Abstract
The association of physical activity (PA) and dietary factors with non-alcoholic fatty liver disease (NAFLD) and NAFLD-related fibrosis have never been examined in a representative sample of U.S. adults using a more precise form of measuring NAFLD. The purpose of this study was to assess the associations of PA and diet quality (Healthy Eating Index [HEI]-2015) with NAFLD and a subset with advanced fibrosis (F3-4) as assessed by vibration-controlled transient elastography with controlled attenuation parameter in a representative sample of U.S. adults. This cross-sectional analysis uses data from 2017–2018 National Health and Nutrition Examination Survey. NAFLD was defined as controlled attenuation parameter ≥285 dB/m, and high likelihood of advanced fibrosis as liver stiffness measurements ≥8.6 kPa. Associations of HEI-2015 from 24-hour dietary recalls and self-reported PA and sedentary behavior were estimated in multivariable-adjusted logistic regression models of NAFLD and advanced fibrosis. In 2,892 adults, the prevalence of NAFLD and advanced fibrosis was 35.6% and 5.6%, respectively. We found that high adherence to U.S. dietary recommendations (highest vs. lowest HEI-2015 tertile) and more PA (middle tertile vs. lowest) were associated with reduced odds of NAFLD (Adjusted OR and 95% CI; 0.60 (0.44, 0.84) and 0.65 (0.42, 0.99), respectively). More PA was inversely associated with advanced fibrosis (Adjusted OR=0.35, 95%CI 0.16, 0.75). Diet quality and PA are associated with reduced odds of NAFLD, and PA may be critical even for those with advanced liver disease. These behaviors should be the focus of targeted public health interventions.
Keywords: physical activity, sedentary behavior, Diet quality, Diet, Non-alcoholic fatty liver disease, fatty liver, fibroscan, fibrosis, advanced fibrosis, National Health and Nutrition Examination Survey
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent liver complication closely associated with obesity,1,2 soon to become the top reason for liver transplants in the U.S.3 Currently, NAFLD prevalence is estimated between 34.1–56.7% among U.S. adults.4 Metabolic dysfunction-associated liver disease (MAFLD) has recently emerged as an alternative nomenclature for this condition. MAFLD is clinically similar to NAFLD and thus they have very similar prevalence estimates in the U.S.5
NAFLD is a spectrum of disease ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) with progressive fibrosis that can culminate in cirrhosis and its complications. Among patients with NAFLD, those with advanced liver fibrosis are at highest risk for succumbing to adverse outcomes, including cardiovascular disease,6 chronic liver failure,6 and hepatocellular carcinoma.7,8 Pharmacologic treatments for NAFLD do not exist. Lifestyle modifications are recommended as the first line treatment for NAFLD.9 This is based on data showing that physical inactivity and poor dietary habits may be associated with progressive NASH fibrosis and changes in these behaviors can lead to improvements in hepatic fat.10,11
Existing research on the association between physical activity and diet with NAFLD and NAFLD progression in the U.S. population has shown an association, but the studies relied on imprecise methods for case definition or did not use representative samples.12–16 The cross-sectional association of physical activity and diet with NAFLD and a subset of those with fibrosis has been previously assessed in a representative sample of U.S. adults using liver enzymes13,14 or ultrasound12 to define NAFLD. However, use of liver enzymes and ultrasound data to define NAFLD may potentially result in misclassification bias: liver enzymes are normal among a large proportion of NAFLD cases and ultrasound can miss mild steatosis17,18 Furthermore, neither is a reliable measure of fibrosis.19 Vibration controlled transient elastography (VCTE) with controlled attenuation, a non-invasive method for quantifying liver fat and stiffness, shows good specificity and sensitivity compared to liver biopsy,20,21 and is a potentially more accurate measure of NAFLD than liver enzymes or ultrasound.22,23 Additional research on lifestyle behaviors associated with NAFLD and advanced fibrosis using this more precise measurement by VCTE measured in a large, population-based in the U.S. could provide data to support clinical management recommendations for liver disease prevention.
The purpose of this study was to assess the association of physical activity, sedentary behavior and diet quality with NAFLD and a subset with advanced fibrosis as measured by VCTE with controlled attenuation, in a representative sample of U.S. adults.
Methods
Study Design and Population
We used data extracted from the 2017–2018 cycle of National Health and Nutrition Examination Survey (NHANES), a stratified, multistage probability sample representative of the civilian non-institutionalized U.S. population.24 A total of 5,265 adults completed both the survey and medical examination (Figure 1). Of these, we included participants with valid VCTE, and excluded those with hepatitis B surface antigen positivity, hepatitis C antibody positivity, significant alcohol consumption (>21 drinks/week in men and >14 drinks/week in women on average),9 or missing diet and/or physical activity data. The final analytic sample included 2,892 participants. Participants provided written consent. NHANES procedures were approved by the National Center for Health Statistics review board and this study was approved by the Institutional Review Board (IRB) of The University of Texas MD Anderson Cancer Center.
Figure 1.
