Abstract
Objective
Our goal was to compare the strength of association and predictive ability of qualitative and quantitative EEG factors with the outcomes of death and neurological disability in pediatric cerebral malaria (CM).
Methods
We enrolled children with a clinical diagnosis of CM admitted to Queen Elizabeth Central Hospital (Blantyre, Malawi) between 2012 and 2017. A routine length EEG was performed within 4 hours of admission. EEG data were independently interpreted using qualitative and quantitative methods by trained pediatric neurophysiologists. EEG interpreters were blinded to patient discharge outcome.
Results
EEG tracings from 194 patients were reviewed. Multivariate modeling revealed several qualitative and quantitative EEG variables that were independently associated with outcomes. Quantitative methods modeled on mortality had better goodness of fit than qualitative ones. When modeled on neurologic morbidity in survivors, goodness of fit was better for qualitative methods. When the probabilities of an adverse outcome were calculated using multivariate regression coefficients, only the model of quantitative EEG variables regressed on the neurologic sequelae outcome showed clear separation between outcome groups.
Conclusions
Multiple qualitative and quantitative EEG factors are associated with outcomes in pediatric CM. It may be possible to use quantitative EEG factors to create automated methods of study interpretation that have similar predictive abilities for outcomes as human-based interpreters, a rare resource in many malaria-endemic areas. Our results provide a proof-of-concept starting point for the development of quantitative EEG interpretation and prediction methodologies useful in resource-limited settings.
Keywords: electroencephalogram, malaria, coma, Africa
Introduction
Malaria continues to have a deep and enduring impact on global health. An estimated 228 million annual cases result in approximately 405,000 deaths, most of which are in children.1, 2 Cerebral malaria (CM) is the most lethal form of malarial disease and is a leading cause of death in children under the age of 5 years in sub-Saharan Africa.1 At hospital discharge, one-third of CM survivors have neurologic sequelae, including epilepsy, motor delays, cognitive impairments, and/or behavioral dysregulation.3, 4
In children with CM, electroencephalography (EEG) is used in real-time to detect and treat subclinical seizures and non-convulsive status epilepticus, both of which are common and may contribute to ongoing coma and secondary brain injury.5-7 Admission EEG features interpreted qualitatively have previously been shown to be associated with discharge outcomes.8 Slower background frequencies, lower average voltages, focal slowing, and lack of reactivity are all associated with death.
Few previously published studies have evaluated associations between quantitative EEG and outcomes or compared the ability of qualitative versus quantitative EEG to predict outcomes in CM. In Malawian children, increased gamma power, determined quantitatively, is associated with an increased risk of the epilepsy within 2 years of the diagnosis of CM; qualitative findings were not associated with this outcome.9 Stochastic modeling (one that incorporates randomness) of the quantitative EEG signal strengthens the association between quantitative measures and outcomes in pediatric CM.10
Though associations between EEG and outcomes are interesting and important, there are several practical limitations to deploying EEG services in low-resource areas. Equipment is expensive, and technician training is necessary to acquire tracings of high quality. Qualitative interpretation of EEG tracings requires a trained neurophysiologist, a scarce commodity in resource-limited malaria endemic settings. Although trained personnel may provide remote EEG interpretation, the model of study acquisition in low-resource areas with interpretation provided from higher-resource environments requires extensive Internet bandwidth, rare in some resource-limited settings. Additionally, geographically separating study acquisition and interpretation often leads to asynchronous communication between clinicians and EEG interpreters, due to varied time zones. Ideally, even in resource-limited environments, EEG interpretation would be performed onsite and near the time of study acquisition. Since trained neurophysiologists are rare in Africa, using automated interpretation methods may improve neurological service delivery to these resource-limited areas.
Software-based interpretation systems, which primarily use quantitative EEG, are an alternative to human-based qualitative readings, and are more amenable to automation. Fast Fourier transformation can decompose raw EEG waveforms into their component frequencies and amplitudes, yielding “power” as an area-under-the-curve measure, and quantifying a frequency of interest. Extracting quantitative EEG data as numeric power values may minimize the amount of training required on the part of the interpreter.
Development and validation of automated methods of EEG interpretation would be valuable both for clinical care and research in resource-limited environments. Since qualitative methods are currently the gold standard in EEG interpretation, a first step in automating interpretation (using quantitative EEG as its basis) is evaluating whether qualitative and quantitative interpretation methods and their associations with outcomes are approximately equivalent.
This study’s goal was to compare qualitative and quantitative EEG interpretation methods and their associations with outcomes at hospital discharge in pediatric CM. If qualitative and quantitative methods have similar outcome predictive abilities, this may represent a potential step toward automating EEG interpretation in resource-limited areas and in clinical conditions that demand real-time interpretation.
Materials and Methods
Patient enrollment
We enrolled children between 6 months and 14 years old with a clinical diagnosis of CM admitted to Queen Elizabeth Central Hospital (Blantyre, Malawi) between January 2012 and June 2017. Standard diagnostic criteria included: coma (Blantyre coma score [BCS] ≤ 2); asexual forms of malaria parasites on peripheral blood smear; and no other explanation for coma (e.g., hypoglycemia, meningitis, trauma, or post-ictal state).
