Abstract
Objective:
To assess the functional role of Hyperpolarization-activated cyclic nucleotide-gated gated channel (HCN) subtypes in the aging bladder phenotype characterized by diminished bladder volume sensation (BVS) with or without the detrusor instability (DI).
Methods:
Expression of HCN subtypes was examined by quantitative RT-PCR and Western blot in aged male Fisher 344 rats (n=15) and young rats (n=15). Nocturnal urination and awake cystometry (CMG) were assessed in presence and absence of a steady state HCN channel blockade achieved with daily oral gavage of vehicle or Ivabradine (HCN blocker) 6mg/kg for 7 days.
Results:
The association of BVS with the age-related downregulation (~30%) of cAMP sensitive HCN1, HCN2 subtypes, and (~50%) upregulation of cAMP insensitive HCN3 subtype is evinced by a doubling in the mean urine volume of nocturnal voids (0.82 ±0.22 mL vs 0.41 ±0.12 mL; n=10; p < 0.05) predicting an age-related rise in micturition volume threshold (p <0.0001) in CMG, raised further by Ivabradine treatment (p <0.0005). Ivabradine also doubled non-voiding contractions (NVC) and maximum voiding pressure (MVP) in young and aged rats, respectively (p <0.0001) to abolish the age-related, innate two -fold elevation in NVC not accompanied with MVP rise in untreated aged rats (p <0.005).
Conclusion:
The age-related HCN downregulation is mechanistically linked to the exhibition of aging bladder phenotype with the manifestation of DI following steady state blockade of HCN channels in Ivabradine treated young rats. The amplification of MVP in aged rats mediated by FDA approved Ivabradine hints at potential repurposing opportunity in detrusor underactivity.
Keywords: Aging Bladder Phenotype, HCN, cAMP, Ivabradine, Bladder volume sensation, non-voiding contraction, Maximum Voiding Pressure
Introduction
It is widely reported that there is an age-related decline in the ability to sense the filled bladder as well as to empty the filled bladder (Chen et al., 2017, Smith et al., 2015), defining characteristics of an aging bladder phenotype. Specifically, detrusor instability (DI) with or without the diminished bladder volume sensitivity (BVS) manifest frequently in the urodynamics of aged patients afflicted with detrusor overactivity, detrusor underactivity, or detrusor hyperactivity with impaired contractility (Chen et al., 2017, Smith et al., 2015) which is also recapitulated to some extent in the cystometry of aged rodents (Hardy et al., 2019, Zhao et al., 2010). But, whether these urodynamic changes are linked to the age-related downregulation of Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Al-Naggar et al., 2019, Mader et al., 2018) is yet to be tested empirically.
We were the first to report that HCN channels, membrane proteins in the soma of bladder afferent nerves, control the electrical excitability of L6-S1 dorsal root ganglia (DRG) (Masuda et al., 2006). Unlike most other voltage-activated ion channels on cell membrane, the pore of HCN channels opens in response to hyperpolarizing voltages rather than depolarizing voltages to conduct a tonic, cationic inward current for maintaining the resting membrane potential close to the firing threshold. In addition, except for HCN3, other HCN subtypes are gated by adenosine-3’,5’-cyclic monophosphate (cAMP) binding to the intracellular carboxy terminal of HCN subtypes (Stieber et al., 2005). Since intracellular levels of cAMP in detrusor smooth muscle, urothelium and interstitial cells of bladder are under the influence of both adrenergic and cholinergic stimulation (Wakabayashi et al., 1995, Wakabayashi et al., 1993), we posited that HCN channels may subserve as a putative “sensor” for the autonomic control of the bladder function (Kashyap et al., 2020, Kashyap et al., 2015). While the cystometry under urethane anesthesia (Masuda et al., 2008) following acute intrathecal administration of HCN channel blocker (ZD7288) demonstrated the functional impact of HCN channels expressed on DRG, understanding the integrated effect of HCN blockade on the mammalian bladder function will be facilitated by a HCN blocker that is potent than ZD7288 (Bucchi et al., 2006) and have improved safety and oral bioavailability.
On that yardstick, Ivabradine is ten times more potent than ZD7288 (Bucchi et al., 2006), was approved as oral drug by Europe medical agency for clinical use in 2005 and exactly10 years later by US FDA for reducing the hospitalization from worsening heart failure. Ivabradine efficiently blocks human HCN1 and HCN4 channels with the IC50 of 2.04 and 2.14 μM, respectively (Novella Romanelli et al., 2016) and the published pharmacokinetic studies in rat (Chen et al., 2015) predicts that the multiple oral doses of Ivabradine 6mg/kg will reach the plasma concentration necessary for a generalized HCN blockade during physiological assays of bladder function. It is likely that our earlier findings on ZD7288 may have prompted the off-label examination of Ivabradine, which reportedly improved the storage symptoms of a bladder outlet obstructed patient refractory to the standard treatment (Stamatiou et al., 2008).
In addition to the expression of HCN channels in the soma of L6-S1 DRG (Masuda et al., 2006), recent studies demonstrate a dense expression of all the four HCN1–4 isoforms in the urothelium, interstitial cells of Cajal, as well as peptidergic and cholinergic nerve endings in the urothelium and detrusor smooth muscle of mammalian urinary bladder (Al-Naggar et al., 2019, Kashyap et al., 2020, Kashyap et al., 2015, Mader et al., 2018). The predominant expression in rat and human bladder (Kashyap et al., 2020, Kashyap et al., 2015, Mader et al., 2018) of HCN1 and HCN4 subtypes with half-activation voltages of −70mv and −100mv, respectively (Novella Romanelli et al., 2016) ensures they are tonically active at the resting membrane potential of −40mV and −14mV for human detrusor and urothelium, respectively (Hashitani and Brading, 2003, Laaris et al., 2012). Indeed, we and others have reported that the tonic activity of HCN channels in the bladder constrains the evoked and spontaneous contractions of isolated rat (Green et al., 1996, Kashyap et al., 2015) and human bladder strips (Kashyap et al., 2020, Mader et al., 2018) which prompted us to hypothesize that the aging bladder phenotype (Chen et al., 2017, Smith et al., 2015) characterized by DI and BVS (Hardy et al., 2019, Zhao et al., 2010) is functionally linked to the age-related downregulation of HCN channels (Al-Naggar et al., 2019, Mader et al., 2018). Here, we tested that hypothesis by first correlating the age dependent HCN expression with the bladder function of unanesthetized rats with or without the pharmacological blockade of HCN channels.
Material and methods
Materials
All the experiments were conducted on aged (n=15) ~28 months male (350–500 gm) and young ~3–4 months male (150–200 g) Fischer F 344 rats (n=15) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. A subset of young (n=5) and aged rats (n=5) received daily oral gavage of vehicle (tap water) or Ivabradine 6mg/kg (AMGEN, Cat No. NDC 55513–800) in volume of 0.5mL for 7 days before physiological experiments.
Nocturnal Urination pattern and awake CMG
Treated or untreated rats were placed in a metabolic cage for 12 hours (from 7 pm to 7 am) with food and water ad libitum for measuring voiding frequency, single void urine volume, and total urine volume.