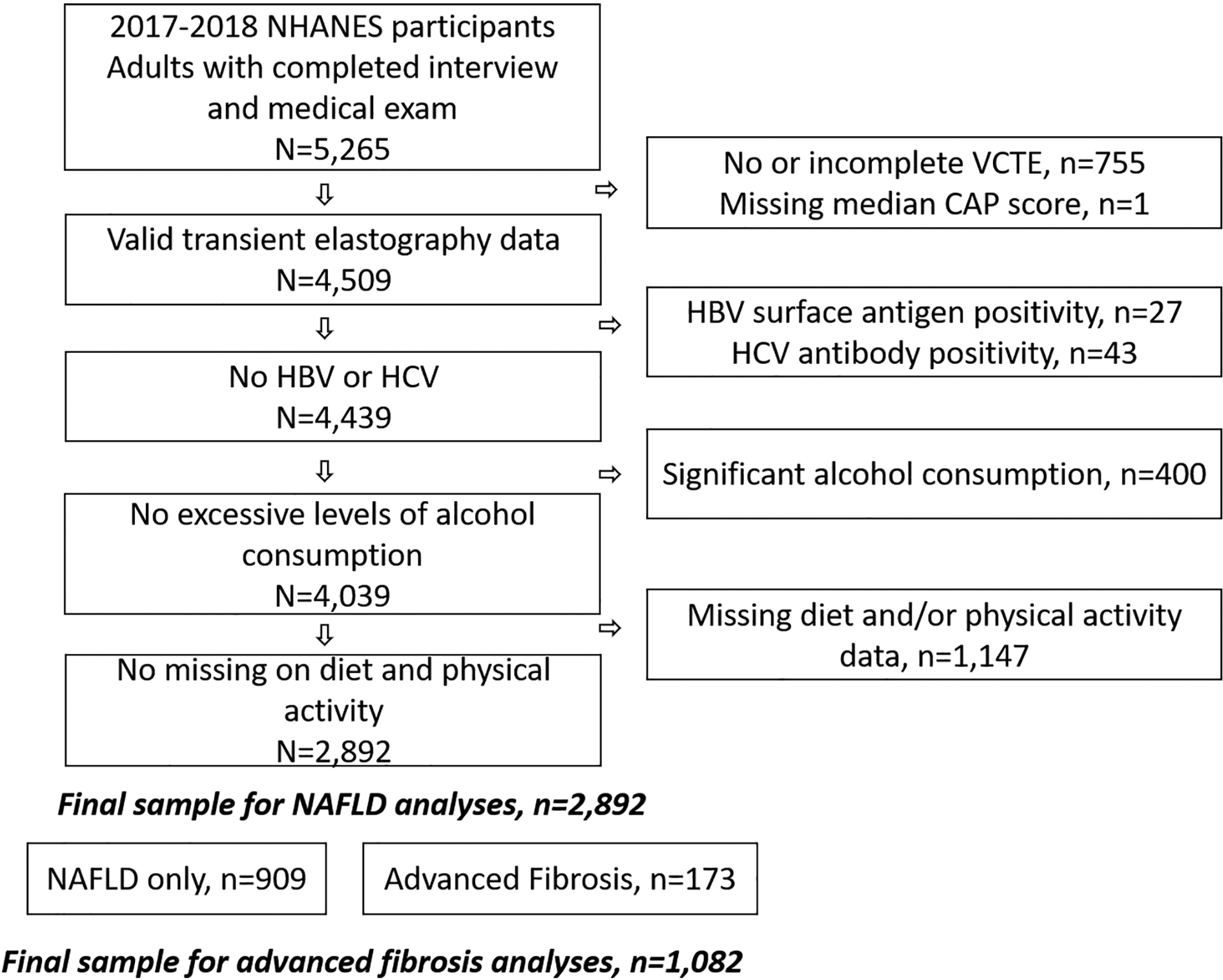
Study Population
Data Collection and Measures
NHANES interviews use a combination of computer-assisted personal interviewing and audio computer-assisted self-interviewing surveys to elicit sociodemographic and behavioral characteristics.24 The examination component consists of medical, dental, and physiological measurements, as well as laboratory tests administered by trained medical personnel.
NAFLD and fibrosis.
Hepatic steatosis and advanced fibrosis were assessed using FibroScan®, which uses the controlled attenuation parameter (CAP) value and VCTE with controlled attenuation to derive liver stiffness measurements (LSM), respectively.25 Exams were considered complete if participants fasted at least 3 hours prior to the exam, there were 10 or more complete LSM, and the liver stiffness IQR/median <30%.25 CAP values range from 100–400 dB/m, with higher values indicating higher amounts of fat in the liver. LSM range from 1.5 kPa to 75 kPa, with higher values indicating a higher probability of advanced fibrosis. We defined NAFLD as a CAP score ≥285 dB/m among the included study population; a high likelihood of advanced fibrosis (F3-4) was defined as LSM ≥ 8.6 kPa.21
Physical activity, sedentary behavior and diet.
