Abstract
Adverse childhood experiences (ACEs) potentially contribute to posttraumatic stress disorder (PTSD) after adult trauma exposure, but underlying brain changes remain unclear. The present study tested relationships between ACEs, whole thalamus and thalamic nuclei volumes, and post-trauma stress symptoms (PTSS) after adult trauma. Trauma survivors (n=101) completed the Childhood Trauma Questionnaire (CTQ), the PTSD checklist-special stressor version 5 (PCL), and a structural magnetic resonance imaging (sMRI) scan within post-trauma 2 weeks. At post-trauma 3 months, survivors completed a second PCL survey and a PTSD diagnosis interview using the Clinician-Administered PTSD Scale (CAPS). CTQ scores significantly positively correlated with PCL scores at post-trauma 2 weeks and 3 months (respective p’s < 0.01 and < 0.001). CTQ scores significantly negatively correlated with whole thalamus and 7 thalamic nuclei volumes at post-trauma 2 weeks in the PTSD (N=50), but not the non-PTSD (N=51) group. Whole thalamus and 22 nuclei volumes significantly negatively correlated with PCL scores at post-trauma 3 months in the PTSD, but not the non-PTSD group. These results suggest ACEs negatively influence early post-trauma thalamic volumes which, in turn, are negatively associated with PTSS in survivors who develop PTSD.
Keywords: Magnetic resonance imaging, the Childhood Trauma Questionnaire, the PTSD checklist-special stressor, FreeSurfer
1. Introduction
Posttraumatic stress disorder (PTSD) is a debilitating condition characterized by post-traumatic stress symptoms (PTSS) that persist for more than one month after trauma (Calhoun et al., 2012; Jorge, 2015). About 7% of Americans suffer from PTSD at some point in their lives; consequently, PTSD is considered to have a major impact on public health (Kessler and Wang, 2008). The factors and brain changes that contribute to PTSD development after an adult traumatic event remain largely unknown.
Relationships between PTSS and organization of brain structure have been a focus of previous work (Bremner, 2006; Xie et al., 2018). The thalamus is a large diencephalic structure composed of heterogeneous nuclei that contribute to diverse functions including sensation, cognition, memory, and fear processing (Beas et al., 2018; Bergmann, 2008; LeDoux, 1986; Penzo et al., 2015; Steriade and Llinas, 1988). In general, alterations in the thalamus have been linked to a range of stress-related processing changes, including incorrect integration of trauma-related sensory inputs (Brewin, 2001), inappropriate attention processing (Suvak and Barrett, 2011), and over-consolidation of traumatic memory (Brewin, 2001; Suvak and Barrett, 2011).
PTSS have been shown to be associated with functional changes in the thalamus (Yan et al., 2013; Yin et al., 2011). In addition, thalamic structural alterations have been reported in chronic PTSD patients. One sMRI study has reported gray matter atrophy in the thalamus was significantly greater in chronic PTSD patients than controls (Cardenas et al., 2011). Trauma-related re-experiencing symptoms also negatively correlated with thalamus volumes in chronic PTSD patients (Shucard et al., 2012). The above findings on thalamic structure focus on chronic effects years after trauma. However, PTSS can emerge immediately after trauma and brain structural effects can be seen during the early post-trauma period. For example, we previously reported that hippocampal volumes are negatively associated with PTSS from 2 weeks to subsequent months after trauma in survivors who develop PTSD (Xie et al., 2018). Potential thalamic structural contributions to early PTSS and PTSD development after acute trauma have received little attention. Interestingly, one recent study reported that whole thalamus grey matter volume interacted with fear-potentiated startle at 2 weeks post trauma to predict PCL score 8 weeks later (Steuber et al., 2021). How these results relate to early post-trauma PTSS severity remains unknown. Recent neuroimaging studies also suggest thalamic nuclei volume changes are related to mental and neurological disorders including psychosis, obsessive-compulsive disorder (OCD), migraine, and epilepsy (Chen et al., 2021; Huang et al., 2020; Shin et al., 2019; Weeland et al., 2021). To our knowledge, no studies have assessed thalamic nuclei volume relationships to early post-trauma PTSS or PTSD development.
It has been suggested that adverse childhood experiences (ACEs) may affect development of brain structure which, in turn, may increase risk for PTSD after adult trauma. ACEs, which affect 7-60% of children, include neglect and physical, emotional, or sexual abuse, (Gilbert et al., 2009). ACEs have been linked to mental health problems, including PTSD, later in life (Brewin et al., 2000; Kessler et al., 2010; McLaughlin et al., 2012; Nemeroff, 2016). For example, 17-23% of young adults who experienced at least one type of ACE were diagnosed with PTSD after trauma exposure later in adult life as compared to 10% of those without an ACE history (Widom, 1999).
Structural changes in a range of cortical and subcortical structures including prefrontal cortex, hippocampus, and amygdala have been reported in chronic PTSD patients with an ACE history (Bremner et al., 1997; De Bellis et al., 2002; Evans et al., 2016; Hart and Rubia, 2012). Few studies have focused on diencephalic structures, including the thalamus. Children exposed to ACEs may have smaller thalamic volumes than children not exposed to ACEs (Hanson et al., 2010). ACE effects on stress systems have been proposed to underlie brain changes, impairment of stress responses, and vulnerability to PTSD (De Bellis and Zisk, 2014). Whether or how ACEs influence thalamic volumes, stress responses, and PTSD development in related ways at early times after adult trauma is unknown.