Awake CMG
First, under isoflurane anesthesia, bladder was exposed through a lower midline abdominal incision for implanting the PE- 50 tubing through the bladder dome. Anesthetized rats were restrained in Ballman cages and allowed to recover from anesthesia and get acclimated to the restraint (1–2h), subsequently saline was infused continuously at the rate of 0.04 mL/min to evoke fluid elimination from the urethral orifice. After an initial stabilization period (60 min), at least three reproducible micturition cycles were recorded during continuous CMG and then the saline infusion was stopped for recording single CMG. Non- voiding contractions (NVC) were defined as abrupt rises in intravesical pressure exceeding 8 cm H2O over the baseline not accompanied by any elimination of fluid from urethra. Volume threshold (VT), defined as the infused fluid volume sufficient to induce the voiding contraction was computed from the product of infusion rate and the average intercontraction time interval between voids during the continuous CMG and from the sum of voided volume and post-void residual volume (PVR) during single CMG. Nocturnal urination and CMG were digitally recorded using Lab chart software (ADInstrument, Milford, MA, USA).
RNA extraction and Real-Time RT-PCR
We harvested the whole bladder from young and aged rat bladders not subjected to CMG and total RNA was isolated with or without separated mucosa (Funahashi et al., 2019) using TRizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. 2 μg of RNA was reverse transcribed into cDNA in 20 μl reaction mix with Superscript III reverse transcriptase kit (Invitrogen) using random hexamers primers. RT-PCR was performed in a 25 μL reaction mix which contained 500 nM of HCN1–4 gene-specific primers (Table 1), 12.5 μL SYBR green mix, 1μL template cDNA in a total volume of 25 μL in RNase-free water. Quantitative RT-PCR was performed using the Mx3000Pâ qPCR detection system and relative expression was determined using threshold Ct value. The thermocycling program consisted of an initial 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. No Template Control was used as negative control and HCN channel expression was normalized to β -Actin using the 2−ΔΔCt method.
Table 1.
Primers used for quantitative RT-PCR.
| Gene | Species | Primer sequence | Accession no. |
|---|---|---|---|
|
| |||
| HCN1 | Rat | F 5’- TCAACGAGGTCTTGGAGGAA -3’ | NM_053375.1 |
| R 5’- ACCAGTGTTCAGATCCTTCTGG -3’ | |||
| HCN2 | Rat | F 5’- GATCATCCACCCCTACAGCG-3’ | NM_053684.1 |
| R 5’- AAGTGATGCCCACAGGGATG-3’ | |||
| HCN3 | Rat | F 5’- GATCATCCACCCCTACAGCG-3’ | NM_053685.1 |
| R 5’-CCACGGGCAGTACTATGAGG -3’ | |||
| HCN4 | Rat | F 5’ CACCCCTACAGCGACTTCAG-3’ | NM_021658.1 |
| R 5’- AAGAAGGTGATGCCCACAGG -3’ | |||
| β-ACTIN | Rat | F 5’- AACCTTCTTGCAGCTCCTCC-3’ | NM_031144.3 |
| R 5’- CGCAGCGATATCGTCATCCA-3’ | |||
F, forward; R, reverse; HCN, hyperpolarization-activated cyclic nucleotide-gated channel.
Western blot analysis
Briefly, bladder tissue was homogenized using CellyticTM MT Mammalian Tissue Lysis Reagent (Sigma, USA) in presence of phenylmethylsulphonyl fluoride (PMSF, 1 mM), dithiothreitol (DTT, 2 mM), and protein inhibitor cocktail (Sigma, USA). After estimating protein content by BCA Protein Assay Kit (Pierce, Rockford, Illinois), 100 μg of denatured proteins were loaded in duplicate onto 10% SDS-polyacrylamide gel and blotted on PVDF membranes using a wet transfer system. After blocking with 5% skimmed milk (2h at RT), membranes were incubated overnight at 4°C with primary antibodies in blocking buffer followed by washing and re-incubation with HRP tagged secondary antibodies for 2h (Santa Cruz Biotechnologies, USA). The blots were developed using luminol (Thermo Scientific, USA) and measured on Versa doc imaging system (Model 4000; Bio Rad, USA) using Alpha Ease FC Stand Alone V. 4.0.0 software for normalizing the HCN band density of β-Actin as an internal control.
Statistical Analysis
The values are presented as mean ± standard deviation. Age-related alterations in the molecular expression between untreated young and aged rats were analyzed by unpaired Student’s t-test and CMG parameters after Ivabradine treatment were analyzed by Two-way ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA). Values at p<0.05 were considered significant.
Results
Aging Bladder Phenotype
The mean nocturnal urine void volume (VV) of untreated aged rats is doubled relative to the untreated young rats (0.82 ±0.22 mL vs 0.41 ±0.12 mL; n=10; p < 0.05; unpaired Student’s t-test Fig. 1A, B) which implies that BVS diminishes with age. Diminished BVS of aged rats is also reflected in the significant rise of micturition volume threshold (VT) (0.45 ±0.09 mL vs 0.22 ±.02 mL, p<0.05; Fig. 1C and D) during continuous awake CMG of aged rats. Age-related rise in DI is evident from the doubling in NVC frequency of untreated aged rats relative to the untreated young rats (0.5 ±0.20/min vs 0.27 ±0.09/min, p<0.05) (Fig. 1E). Lower micturition frequency of aged rats in the nocturnal urination tracing (Fig. 1A) is corroborated by the longer inter contraction interval in CMG tracing (Fig. 1A and C, bar graphs are not shown). Unlike anesthetized CMG, only a negligible PVR elevation in awake CMG of aged rats (0.060 ±0.005 mL; data not shown) implies that the age-dependent rise in VT also reflects a rise in the voided volume and bladder capacity (Fig. 1 C, D). Apart from PVR, we also did not observe any age-related change in the bladder compliance or the maximum voiding pressure (MVP), as described subsequently with vehicle and Ivabradine treated animal groups.