The NHANES survey included the Global Physical Activity Questionnaire,26 which assesses time spent sitting and time spent engaged in typical physical activity over the past week. Questions captured time spent doing physical activity in various domains and by intensity, including vigorous and moderate activity at work, transport activity, and vigorous and moderate activity during leisure time. We calculated metabolic equivalent of task (MET) minutes using NHANES-recommended conversions. We defined total physical activity as total MET hours per week, summed across all physical activity questions. Sedentary behavior was defined as total time spent sitting, in hours per day.
Participants completed a 24-hour recall of all food and drink consumed during the day prior to the interview (midnight to midnight), as well as a second follow-up 24-hour recall 3–10 days later. Using the United States Department of Agriculture Food Patterns Equivalents Database, we calculated the Health Eating Index (HEI)-2015.27 When two dietary recalls were available (n=2,530, approximately 87% of our sample,), HEI-2015 scores calculated from each assessment were averaged, otherwise data from one recall was used (n=362; approximately 13% of our sample). HEI-2015 measures overall diet quality and alignment with the current Dietary Guidelines for Americans 2015–2020,28 with higher scores indicating higher diet quality. HEI-2015 includes 13 components, assessing the adequacy of the consumption of foods recommended as part of a healthy diet, including total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant protein, as well the consumption of foods that should be moderated or consumed sparingly, including refined grains, sodium, and added sugars. Fatty acids are represented as a ratio of monounsaturated and polyunsaturated fatty acids to saturated fatty acids. Each component is assigned points, with the overall HEI-2015 score having a possible range of zero to 100. Higher HEI-2015 scores have been associated with lower risk of NAFLD,15 as well as lower risk of all-cause, cardiovascular disease and cancer-related mortality.29
Covariates.
NHANES interviews elicit sociodemographic and behavioral characteristics, including age, sex, race/ethnicity, education, household income, smoking, and alcohol use.24 After excluding significant alcohol consumers from the sample to remove patients at risk for alcohol-related fatty liver,9 we created a categorical variable representing the average daily use of alcohol over the past year. Alcohol use was categorized no past year use (no alcoholic drink ever or in the past year), light to-moderate (≤2 drinks/day for men and ≤1 drink/day for women, on average during the past year) and heavy (>2 drinks/day for men and >1 drink/day for women, on average during the past year). Trained NHANES staff assessed weight, height and waist circumference. We calculated body mass index (BMI) as weight divided by height squared (kg/m2) and categorized into underweight and normal (BMI< <25 kg/m2), overweight (BMI 25–29.9 kg/m2) and Class 1 obesity (BMI ≥ 30 kg/m2), Class 2 obesity (35–39.9 kg/m2) and Class 3 obesity (>40 kg/m2). Metabolic syndrome was determined by the presence of three of the five Adult Treatment Panel (ATP) III criteria,30 including abdominal obesity (waist circumference >102 cm in men and >88 cm in women); high blood pressure/hypertension (systolic blood pressure ≥ 130, diastolic blood pressure ≥ 85); high triglycerides (≥150 mg/dl), low HDL cholesterol (<40 mg/dl in men and <50 mg/dl in women); and high fasting glucose (≥110 mg/dl). Type 2 diabetes was categorized as normal (HgbA1C <5.7% and no self-reported diabetes), pre-diabetes (HgbA1C 5.7–6.4% and no self-report diabetes), and diabetes (HgbA1C ≥6.5% or self-report diabetes).
Statistical Analyses
Primary exposure variables were stratified into tertiles based on the distribution in disease-free individuals (i.e., those without NAFLD). We used Chi-Square test or Fisher’s exact test for categorical variables to examine for differences between participants with and without NAFLD and advanced fibrosis. We used multivariable (MV)-adjusted logistic regression models to estimate the associations of physical activity, sedentary behavior, and dietary variables with the presence of NAFLD or advanced fibrosis, in a series of models controlling for potential confounders. We first controlled for sex, age, and race/ethnicity (Model 1). We then added covariates significantly associated (p<0.05) with the outcomes in univariate analyses. However, given issues of multicollinearity between metabolic syndrome, BMI and type 2 diabetes, we controlled only for metabolic syndrome, given the overlap in its definition. Thus, in Model 2 for NAFLD, we controlled for metabolic syndrome, while in Model 2 for advanced fibrosis we controlled for metabolic syndrome and smoking status. In the fully adjusted model (Model 3), we additionally controlled for total energy intake in physical activity and sedentary behavior models, and total energy intake, physical activity and alcohol use in HEI models. Lastly, we stratified by type 2 diabetes status and tested an interaction term between each behavior and type 2 diabetes. Weighted analyses were carried out using survey weights, which is fundamental to NHANES. These weights are used to account for the complex survey design, survey non-response, post-stratification, and oversampling. The NHANES methodology for weighting is applied to ensure the sample is representative of the U.S. non-institutionalized population.31 SAS 9.4 (SAS Institute INC, Cary, NC) was used for all analyses (conducted in 2021) with a p-value of less than 0.05 indicative of statistical significance.
