Abstract
Since 2012, cervical cancer screening guidelines allow for choice of screening test for women age 30–65 years (i.e., Pap every 3 years or Pap with human papillomavirus co-testing every 5 years). Intended to give patients and providers options, this flexibility reflects a trend in the growing complexity of screening guidelines. Our objective was to characterize variation in cervical screening at the individual, provider, clinic/facility, and healthcare system levels. The analysis included 296,924 individuals receiving screening from 3,626 providers at 136 clinics/facilities in three healthcare systems, 2010 to 2017. Main outcome was receipt of co-testing vs. Pap alone. Co-testing was more common in one healthcare system before the 2012 guidelines (adjusted odds ratio (AOR) of co-testing at the other systems relative to this system 0.00 and 0.50) but was increasingly implemented over time in a second with declining uptake in the third (2017: AORs shifted to 7.32 and 0.01). Despite system-level differences, there was greater heterogeneity in receipt of co-testing associated with providers than clinics/facilities. In the three healthcare systems, providers in the highest quartile of co-testing use had an 8.35, 8.81, and 25.05-times greater odds of providing a co-test to women with the same characteristics relative to the lowest quartile. Similarly, clinics/ facilities in the highest quartile of co-testing use had a 4.20, 3.14, and 6.56-times greater odds of providing a co-test relative to the lowest quartile. Variation in screening test use is associated with health system, provider, and clinic/facility levels even after accounting for patient characteristics.
Keywords: cervical cancer screening, guideline implementation
Introduction
Over the past decade, cancer screening guidelines have moved from a standard “one size fits all” approach to recommendations tailored to an individual’s characteristics and preferences. While tailoring supports individual-centered care, the complexity of the resulting screening guidelines may challenge individuals, clinicians, clinics and healthcare systems trying to follow the recommendations to achieve optimal outcomes.
Cervical cancer screening guidelines exemplify this shift. Since 2012, the US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) cervical cancer screening guidelines have endorsed two options for average risk women age 30–65 years: Pap testing every 3 years or Pap test with the human papilloma virus (HPV) test (i.e., co-testing) every 5 years.1,2 Offering this choice is a notable departure from the prior longstanding, simple practice of an annual Pap test that resulted in the dramatic reduction in cervical cancer incidence and mortality during the 20th century.3
The choice of a particular screening test is generally made by the provider, but may be influenced by factors from multiple levels, including: (1) a woman’s preferences about screening interval, harms and benefits; (2) provider knowledge, beliefs and time available to discuss differences between the two options; (3) clinic/ facility resource constraints such as the cost of HPV testing or the availability of providers to conduct screening; and (4) healthcare system policies and protocols outlining leadership’s preference for a particular screening modality and resources such as availability of high-throughput systems to batch process samples. While several studies suggest that there was broad early adoption of co-testing, there is limited information about the implementation of co-testing in diverse healthcare systems, across clinics/ facilities, or by different types of providers for diverse populations.4–7
We sought to identify and characterize variation in the receipt of co-testing at the woman, provider, clinic/ facility, and healthcare system levels using longitudinal clinical and administrative data from three diverse healthcare systems. Understanding the sources and magnitude of variation may suggest opportunities for intervention if variation is not desired.8 This approach of examining variation at each of these levels has implications for the implementation of screening guidelines more broadly.
Methods
Setting, Study Population, and Data Collection
Our study was conducted within the MultilEvel opTimization of the ceRvIcal Cancer Screening process in diverse Settings & populations (METRICS), part of the Population-based Research to Optimize the Screening Process (PROSPR II) consortium.9 METRICS has three data-contributing sites that represent diverse healthcare settings: Mass General Brigham (MGB); Kaiser Permanente Washington (KPWA); and Parkland Health & Hospital System-University of Texas, Southwestern (PHHS-UTSW). MGB is an integrated delivery system that includes two academic medical centers—Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH)—and their affiliated primary care networks, in Massachusetts); KPWA is a mixed model healthcare system in Washington State; PHHS-UTSW is an integrated safety-net healthcare system for under- and uninsured in Dallas County, Texas). This work was approved by the institutional review boards of the participating institutions.
The METRICS cohort includes women ages 18–89 years. MGB and PHHS-UTSW included women with at least one visit to a primary care or women’s health clinic anytime between January 1, 2010 to December 31, 2017. KPWA included women who were enrolled in the health plan and who selected, were assigned, or were attributed to a KPWA primary care provider during this time period. All healthcare systems collected comprehensive cervical cancer screening process data on their cohorts at the woman- and provider-levels using a rich array of electronic clinical information systems and administrative databases.10 Additionally, women and providers are attributed to clinics/ facilities within each of the three healthcare systems.
Study Population
For this analysis, we identified METRICS cohort members who were between the ages of 30–65 years at some point between 2010–2017. As the focus of this analysis was on screening, we used algorithms to identify and exclude Pap tests that were done for surveillance of a prior abnormality or concurrent with diagnostic evaluation via colposcopy. We excluded women: with a prior hysterectomy, prior history of cervical cancer, or who required an alternate screening schedule because of elevated risk of cervical cancer. We also excluded KPWA members who did not visit a primary care provider (PCP) during any of the study years to approximate the MGB and PHHS-UTSW cohort definitions. Since we were interested in examining variation in selection of screening test, we excluded women from all sites who did not receive a screening test at any time during the study period.