Fig. 1:
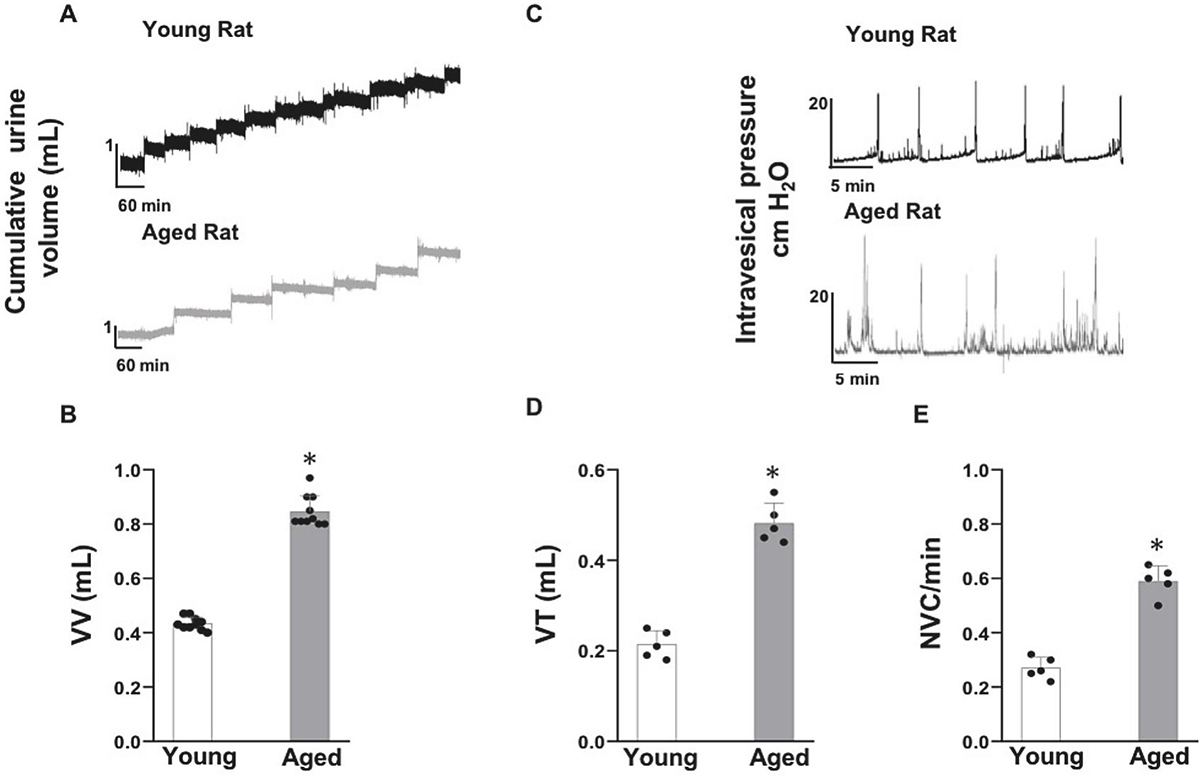
Aging bladder Phenotype exhibited by the nocturnal micturition pattern and awake cystometry (CMG) of young and aged rat: Panel A-B- Nocturnal micturition pattern demonstrated a significant age-associated rise in the mean voided volume (VV) (n=10 each) as graphed in panel B. Panel C-E- Awake continuous CMG of aged male Fisher 344 rats confirmed the aging bladder phenotype with the exhibition of a significant rise in the micturition volume threshold (VT) accompanying a significant rise in the frequency of non-voiding contractions (NVC), a hallmark of detrusor instability (DI) (n=5 each). Values are Mean ± SD; *p < 0.05; Unpaired Student’s t-test. There were no age-related alterations in the CMG parameters of bladder compliance and maximum voiding pressure.
Age-related differential in HCN channel expression
Whole bladder:
We noted a significant downregulation of HCN1 and HCN2 subtypes mRNA (~30%) and an upregulation of HCN3 mRNA (~50%) in aged rats with a negligible change in the expression of HCN4 subtype relative to the housekeeping gene, β-actin (Fig. 2A). Subsequently, we probed SDS-PAGE gel with different HCN channel antibodies using β-actin as the loading control (Fig. 2B) to validate the RT-PCR data with the relative protein expression of HCN1–4 isoforms, as shown in Fig.2C.
Fig. 2:
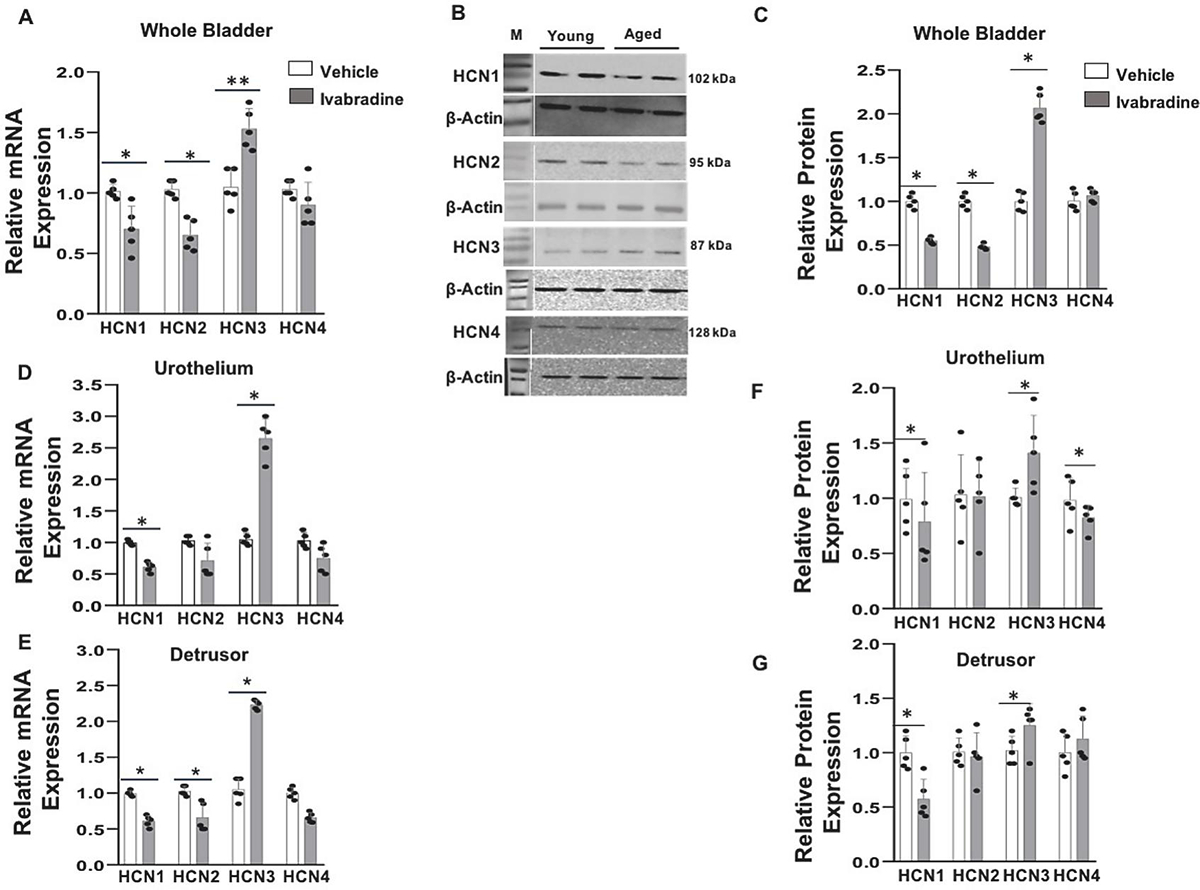
Age-related altered bladder expression of HCN channel subtypes: Expression of HCN channel in young and aged rat bladder was analyzed by quantitative RT-PCR and Western blot analysis. Panel A- Relative mRNA expression of HCN1–4 isoforms was measured with gene specific primers using β-actin as internal control (n=5 each). Panel B- Protein expression of HCN1–4 subtypes was measured in duplicate using β-actin as loading control for each lane in Western blot analysis. Panel C - Relative protein expression was normalized to β-actin in densitometry analysis (n=5 each). *p < 0.05, **p <0.01; unpaired Student’s t-test).: Age related altered region-wise expression of HCN channel subtypes: Panel D, E - Relative mRNA expression of different HCN subtypes in separated urothelium and detrusor layers of young and aged rats was investigated using gene specific primers and β-actin as internal control (n=5). (F, G) Western blot analysis validated the altered expression of HCN1–4 subtypes normalized to β-actin in densitometry analysis (n=5 each). M- Size marker. Values are Mean ± SD; *p < 0.05, **p <0.01; unpaired Student’s t-test)
Mucosa (containing urothelium) vs Detrusor:
we repeated the molecular experiments of the whole bladder on physically separated mucosal tissue to confirm that the upregulation of HCN3 mRNA and protein in both layers is accompanied by a significant downregulation of HCN1 in the mucosa and the downregulation of HCN1 & 2 subtypes in the detrusor (Fig.2D, E). The lack of any HCN2 mRNA expression in the separated mucosa rules out the contamination of detrusor smooth muscle in mucosal specimens and of mucosal contamination in the detrusor specimens. Since HCN4 mRNA expression levels were comparable for young and aged rats, a modest downregulation of HCN4 protein (~10%) in aged urothelium suggests a role for an age-related increase in the protein degradation (Fig. 2F).