A routine length (minimum 30 minutes) EEG was performed within 4 hours of admission, after clinical stabilization and the start of intravenous antimalarials. Electrodes were applied by trained technicians using the standard 10-20 system and recorded on a CeeGraph digital machine (Biologic/Natus, Pleasanton, California) through 2016 and on an XLTEC 32 channel machine (Natus Medical Corporation, Pleasanton, California) in 2017, both with a sampling frequency of 200 Hz.
All patients received intensive supportive therapy and current standard of care therapies for children with CM in a specialized clinical care unit. Although no formalized protocols for the treatment of acute seizures were in place, local clinical practice was to treat clinical seizures with benzodiazepines and, if necessary, escalate to intravenous phenobarbital followed by maintenance therapy. Intravenous phenytoin was administered in patients whose seizures did not respond to phenobarbital. Mechanical ventilation was not available throughout the enrollment period. At hospital discharge, children who survived were classified as being without or with neurologic sequelae (motor, speech, developmental, acute onset of epilepsy) based on neurologic and physical examinations and caretaker interview. Data concerning long-term neurological follow-up were unavailable.
Brain magnetic resonance imaging (MRI) was performed at the time of admission using a Signa Ovation Excite 0.35 T MRI scanner (General Electric). A brain volume score was assigned by two independent staff radiologists blinded to outcome. A score of 7 indicated substantially increased brain volume with sulcal and cisternal effacement but without signs of uncal hernation. A score of 8 included signs of herniation. Substantially increased brain volume on MRI (scores of 7 or 8) is strongly associated with death in children with cerebral malaria.11
EEG Processing
Raw EEG data were independently interpreted for the qualitative and quantitative analyses by trained pediatric neurophysiologists. EEG interpreters were blinded to patient outcome.
Assessed qualitative EEG variables included background voltage, predominant frequency, presence of sleep transients, anterior-posterior frequency and amplitude gradient (AP gradient), continuity of the background, presence of focal slowing, symmetry, variability, and reactivity to a painful stimulus. Dominant frequency and maximum voltage were determined based on visual scanning of the entire record. Average voltage was estimated based on the amplitude of the predominant background activity. Epileptiform discharges and electrographic seizures were noted. The American Clinical Neurophysiology Society Standardized Critical Care EEG terminology was used in classification of background voltage, continuity of the background, degree of asymmetry, reactivity, and prevalence of epileptiform discharges.12
Quantitative analysis was performed using Persyst 13 (Persyst Corporation, Prescott, Arizona) software as previously described.13 Under conditions of artifact reduction, outputs included: absolute power (μV2) of frequencies of interest, including delta (0.5-3.9 Hz), theta (4.0-7.9 Hz), and alpha (8.0-12.9 Hz); total power of all frequency bands; relative spectral power of frequencies of interest (the power of the frequency of interest divided by total power); and power ratios of frequencies of interest (the power of the frequency of interest divided by delta power). The low delta/delta ratio was defined as power in the lowest delta range (0.5-1.9 Hz) divided by total delta (0.5-3.9 Hz) power. The peak envelope and absolute asymmetry index (EASI) were derived from proprietary Persyst algorithms to measure the median waveform amplitude and the quantified percent asymmetry between the cerebral hemispheres, respectively. Theta-alpha variability was calculated using a 2-second time window placed by visual inspection at the maximum and minimum theta plus alpha/delta ratio (TADR) values and calculating the maximum percent change between the two values, as previously described.13
Quantitative EEG variables of power and amplitude were assessed in two ways, as a mean of five 2-second samples and as a continuous sample of the first five artifact-free minutes. We obtained the five 2-second samples systematically. We selected the two time points of the maximum and minimum TADR (used to calculate theta-alpha variability13) and three other 2- second samples taken at the 5- 10- and 15-minute marks. Quantitative variables were also assessed by sampling the first 5 artifact-free minutes of the recording. Sampling in this manner allowed comparisons of the most common methods of quantitative data acquisition: using 2 second samples at pre-determined intervals compared to a single 5-minute sample at or near recording onset.
Any segment of EEG that included periodic epileptiform discharges, non-cerebral artifact, and/or electrographic seizure activity was excluded from quantitative sampling. When electrographic seizure activity was present, EEG samples were selected prior to seizure onset. No samples were taken from the post-ictal recording. When periodic or sporadic epileptiform discharges occupied a substantial portion of the EEG, that portion of the record was not evaluated. If discharges occupied the entire recording, the continuous 5-minute sampling values were not measured. Rather, 2-second samples were taken in manually selected intervals of EEG tracing identified as discharge-free. Excluded studies were determined by consensus (TZ, AA, and DP).
After collecting quantitative EEG data in this manner, a preliminary analysis with Pearson Correlation Coefficients showed that measures of absolute power by frequency band of interest, amplitude, and symmetry had high correlation between the 2-second and 5-minute values (Figure 1).
