on calcineurin-NFAT signaling in cardiac hypertrophy. It revealed that harmine dramatically downregulated the expression level of NFAT3 but not calcineurin in NE-induced hypertrophy of hESCs-derived cardiomyocytes (Supplementary Fig. 7).
Discussion
The heart develops a series of adaptive responses to environmental stress, including changing in chamber volume, systolic and diastolic function, heart rate, as well as muscle mass, to maintain a normal circulatory need for the body [52]. In the early stage when cardiac output increases, hypotrophy mainly plays a compensatory role. However, the sustained pathological hypertrophy will eventually lead to ventricular dilation, dysfunction, and even heart failure [53, 54]. Although we have a progressive understanding of the pathogenesis in cardiac hypertrophy, which involves cardiomyocytes proliferation and apoptosis, fibrosis, inflammation, and electrophysiological remodeling [55, 56], there is still few specific drugs for the clinical treatment of cardiac hypertrophy. One possible reason is that cardiac hypertrophy is regulated by multiple pathways and complex mechanisms, leading to a poor treatment outcome with a single-target therapy.
In this study, we investigated the cardioprotective effects and underlying mechanisms of harmine on cardiac hypertrophy. Previous studies have shown a potential pharmacological effect of harmine on vascular system. Here, we firstly validated the effect of harmine on cardiac hypertrophy in vivo using the SHR model. Our results showed that both captopril and harmine effectively alleviated cardiac hypertrophy in SHRs. The stronger anti-hypertrophy effect of captopril maybe resulted from its anti-hypertension effects (shown in supplemental Fig. 1e, f). Of note, previous studies indicated that harmine presented a vasorelaxant effect in cellular experiments [30, 31]. However, in our present study, it is interesting to see that the blood pressure of SHRs was not affected by oral administration of harmine. Several factors may account for this inconsistency. First, more complex situations are involved in the in vivo environment, which may cause our results found in SHRs different with previous reports derived from only in vitro studies. Second, the concentration of harmine used in our study (0.05% added ratio in feedstuff) might not reach the effective plasma concentration for lowering the blood pressure in SHRs. Meanwhile, this also indicated that alleviation of cardiac hypertrophy in SHRs by harmine at the concentration used in our study was not dependent on lowering the blood pressure.
It is known that harmine is a competitive inhibitor of dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) [57], which plays a key role in embryonic heart development. Overexpressing of DYRK1A in mice was characterized by heart birth defects and heart failure [58]. DYRK1A is able to antagonize the calcineurin-NFAT signaling by phosphorylating NFATs [59–61]. However, the role of DYRK1A in cardiac hypertrophy remains controversial. Kuhn et al. found that overexpression of DYRK1A had a protective effect in phenylephrine-induced cardiac hypertrophy in right ventricular cardiomyocytes, while harmine treatment (at 1 μM) could promote cardiac hypertrophy [59]. On the contrary, Grebe et al. reported that overexpression of DYRK1A failed to prevent cardiac hypertrophy in transverse aortic constriction and the transgenic calcineurin mouse models [60]. Here, our data demonstrated that a higher concentration (10 μM) of harmine alleviated cardiac hypertrophy in hESCs-derived cardiomyocytes. To address this conflicting finding, we proceeded to study the regulation of harmine on calcineurin-NFAT signaling. Our data suggest that harmine suppressed the expression of NFAT3 in NE-challenged cardiomyocytes, which may further contribute to harmine’s protective effect in cardiac hypertrophy in vitro. However, the dual directional effect of harmine on cardiomyocyte remodeling under lower and higher doses requires further investigation to identify discrepant targets and mechanisms of harmine in vivo. Whether harmine can alleviate inherited hypertrophic cardiomyopathy caused by genetic mutations also need to be further explored.