HCN channel blockade in young and aged rats
To investigate the mechanistic link between the age-related decline in BVS, and the elevation in DI (Fig. 1) with the age-related downregulation of HCN subtypes (Fig.2), we queried the impact of steady-state HCN channels blocker on the nocturnal micturition and on the awake single CMG of young and rats (Fig. 3). HCN channel blockade was achieved with multiple oral dosing of Ivabradine 6mg/kg for 7 days by daily oral gavage (Chen et al., 2015). Since variable urine production obscured the discernible effect of Ivabradine on the nocturnal urination pattern (data not shown), we relied on the constant bladder infusion of room temperature saline during awake CMG to resolve the effect of age and Ivabradine on bladder function.
Fig. 3: Ivabradine amplifies NVC and MVP in young and aged rat, respectively:
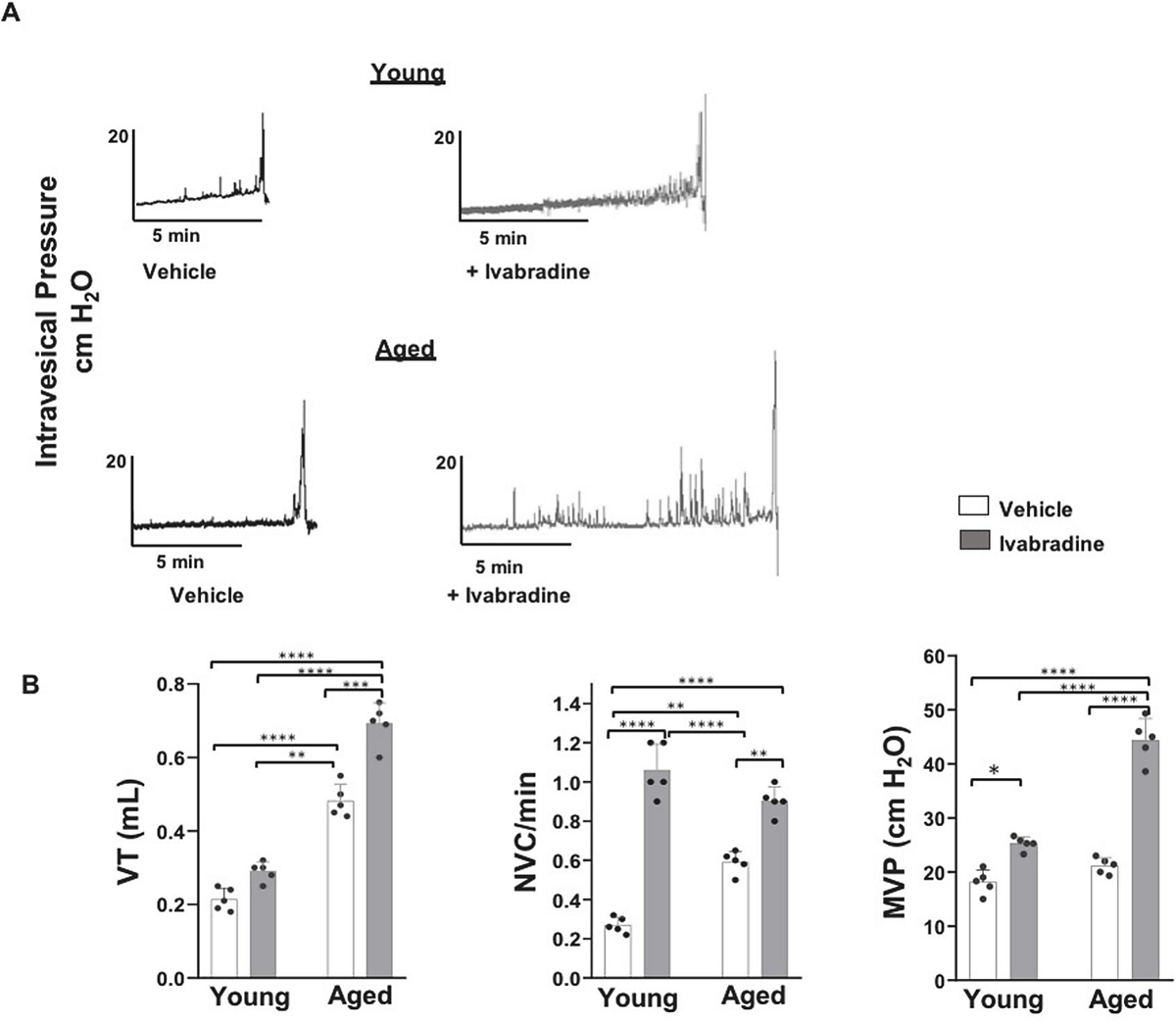
Panel A, B - Representative awake single CMG traces and bar graphs for vehicle and Ivabradine treated (6 mg/kg) young and aged rats (n=5). Ivabradine treatment generates a moderate but a significant rise in VT of aged rats but replaced a two-fold elevation in the frequency of NVC of vehicle treated young and aged rats by a lack of difference while replacing a lack of difference in MVP of vehicle treated young and aged rats by a two-fold elevation in MVP of aged rats (Values are Mean ± SD; ***p <0.0001; Two-way ANOVA followed by Tukey’s multiple comparison test.
The two-fold elevation of NVC in untreated aged rats (Fig.1E) was replicated by the vehicle treated aged rats (Fig.3 B) relative to untreated young and vehicle treated young rats. This age-related difference in the NVC was abolished following Ivabradine treatment in young rats (1.0 ±0.07/min vs 0.9 +/− 0.05/min; p >0.05; Tukey’s test) because of a ~333% rise in the NVC of young rats relative to the vehicle treated young rats (0.27 ±0.09/min; n=5; p<0.0001; Tukey’s test) compared to only a moderate ~55% rise in the Ivabradine treated aged rats (0.58 +/− 0.25/min; p<0.01) relative to the vehicle treated aged rats.
