Abstract
Objectives
Hepatitis B virus (HBV) can reactivate among rheumatology patients initiating tocilizumab or tofacitinib. HBV screening is recommended by the Centers for Disease Control and Prevention (CDC), the American Association for the Study of Liver Diseases (AASLD), and the Canadian Rheumatology Association but is not explicitly recommended by the American College of Rheumatology.
Methods
We conducted a cross-sectional study to characterize HBV screening practices for adult rheumatology patients initiating tocilizumab or tofacitinib before December 31, 2018, in the Greater Boston area. We classified appropriate HBV screening patterns prior to tocilizumab or tofacitinib (i.e., HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb]) as: complete (all 3 tested), partial (any 1 or 2 tests), or none. We determined the frequency of inappropriate HBV testing (HBeAg, anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb) and used multivariable regression to assess factors associated with complete HBV screening.
Results
Among 678 subjects initiating tocilizumab, 194 (29%) completed appropriate HBV screening, 307 (45%) had partial screening, and 177 (26%) had none. Among 391 subjects initiating tofacitinib, 94 (24%) completed appropriate HBV screening, 195 (50%) had partial screening, and 102 (26%) had none. Inappropriate testing was performed in 22% of subjects. Race was associated with complete HBV screening (white versus non-white, OR 0.74; 95%CI: 0.57-0.95) while prior immunosuppression was not (csDMARDs, OR 1.05, 95%CI: 0.72-1.55; bDMARDs, OR 0.73, 95%CI: 0.48-1.12).
Conclusion
Patients initiating tocilizumab or tofacitinib are infrequently screened for HBV despite recommendations from AASLD and CDC.
Keywords: hepatitis B virus, Monoclonal antibodies, Januse kinase inhibitors, rheumatoid arthritis, latent infection, vasculitis
Introduction
Reactivation of hepatitis B virus (HBV) is characterized by increased HBV replication, which can cause acute liver failure and death among people taking immunosuppressive drug therapy(1). Individuals with past HBV infection have persistent HBV DNA in hepatocytes even without circulating HBV surface antigen (HBsAg) or a detectable serum HBV DNA. They remain at risk of HBV reactivation when treated with immunosuppressive drug therapy, despite having HBV surface antibody (HBsAb)(2). For adults with past or active HBV infection, HBV reactivation risk varies based on the immunosuppressive drug and the HBV serologic profile. HBV reactivation occurs in >25% of people with active HBV infection (HBsAg-positive) undergoing chemotherapy for cancer(2,3). In people with past HBV infection (HBsAg-negative with anti-HBV core antibody [anti-HBcAb]), HBV reactivation occurs in 3-41% with rituximab and up to 5% with tumor necrosis factor-alpha (TNF) inhibitors(4). HBV reactivation can be prevented with antiviral therapy; patients who are treated prophylactically have fewer episodes of hepatitis, liver failure, and interruptions in immunosuppression(3).
Despite the risk of serious illness and the opportunity for prevention, many people are not screened for HBV prior to initiating immunosuppressive drug therapy(5). Guidelines from the Centers for Disease Control and Prevention (CDC) and the American Association for the Study of Liver Diseases (AASLD) recommend screening for HBsAg, anti-HBcAb, and HBsAb prior to initiation of immunosuppressive drug therapy, “including chemotherapy, organ transplantation, and immunosuppression for rheumatologic diseases or gastroenterological disorders(6,7).” The Canadian Rheumatology Association (CRA) also recommends HBV screening prior to biologic therapy(8). In contrast, the American College of Rheumatology (ACR) guidelines for management of rheumatoid arthritis do not explicitly recommend HBV screening prior to immunosuppression, though they provide guidance on HBV DNA monitoring for those with diagnosed HBV(9).