To investigate the potential associations between ACEs, subsequent early post-trauma whole thalamus and thalamic nuclei volumes, and PTSS and PTSD development, the present study tracked self-reported ACEs, and whole thalamus and thalamic nuclei volumes at 2 weeks, PTSS at 2 weeks and 3 months, and PTSD diagnosis at 3 months after an adult trauma. This provided original temporal analyses of potential linkages between early life ACEs, subsequent early post-trauma thalamic volumes, and PTSS and PTSD development.
2. Methods and Materials
2.1. Subject enrollment
Adult subjects (18–60 years of age) who were admitted to the hospital Emergency Department (ED) immediately following a traumatic experience were recruited within 48 hours after trauma. Traumatic experience included motor vehicle collision (MVC) (n= 53), physical assault (n= 40), sexual assault (n= 7), or other trauma (n= 1). All subjects required immediate medical treatment in the ED and were then discharged from the ED. Excluded from the study were survivors who: 1) had severe injuries, i.e., Abbreviated Injury Scale (AIS) > 2 (Gennarelli and Wodzin, 2006), requiring surgical procedures which precluded MRI scanning within the planned time-frame, 2) experienced MVC with low pain (Numeric Pain Rating Scale (NPRS) < 6) (Kahl and Cleland, 2005), 3) showed indications or history of moderate or severe traumatic brain injury, 4) could not read and write English, 5) were diagnosed with severe neurological, psychiatric, or mental problems, 6) were under the influence of alcohol or other substances when the trauma happened, and/or 7) had contraindications for MRI scans, e.g. pregnancy, claustrophobia, or ferrous materials within body tissues. All studied survivors gave written informed consent. Consented subjects immediately completed the PTSD Checklist-Stressor Specific (PCL) for DSM-V to evaluate post-trauma stress level (Blevins et al., 2015), and survivors who experienced high post-trauma stress (PCL score ≥ 28) were recruited. All study procedures were approved by the Institutional Review Board of the University of Toledo.
2.2. Psychological assessments
At post-trauma 2 weeks, all survivors completed the 28-item self-report Childhood Trauma Questionnaire (CTQ) for ACE assessment (Thombs et al., 2007), and self-report PCL survey for PTSS severity. Total CTQ score was used for cumulative ACE assessment. At post-trauma 3 months, survivors completed a second PCL survey as a follow-up PTSS severity assessment; in addition, at this time survivors were also interviewed by an experienced clinical psychologist for PTSD diagnosis using the Clinician-Administered PTSD Scale (CAPS). Diagnosis of PTSD required at least 1 re-experiencing, 1 avoidance, 2 negative feelings, and 2 hyperarousal symptoms as specified by DSM-V criteria (American Psychiatric Association, 2013). A diagnosis of partial PTSD required at least 1 symptom in each of these symptom clusters. Partial PTSD was identified because partial PTSD with impairment of social functioning often requires clinical intervention (Mylle and Maes, 2004).
2.3. sMRI acquisition, image processing and measures of whole thalamus and thalamic nuclei volumes
Within 2 weeks after trauma, survivors were scanned using a 3T General Electric Signa HDx MRI scanner (GE Healthcare, Chicago, IL, USA). High-resolution T1-weighted sMRI brain images were obtained using a validated three-dimensional volume inversion recovery fast spoiled gradient recall echo protocol (repetition time =7.9 ms, echo time = 3 ms, inversion time = 650 ms, field of view = 25.6 x 25.6 cm, matrix = 256 x 256, slice thickness =1 mm, voxel dimensions = 1 x 1 x 1 mm, 164 contiguous axial slices) (Xie et al., 2018). Reviews of sMRI images by a radiologist indicated no qualitative brain abnormalities. sMRI images were processed using FreeSurfer version 6 (https://surfer.nmr.mgh.harvard.edu) and, subsequently volume measures of whole thalamus and 25 thalamic nuclei1 on each side were made using the automated thalamic nuclei segmentation function in FreeSurfer version 7.1 (https://freesurfer.net/fswiki/ThalamicNuclei) (Fischl, 2012; Iglesias et al., 2018). This thalamic segmentation has been recently used in several studies examining thalamic nuclei involvement in mental disorders (Chen et al., 2021; Jurng et al., 2021).
2.4. Statistical analyses
Univariate analyses were used to test for differences in CTQ and PCL scores at post-trauma 2 weeks and 3 months for PTSD vs non-PTSD. Effects of age and sex were adjusted in all analyses. A one-way ANOVA test was used to compare age for the PTSD vs non-PTSD. Sex (male/female) composition was compared using a Chi-Square test. Univariate analyses were used to test possible effects of trauma type on thalamic volumes for assault trauma survivors (N=47: combined physical (N=40) and sexual assault (N=7) survivors) vs MVC trauma survivors (N=53), controlling age and sex. Trauma type compositions in PTSD vs non-PTSD groups were compared using a Chi-Square test.
Relationships between (a) CTQ scores vs PCL scores at post-trauma 2 weeks and 3 months, (b) CTQ scores vs early post-trauma volumes of whole thalamus and thalamic nuclei, and (c) volumes of whole thalamus and thalamic nuclei vs PCL scores at 2 weeks and 3 months were tested using partial correlations, with adjustments for age, sex, and intracranial volume (ICV). False detection rate (FDR) was applied for multiple comparison correction in thalamic nuclei analyses. Fisher r-to-z transform and correlation coefficient comparisons were used to test for correlation differences between the PTSD vs non-PTSD groups. Statistical analyses were conducted using SPSS version 26 (IBM Corp., Armonk, NY). Data are reported as mean ± standard deviation (SD), with p<0.05 as statistical significance.