Outcome Classification
A sequential algorithm was used to assign screening test type. If no HPV test occurred within 14 days of a screening Pap test, then “Pap alone” was assigned. If an HPV test indication was noted to be a co-test, this status was assigned. If the HPV test indication was missing and an HPV test was done within 14 days of an ASCUS cytology result, then the test was considered to be a reflex HPV and their outcome status was assigned as “Pap alone.” If the HPV test indication was missing and an HPV test was done within 14 days of a normal cytology result or an abnormal cytology result of LSIL or worse, an outcome of “co-test” was assigned.
Covariates
Woman-level data included age, race/ ethnicity (non-Hispanic-White (hereafter “White”), non-Hispanic Black (hereafter “Black”), Hispanic, Asian/ Pacific Islander, or other/ multiracial/ unknown), and health insurance (commercial, public (Medicare, Medicaid, other government), uninsured or medical assistance, or multiple insurance/ other/ unknown). We differentiated whether a woman was known to be at average risk of cervical cancer based on a documented prior normal screening history or had an unknown risk because the electronic medical record data did not include any documentation of prior screening history. Provider-level data included the type of provider who performed the Pap or co-test (physician [MD/DO], nurse/ nurse practitioner / physician’s assistant [Nurse/NP/PA], or other) and the specialty of the provider who performed the Pap or co-test (family medicine, internal medicine, obstetrics/ gynecology [ob-gyn], other or unknown); non-physician providers were assigned the specialty category based on the clinic setting in which they practiced. Based on where the Pap test was performed, each woman and provider was attributed to a clinic (MGB and PHHS-UTSW) or a facility (KPWA, defined as distinct buildings or buildings that are co-located [i.e., medical centers] and share resources).
Data Analysis
To assess variation in receipt of screening test type and estimate associations with measured factors at multiple levels, we fit a series of mixed-effects multilevel logistic models regressing screening test type (Pap alone vs. co-testing) on fixed and random effects accounting for woman, provider, clinic/ facility-level factors and healthcare system. Because of the complexity of the models, we randomly selected one screening test per woman if a woman had multiple screening tests during the study period (n= 193,227 screening tests). To assess differences in the odds of co-testing across healthcare systems in 2010, 2013 and 2017, as well as year-to-year trends in each system, we initially fit an overall unadjusted model, with fixed effects for healthcare system, calendar year of the Pap or co-test, and the interaction between healthcare system and year, irrespective of other factors. We then included fixed effects for additional covariates, including age at screening, race/ethnicity, insurance type, and provider specialty to assess differences in the odds of co-testing after accounting for variation from these covariates.
Separate mixed-effects logistic regression models including calendar year, the additional covariates, and random effects for provider and clinic/ facility were fit by healthcare system. We used these stratified models to assess the degree of variability across providers and facilities at each healthcare system by comparing the odds for a woman to receive a co-test by providers and facilities with the highest vs. lowest quartile of random intercept, calculated based on the variance component estimates. For example, an OR of 2 based on the provider random effect would indicate that a woman is estimated to have twice the odds of having a co-test when receiving care from providers at the 75th percentile of co-test use vs. 25th percentile, after accounting for other factors in the model.
To visualize the estimated trends across providers by healthcare system, we fit models stratified by healthcare system using data for average risk women by provider specialty. We then plotted the estimated co-testing propensity over time using the most common screening population as a reference (age 45, White women with commercial insurance), assuming an average clinic/ facility-level random effect and provider-level random effects at the 25th, 50th, and 75th percentiles for each healthcare system. To assess the generalizability of these findings in women with other characteristics, we performed an additional analysis for Black women, age 45 with public insurance as the reference. Analyses were conducted using SAS (version 9.4) for analyses and R (version 4.0.2, “lme4” package). Institutional Review Boards at each site approved study activities.
Results
Characteristics of Screened Women, Age 30 – 65 years
Our study sample included 296,924 women receiving care from 3,626 providers at 136 clinics/facilities across the three healthcare systems (Table 1). The median age was 44 years. Across systems, the sample was 48.4% White, 27.2% Hispanic, 11.3% Black, 8.5% Asian/ Pacific Islander and 4.6% had race coded as other or unknown. Race/ ethnicity varied by healthcare system. Overall, most (59.6%) women were commercially insured, 26.5% had public insurance and 13.5% were uninsured or on medical assistance. Insurance status varied by healthcare system. Overall, 56.9% of women had information available to determine their cervical cancer risk and 43.1% had no documented prior screening history. Provider specialty varied by healthcare system, with internal medicine, family practice and ob-gyn providers most commonly performing cervical cancer screening at MGB, KPWA and PHHS-UTSW respectively.
Table 1.