Without uniform recommendations for HBV screening, clinical practice may vary by the specific type of immunosuppressive drug and the perceived risks of HBV reactivation. HBV screening practices have been previously described for TNF inhibitors and rituximab(10–12), but practices may differ with more recently approved agents, such as IL-6 receptor antagonists and oral Janus kinase (JAK) inhibitors. Recent reports suggest that the IL-6 receptor antagonist, tocilizumab, has an equivalent HBV reactivation risk to other biologic agents (i.e., up to 5% in past HBV infection)(13,14). HBV reactivation has also been observed in post-marketing studies of the oral JAK inhibitor, tofacitinib, which remains the first-line JAK inhibitor of choice in the treatment of rheumatoid arthritis(14). These two medication classes are being used with increasing frequency as first-line agents for an expanding number of indications in rheumatic diseases and other disorders, but HBV screening practices are not well described. We previously reported the prevalence of resolved and active HBV infection in this population(15). In this analysis, we sought to characterize HBV screening among people initiating tocilizumab or tofacitinib for rheumatologic indications and to assess factors associated with complete HBV screening in a large academic U.S. healthcare system.
Materials and Methods
We conducted a retrospective cross-sectional study of all adult patients who initiated tocilizumab or tofacitinib prior to December 31, 2018, while engaged in rheumatology care within the Mass General Brigham health system (MGB) in the Greater Boston area. We studied clinical practices at two quaternary care hospitals, three community hospitals, and their affiliated outpatient clinics.
We identified eligible patients using the Research Patient Data Registry (RPDR), a comprehensive clinical care database of electronic data. This study was approved by MGB Human Research Committee (institutional review board) as exempt; informed consent was waived as the research involved retrospective use of information from healthcare operations and medical records (protocol #2019P000836).
Tocilizumab and tofacitinib were approved for use in the U.S. on January 11, 2010, and November 6, 2012, respectively. We queried the RPDR database to identify all adult patients who received at least one medication order or prescription for tocilizumab or tofacitinib from the time of their approved use until December 31, 2018. We included patients >18 years old with at least one outpatient rheumatology encounter in the MGB health system 90 days prior to or 30 days following the initiation of tocilizumab or tofacitinib. We stratified patients based on their past history of receiving conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs), or neither (i.e., prior to tocilizumab or tofacitinib).
We classified appropriate HBV screening patterns into three mutually exclusive categories: complete (HBsAg, total anti-HBcAb, and HBsAb), partial (any 1 or 2 of these tests), or none (zero of these tests). We classified inappropriate HBV testing as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb. For both tocilizumab and tofacitinib, we determined the frequency of inappropriate HBV testing among subjects in each category of appropriate HBV screening (complete, partial, and none).
We used the RPDR to extract demographic information, medical encounters, laboratory data, and medications for all subjects between January 1, 1995, and December 31, 2018. To determine the primary indication for tocilizumab and tofacitinib, we identified the most commonly coded principal diagnosis for any rheumatology encounter in the RPDR dataset for each patient. We used accepted International Classification of Diseases (ICD)-9 and ICD-10 codes for rheumatoid arthritis (ICD-9 714, except 714.3; ICD-10 M05-M07), psoriatic arthritis (ICD-9 696; ICD-10 L40), vasculitis (ICD-9 446.5; ICD-10 M31.5), and other arthritis (ICD-9 713, 714.3, 715-719; ICD-10 M08, M12-M25). For subjects whose charted diagnosis differed from common rheumatologic indications, we conducted a manual chart review to confirm the primary indication for drug therapy.
We determined the date of HBV laboratory testing in reference to the date of the earliest prescription for tocilizumab or tofacitinib. We classified subjects as having completed HBV screening if testing occurred any time before or up to thirty days following the date of the first tocilizumab or tofacitinib prescription. We extracted the site of care and the prescribing clinician for tocilizumab and tofacitinib. For a randomly selected 5% of patients, we used chart review to confirm that the RPDR search strategy produced a relevant study population and that laboratory data captured in RPDR accurately reflected the information available to rheumatology clinicians initiating tocilizumab and tofacitinib.
