Abstract
Introduction:
Unbalanced dietary intake has been increasingly recognized as an important modifiable risk factor for asthma. In this study we assessed whether a pro-inflammatory diet is associated with higher asthma burden in three steps: 1) identification of asthma latent classes (LC) based on symptoms, indoor exposures, and pulmonary function; 2) identification of risk factors associated with LC membership; 3) estimation of the probabilities of LC membership with variation in DII.
Methods:
Cross-sectional study on 415 children aged 5–14 years (266 with persistent asthma and 149 controls). LC analysis was performed in asthmatic children. The DII was calculated based on a semi-quantitative food frequency questionnaire. Elastic net logistic regression was used to investigate whether increasing DII was associated with worse asthma burden.
Results:
Two LCs were identified. Children in Class 1, “high burden”, had higher symptom burden and worse lung function. Children in Class 2, “low burden”, had lower symptom burden and less impaired lung function, but were more subject to indoor exposures. DII was the only risk factor significantly associated with Class 1 membership. As the DII increased (from −4.0 to +4.0), the probability of Class 1 membership increased from 32% to 65% when compared to control group, while it increased from 41% to 72% when compared to Class 2.
Conclusions:
We identified two phenotypes of persistent asthma associated with different disease burden linked to indoor exposures. An increasing DII was associated with high-burden asthma, providing further evidence about the role of a pro-inflammatory diet in asthma morbidity.
Keywords: asthma, children, dietary inflammatory index, burden, latent class analysis, lung function, indoor exposures
Introduction
Asthma is a major non-communicable disease imposing a relevant burden on children (1). Indeed, although most children with persistent asthma can be managed with low to medium doses of inhaled corticosteroids, some children can experience frequent and severe asthma attacks and poor lung function (2). There is also evidence that comorbid conditions may affect asthma outcomes among children, contributing to the increased burden of the disease (3). Modifiable risk factors, such as indoor environmental exposure and unhealthy dietary habits, may play an important role in increasing airway inflammation and the risk of asthma exacerbations (4,5). In particular, an unbalanced dietary intake has been increasingly recognized as an important modifiable risk factor for asthma (6), as it may contribute to an increased allergic airway inflammation by modulating innate and adaptive immune responses (7).
The Dietary Inflammatory Index (DII) is a score that defines an individual’s diet on a continuum, from the most anti-inflammatory to the most pro-inflammatory. It encompasses the whole diet of an individual, rather than isolated nutrients or food items (8). A previous study in Hispanic adults reported an association between a pro-inflammatory diet characterized by a high DII score, current asthma symptoms, and lower lung function as assessed by spirometry (8). A higher DII has also been associated with an increased odds of current wheeze in adults and children with atopic wheeze from the U.S. National Health and Nutrition Examination Survey (NHANES) (9).
The phenotypic characterization of asthmatic children based on risk factors, clinical outcomes and lung function may not be accurate enough to characterize children at high risk for poor outcomes. Previous studies aimed to provide a deeper characterization of childhood asthma at a phenotypic/endotypic level (10). In particular, two recent studies used data-driven approaches, such as Latent Class Analysis (LCA), to identify children at risk for asthma exacerbations (11,12).
Previous studies have demonstrated that a pro-inflammatory diet may increase the harmful effect of indoor pollutants on respiratory symptoms and lung function in children with asthma, suggesting that such modifiable exposures can synergistically worsen asthma outcomes (7,13). However, to date no studies have investigated the role of DII as a risk factor for asthma burden (i.e. exacerbations, comorbidities and lung function) linked to indoor exposures in children.
To address this knowledge gap, we applied LCA in a sample of children with persistent asthma. The aim of this study was to assess whether a pro-inflammatory diet was associated with worse asthma burden in three steps: 1) to identify asthma latent classes based on symptom history, indoor exposure and pulmonary function; 2) to identify the risk factors associated with latent class membership; 3) to estimate the probabilities of latent class membership with variation in DII.
Methods
Study design and study population
In the present cross-sectional study carried out between September 2013 and January 2018, 266 children aged 5–14 years with persistent asthma (cases) and 149 children without asthma (controls), were consecutively recruited as a part of the ongoing CHildhood ASthma and Environment Research Study-CHASER Study (ClinicalTrials.gov ID: NCT02433275), carried out at the outpatient clinic of Paediatric Allergology & Pulmonology of Institute of Research and Biomedical Innovation - National Research Council (IRIB - CNR) of Palermo, Italy.
All children were clinically evaluated by well-trained physicians (VM, SLG, GF). Diagnosis of persistent asthma was performed according to Global Initiative for Asthma (GINA, www.ginasthma.org) (14) and exacerbations requiring emergency visits in the last 12 months were also collected. All outpatient children who did not receive an asthma diagnosis served as controls.
The study was approved by local Ethics Committee and all participants provided written informed consent.
Procedures
Both children with asthma and controls were assessed with the following procedures. Atopy was defined as at least one positive (wheal ≥3 mm) skin prick tests to a panel of common aeroallergens (Dermatophagoides mix, cat, dog, pellitory, olive, cypress, mixed grasses, alternaria). Asthma control status was determined according to GINA.
Parents or legal guardians were interviewed through a modified version of the Italian Studies on Respiratory Disorders in Children and the Environment (SIDRIA) questionnaire (15). Maternal and paternal education, parental history of asthma, breastfeeding, physical activity, and current indoor exposures (mold, pets and second-hand smoke) were obtained through the questionnaires completed by parents. Rhino-conjunctivitis was defined as a positive answer to both the questions: “Have you ever had a problem with sneezing, or runny, or blocked nose apart from common cold or flu in the last 12 months?” and “In the past 12 months, has this nose problem been accompanied by itching and/or watering eyes?”. Eczema was defined as positive answers to both the following questions: “In the last 12 months, have you had an itchy rush which was coming and going for at least 6 months?” and “Has this itchy rush at any time affected any of the following places: folds of elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or eyes?”. The crowding index was defined as the total number of co-residents per household, divided by the total number of rooms, excluding the kitchen and bathrooms. The Family Affluence Scale (FAS) was computed (16).
Children’s dietary habits were assessed through a semi-quantitative 70-item Food Frequency Questionnaire (FFQ) filled in by parents during the visit. Details are provided in Appendix A – Methodological details.