Characteristics of Screened Women age 30 – 65 years, Overall and by Healthcare System
| All | Mass General Brigham | Kaiser Permanente, Washington | PHHS-UTSW | |||||
|---|---|---|---|---|---|---|---|---|
| Number of women | 296,924 | 97,464 | 109,376 | 90,084 | ||||
| Number of providers | 3,626 | 1,768 | 731 | 1,127 | ||||
| Number of clinics/facilities | 136 | 75 | 21 | 40 | ||||
| Median number (range) of women per provider | 12 | (1–1,957) | 6 | (1–927) | 75 | (1–1,773) | 8 | (1–1,957) |
| Median number (range) of women per clinic | 867 | (1–32,493) | 648 | (1–6,968) | 4,018 | (1,514–14,982) | 174 | (1–32,493) |
| Median Age, years (std. deviation) | 44 (10.5) | 46 (10.7) | 46 (10.7) | 40 (9.4) | ||||
| N | % | N | % | N | % | N | % | |
| Race/ Ethnicity | ||||||||
| Asian/ Pacific Islander | 25,281 | 8.5 | 6,835 | 7.0 | 15,694 | 14.3 | 2,752 | 3.1 |
| Hispanic | 80,748 | 27.2 | 12,268 | 12.6 | 6,984 | 6.4 | 61,496 | 68.3 |
| Non-Hispanic Black | 33,520 | 11.3 | 9,072 | 9.3 | 5,437 | 5.0 | 19,011 | 21.1 |
| Non-Hispanic White | 143,752 | 48.4 | 65,488 | 67.2 | 71,949 | 65.8 | 6,315 | 7.0 |
| Other/ Unknown | 13,623 | 4.6 | 3,801 | 3.9 | 9,312 | 8.5 | 510 | 0.6 |
| Insurance | ||||||||
| Commercial | 176,844 | 59.6 | 70,394 | 72.2 | 101,941 | 93.2 | 4,509 | 5.0 |
| Uninsured/ Medical Assistance | 40,208 | 13.5 | 822 | 0.8 | 0 | 0 | 39,386 | 43.7 |
| Public (Medicare/ Medicaid/ Other Government) | 78,576 | 26.5 | 26,050 | 26.7 | 7,435 | 6.8 | 45,091 | 50.1 |
| Multiple Insurance/ Other/ Unknown | 1,296 | 0.4 | 198 | 0.2 | 0 | 0 | 1,098 | 1.2 |
| Risk of Cervical Cancer | ||||||||
| Average Risk | 168,984 | 56.9 | 69,615 | 71.4 | 55,006 | 50.3 | 44,363 | 49.2 |
| Unknown Risk | 127,940 | 43.1 | 27,849 | 28.6 | 54,370 | 49.7 | 45,721 | 50.8 |
| Provider Specialty | ||||||||
| Family Medicine | 101,451 | 34.2 | 1,591 | 1.6 | 84,730 | 77.5 | 15,130 | 16.8 |
| Internal Medicine | 72,948 | 24.6 | 52,863 | 54.2 | 5,254 | 4.8 | 14,831 | 16.5 |
| OB/GYN | 97,074 | 32.7 | 25,181 | 25.8 | 18,099 | 16.5 | 53,794 | 59.7 |
| Other | 7,751 | 2.6 | 1,001 | 1.0 | 1,293 | 1.2 | 5,457 | 6.1 |
| Unknown | 17,700 | 6.0 | 16,828 | 17.3 | 0 | 0 | 872 | 1.0 |
| Provider Type * | ||||||||
| MD/DO | 188,548 | 63.5 | 70,306 | 72.1 | 84,301 | 77.1 | 33,941 | 37.7 |
| Nurse/NP/PA | 82,411 | 27.8 | 9,497 | 9.7 | 17,913 | 16.4 | 55,001 | 61.1 |
| Other | 25,965 | 8.7 | 17,661 | 18.1 | 7,162 | 6.5 | 1,142 | 1.3 |
Notes:
Non-physician providers were assigned the specialty category based on the clinic setting in which they practiced.
Receipt of Co-Testing versus Pap alone by Healthcare System, 2010–2017
Receipt of co-testing versus Pap-alone varied considerably over time across the three healthcare systems (Figure 1). In 2010, the receipt of co-testing was similar at MGB and PHHS-UTSW (22.4%) as compared to KPWA (0.4%). At MGB, co-testing use increased gradually, reaching 75.9% by 2017. At KPWA, receipt of co-testing remained low until after the 2012 guidelines were introduced and then quickly increased such that almost all women were receiving co-testing by 2017 (95.6%). At PHHS-UTSW, receipt of co-testing declined after 2012, with a co-testing proportion of 12.5% in 2017.
Figure 1.