We report continuous variables as means and standard deviations (SD) and categorical variables as frequencies and percentages. We used chi-square tests to compare the proportion of subjects with complete, incomplete, absent, and inappropriate HBV screening among those receiving tocilizumab or tofacitinib. We used multivariable logistic regression to determine factors associated with complete appropriate HBV screening. We accounted for provider-level and site-level clustering using generalized estimating equations. We adjusted for covariates thought to be relevant a priori, including sex, drug (tocilizumab or tofacitinib), race, and prior receipt of bDMARD or csDMARD therapy. In these analyses, subjects who received both tocilizumab and tofacitinib were categorized by the drug they received first. We used a p-value of <0.05 as the predefined threshold of statistical significance. We used SAS 9.4 (SAS Institute, Cary, North Carolina) using the GENMOD procedure for generalized estimating equations.
Results
We identified 913 subjects initiating tocilizumab and 630 subjects initiating tofacitinib at MGB before December 31, 2018 (Figure 1). After excluding subjects <18 years (tocilizumab, n=2; tofacitinib, n=3) and those without outpatient rheumatology encounters (tocilizumab, n=233; tofacitinib, n=236), the final study population included 678 subjects initiating tocilizumab and 391 subjects initiating tofacitinib.
Figure 1: Flowchart of HBV screening among patients treated with tocilizumab and tofacitinib.
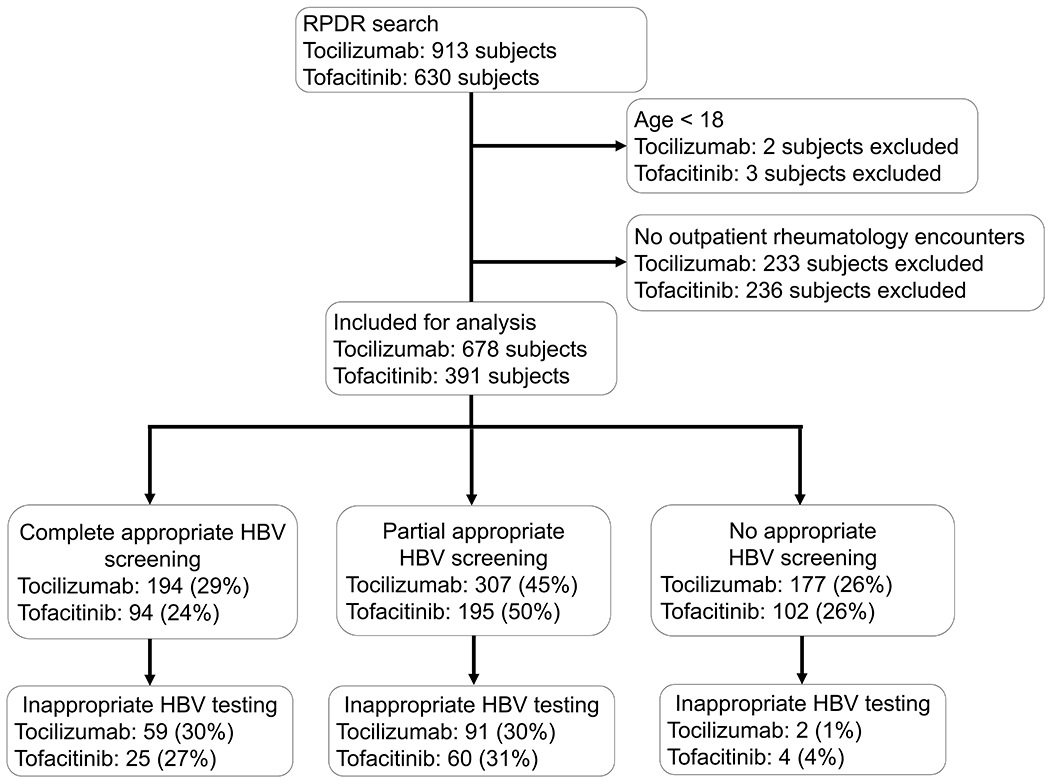
Abbreviations: RPDR = Research Patient Data Registry; HBV = Hepatitis B virus.
