Abstract
D-mannose (D-m) is a glucose epimer found in natural products, especially fruits. In mouse models of diabetes and airway inflammation, D-m supplementation via drinking water attenuated pathology by modifying cellular energy metabolism, leading to the activation of latent transforming growth factor beta (TGF-β), which in turn induced T regulatory cells (Tregs). Given that Tregs are important in controlling neuroinflammation in experimental autoimmune encephalomyelitis (EAE) and likely in multiple sclerosis (MS), we hypothesized that D-m could also suppress EAE. We found that D-m delayed disease onset and reduced disease severity in two models of EAE. Importantly, D-m treatment prevented relapses in a relapsing-remitting model of EAE, which mimics the most common clinical manifestation of MS. EAE suppression was accompanied by increased frequency of CD4+FoxP3+ Tregs in the central nervous system, suggesting that EAE suppression resulted from Treg cell induction by D-m. These findings suggest that D-m has the potential to be a safe and low-cost complementary therapy for MS.
Keywords: D-mannose, Experimental Autoimmune Encephalomyelitis, Multiple sclerosis, Regulatory T cells
1. Introduction
D-mannose (D-m) is an epimer of glucose commonly found in natural products. D-m is present in the serum of mice and humans, typically at 0.1 mM levels, and long-term supplementation in drinking water raises its concentration to ~0.9 mM without adverse effects (Davis and Freeze, 2001), as levels of ~2 mM can be maintained without toxicity (Mayatepek et al., 1997). Treatment with D-m has beneficial effects in diseases such as disorders of glycosylation type Ib (Schneider et al., 2011; de Lonlay and Seta, 2009) and in bacterial urinary tract infections (Kranjcec et al., 2014; Michaels et al., 1983). In mouse models of diabetes and airway inflammation, D-m supplementation through drinking water attenuated pathology by modifying cellular energy metabolism, leading to the activation of latent TGF-β, which in turn induced Tregs (Zhang et al., 2017). Given that Tregs are believed to control neuroinflammation in MS and are proven to control neuroinflammation in experimental autoimmune encephalomyelitis (EAE) (Fletcher et al., 2010; Hilliard et al., 2000; McHugh and Shevach, 2002; O’Connor and Anderton, 2008; Lowther and Hafler, 2012; Kleinewietfeld and Hafler, 2014), we hypothesized that D-m could also induce Tregs in EAE and suppress disease. In the following study, we tested the effects of oral D-m treatment in chronic and relapsing-remitting EAE, which are models of MS.
2. Methods and Materials
Mice
Mice used in this study were on C57BL/6J and SJL/J genetic backgrounds. All mice were females. Mice were obtained from The Jackson Laboratories (Bar Harbor, Maine). All experimental procedures were approved by the IACUC of Thomas Jefferson University.
Isolation of Immune Cells from the CNS
Mice were anesthetized and blood was removed by intracardial perfusion with 60 mL PBS. Brains and spinal cords were collected and digested in 0.7 mg/mL Liberase TL in RPMI (Roche, Basel, Switzerland) for 30 min at 37°C. Tissue was dissociated through a 100 μm filter and resuspended in 25 mL of 70% 1x Percoll-PBS (90% Percoll, 10% 10x PBS). 25 mL of 30% Percoll-PBS was gently overlayed onto the 70% layer and was centrifuged at 2000 RPM without brake at room temperature for 30 min. Cells that pooled at the interface of 30/70% layers were then collected.