Figure 1:
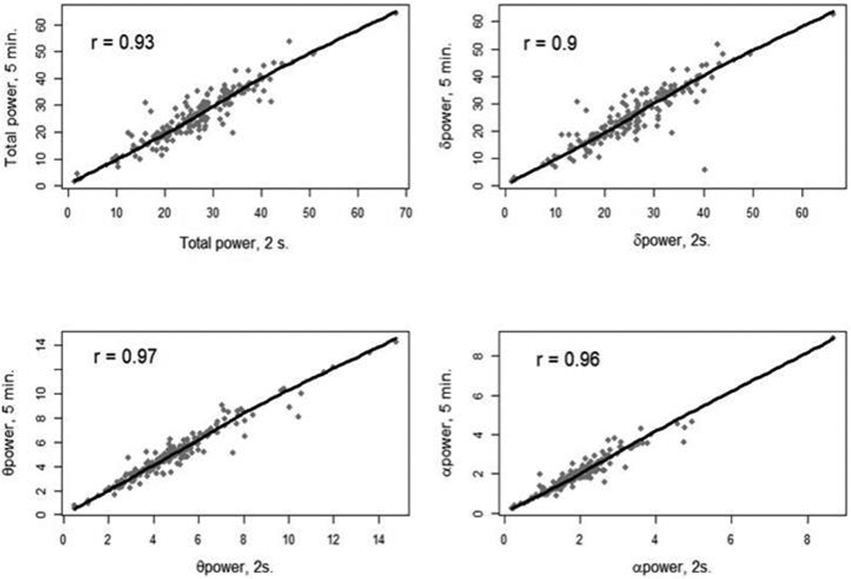
Correlation between 2-second and 5-minute average sampling methods. Plots and numeric Pearson correlation coefficients for power of frequency bands (total, delta, theta, and alpha) comparing the two sampling methods
Given that 5-minute average sampling allows for less visual selection and thus greater promise of automation, the 5-minute data were used in all further analyses (except for fast frequency variability, which required comparison of a maximum and minimum of the TADR13).
Ethical review and approval of the parent study were provided by Michigan State University (USA) and the University of Malawi College of Medicine. At the time of enrollment in the parent study (an observational study of cerebral malaria pathogenesis), patient families consented to the secondary analysis of de-identified data, which were used in this study.
Statistical analyses
We summarized baseline and demographic characteristics, EEG quantitative variables, and EEG qualitative variables for patients in the three outcome groups: survived without sequelae, survived with neurologic sequelae, and died. A Shapiro-Wilk Test of normative distribution prompted reporting of demographic variables as medians (Table 1). Univariate analysis evaluated the association of each EEG factor with mortality or sequelae among survivors. P-values were calculated using ANOVA or Wilcoxon -Mann-Whitney tests for continuous variables; chi square was used to test for associations between categorical data and outcomes..
Table 1:
Demographic characteristics (including admission laboratory values) and outcomes, univariate pairwise analysis
| Demographic variable |
Died (n=28) |
Survived with neurologic sequelae (n=23) |
Survived without neurologic sequelae (n=143) |
P-value comparing those who died vs. survivors |
P-value survival with vs. without sequelae |
|
|---|---|---|---|---|---|---|
| Age (months): median (IQR) | 36 (24.5 – 49.5) | 36 (18 – 74) | 55 (36 – 78) | 0.0067* | 0.04* | |
| Blantyre coma score: count (%) | ||||||
| 0 | 7 (25%) | 5 (21.7%) | 7 (4.9%) | 0.001* | <0.001* | |
| 1 | 15 (53.6%) | 8 (34.8%) | 62 (42.0%) | |||
| 2 | 6 (21.4%) | 10 (43.5%) | 76 (53.1%) | |||
| Glucose (mg/dL): median (IQR) | 86.4 (45.0 – 117.0) | 104.4 (66.6 – 138.6) | 104.4 (84.6 – 129.6) | 0.015* | 1.0 | |
| Platelets (x103)/ml: median (IQR) | 48 (9.3 – 85.9) | 43.4 (7.6 - 304) | 1.7 (0.3 – 49.8) | 0.089 | 0.084 | |
| Hematocrit (%):median (IQR) | 26.1 (21 – 30.1) | 20.5 (14.9 – 24) | 23.4 (18.9 – 27) | 0.057 | 0.048* | |
| Time from onset of fever to hospital admission (hours): median (IQR) | 48 (14 – 72) | 48 (24 -72) | 72 (48 – 72) | 0.13 | 0.50 | |
| Time from onset of coma to hospital admission (hours): median (IQR) | 10 (7 – 18) | 24 (21 -48) | 12 (7-24) | 0.26 | 0.0006* | |
| Time from last clinical convulsion until hospital admission (hours): median (IQR) | 10 (5 – 24) | 7 (0 – 24) | 5 (0 - 15.5) | 0.09 | 0.33 | |
| History of pre-hospital administration of benzodiazepines or barbiturates (%) | 17 (60.7%) | 12 (52.2%) | 75 (53.2%) | 0.45 | 0.93 | |
| Brain volume score | ||||||
| 1-6 | 12 (44.4%) | 20 (87%) | 114 (80.3%) | <0.001* | 0.45 | |
| 7-8 | 15 (55.6%) | 3 (13%) | 28 (19.7%) | |||
Variables expressed as median (IQR) for continuous variables, counts (percent) for categorical variables. P-values using ANOVA or Wilcoxon -Mann-Whitney test for continuous variables, chi square for categorical; SD = standard deviation.