RPDR query identified all patients receiving tocilizumab and tofacitinib from the time of their approval to December 31, 2018. The cohort for this analysis included adult patients with outpatient rheumatology encounters within 90 days before or 30 days after their first tocilizumab or tofacitinib prescription. Complete appropriate screening includes all three of HBV surface antigen, HBV surface antibody, and total anti-HBV core antibody; partial appropriate screening includes any 1 or 2 of these tests; no appropriate screening includes zero of these tests. Inappropriate HBV testing includes HBV e-antigen, anti-HBV core IgM, or HBV DNA without a positive HBV surface antigen or total anti-HBV core antibody.
Mean age was 61 (SD, 16) years (tocilizumab) and 60 (SD, 13) years (tofacitinib), and the majority of patients were female and non-Hispanic white (Table 1). Nearly all subjects (89%) were followed in our health system for at least a year prior to their first prescription of tocilizumab or tofacitinib. The most common indication was rheumatoid arthritis (tocilizumab: 362 [53%]; tofacitinib: 301 [77%]). Most subjects were previously treated with csDMARDs or bDMARDs; 152 (22%) of those initiated on tocilizumab and 16 (4%) on tofacitinib had received no prior treatment with csDMARDs or bDMARDs.
Table 1:
Baseline characteristics of subjects initiating tocilizumab or tofacitinib at MGB prior to December 31, 2018*
| Variable | Tocilizumab (n=678) | Tofacitinib (n=391) |
|---|---|---|
| Age, years, mean (standard deviation) | 61 (16) | 60 (13) |
| Female, n (%) | 529 (78%) | 343 (88%) |
| Race/Ethnicity, n (%) | ||
| White, non-Hispanic | 568 (84%) | 328 (84%) |
| Black, non-Hispanic | 32 (5%) | 17 (4%) |
| Hispanic | 25 (4%) | 19 (5%) |
| Asian | 21 (3%) | 9 (2%) |
| Other | 17 (3%) | 7 (2%) |
| Not reported | 15 (2%) | 11 (3%) |
| Diagnosis, n (%) | ||
| Rheumatoid arthritis | 362 (53%) | 301 (77%) |
| Vasculitis | 151 (22%) | 2 (1%) |
| Other inflammatory arthritis | 119 (18%) | 44 (11%) |
| Psoriatic arthritis | 9 (1%) | 24 (6%) |
| Other diagnosis | 37 (5%) | 20 (5%) |
| Past Medication History, n (%) | ||
| csDMARD only | 88 (13%) | 44 (11%) |
| bDMARD only | 53 (8%) | 51 (13%) |
| csDMARD and bDMARD | 385 (57%) | 280 (72%) |
| No prior csDMARD or bDMARD | 152 (22%) | 16 (4%) |
Abbreviations: MGB=Mass General Brigham; csDMARD=conventional synthetic disease-modifying anti-rheumatic drug; bDMARD=biologic disease-modifying anti-rheumatic drug
Of the 678 subjects initiating tocilizumab, 194 (29%) underwent complete appropriate HBV screening, 307 (45%) underwent partial appropriate HBV screening, and 177 (26%) had no appropriate HBV screening (Figure). Of the 152 subjects who received tocilizumab without a prior history of csDMARD or bDMARD therapy, 51 (33%) underwent complete appropriate HBV screening, 47 (31%) underwent partial appropriate HBV screening, and 54 (36%) had no appropriate HBV screening. In the remaining 526 subjects who received tocilizumab with a history of csDMARD and/or bDMARD therapy, 143 (27%) had complete appropriate HBV screening, 260 (49%) had partial appropriate HBV screening, and 123 (23%) had no appropriate HBV screening. Compared to subjects without prior immunosuppression, those with a history of csDMARD and/or bDMARD use were no more likely to undergo complete appropriate HBV screening (p=0.313), but they more frequently had partial appropriate HBV screening (p<0.001). Of the 152 (22%) subjects receiving tocilizumab who met our definition for inappropriate HBV testing, 59 had complete appropriate HBV screening while 91 had undergone partial appropriate HBV screening, and 2 had no appropriate HBV screening (Figure).