Results
Table 1 presents characteristics of the overall sample, as well as characteristics by NAFLD classification (absence vs. presence) and advanced NAFLD fibrosis classification (no/mild fibrosis vs. advanced fibrosis). The overall sample was n=2,892, with 1,082 (weighted %, 35.6%) classified as having NAFLD and 173 (weighted %, 5.6%) classified as having advanced fibrosis related to NAFLD. As compared to those without NAFLD, those with NAFLD has a significantly higher proportion of individuals who were 40 years of age or older (72.1% vs. 52.1%), male (55.7% vs. 47%), and Hispanic (20.3 vs. 15.7%). As expected, those with NAFLD had a higher BMI, less physical activity, and were more likely to have diabetes and metabolic syndrome than those without NAFLD. Among the subset of people with NAFLD, those with advanced fibrosis were more likely to be male, nonsmokers or former smokers, and have a higher BMI, type 2 diabetes, and metabolic syndrome than those with no or mild NAFLD fibrosis. As compared to those with NAFLD and none or mild fibrosis, those with advanced fibrosis had a significantly higher proportion of individuals with type 2 diabetes (47.5% vs 21.8%, respectively) and metabolic syndrome (64.8% vs. 45.6%, respectively).
Table 1.
Characteristics for total sample, and by non-alcoholic fatty liver disease (NAFLD) and subset with advanced fibrosis
| Variables | Total n=2892 | NAFLD (CAP score ≥285 dB/m) | Advanced fibrosis (LSM≥ 8.6 kPa) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes n=1082 | No=1810 | p-value | Yes n=173 | No n=909 | p-value | ||||||||||||
| Weighted % ± SE | Weighted % ± SE | Weighted % ± SE | Weighted % ± SE | Weighted % ± SE | |||||||||||||
| Age | |||||||||||||||||
| 20–39 | 68 | 0.8 | 48 | 7.9 | 20 | 7.9 | < 0.0001 | 0 | 3.6 | 08 | 6.8 | 0.23 | |||||
| 40–59 | 06 | 4.3 | 89 | 0.1 | 17 | 1.1 | 8 | 9.9 | 31 | 1.2 | |||||||
| 60–89 | 018 | 4.9 | 45 | 2.0 | 73 | 1.0 | 5 | 6.5 | 70 | 3.0 | |||||||
| Sex | |||||||||||||||||
| Male | 444 | 0.1 | 08 | 5.7 | 36 | < 0.001 | 09 | 6.4 | 99 | 3.8 | 0.01 | ||||||
| Female | 448 | 9.9 | 74 | 4.3 | 74 | 4 | 3.6 | 10 | 6.2 | ||||||||
| Race/Ethnicity | |||||||||||||||||
| Non-Hispanic White | 59 | 0.9 | 86 | 1.9 | 73 | 0.4 | < 0.0001 | 4 | 0.6 | 22 | 2.1 | 0.90 | |||||
| Non-Hispanic Black | 88 | 1.9 | 90 | .3 | 98 | 3.8 | 7 | .4 | 63 | .5 | |||||||
| Hispanic | 20 | 7.4 | 19 | 0.3 | 01 | 5.7 | 4 | 2.2 | 65 | 9.9 | |||||||
| Other | 25 | .9 | 87 | .6 | 38 | 0.0 | 8 | .8 | 59 | .5 | |||||||
| Education | |||||||||||||||||
| <12th grade | 55 | 1.1 | 13 | 1.6 | 42 | 0.8 | 0.33 | 78 | 1.0 | 5 | 4.6 | 0.30 | |||||
| High school + | 333 | 8.9 | 66 | 8.4 | 467 | 9.2 | 28 | 9.0 | 38 | 5.4 | |||||||
| Household income | |||||||||||||||||
| <$55,000 | 417 | 2.2 | 32 | 1.8 | 85 | 2.4 | 0.85 | 7 | 2.9 | 45 | 1.6 | 0.80 | |||||
| ≥$55,000 | 244 | 7.8 | 63 | 8.2 | 81 | 7.6 | 5 | 7.1 | 88 | 8.4 | |||||||
| Smoking | |||||||||||||||||
| Nonsmoker | 724 | 9.6 | 04 | 9.4 | 120 | 9.9 | 0.61 | 01 | 3.0 | 03 | 8.7 | 0.02 | |||||
| Former smoker | 08 | .8 | 6 | .9 | 2 | .3 | 0.0 | 0 | .1 | ||||||||
| Current smoker | 82 | 5.4 | 20 | 4.8 | 62 | 5.8 | 3 | .0 | 07 | 6.2 | |||||||
| Alcohol Use | |||||||||||||||||
| No past year use | 02 | 9.9 | 61 | 5.7 | 41 | 2.9 | 0.28 | 0 | 6.7 | 01 | 5.5 | 0.52 | |||||
| Light-to-Moderate | 078 | 0.6 | 17 | 2.2 | 61 | 9.7 | 1 | 7.2 | 46 | 3.1 | |||||||
| Heavy Use | 11 | 5.5 | 04 | 2.1 | 07 | 7.3 | 2 | 6.0 | 62 | 1.4 | |||||||
| Body Mass Index | |||||||||||||||||
| Under weight/Normal (<25kg/m2) | 44 | 6.4 | 5 | .8 | 79 | 8.9 | < 0.0001 | .6 | 1 | .2 | < 0.0001 | ||||||
