Abstract
Purpose
The coronavirus disease 2019 (COVID-19) pandemic has significantly impacted the rates of screening, case identification, and referral for cancer diagnosis. We investigated the diagnosis and surgery status of breast cancer before and after the COVID-19 pandemic at a multi-institutional level.
Methods
We collected breast cancer data from the clinical data warehouse which contained the medical records of patients from six academic institutions in South Korea. Patients were divided into two groups: February to April (period A) and May to July (period B). The data from the two groups were then compared against the same periods in 2019 and 2020. The primary objective was to investigate the differences in breast cancer stages before and after the COVID-19 pandemic.
Results
Among 3,038 patients, there was a 9.9% reduction in the number of diagnoses in 2020. This decrease was more significant during period A than period B. The breast cancer stage was not statistically different in period A (p = 0.115), but it was in period B (p = 0.001). In the subset analysis according to age, there was a statistical difference between 2019 and 2020 in period B for patients under the age of 65 years (p = 0.002), but no difference was observed in the other groups.
Conclusion
The number of breast cancer cases declined during the pandemic, and the staging distribution has changed after the pandemic peak.
Keywords: Breast Neoplasms, Carcinoma, COVID-19, Early Detection of Cancer, SARS-CoV-2
INTRODUCTION
More than one year has passed since the first coronavirus disease 2019 (COVID-19) case was confirmed on January 20, 2020, in South Korea. Screening, case identification, and referral for symptomatic cancer diagnosis have all been affected by the COVID-19 pandemic [1,2]. Specifically, most routine preventive activities have been reduced, including active screening programs [3]. Analyzing data from a network of 20 institutions with 28 million patients suggested a significant reduction in the number of breast cancer patients [1]. In addition, breast cancer screening has declined drastically by 89.2% [1].
Few guidelines have been found on breast cancer screening and new diagnosis, in contrast to guidelines for triage, prioritization, and treatment [4,5]. Specific recommendations regarding cancer screening and diagnostic testing during the pandemic should be based on the state of COVID-19 cases in a community as well as the availability of resources. Although screening for breast cancer is widely advocated in many countries, some have shut down their cancer screening programs during the pandemic [6]. As of August 2020, the American Society for Clinical Oncology (ASCO) guidelines recommended postponing most cancer screening procedures to conserve health system resources and reduce patient contact with healthcare facilities unless clinically relevant cancer is suspected [7]. However, as the COVID-19 pandemic extends over the past year, postponing screening and diagnosis can be increasingly dangerous. Concerns have been raised about the ramifications of halting national screening programs during the pandemic [8,9].
Telemedicine can be accessed directly from home and may reduce the risk of contracting COVID-19 by restricting person-to-person contact. Telemedicine and virtual visits have expanded across oncologic care during the COVID-19 pandemic [10], and the European Society for Medical Oncology (ESMO) and ASCO suggested that patients who do not require direct examination, treatment, or diagnostics should participate in telemedicine during the pandemic. However, the difficulty in conducting in-office visits can be a hurdle for newly identified breast cancer. In addition, breast cancer screening and diagnosis require imaging modalities and procedures.
Due to the conditions that existed during the pandemic, it has become known that breast cancer diagnoses may have been delayed [11,12]. The risk of a delayed cancer diagnosis should be compared with serious complications or mortality from COVID-19. The goal of this study was to determine whether breast cancer screening and diagnosis have indeed been delayed significantly enough to result in the stage migration of cancer patients. We hypothesized that there would be a shift to a more advanced stage among patients diagnosed during the pandemic period, relative to those diagnosed beforehand. In addition, we assessed whether there were differences according to age in order to evaluate the competing risks of cancer versus infection. This is because the age of 65 years is an established risk factor for severe COVID-19 [13,14,15,16]. Older people are more likely to develop serious complications of COVID-19 and have a higher mortality rate [15,16].