Of the 391 subjects initiating tofacitinib, 94 (24%) underwent complete appropriate HBV screening, 195 (50%) experienced partial appropriate HBV screening, and 102 (26%) had no appropriate HBV screening (Figure). Only 16 subjects received tofacitinib without prior csDMARD or bDMARD therapy. Of the 89 (23%) subjects with inappropriate HBV testing, 25 had undergone complete appropriate HBV screening while 60 had undergone partial appropriate HBV screening, and 4 had no appropriate HBV screening (Figure 1).
In a multivariable analysis, we used generalized estimating equations to account for provider- and hospital-level clustering while adjusting for sex, race/ethnicity, drug (tocilizumab or tofacitinib), and use of prior csDMARDs or bDMARD (Table 2). White subjects were less likely to undergo complete HBV screening (OR 0.74, 95%CI: 0.57-0.95) compared to non-white subjects. Neither tocilizumab nor tofacitinib was more associated with complete appropriate HBV screening (tocilizumab versus tofacitinib OR: 1.08, 95%CI: 0.87-1.34). Subjects with prior use of immunosuppression were not more likely to have complete appropriate HBV screening (csDMARD only: OR 1.05, 95%CI: 0.72-1.55; bDMARD with or without prior csDMARD: OR 0.73, 95%CI: 0.48-1.12).
Table 2:
Factors associated with complete appropriate HBV screening prior to receipt of tocilizumab or tofacitinib in multivariable logistic regression using generalized estimating equations*
| Variable | Adjusted OR (95%CI) |
|---|---|
| Drug Therapy | |
| Tofacitinib | Reference |
| Tocilizumab | 1.08 (0.87, 1.34) |
| Sex | |
| Female | Reference |
| Male | 0.93 (0.70, 1.24) |
| Race/Ethnicity | |
| Non-white | Reference |
| White | 0.74 (0.57, 0.95) † |
| Immunosuppression History | |
| No prior csDMARD or bDMARD | Reference |
| Prior csDMARD only | 1.05 (0.72, 1.55) |
| Prior bDMARD (with or without prior csDMARD) | 0.73 (0.48, 1.12) |
Complete appropriate screening includes all three of HBV surface antigen, HBV surface antibody, and total anti-HBV core antibody. Abbreviations: csDMARD = conventional synthetic disease-modifying anti-rheumatic drug; bDMARD = biologic disease-modifying anti-rheumatic drug
p<0.05
Discussion
HBV reactivation is a preventable complication of immunosuppressive drug therapy, yet fewer than one third of rheumatology patients initiating tocilizumab or tofacitinib at a large health system completed appropriate HBV screening. We focused on these two agents as they are being used with increasing frequency as first-line agents for an expanding number of rheumatologic indications. The majority of patients had previously received other csDMARD or bDMARD therapy, but they were no more likely to have complete appropriate HBV screening than those without prior use of immunosuppression. Twenty-two percent of the subjects in this study underwent inappropriate HBV testing, frequently without completing appropriate HBV screening.
The first important finding of this study is the low frequency of pre-treatment HBV screening. While we focused on tocilizumab or tofacitinib, we noted that most subjects had previously received other csDMARD or bDMARD agents, which have been definitively associated with HBV reactivation(14). These findings suggest that HBV screening is too infrequent among patients with rheumatic diseases on immunosuppressive therapy regardless of the immunosuppressive medications. An analysis of a national registry of patients with rheumatologic conditions similarly found that only 28.8% of patients had completed HBsAg and total anti-HBcAb testing prior to immunosuppression with any DMARD(5). Perhaps not unexpectedly, these screening patterns conform more with recommendations from the ACR, which do not explicitly stipulate universal HBV screening, compared with guidelines from CDC or AASLD that recommend universal HBV screening for patients initiating immunosuppressive drug therapy or the CRA which recommends screening prior to certain medications. Furthermore, the FDA package inserts of tocilizumab and tofacitinib do not include specific recommendations for HBV screening, even though HBV reactivation has been observed in post-marketing studies of both IL-6 receptor antagonists and oral JAK inhibitors(13,16). Consistent and explicit recommendation for HBV screening across societal recommendations could improve HBV screening frequency.