Flow Cytometry
Cells isolated from the CNS were stimulated with PMA (500 ng/mL; Sigma Aldrich, St. Louis, MO), ionomycin (50 ng/mL; Sigma Aldrich, St. Louis, MO), and 1 μL/mL Golgiplug (BD Biosciences, Franklin Lakes, NJ) for 4 h at 37°C. Cells were washed with PBS containing 3% FBS. Surface antigens were stained with Abs in 100 μL of PBS/3% FBS for 20 min at 4°C. Cells were then fixed with 100 μL Fix and Perm Medium A (Thermo Fisher, Waltham, MA) for 20 min at room temperature and then permeabilized with Fix and Perm Medium B (Thermo Fisher, Waltham, MA) and stained with Abs against intracellular antigens in 100 μL Fix and Perm Medium B and 100 μL PBS/3%FBS for 1 h. Cells were then washed twice, resuspended in 500 μL PBS and analyzed on a BD FACSAria Fusion flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Induction and Scoring of EAE
EAE in C57BL/6J mice was induced by immunization with complete Freund’s adjuvant (CFA) containing 5 mg/mL heat killed M. tuberculosis (BD Biosciences, Franklin Lakes, NJ) and 1 mg/mL MOG35–55 peptide (Genscript, Piscataway, NJ). SJL/J mice were immunized with CFA containing 0.75 mg/mL PLP139–151. Mice were immunized on the both flanks by subcutaneous injection of the emulsion for a total of 200 μL. Pertussis toxin was i.p. injected on days 0 and 2 post-immunization at 200 ng per dose. Mice were scored according to the following scale: 0 - No clinical symptoms. 0.5 - Partial paralysis of the tail or waddling gait. 1.0 - Full paralysis of the tail. 1.5 - Full paralysis of the tail and waddling gait. 2.0 - Partial paralysis in one leg. 2.5 - Partial paralysis in both legs or one leg paralyzed. 3.0 - Both legs paralyzed. 3.5 - Ascending paralysis. 4.0 - Paralysis above the hips. 4.5 – Moribund; mouse being unable to right itself for 30 seconds. 5.0 - Death.
Treatment with D-m
D-m (MilliporeSigma, Burlington, MA) was dissolved in ddH2O at 0.55 or 1.1 mM and provided ad libitum in place of regular drinking water for mice.
3. Results
3.1. Prophylactic treatment with D-m suppresses both chronic and relapsing-remitting EAE.
Immunization of C57BL/6J mice with MOG35–55 results in a chronic EAE disease course (C-EAE). Providing C-EAE mice with water containing D-m attenuated clinical disease (Fig. 1A). The effect of D-m on EAE was dose dependent, as 0.55M D-m did not ameliorate disease (Fig 1B). We also tested the effect of D-m in relapsing-remitting EAE (RR-EAE) in SJL mice. D-m-treated mice had a markedly suppressed disease course compared to control animals, which suffered from severe disease that led to the death of all animals by the first relapse of disease (Figs. 1C, D). Surprisingly, after stopping D-m-treatment (day 27 post immunization) mice did not relapse for an additional 10 days, when the experiment was ended. This indicates that the suppressive effect of D-m in EAE lasts beyond the treatment period, which would be a highly beneficial therapeutic feature in MS.
Figure 1. Prophylactic treatment with D-m suppresses both C-EAE and RR-EAE.
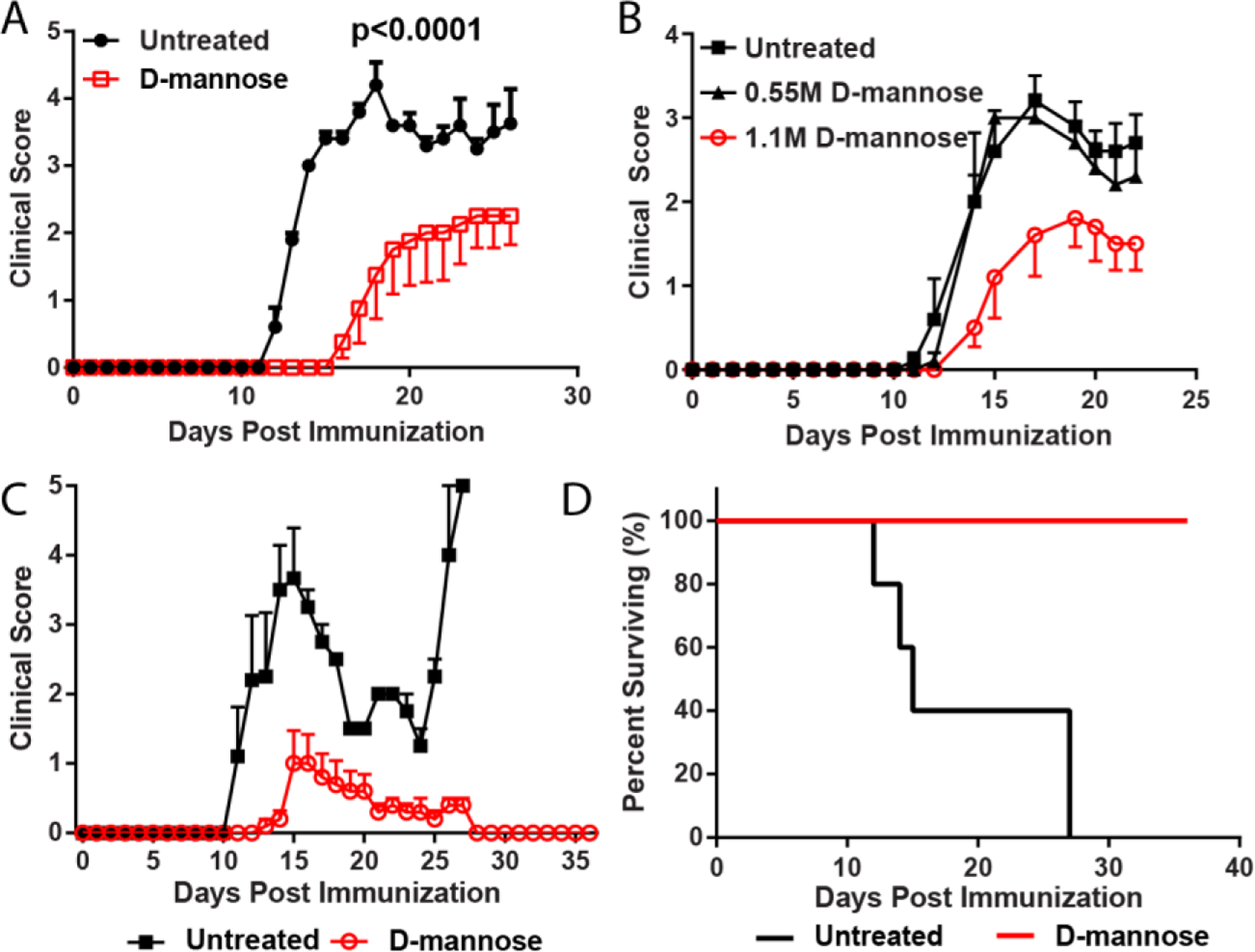