3. Results
3.1. Behavior and brain measures
101 adult trauma survivors completed this longitudinal study. 50 survivors comprising the PTSD group met full (N= 38) or partial (n= 12) PTSD diagnosis at 3 months. This included 23 MVC and 27 assault survivors. 51 survivors comprising the non-PTSD group did not meet full or partial PTSD diagnosis at 3 months. This group had 30 MVC, 20 assault, and 1 other trauma survivors. There was no significant difference in PTSD diagnosis in MVC vs assault survivors (χ2= 1.967, p=0.161). There was no significant trauma type effect on left or right whole thalamus volumes (left: F=0.546, p=0.455; right: F=0.399, p=0.529), or on interaction of trauma type x PTSD diagnosis on thalamus volume (left: F=0.024, p=0.876; right: F=0.074, p=0.787). Consequently, to increase statistical test power, survivors with all types of trauma were pooled in further analyses. PTSD vs non-PTSD groups did not differ in age (respectively 32.4 ± 9.4 vs. 32.9 ± 10.8 years, F= 0.057, P= 0.812) or sex composition (respectively 36 female/14 male vs. 32 female/19 male, χ2= 0.983, P= 0.321). There were no significant differences in volumes of whole thalamus (Table 1) and thalamic nuclei (Supplement Table 1) for the PTSD vs non-PTSD groups.
Table 1.
Behavioral and Brain Measures
| All survivors (n=101) |
PTSD (n=50) |
non-PTSD (n=51) |
|
|---|---|---|---|
| CTQ | 57.5 ± 22.9 | 59.2 ± 23.5 | 55.7 ± 22.4 |
| PCL | |||
| 2 weeks | 48.4 ± 15.8 | 52.5 ± 11.6* | 44.3 ± 18.2 |
| 3 months | 35.6 ± 18.3 | 44.3 ± 15.1* | 27.0 ± 17.1 |
| Thalamic volumes (mm3) | |||
| left (mm3) | 6601.4 ± 748.9 | 6563.9 ± 833.5 | 6638.2 ± 661.9 |
| right (mm3) | 6347.7 ± 729.2 | 6312.6 ± 760.1 | 6382.2 ± 703.1 |
PTSD group and non-PTSD group were significantly different at p<0.05 level
3.2. Associations between CTQ and PCL scores at post-trauma 2 weeks and 3 months
Mean (± SD) CTQ scores, and PCL scores at post-trauma 2 weeks and 3 months, for all survivors and for the PTSD and non-PTSD groups are reported in Table 1. CTQ scores did not significantly differ for the PTSD vs non-PTSD groups (F= 0.319, P= 0.574). PCL scores at both 2 weeks and 3 months after trauma were significantly higher in the PTSD group than non-PTSD group (F= 6.906, p= 0.010 at 2 weeks; F= 27.482, p< 0.001 at 3 months, Table 1).
Across all survivors, CTQ scores were significantly positively correlated with PCL scores at post-trauma 2 weeks (r= 0.258, p< 0.01, df= 97) and 3 months (r= 0.345, p< 0.001, df= 97) (Figure 1). Further analysis revealed that CTQ scores were positively correlated with PCL scores at 2 weeks and at 3 months in the PTSD group (2 weeks: r= 0.306, p= 0.034, df= 46; 3 months: r= 0.288, p= 0.047, df= 46). In contrast, CTQ score was positively correlated with PCL scores only at 3 months in the non-PTSD group (r= 0.440, p= 0.002, df= 47).
Figure 1:
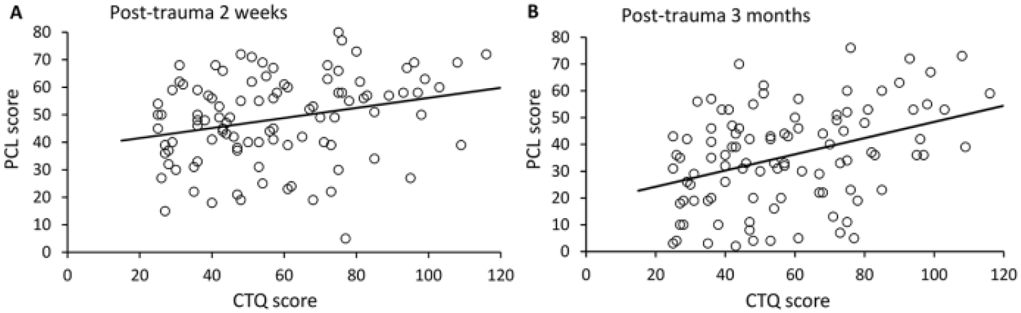
CTQ scores were significantly positively correlated with PCL scores at (A) 2 weeks and (B) 3 months after adult trauma.
3.3. Associations between CTQ scores and volumes of whole thalamus and thalamic nuclei at post-trauma 2 weeks
Across all survivors, CTQ scores were significantly negatively correlated with both left and right whole thalamus volumes (left, r= −0.290, p= 0.004; right, r= −0.283, p= 0.005; df= 96). Further CTQ analysis of each group revealed that significant negative correlations with left and right whole thalamus volumes held for the PTSD group (left, r= −0.441, p= 0.002; right, r= −0.445, p= 0.002; df= 45), but not the non-PTSD group (left, r= −0.116, p= 0.432; right r= −0.123, p= 0.405; df= 46) (Figure 2). Correlation coefficient comparisons for the PTSD vs non-PTSD groups significantly differed for both left and right whole thalamus volumes (left and right respectively: Z= −1.739, p= 0.041, and Z= −1.729, p= 0.042).