Pulmonary function tests were performed. Respiratory impedance (Forced Oscillation Technique, FOT) was measured using the Quark i2m® Forced Oscillation Measurement system (Cosmed, Italy) based on a pseudo-random noise signal between 4 and 48 Hz, according to the ATS/ERS recommendations (17). The mean value of three valid measurements was retained, Z-scores were computed according to Calogero et al. (18). Spirometry was performed through a portable flow turbine spirometer (Pony FX portable spirometer, Cosmed, Italy), in accordance with ATS/ERS guidelines (19). The mean value of three valid measurements was retained; Z-scores were computed according to Global Lung Initiative 2012 equations that account for age, sex, race/ethnicity, and height (20).
Dietary Inflammatory Index (DII)®
To reflect rapid increases in understanding of the role of inflammation and diet in health, Hébert et al. created the DII, which reflects a robust literature base and standardization of individual intakes to global reference values (21,22). In brief, the investigators reviewed and scored 1,943 articles linking inflammation to 45 individual food parameters published between 1950 and 2010; points were then assigned to each parameter according to whether they increased (+1), decreased (−1), or had no effect (0) on six biomarkers of inflammation. An overall inflammatory effect score was then calculated for each food parameter (Table S1) (21), based on the ratio of the total weighted number of articles to the weighted pro- and anti-inflammatory articles for that parameter, followed by subtracting the anti- from the pro-inflammatory fraction. Parameters with a number of weighted articles greater than the median of 236 were assigned the full value of that score. Parameters with a total article weight less than 236 were adjusted by dividing it by 236, and then multiplying this fraction by the previously defined inflammatory effect score. Dietary intake data were adjusted against a reference global daily mean and standard deviation intake for each parameter to obtain Z-scores and centered percentiles. The global intake data were based on consumption data from 11 countries worldwide. Z-scores were then converted to centered proportions, which were then multiplied by food parameter–specific inflammation effect score to obtain food parameter–specific DII scores. These were then summed to get the overall DII score. Since its inception, the DII has been applied in a wide variety of populations to study the association between a pro-inflammatory diet and chronic conditions and health outcomes (23).
In our study, intake for each nutrient was estimated from the FFQ administered in CHASER. Out of the 45 dietary parameters encompassed by the DII, 26 were available (Table S1): carbohydrates; proteins; fiber; cholesterol; total, saturated, monounsaturated (MUFA), and polyunsaturated fats (PUFAs); omega-3 and omega-6 PUFAs; niacin; vitamins A, B1, B2, B6, B12, C, D, and E; iron; magnesium; zinc; selenium; folic acid; β-carotene; and total energy. Higher scores indicate a pro-inflammatory diet, while lower scores indicate an anti-inflammatory diet.
Questionnaires for asthmatic children
Children with asthma completed the Childhood Asthma Control Test (C-ACT, age 5–11 years) or Asthma Control Test (ACT, age 12–14 years) (24) for evaluating symptoms control, the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) (25) to assess disease related quality of life, and the Pittsburgh Sleep Quality Index (PSQI) (26) for assessing sleep quality. Higher ACT/C-ACT or PAQLQ scores represents better asthma control or better quality of life, while higher PSQI scores represent worse sleep quality.
Statistical analysis
Data were presented as absolute and percentage frequencies or as mean and standard deviation. Differences of categorical variables were compared using the X2 test, quantitative variables were compared using the Kruskal Wallis test to avoid distributional assumptions. Pairwise p-values were adjusted using the Benjamini & Hochberg method. LCA was used for identifying asthma phenotypes, using the R poLCA package. The variables included in the LCA based on literature (11,12) were: ≥3 exacerbations in the last 12 months (yes or no), comorbidity (yes, when the co-occurrence of at least one disease among rhino-conjunctivitis, eczema was reported; no, otherwise), indoor exposure (yes, when current exposure to at least one among mold, pets and second-hand smoke was reported; no, otherwise), FEV1/FVC <95% (yes or no) as synthetic spirometry parameter, Z-score of the area under the reactance curve (AX) >1.64 (yes or no) as synthetic FOT parameter. Further methodological details are provided in the Supplementary Material.
Three elastic net (EN) logistic regression models were used to identify the risk factors associated with latent class membership. In addition to DII, explanatory variables included in the models were: age (continuous variable); gender (male vs female); BMI (continuous variable); crowding index (continuous variable); maternal history of asthma (Y vs N); paternal history of asthma (Y vs N); maternal education level (>8 years vs ≤8 years); paternal education level (>8 years vs ≤8 years); breastfeeding ≥3 months (Y vs N); atopy (Y vs N); physical activity >3 times/week (Y vs N); and FAS (high/medium vs low).
The EN is a regularized regression method that linearly combines the L1 and L2 penalties, overcoming variable selection and multicollinearity problems. Although EN models are typically used for prediction, they can also be applied to make inference, ensuring good performances (27). Details concerning elastic net objective function are provided in the Supplementary Material. Although LASSO regression represents a very elegant and relatively widespread solution to carry out variable selection and parameter estimation simultaneously, as it is well known, it also exhibits some drawbacks deriving from the non-smoothness of the objective function. Consequently, some problems such as computation of standard errors may occur. In order to overcome these drawbacks and to carry out inference, the LASSO penalty was replaced with a new smooth version (IS-LASSO) in which the L1 penalty is replaced by a smooth approximation justified by the induced smoothing paradigm (28), as direct consequence of smoothing out the LASSO penalty, point estimates from IS-LASSO will never be exactly zero. Therefore, the model strategy applied allows to include all available confounders. The elastic net logistic regression models were computed using the islasso R package (29). In the implemented algorithm the penalization term is defined as
where α and λ are the tuning parameters, 0≤ ≤1 and =1 is the IS-LASSO penalty, and α =0 implies the ridge penalty. is the norm 1 of βs i.e. and is the norm 2 of βs i.e.. For each model, the best α was chosen based on Akaike Information Criterion (AIC) minimization, while the best was chosen using the cross-validation procedure. Estimates were expressed as log-odds ratios, and controls were used as the reference group. Probabilities of membership in Class 1 conditional to DII (for fixed values, average values for quantitative variables and reference group for categorical variables, of the other risk factors) were estimated as follows:
Analyses were performed with R 3.6.2 software. A p-value lower than 0.05 was considered statistically significant.
Results
Characteristics of study population
Characteristics of the study population by DII level are summarized in Table 1. Physical activity was more frequent in children with a DII between −1.29 and 0.58.