METHODS
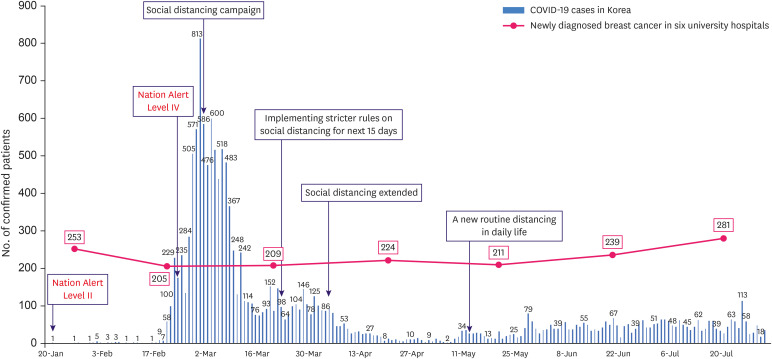
Data on patients diagnosed with breast cancer from February 2019 to July 2020 were collected retrospectively from the clinical data warehouse (CDW), which contained the medical records of patients from academic institutions of the Catholic Medical Center in South Korea (http://cohort.cmcnu.or.kr). In total 3,038 patients were included in the CDW. Medical records were analyzed from the data extracted from the CDW in text format. This study collected de-identified data from female patients aged 18 years and older with pathologically confirmed invasive or in situ breast malignancy at six academic institutions: Incheon St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, St. Vincent’s Hospital, Seoul St. Mary’s Hospital, and Bucheon St. Mary’s Hospital. We also obtained demographic data, clinical manifestations, and laboratory findings, including polymerase chain reaction (PCR) test results for COVID-19 and radiologic images of the enrolled patients by reviewing their medical records. The number of patients was analyzed by the data from the CDW. Study participants were divided into two groups according to their initial date of malignancy diagnosis: from February 1 to April 30 (period A) and from May 1 to July 31 (period B). We set period A from February to April, which was when the country’s crisis alert level was raised to level IV (the highest level) and the number of COVID-19 cases rapidly increased in 2020. The government provided a plan for stronger social distancing on March 22. On April 20, it was announced that social distancing would continue in a somewhat relaxed form until May 5. A new routine of distancing in daily life was applied on May 6. This was a sub-category of social distancing, the lowest phase-one social distancing. In April, the number of COVID-19 patients decreased to less than 100, but the social activity and mood changed as of May. Therefore, period B was set to the same period of three months from May to July. Figure 1 shows the trend in COVID-19 occurrence in South Korea from the date of the first-identified case to July 2020 and the number of newly identified breast cancer patients per month in the six university hospitals. Data from the two groups were then compared with those from the corresponding periods in 2019 and 2020. The tumor stage was based on the tumor-node-metastasis (TNM) classification of the American Joint Committee on Cancer eighth edition. Clinical characteristics and stage migrations were analyzed using data from the medical records. A total of 2,398 patients were included in this study, including 1,090 in period A and 1,308 in period B. The cohorts from periods A and B had similar characteristics, including age, stage, and region (Table 1).
Figure 1. Timeline of the COVID-19 pandemic in South Korea and the number of breast cancer diagnoses.
COVID-19 = coronavirus disease 2019.
Table 1. Characteristics from electrical medical records database (n = 2,398).
| Characteristics | All patients (n = 2,398) | Period A (n = 1,090) | Period B (n = 1,308) | p-value | |
|---|---|---|---|---|---|
| Age (yr) | 0.076 | ||||
| Median | 53 | 53 | 53 | ||
| Range | 19–96 | 21–91 | 19–96 | ||
| Age group (yr) | 0.164 | ||||
| ≤ 65 | 1,969 (82.1) | 907 (83.1) | 1,062 (81.2) | ||
| > 65 | 427 (17.8) | 183 (16.8) | 246 (18.8) | ||
| Unknown | 2 (0.1) | 2 (0.2) | 0 (0.0) | ||
| Year | 0.032 | ||||
| 2019 (pre-COVID-19) | 1,267 (52.8) | 602 (55.2) | 665 (50.8) | ||
| 2020 (COVID-19) | 1,131 (47.2) | 488 (44.8) | 643 (49.2) | ||
| cT stage | 0.195 | ||||
| 0 | 420 (17.5) | 186 (17.1) | 234 (17.9) | ||
| 1 | 696 (29.0) | 346 (31.7) | 350 (26.8) | ||
| 2 | 676 (28.2) | 297 (27.2) | 379 (29.0) | ||
| 3 | 140 (5.8) | 65 (6.0) | 75 (5.7) | ||
| 4 | 24 (1.0) | 13 (1.2) | 11 (0.8) | ||
| Unknown | 442 (18.4) | 183 (16.8) | 259 (19.8) | ||
| cN stage | 0.338 | ||||
| 0 | 1,055 (44.0) | 516 (47.3) | 539 (41.2) | ||
| 1 | 472 (19.7) | 210 (19.3) | 262 (20.0) | ||
| 2 | 7 (0.3) | 4 (0.4) | 3 (0.2) | ||
| 3 | 45 (1.9) | 19 (1.7) | 26 (2.0) | ||
| Unknown | 819 (34.15) | 341 (31.3) | 478 (36.5) | ||
| Location | 0.092 | ||||
| Seoul | 548 (22.9) | 235 (21.6) | 313 (23.9) | ||
| Incheon | 455 (19.0) | 205 (18.8) | 250 (19.1) | ||
| Gyeonggi-do (east) | 548 (22.9) | 243 (22.3) | 305 (23.3) | ||
| Gyeonggi-do (west) | 470 (19.6) | 212 (19.4) | 258 (19.7) | ||
| Others | 359 (15.0) | 183 (16.8) | 176 (13.5) | ||
| Unknown | 18 (0.8) | 12 (1.1) | 6 (0.5) | ||
Values are presented as number of patients (%) not otherwise specified.