Figure 2:
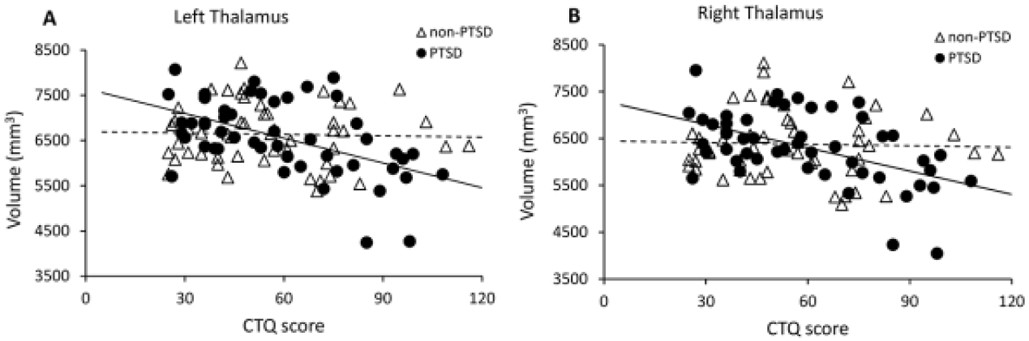
CTQ scores were significantly negatively correlated with (A) left, and (B) right whole thalamic volumes after adult trauma in the PTSD but not non-PTSD group.
Relationships between CTQ scores and thalamic nuclei volumes were tested separately for the PTSD and non-PTSD groups. In the PTSD group, after FDR correction, significant negative correlations between CTQ and thalamic nuclei volumes were found for the following 22 nuclei on both sides that spanned ventral (VPL, VLa, VLp, VA, VAmc), medial (MDm, Pt), intralaminar (Pc, CeM, CM), and posterior (PuA) groups (Supplement Table 1). In addition, significant negative correlations were also seen for 4 nuclei on the left side including PuM, LGN, Pf and MV-re that were in posterior, intralaminar and medial groups respectively (Supplement Table 1). For the non-PTSD group, in contrast, no correlations were significant after FDR correction (Supplement Table 1). For the above thalamic nuclei with significant correlations with CTQ, correlation coefficient comparisons showed significant correlation differences between the PTSD and non-PTSD group for left LGN, PuM, MDm, VAmc, and right VLa, VA and Pc (z scores: −2.265 to −1.756, p value: 0.012 to 0.040, Supplement Table 1).
3.4. Associations between thalamic volumes at post-trauma 2 weeks and PCL scores at post-trauma 2 weeks and 3 months
Correlations between left and right thalamus volumes with PCL scores at post-trauma 2 weeks were not significant for either the PTSD (left: r= −0.223, p= 0.132; right r= −0.158, p= 0.290; df= 45) or non-PTSD group (left: r= 0.051, p= 0.731; right r= 0.083, p= 0.576; df= 46).
In contrast, both left and right thalamic volumes were significantly negatively correlated with PCL scores at post-trauma 3 months for the PTSD group (left, r= −0.393, p= 0.006; right, r= −0.344, p= 0.018; df= 45), but not the non-PTSD group (left: r= 0.156, p= 0.289; right r= 0.244, p= 0.095; df= 46) (Figure 3). Correlation coefficients significantly differed for the two groups (left and right respectively: Z= −2.790, p= 0.003, and Z= −2.961, p= 0.002).
Figure 3:
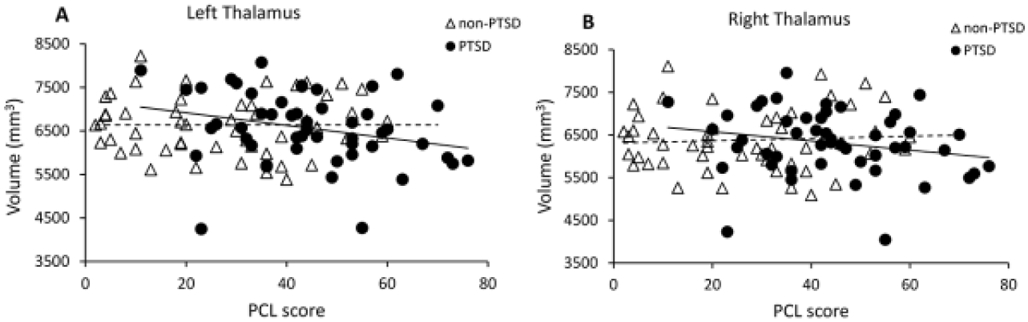
(A) Left and (B) right post-trauma 2 week thalamus volumes significantly negatively correlated with PCL scores at post-trauma 3 months in the PTSD group, but not non-PTSD group.
For thalamic nuclei, after FDR correction, significant negative correlations were found in the PTSD group for 18 nuclei on both sides that spanned ventral (VPL, VLa, VLp, VA, VM), medial (MDI), and intralaminar (Pf, CeM, CM) groups; 4 nuclei on the left side in ventral (MDm, VAmc) and intralaminar (CL, Pc) groups; and 3 nuclei on the right side in the posterior (L-SG and PuL) and medial (MV-re) groups (Supplement Table 2). No correlations were significant after FDR correction for the non-PTSD group (Supplement Table 2). With exception of left Pc, and right MDI and MV-re, correlation coefficients significantly differed between PTSD vs non-PTSD groups for all the above nuclei that had significant correlations (z score: −3.572 to −1.867, p: <0.001 to 0.031, Supplement Table 2).