Table 1.
Subject characteristics by DII level expressed as tertiles.
| DII 1st tertile | DII 2nd tertile | DII 3rd tertile | p-value | |
|---|---|---|---|---|
| ≤ −1.29 | −1.29-0.58 | >0.58 | ||
| n | 136 | 137 | 141 | |
| Age, years | 9.19 ± 2.58 | 9.21 ± 2.30 | 9.28 ± 2.40 | 0.892 |
| Gender: Male | 87 (63.97) | 94 (68.61) | 93 (65.96) | 0.718 |
| BMI, Kg/m2 | 22.70 ± 8.18 | 22.72 ± 7.88 | 22.81 ± 8.44 | 0.976 |
| Physical activity (>3 times/week) | 74 (54.41) | 82 (60.29) | 60 (43.80) | 0.022 |
| Maternal history of asthma | 21 (15.79) | 20 (14.60) | 22 (15.94) | 0.945 |
| Paternal history of asthma | 25 (18.52) | 26 (18.98) | 17 (12.32) | 0.253 |
| Maternal education level (>8 years) | 112 (82.96) | 114 (83.21) | 114 (82.61) | 0.991 |
| Paternal education level (>8 years) | 106 (79.10) | 101 (74.26) | 107 (78.68) | 0.574 |
| Atopy, Y/N | 94 (83.19) | 101 (84.17) | 97 (78.23) | 0.436 |
| Crowding Index | 1.11 ± 0.43 | 1.07 ± 0.56 | 1.04 ± 0.44 | 0.307 |
| Breastfeeding (≥3 months), Y/N | 89 (71.20) | 77 (61.11) | 90 (69.23) | 0.195 |
| FAS | 0.549 | |||
| Low | 21 (18.10) | 21 (16.80) | 13 (10.66) | |
| Medium | 62 (53.45) | 67 (53.60) | 69 (56.56) | |
| High | 33 (28.45) | 37 (29.60) | 40 (32.79) |
Data are expressed as n (%) or mean ± SD, DII: Dietary Inflammatory Index. FAS: Family Affluence Scale. p-values come from Kruskal-Wallis test for quantitative variables and X2 test for categorical variables; p-values in bold are significant.
Latent Class Analysis (LCA)
Figure S1 represents the AIC obtained by 5 models; the 2-class solution was chosen as the best given the lowest AIC.
Figure 1 illustrates the two identified classes using the LCA. Class 1 (n=120, 45%) was labelled as “high burden” asthma. It was mainly composed of asthmatic children with ≥3 exacerbations during the last 12 months (90%). All the children in this class had at least one comorbidity; 53.8% had FEV1/FVC ≤95%. Moreover, 23.3% of children had Z-score AX >1.64, and 51.3% was subject to indoor exposures.
Figure 1. Asthma characteristics and response probabilities among the two latent classes.
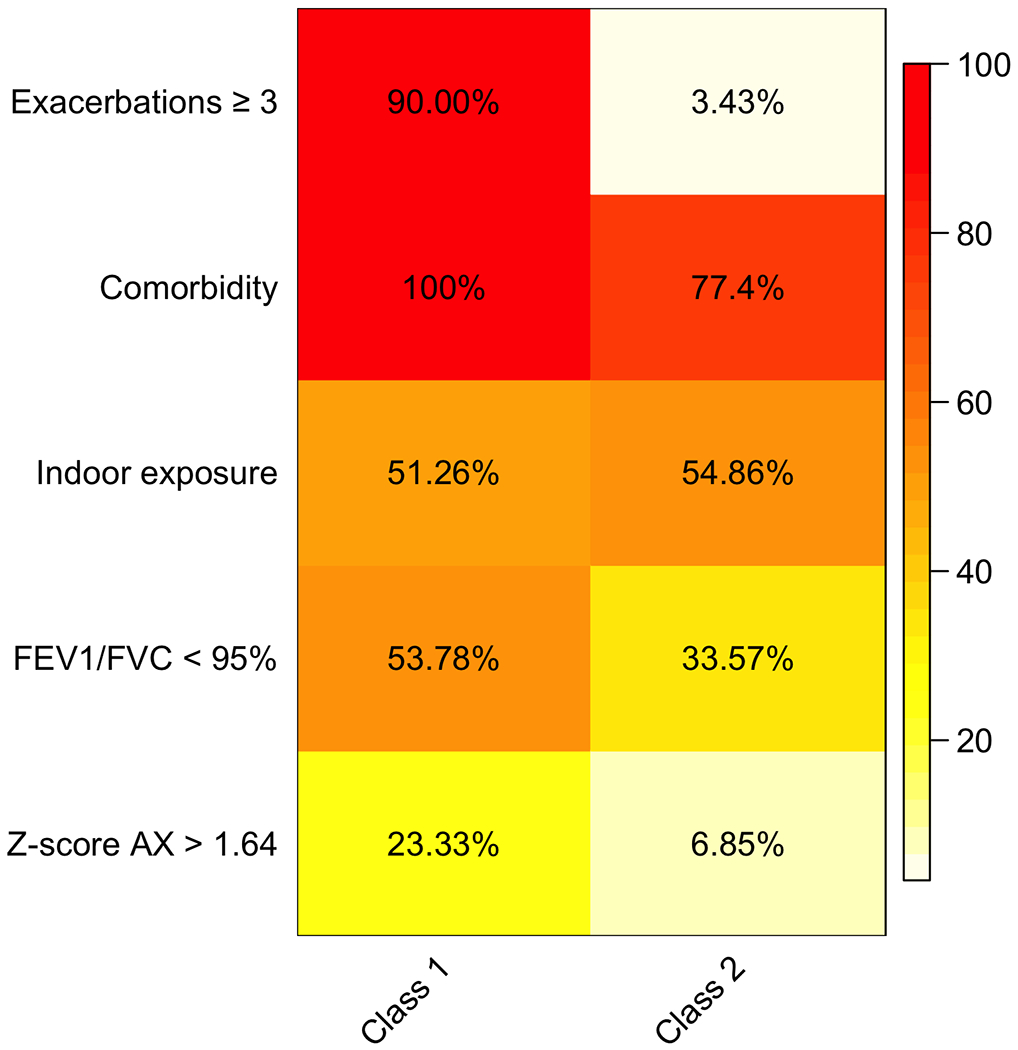
The figure illustrates the two classes identified by means of LCA. The proportion of each variable in each class is represented with a color scale spanning from white (0%) to red (100%).