| Overweight (2529.9kg/m2) | 29 | 1.3 | 01 | 4.7 | 28 | 4.9 | 2 | .8 | 79 | 7.5 | |||||||
| Class 1 obesity (3034.9 kg/m2) | 34 | 1.6 | 12 | 8.5 | 22 | 7.7 | 4 | 5.1 | 76 | 1.0 | |||||||
| Class 2 obesity (3539.9 kg/m2) | 14 | 1.4 | 03 | 2.1 | 11 | .4 | 7 | 0.7 | 66 | 2.4 | |||||||
| Class 3 obesity (>40 kg/m2) | 55 | .4 | 97 | 0.8 | 8 | .0 | 6 | 2.9 | 21 | 4.9 | |||||||
| Diabetes | |||||||||||||||||
| Normal | 535 | 6.6 | 00 | 6.7 | 135 | 7.5 | < 0.0001 | 0 | 6.0 | 60 | 8.6 | < 0.0001 | |||||
| Pre-diabetes | 88 | 0.4 | 10 | 7.6 | 78 | 6.4 | 4 | 6.5 | 76 | 9.6 | |||||||
| Diabetes | 09 | 3 | 17 | 5.6 | 92 | .1 | 8 | 7.5 | 29 | 1.8 | |||||||
| Metabolic Syndrome | |||||||||||||||||
| Yes | 47 | 6.2 | 89 | 8.6 | 58 | 3.6 | < 0.001 | 5 | 4.8 | 94 | 5.6 | < 0.0001 | |||||
| No | 867 | 3.8 | 97 | 1.4 | 370 | 6.4 | 0 | 5.2 | 37 | 4.4 | |||||||
| Total energy intake | |||||||||||||||||
| T1 (<1591 kcal) | 11 | 0.1 | 90 | 8.8 | 21 | 0.8 | 0.82 | 6 | 5.4 | 44 | 9.4 | 0.35 | |||||
| T2 (1591–2273 kcal) | 78 | 4.9 | 50 | 5.6 | 28 | 4.5 | 4 | 9.3 | 06 | 6.6 | |||||||
| T3 (≥2273 kcal) | 41 | 5.0 | 06 | 5.6 | 35 | 4.6 | 7 | 5.3 | 49 | 4.0 | |||||||
| Physical activity | |||||||||||||||||
| T1 (<4.67 MET hours/week) | 050 | 0.3 | 46 | 5.7 | 04 | 7.3 | < 0.01 | 7 | 5.5 | 79 | 5.8 | 0.79 | |||||
| T2 (4.67 – 60 MET hours/week) | 17 | 5.1 | 19 | 4.0 | 98 | 5.8 | 3 | 7.9 | 56 | 3.2 | |||||||
| T3 (≥60 MET hours/week) | 25 | 4.5 | 17 | 0.3 | 08 | 6.9 | 3 | 6.6 | 74 | 0.9 | |||||||
| Sedentary Behavior | |||||||||||||||||
| T1 (<4 hours/day) | 06 | 6.4 | 13 | 3.5 | 93 | 8.1 | 0.11 | 2 | 1.9 | 71 | 3.8 | 0.18 | |||||
| T2 (4–6 hours/day) | 59 | 6.0 | 79 | 5.9 | 80 | 6.1 | 4 | 8.7 | 45 | 7.3 | |||||||
| T3 (≥ 6 hours/day) | 227 | 7.5 | 90 | 0.6 | 37 | 5.9 | 7 | 9.4 | 93 | 9.0 | |||||||
| Healthy Eating Index (HEI) | |||||||||||||||||
| T1 (<45.6) | 45 | 4.4 | 40 | 8.2 | 05 | 2.4 | 0.18 | 7 | 2.6 | 83 | 7.5 | 0.78 | |||||
| T2 (45.6–58.5) | 41 | 5.5 | 00 | 5.3 | 41 | 5.6 | 8 | 1.6 | 52 | 5.9 | |||||||
| T3 (≥58.5) | 44 | 0.1 | 06 | 6.5 | 38 | 2.1 | 2 | 5.8 | 64 | 6.6 | |||||||
Notes:
Abbreviations: NAFLD: non-alcoholic fatty liver disease, CAP: controlled attenuation parameter, LSM: liver stiffness measurements, SE: standard error, T: tertile, kcal: kilocalorie, MET: metabolic equivalent.
Models: Chi-Square test or Fisher’s exact test
Table 2 shows MV-adjusted associations for diet and physical activity with NAFLD and advanced fibrosis. Higher adherence to current U.S. dietary recommendations, as defined by the HEI-2015 diet quality score, was significantly associated with NAFLD. In fully adjusted models considering demographics, metabolic syndrome and other lifestyle behaviors, the highest vs lowest tertile of the HEI-2015 score was associated with 40% lower odds of NAFLD (adjusted OR=0.60, 95% CI 0.44, 0.84). We found some evidence that the association of HEI with NAFLD is modified by type 2 diabetes (Supplementary Table 1). There was no clear linear association of increased physical activity with NAFLD. As compared to participants in the lowest tertile of physical activity, participants in the middle tertile had statistically significant lower odds of NAFLD (adjusted OR=0.65, 95% CI 0.42, 0.99), while there was no association with individuals in the highest tertile (adjusted OR=0.98, 95%CI 0.66, 1.47). In the subset of the sample with NAFLD, the HEI-2015 diet quality score was not associated with advanced fibrosis, while participants in the highest tertile of physical activity had 65% lower odds of advanced fibrosis (adjusted OR=0.35, 95%CI 0.16, 0.75). No significant associations were observed for sedentary behavior.