Interestingly, the volumes of left MDm and VAmc, and right VLa and VA nuclei were significantly negatively correlated with both preceding CTQ and post-trauma 3 month PCL scores in the PTSD but not non-PTSD group, and all correlation coefficients significantly differed for PTSD vs non-PTSD groups (Table 2). For example, left MDm volume was significantly negatively correlated with both CTQ and post-trauma 3-month PCL scores in the PTSD but not non-PTSD group, and both correlation coefficients significantly differed for PTSD vs non-PTSD groups (Figure 4). This suggests that volumes of these nuclei may be affected by early life ACEs and contribute to development of PTSS in PTSD patients with later adult trauma.
Table 2.
Thalamic nuclei with correlations between volumes and both CTQ and post-trauma 3 month PCL scores
| Group | nucleus | Correlation with CTQ scores | Correlation with post-trauma 3 month PCL scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD (N=50) | non-PTSD (N=51) | Corr. coef. comp. | PTSD (N=50) | non-PTSD (N=51) | Corr. coef. comp. | ||||||||
| r | FDR P | r | FDR P | z | p | r | FDR P | r | FDR P | z | p | ||
| Left | |||||||||||||
| Medial | MDm | −0.369 | 0.025 # | −0.020 | 0.940 | −1.790 | 0.037 * | −0.338 | 0.042 # | 0.226 | 0.769 | −2.835 | 0.002 * |
| Ventral | VAmc | −0.518 | 0.005 # | −0.210 | 0.345 | −1.756 | 0.040 * | −0.399 | 0.014 # | −0.004 | 0.976 | −2.039 | 0.021 * |
| Right | |||||||||||||
| Ventral | VA | −0.376 | 0.023 # | −0.029 | 0.917 | −1.785 | 0.037 * | −0.358 | 0.039 # | 0.088 | 0.729 | −2.255 | 0.012 * |
| VLa | −0.433 | 0.010 # | −0.094 | 0.658 | −1.800 | 0.036 * | −0.462 | 0.013 # | 0.229 | 0.397 | −3.572 | <0.001 * | |
FDR P<0.05, partial correlation
P<0.05, correlation coefficient comparison.
Figure 4:
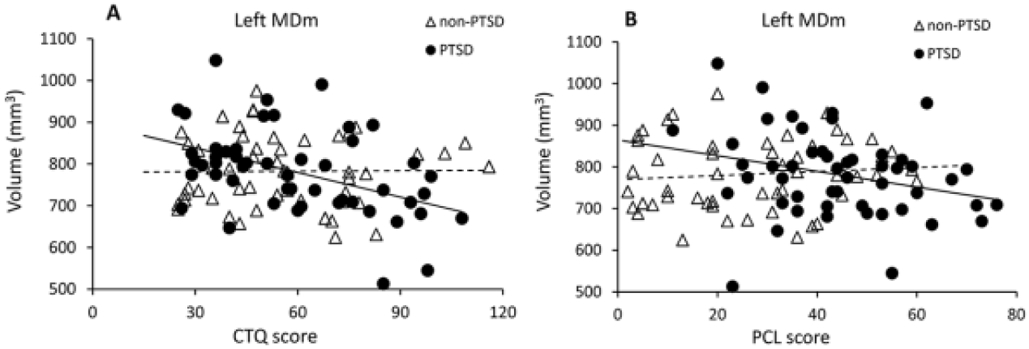
Volume of left MDm at post-trauma 2 weeks significantly negatively correlated with (A) preceding CTQ scores, and (B) subsequent post-trauma 3 month PCL scores in the PTSD, but not non-PTSD group.
4. Discussion
We find that ACEs are positively associated with PTSS severity in the early weeks to months after subsequent adult trauma. We also report that ACEs are inversely associated with whole thalamus volumes within the first 2 weeks after adult trauma, which, in turn, were inversely associated with subsequent PTSS severity at post-trauma 3 months in survivors who developed PTSD. Finally, with respect to specific thalamic nuclei, volumes of left MDm and VAmc, and right VLa and VA were inversely associated with both CTQ and PTSS at post-trauma 3 months in survivors who developed PTSD. These findings suggest that early life ACEs and associated effects on early post-trauma whole thalamus and thalamic nuclei volumes influence PTSS and PTSD development after adulthood trauma.
4.1. ACE relationships with PTSS at early times after adult trauma
The finding of a positive association between ACEs and PCL scores at 2 weeks and 3 months after adult trauma adds to existing work that suggests ACEs increase risk for chronic PTSD after adult trauma (Brewin et al., 2000). Neuropsychological studies suggest an association of ACEs with both maladaptive emotional regulation (Kalia and Knauft, 2020) and deficits in attention, learning, and memory (Pine et al., 2005; Pollak and Tolley-Schell, 2003; Samuelson et al., 2010). ACEs may have prolonged effects that lead to PTSD after adult trauma (Breslau et al., 1991; Brewin et al., 2000; Widom, 1999). Our findings provide new evidence and insight that indicates ACEs can also be associated with increased PTSS during initial weeks to months after adult trauma. To our knowledge, the present study is the first to examine early post-trauma PTSS and its relationship to ACEs. The results suggest that ACEs have modulatory effects on post-trauma stress at early times after adult trauma, particularly in trauma survivors who later develop PTSD. This provides a new perspective that ACE can increase risk for PTSD.
4.2. ACE relationships with early post-trauma whole thalamus and thalamic nuclei volumes in adulthood
From existing studies, ACEs may influence thalamic development to lead to changes in thalamic structure and/or function (Duarte et al., 2016; Hanson et al., 2010; Yoshii, 2021). Our findings of negative correlation between ACEs and thalamus volumes in adulthood may support these early results. The current study further finds that ACEs were negatively associated with volumes of 26 out of 50 left and right thalamic nuclei in trauma survivors who developed PTSD later. Among these nuclei, correlation coefficients of 7 nuclei significantly differed between the PTSD vs non-PTSD groups. These findings suggest that ACE-related influences on multiple thalamic nuclei may contribute to early development of PTSD after adult trauma.