Class 2 (n=146, 55%) was labelled as “low burden” asthma. Only 3.4% of children in this class had ≥3 exacerbations during the last 12 months; 77.4% of children had at least one comorbidity, 54.9% was subject to indoor exposures, 33.5% had FEV1/FVC <95%, and 6.5% had Z-score AX >1.64.
Comparisons between LCA classes and controls
Maternal history of asthma was more frequently observed in Class 1 than in Class 2 and controls. Children in Class 2 were younger. They had a higher crowding index and were more frequently atopic than children in Class 1 and controls (Table 2). Figure 2 reports the distribution of DII in LCA classes and controls. Children in Class 1 had a higher DII than children in Class 2. Children in Class 1 had more frequently persistent moderate/severe asthma with poorer control level, and they reported lower and higher scores of C-ACT and PSQI, respectively (Table 2). The distribution of children with FEV1 and FEV1/FVC below the LLN stratified by treatment assignment and latent class membership is shown in Figure S2.
Table 2.
Comparison of demographic and clinical characteristics among controls and LCA classes.
| controls | Class 1 | Class 2 | p-value | Class 1 vs controls | Class 2 vs controls | Class 2 vs Class 1 | |
|---|---|---|---|---|---|---|---|
| n | 149 | 120 | 146 | ||||
| Age, years | 9.70 ± 2.60 | 9.13 ± 2.38 | 8.84 ± 2.21 | 0.017 | 0.129 | 0.006 | 0.589 |
| Gender: Male | 92 (61.74) | 81 (67.50) | 101 (69.18) | 0.372 | 0.592 | 0.592 | 0.873 |
| BMI, Kg/m2 | 23.17 ± 8.29 | 22.52 ± 7.86 | 22.52 ± 8.27 | 0.673 | 0.791 | 0.773 | 1.000 |
| DII | 0.01 ± 1.89 | 0.26 ± 2.00 | −0.31 ± 1.84 | 0.065 | 0.555 | 0.308 | 0.042 |
| Physical activity (>3 times/week) | 83 (56.85) | 56 (47.46) | 77 (52.74) | 0.315 | 0.489 | 0.557 | 0.557 |
| Maternal history of asthma | 14 (9.46) | 26 (22.22) | 24 (16.67) | 0.016 | 0.020 | 0.147 | 0.329 |
| Paternal history of asthma | 21 (14.19) | 27 (23.08) | 20 (13.70) | 0.079 | 0.132 | 1.000 | 0.132 |
| Maternal education level (>8 years) | 125 (84.46) | 96 (81.36) | 120 (82.76) | 0.797 | 0.894 | 0.894 | 0.894 |
| Paternal education level (>8 years) | 116 (78.91) | 88 (75.21) | 111 (77.62) | 0.773 | 0.902 | 0.902 | 0.902 |
| Atopy, Y/N | 79 (68.10) | 99 (84.62) | 114 (91.20) | <0.001 | 0.007 | <0.001 | 0.168 |
| Crowding Index | 0.99 ± 0.41 | 1.06 ± 0.57 | 1.17 ± 0.45 | <0.001 | 0.455 | 0.006 | 0.194 |
| FAS | 0.483 | 0.576 | 0.576 | 0.576 | |||
| Low | 17 (12.88) | 19 (17.76) | 19 (15.20) | ||||
| Medium | 69 (52.27) | 56 (52.34) | 74 (59.20) | ||||
| High | 46 (34.85) | 32 (29.91) | 32 (25.60) | ||||
|
|
|||||||
| Persistent Asthma | 0.034 | ||||||
| Mild | n/a | 79 (65.83) | 114 (78.08) | ||||
| Moderate/Severe | n/a | 41 (34.17) | 32 (21.92) | ||||
| GINA Control | 0.001 | ||||||
| Controlled | n/a | 27 (22.50) | 56 (39.44) | ||||
| Partially controlled/Uncontrolled | n/a | 93 (77.50) | 86 (60.56) | ||||
| Asthma duration (yrs) | n/a | 4.75 ± 3.01 | 4.39 ± 2.91 | 0.357 | |||
| Assigned treatment | 0.001 | ||||||
| ICS§ | n/a | 90 (75.00) | 127 (86.99) | ||||
| ICS-LABA | n/a | 30 (27.02) | 19 (16.67) | ||||
| Median ICS dose μg·day−1§ | n/a | 200.00 (150.00) | 100.00 (100.00) | <0.001 | |||
| Emergency visits* | n/a | 25 (22.32) | 17 (14.91) | 0.207 | |||
| C-ACT/ACT total score | n/a | 17.12 ± 4.44 | 18.53 ± 3.77 | 0.011 | |||
| C-ACT/ACT ≤19 | n/a | 77 (65.81) | 61 (55.96) | 0.167 | |||
| PAQLQ total score | n/a | 5.21 ± 1.22 | 5.37 ± 1.32 | 0.469 | |||
| PAQLQ ≤5 | n/a | 30 (40.54) | 25 (37.31) | 0.826 | |||
| PSQI total score | n/a | 5.37 ± 2.39 | 4.73 ± 2.08 | 0.048 | |||
| PSQI ≥5 | n/a | 61 (59.80) | 47 (50.54) | 0.248 | |||
Data are expressed as n (%) or mean ± SD
last 12 months; C-ACT: Childhood-Asthma Control Test; FAS: Family Affluent Scale; ICS: Inhaled Corticosteroids; LABA: Long-Acting Beta Agonists; DII: Dietary Inflammatory Index; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PSQI: Pittsburgh Sleep Quality Index. p-values come from Kruskal-Wallis test for quantitative variables and from X2 for categorical variables; p-values in bold are significant ; pairwise p-values were adjusted using Benjamini & Hochberg method.
Figure 2.