COVID-19 = coronavirus disease 2019; cT = clinical tumor stage; cN = clinical nodal stage.
The main goal of this study was to determine whether there were any differences in the clinical stage. We employed a cross-tabulation analysis for the clinical stage using data from the pre-pandemic and pandemic periods. For staging comparison, patients whose clinical stage could be assessed without any associated missing data were enrolled and analyzed. Subgroups divided according to time course and age, were also analyzed for clinical stage migration. We set a cutoff age of 65 years because that age is an established risk factor for severe COVID-19 [13,14,15,16]. Using CDW data, we compared the differences in the number of patients with breast cancer screening, newly identified disease, and surgery between 2019 and 2020. The number of breast cancer screenings was defined as mammography or breast ultrasonography in the asymptomatic group. The screening data consisted of people who had undergone mammography or breast sonography in the health care screening center; those who had taken images in an outpatient setting and those who were hospitalized or admitted to the emergency department were excluded. Primary imaging modalities for breast cancer were also tracked by assessing the number of mammography or breast ultrasonography examinations in the asymptomatic and diagnostic groups, including those at the outpatient clinic. The number of mammography and breast sonography was counted separately and summed. Cancer surgeries were compared by stratifying the patients into breast and axillary operations. Reconstruction surgeries were also performed for patients with breast cancer. Breast diagnoses and screening were analyzed according to age. This study was approved by the Institutional Review Board of the Catholic Medical Center (IRB No. XC20WIDI0138) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was not required from study participants.
Continuous variables are expressed as mean (standard deviation) values, and categorical variables are expressed as percentages (frequency). Continuous variables were analyzed using the unpaired t-test or analysis of variance, and categorical variables were analyzed using the χ2 test or Fisher’s exact test. Pearson’s χ2 test was applied for dichotomous variables if the expected frequency was greater than five, whereas Fisher’s exact test was applied if it was not. The values from two-sided tests are presented, and a p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for the Social Sciences, version 26.0 (IBM Corporation, Armonk, USA).
RESULTS
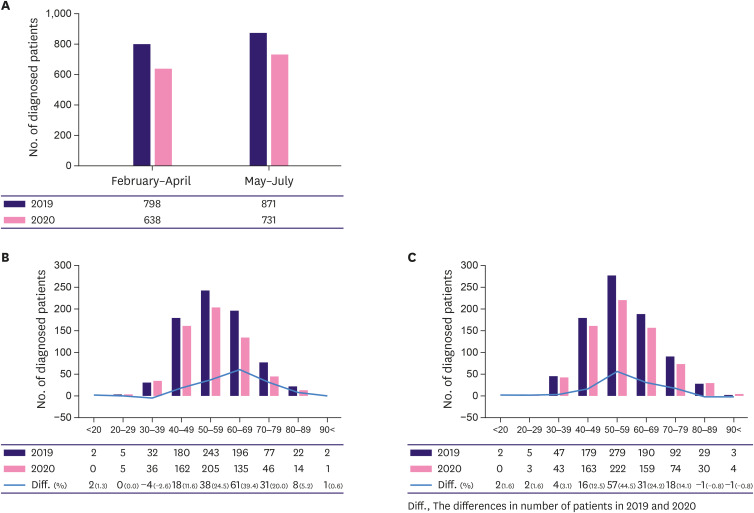
Overall, the number of newly diagnosed breast cancer cases was 1,669 from February to July 2019 and 1,369 in the same period in 2020 (a decrease of 9.9%). More specifically, the number of new cases decreased by 11.2% in period A and 8.8% in period B relative to 2019, showing a 2.4% greater reduction in period A than in period B (Figure 2A, Supplementary Table 1). In comparing the difference between the number of patients in the two periods by age, the decrease in diagnosis in 2020 was seen mainly among patients in their 40s to 70s in both periods. In period A, the differences were 11.6%, 24.5%, 39.4%, and 20.0% in those in their 40s, 50s, 60, and 70s, respectively. In period B, 12.5%, 44.5%, 24.2%, and 14.1% in those in their 40s, 50s, 60, and 70s, respectively. (Figure 2B and C, Supplementary Table 2).
Figure 2. Numbers of newly diagnosed breast cancer patients before and after the COVID-19 pandemic at six university hospitals.
(A) During periods A (February to April) and B (May to July). (B) According to age during period A. (C) According to age during period B.