4.3. Early post-trauma thalamic volume relationships with PTSD development
Inverse relationships between thalamic volume and PTSS have been reported in chronic PTSD patients (Shucard et al., 2012). For example, gray matter atrophy in the thalamus was found in chronic PTSD patients compared to a control group without PTSD (Cardenas et al., 2011). Re-experiencing symptoms negatively correlate with left thalamus volume (Shucard et al., 2012), suggesting that thalamic volume may influence post-trauma sensory processing. It has also been proposed that reduced thalamic activity may be related to the reduced ability to cope with stress (Zhang et al., 2019). From these chronic PTSD findings, it is possible that impairments in sensory processing and ability to cope with stress, due to thalamus structural effects, contribute to PTSD development. Our findings appear consistent with the relationship between whole thalamus volume and PTSS. We further found significant inverse relationships between volumes of 25 of 50 thalamic nuclei vs 3 month post-trauma PTSS severity in survivors who developed PTSD. In addition, correlation coefficients of 22 of the above nuclei significantly differed in the PTSD vs non-PTSD group. This suggests that multiple thalamic nuclei may contribute to PTSS in patients who developed PTSD. Interestingly, thalamus volumes at 2 weeks post-trauma were not significantly associated with PTSS at early weeks, but were significantly negatively associated with PTSS severity at 3 months post-trauma in the PTSD group. The lack of association between thalamic volumes and PTSS in the initial weeks may reflect a number of factors, possibly including trait anxiety (Suliman et al., 2013) or that influences of other parts of the brain may have greater effects on PTSS at this early time. Further studies are needed to evaluate alternative causal models
4.4. ACE-vulnerable thalamic nuclei may contribute to PTSS and PTSD development
It is known that the thalamus is involved in multiple brain functions through its complex connectivity with cortical neurocircuitries (Dolleman-van der Weel et al., 2019; Wolff and Vann, 2019). Specific thalamic nuclei have been linked to mental and neurological disorders (Hoang et al., 2021; Huang et al., 2020; Jurng et al., 2021; Shin et al., 2019). However, specific contributions of thalamic nuclei to PTSD in human studies remain poorly understood. The present results provide initial evidence that volumes of thalamic nuclei including left MDmc and VAmc and right VLa and VA may be affected by early life ACEs and, as a consequence, contribute to subsequent development of PTSS in patients who develop PTSD after later adult trauma. Evidence that MD, VA, and VLa connect with prefrontal, cingulate, hippocampus, and amygdala to perhaps affect attention, threat memories, fear responses, or social cognition (Abivardi and Bach, 2017; de Bourbon-Teles et al., 2014; Grodd et al., 2020) suggests that these nuclei contribute to circuits involved in PTSD development. It is plausible, for example, that ACE in early life may negatively impact MDmc, VAmc, VLa and VA and lead to deficits in threat memories, fear responses, or social cognition, adversely influencing recovery after adult trauma and promoting early development of PTSD. Properties of medial and ventral thalamic nuclei may prove useful for predicting PTSD development in adult trauma survivors with an ACE history.
4.5. Limitations
This study has the following limitations. First, we studied trauma patients with high stress and pain at the time of trauma. Generalization of the findings to all trauma survivors needs further tests. Second, ACE is associated with mental disorders including depression, anxiety, and addiction, which can be comorbid factors for PTSD. These comorbidities should be considered in future work. Third, age at which ACE occurred, which was not addressed, may be a factor that influences ACE outcomes. Fourth, the findings are based on correlation analyses of measures with a temporal sequence, but do not prove causal-effect relationships. Fifth, we did not find differences in effects of different trauma, e.g. MVC and assault, as recently reported (Geoffrion et al., 2020). This merits further attention with larger samples.
4.6. Conclusions
The current study reports that whole thalamus and specific thalamic nuclei volumes at 2 weeks after trauma are negatively associated with both preceding ACEs and subsequent PTSS at 3 months after trauma in patients who develop PTSD. These associations may mediate further positive associations between ACEs and early post-trauma PTSS severity and shed light on thalamic contributions to increased risk for PTSD after ACE.
Supplementary Material
Highlights.
CTQ scores positively correlated with PCL scores at both 2 weeks and 3 months post-trauma
CTQ scores negatively correlated with whole thalamus and 7 nuclei volumes at post-trauma 2 weeks in the PTSD group, but not the non-PTSD group
Whole thalamus and 22 nuclei volumes negatively correlated with subsequent PCL scores at 3 months in the PTSD, but not the non-PTSD group
Volumes of left MDm and VAmc, and right VLa and VA inversely associated with both CTQ and post-trauma 3 month PTSS in survivors who developed PTSD
Acknowledgements
This work was supported by a grant from the National Institutes of Mental Health (NIMH), Grant R01MH110483.