A) C57BL/6 mice were immunized to induce EAE and treated with 1.1 M D-m. Clinical course of EAE is shown. N = 4–5 mice per group. Representative data from 1 of 2 independent experiments is shown. B) Clinical course for untreated, 0.55 M D-m treated and 1.1 M D-m treated mice. N = 5 mice per group. Experiment was performed once. C) SJL mice were immunized to induce RR-EAE. N = 5 mice per group. Representative data from 1 of 2 independent repeats. Clinical course of EAE is shown. D) Kaplan–Meier plot depicting survival over time for mice in C. Statistical significance was calculated using two-way ANOVA.
We also tested whether treatment with D-m initiated after onset of disease could have a suppressive effect but found no suppressive effect when D-m was given after disease onset (Fig. 2). However, this conclusion should be accepted with a caution, because D-m has a very bitter taste and at disease onset, mice regularly reduce an intake of food and water, which is reflected in a rapid loss of their body weight. In order to circumvent this problem, we experimented with masking the bitterness with artificial sweeteners and flavors, and with including D-m into various types of mouse food, instead of into water, but these efforts did not increase D-m consumption to the levels that mice consume pre-disease onset (data not shown). We also attempted to deliver D-m by oral gavage, but because of large quantity of D-m that needs to be delivered, this approach was not feasible (data not shown). Hence, it remains to be determined with certainty whether D-m can suppress ongoing disease when treatment is started after disease onset with mice achieving and maintaining an optimal intake of D-m.
Figure 2. Therapeutic treatment with D-m did not suppress ongoing EAE.

C57BL/6 mice with C-EAE were treated with D-m after disease onset on day 11 p.i. Clinical course of EAE in untreated and 1.1 M D-m-treated mice is shown. N = 5 mice per group. Representative data from 1 of 2 independent repeats is shown.
3.2. D-m treated mice with EAE have reduced immune cell infiltration and increased numbers of Treg cells.
We determined how D-m treatment influenced the immune response in the CNS of mice with C-EAE. D-m-treated mice had fewer CD45+ cells in their CNS during EAE (Fig. 3A). This reduction was primarily caused by decreased number of CD11b+ cells (Fig. 3B). Notably, numbers of CD4+ cells were not impacted. Given that D-m has been shown to induce Treg cells, we quantified the distribution of CD4+ T cells subsets in the CNS during EAE. We found increased frequency of CD4+FoxP3+ Treg cells, including FoxP3+IL-10+ cells (Fig. 3C–E), indicating that D-m confers protection against EAE via a Treg-dependent mechanism. This is in agreement with published findings that D-m suppresses some other autoimmune diseases via Treg-dependent mechanism. It is worth noting however that absolute numbers of Tregs were increased on average but not sufficiently to reach statistical significance (Fig. 3F,G), likely due to the overall reduction in neuroinflammation and therefore immune cell infiltration in D-m-treated mice.