COVID-19 = coronavirus disease 2019.
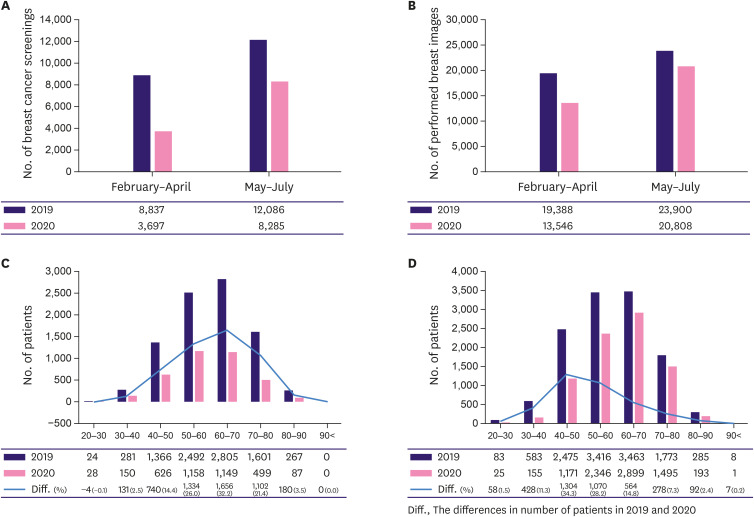
The total number of breast cancer screenings was reduced by 27.4% in 2020 and was even lower in period A than in period B. A steep decline of 41% was observed in period A when the number of COVID-19 cases surged (Figure 3A, Supplementary Table 1). The total number of mammograms and breast ultrasonography examinations also decreased in 2020 (Figure 3B, Supplementary Table 1), with decreases of 17.7% and 6.9% in periods A and B, respectively. According to age, the screening analysis showed a further decline in the older group during period A. The decline was most prominent in patients in their 50s and 60s, whereas, in period B, there was a greater decrease in the younger group (Figure 3C and D, Supplementary Table 3).
Figure 3. Numbers of breast imaging modalities (mammogram and breast sonography).
(A) Numbers of breast cancer screening (asymptomatic group). (B) Numbers of primary breast imaging modalities (asymptomatic and diagnostic groups). (C) Numbers of breast cancer screenings according to age during period A (February to April). (D) Numbers of breast cancer screenings according to age during period B (May to July).
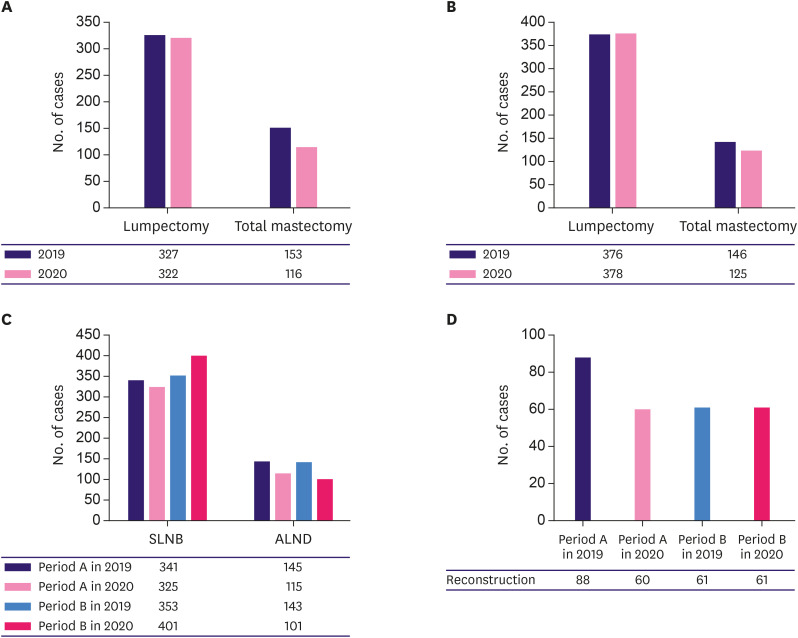
The absolute number of surgeries decreased in both periods, but the number of lumpectomy cases in 2019 and 2020 were comparable; only the number of total mastectomy procedures was reduced in 2020 (Figure 4A and B, Supplementary Table 1). Statistical analysis showed no difference in breast surgery in period A (p = 0.212) but a significant difference in period B (p = 0.003). The number of lymph node surgeries was statistically different in both periods (p = 0.026 in period A and p < 0.001 in period B). The number of sentinel biopsies decreased in 2020 during period A but was performed more frequently during period B (Figure 4C, Supplementary Table 1). Meanwhile, the absolute number of axillary lymph node dissections decreased in both periods, and the rate of reconstruction surgeries decreased in period A but recovered in period B (Figure 4C and D, Supplementary Table 1).