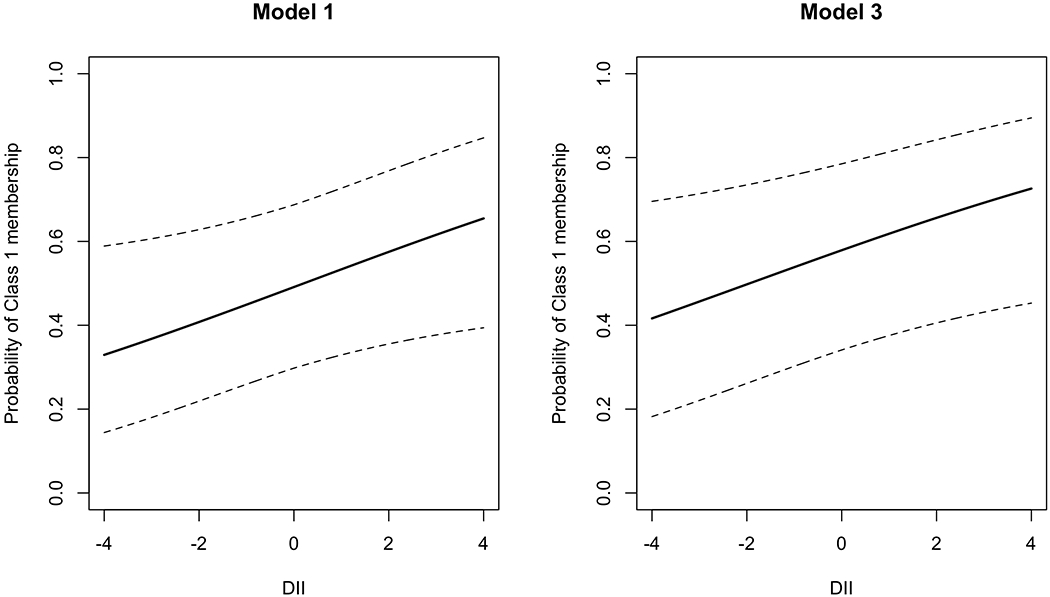
Distribution of DII score in controls and classes
Significant differences were observed for all the spirometry and FOT parameters between Class 1 and controls (Table 3). The FOT parameters were not significantly different between Class 2 and controls.
Table 3.
Comparison of spirometry and Forced Oscillation Technique (FOT) parameters among controls and LCA classes.
| controls | Class 1 | Class 2 | p-value | Class 1 vs controls | Class 2 vs controls | Class 2 vs Class1 | |
|---|---|---|---|---|---|---|---|
| n | 149 | 120 | 146 | ||||
| Spirometry parameters | |||||||
|
| |||||||
| FEV1 | 1.97 ± 0.65 | 1.72 ± 0.63 | 1.75 ± 0.53 | <0.001 | 0.003 | 0.008 | 0.886 |
| Z-score FEV1 | 0.19 ± 1.94 | −0.35 ± 1.61 | −0.20 ± 1.24 | 0.014 | 0.019 | 0.103 | 0.723 |
| FVC | 2.23 ± 0.77 | 2.06 ± 0.76 | 2.02 ± 0.63 | 0.030 | 0.121 | 0.033 | 0.906 |
| Z-score FVC | 0.15 ± 1.82 | 0.09 ± 1.57 | −0.01 ± 1.35 | 0.986 | 0.945 | 0.676 | 0.881 |
| FEV1/FVC | 0.89 ± 0.07 | 0.84 ± 0.08 | 0.87 ± 0.07 | <0.001 | <0.001 | 0.064 | 0.009 |
| Z-score FEV1/FVC | 0.04 ± 1.15 | −0.71 ± 1.23 | −0.28 ± 1.20 | <0.001 | <0.001 | 0.054 | 0.013 |
| FEF25–75 | 2.31 ± 0.88 | 1.82 ± 0.79 | 1.96 ± 0.70 | <0.001 | <0.001 | 0.001 | 0.346 |
| Z-score FEF25–75 | −0.32 ± 1.32 | −0.94 ± 1.21 | −0.63 ± 0.93 | <0.001 | <0.001 | 0.064 | 0.082 |
|
| |||||||
| FOT parameters | |||||||
|
| |||||||
| Rrs6 | 5.73 ± 2.36 | 6.91 ± 2.30 | 6.04 ± 1.90 | <0.001 | <0.001 | 0.458 | 0.004 |
| Z-score Rrs6 | −0.50 ± 1.40 | 0.27 ± 1.34 | −0.26 ± 1.38 | <0.001 | <0.001 | 0.278 | 0.005 |
| Rrs8 | 5.47 ± 2.10 | 6.62 ± 2.15 | 5.91 ± 1.88 | <0.001 | <0.001 | 0.157 | 0.013 |
| Z-score Rrs8 | −0.59 ± 1.39 | 0.22 ± 1.32 | −0.25 ± 1.46 | <0.001 | <0.001 | 0.102 | 0.019 |
| Rrs10 | 5.28 ± 1.97 | 6.41 ± 2.05 | 5.74 ± 1.79 | <0.001 | <0.001 | 0.104 | 0.015 |
| Z-score Rrs10 | −0.48 ± 1.45 | 0.36 ± 1.34 | −0.12 ± 1.51 | <0.001 | <0.001 | 0.073 | 0.020 |
| Xrs6 | −2.03 ± 1.14 | −2.66 ± 1.44 | −2.31 ± 2.67 | <0.001 | 0.021 | 0.416 | 0.303 |
| Z-score Xrs6 | 0.14 ± 1.01 | −0.41 ± 1.25 | −0.04 ± 2.14 | <0.001 | 0.012 | 0.568 | 0.138 |
| Xrs8 | −1.63 ± 1.06 | −2.28 ± 1.29 | −1.85 ± 1.97 | <0.001 | 0.002 | 0.448 | 0.054 |
| Z-score Xrs8 | 0.09 ± 0.96 | −0.52 ± 1.16 | −0.07 ± 1.79 | <0.001 | 0.001 | 0.582 | 0.018 |
| Xrs10 | −1.34 ± 1.07 | −2.00 ± 1.26 | −1.59 ± 1.74 | <0.001 | <0.001 | 0.299 | 0.042 |
| Z-score Xrs10 | 0.26 ± 0.96 | −0.35 ± 1.11 | 0.07 ± 1.61 | <0.001 | <0.001 | 0.417 | 0.018 |
| AX | 19.11 ± 21.71 | 31.79 ± 24.15 | 22.66 ± 34.33 | <0.001 | 0.001 | 0.508 | 0.020 |
| Z-score AX | −0.26 ± 1.22 | 0.60 ± 1.31 | −0.04 ± 1.52 | <0.001 | <0.001 | 0.351 | 0.001 |
| FRES | 20.00 ± 6.72 | 24.57 ± 7.62 | 21.87 ± 6.62 | <0.001 | <0.001 | 0.056 | 0.005 |
| Z-score FRES | −0.31 ± 1.20 | 0.49 ± 1.39 | −0.01 ± 1.24 | <0.001 | 0.464 | 0.657 | 0.934 |
Data are expressed as n (%) or mean ± SD. AX: area under the reactance curve; BDR: bronchodilator response; FEV1: forced expiratory volume in 1 s; FRES: resonant frequency; FVC: forced vital capacity; FEF25–75: forced expiratory flow at 25–75% of the FVC; LLN: lower limit of normal. Rrs6: resistance at 6 Hz; Rrs8: resistance at 8 Hz; Rrs10: resistance at 10 Hz; Xrs6: reactance at 6 Hz; Xrs8: reactance at 8 Hz; Xrs10: reactance at 10 Hz; p-values come from Kruskal-Wallis test for quantitative variables and from X2 for categorical variables; p-values in bold are significant; pairwise p-values were adjusted using Benjamini & Hochberg method.