Ivabradine treatment also caused an insignificant rise in the VT of young rats but a significant change in the VT of Ivabradine treated aged rats (0.69 ±0.07 mL vs 0.48 ±0.08 mL; p<0.005; Tukey’s test) relative to vehicle treated aged rats. An insignificant difference between the MVP of vehicle treated aged and young rats (21.16 ±2.5 cm H2O vs 16.22 ±1.8 cm H2O; p >0.05) also reflects the absence of a difference in MVP of untreated aged and young rats (data not shown). However, Ivabradine treatment of aged rats generated nearly a two-fold rise in MVP relative to the vehicle treated aged rats (44 ±7.0 vs 25.0 ±0.47 cm H2O; p<0.0001; Tukey’s test; Fig. 3 A, B) which suggests that Ivabradine mediated withdrawal of the tonic constraint on neuroexocytosis (Huang et al., 2017, Kashyap et al., 2020) amplifies the efferent discharge (Zeng et al., 2012) evoked reflexively by a slight rise in VT of a highly compliant aged bladder.
Discussion
Here, we provide molecular and pharmacological evidence to support that the age-related downregulation of HCN channels at the mRNA and protein levels could be a potential molecular signature for the aging bladder phenotype characterized by a decline in BVS and NVC in untreated aged rats. Moreover, the pharmacological blockade of HCN channels by Ivabradine in young rats makes their NVC comparable to untreated aged rats whereas the combination of age related HCN channel downregulation with the pharmacological blockade of HCN channels generated a significant rise in NVC and VT together with a two-fold rise in MVP of aged rats to support HCN channels as a critical molecule in aging bladder phenotype.
Previously, we and others reported that HCN channels are expressed prejunctionally on peptidergic and cholinergic nerve endings in bladder (Kashyap et al., 2020, Mader et al., 2018) and the tonic activity of HCN channels is known to reduce the input resistance for constant opposition of negative as well as positive fluctuations in the membrane potential- voltage-dependent hysteresis (use-dependent attenuation) (Barthel et al., 2016). The neuronally expressed HCN channels have been shown to tonically constrain the neuroexocytosis of neurotransmitters (Huang et al., 2017, Kashyap et al., 2020) and the upregulation of HCN channels is an established molecular hallmark for the maturation of sensory neurons during post-natal development (Hulme et al., 2020, Kanyshkova et al., 2009). Therefore, the age related downregulation of HCN channels in mammalian bladder reported here and by others (Al-Naggar et al., 2019, Mader et al., 2018) could be a putative molecular signature for the extensively reported neurodegenerative changes in the aging bladder (Al-Naggar et al., 2019, Frazier et al., 2006, Hotta et al., 1995, Lluel et al., 2000, Mizuno et al., 2007, Zhao et al., 2010). Moreover, the neurodegenerative changes of aged rat bladder are coincident with a decrease in the sensitivity of bladder sensory nerves or BVS measured here by elevation of VT in awake cystometry and by a larger mean voided urine volume of aged rats. Awake cystometry not only supported the previously reported age-related decline in BVS (Zhao et al., 2010) but also excluded the concern of polyuria generating the observed rise in the mean voided urine volume of aged rats housed in metabolic cage.
Apart from nerve endings in bladder, HCN channels are also channels expressed post-junctionally on detrusor smooth muscles (DSM)(Xue et al., 2012) (Fig. 4) and based on the evidence published by us and other groups (Al-Naggar et al., 2019, Green et al., 1996, Kashyap et al., 2020, Kashyap et al., 2015, Mader et al., 2018) including spontaneous contractility measured in presence of tetrodotoxin which eliminates the contribution of pre-junctional HCN channels, we posited that the HCN tonically accelerate the decay in the spread of spontaneous action potentials between DSM, a prerequisite for spontaneous detrusor contractions. Since HCN channels in bladder are expressed at pre-junctional and post-junctional sites of the neuroeffector junction, their pharmacological blockade is expected to generate a panoply of excitatory effects as evident from the detrusor excitability ex-vivo due to a post-junctional action of HCN blocker (Al-Naggar et al., 2019, Green et al., 1996, Kashyap et al., 2020, Kashyap et al., 2015, Mader et al., 2018) foreshadowing a dramatic rise in NVC with Ivabradine in vivo whereas the pre-junctional excitatory effect (Fig. 4) of HCN blocker raises the amplitude of evoked detrusor contractions (Kashyap et al., 2020) and a two-fold rise in MVP of Ivabradine treated aged rats. Overall, a reduction in the activity of HCN channels secondary to the age-related downregulation of HCN1 and HCN2 subtypes can be successfully recapitulated in young rats with the multiple oral dosing of Ivabradine, a HCN channel blocker.
Fig. 4: Mechanistic link of HCN and Aging Bladder Phenotype:
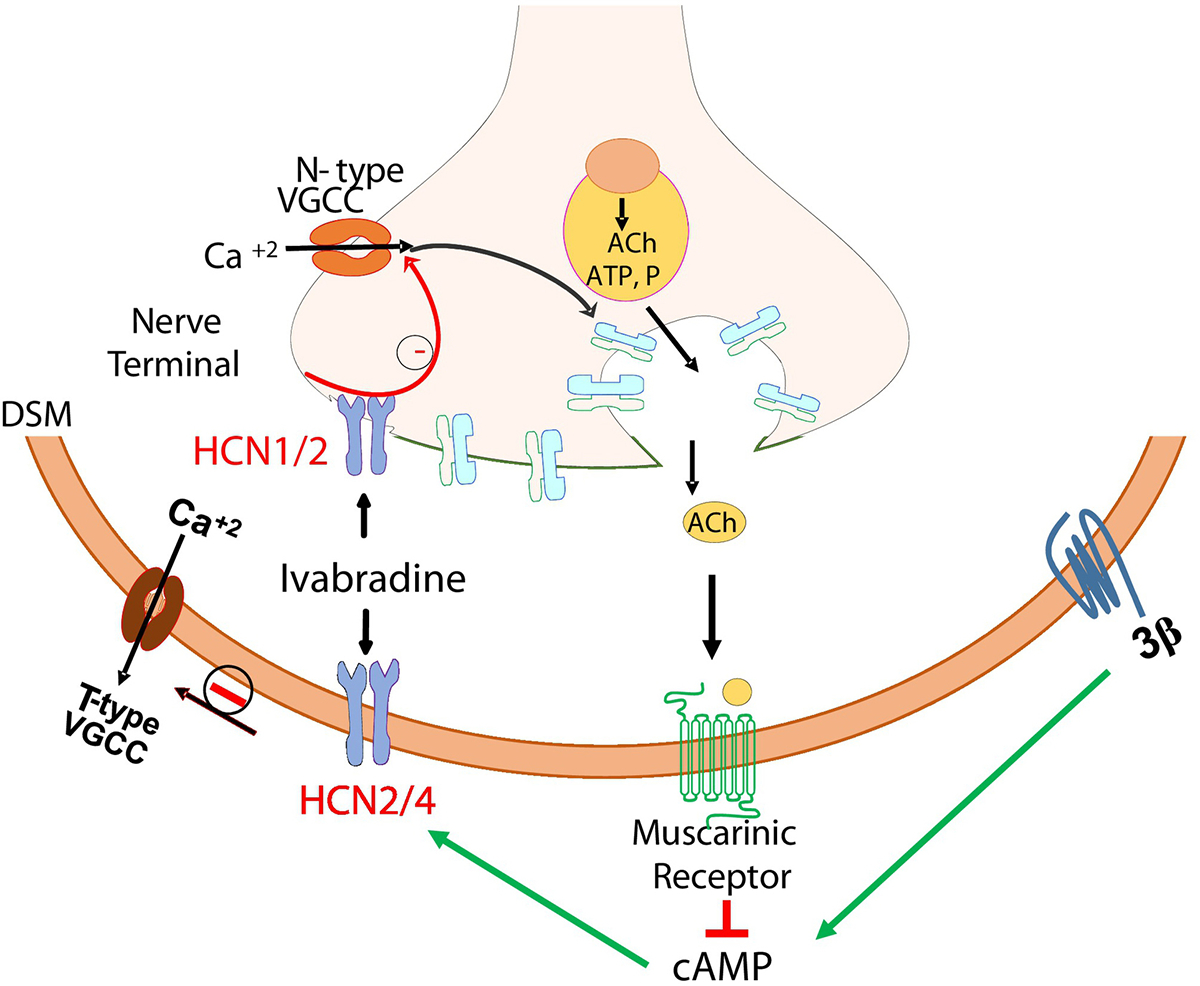
The age-related downregulation of HCN1, HCN2 subtypes and the upregulation of HCN3 subtype is mechanistically linked to the exhibition of aging bladder phenotype by the demonstration of DI in young rats upon steady state blockade of HCN channels by Ivabradine. The amplification of MVP in aged rats implies that HCN channels expressed on detrusor smooth muscle and intramural nerves tonically restrain the detrusor excitability by accelerating the decay of NVC and the coordination of nerve evoked detrusor contraction for bladder emptying. The pharmacological blockade of prejunctional HCN channels by Ivabradine removes the tonic restraint on the activity of N-Type calcium channels to increase the neuroexocytosis, prerequisite for the rise in MVP, whereas the blockade of post-junctional HCN channels removes the tonic restraint on the opening of low-voltage gated calcium channels (T-type), a prerequisite for high voltage gated calcium channels (L-Type) to open and drive myogenic NVC. NVC- Non-Voiding Contraction; VT- Voiding threshold; MVP- Maximum Voiding Pressure.