Figure 4. Numbers of breast cancer surgeries.
(A) Breast surgeries during period A (February to April). (B) Breast surgeries during period B (May to July). (C) Numbers of axillary surgeries. (D) Numbers of reconstruction surgeries.
SLNB = sentinel lymph node biopsy; ALND = axillary lymph node dissection.
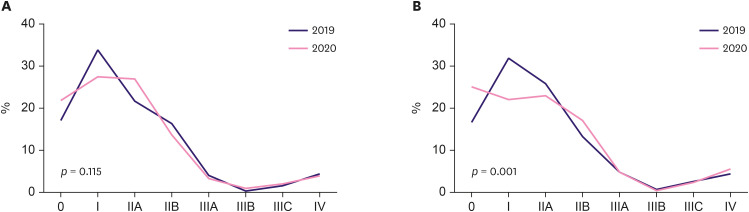
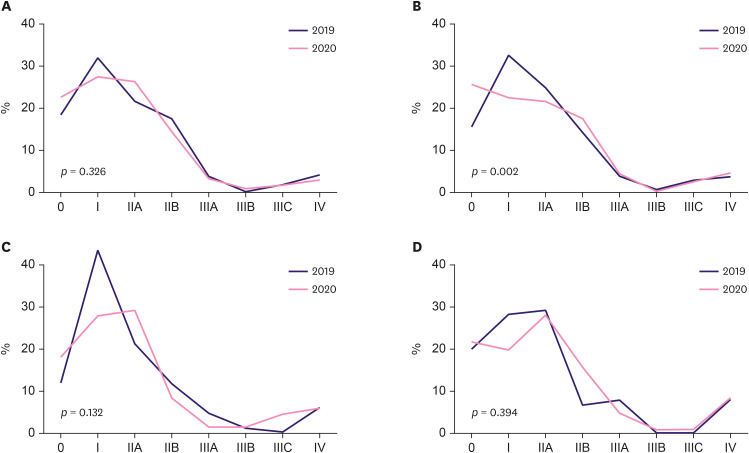
Looking at the stage distribution relative to that of the previous year, there was no statistically significant difference in period A (Figure 5A, Table 2) (p = 0.115). However, in period B, the stage distribution differed between 2019 and 2020 (Figure 5B, Table 2) (p = 0.001). In period B, the ratios were 31.9% in 2019 and 22.1% in 2020 for stage I, and 25.8% in 2019 and 22.9% in 2020 for stage IIA. The proportion of patients with stage IV disease was 4.5% and 5.6% in 2019 and 2020, respectively. Although stages I and IIA invasive cancer were more frequently diagnosed during the pre-pandemic period, the rate of stages IIB (13.2% in 2019, 17.01% in 2020) and IV diagnoses increased during the pandemic. The rate of in situ cases was also higher during the pandemic (16.7% in 2019 and 25.0% in 2020). The stage distribution was analyzed according to age (younger or older than 65 years). There was a statistical difference only in period B in patients under the age of 65 years (p = 0.002), but no difference was observed in the other groups (Figure 6, Table 3).
Figure 5. Clinical stage distribution of breast cancer.
(A) During period A (February to April). (B) During period B (May to July).
Table 2. Clinical stage distribution of breast cancer according to period.
| Periods | Years | cStage | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | IIA | IIB | IIIA | IIIB | IIIC | IV | Total | |||
| Period A | 2020 | 98 (22.0) | 123 (27.6) | 120 (26.9) | 61 (13.5) | 14 (3.1) | 4 (0.9) | 9 (2.0) | 18 (4.0) | 447 | 0.115 |
| 2019 | 88 (17.4) | 171 (33.9) | 109 (21.6) | 84 (16.4) | 21 (4.2) | 2 (0.4) | 8 (1.6) | 22 (4.6) | 505 | ||
| Period B | 2020 | 144 (25.0) | 127 (22.1) | 132 (22.9) | 99 (17.01) | 27 (4.7) | 3 (0.5) | 13 (2.3) | 31 (5.6) | 576 | 0.001 |
| 2019 | 90 (16.7) | 172 (31.9) | 139 (25.8) | 71 (13.2) | 25 (4.6) | 4 (0.7) | 14 (2.6) | 24 (4.5) | 539 | ||
Values are presented as number of patients (%) not otherwise specified.
cStage = clinical stage.
Figure 6. Clinical stage distribution of breast cancer according to age.
(A) Patients younger than 65 years in period A (February to April). (B) Patients younger than 65 years in period B (May to July). (C) Patients older than 65 years in period A. (D) Patients older than 65 years in period B.