We evaluated the independent association of EEG variables (quantitative variables only, or qualitative variables only) with each outcome using multivariable logistic regression models adjusting for demographic and clinical factors shown to be outcome predictors on univariate analysis (age, Blantyre coma score, admission glucose, admission hematocrit, and brain volume score based on MRI). Because the number of events for each outcome was low compared to the number of potential predictors, we used model selection with stepwise regression to select the model with the lowest Akaike Information Criteria (AIC). For each final model, a Wald 95% confidence interval was calculated. Finally, for each outcome, we compared the goodness of fit and predictive ability of the two EEG models (quantitative variables with statistically significant demographic characteristics associated with outcomes, qualitative variables with statistically significant demographic characteristics associated with outcome) with the AICs and the fitted probabilities relative to the observed outcomes.
Statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). In all analyses, a p value less than 0.05 was considered a statistically significant difference between groups.
Results
EEG tracings from 195 patients were available for review. The tracing of one patient was occupied by continuous seizure activity and was excluded from analysis. The EEG records of 7 patients had periodic or abundant discharges and/or substantial artifact that precluded 5-minute quantitative analysis (e.g., no continuous 300 second period of quality EEG data), thus their data were imputed by sampling five 2-second epochs devoid of artifact and/or discharges.
Of the 194 included patients, 28 died, 23 survived with neurologic sequelae at time of discharge, and 143 survived with no neurologic sequelae. Children who died were more likely to be younger, to have lower admission Blantyre coma score, to have lower glucose, and to have a higher brain volume score than those who survived (Table 1). Younger age, lower admission Blantyre coma score, lower hematocrit, and longer time from onset of coma to hospital admission were associated with neurologic sequelae in survivors (Table 1).
Univariate analyses of qualitative EEG variables and their association with outcomes revealed the following associations with death: lower background voltage, absent or reversed anterior-posterior gradient, background asymmetry, lack of variability, lack of reactivity, lack of sleep spindles, and presence of electrographic seizures (Table 2). All patients who died had an abnormal (reversed or absent) anterior-posterior gradient of voltage and/or frequency. Absent sleep spindles were significantly associated with the presence of neurologic sequelae in survivors.
Table 2:
Associations between qualitative and quantitative EEG variables and outcomes stratified by outcome, univariate analysis
| EEG variable | Died (n=28) | Survived with neurologic sequelae (n=23) |
Survived without neurologic sequelae (n=143) |
P-value comparing those who died vs. survivors |
P-value survival with vs. without sequelae |
|
|---|---|---|---|---|---|---|
| Qualitative EEG variables | ||||||
| Average voltage (μv): mean (SD) | 69.25 (51.32) | 91.52 (27.57) | 101.22 (33.39) | <0.001* | 0.19 | |
| Maximum voltage (μv): median (IQR) | 122.5 (60 - 230) | 180 (130 – 240) | 200 (150 – 250) | <0.001* | 0.13 | |
| Dominant frequency (Hz) median (IQR) | 3 (2.25 – 4.25) | 3 (2 – 4) | 3 (2.5 – 3.5) | 0.73 | 0.62 | |
| Absent variability: number (%) | 4 (14.3) | 0 (0.0) | 3 (2.1) | 0.006* | Not applicable | |
| Asymmetric background: number (%) | 12 (42.9) | 4 (17.4) | 12 (8.4) | <0.001* | 0.33 | |
| Periodic discharges: number (%) | 2 (7.1) | 1 (4.3) | 0 (0.0) | 0.08 | 0.29 | |
| Reactivity to painful stimulus: number (%) | ||||||
| Absent | 14 (50.0) | 11 (47.8) | 61 (42.7) | <0.001* | 0.46 | |
| Present | 6 (21.4) | 12 (52.2) | 73 (51.0) | |||
| Not assessed | 8 (28.6) | 0 (0.0) | 9 (6.3) | |||
| Anterior-posterior gradient: number (%) | ||||||
| Normal | 0 (0.0) | 6 (26.1) | 23 (16.1) | 0.04* | 0.16 | |
| Reversed | 3 (10.7) | 5 (21.7) | 17 (11.9) | |||
| None | 25 (89.3) | 12 (52.2) | 103 (72.0) | |||
| Sleep spindles present: number (%) | 7 (25.0) | 3 (13.0) | 77 (53.8) | 0.04* | 0.001* | |