More than a threefold rise in the NVC of young rats following pharmacological blockade of HCN channels by Ivabradine may also shed light on the coincidence between the age-related downregulation of cAMP sensitive HCN channels and the age-related rise in DI observed here and in other reports (Lluel et al., 2000). When taken together, the association between the downregulation of cAMP sensitive HCN channels and the reported degeneration of intramural adrenergic nerves in aged bladder (Al-Naggar et al., 2019, Frazier et al., 2006) and the known mechanism of action of Ivabradine in heart (Novella Romanelli et al., 2016) makes it plausible for Ivabradine to attenuate the adrenergic mediated detrusor relaxation during storage phase for generating a three-fold rise in the NVC of young rat. The mechanistic findings of a recently reported in vitro study (Zhong et al., 2019) support the putative link because Ivabradine 10 μM suppressed the nerve growth factor induced differentiation of PC12 cells into sympathetic neurons (Zhong et al., 2019). In addition to hampering the adrenergic neurotransmission, the pharmacological blockade of HCN channels could also hamper the activity of bladder sensory neurons which manifest itself as increase in voided volume and VT (BVS) during metabolic cage and awake cystometry, respectively. Indeed, the localized blockade of HCN channels has been demonstrated to dose dependently attenuate the mechanoreceptor sensitivity of rodent bladder (Masuda et al., 2008) and of rodent paw (Miyake et al., 2019). While the injection of Ivabradine [20–50μM] into rat paw suppressed the somatic pain sensitivity, the intrathecal administration of HCN blocker, ZD7288 (Masuda et al., 2008) generated a dose dependent decline in the sensitivity of bladder afferent neurons to raise the intercontractile interval (surrogate of BVS) and even impair the reflex voiding at higher doses through a complete suppression of the BVS. Therefore, multiple lines of evidence reinforce the concept that HCN channels are a key molecular determinants in the age related decline in the BVS (Chai et al., 2000, Smith et al., 2015, Smith et al., 2012) and the rise in DI (Lluel et al., 2000, Zhao et al., 2010). Hence, we inferred that pharmacologically engendered decrease in the functional activity of HCN channels hampers the adrenergic and sensory neurotransmission of voiding reflex and the age-related downregulation of HCN channel gene expression raises DI through the reported degeneration of intramural adrenergic nerves (Al-Naggar et al., 2019, Frazier et al., 2006) while HCN downregulation associated with the degeneration of bladder sensory nerve endings (Hotta et al., 1995, Lluel et al., 2000, Mizuno et al., 2007, Zhao et al., 2010) leads to a lower BVS.
The converse pharmacological action of Ivabradine and Lamotrigine on HCN channels (Bucchi et al., 2006, Kashyap et al., 2020, Mader et al., 2018, Miyake et al., 2019) was used to reverse Ivabradine action on somatic mechanoreceptors by coadministration with Lamotrigine, a HCN activator (Miyake et al., 2019) and to explain the excitatory effect of Ivabradine on NVC, VT, and MVP of disease free young and aged rats (in vivo), reported here. Relative to Ivabradine, the converse mechanism of action for Lamotrigine (chronic dose of 3mg/kg) explains its inhibitory effect on NVC and MVP of spinal cord injured rats (Loutochin et al., 2012) and spontaneously hypertensive rats (Clouse et al., 2012). The activation of HCN channels is responsible for the inhibitory effect of Lamotrigine on the spontaneous (Kashyap et al., 2020, Kashyap et al., 2015, Mader et al., 2018) and the evoked contractions of bladder strips (ex vivo) whereas the blockade of HCN channels generates the excitatory effect on spontaneous and evoked contractions (Green et al., 1996, Kashyap et al., 2020, Kashyap et al., 2015, Mader et al., 2018).
Our molecular investigations performed after separating the mucosa from detrusor uncovered hitherto unreported significant upregulation of HCN3 subtype and the downregulation of HCN1 and HCN2 isoform at the mRNA and protein level in aged rat. HCN channel subtypes vary in their sensitivity to the levels of intracellular cAMP that shifts the voltage dependence to more depolarized potentials for the activation of HCN2 and HCN4 subtypes (Stieber et al., 2005) but not for the HCN3 subtype. The context for the understanding the functional importance of cAMP insensitive HCN3 subtype upregulation by aged bladder in conjunction with the downregulation of HCN1 and 2 subtypes is constructed by the reported expression of cAMP sensitive HCN subtypes in heart (Novella Romanelli et al., 2016) and of cAMP insensitive HCN3 subtype expression by renal-pelvis region (Hurtado and Smith, 2016). While the modulation of heart rate rhythm by autonomic nervous system is critically dependent on the cardiac expression of cAMP sensitive HCN subtypes (Novella Romanelli et al., 2016), the initiation of ureter peristalsis is independent of the autonomic control owing to cAMP insensitive HCN3 subtype expression in the renal-pelvis region (Hurtado and Smith, 2016). Hence, it can be argued that the upregulation of HCN3 subtype (Stieber et al., 2005) in the aged mammalian bladder (Al-Naggar et al., 2019, Mader et al., 2018) represents a counter-response to the cAMP deficit generated by the age-related neurodegeneration of intramural adrenergic nerves (Al-Naggar et al., 2019) and the downregulation of β-adrenergic receptors (Frazier et al., 2006). Since HCN channels are the purported mediator for the homeostasis of bladder function (Al-Naggar et al., 2019), the ratio of HCN3/HCN4 expression (ratio of cAMP insensitive and cAMP sensitive HCN subtypes) in aging human bladder could be a potential molecular fingerprint for the status of adrenergic control during storage phase of aging bladder phenotype.