Table 3. Clinical stage distribution of breast cancer according to age.
| Periods | Years | cStage | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | IIA | IIB | IIIA | IIIB | IIIC | IV | Total | ||||
| Patients younger than 65 years | ||||||||||||
| Period A | 2020 | 85 (22.7) | 103 (27.5) | 99 (26.4) | 55 (14.7) | 13 (3.5) | 3 (0.8) | 6 (1.6) | 11 (2.9) | 375 | 0.326 | |
| 2019 | 78 (18.6) | 134 (31.9) | 91 (21.7) | 74 (17.6) | 17 (4.1) | 1 (0.2) | 8 (1.9) | 17 (4.1) | 420 | |||
| Period B | 2020 | 120 (25.8) | 105 (22.5) | 101 (21.7) | 82 (17.6) | 22 (4.7) | 2 (0.4) | 12 (2.6) | 22 (4.7) | 466 | 0.002 | |
| 2019 | 72 (16.0) | 147 (32.7) | 113 (25.1) | 65 (14.4) | 18 (4.0) | 4 (0.9) | 14 (3.1) | 17 (3.8) | 450 | |||
| Patients older than 65 years | ||||||||||||
| Period A | 2020 | 13 (18.1) | 20 (27.8) | 21 (29.2) | 6 (8.3) | 1 (1.4) | 1 (1.4) | 3 (4.2) | 7 (5.7) | 72 | 0.132 | |
| 2019 | 10 (11.8) | 37 (43.5) | 18 (21.2) | 10 (11.8) | 4 (4.7) | 1 (1.2) | 0 (0.0) | 5 (5.9) | 85 | |||
| Period B | 2020 | 24 (21.8) | 22 (20.0) | 31 (28.2) | 17 (15.5) | 5 (4.6) | 1 (0.9) | 1 (0.9) | 9 (8.2) | 110 | 0.394 | |
| 2019 | 18 (20.2) | 25 (28.1) | 26 (29.2) | 6 (6.7) | 7 (7.9) | 0 (0.0) | 0 (0.0) | 7 (7.9) | 89 | |||
Values are presented as number of patients (%) not otherwise specified.
cStage = clinical stage.
DISCUSSION
The healthcare system for cancer was not overloaded, and restrictions on visiting healthcare facilities were minimal in South Korea during the COVID-19 pandemic. However, the number of breast cancer screenings, diagnoses, and surgeries decreased relative to that of the pre-pandemic period. It could be assumed that patients became more afraid to visit hospitals because of the fear of nosocomial transmission of this novel disease. These fears affected societies in most countries and were potentially associated with the reduced utilization of oncology services. Moreover, the degree of decline was more noticeable in the older patients. This study verified stage differences in breast cancer after the start of the COVID-19 pandemic, particularly among patients younger than 65 years of age.
In comparing the differences between the number of breast cancer patients in the two periods by age, a decrease was seen mainly among patients in their 40s to 70s in both periods. The age groups with the most significant decrease were those in their 60s (period A) and 50s (period B). A more profound decrease was observed in the older age groups (those in their 50s to 70s) in period A than in period B; this was especially noticeable in those in their 50s and 60s. As there was a difference even in 80–89 age group in period A, there was a relatively greater overall decrease in older patients in this period than in period B. There was no discernible difference in those aged < 40 years who were not included in the national cancer screening program [17]. In addition, there was a minor difference in patients in their 80s, which was assumed to be the cause of lower activity in the breast cancer tests [17].
In period B, lower rates of stages I and IIA were observed in 2020, while stages IIB and IV were observed at a higher rate in 2020. For stages IIB, IIIA, and IIIB, the number of newly diagnosed patients was small, with the differences in patient numbers between 2019 and 2020 being 2, 1, and 1, respectively. Additionally, although the percentage of in situ cases was higher in the pandemic period, the absolute number of in situ cases was small. The clinical stage, but not the pathologic stage, of breast cancer was compared in this study. Since 2010, the proportion of neoadjuvant chemotherapy has increased regardless of the tumor subtype [18]. Many patients with estrogen receptor (ER)-positive and human epidermal growth factor receptor 2 (HER2)-negative primary breast cancer attempted to postpone surgery in favor of neoadjuvant endocrine therapy during the COVID-19 pandemic [19]. In this study, patients younger than 65 years exhibited more prominent cancer stage migration, despite their reduced reluctance to visit a hospital during the pandemic, relative to older patients. The reason for this finding has not yet been ascertained. Young patients tend to present at more advanced stages, and their tumors tend to be of higher grades, be hormone receptor-negative, have increased HER2 overexpression, and display more lymphovascular invasion [20,21].