A second important finding is that 22% of the subjects underwent inappropriate HBV testing, frequently in the absence of completing appropriate HBV screening. An analysis of HBV laboratory tests conducted among all patients in a health system in Canada found that >70% of patients who underwent testing with HBeAg, HBV e-antibody, or HBV DNA did not have evidence of past or present infection(17). Those investigators estimated that 30% of HBV testing costs could be saved in their health system if clinicians only pursued HBeAg, HBV e-antibody, and HBV DNA testing based on positive results of HBsAg or total anti-HBcAb. Relative to the lifetime costs of DMARD therapy, the costs of one-time HBV tests are negligible. However, we found that inappropriate HBV testing was often conducted in lieu of, rather than in addition to, HBsAg or total anti-HBcAb testing. We worry that clinicians may be erroneously reassured by negative HBeAg, HBcAb IgM, or HBV DNA results without accurately confirming the absence of latent infection in their patients. Clinical decision support systems may help to reduce inappropriate laboratory testing (18).
Our findings should be interpreted within limitations of the study design. The data in this study were retrospectively collected. Some subjects may have had HBV screening in laboratories outside of the MGB health system or prior to 1995 (i.e., the earliest date of testing data captured in our study) or greater than 30 days after initiating tocilizumab or tofacitinib. Some subjects may have initiated tocilizumab or tofacitinib by clinicians outside the MGB system; however, this rarely occurred in a manual chart review of 5% of the subjects. We were unable to characterize the dose or duration of corticosteroid use in this dataset, so we were unable to include that important factor as a covariate. Our findings may not be generalizable to non-rheumatologic settings or other immunomodulators, although studies in other patient populations (e.g., solid-organ cancer, inflammatory bowel disease) have identified similarly low HBV screening frequencies(1,19,20).
We propose at least three reasons why universal HBV screening should be reconsidered in clinical society recommendations. First, HBV risk factors are frequently unreported by patients and unrecognized by physicians. In one study, fewer than 3% of patients from HBV-endemic regions were correctly identified by their rheumatologists as being at increased risk for HBV(21). Second, HBV prevalence may be rising due to the opioid epidemic, despite recommendations for universal childhood vaccination(22). Reported outbreaks of acute HBV, particularly in young, non-urban populations affected by the opioid epidemic, may only be the tip of the iceberg of national trends of rising HBV transmission; if so, there may soon be a growing population of patients with prior HBV infection who would be at risk of reactivation if exposed to immunosuppressive drug therapy(22). Third, the true incidence of HBV reactivation related to immunosuppressive drug therapy may be under-reported in observational cohorts because of ascertainment bias due to the low prevalence of HBV testing, even among those at high risk of HBV infection.
Conclusion
Fewer than one third of people completed appropriate HBV screening prior to initiating tocilizumab or tofacitinib for rheumatic diseases, and many underwent inappropriate HBV testing in lieu of HBsAg or total anti-HBcAb testing. Consistency in HBV screening recommendations across society recommendations may improve HBV detection prior to initiation of tocilizumab and tofacitinib and decrease untoward outcomes related to HBV reactivation.
ACKNOWLEDGEMENT
We thank Giulia Park and Stephanie Lee for their assistance preparing the final manuscript.