The pharmacodynamic action of Ivabradine on HCN3 subtype will be determined by the pharmacokinetics of Ivabradine at the 6mg/kg oral dose in rat. Since the expected plasma concentration (Chen et al., 2015) with the 6mg/kg dose of Ivabradine is far lower than the IC50 of 2.5 μM required for HCN3 inhibition (Novella Romanelli et al., 2016), we do not expect the orally administered Ivabradine to act on the HCN3 subtype in upper (Hurtado and Smith) and lower urinary tract (Kashyap et al., 2020, Kashyap et al., 2015). However, the 6mg/kg oral dose of Ivabradine can easily achieve plasma levels <2.5 μM for the half-maximal blockade of HCN1, HCN2 and HCN4 channels expressed on nerve endings in bladder (Novella Romanelli et al., 2016). Hence, we inferred that the Ivabradine action on intramural nerve endings in bladder (Hotta et al., 1995, Mizuno et al., 2007) is the primary mode and a direct action on DSM (Kashyap et al., 2020, Kashyap et al., 2015) only subserves the neurogenic action of Ivabradine for generating the rise in MVP.
Irrespective of the rat age, our findings on the awake cystometry of Ivabradine treated rats reflects the integrated effect of Ivabradine action on the HCN channels expressed at prejunctional and post-junctional sites of neuroeffector junctions in the micturition reflex, however, the relative contribution of Ivabradine action at different sites of the micturition reflex will require future studies where Ivabradine is directly administered at bladder, spinal and supraspinal centers of micturition reflex in addition to the reported ex vivo studies performed at concentrations that were >10–30 times the IC50 of Ivabradine for HCN channels (Aydin et al., 2018). It can be challenging to deduce the contribution of HCN channels in ex vivo studies performed at high concentrations of Ivabradine [30–90μM], as the concentration is beyond the range of IC50 for HCN channels (Bucchi et al., 2006) and non-specific effect of Ivabradine at other channels and receptors could not be ruled out. Moreover, there are inherent limitations in the readout in ex vivo contractility studies for investigating the biological function of HCN channels that are tonically active at the resting membrane potential of urothelium and detrusor. Nonetheless, our in vivo findings were generated with the Ivabradine plasma concentration <2.5 μM within the IC50 range for the inhibition of HCN channels by Ivabradine (Bucchi et al., 2006).
The published findings on 28 months old male Fisher 344 rats voiding at a lower frequency with larger urine volume in each void (Lluel et al., 2000) when housed overnight in metabolic cages were reproduced here. Instead of anesthetized cystometry, the use of awake cystometry in our studies showed a negligible age- related rise in PVR to underscore a critical role for local bladder changes in the manifestation of aging bladder phenotype. The anesthesia can magnify the importance of centrally-mediated adaptive failures to the aging stressors in mice (Hardy et al., 2019) as urethane can mask the homeostatic adaptations in the afferent arm (Maggi and Conte, 1990) while the adaptations in the efferent arm of micturition reflex remain unmasked to preserve the detrusor expulsive strength in aged mice (Hardy et al., 2019). A dominant role for extra-bladder factors in the aging bladder phenotype is also contested by the alleviation of urge incontinence in patients with a mean age of 65 years following intradetrusor injection of botulinum toxin or of plasmid encoding potassium channels targeting the detrusor smooth muscle hyperpolarization (Rovner et al., 2020).
Overall, we used pharmacological blockade of HCN channels to probe the functional implications of age-related downregulation of HCN channels in bladder (Al-Naggar et al., 2019, Mader et al., 2018). Our findings also provide a mechanistic explanation for the therapeutic benefit of Ivabradine on voiding and storage symptoms (Stamatiou et al., 2008) through a use-dependent attenuation of HCN channel function (Barthel et al., 2016) instead of muscarinic agonist like Bethanechol (Singh et al., 2020). Therefore, findings support the repurposing of Ivabradine for increasing the strength of detrusor contraction in DU patients with or without bladder outlet obstruction.
Conclusions
The age-related downregulation of HCN subtypes is mechanistically linked to the exhibition of aging bladder phenotype by the demonstration of DI in young rats following the steady-state blockade of HCN channels by Ivabradine. The Ivabradine mediated rise in MVP of aged rats highlights the therapeutic potential of Ivabradine for its repurposing on detrusor underactivity.
Acknowledgements
This project was supported by a grant awarded by NIA- AG062971
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Naggar IM, Hardy CC, Taweh OG, Grabauskas T, Mulkey DK, Kuchel GA, et al. HCN as a Mediator of Urinary Homeostasis: Age-Associated Changes in Expression and Function in Adrenergic Detrusor Relaxation. J Gerontol A Biol Sci Med Sci. 2019;74:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin HR, Turgut H, Kurt A, Sahan R, Kalkan OF, Eren H, et al. Ivabradine inhibits carbachol-induced contractions of isolated rat urinary bladder. Adv Clin Exp Med. 2018;27:893–7. [DOI] [PubMed] [Google Scholar]
- Barthel L, Reetz O, Strauss U. Use Dependent Attenuation of Rat HCN1-Mediated Ih in Intact HEK293 Cells. Cell Physiol Biochem. 2016;38:2079–93. [DOI] [PubMed] [Google Scholar]
- Bucchi A, Tognati A, Milanesi R, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol. 2006;572:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai TC, Andersson KE, Tuttle JB, Steers WD. Altered neural control of micturition in the aged F344 rat. Urol Res. 2000;28:348–54. [DOI] [PubMed] [Google Scholar]
- Chen SF, Jiang YH, Kuo HC. Urinary biomarkers in patients with detrusor underactivity with and without bladder function recovery. Int Urol Nephrol. 2017;49:1763–70. [DOI] [PubMed] [Google Scholar]
- Chen XP, Zheng HT, Cai WW, Li MK, Zhang JW, Hu J. The Effect of Silibinin on the Pharmacokinetics of Ivabradine and N-Desmethylivabradine in Rats. Pharmacology. 2015;96:107–11. [DOI] [PubMed] [Google Scholar]
- Clouse AK, Jugus MJ, Eisennagel SH, Laping NJ, Westfall TD, Thorneloe KS. Voltage-gated Na+ channel blockers reduce functional bladder capacity in the conscious spontaneously hypertensive rat. Urology. 2012;79:1410 e1–6. [DOI] [PubMed] [Google Scholar]
- Frazier EP, Schneider T, Michel MC. Effects of gender, age and hypertension on beta-adrenergic receptor function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:300–9. [DOI] [PubMed] [Google Scholar]