Small animal models are commonly acceptable tools for modeling human heart diseases and testing the efficacy of new drugs. However, the marked differences are inevitable in cardiac physiology between mice/rats and humans, not to mention that there exist inter-individual variations in the pharmacological response due to multiple factors such as ages, organ function, genetics, drug interaction, and underlying diseases. Therefore, here in our study, we further focused on the pharmacological effect of harmine on human cardiomyocytes. Considering chronic adrenergic stimulation as one of the pathogenic triggers leading to hypertrophy, previous data showed that exposure to isoproterenol or phenylephrine induced hypotrophy in pluripotent stem cell-derived cardiomyocytes (hPSC derived cardiomyocytes) [62–64]. Here, we successfully established the in vitro cardiac hypertrophy model by using hESCs-derived cardiomyocytes induced by NE treatment. NE is another commonly used stimulator that can cause cardiomyocyte hypertrophy and even apoptosis [65]. It has been reported that NE can promote hypertrophy in primary cardiomyocytes and animals [66, 67]. Here, we found that NE treatment led to increased cell size, contractility, beating frequency, calcium activity, as well as upregulated hypertrophic marker genes in hESCs-derived cardiomyocytes, which were consistent with previous studies [68–70]. The hPSC-derived cardiomyocytes are thought to have great promise for individual pharmaceutical test and a stable in vitro model resembles the similar gene expression pattern, morphological, electrophysiological, and contractile properties compared to adult human cardiomyocytes [71–73]. With the obvious hypertrophic response of hESCs-derived cardiomyocytes to NE treatment in our study, this hypertrophic cell model can reflect the sensitivity of human cardiomyocytes against harmine. Angiotensin II and other catecholamine hormone can activate intracellular Ca2+ channel in cardiomyocytes, further activate calmodulin kinase (CaMK) and NFAT signaling pathway, and then cause cardiomyocyte hypertrophy [43, 74, 75]. Our data demonstrated that harmine effectively suppressed the NE-triggered elevation of Ca2+ handling, contractility and NFAT abundance of hESCs-derived cardiomyocytes. And the observed alleviation of hypertrophic phenotypes by harmine treatment was not due to cytotoxicity on cardiomyocytes. The consistent and effectively inhibitory effect of harmine on SHR model in vivo and on NE-induced hypertrophy model of hESCs-derived cardiomyocytes in vitro indicated that harmine deserves further examination for future clinical treatment of cardiac hypertrophy.
Of note, increasing evidences showed that inflammation was involved in the pathophysiological process of heart remodeling during cardiac hypertrophy. Overexpression of TNF-α induced cardiac hypertrophy and re-expression of fetal gene program [76, 77]. It has also been confirmed that TNF-α promoted the abundance of IL-1 and IL-6 which further stimulate hypertrophy growth response [78]. Overexpression of IL-6 and its receptor led to concentric cardiac hypertrophy, fibrosis, and diastolic dysfunction in mice [79], while deletion of IL-6 ameliorated angiotensin II-induced cardiac hypertrophy, inflammation, and fibrosis [80, 81]. Previous evidences supported that IL-1β and TNF-α directly promoted expression of hypertrophic-related genes in cultured cardiomyocytes [82–84]. Higher level of ICAM-1, VCAM-1, and other adhesion molecules were seen in patients of left ventricular hypertrophy [85]. Emerging evidence supported the chemokine-mediated monocyte infiltration during cardiac remodeling [86, 87]. Here we also observed that the mRNA abundance of representative pro-inflammatory cytokines, chemokines, and adhesion molecules were markedly increased in SHRs. Interestingly, discrepant effects of captopril and harmine on the expression of the pro-inflammatory factors after cardiac hypertrophy were observed in our study. Captopril synergized the stimulatory effect on TNF-α, CCR2, and CXCL1, while suppressed the abundance of other factors. Meanwhile, we noticed that harmine showed a strong inhibitory regulation on all the targeted factors we tested. All these data suggested a different protective mechanism of harmine from captopril in cardiac hypertrophy.
It is well known that NF-κB is a key transcription regulator of many pro-inflammatory adhesion molecules involved in various inflammation-related pathological processes and cardiovascular diseases [88]. Studies have shown that activation of NF-κB played an important role in the development of cardiac hypertrophy in patients with heart failure as well as in SHRs [89]. NF-κB interacts with the MAPK signal pathway, which jointly promotes the development of cardiac hypertrophy [90]. As a powerful anti-inflammatory molecule, harmine can inhibit inflammation by downregulating NF-κB [50]. Studies have also demonstrated that harmine could inhibit inflammation through TLR4-NF-κB/NLRP3 to improve LPS-induced renal injury [51]. In our study, we demonstrated that harmine showed a stronger inhibitory effect than captopril on phosphorylation of NF-κB, which further suppressed the inflammation and cardiac hypertrophy in SHRs and hESCs-derived cardiomyocytes in vitro.