Funding:
AMM is supported by the NIH/NIAID [T32AI007433]. NSB is supported by NIH [T32AR007258]. AYK is supported by NIH/NIAID [AI082630]. EPH is supported by NIH/NHLBI [K01HL123349]. ZSW is funded by NIH/NIAMS [K23AR073334 and L30AR070520]. The National Institute of Health had no role in the design or authorship of this publication. The article contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Lok ASF, Ward JW, Perrillo RP, McMahon BJ, Liang TJ. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med 2012;156:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann Intern Med 2016;164:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008;148:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221–244.e3. [DOI] [PubMed] [Google Scholar]
- 5.Schmajuk G, Li J, Evans M, Anastasiou C, Izadi Z, Kay JL, et al. RISE registry reveals potential gaps in medication safety for new users of biologics and targeted synthetic DMARDs. Semin Arthritis Rheum 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Recomm Rep Morb Mortal Wkly Rep 2008;57:1–20. [PubMed] [Google Scholar]
- 7.Terrault NA, Lok ASF, McMahon BJ, Chang K-M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic Drugs. J Rheumatol 2012;39:1559–82. [DOI] [PubMed] [Google Scholar]
- 9.Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis: ACR RA Treatment Recommendations. Arthritis Care Res 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 10.Stine JG, Khokhar OS, Charalambopoulos J, Shanmugam VK, Lewis JH. Rheumatologists’ awareness of and screening practices for Hepatitis B virus infection prior to initiating immunomodulatory therapy. Arthritis Care Res 2010;62:704–11. [DOI] [PubMed] [Google Scholar]
- 11.Lin T-C, Hashemi N, Kim SC, Yang Y-HK, Yoshida K, Tedeschi S, et al. Practice pattern of hepatitis B testing in rheumatoid arthritis patients: a cross-national comparison between the US and Taiwan. Arthritis Care Res 2018;70:30–8. [DOI] [PubMed] [Google Scholar]
- 12.Méndez-Navarro J, Corey KE, Zheng H, Barlow LL, Jang JY, Lin W, et al. Hepatitis B screening, prophylaxis and re-activation in the era of rituximab-based chemotherapy: Rituximab and HBV re-activation. Liver Int 2011;31:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L-F, Mo Y-Q, Jing J, Ma J-D, Zheng D-H, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis 2017;20:859–69. [DOI] [PubMed] [Google Scholar]
- 14.Chiu Y-M, Chen D-Y. Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics. Expert Rev Clin Immunol 2020;16:207–28. [DOI] [PubMed] [Google Scholar]
- 15.Serling-Boyd N, Mohareb AM, Kim AY, Hyle EP, Wallace ZS. The use of tocilizumab and tofacitinib in patients with resolved hepatitis B infection: a case series. Ann Rheum Dis 2021;80:274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y-M, Huang W-N, Wu Y-D, Lin C-T, Chen Y-H, Chen D-Y, et al. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis 2018;77:780–2. [DOI] [PubMed] [Google Scholar]
- 17.Lawandi A, Cheng MP, Lee TC. Hepatitis B testing practices at a tertiary care centre and their associated costs: A retrospective analysis. Khudyakov YE, editor. PLOS ONE 2019;14:e0219347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottheil S, Khemani E, Copley K, Keeney M, Kinney J, Chin-Yee I, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Qual Improv Rep 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JP, Fisch MJ, Zhang H, Kallen MA, Routbort MJ, Lal LS, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract 2012;8:e32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul S, Shuja A, Tam I, Kim EM, Kang S, Kapulsky L, et al. Gastroenterologists have suboptimal hepatitis B virus screening rates in patients receiving immunosuppressive therapy. Dig Dis Sci 2016;61:2236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visram A, Chan KKW, McGee P, Boro J, Hicks LK, Feld JJ. Poor recognition of risk factors for hepatitis B by physicians prescribing immunosuppressive therapy: a call for universal rather than risk-based screening. Chemin IA, editor. PLOS ONE 2015;10:e0120749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gish RG, Cohen CA, Block JM, Brosgart CL, Block TM, Clary R, et al. Data supporting updating estimates of the prevalence of chronic hepatitis B and C in the United States. Hepatology 2015;62:1339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