Table 2.
Multivariate Analyses for non-alcoholic fatty liver disease (NAFLD) and subset with advanced fibrosis
| NAFLD (CAP score ≥285 dB/m) | Advanced fibrosis (LSM≥ 8.6 kPa) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Physical activity | ||||||
| T1 (Reference) | ||||||
| T2 | 0.67 (0.46, 0.98) | 0.65 (0.42, 0.99) | 0.65 (0.42, 0.99) | 0.83 (0.35, 1.96) | 1.08 (0.39, 3.02) | 1.11 (0.41, 2.98) |
| T3 | 0.87 (0.60, 1.26) | 0.98 (0.56, 1.47) | 0.98 (0.66, 1.47) | 0.54 (0.31, 0.95) | 0.36 (0.18, 0.72) | 0.35 (0.16, 0.75) |
| Sedentary behavior | ||||||
| T1 (Reference) | ||||||
| T2 | 0.95 (0.65, 1.39) | 0.98 (0.65, 1.49) | 0.99 (0.65, 1.50) | 0.72 (0.29, 1.79) | 0.68 (0.26, 1.82) | 0.57 (0.24, 1.39) |
| T3 | 1.19 (0.87, 1.63) | 1.15 (0.81, 1.64) | 1.16 (0.82, 1.64) | 1.38 (0.57, 3.36) | 1.23 (0.53, 2.84) | 1.03 (0.47, 2.21) |
| Health Eating Index (HEI) | ||||||
| T1 (Reference) | ||||||
| T2 | 0.80 (0.49, 1.30) | 0.75 (0.50, 1.15) | 0.75 (0.50, 1.13) | 0.81 (0.29, 2.28) | 0.62 (0.20, 1.91) | 0.69 (0.20, 2.34) |
| T3 | 0.57 (0.40, 0.82) | 0.61 (0.43, 0.85) | 0.60 (0.44, 0.84) | 0.96 (0.37, 2.47) | 0.84 (0.28, 2.49) | 0.91 (0.29, 2.82) |
Notes:
Abbreviations: NAFLD: non-alcoholic fatty liver disease, CAP: controlled attenuation parameter, LSM: liver stiffness measurements, CI: confidence interval, T: tertile
Models: Multivariable-adjusted logistic regression
Model 1: Adjusted for age, sex, race/ethnicity
Model 2 for NAFLD: Model 1 + Metabolic Syndrome
Model 2 for advanced fibrosis: Model 1 + Metabolic Syndrome + smoking
Model 3 for Physical Activity and Sedentary Behavior: Model 2 + total energy
Model 3 for HEI: Model 2 + total energy, physical activity, alcohol use
Discussion
In this representative sample of U.S. adults, we assessed the association of physical activity, sedentary behavior, and diet quality with the presence of NAFLD and advanced fibrosis using VCTE with controlled attenuation. We found that higher diet quality scores and moderate levels of physical activity were associated with lower odds of NAFLD, while, among patients with NAFLD, physical activity was inversely associated with advanced fibrosis.
Our results indicate that higher diet quality, as represented by increased adherence to the current Dietary Guidelines for Americans (higher HEI-2015 score) was associated with lower odds of NAFLD. These findings align with a previous NHANES 1998–1994 analysis using ultrasound to define NAFLD and a prior version of the HEI.12 Similarly, in the Multiethnic Cohort, diet quality was inversely associated with NAFLD (ascertained through Medicare claims).15 Although it did not reach statistical significance, our findings indicate that HEI may be more strongly associated with lower odds of NAFLD in those without type 2 diabetes, although the reasons for this are unclear. We did not find an association of HEI with advanced fibrosis, in contrast to previous studies.15 Higher diet quality does not hinge on any single component in the diet, but rather indicates a greater conformity with overall healthful eating patterns emphasizing that American’s fill their plates with a variety of fiber-rich plant foods and lean protein while limiting intake of added sugars and fats from processed foods and sugar-sweetened beverages.
We found that moderate amounts of physical activity (middle tertile; between 4.67 and 60 MET hours/week) was associated with lower odds of NAFLD, though there was no protective effect among those in the highest tertile who had ≥60 MET hours/week. These findings are similar to a previous NHANES paper that used noninvasive panels to define NAFLD.14 We also found that the highest levels of physical activity were associated with significantly lower odds of advanced fibrosis. Again, these findings align with previous NHANES findings using noninvasive panels to define fibrosis,14 as well as findings using biopsy-ascertained fibrosis in non-representative samples.16 Physical activity has been identified as an important method for preventing progression of NAFLD.10 Importantly, our findings show that it may be important to promote physical activity both for those with early disease, i.e. NAFLD-only, as well as those with more advanced liver disease. We did not find an association between sedentary behavior and NAFLD. This finding is in contrast to previous NHANES work where sedentary behavior was significantly predictive of NAFLD.14 More research is needed on the association of domain-specific physical activity with NAFLD, as well as to establish a prospective association of sedentary behavior and NAFLD.