Overall, our study showed that harmine was a small molecule effective in reducing hypertrophy both in the in vivo cardiac hypertrophy model of SHRs and in the NE-induced in vitro hypertrophy model of human cardiomyocytes. This anti-hypertrophy effect of harmine was not dependent on blood pressure change. Harmine displayed a more obvious anti-inflammatory effect on cardiac hypotrophy model than captopril via phosphorylation of NF-κB which could be one of the potential underlying mechanisms. Harmine could be a novel therapeutic agent for cardiac hypertrophy and used in combination with other medicines controlling blood pressure for cardiac hypertrophy prevention.
Supplementary information
Acknowledgements
We thank Chemical Biology Core Facility of SIBCB for technical supports. We also thank Ming Chen, Shu-jue Lan, Shuai Han, Hong-wei Zhao, Yun-qing Ci, and Rong-chao Yang (SIBCB) for their experimental assistance. This work was supported by the National Natural Science Foundation of China #82070391 (to NS), #81500241(to CX); by the Innovative Research Team of High-Level Local Universities in Shanghai; by the National Key R&D Program of China 2018YFC2000202, the Haiju program of National Children’s Medical Center EK1125180102.
Author contributions
JH, NS, CX, and HYC designed the research and wrote the manuscript; JH, YL, JXC, XYL, WJZ, LQ, and ZXX performed the experiment; YL and HYC contributed equipment and analytic tools; JH, JXC, XYL, LQ, ZXX, QYZ, and EML analyzed the data.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jie Huang, Yang Liu
Contributor Information
Ning Sun, Email: sunning@fudan.edu.cn.
Chen Xu, Email: xu_chen85@fudan.edu.cn.
Hai-yan Chen, Email: chen.haiyan@zs-hospital.sh.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00639-y.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 3.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol. 2005;289:H8–h16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 4.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–9. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–55. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K, Murakami M, Miura D, Yunoki K, Enko K, Tanaka M, et al. Beta-blockers and oxidative stress in patients with heart failure. Pharmaceuticals. 2011;4:1088–100. doi: 10.3390/ph4081088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 9.Ma H, Yu S, Liu X, Zhang Y, Fakadej T, Liu Z, et al. Lin28a regulates pathological cardiac hypertrophic growth through Pck2-mediated enhancement of anabolic synthesis. Circulation. 2019;139:1725–40. doi: 10.1161/CIRCULATIONAHA.118.037803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aksu T, Güler TE, Yalın K, Gölcük ŞE, Özcan KS. Role of endocardial septal ablation in the treatment of hypertrophic obstructive cardiomyopathy. Anatol J Cardiol. 2016;16:707–12. doi: 10.14744/AnatolJCardiol.2016.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 12.Kuusisto J, Kärjä V, Sipola P, Kholová I, Peuhkurinen K, Jääskeläinen P, et al. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart. 2012;98:1007–13. doi: 10.1136/heartjnl-2011-300960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderheyden M, Paulus WJ, Voss M, Knuefermann P, Sivasubramanian N, Mann D, et al. Myocardial cytokine gene expression is higher in aortic stenosis than in idiopathic dilated cardiomyopathy. Heart. 2005;91:926–31. doi: 10.1136/hrt.2004.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, et al. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–45. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 16.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41:532–43. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 17.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 18.Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, et al. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005;111:2319–25. doi: 10.1161/01.CIR.0000164237.58200.5A. [DOI] [PubMed] [Google Scholar]