We thank Cindy Grey, Lindsey Katschke, Danielle Perry, and the Department of Radiology at the University of Toledo for clinical and technical support, ProMedica Health System for subject recruitment, and Carol Brikmanis for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors report no biomedical financial interests or other potential conflicts of interest.
anteroventral (AV), laterodorsal (LD), lateral posterior (LP), ventral anterior (VA), ventral anterior magnocellular (VAmc), ventral lateral anterior (VLa), ventral lateral posterior (VLp), ventral posterolateral (VPL), ventromedial (VM), central medial (CeM), central lateral (CL), paracentral (Pc), centromedian (CM), parafascicular (Pf), paratenial (Pt), medial ventral reuniens (MV-re), mediodorsal medial (MDm), mediodorsal lateral parvocellular (MDl), lateral geniculate (LGN), medial geniculate (MGN), limitans - suprageniculate (L-SG), pulvinar anterior (PuA), pulvinar medial (PuM), pulvinar lateral (PuL), and pulvinar inferior (PuI)
References
- Abivardi A, Bach DR, 2017. Deconstructing white matter connectivity of human amygdala nuclei with thalamus and cortex subdivisions in vivo. Hum. Brain. Mapp 38, 3927–3940. 10.1002/hbm.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Beas BS, Wright BJ, Skirzewski M, Leng Y, Hyun JH, Koita O, Ringelberg N, Kwon HB, Buonanno A, Penzo MA, 2018. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci 21, 963–973. 10.1038/s41593-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann U, 2008. The Neurobiology of EMDR: Exploring the Thalamus and Neural Integration. J. EMDR Prac. Res 2, 300–314. 10.1891/1933-3196.2.4.300. [DOI] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL, 2015. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress 28, 489–498. 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Bremner JD, 2006. Traumatic stress: effects on the brain. Dialogues Clin. Neurosci 8, 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS, 1997. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol. Psychiatry 41, 23–32. 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, 2001. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav. Res. Ther 39, 373–393. 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD, 2000. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J. Consult. Clin. Psychol 68, 748–766. 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Hertzberg JS, Kirby AC, Dennis MF, Hair LP, Dedert EA, Beckham JC, 2012. The Effect of Draft DSM-V Criteria on Posttraumatic Stress Disorder Prevalence. Depress. Anxiety 29, 1032–1042. 10.1002/da.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Samuelson K, Lenoci M, Studholme C, Neylan TC, Marmar CR, Schuff N, Weiner MW, 2011. Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res. 193, 93–100. 10.1016/j.pscychresns.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fallon N, Kreilkamp BAK, Denby C, Bracewell M, Das K, Pegg E, Mohanraj R, Marson AG, Keller SS, 2021. Probabilistic mapping of thalamic nuclei and thalamocortical functional connectivity in idiopathic generalised epilepsy. Hum. Brain. Mapp n/a, 1–17. 10.1002/hbm.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G, 2002. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol. Psychiatry 52, 1066–1078. 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A, 2014. The biological effects of childhood trauma. Child Adolesc. Psychiatr Clin. N. Am 23, 185–222. 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, Egner T, Husain M, Soto D, 2014. Thalamic Control of Human Attention Driven by Memory and Learning. Curr. Biol 24, 993–999. 10.1016/j.cub.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Griffin AL, Ito HT, Shapiro ML, Witter MP, Vertes RP, Allen TA, 2019. The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior. Learn Mem. 26, 191–205. 10.1101/lm.048389.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte DG, Neves Mde C, Albuquerque MR, de Souza-Duran FL, Busatto G, Corrêa H, 2016. Gray matter brain volumes in childhood-maltreated patients with bipolar disorder type I: A voxel-based morphometric study. J Affect Disord. 197, 74–80. 10.1016/j.jad.2016.02.068. [DOI] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, Angstadt M, Phan KL, Xie H, Liberzon I, 2016. Childhood Cumulative Risk Exposure and Adult Amygdala Volume and Function. J. Neurosci. Res 94, 535–543. 10.1002/jnr.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. Neuroimage. 62, 774–781. 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, Wodzin E, 2006. AIS 2005: a contemporary injury scale. Injury. 37, 1083–1091. 10.1016/j.injury.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Geoffrion S, Goncalves J, Robichaud I, Sader J, Giguère C-É, Fortin M, Lamothe J, Bernard P, Guay S, 2020. Systematic Review and Meta-Analysis on Acute Stress Disorder: Rates Following Different Types of Traumatic Events. Trauma Violence Abuse. June 26, 1524838020933844. 10.1177/1524838020933844. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S, 2009. Burden and consequences of child maltreatment in high-income countries. The Lancet. 373, 68–81. 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Grodd W, Kumar VJ, Schüz A, Lindig T, Scheffler K, 2020. The anterior and medial thalamic nuclei and the human limbic system: tracing the structural connectivity using diffusion-weighted imaging. Sci. Rep 10, 10957. 10.1038/s41598-020-67770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak D, 2010. Early Stress Is Associated with Alterations in the Orbitofrontal Cortex: A Tensor-Based Morphometry Investigation of Brain Structure and Behavioral Risk. J. Neurosci 30, 7466–7472. 10.1523/Jneurosci.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K, 2012. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci 6, 52. 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang D, Lizano P, Lutz O, Zeng V, Raymond N, Miewald J, Montrose D, Keshavan M, 2021. Thalamic, Amygdalar, and hippocampal nuclei morphology and their trajectories in first episode psychosis: A preliminary longitudinal study. Psychiatry Res. Neuroimaging 309, 111249. 10.1016/j.pscychresns.2021.111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Rogers B, Sheffield J, Jalbrzikowski M, Anticevic A, Blackford J, Heckers S, Woodward N, 2020. Thalamic Nuclei Volumes in Psychotic Disorders and in Youths With Psychosis Spectrum Symptoms. Am. J. Psychiatry 177, appiajp202019101099. 10.1176/appi.ajp.2020.19101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, van der Kouwe A, Fischl B, Caballero-Gaudes C, Paz-Alonso PM, 2018. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage. 183, 314–326. 10.1016/j.neuroimage.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, 2015. Posttraumatic stress disorder. Continuum (Minneap Minn). 21, 789–805. 10.1212/01.CON.0000466667.20403.b1. [DOI] [PubMed] [Google Scholar]