| Electrographic seizures present: number (%) | 5 (17.9) | 2 (8.7) | 3 (2.1) | 0.005* | 0.29 | |
| Suppressed or attenuated background: number (%) | 14 (50) | 2 (8.7) | 7 (4.9) | <0.001* | 0.80 | |
| Quantitative EEG variables | ||||||
| Total power: mean (SD) | 21.09 (13.44) | 25.96 (10.17) | 26.72 (9.25) | 0.01* | 0.73 | |
| Delta power: mean (SD) | 20.56 (13.47) | 25.09 (9.95) | 25.70 (9.43) | 0.02* | 0.79 | |
| Theta power: median (IQR) | 3.56 (1.66 – 5.24) | 4.18 (2.89 – 5.74) | 4.84 (3.76 – 6.14) | <0.001* | 0.18 | |
| Alpha power: median (IQR) | 1.54 (0.74 – 1.84) | 1.71 (1.04 – 2.61) | 2.02 (1.58 – 2.5) | 0.001* | 0.24 | |
| Peak envelope: median (IQR) | 25.69 (14.05 – 35.92) | 30.66 (21.98 – 40.57) | 35.32 (27.22 – 42.73) | 0.002* | 0.24 | |
| Low delta/delta ratio (0.5-1.9Hz / 0.5-3.9Hz) | 0.61 (0.58 – 0.67) | 0.61 (0.57 – 0.64) | 0.58 (0.51 – 0.63) | 0.02* | 0.087 | |
| Theta-alpha/delta ratio (4.0-12.9Hz / 0.5-3.9Hz) | 0.33 (0.22 – 0.44) | 0.27 (0.22 – 0.47) | 0.33 (0.25 – 0.44) | 0.84 | 0.50 | |
| Asymmetry index (EASI) | 26.84 (20.91 – 33.3) | 24.06 (19.13 – 29.49) | 20.91 (18.43 – 26.09) | 0.004* | 0.20 | |
| Theta-alpha variability | 0.15 (0.1 – 0.29) | 0.22 (0.12 – 0.37) | 0.37 (0.24 – 0.49) | <0.001* | 0.006* | |
p-value ≤ 0.05
On univariate pairwise analysis of associations of quantitative EEG variables and outcomes, the following were significantly associated with death: lower total power, lower delta power, lower theta power, lower alpha power, lower peak envelope (median waveform amplitude), higher low delta/delta ratio, higher asymmetry index (EASI), and lower theta-alpha variability (Table 2). Lower theta-alpha variability was also associated with the presence of neurologic sequelae in survivors (Table 2).
Multivariate modeling revealed several qualitative and quantitative EEG variables independently associated with outcomes (Tables 3 and 4).
Table 3:
Multivariable association of qualitative and quantitative EEG and mortality
| Clinical factors/ EEG variables | Odds Ratio |
95% confidence interval |
P value |
|---|---|---|---|
| Qualitative EEG analysis (AIC* = 100.1) | |||
| Standardized age | 0.46 | 0.25, 0.86 | 0.015 |
| Brain volume score 7 or 8 | 9.14 | 2.58, 32.4 | 0.001 |
| Average background voltage | 0.48 | 0.25, 0.93 | 0.029 |
| Asymmetry present | 5.47 | 1.56, 19.2 | 0.008 |
| Background attenuated or suppressed | 13.8 | 3.14, 60.7 | 0.001 |
| Quantitative EEG analysis (AIC = 91.3) | |||
| Standardized age | 0.37 | 0.17, 0.77 | 0.008 |
| Brain volume score 7 or 8 | 14.3 | 3.47, 59.1 | <0.001 |
| Low delta to total delta ratio | 2.02 | 0.92, 4.40 | 0.079 |
| Alpha Power | 0.21 | 0.07, 0.58 | 0.003 |
| Theta-plus-alpha to delta ratio | 3.10 | 1.37, 6.98 | 0.006 |
| Theta-alpha variability | 0.32 | 0.15, 0.70 | 0.004 |
AIC quantifies strength of fit between a group of independent variables and the dependent variable. Models including many independent variables are penalized, thus avoiding overcomparison. When comparing models, the one with the lower AIC value has better goodness of fit.
Table 4:
Multivariable association and qualitative and quantitative EEG and the presence of neurological sequelae in cerebral malaria survivors
| Clinical factors/ EEG variables |
Odds ratio | 95% confidence interval |
P value |
|---|---|---|---|
| Qualitative EEG analysis (AIC = 111.7) | |||
| Age (standardized) | 0.57 | 0.33, 0.97 | 0.040 |
| Admission hematocrit | 0.62 | 0.37, 1.04 | 0.071 |
| Maximum background voltage | 0.99 | 0.99, 1.00 | 0.100 |
| Sleep spindles present | 0.10 | 0.03, 0.36 | 0.001 |
| Quantitative EEG analysis (AIC = 115.7) | |||
| Age (standardized) | 0.61 | 0.35, 1.07 | 0.084 |
| Admission hematocrit | 0.63 | 0.36, 1.10 | 0.106 |
| Theta power | 0.68 | 0.46, 1.00 | 0.052 |
| Alpha power | 1.81 | 0.97, 3.36 | 0.061 |
| Theta-alpha variability | 0.51 | 0.29, 0.88 | 0.015 |
For the outcome of mortality, qualitative interpretation revealed that background asymmetry and lower background voltage- particularly an attenuated or suppressed background- were independently associated with death. Using quantitative EEG interpretation, lower alpha power, lower theta-alpha variability, and higher theta-plus-alpha to delta ratio were independently associated with death. The AIC for quantitative methods modeled on mortality was lower than when using qualitative interpretation methods, indicating better goodness of fit.