According to C. Calogero et al., Respiratory impedance and bronchodilator responsiveness in healthy children aged 2–13 years, Pediatr. Pulmonol. 48 (2013) 707–715
Multivariable analyses
Results from the three elastic net logistic regression models are shown in Table 4. Risk factors associated with membership to Class 1 when compared to the control group (EN-Model 1) were: maternal history of asthma, atopy and DII. Likewise, risk factors associated with Class 2 membership when compared to the control group (EN-Model 2) were: crowding index and atopy. The only risk factor significantly associated with membership to Class 1 when compared to Class 2 was DII (EN-Model 3).
Table 4.
Elastic net regression models: risk factors for class membership.
| MOD 1: Class1 vs controls | MOD 2: Class 2 vs controls | MOD 3: Class 1 vs Class 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-value | Estimate | Std. Error | p-value | Estimate | Std. Error | p-value | |
| (Intercept) | 0.678 | 0.762 | 0.373 | −0.069 | 0.82 | 0.933 | −0.13 | 0.762 | 0.864 |
| Age (unit increase) | −0.171 | 0.091 | 0.06 | −0.269 | 0.1 | 0.007 | 0.078 | 0.093 | 0.402 |
| Gender: Male | 0.129 | 0.216 | 0.551 | 7.62e-06 | 0.000287 | 0.979 | −4.49e-11 | 2.59e-09 | 0.986 |
| BMI (unit increase) | 0.022 | 0.028 | 0.426 | 0.042 | 0.028 | 0.142 | −0.009 | 0.027 | 0.745 |
| Crowding Index (unit increase) | 0.21 | 0.213 | 0.325 | 0.693 | 0.319 | 0.03 | −1.10e-04 | 0.004 | 0.978 |
| Maternal History of asthma, Y/N | 0.444 | 0.225 | 0.049 | 6.79e-06 | 0.000260 | 0.979 | 0.213 | 0.334 | 0.524 |
| Paternal History of Asthma, Y/N | 0.152 | 0.222 | 0.495 | −5.59e-06 | 0.000245 | 0.982 | 0.388 | 0.325 | 0.232 |
| Maternal education level (>8 years) | −0.252 | 0.215 | 0.241 | −5.31e-06 | 0.000243 | 0.983 | −2.09e-09 | 7.47e-08 | 0.978 |
| Paternal education level (>8 years) | −0.056 | 0.212 | 0.79 | 3.67e-06 | 0.000239 | 0.988 | −2.24e-04 | 0.008 | 0.978 |
| Breastfeeding (≥3 months), Y/N | 0.26 | 0.218 | 0.233 | −0.103 | 0.296 | 0.727 | 0.584 | 0.301 | 0.052 |
| Atopy, Y/N | 0.61 | 0.22 | 0.005 | 1.298 | 0.368 | <0.001 | −0.819 | 0.431 | 0.058 |
| Physical activity (>3 times/week) | −0.463 | 0.213 | 0.03 | −0.042 | 0.293 | 0.885 | −0.314 | 0.263 | 0.231 |
| DII (unit increase) | 0.169 | 0.083 | 0.042 | −0.03 | 0.089 | 0.734 | 0.164 | 0.079 | 0.038 |
| FAS: Medium vs Low | −0.045 | 0.199 | 0.82 | −2.81e-09 | 0.000239 | 1 | −0.061 | 0.294 | 0.835 |
| FAS: High vs Low | −0.233 | 0.197 | 0.236 | −0.065 | 0.305 | 0.831 | −2.95e-11 | 2.42e-09 | 0.99 |
|
| |||||||||
| α | 0 | 0.70 | 0.80 | ||||||
| λ | 4.87 | 3.55 | 1.98 | ||||||
Estimates are expressed as log-odds ratio.
As the DII increased from −4.0 to +4.0 (as the diet becomes more pro-inflammatory), the probability of membership to Class 1 rather than to the control group (EN-Model 1) increased from 0.33 to 0.65, while the probability of membership to Class 1 rather than to Class 2 (EN-Model 3) increased from 0.42 to 0.73 (Figure 3).
Figure 3. Estimated probabilities of asthma class by DII score.