Figure 3. D-m reduced immune cells infiltration in the CNS of mice with EAE.

Untreated and D-m-treated mice with EAE were sacrificed on day 22 p.i. and CNS mononuclear cells were isolated. A) Number of CD45+ cells. B) Number of CD11b+ and CD4+ cells. C) Frequency of FoxP3+, IL-17A+, IL-17A+IFN-γ+, IFN-γ+ and GM-CSF+ cells among CD4+ cells. D) Quantification of IL-10+FoxP3+ cells among CD4+ cells. E) Flow cytometry depicting FoxP3, IL-10, GM-CSF, IFN-γ and IL-17 expression among CD4+ cells. F) Quantification of numbers of CD4+FoxP3+ cells. G) Quantification of numbers of CD4+IL-10+ and CD4+IL-10− cells. Statistical significance was determined by Unpaired Student’s t test. Representative data from 1 of 2 experiments are shown (N = 4 mice per group).
4. Discussion
Our studies show that oral D-m treatment can suppress both C-EAE and RR-EAE. It is especially noteworthy that D-m treatment can suppress RR-EAE, as this is the predominant clinical manifestation of MS (Berkovich, 2016). Moreover, our data show that even after cessation of D-m treatment, mice with RR-EAE did not relapse, indicating that treatment with D-m has a lasting effect. This is particularly relevant as it has been a long-standing goal of MS therapy to prevent relapses, as continued episodes of disease eventually lead to accumulation of permanent neurologic deficits. However, current treatments that reduce MS relapse incidence have several drawbacks. For example, natalizumab treatment has been shown to be effective in reducing frequency of MS relapse (Clerico et al., 2017), but it suffers from safety concerns, as it increased the risk of progressive multifocal leukoencephalopathy (Linda et al., 2009). Lastly, rituximab, an anti-CD20 therapy that depletes B cells was effective at reducing relapses, but notably at the cost of circulating B cells (Ineichen et al., 2020), which may increase the chance of opportunistic infections among other effects. Thus, therapies that can prevent or lessen the frequency of disease relapse are particularly needed in the field. D-m treatment, if proven efficacious in reducing relapse frequency, may offer a safe alternative to current treatment options. Going forward, D-m treatment in RR-EAE initiated after remission are needed to further determine if D-m does indeed have potential in reducing relapses.
Treatment initiated after onset of clinical symptoms were not effective in suppressing disease in the C-EAE model. We attributed this to insufficient intake of D-m during the acute phase of EAE. Moreover, it remains untested whether therapeutic treatments in RR-EAE would suppress disease. It is possible that treatment initiated during remission would be better tolerated by mice, as the temporary cessation of clinical symptoms may allow for normal feeding behavior. Overall, if D-m treatments do have a therapeutic effect, patient compliance is unlikely to be a drawback, as palatable formulations of D-m would likely be available.
Lastly, our characterization of CNS infiltrating immune cells in EAE show a marked increase in the frequency of Treg cells, suggesting that EAE suppression is caused by this increase. This aligns well with both the essential role of Tregs in curtailing CNS neuroinflammation in EAE (Kleinewietfeld and Hafler, 2014), as well as with the anti-inflammatory effects of D-m. Indeed, it has been shown that D-m can direct T cell differentiation toward the Treg lineage by altering energy metabolism during their differentiation, resulting in increased TGF-β activation and thus promoting differentiation into Tregs (Zhang et al., 2017). Increased frequency of Tregs were concomitant with decreases in the number of inflammatory cells, particularly inflammatory myeloid cells, which are the most prevalent cell type causing damage in the CNS in EAE (Croxford et al., 2015; Fife et al., 2000; Giles et al., 2018) and MS (Ramaglia et al., 2019; Zrzavy et al., 2017; Machado-Santos et al., 2018).
5. Conclusion
Our data how that oral D-m treatment suppresses both C-EAE and RR-EAE. Taken together, the above findings suggest that D-m has potential as a safe and low-cost complementary therapy for MS.
Highlights.
D-mannose (D-m) is a naturally occurring epimer of glucose with known immunoregulatory properties.
Oral treatment with D-m suppressed both chronic and relapsing-remitting experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS).