- Jurng J, Park H, Kim T, Park I, Moon S-Y, Lho SK, Kim M, Kwon JS, 2021. Smaller volume of posterior thalamic nuclei in patients with obsessive–compulsive disorder. Neuroimage Clin. 30, 102686. 10.1016/j.nicl.2021.102686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl C, Cleland JA, 2005. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: an overview of psychometric properties. Phys. Ther. Rev 10, 123–128. 10.1179/108331905X55776. [DOI] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, Haro JM, Hu CY, Karam EG, Kawakami N, Lee S, Lépine JP, Ormel J, Posada-Villa J, Sagar R, Tsang A, Ustün TB, Vassilev S, Viana MC, Williams DR, 2010. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 197, 378–385. 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wang PS, 2008. The Descriptive Epidemiology of Commonly Occurring Mental Disorders in the United States. Annu. Rev. Public Health 29, 115–129. 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 1986. Sensory systems and emotion: A model of affective processing. Integr. Psychiatry 4, 237–243. [Google Scholar]
- McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC, 2012. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry 69, 1151–1160. 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylle J, Maes M, 2004. Partial posttraumatic stress disorder revisited. J. Affect. Disord 78, 37–48. 10.1016/s0165-0327(02)00218-5. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, 2016. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron. 89, 892–909. 10.1016/j.neuron.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B, 2015. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 519, 455–459. 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KJ, Lee H-J, Park KM, 2019. Alterations of individual thalamic nuclei volumes in patients with migraine. J. Headache Pain 20, 112. 10.1186/s10194-019-1063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shucard JL, Cox J, Shucard DW, Fetter H, Chung C, Ramasamy D, Violanti J, 2012. Symptoms of posttraumatic stress disorder and exposure to traumatic stressors are related to brain structural volumes and behavioral measures of affective stimulus processing in police officers. Psychiatry Res. 204, 25–31. 10.1016/j.pscychresns.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR, 1988. The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev 68, 649–742. 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Steuber ER, Seligowski AV, Roeckner AR, Reda M, Lebois LAM, van Rooij SJH, Murty VP, Ely TD, Bruce SE, House SL, Beaudoin FL, An X, Zeng D, Neylan TC, Clifford GD, Linnstaedt SD, Germine LT, Rauch SL, Lewandowski C, Sheikh S, Jones CW, Punches BE, Swor RA, McGrath ME, Hudak LA, Pascual JL, Chang AM, Pearson C, Peak DA, Domeier RM, O'Neil BJ, Rathlev NK, Sanchez LD, Pietrzak RH, Joormann J, Barch DM, Pizzagalli DA, Elliott JM, Kessler RC, Koenen KC, McLean SA, Ressler KJ, Jovanovic T, Harnett NG, Stevens JS, 2021. Thalamic volume and fear extinction interact to predict acute posttraumatic stress severity. J Psychiatr Res 141, 325–332. 10.1016/j.jpsychires.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman S, Troeman Z, Stein DJ, Seedat S, 2013. Predictors of acute stress disorder severity. J. Affect. Disord 149, 277–281. 10.1016/j.jad.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Suvak MK, Barrett LF, 2011. Considering PTSD from the perspective of brain processes: a psychological construction approach. J. Trauma. Stress 24, 3–24. 10.1002/jts.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs BD, Lewis C, Bernstein DP, Medrano MA, Hatch JP, 2007. An evaluation of the measurement equivalence of the Childhood Trauma Questionnaire—Short Form across gender and race in a sample of drug-abusing adults. J. Psychosom. Res 63, 391–398. 10.1016/j.jpsychores.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Weeland CJ, Vriend C, van der Werf Y, Huyser C, Hillegers M, Tiemeier H, White T, van den Heuvel OA, 2021. Thalamic Subregions and Obsessive-Compulsive Symptoms in 2,500 Children From the General Population. J. Am. Acad. Child Adolesc. Psychiatry 10.1016/j.jaac.2021.05.024. [DOI] [PubMed] [Google Scholar]
- Widom CS, 1999. Posttraumatic stress disorder in abused and neglected children grown up. Am. J. Psychiatry 156, 1223–1229. 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Wolff M, Vann SD, 2019. The Cognitive Thalamus as a Gateway to Mental Representations. J. Neurosci 39, 3–14. 10.1523/jneurosci.0479-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Claycomb Erwin M, Elhai JD, Wall JT, Tamburrino MB, Brickman KR, Kaminski B, McLean SA, Liberzon I, Wang X, 2018. Relationship of Hippocampal Volumes and Posttraumatic Stress Disorder Symptoms Over Early Posttrauma Periods. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 968–975. 10.1016/j.bpsc.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, Shalev A, Wolkowitz OM, Hamilton SP, Yehuda R, Sodickson DK, Weiner MW, Marmar CR, 2013. Spontaneous brain activity in combat related PTSD. Neurosci. Lett 547, 1–5. 10.1016/j.neulet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Chen H, Feng B, Jiang T, Jin H, Wong C, Gong Q, Li L, 2011. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 1411, 98–107. 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Yoshii T, 2021. The Role of the Thalamus in Post-Traumatic Stress Disorder. Int J Mol Sci. 22. 10.3390/ijms22041730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li X, Steffens DC, Guo H, Wang L, 2019. Dynamic changes in thalamic connectivity following stress and its association with future depression severity. Brain Behav. 9, e01445. 10.1002/brb3.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