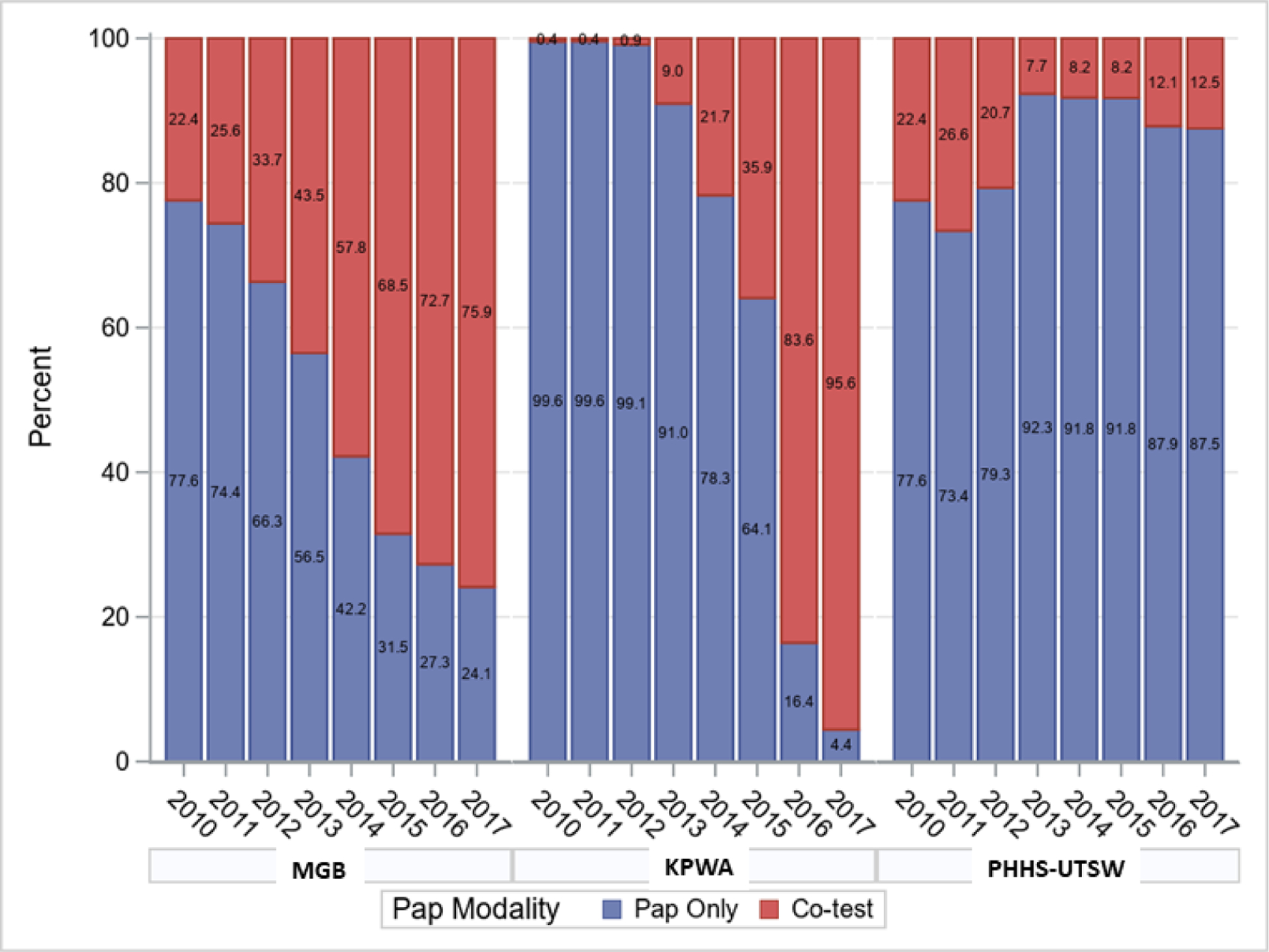
Receipt of Co-Testing versus Pap alone Between 2010–2017, by Healthcare System (unadjusted).
Numbers represent the percentage in each year with Pap only or co-test.
MGB: Mass General Brigham
KPWA: Kaiser Permanente Washington
PHHS-UTSW: Parkland Health & Hospital System-University of Texas Southwestern
Multilevel Characteristics Associated with Receipt of Co-Testing versus Pap alone
Multilevel logistic regression models showed that healthcare system and year of screening were associated with receipt of co-testing compared to a Pap alone (Table 2). For example, among women receiving Pap tests in 2013, the adjusted odds of receiving a co-test vs. Pap alone was significantly lower at KPWA (adjusted odds ratio (AOR)=0.02, 95% confidence interval (CI) 0.01–0.04) and PHHS-UTSW (0.08, 0.04–0.14) relative to MGB. By 2017, however, the AOR was greater at KPWA (7.32, 3.69–14.54) and lower at PHHS-UTSW (0.01, 0.00–0.01) relative to MGB.
Table 2.
Receipt of Co-testing versus Pap Alone by year, in three US health care systems (2010–2017).
| Patient Count | Unadjusted | Adjusted * | ||||
|---|---|---|---|---|---|---|
| Co-test | Pap-only | OR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Healthcare system, 20101 | ||||||
| MGB | 3,484 | 9,389 | (ref) | - | (ref) | - |
| KPWA | 91 | 17,396 | 0.00 (0.00,0.00) | <0.001 | 0.00 (0.00,0.00) | <0.001 |
| PHHS-UTSW | 2,547 | 8,533 | 0.43 (0.24,0.75) | 0.003 | 0.50 (0.28,0.89) | 0.018 |
| Healthcare system, 20131 | ||||||
| MGB | 5,498 | 5,415 | (ref) | - | (ref) | - |
| KPWA | 1,222 | 11,202 | 0.02 (0.01,0.03) | <0.001 | 0.02 (0.01,0.04) | <0.001 |
| PHHS-UTSW | 996 | 11,280 | 0.07 (0.04,0.11) | <0.001 | 0.08 (0.04,0.14) | <0.001 |
| Healthcare system, 20171 | ||||||
| MGB | 9,567 | 2,516 | (ref) | - | (ref) | - |
| KPWA | 10,219 | 467 | 5.63 (2.99,10.58) | <0.001 | 7.32 (3.69,14.54) | <0.001 |
| PHHS-UTSW | 1,446 | 9,703 | 0.01 (0.00, 0.01) | <0.001 | 0.01 (0.00,0.01) | <0.001 |
| Annual change, by healthcare system2 | ||||||
| MGB | - | - | 1.70 (1.68,1.71) | <0.001 | 1.69 (1.68,1.71) | <0.001 |
| KPWA | - | - | 7.20 (6.97,7.43) | <0.001 | 7.20 (6.97,7.43) | <0.001 |
| PHHS-UTSW | - | - | 0.91 (0.89,0.93) | <0.001 | 0.91 (0.89,0.93) | <0.001 |
Notes:
Estimates based on overall fixed effect model fitted to data from all three healthcare systems including main effects for healthcare system, calendar year of Pap or co-test, their interactions, main effects for covariates in adjusted models, and random effects for provider and clinic/facilities.