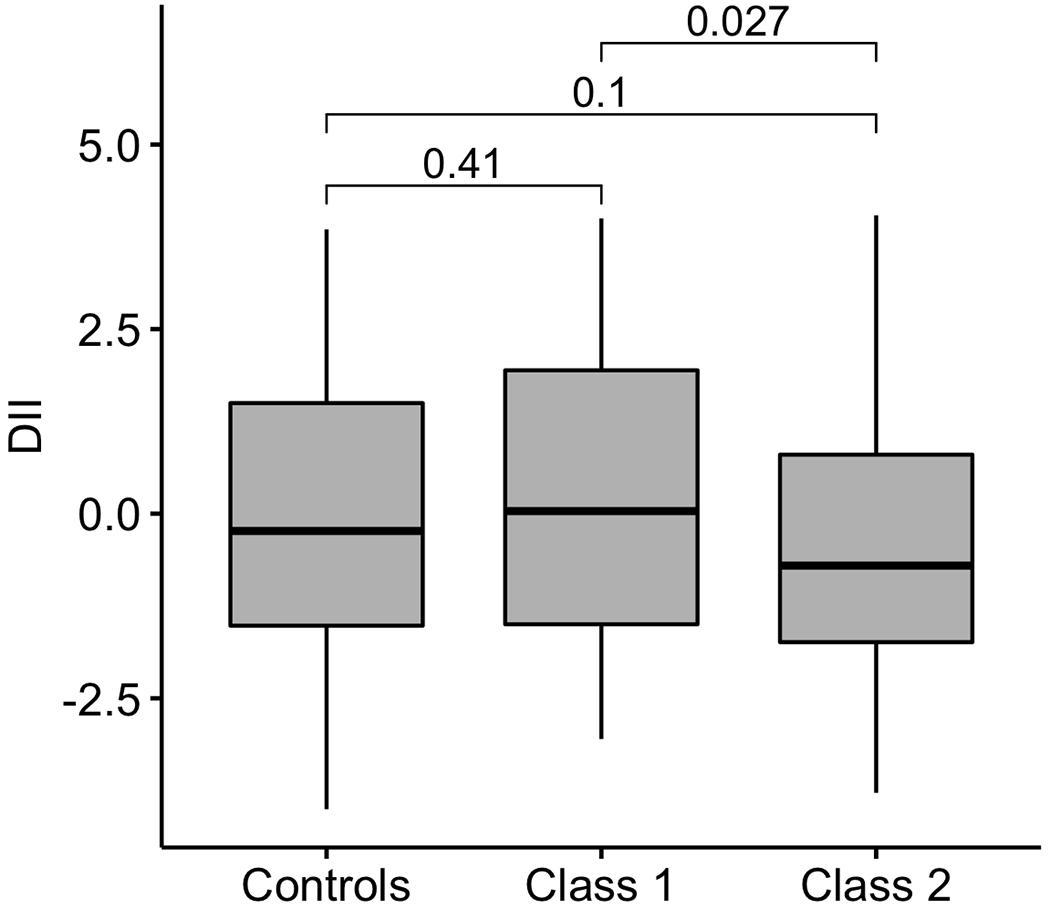
Probability curves for the class 1 membership with variation in DII based on model 1 (left panel) and model 3 (right panel)
Discussion
In this study, we used LCA for a deeper characterization of childhood asthma at a phenotypic level. We identified two classes of children with persistent asthma with different disease burden (exacerbations, comorbidities and lung function) linked to indoor exposures. Additionally, we demonstrated the role of a pro-inflammatory diet as a risk factor for high asthma burden linked to indoor exposures.
Children in Class 1, “high burden”, had more frequently exacerbations and comorbidity and worse lung function parameters. Children in Class 2, “low burden”, had less exacerbations and less impaired lung function. Moreover, children in Class 1 had a higher DII score than children in Class 2. A higher DII score in this class suggests that a pro-inflammatory diet could contribute to a higher burden of asthma and is in agreement with a previous study in adults showing an association among DII score, increased systemic inflammation and lower lung function (30). Furthermore, the finding of more frequent comorbidity in this class is in line with previous data in children (31) highlighting that coexisting health conditions have a significant impact on individuals with asthma putting them at higher risk of exacerbations and healthcare utilization. Unsurprisingly, with respect to children in Class 2, children in Class 1 had more frequently persistent moderate/severe asthma with poorer control level and reported lower scores of C-ACT and higher scores of PSQI. This confirms that higher severity of asthma symptoms as well as lower level of control may lead to a higher burden of disease in these children (32) as also reflected by patients’ own perception of poor controlled symptoms (33) and sleep quality (34). The higher asthma burden in Class 1 is also evidenced by the significantly greater proportion of children with FEV1 and FEV1/FVC below LLN, who mostly were assigned an ICS+LABA controller therapy. Furthermore, our analysis of FOT parameters showed worse values in children in Class 1, suggesting the occurrence of a more pronounced inflammatory involvement of peripheral airways in this group. This finding provides additional evidence for the role of FOT in the phenotypic characterization of children with asthma.
Results from multivariable analyses allowed us to confirm the role of a high DII score as a risk factor for Class 1 membership. Moreover, we found that the probability of Class 1 membership increased when the DII score increased, emphasizing that a pro-inflammatory diet might be considered a key factor in the systemic inflammation of children with high burden of asthma.
Literature evaluating the association between dietary exposures and asthma morbidity has provided mixed results. A previous population-based case–control study of Puerto Rican children did not find significant associations between dietary pattern (evaluated by means of a scoring system other than DII) and asthma exacerbations (35). These results are not consistent with our findings because the authors did not consider DII in their analyses; moreover, the outcome of interest was asthma exacerbations, which could be considered as a component of asthma burden. More recently, a cross-sectional study on Turkish adults with asthma showed a significant inverse correlation between DII computed on a 24-hour recall questionnaire and asthma control (36). Despite relevant differences in study population and design, these results are in line with our findings, highlighting the adverse effect of a pro-inflammatory diet on asthma control, which can be considered a component for asthma burden.
When considering the burden of asthma linked to indoor exposures, our findings are in line with a previous U.S. study finding that a pro-inflammatory diet (higher omega-6 intake) was associated with amplified effect of indoor PM2.5 on asthma morbidity (more severe symptoms and reduced lung function) (13), although several differences in asthma definition (self-reported diagnosis), study population (minority schoolchildren, mostly with moderate/severe asthma), design and exposures assessment. In particular, the authors did not consider DII in their analyses; omega-3 and omega-6 intake were derived through dietary intake assessed weekly via a quantitative 7-day recall food frequency questionnaire. Similarly, a community-based survey on Portuguese schoolchildren reported that the exposure effect of PM2.5 and PM10 on those with asthma was higher for those having a pro-inflammatory diet compared to those having an anti-inflammatory diet, as assessed through DII computed on a 24-hour recall questionnaire answered by children. Again, differences in asthma definition (medical diagnosis; medical diagnosis or positive bronchodilation (+BD); medical diagnosis with asthma symptoms or +BD; medical diagnosis and asthma treatment), study population (general population of schoolchildren), study setting (school) and exposures assessment have to be considered (7). It should be noted that in both the aforementioned studies, results were obtained on a small sample of children with asthma and therefore need to be confirmed in larger populations.