Although COVID-19 itself is a problem, delays in cancer diagnosis due to the COVID-19 pandemic are also expected to lead to many problems, including increased mortality rates. In a breast cancer modeling study, Sud et al. [22] contended that a three-month delay in patient presentation would result in 734 additional deaths and 15,339 life-years lost. Attributable life-years lost were the highest for breast cancer. The National Cancer Institute also predicts that 10,000 excess deaths from breast and colorectal cancers alone will occur over the next decade in the United States [23]. Similarly, Maringe et al. [12] estimated that there might be an increase in cancer deaths due to diagnostic delays over the next 5 years, including 9.6% in patients with breast cancer.
Since 2000, we have experienced epidemics and pandemics of several novel infectious diseases, including severe acute respiratory syndrome (SARS), H5N1, and Middle East respiratory syndrome (MERS). South Korea stood out in the global COVID-19 response, with fewer than 80,000 cases and 1,500 deaths a year after the first case was reported [24]. Despite such conditions, the outbreak of COVID-19 as an infectious disease has made people more reluctant to visit hospitals. Decreased health care utilization has also been observed in the context of SARS and MERS, respectively [25,26]. Multiple variants of SARS-CoV-2 have been documented globally during the current pandemic. The delta COVID-19 variant (B.1.617.2) has grown globally as the most dominant strain [27]. This variant has numerous mutations, some of which are concerning. Recently, Omicron (B.1.1.529) has spread worldwide [28]. Diagnostic delays for breast cancer could have occurred before returning to normal in these situations. As a result, we can expect a surge in the number of breast cancer patients at an advanced stage. Short-term gains achieved for healthcare systems by restricting oncologic services may later lead to longer-term misery, as significant reductions in mortality have been attributed to the implementation of screening and diagnostic services.
There were a few missing values among the extracted data in the CDW, which led to several limitations. Pathological data were incomplete in 1,966 of the 2,398 patients. We assessed molecular subtypes in 245 patients. Accordingly, pathological stage and molecular subtype analyses were not performed. At the 2020 San Antonio Breast Cancer Symposium, a retrospective study reported that more patients had advanced and aggressive types of breast cancer at the beginning of the COVID-19 pandemic [11]. Fewer patients had hormone receptor-positive, HER2-negative breast cancer, whereas the rate of triple-negative breast cancer increased during the COVID-19 pandemic. The study results also showed that more symptomatic patients were diagnosed in 2020 [11]. However, this study did not compare the rate of patients with symptomatic breast cancer. Records of the patients’ symptoms were inaccurate, and few records were documented. Another limitation was that screening was reduced during the pandemic, but the rate of in situ screening was increased compared with the pre-pandemic rate. We could not determine why the in situ lesions did not cause upstage migration during the pandemic. Assessing the pathologic stage and molecular subtypes might be instrumental in understanding the reasons, but the difficulties of the analysis due to the lack of data were considered a limitation. Additionally, the rates of neoadjuvant and adjuvant systemic therapies were not analyzed. The differences in the rate of neoadjuvant systemic therapies have not been analyzed based on recent trends in breast cancer treatment. Furthermore, the time from diagnosis to surgery was expected to be delayed; however, this could not be analyzed due to insufficient data. Further studies are needed to analyze pathologic stage differences, stage migration on subtypes, symptoms, and the rate of neoadjuvant systemic therapy.
In 2021, multiple safe and effective vaccines for COVID-19 have been developed [29]. Based on these achievements and the aforementioned problems, several national professional societies, including the ASCO and American Cancer Society, have modified their guidelines [30]. Therefore, it is necessary to discuss the potential long-lasting deleterious effects of the COVID-19 pandemic on cancer diagnosis and management. In addition, we should prepare to deal with the backlog of cases caused by the COVID-19 pandemic and other diagnostic delay situations. Individuals who have risk factors such as a family history of cancer should be encouraged to accept vaccination and not avoid putting off routine screenings and immediately seek medical care if they experience cancer symptoms. These results should be of particular interest to those younger than 65 years who might be affected by diagnostic delays and who have relatively lower mortality rates from infectious diseases.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kang YJ, Oh SJ.
- Data curation: Kang YJ, Baek JM, Kim YS, Jeon YW, Yoo TK, Rhu J, Shin CH, Cho S, Choi H.
- Formal analysis: Kang YJ, Baek JM, Kim YS, Jeon YW, Yoo TK, Rhu J, Shin CH, Cho S, Choi H.
- Investigation: Kang YJ, Baek JM, Kim YS, Jeon YW, Yoo TK, Rhu J.
- Methodology: Kang YJ, Baek JM, Kim YS, Jeon YW, Yoo TK, Rhu J, Shin CH, Cho S, Choi H.
- Supervision: Oh SJ.