- 19.Patel K, Gadewar M, Tripathi R, Prasad SK, Patel DK. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid ‘Harmine'. Asian Pac J Trop Biomed. 2012;2:660–4. doi: 10.1016/S2221-1691(12)60116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013;7:199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol. 2017;35:56–68. doi: 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho A, Chu J, Meinguet C, Kiss R, Vandenbussche G, Masereel B, et al. A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis. Eur J Pharmacol. 2017;805:25–35. doi: 10.1016/j.ejphar.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XF, Sun RQ, Jia YF, Chen Q, Tu RF, Li KK, et al. Synthesis and mechanisms of action of novel harmine derivatives as potential antitumor agents. Sci Rep. 2016;6:33204. doi: 10.1038/srep33204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Wu J, Gong Y, Wang P, Zhu L, Tong L, et al. Harmine produces antidepressant-like effects via restoration of astrocytic functions. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:258–67. doi: 10.1016/j.pnpbp.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Filali I, Bouajila J, Znati M, Bousejra-El Garah F, Ben Jannet H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J Enzym Inhib Med Chem. 2015;30:371–6. doi: 10.3109/14756366.2014.940932. [DOI] [PubMed] [Google Scholar]
- 26.Lala S, Pramanick S, Mukhopadhyay S, Bandyopadhyay S, Basu MK. Harmine: evaluation of its antileishmanial properties in various vesicular delivery systems. J Drug Target. 2004;12:165–75. doi: 10.1080/10611860410001712696. [DOI] [PubMed] [Google Scholar]
- 27.Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–82. doi: 10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Tian X, Zou X, Xu S, Wang H, Zheng N, et al. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-κB pathway. Int Immunopharmacol. 2018;60:111–20. doi: 10.1016/j.intimp.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 29.Quintana VM, Piccini LE, Panozzo Zénere JD, Damonte EB, Ponce MA, Castilla V. Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antivir Res. 2016;134:26–33. doi: 10.1016/j.antiviral.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Aarons DH, Rossi GV, Orzechowski RF. Cardiovascular actions of three harmala alkaloids: harmine, harmaline, and harmalol. J Pharm Sci. 1977;66:1244–8. doi: 10.1002/jps.2600660910. [DOI] [PubMed] [Google Scholar]
- 31.Berrougui H, Martin-Cordero C, Khalil A, Hmamouchi M, Ettaib A, Marhuenda E, et al. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol Res. 2006;54:150–7. doi: 10.1016/j.phrs.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Karaki H, Kishimoto T, Ozaki H, Sakata K, Umeno H, Urakawa N. Inhibition of calcium channels by harmaline and other harmala alkaloids in vascular and intestinal smooth muscles. Br J Pharmacol. 1986;89:367–75. doi: 10.1111/j.1476-5381.1986.tb10269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Yao J, Sheng H, Zhu J. Involvement of the dual-specificity tyrosine phosphorylation-regulated kinase 1A-alternative splicing factor-calcium/calmodulin-dependent protein kinase IIδ signaling pathway in myocardial infarction-induced heart failure of rats. J Card Fail. 2015;21:751–60. doi: 10.1016/j.cardfail.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–64. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 37.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–75. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishikawa J, Kario K, Matsui Y, Shibasaki S, Morinari M, Kaneda R, et al. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res. 2005;28:995–1001. doi: 10.1291/hypres.28.995. [DOI] [PubMed] [Google Scholar]
- 39.Jan MI, Khan RA, Sultan A, Ullah A, Ishtiaq A, Murtaza I. Analysis of NT-proBNP and uric acid due to left ventricle hypertrophy in the patients of aortic valve disease. Pak J Med Sci. 2019;35:183–8. doi: 10.12669/pjms.35.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber M, Arnold R, Rau M, Elsaesser A, Brandt R, Mitrovic V, et al. Relation of N-terminal pro B-type natriuretic peptide to progression of aortic valve disease. Eur Heart J. 2005;26:1023–30. doi: 10.1093/eurheartj/ehi236. [DOI] [PubMed] [Google Scholar]
- 41.Saleem N, Prasad A, Goswami SK. Apocynin prevents isoproterenol-induced cardiac hypertrophy in rat. Mol Cell Biochem. 2018;445:79–88. doi: 10.1007/s11010-017-3253-0. [DOI] [PubMed] [Google Scholar]
- 42.Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, et al. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res. 2014;101:373–82. doi: 10.1093/cvr/cvt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dewenter M, von der Lieth A, Katus HA, Backs J. Calcium signaling and transcriptional regulation in cardiomyocytes. Circ Res. 2017;121:1000–20. doi: 10.1161/CIRCRESAHA.117.310355. [DOI] [PubMed] [Google Scholar]
- 44.Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol. 2011;3:a004242. doi: 10.1101/cshperspect.a004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–80. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riehle C, Bauersachs J. Key inflammatory mechanisms underlying heart failure. Herz. 2019;44:96–106. doi: 10.1007/s00059-019-4785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erten Y, Tulmac M, Derici U, Pasaoglu H, Altok Reis K, Bali M, et al. An association between inflammatory state and left ventricular hypertrophy in hemodialysis patients. Ren Fail. 2005;27:581–9. doi: 10.1080/08860220500200072. [DOI] [PubMed] [Google Scholar]