Healthcare system ORs are odds ratios for receiving co-test vs. Pap alone for women at each healthcare system relative to women at MGB, in the specified year, assuming common unobserved provider and clinic/facility effects and adjustment for a woman’s age, race, insurance type, risk status, and provider type, for adjusted estimates.
Annual change ORs are odds ratios for women in one year relative to the prior year, at each healthcare system, assuming common unobserved provider and clinic/facility effects and adjustment for individual- and provider-level covariates in the adjusted estimates.
Abbreviations:
MGB: Mass General Brigham
KPWA: Kaiser Permanente Washington
PHHS-UTSW: Parkland Health & Hospital System-University of Texas Southwestern
OR: Odds Ratio
95% CI: 95% confidence interval
These models were also used to examine associations between receipt of co-testing and women’s characteristics and provider specialty (Table 3). At MGB, the odds of the screening test being a co-test was greater for Hispanic women compared to White women (1.37, 1.28–1.45) and for women with public coverage compared to women with commercial insurance (1.12, 1.07–1.16). At KPWA, the odds of the screening test being a co-test was lower for women with public coverage compared to women with commercial insurance (0.61, 0.54–0.68). At PHHS-UTSW, the odds of the screening test being a co-test was greater for women with commercial insurance compared to those with public coverage (1.23, 1.06–1.42).
Table 3.
Patient characteristics and provider specialty in relation to receipt of co-testing versus Pap alone in three US health care systems (2010–2017).
| MGB | KPWA | PHHS-UTSW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Count (%) | OR * (95% CI) | p-value | Count (%) | OR * (95% CI) | p-value | Count (%) | OR * (95% CI) | p-value | |
| Age | - | 0.99 (0.99,0.99) | <0.001 | - | 1.00 (1.00,1.00) | 0.73 | - | 1.00 (1.00,1.00) | 0.94 |
| Race | |||||||||
| White | 34,117 (52.1%) | (ref) | - | 16,989 (23.6%) | (ref) | - | 1,215 (19.2%) | (ref) | - |
| Asian | 3,927 (57.5%) | 0.99 (0.93,1.06) | 0.82 | 4,126 (26.3%) | 0.92 (0.85,1.00) | 0.06 | 404 (14.7%) | 1.09 (0.91,1.32) | 0.35 |
| Black | 4,561 (50.3%) | 0.94 (0.89,1.00) | 0.05 | 1,363 (25.1%) | 0.94 (0.82,1.06) | 0.32 | 4,367 (23%) | 0.99 (0.88,1.11) | 0.86 |
| Hispanic | 8,030 (65.5%) | 1.37 (1.28,1.45) | <0.001 | 1,824 (26.1%) | 1 (0.90,1.12) | 0.97 | 7,831 (12.7%) | 0.93 (0.84,1.04) | 0.19 |
| Other | 2,032 (53.5%) | 0.89 (0.81,0.97) | 0.01 | 2,212 (23.8%) | 1.04 (0.94,1.16) | 0.42 | 74 (14.5%) | 0.83 (0.58,1.20) | 0.33 |
| Insurance | |||||||||
| Commercial | 37,340 (53%) | (ref) | - | 24,954 (24.5%) | (ref) | - | 727 (16.1%) | 1.23 (1.06,1.42) | 0.01 |
| Medicare/Medicaid/Other Government | 14,931 (57.3%) | 1.12 (1.07,1.16) | <0.001 | 1,560 (21%) | 0.61 (0.54,0.68) | <0.001 | 3,227 (7.2%) | (ref) | - |
| Multiple/Other/Unknown | 125 (63.1%) | 1.23 (0.86,1.76) | 0.25 | 0 (0%) | - | - | 59 (5.4%) | 1.18 (0.80,1.73) | 0.40 |
| Uninsured/Medical Assistance | 271 (33%) | 0.71 (0.59,0.86) | <0.001 | 0 (0%) | - | - | 9,878 (25.1%) | 1.01 (0.94,1.08) | 0.84 |
| Risk Group | |||||||||
| Average Risk | 35,608 (51.1%) | (ref) | - | 11,583 (21.1%) | (ref) | - | 5,227 (11.8%) | (ref) | - |
| Unknown Risk ** | 17,059 (61.3%) | 1.13 (1.09,1.18) | <0.001 | 14,931 (27.5%) | 1.04 (0.99,1.10) | 0.1510 | 8,664 (18.9%) | 1.25 (1.17,1.32) | <0.001 |
| Specialty | |||||||||
| Family Medicine | 1,000 (62.9%) | (ref) | - | 19,873 (23.5%) | (ref) | - | 4,721 (31.2%) | (ref) | - |
| Internal Medicine | 27,550 (58.1%) | 2.07 (1.19,3.59) | 0.01 | 1,546 (29.4%) | 0.92 (0.44,1.91) | 0.82 | 4,763 (36.5%) | 0.78 (0.37,1.64) | 0.51 |
| OB/GYN | 14,380 (57.1%) | 1.37 (0.74,2.52) | 0.32 | 4,984 (27.5%) | 5.44 (3.46,8.53) | <0.001 | 2,480 (4.6%) | 0.16 (0.09,0.29) | <0.001 |
| Other | 4,575 (70.8%) | 2.09 (1.15,3.80) | 0.02 | 111 (8.6%) | 0.65 (0.20,2.11) | 0.47 | 1,806 (24.9%) | 0.25 (0.12,0.52) | <0.001 |
| Unknown | 5,162 (30.7%) | 1.23 (0.59,2.58) | 0.58 | 0 (0%) | - | - | 121 (13.9%) | 0.48 (0.15,1.52) | 0.21 |
Notes:
Estimates based on mixed-effects multilevel logistic models fit to data specific to each healthcare system with main effects for calendar year and age at screening, individual race/ethnicity, insurance type, and provider specialty and random intercepts for provider and facility.