Using neurological morbidity in CM survivors as the outcome of interest, the results of multivariate modeling changed. During qualitative interpretation, the presence of sleep spindles independently decreased the risk of an adverse neurological outcome in CM survivors. Using quantitative interpretation, only reduced theta-alpha variability was significantly associated with an increased risk of an adverse neurological outcome in a CM survivor. When modeled on the presence of neurologic morbidity in survivors, the AIC was lower using qualitative interpretation methods. (All models excluded a reduced or absent anterior-posterior gradient, periodic discharges, and absent variability, all of which were completely predictive, producing data separation.)
Predictive modeling using either qualitative or quantitative EEG variables yielded mixed results. When the probabilities of an adverse outcome were calculated using multivariate regression coefficients and patient clinical data (age, Blantyre coma score), only the model of qualitative EEG variables regressed on the neurologic sequelae outcome showed clear separation between outcome groups (Figure 2).
Figure 2:
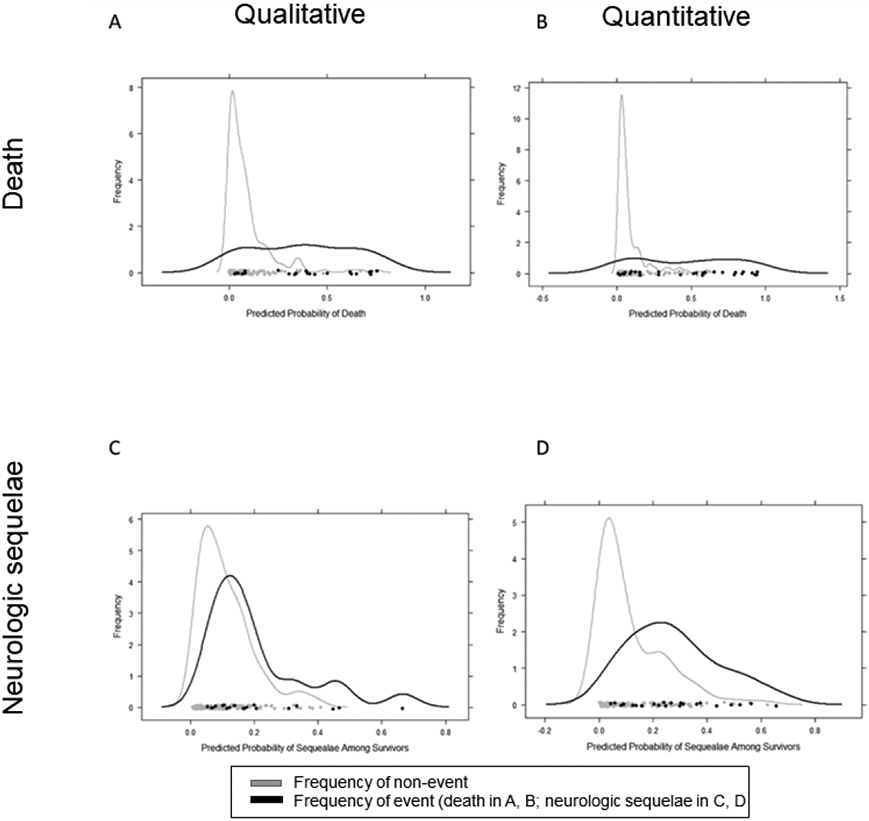
Probability density curves predicting death using qualitative (A) or quantitative (B) EEG interpretation methods; predicting neurologic sequelae in survivors using qualitative (C) or quantitative (D) methods. Separation of two defined peaks indicates superior predictive ability.
Conclusions:
Multiple qualitative and quantitative EEG factors are associated with outcomes in pediatric CM. Clinical variables known to be associated with outcome- age, Blantyre Coma Score, hematocrit, glucose, and brain volume score on MRI- remained in the multivariate models. While duration of coma correlated with outcome of neurologic sequelae, this variable was not commonly available in the total patient population and was therefore excluded. When controlling for these clinical variables, multiple quantitative and qualitative EEG factors were associated with both mortality and neurological sequelae in CM survivors. Comparison of qualitative and quantitative EEG methods and their associations with outcome reveals that the goodness of fit of either method is similar, with currently available qualitative analyses superior when fitting models to adverse neurologic outcomes in survivors and quantitative analyses better when modeling the outcome of death
In the multivariate model predicting death, qualitative attenuation or suppression of the background yielded higher odds of an adverse outcome than did the brain volume score, which is a well-established risk marker for mortality.11 Quantitative decline in fast frequency (alpha) power along with lower variability of faster frequencies (theta-alpha variability) similarly yielded lower odds of survival. Presence of a higher theta-plus-alpha to delta ratio in the multivariate model predicting death is counterintuitive. It may be the case that delta power is underrepresented in the background of the most attenuated and suppressed records, and therefore that a percent-change measure (theta-alpha variability) eliminating the absolute value of delta frequency power more accurately reflects what is seen visually on the EEG.