- 48.Samak M, Fatullayev J, Sabashnikov A, Zeriouh M, Schmack B, Farag M, et al. Cardiac hypertrophy: an introduction to molecular and cellular basis. Med Sci Monit Basic Res. 2016;22:75–9. doi: 10.12659/MSMBR.900437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen D, Su A, Fu Y, Wang X, Lv X, Xu W, et al. Harmine blocks herpes simplex virus infection through downregulating cellular NF-κB and MAPK pathways induced by oxidative stress. Antivir Res. 2015;123:27–38. doi: 10.1016/j.antiviral.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Li M, Tan S, Wang C, Fan S, Huang C. Harmine is an inflammatory inhibitor through the suppression of NF-κB signaling. Biochem Biophys Res Commun. 2017;489:332–8. doi: 10.1016/j.bbrc.2017.05.126. [DOI] [PubMed] [Google Scholar]
- 51.Niu X, Yao Q, Li W, Zang L, Li W, Zhao J, et al. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice. Eur J Pharmacol. 2019;849:160–9. doi: 10.1016/j.ejphar.2019.01.062. [DOI] [PubMed] [Google Scholar]
- 52.Li L, Xu J, He L, Peng L, Zhong Q, Chen L, et al. The role of autophagy in cardiac hypertrophy. Acta Biochim Biophys Sin. 2016;48:491–500. doi: 10.1093/abbs/gmw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89:1401–38. doi: 10.1007/s00204-015-1477-x. [DOI] [PubMed] [Google Scholar]
- 54.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–21. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 55.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie M, Burchfield JS, Hill JA. Pathological ventricular remodeling: therapies: part 2 of 2. Circulation. 2013;128:1021–30. doi: 10.1161/CIRCULATIONAHA.113.001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glennon RA, Dukat M, Grella B, Hong S, Costantino L, Teitler M, et al. Binding of beta-carbolines and related agents at serotonin (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug Alcohol Depend. 2000;60:121–32. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 58.Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 59.Kuhn C, Frank D, Will R, Jaschinski C, Frauen R, Katus HA, et al. DYRK1A is a novel negative regulator of cardiomyocyte hypertrophy. J Biol Chem. 2009;284:17320–7. doi: 10.1074/jbc.M109.006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grebe C, Klingebiel TM, Grau SP, Toischer K, Didie M, Jacobshagen C, et al. Enhanced expression of DYRK1A in cardiomyocytes inhibits acute NFAT activation but does not prevent hypertrophy in vivo. Cardiovasc Res. 2011;90:521–8. doi: 10.1093/cvr/cvr023. [DOI] [PubMed] [Google Scholar]
- 61.Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med. 2015;21:383–8. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foldes G, Mioulane M, Wright JS, Liu AQ, Novak P, Merkely B, et al. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy? J Mol Cell Cardiol. 2011;50:367–76. doi: 10.1016/j.yjmcc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gesmundo I, Miragoli M, Carullo P, Trovato L, Larcher V, Di Pasquale E, et al. Growth hormone-releasing hormone attenuates cardiac hypertrophy and improves heart function in pressure overload-induced heart failure. Proc Natl Acad Sci USA. 2017;114:12033–8. doi: 10.1073/pnas.1712612114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin TP, Hortigon-Vinagre MP, Findlay JE, Elliott C, Currie S, Baillie GS. Targeted disruption of the heat shock protein 20-phosphodiesterase 4D (PDE4D) interaction protects against pathological cardiac remodelling in a mouse model of hypertrophy. FEBS Open Bio. 2014;4:923–7. doi: 10.1016/j.fob.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu YN, Wang Y, Bo S, Jiang Y, Liu XD, Shen XC. Ameliorated effects of cyclovirobuxine D on oxidative stress and energy metabolism in experimental cardiac injured rats induced by sympathetic overactivity in vivo. Zhong Yao Cai. 2014;37:1213–7. [PubMed] [Google Scholar]