Women whose electronic medical record data did not include any documentation of prior screening history were categorized as unknown risk.
Abbreviations:
MGB: Mass General Brigham
KPWA: Kaiser Permanente Washington
PHHS-UTSW: Parkland Health & Hospital System-University of Texas, Southwestern
Count: Number of patients receiving co-testing
OR: Odds Ratio
95% CI: 95% confidence interval
We observed associations with provider specialty that varied across sites. At MGB, the odds of the screening test being a co-test were greater for those receiving care from internal medicine compared to family practice providers (2.07, 1.19–3.59). At KPWA, the odds of the screening test being a co-test were greater for those receiving care from ob-gyn compared to family practice providers (5.44, 3.46–8.53). In contrast, at PHHS-UTSW the odds of the screening test being a co-test were lower for those receiving care from ob-gyn compared to family practice providers (0.16, 0.09–0.29).
Relative Variation in the Receipt of Cervical Cancer Screening at the Provider and Clinic/ Facility Levels
There was greater heterogeneity in type of cervical cancer screening across providers than across clinics/ facilities within each healthcare system. Based on multi-level logistic regression models stratified by healthcare system, providers in the highest quartile of co-testing use were estimated to have 8.35, 11.76, and 33.21 times greater odds of providing a co-test to women with the same characteristics relative to providers in the lowest quartile at MGB, KPWA, and PHHS-UTSW, respectively. Clinics/ facilities in the highest quartile are estimated to have 4.44, 3.41, and 8.11 times greater odds of providing a co-test to women with the same observed characteristics relative to clinics/ facilities in the lowest quartile, after adjustment for provider-level effects at MGB, KPWA, and PHHS-UTSW, respectively. PHHS-UTSW has the largest variation between the highest and lowest quartile among providers and clinics.
Estimated Trends in Receipt of Co-testing by Healthcare System by Provider Specialty
Temporal trends for receipt of co-testing varied greatly between institutions and, to some degree, provider specialties within each institution (Figure 2). Lines that are farther apart at a given time indicate that providers at the different percentiles of co-test use have greater differences in the rates of performing a co-test. At MGB, the probability of receiving a co-test vs. Pap alone exhibited heterogeneity across providers over the entire study period. Heterogeneity among internal medicine providers was largest in the middle years of the study, around the time of the 2012 guideline revisions, with differences in the probability of co-testing by provider remaining in 2017. A similar pattern was observed for ob-gyn providers. For women at KPWA, there was minimal provider heterogeneity in receipt of co-testing prior to 2012, particularly for family practice providers. Like MGB, at KPWA, the greatest heterogeneity in co-testing use by providers occurred after 2012. By 2017, these differences at KPWA had narrowed and are smaller than that those observed at MGB. For most providers at PHHS-UTSW, the probability of co-testing remained quite low even after the guidelines were implemented, with heterogeneity between providers narrowing for primary care and widening for ob-gyn. Similar trends by health system and year were seen for Black women, age 45 with public insurance as the reference (not shown).
Figure 2.