Notably, our study is based on cross-sectional analyses, and therefore we cannot determine causality. More specifically, we cannot determine if prevalent disease impacts lifestyle behaviors, that is, if those with impaired liver function are able to do engage in sufficient physical activity and/or eat a healthy and diverse diet, or whether have already changed their behavior based on symptoms or a diagnosis. We were limited to the data available from NHANES 2017–2018, which does not currently include accelerometry-based physical activity. Therefore, physical activity was assessed with self-report, which is subject to measurement error and bias,32 including social desirability and recall bias, though we used a well-validated measure of self-reported physical activity. Similarly, the interviewer-administered 24-hour dietary recall was the basis for our HEI calculation, a form of dietary assessment where underreporting can be an issue.33 However, interviewer-administered 24-hour dietary recalls are considered a gold-standard method, as compared to food frequency questionnaires;34,35 and their validity is strengthened with the use of multiple recalls and vast majority (87%) of our sample completed two recalls.36 Measurement error for HEI-2015 scores based on 24-hour dietary recalls is generally small and use of a single recall is an acceptable estimate of mean dietary intake at the population-level.37 Furthermore, because case-control status was unknown at the time of survey administration, any recall bias would likely be non-differential between those with and without NAFLD. While VCTE is more accurate than liver enzymes and ultrasound, there are no well-established cut-offs for LSM or CAP values. However, we relied on previous studies that compared VCTE with liver biopsy to identify acceptable cut-off values.21 We excluded participants for this study without complete data on primary predictor and outcome variables, as well as those who may have had elevated CAP or LSM due to other clinical reasons. These exclusions may have created selection bias. We performed sensitivity analyses to assess differences between our final sample and those from the overall NHANES 2017–2018 sample that were excluded from our study. We found that our analytic sample was significantly younger (46 years of age for included vs.51 years of age in excluded sample), included a larger proportion of males (50% of the sample in included vs. 46% in excluded sample), and included a smaller proportion of non-Hispanic white participants (61% included vs. 64% excluded). While not a primary focus of this study and limited by small numbers of persons who smoke and have advanced fibrosis, we found an inverse relationship with smoking and advanced fibrosis, which has been found in prior analyses of NHANES.38–40 While this finding is contrary to the expected relationship, future studies are needed as data from prospective studies provide strong evidence that smoking is a risk factor for NAFLD and advanced fibrosis.41,42”
Lastly, there may be residual confounders if there were factors associated with diet, physical activity and NAFLD that remained uncontrolled in this sample. The strengths of this study include the use of well-validated questionnaires and physiological assessments collected by trained personnel using a large cohort of participants representative of the non-institutionalized U.S. population. This is also one of the largest studies to date using VCTE to assess the association of lifestyle behaviors with NAFLD.
Conclusion
Our findings point to the important association of diet quality and physical activity with NAFLD in a representative sample of U.S. adults. Furthermore, physical activity was the only behavior associated with reduced odds for NAFLD-associated advanced fibrosis, pointing to the need to focus on this behavior as part of NAFLD treatment. These findings help identify aspects of lifestyle that need to be targeted among the general population to potentially prevent NAFLD and advanced fibrosis.
Supplementary Material
Highlights.
High adherence to U.S. dietary recommendations associated with reduced odds of non-alcoholic fatty liver disease (NAFLD)
More physical activity associated with reduced odds of NAFLD
More physical activity inversely associated with advanced fibrosis
Financial disclosure
NH was supported by funding from the Prevent Cancer Foundation. XZ was supported by a training grant from the Cancer Prevention and Research Institute of Texas (CPRIT; RP160097). JPH reports research funding from CPRIT (RP190513) and Merck. CRD and LHM were supported by the National Cancer Institute/National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30CA016672) and CRD was supported by a SPORE in Hepatocellular Carcinoma (P50CA217674).
Conflict of interest statement
The authors report that they have received funding from the Prevent Cancer Foundation, National Institutes of Health, Cancer Prevention and Research Institute of Texas, and Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Natalia I. Heredia: conceptualization, investigation, methodology, supervision, visualization and writing-original draft.
Xiaotao Zhang: conceptualization, data curation, formal analysis, methodology, validation, visualization, writing-review & editing.
Maya Balakrishnan: conceptualization, methodology, writing- review & editing.
Carrie Daniel: conceptualization, methodology, supervision, validation, writing- review & editing.
Jessica Hwang: conceptualization, methodology, writing- review & editing.
Lorna McNeill: conceptualization, writing- review & editing.
Aaron Thrift: conceptualization, methodology, formal analysis, resources, supervision, writing- review and editing.