It may be possible to use quantitative EEG factors to create automated methods of EEG interpretation that have similar predictive abilities as human-based interpreters. While quantitative EEG methods have revealed factors associated with long-term epileptogenesis in pediatric CM, our results reveal that quantitative methods may be useful in predicting death or neurological disability at hospital discharge.9, 10 Predicting a higher likelihood of these outcomes at admission may inform acute therapeutic and supportive measures for children with CM. Scarce resources or therapeutic interventions could be preferentially focused on those at higher risk of death or neurological sequelae. Though ‘tele-neurology’ may increase access to neurologic care in remote and resource-limited areas, optimization of care for patients with CM may be aided by using automated EEG interpretation systems operating on site in real time.14
Our findings are congruent with previously published studies evaluating associations between quantitative EEG variables and outcome in non-malarial critical illnesses. Lower power across most frequency bands (total, delta, theta, alpha) has been associated with adverse outcomes for adults after cardiac arrest,15 and construction of a cerebral recovery index using quantitative EEG methods is useful in predicting outcomes in these patients.16 A pediatric cardiac arrest predictive model constructed with a random forest algorithm found patient age and eight quantitative EEG parameters (normalized power bands of delta, theta and alpha among them) to be risk predictors with a positive predictive value of 0.79 and a negative predictive value of 0.80.17
Quantitative and qualitative EEG have been previously compared with respect to their associations with outcome in other critical illnesses. Quantifying EEG reactivity using measures of entropy and power performs equally well compared to expert review in predicting favorable or unfavorable outcomes in adult cardiac arrest.18 Quantitative variables of total power and theta/delta ratio predict survival as well as need for liver transplant in pediatric acute liver failure, but visual grading of EEG abnormalities does not.19 In our population, similar to adults with cardiac arrest, both qualitative and quantitative interpretation methods yielded regression results with similar goodness of fit. Quantitative methods, which extract information from the EEG background, appear to be as useful as qualitative methods in predicting outcome and may be more useful in resource limited settings where trained neurophysiologists are rare.
In our analysis, the presence of electrographic seizures on qualitative interpretation was associated with an adverse outcome. Our analysis did not attempt to improve on existing methods for the detection of electrographic seizures using quantitative methods. Quantitative EEG outputs have been used by non-neurophysiologist medical staff (such as neuro-ICU nurses) in seizure recognition with low false-positive rates.20, 21 The development and validation of similar methods for children has precedent, but is not currently validated in clinical settings such as the one in this study.22-24 Deployment of automated seizure detection methods in pediatric CM is warranted and will likely improve the clinical use of EEG in the care of these critically ill children in resource-limited areas.
Limitations of the present study include a lack of standardized measures of neurologic outcomes. The presence or absence of neurologic sequelae in survivors was determined at hospital discharge by examination and caregiver interview. If neurologic sequelae spontaneously resolve or appear after a delay following hospitalization, a longer follow up period may modify the associations between EEG factors and the presence of adverse neurologic outcomes in survivors. Our study relied on the use of a single routine EEG at hospital admission, which represents shorter data acquisition than is customary in the evaluation of comatose patients in high-resource environments. In the resource-limited area in which this study was conducted, continuous EEG was unavailable. Visual inspection of the first 5 minutes of the raw EEG to select quality samples for quantitative analysis, while minimizing the technical demand on the data-gatherer, still leaves open the possibility of inter-operator variability. Methods that further minimize this variability may take the form of machine learning to ascertain more subtle predictive features in the quantitative EEG data. Finally, the extent to which antiseizure medication administration may impact fast frequency power in this cohort is unknown as complete and reliable pre-hospital medical records are extremely rare in Malawi. For those records where this information was available, there were not substantial differences in pre-hospital medication exposure between outcome groups (Table 1). We excluded the beta frequency band (13-30 Hz) from analysis to attempt to mitigate this uncertainty.
This study provides a proof-of-concept starting point for the development of quantitative EEG interpretation and prediction methodologies useful in resource-limited settings. Future studies should explore creation of other quantitative EEG variables (such as theta-alpha variability) that may increase the clinical use and predictive value of quantitative EEG parameters in critically ill children with CM. Automating quantitative interpretations may be a solution for the shortage of qualified EEG interpreters in resource-limited areas, while foreknowledge of outcomes may help identify and channel limited services (MRI, physical therapy) to the patients at highest risk of death or neurological sequelae.