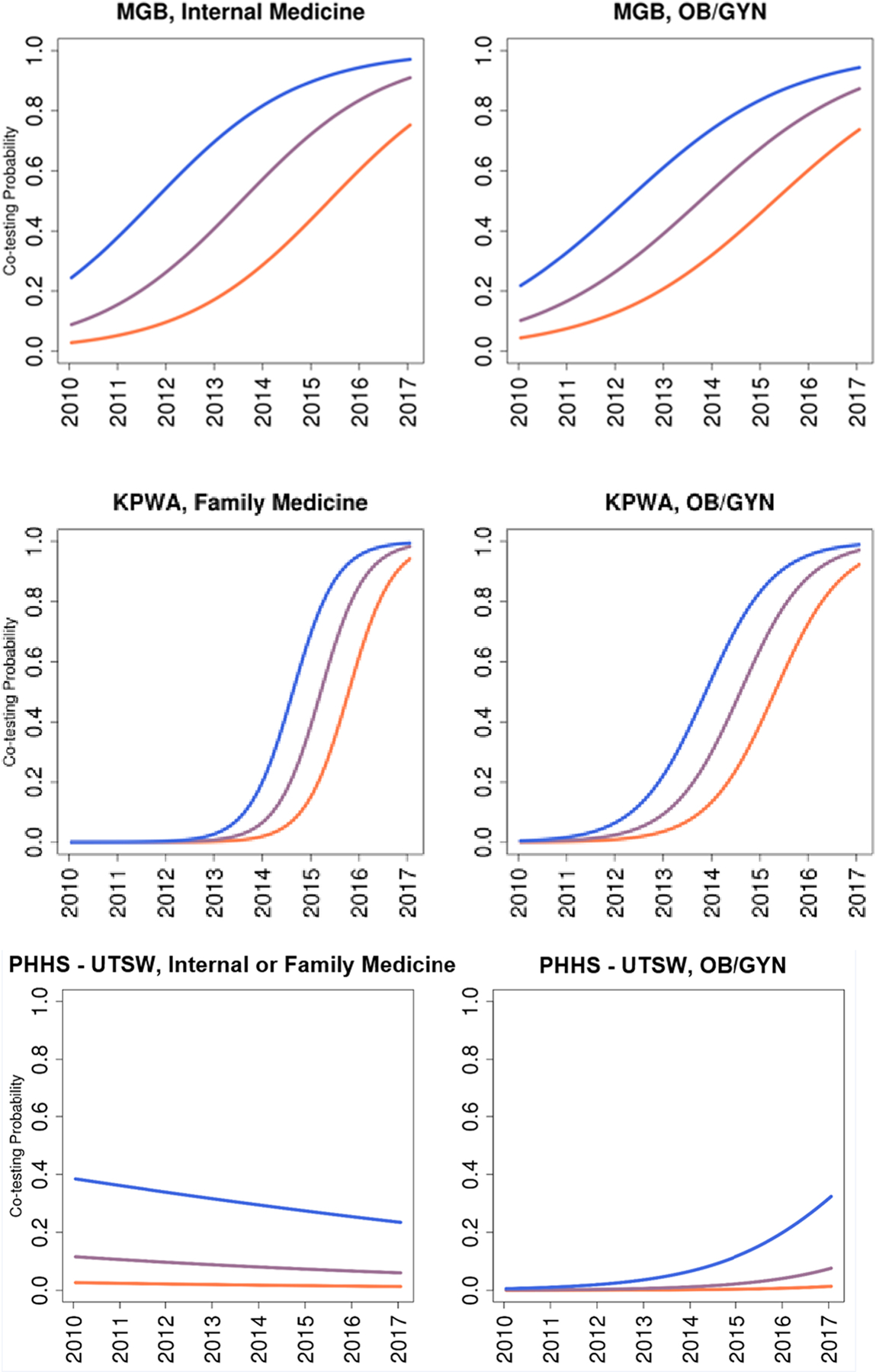
Estimated Trends in Co-Testing Use by Provider Specialty and Healthcare System.
The different colors represent the estimated co-testing propensity over time for the most common screening population (45-year-old White woman with commercial insurance assuming an average facility-level random effect) and provider-level random effects at the 25th, 50th, and 75th percentiles.
MGB: Mass General Brigham
KPWA: Kaiser Permanente Washington
PHHS-UTSW: Parkland Health & Hospital System-University of Texas, Southwestern
Discussion
Although conceptual models promote the importance of understanding multi-level influences on the cancer care continuum,11–13 this study is one of the first to empirically examine the multilevel influences of individual, provider, clinic/ facility and health system characteristics on cancer screening.14 We examined the receipt of co-testing versus Pap alone among women aged 30–65 years in three diverse healthcare systems between 2010 and 2017 – a period overlapping with the dissemination of the guideline revisions offering two options (Pap alone or co-testing).1
Our findings suggest that unmeasured policies and organizational characteristics of the healthcare systems may influence the receipt of co-testing for women age 30–65 as compared to Pap alone in these diverse healthcare systems. The differences observed between these healthcare systems are potentially illustrative of the goals and barriers of these systems. For example, the broad and rapid increase of co-testing at KPWA reflects that this healthcare system uses the USPSTF guidelines as the standard of care and organizational leaders adopted co-testing as the preferred screening option following the release of the 2012 USPSTF guidelines. Alternatively, PHHS-UTSW leaders use Pap alone every 3 years as the default screening strategy because this approach has been demonstrated to be the most cost-effective strategy,15 and enables the health system to cover more uninsured women via public payer programs like the National Breast and Cervical Cancer Early Detection Program and family planning block grants.16
Further, it simplifies the process of screening and triage of results for providers and clinic staff, and it may encourage patients to engage in broader preventive care (e.g., breast cancer screening, cardiovascular risk assessment) at least every 3 years. At MGB, women and providers may value having broader discretion to choose between co-testing or Pap alone. If the observed healthcare system variation is not intended, system-level policies should be considered to align screening patterns with the goals of the organization.
The provider level accounted for more variation in the receipt of co-testing than individual-level factors or clinic/ facility-level. This finding held across all three of these diverse healthcare systems. Provider-level variation increased following release of the 2012 guidelines, which allowed for choice among two screening options. This variation in response to the guidelines suggests healthcare systems should explicitly consider first, whether heterogeneity in provider behavior is beneficial or not, and if not, whether organizational strategies to reduce variation may be desirable.17 Such organizational strategies could include “smart-set” orders that make it easier for providers to use the organization’s preferred strategy. Further, our findings suggest that interventions designed to change the receipt of co-testing within a healthcare system may be less effective if targeted towards women (e.g., education or outreach) or clinics/ facilities (e.g., education, local policies) than providers. Future work should explore the sources of provider-level heterogeneity, including whether it is related to reimbursement policies, knowledge and beliefs about co-testing, or time available to discuss the differences between screening modalities.18 Individual-level factors (e.g., age, race, risk status, insurance status), generally were less strongly associated with receipt of co-testing compared to provider-, clinic/ facility-, and system-level factors. Since the guideline presents two options for screening, ideally a woman’s preferences for the frequency, harms and benefits of screening would play a more important role in the selection of screening approach than provider and site-level factors.