References
- 1.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1):S47–S64. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nature reviews Gastroenterology & hepatology. 2013;10(11):686–690. [DOI] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Heredia NI, Balakrishnan M, Thrift AP. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017–2018. PLoS One. 2021;16(6):e0252164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver International. 2021;41(6):1290–1293. [DOI] [PubMed] [Google Scholar]
- 6.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature reviews Gastroenterology & hepatology. 2013;10(6):330. [DOI] [PubMed] [Google Scholar]
- 7.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. [DOI] [PubMed] [Google Scholar]
- 11.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World journal of gastroenterology: WJG. 2011;17(29):3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo ER, Kim D, Vazquez-Montesino LM, et al. Diet quality and its association with nonalcoholic fatty liver disease and all-cause and cause-specific mortality. Liver International. 2020;40(4):815–824. [DOI] [PubMed] [Google Scholar]
- 13.Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36(8):772–781. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Vazquez-Montesino LM, Li AA, Cholankeril G, Ahmed A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology. 2020;72(5):1556–1568. [DOI] [PubMed] [Google Scholar]
- 15.Park S-Y, Noureddin M, Boushey C, Wilkens LR, Setiawan VW. Diet quality association with nonalcoholic fatty liver disease by cirrhosis status: The multiethnic cohort. Current developments in nutrition. 2020;4(3):nzaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kistler KD, Brunt EM, Clark JM, et al. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. The American journal of gastroenterology. 2011;106(3):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. [DOI] [PubMed] [Google Scholar]
- 18.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. [DOI] [PubMed] [Google Scholar]
- 19.Balakrishnan M, Loomba R. The role of noninvasive tests for differentiating NASH from NAFL and diagnosing advanced fibrosis among patients with NAFLD. J Clin Gastroenterol. 2020;54(2):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver International. 2012;32(6):902–910. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156–163. e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jun BG, Park WY, Park EJ, et al. A prospective comparative assessment of the accuracy of the FibroScan in evaluating liver steatosis. PLoS One. 2017;12(8):e0182784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501. [DOI] [PubMed] [Google Scholar]
- 24.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital and health statistics Series 2, Data evaluation and methods research. 2013(161):1–24. [PubMed] [Google Scholar]
- 25.Centers for Disease Control & Prevention. National Health and Nutrition Examination Survey. 2017–2018. Data Documentation, Codebook, and Frequencies. Liver Ultrasound Transient Elastography. https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/LUX_J.htm. Accessed March 4, 2021. [Google Scholar]
- 26.Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). Journal of Public Health. 2006;14(2):66–70. [Google Scholar]
- 27.Reedy J, Lerman JL, Krebs-Smith SM, et al. Evaluation of the healthy eating index-2015. J Acad Nutr Diet. 2018;118(9):1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSalvo KB, Olson R, Casavale KO. Dietary guidelines for Americans. JAMA. 2016;315(5):457–458. [DOI] [PubMed] [Google Scholar]
- 29.Panizza CE, Shvetsov YB, Harmon BE, et al. Testing the predictive validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: is the score associated with a reduced risk of all-cause and cause-specific mortality? Nutrients. 2018;10(4):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults,. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486. [DOI] [PubMed] [Google Scholar]
- 31.Chen TC CJ, Riddles MK, Mohadjer LK, Fakhouri THI. . National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation Procedures. Vital Health Stat. 2020;2(184). [PubMed] [Google Scholar]
- 32.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(sup2):1–14. [DOI] [PubMed] [Google Scholar]
- 33.Novotny JA, Rumpler WV, Riddick H, et al. Personality characteristics as predictors of underreporting of energy intake on 24-hour dietary recall interviews. J Am Diet Assoc. 2003;103(9):1146–1151. [DOI] [PubMed] [Google Scholar]
- 34.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. [DOI] [PubMed] [Google Scholar]
- 35.Kirkpatrick SI, Subar AF, Douglass D, et al. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. The American journal of clinical nutrition. 2014;100(1):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkpatrick SI, Dodd KW, Potischman N, et al. Healthy Eating Index-2015 Scores Among Adults Based on Observed vs Recalled Dietary Intake. J Acad Nutr Diet. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arshad T, Golabi P, Paik J, Mishra A, Younossi ZM. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatology communications. 2019;3(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jinjuvadia R, Antaki F, Lohia P, Liangpunsakul S. The association between nonalcoholic fatty liver disease and metabolic abnormalities in United States population. J Clin Gastroenterol. 2017;51(2):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciardullo S, Muraca E, Zerbini F, Manzoni G, Perseghin G. NAFLD and liver fibrosis are not associated with reduced femoral bone mineral density in the general US population. The Journal of Clinical Endocrinology & Metabolism. 2021. [DOI] [PubMed] [Google Scholar]
- 41.Hamabe A, Uto H, Imamura Y, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46(6):769–778. [DOI] [PubMed] [Google Scholar]
- 42.Jung H-S, Chang Y, Kwon M-J, et al. Smoking and the risk of non-alcoholic fatty liver disease: a cohort study. Official journal of the American College of Gastroenterology|ACG. 2019;114(3):453–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