- 66.Chiarello C, Bortoloso E, Carpi A, Furlan S, Volpe P. Negative feedback regulation of Homer 1a on norepinephrine-dependent cardiac hypertrophy. Exp Cell Res. 2013;319:1804–14. doi: 10.1016/j.yexcr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Li X, Kong M, Jiang D, Dong A, Shen Z, et al. Cardiac-targeting magnetic lipoplex delivery of SH-IGF1R plasmid attenuate norepinephrine-induced cardiac hypertrophy in murine heart. Biosci Rep. 2014;34:e00140. doi: 10.1042/BSR20130107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo JD, Xie F, Zhang WW, Ma XD, Guan JX, Chen X. Simvastatin inhibits noradrenaline-induced hypertrophy of cultured neonatal rat cardiomyocytes. Br J Pharmacol. 2001;132:159–64. doi: 10.1038/sj.bjp.0703792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–91. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 70.Zhang C, Shan XL, Liao YL, Zhao P, Guo W, Wei HC, et al. Effects of stachydrine on norepinephrine-induced neonatal rat cardiac myocytes hypertrophy and intracellular calcium transients. BMC Complement Alter Med. 2014;14:474. doi: 10.1186/1472-6882-14-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grubb S, Vestergaard ML, Andersen AS, Rasmussen KK, Mamsen LS, Tuckute G, et al. Comparison of cultured human cardiomyocyte clusters obtained from embryos/fetuses or derived from human embryonic stem cells. Stem Cells Dev. 2019;28:608–19. doi: 10.1089/scd.2018.0231. [DOI] [PubMed] [Google Scholar]
- 72.Blazeski A, Zhu R, Hunter DW, Weinberg SH, Boheler KR, Zambidis ET, et al. Electrophysiological and contractile function of cardiomyocytes derived from human embryonic stem cells. Prog Biophys Mol Biol. 2012;110:178–95. doi: 10.1016/j.pbiomolbio.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colella M, Grisan F, Robert V, Turner JD, Thomas AP, Pozzan T. Ca2+ oscillation frequency decoding in cardiac cell hypertrophy: Role of calcineurin/NFAT as Ca2+ signal integrators. Proc Natl Acad Sci USA. 2008;105:2859–64. doi: 10.1073/pnas.0712316105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lattion AL, Michel JB, Arnauld E, Corvol P, Soubrier F. Myocardial recruitment during ANF mRNA increase with volume overload in the rat. Am J Physiol. 1986;251:H890–6. doi: 10.1152/ajpheart.1986.251.5.H890. [DOI] [PubMed] [Google Scholar]
- 76.Shiota N, Rysa J, Kovanen PT, Ruskoaho H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1935–44. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 77.Kubota T, McTiernan CF, Frye CS, Demetris AJ, Feldman AM. Cardiac-specific overexpression of tumor necrosis factor-alpha causes lethal myocarditis in transgenic mice. J Card Fail. 1997;3:117–24. doi: 10.1016/s1071-9164(97)90045-2. [DOI] [PubMed] [Google Scholar]
- 78.Turner NA, Mughal RS, Warburton P, O'Regan DJ, Ball SG, Porter KE. Mechanism of TNFα-induced IL-1α, IL-1β and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc Res. 2007;76:81–90. doi: 10.1016/j.cardiores.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–31. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–25. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meier H, Bullinger J, Marx G, Deten A, Horn LC, Rassler B, et al. Crucial role of interleukin-6 in the development of norepinephrine-induced left ventricular remodeling in mice. Cell Physiol Biochem. 2009;23:327–34. doi: 10.1159/000218180. [DOI] [PubMed] [Google Scholar]
- 82.de Bold AJ. Cardiac natriuretic peptides gene expression and secretion in inflammation. J Investig Med. 2009;57:29–32. doi: 10.2310/JIM.0b013e3181948b37. [DOI] [PubMed] [Google Scholar]
- 83.Meirovich YF, Veinot JP, de Bold ML, Haddad H, Davies RA, Masters RG, et al. Relationship between natriuretic peptides and inflammation: proteomic evidence obtained during acute cellular cardiac allograft rejection in humans. J Heart Lung Transpl. 2008;27:31–7. doi: 10.1016/j.healun.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 84.Fish-Trotter H, Ferguson JF, Patel N, Arora P, Allen NB, Bachmann KN, et al. Inflammation and circulating natriuretic peptide levels. Circ Heart Fail. 2020;13:e006570. doi: 10.1161/CIRCHEARTFAILURE.119.006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masiha S, Sundstrom J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens. 2013;27:13–7. doi: 10.1038/jhh.2011.113. [DOI] [PubMed] [Google Scholar]
- 86.Wang L, Zhang YL, Lin QY, Liu Y, Guan XM, Ma XL, et al. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39:1818–31. doi: 10.1093/eurheartj/ehy085. [DOI] [PubMed] [Google Scholar]
- 87.Fang L, Ellims AH, Beale AL, Taylor AJ, Murphy A, Dart AM. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res. 2017;9:5063–73. [PMC free article] [PubMed] [Google Scholar]
- 88.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–3. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 89.Gupta S, Young D, Sen S. Inhibition of NF-kappaB induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H20–9. doi: 10.1152/ajpheart.00082.2005. [DOI] [PubMed] [Google Scholar]
- 90.Rauvala H, Rouhiainen A. RAGE as a receptor of HMGB1 (Amphoterin): roles in health and disease. Curr Mol Med. 2007;7:725–34. doi: 10.2174/156652407783220750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.