Our work is consistent with an earlier study examining variation in adherence with breast cancer screening that found that primary care providers and healthcare systems accounted for most of the variance in screening use.14 Our study extends the findings from studies of single healthcare systems that have examined trends in the receipt of co-testing following the 2012 USPSTF guideline change.4–6 Our sample is large and includes multi-level data elements across diverse healthcare settings. Our results should be interpreted within the context of study limitations. We have limited information about the characteristics of the providers and clinics/facilities that are associated with the observed heterogeneity. While the settings represent distinct models of the US healthcare system, our study results may not be generalizable to other healthcare systems, practice organizations, providers, or women. Future work should examine the multi-level effect of the 2018 USPSTF guidelines and the 2020 ACS guidelines on cervical screening practices.19,20 While the ACS notes that primary HPV screening is the preferred option with co-testing and Pap alone as acceptable alternatives, the USPSTF expresses no preference among the three test options for women of this age.
Conclusion
Current cervical cancer screening guidelines are associated with variation in screening test use at the healthcare system, provider and clinic/facility levels. This suggests that improvements in cervical cancer screening may require moving away from individual-level interventions and instead emphasize healthcare system policies, and provider-targeted interventions to reduce healthcare system and provider variation.
Highlights.
There are options for cervical screening (co-testing vs Pap) for women 30–65 years.
Largest variation was seen at the levels of the health system and provider.
Provider variation was 2-to-4 fold the variation at the level of the clinic.
Least variation was observed at the patient level.
Acknowledgements:
Contributors:
The authors wish to thank the participating METRICS sites for the data they have provided for this study. A list of the METRICS investigators and contributing research staff is provided at: https://utsouthwestern.edu/labs/prospr-metrics.
Disclosure of Funding and Conflicts of Interest:
This work was supported by the National Cancer Institute (NCI grant number UM1CA221940). NCI project scientists collaborated on the study’s design, conduct, and reporting. None of the authors report a conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- 1.Moyer VA, for the US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891, W312. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annual Rates of New Cancers, 1999–2017. https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed December 12, 2020.
- 4.Silver MI, Rositch AF, Phelan-Emrick DF, Gravitt PE. Uptake of HPV testing and extended cervical cancer screening intervals following cytology alone and Pap/HPV cotesting in women aged 30–65 years. Cancer Causes Control. 2018;29(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLaughlin KL, Jacobson RM, Radecki Breitkopf C, et al. Trends Over Time in Pap and Pap-HPV Cotesting for Cervical Cancer Screening. J Womens Health (Larchmt). 2019;28(2):244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson M, Benard V, Flagg EW. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003–2014. Prev Med Rep. 2018;9:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44–50. [DOI] [PubMed] [Google Scholar]
- 8.Cervical Cancer Screening. National Cancer Institute. Cancer Trends Progress Report Web site. https://progressreport.cancer.gov/detection/cervical_cancer. Published 2020. Accessed Feb 15, 2021.
- 9.Beaber EF, Kim JJ, Schapira MM, et al. Unifying screening processes within the PROSPR consortium: a conceptual model for breast, cervical, and colorectal cancer screening. J Natl Cancer Inst. 2015;107(6):djv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamineni A, Tiro JA, Beaber EF, et al. Cervical cancer screening research in the PROSPR I consortium: Rationale, methods and baseline findings from a US cohort. Int J Cancer. 2019;144(6):1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zapka J, Taplin SH, Price RA, Cranos C, Yabroff R. Factors in quality care--the case of follow-up to abnormal cancer screening tests--problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010(40):58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4–13. [PubMed] [Google Scholar]
- 13.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onega T, Tosteson TD, Weiss J, et al. Multi-level Influences on Breast Cancer Screening in Primary Care. J Gen Intern Med. 2018;33(10):1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawaya GF, Sanstead E, Alarid-Escudero F, et al. Estimated Quality of Life and Economic Outcomes Associated With 12 Cervical Cancer Screening Strategies: A Cost-effectiveness Analysis. JAMA Intern Med. 2019;179(7):867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corley DA, Haas JS, Kobrin S. Reducing Variation in the “Standard of Care” for Cancer Screening: Recommendations From the PROSPR Consortium. JAMA. 2016;315(19):2067–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabroff KR, Zapka J, Klabunde CN, et al. Systems strategies to support cancer screening in U.S. primary care practice. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith-McCune K Choosing a screening method for cervical cancer: Papanicolaou testing alone or with human papillomavirus testing. JAMA internal medicine. 2014;174(7):1027–1028. [DOI] [PubMed] [Google Scholar]
- 19.Force USPST, Curry SJ, Krist AH, et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674–686. [DOI] [PubMed] [Google Scholar]
- 20.Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321–346. [DOI] [PubMed] [Google Scholar]