- Funahashi Y, Takahashi R, Mizoguchi S, Suzuki T, Takaoka E, Ni J, et al. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol. 2019;597:2063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ME, Edwards G, Kirkup AJ, Miller M, Weston AH. Pharmacological characterization of the inwardly-rectifying current in the smooth muscle cells of the rat bladder. Br J Pharmacol. 1996;119:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CC, Keilich SR, Harrison AG, Knight BE, Baker DS, Smith PP. The aging bladder phenotype is not the direct consequence of bladder aging. Neurourol Urodyn. 2019;38:2121–9. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003;140:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta H, Morrison JF, Sato A, Uchida S. The effects of aging on the rat bladder and its innervation. Jpn J Physiol. 1995;45:823–36. [DOI] [PubMed] [Google Scholar]
- Huang Z, Li G, Aguado C, Lujan R, Shah MM. HCN1 channels reduce the rate of exocytosis from a subset of cortical synaptic terminals. Sci Rep. 2017;7:40257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme AJ, McArthur JR, Maksour S, Miellet S, Ooi L, Adams DJ, et al. Molecular and Functional Characterization of Neurogenin-2 Induced Human Sensory Neurons. Front Cell Neurosci. 2020;14:600895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado R, Smith CS. Hyperpolarization-activated cation and T-type calcium ion channel expression in porcine and human renal pacemaker tissues. J Anat. 2016;228:812–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, et al. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci. 2009;29:8847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap M, Singh N, Yoshimura N, Chermansky C, Tyagi P. Constitutively active HCN channels constrain detrusor excitability and modulate evoked contractions of human bladder. Am J Clin Exp Urol. 2020;8:163–76. [PMC free article] [PubMed] [Google Scholar]
- Kashyap M, Yoshimura N, Smith PP, Chancellor M, Tyagi P. Characterization of the role of HCN channels in beta3-adrenoceptor mediated rat bladder relaxation. Bladder (San Franc). 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Sun Y, Chai T. Characterization of resting membrane potential in rat and human bladder urothelial cells. J Urol. 2012;187:e202. [Google Scholar]
- Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, et al. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R964–72. [DOI] [PubMed] [Google Scholar]
- Loutochin O, Al Afraa T, Campeau L, Mahfouz W, Elzayat E, Corcos J. Effect of the anticonvulsant medications pregabalin and lamotrigine on urodynamic parameters in an animal model of neurogenic detrusor overactivity. Neurourol Urodyn. 2012;31:1197–202. [DOI] [PubMed] [Google Scholar]
- Mader F, Muller S, Krause L, Springer A, Kernig K, Protzel C, et al. Hyperpolarization-Activated Cyclic Nucleotide-Gated Non-selective (HCN) Ion Channels Regulate Human and Murine Urinary Bladder Contractility. Front Physiol. 2018;9:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Conte B. Effect of urethane anesthesia on the micturition reflex in capsaicin-treated rats. J Auton Nerv Syst. 1990;30:247–51. [DOI] [PubMed] [Google Scholar]
- Masuda N, Hayashi Y, Matsuyoshi H, Chancellor MB, de Groat WC, Yoshimura N. Characterization of hyperpolarization-activated current (Ih) in dorsal root ganglion neurons innervating rat urinary bladder. Brain Res. 2006;1096:40–52. [DOI] [PubMed] [Google Scholar]
- Masuda N, Masuda H, Matsuyoshi H, Chancellor MB, de Groat WC, Yoshimura N. Effects of intrathecal injection of a hyperpolarization-activated channel (Ih) inhibitor ZD7288 on bladder function in urethane-anesthetized rats. Neurourol Urodyn. 2008;27:838–44. [DOI] [PubMed] [Google Scholar]
- Miyake S, Higuchi H, Honda-Wakasugi Y, Fujimoto M, Kawai H, Nagatsuka H, et al. Locally injected ivabradine inhibits carrageenan-induced pain and inflammatory responses via hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. PLoS One. 2019;14:e0217209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno MS, Pompeu E, Castelucci P, Liberti EA. Age-related changes in urinary bladder intramural neurons. Int J Dev Neurosci. 2007;25:141–8. [DOI] [PubMed] [Google Scholar]
- Novella Romanelli M, Sartiani L, Masi A, Mannaioni G, Manetti D, Mugelli A, et al. HCN Channels Modulators: The Need for Selectivity. Curr Top Med Chem. 2016;16:1764–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner E, Chai TC, Jacobs S, Christ G, Andersson KE, Efros M, et al. Evaluating the safety and potential activity of URO-902 (hMaxi-K) gene transfer by intravesical instillation or direct injection into the bladder wall in female participants with idiopathic (non-neurogenic) overactive bladder syndrome and detrusor overactivity from two double-blind, imbalanced, placebo-controlled randomized phase 1 trials. Neurourol Urodyn. 2020;39:744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Mizoguchi S, Suzuki T, Zabbarova I, Ikeda Y, Kanai A, et al. Excitatory effect of acotiamide on rat and human bladder: Implications for underactive bladder treatment. Life Sci. 2020;258:118179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PP, Chalmers DJ, Feinn RS. Does defective volume sensation contribute to detrusor underactivity? Neurourol Urodyn. 2015;34:752–6. [DOI] [PubMed] [Google Scholar]
- Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol. 2012;302:R577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatiou K, Heretis I, Skoumbourdis E. Does ivabradine exhibit a role in the reduction of bladder overactivity? Int Urol Nephrol. 2008;40:333–4. [DOI] [PubMed] [Google Scholar]
- Stieber J, Stockl G, Herrmann S, Hassfurth B, Hofmann F. Functional expression of the human HCN3 channel. J Biol Chem. 2005;280:34635–43. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Kojima Y, Makiura Y, Tomoyoshi T, Maeda T. Acetylcholinesterase-positive afferent axons in mucosa of urinary bladder of adult cats: retrograde tracing and degeneration studies. Histol Histopathol. 1995;10:523–30. [PubMed] [Google Scholar]
- Wakabayashi Y, Makiura Y, Tomoyoshi T, Kitahama K, Maeda T. Immuno-electron microscopic study of tyrosine hydroxylase in the cat urinary bladder and proximal urethra. J Auton Nerv Syst. 1993;44:243–52. [DOI] [PubMed] [Google Scholar]
- Xue L, Li Y, Han X, Yao L, Yuan J, Qin W, et al. Investigation of hyperpolarization-activated cyclic nucleotide-gated channels in interstitial cells of Cajal of human bladder. Urology. 2012;80:224 e13–8. [DOI] [PubMed] [Google Scholar]
- Zeng J, Pan C, Jiang C, Lindstrom S. Cause of residual urine in bladder outlet obstruction: an experimental study in the rat. J Urol. 2012;188:1027–32. [DOI] [PubMed] [Google Scholar]
- Zhao W, Aboushwareb T, Turner C, Mathis C, Bennett C, Sonntag WE, et al. Impaired bladder function in aging male rats. J Urol. 2010;184:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong LY, Fan XR, Shi ZJ, Fan ZC, Luo J, Lin N, et al. Hyperpolarization-Activated Cyclic Nucleotide-Gated Ion (HCN) Channels Regulate PC12 Cell Differentiation Toward Sympathetic Neuron. Front Cell Neurosci. 2019;13:415. [DOI] [PMC free article] [PubMed] [Google Scholar]