Treatment with D-m reduced numbers of immune cells in the brain and spinal cord of mice with EAE.
There was striking reduction in numbers of myeloid cells present in the central nervous system (CNS) of mice with EAE treated with D-m.
D-m treatment resulted in higher frequency of regulatory T (Treg) cells in the CNS, suggesting that induction of Treg cells was the mechanism of disease suppression.
Funding
This work was supported by the National Multiple Sclerosis Society, Grant ID: PP-1812-33060. This work was also partially supported by the National Institutes of Health T32 training grant (T32AI134646, NIAID).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berkovich RR 2016. Acute Multiple Sclerosis Relapse. Continuum (Minneap Minn), 22(3), pp 799–814. [DOI] [PubMed] [Google Scholar]
- Clerico M, Artusi CA, Liberto AD, Rolla S, Bardina V, Barbero P, Mercanti SF & Durelli L 2017. Natalizumab in Multiple Sclerosis: Long-Term Management. Int J Mol Sci, 18(5), pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M & Becher B 2015. The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity. Immunity, 43(3), pp 502–14. [DOI] [PubMed] [Google Scholar]
- Davis JA & Freeze HH 2001. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta, 1528(2–3), pp 116–26. [DOI] [PubMed] [Google Scholar]
- de Lonlay P & Seta N 2009. The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim Biophys Acta, 1792(9), pp 841–3. [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA & Karpus WJ 2000. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med, 192(6), pp 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N & Mills KH 2010. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol, 162(1), pp 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DA, Duncker PC, Wilkinson NM, Washnock-Schmid JM & Segal BM 2018. CNS-resident classical DCs play a critical role in CNS autoimmune disease. J Clin Invest, 128(12), pp 5322–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard BA, Kamoun M, Ventura E & Rostami A 2000. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol, 68(1), pp 29–37. [DOI] [PubMed] [Google Scholar]
- Ineichen BV, Moridi T, Granberg T & Piehl F 2020. Rituximab treatment for multiple sclerosis. Mult Scler, 26(2), pp 137–152. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M & Hafler DA 2014. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev, 259(1), pp 231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranjcec B, Papes D & Altarac S 2014. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol, 32(1), pp 79–84. [DOI] [PubMed] [Google Scholar]
- Linda H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T & Martin C 2009. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med, 361(11), pp 1081–7. [DOI] [PubMed] [Google Scholar]
- Lowther DE & Hafler DA 2012. Regulatory T cells in the central nervous system. Immunol Rev, 248(1), pp 156–69. [DOI] [PubMed] [Google Scholar]
- Machado-Santos J, Saji E, Troscher AR, Paunovic M, Liblau R, Gabriely G, Bien CG, Bauer J & Lassmann H 2018. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain, 141(7), pp 2066–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayatepek E, Schroder M, Kohlmuller D, Bieger WP & Nutzenadel W 1997. Continuous mannose infusion in carbohydrate-deficient glycoprotein syndrome type I. Acta Paediatr, 86(10), pp 1138–40. [DOI] [PubMed] [Google Scholar]
- McHugh RS & Shevach EM 2002. The role of suppressor T cells in regulation of immune responses. J Allergy Clin Immunol, 110(5), pp 693–702. [DOI] [PubMed] [Google Scholar]
- Michaels EK, Chmiel JS, Plotkin BJ & Schaeffer AJ 1983. Effect of D-mannose and D-glucose on Escherichia coli bacteriuria in rats. Urol Res, 11(2), pp 97–102. [DOI] [PubMed] [Google Scholar]
- O’Connor RA & Anderton SM 2008. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol, 193(1–2), pp 1–11. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, Sheikh-Mohamed S, Legg K, Park C, Rojas OL, Zandee S, Fu F, Ornatsky O, Swanson EC, Pitt D, Prat A, McKee TD & Gommerman JL 2019. Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. Elife, 8( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Thiel C, Rindermann J, DeRossi C, Popovici D, Hoffmann GF, Grone HJ & Korner C 2011. Successful prenatal mannose treatment for congenital disorder of glycosylation-Ia in mice. Nat Med, 18(1), pp 71–3. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, Konkel JE, Nakatsukasa H, Zanvit P, Goldberg N, Chen Q, Sun L, Chen ZJ & Chen W 2017. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med, 23(9), pp 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL & Lassmann H 2017. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain, 140(7), pp 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]