- Validation: Kang YJ, Oh SJ.
- Visualization: Kang YJ.
- Writing - original draft: Kang YJ.
- Writing - review & editing: Kang YJ, Oh SJ.
SUPPLEMENTARY MATERIALS
The number of patients sourced from the clinical data warehouse
Numbers of patients diagnosed with breast cancer according to age
Numbers of breast cancer screenings according to age
References
- 1.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7:458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN) NCCN coronavirus disease 2019 (COVID-19) resources for the cancer care community. [Accessed August 10th, 2020]. http://www.nccn.org/covid-19/default.aspx .
- 5.European Society of Medical Oncology (ESMO) ESMO management and treatment adapted recommendations in the COVID-19 era: breast cancer. [Accessed August 10th, 2020]. https://www.esmo.org/guidelines/breast-cancer/breast-cancer-in-the-covid-19-era . [DOI] [PMC free article] [PubMed]
- 6.Cancer Research UK. How coronavirus is impacting cancer services in the UK. [Accessed April 26th, 2020]. https://news.cancerresearchuk.org/2020/04/21/how-coronavirus-is-impacting-cancer-services-in-the-uk/
- 7.American Society of Clinical Oncology. Cancer screening, diagnosis, staging & surveillance. [Accessed August 10th, 2020]. https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-screening-diagnosis-staging .
- 8.Tan KK, Lau J. Cessation of cancer screening: an unseen cost of the COVID-19 pandemic? Eur J Surg Oncol. 2020;46:2154–2155. doi: 10.1016/j.ejso.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise J. Covid-19: Cancer mortality could rise at least 20% because of pandemic, study finds. BMJ. 2020;369:m1735. doi: 10.1136/bmj.m1735. [DOI] [PubMed] [Google Scholar]
- 10.Mulvey TM, Jacobson JO. COVID-19 and cancer care: ensuring safety while transforming care delivery. J Clin Oncol. 2020;38:3248–3251. doi: 10.1200/JCO.20.01474. [DOI] [PubMed] [Google Scholar]
- 11.Chang SB, Savitz AC, Vuong B, Tang A, Mentakis M, Miller AM, et al. Abstract SS2-06: Characterization of breast cancer management during the COVID 19 pandemic in a large integrated healthcare delivery system: Stage at diagnosis and timing/modality of first treatment. Cancer Res. 2021;81:SS2-06 [Google Scholar]
- 12.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by age group. [Accessed March 15th, 2021]. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html .
- 17.National Cancer Center. Title. [Accessed December 8th, 2021]. https://www.cancerdata.kr/surveillance/data;jsessionid=E193331CCE90F2962B3B9C0737320FFF?menuId=38#n .
- 18.Riedel F, Hoffmann AS, Moderow M, Heublein S, Deutsch TM, Golatta M, et al. Time trends of neoadjuvant chemotherapy for early breast cancer. Int J Cancer. 2020;147:3049–3058. doi: 10.1002/ijc.33122. [DOI] [PubMed] [Google Scholar]
- 19.Dowsett M, Ellis MJ, Dixon JM, Gluz O, Robertson J, Kates R, et al. Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic. NPJ Breast Cancer. 2020;6:21. doi: 10.1038/s41523-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(Suppl 1):S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29:e18–e20. doi: 10.1200/JCO.2010.28.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sud A, Torr B, Jones ME, Broggio J, Scott S, Loveday C, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 24.Our World In Data. Emerging COVID-19 success story: South Korea learned the lessons of MERS. 2020. [Accessed August 31st, 2021]. https://ourworldindata.org/covid-exemplar-south-korea?country=
- 25.Lee SY, Khang YH, Lim HK. Impact of the 2015 Middle East Respiratory Syndrome outbreak on emergency care utilization and mortality in South Korea. Yonsei Med J. 2019;60:796–803. doi: 10.3349/ymj.2019.60.8.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiber M, Lou WY. Effect of the SARS outbreak on visits to a community hospital emergency department. CJEM. 2006;8:323–328. doi: 10.1017/s148180350001397x. [DOI] [PubMed] [Google Scholar]
- 27.Public Health England. Variants: distribution of case data. [Accessed June 11th, 2021]. https://www.gov.uk/government/publications/covid-19-variants-genomically-confirmed-case-numbers/variants-distribution-of-case-data-11-june-2021 .
- 28.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. [Accessed December 8th, 2021]. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern .
- 29.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 30.American Society of Clinical Oncology. Cancer screening, diagnosis, staging & surveillance in ASCO. [Accessed March 19th, 2021]. https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-screening-diagnosis-staging .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of patients sourced from the clinical data warehouse
Numbers of patients diagnosed with breast cancer according to age
Numbers of breast cancer screenings according to age