Acknowledgements:
The authors acknowledge the assistance of Ms. Elizabeth Kalanga and Ms. Monica Sapuwa, EEG technicians in the Pediatric Research Ward at Queen Elizabeth Central Hospital. Without their hard work and attention to detail, this study would not have been possible. We thank Persyst Corporation who provided EEG interpretation software for this study’s use.
Funding sources:
Drs. Postels and Zelleke received salary support for this project via Interagency Personnel Agreements with the National Institute of Allergy and Infectious Diseases. The Division of Neurology at Children’s National Medical Center funded the statistical analyses. The sponsors had no role in: study design; collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declarations of Interest:
Drs. Postels and Zelleke received salary support for this project via Interagency Personnel Agreements with the National Institute of Allergy and Infectious Diseases. No other authors had potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.World Malaria Report: World Health Organization, 2019.
- 2.Barcus MJ, Basri H, Picarima H, et al. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg 2007;77:984–991. [PubMed] [Google Scholar]
- 3.Birbeck GL, Molyneux ME, Kaplan PW, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol;9:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postels DG, Taylor TE, Molyneux M, et al. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology 2012;79:1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 2005;4:827–840. [DOI] [PubMed] [Google Scholar]
- 7.Idro R, Ndiritu M, Ogutu B, et al. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. Jama 2007;297:2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postels DG, Wu X, Li C, et al. Admission EEG findings in diverse paediatric cerebral malaria populations predict outcomes. Malar J 2018;17:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel AA, Jannati A, Dhamne SC, et al. EEG markers predictive of epilepsy risk in pediatric cerebral malaria - A feasibility study. Epilepsy Behav 2020;113:107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veretennikova MA, Sikorskii A, Boivin MJ. Parameters of stochastic models for electroencephalogram data as biomarkers for child's neurodevelopment after cerebral malaria. Journal of statistical distributions and applications 2018;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seydel KB, Kampondeni SD, Valim C et al. Brain Swelling and Death in Children with Cerebral Malaria. N Engl J Med 2015;372:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 13.Andrews A, Zelleke T, Harrar D, Izem R, Gai J, Postels D. Theta-alpha variability on admission EEG is associated with outcome in pediatric cerebral malaria. Journal of Clinical Neurophysiology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavin B, Dormond C, Scantlebury MH et al. Bridging the healthcare gap: Building the case for epilepsy virtual clinics in the current healthcare environment. Epilepsy Behav 2020;111;107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiley SL, Razavi B, Krishnamohan P, et al. Quantitative EEG Metrics Differ Between Outcome Groups and Change Over the First 72 h in Comatose Cardiac Arrest Patients. Neurocrit Care 2018;28:51–59. [DOI] [PubMed] [Google Scholar]
- 16.Tjepkema-Cloostermans MC, van Meulen FB, Meinsma G, van Putten MJ. A Cerebral Recovery Index (CRI) for early prognosis in patients after cardiac arrest. Critical care (London, England) 2013;17:R252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Zhao X, Davis KA, Topjian AA, Litt B, Abend NS. Quantitative EEG predicts outcomes in children after cardiac arrest. Neurology 2019;92:e2329–e2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amorim E, Williamson CA, Moura L, et al. Performance of Spectrogram-Based Seizure Identification of Adult EEGs by Critical Care Nurses and Neurophysiologists. J Clin Neurophysiol 2017;34:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Press CA, Morgan L, Mills M, et al. Spectral Electroencephalogram Analysis for the Evaluation of Encephalopathy Grade in Children With Acute Liver Failure. Pediatr Crit Care Med 2017;18:64–72. [DOI] [PubMed] [Google Scholar]
- 20.Kang JH, Sherill GC, Sinha SR, Swisher CB. A Trial of Real-Time Electrographic Seizure Detection by Neuro-ICU Nurses Using a Panel of Quantitative EEG Trends. Neurocrit Care 2019;31:312–320. [DOI] [PubMed] [Google Scholar]
- 21.Swisher CB, White CR, Mace BE, et al. Diagnostic Accuracy of Electrographic Seizure Detection by Neurophysiologists and Non-Neurophysiologists in the Adult ICU Using a Panel of Quantitative EEG Trends. J Clin Neurophysiol 2015;32:324–330. [DOI] [PubMed] [Google Scholar]
- 22.Topjian AA, Fry M, Jawad AF, et al. Detection of electrographic seizures by critical care providers using color density spectral array after cardiac arrest is feasible. Pediatr Crit Care Med 2015;16:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalgudi Ganesan S, Stewart CP, Atenafu EG, et al. Seizure Identification by Critical Care Providers Using Quantitative Electroencephalography. Critical care medicine 2018;46:e1105–e1111. [DOI] [PubMed] [Google Scholar]
- 24.Du Pont-Thibodeau G, Sanchez SM, Jawad AF, et al. Seizure Detection by Critical Care Providers Using Amplitude-Integrated Electroencephalography and Color Density Spectral Array in Pediatric Cardiac Arrest Patients. Pediatr Crit Care Med 2017;18:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