Our study has some limitations. We could not include data about local and/or systemic inflammation, although the endotype characterization of this population of children goes beyond the aims of the current study. Children’s dietary habits were based on a semi-quantitative FFQ, consisting of a year based pre-specified food list and serving size, which may not exactly reflect each child’s eating pattern at the time of compilation. However, a longitudinal study reported that the DII is relatively constant over long periods (37). Moreover, misclassification of food frequency consumption by the participants’ parents might have been occurred due to recall bias. Nonetheless, parental report of FFQ among children has been shown to be accurate (38). Out of the 45 DII food parameters, only 26 were available in the current study population; however, we were able to include in our analyses data about some of the most relevant macro and micronutrients for which a role in asthma has been described (39). Another limitation is the cross-sectional design; based on our current findings, future studies with a longitudinal design will be important to address the temporal sequence of exposure and outcome. Convenience sampling may impose various types of bias; however, consecutive sampling includes all patients who are accessible within the defined study time period, and the resulting sample is thus more likely to represent the target population than samples resulting from other convenience sampling schemes (40). Finally, our findings in Italian children may limit the generalizability of the current study results in other populations.
At the same time, this study has considerable strengths, including a large sample of well characterized children with persistent asthma. Furthermore, the current study benefited from an advanced statistical analysis, applying unsupervised methods by using LCA. LCA offers the advantage that respondents are assigned into classes based on their characteristics, without any interference from the researcher. Although our results do not provide a comprehensive characterization of persistent asthma phenotypes in children, they contribute to provide some insight into the potential role of dietary habits in increasing the overall burden of the disease. An additional strength is that we applied three elastic net logistic regression models to investigate which independent variables influenced LC membership. Indeed, such models combine LASSO penalty with ridge penalty, allowing variable selection and avoiding multicollinearity problems at the same time. Moreover, using the IS-LASSO estimator allowed us to estimate parameter uncertainty and consequently to make inference.
The relationship between DII, as a novel marker of dietary inflammation, with asthma outcomes, has not been fully clarified so far. Children with asthma are highly vulnerable to modifiable environmental exposures such as diet and pollution that may concur to worsen asthma morbidity. Therefore, it is critical to thoroughly examine the role of diet in the context of environmental exposures linked to asthma morbidity. The current study identified two classes of children with persistent asthma with different disease burden (exacerbations, comorbidities and lung function) linked to indoor exposures, providing further support to the role of a pro-inflammatory diet as a risk factor for higher asthma burden in children. Longitudinal studies are needed to confirm our results over time in order to evaluate whether change toward healthier dietary patterns improves asthma outcomes. The prospective evaluation would also give the opportunity to assess whether controller therapy may have an impact on the relationship between DII and asthma burden over time. Lastly, given the multidimensional nature of asthma, a comprehensive approach considering both dietary and indoor air exposures should be encouraged in order to improve the burden of disease in children.
Supplementary Material
Key message.
We identified two latent classes of children with persistent asthma associated with different disease burden. An increasing Dietary Inflammatory Index was associated with high-burden asthma linked to indoor exposures, providing further evidence about the role of a pro-inflammatory diet in asthma morbidity.
Acknowledgments
We thank all participating children and their families for their invaluable contribution to the study.
Funding
Dr Forno’s contribution was funded in part by grant HL149693 from the U.S. National Institutes of Health (NIH).
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Ethical Approval and Trial Registration Statements
The study was approved by local Ethics Committee (Protocol N. 08/2014) and registered on ClinicalTrials.gov (ID: NCT02433275).
REFERENCES
- 1.Ferrante G, La Grutta S. The Burden of Pediatric Asthma. Front Pediatr 2018: 6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijnenburg MW, Fleming L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir Med 2020. [DOI] [PubMed] [Google Scholar]
- 3.Mirabelli MC, Hsu J, Gower WA. Comorbidities of asthma in US children. Respir Med 2016: 116:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cibella F, Cuttitta G, Della Maggiore R et al. Effect of indoor nitrogen dioxide on lung function in urban environment. Environ Res 2015: 138:8–16. [DOI] [PubMed] [Google Scholar]
- 5.Corbo GM, Forastiere F, De Sario M et al. Wheeze and asthma in children: associations with body mass index, sports, television viewing, and diet. Epidemiology 2008: :747–755. [DOI] [PubMed] [Google Scholar]
- 6.Han Y-Y, Forno E, Holguin F et al. Diet and asthma: an update. Curr Opin Allergy Clin Immunol 2015: 15:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Castro Mendes F, Paciência I, Cavaleiro Rufo J et al. The inflammatory potential of diet impacts the association between air pollution and childhood asthma. Pediatr Allergy Immunol 2020: 31:290–296. [DOI] [PubMed] [Google Scholar]
- 8.Han Y-Y, Jerschow E, Forno E et al. Dietary patterns, asthma, and lung function in the Hispanic Community Health Study/Study of Latinos. Ann Am Thorac Soc 2020: 17:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Y-Y, Forno E, Shivappa N et al. The dietary inflammatory index and current wheeze among children and adults in the United States. J Allergy Clin Immunol Pract 2018: 6:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagliardo R, La Grutta S, Chanez P et al. Non-invasive markers of airway inflammation and remodeling in childhood asthma. Pediatr Allergy Immunol 2009: 20:780–790. [DOI] [PubMed] [Google Scholar]
- 11.Grunwell JR, Gillespie S, Morris CR et al. Latent Class Analysis of School-Age Children at Risk for Asthma Exacerbation. J Allergy Clin Immunol Pract 2020: 8:2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick AM, Bacharier LB, Jackson DJ et al. Heterogeneity of mild to moderate persistent asthma in children: confirmation by latent class analysis and association with 1-year outcomes. J Allergy Clin Immunol Pract 2020: 8:2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigham EP, Woo H, McCormack M et al. Omega-3 and omega-6 intake modifies asthma severity and response to indoor air pollution in children. Am J Respir Crit Care Med 2019: 199:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2016 GINA Report, Global Strategy for Asthma Management and Prevention. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA). https://ginasthma.org/wp-content/uploads/2016/05/WMS-GINA-2016-main-Pocket-Guide.pdf [Google Scholar]
- 15.Galassi C, De Sario M, Biggeri A et al. Changes in prevalence of asthma and allergies among children and adolescents in Italy: 1994–2002. Pediatrics 2006: 117:34–42. [DOI] [PubMed] [Google Scholar]
- 16.Currie C, Molcho M, Boyce W et al. Researching health inequalities in adolescents: the development of the Health Behaviour in School-Aged Children (HBSC) family affluence scale. Soc Sci Med 2008: 66:1429–1436. [DOI] [PubMed] [Google Scholar]
- 17.Beydon N, Davis SD, Lombardi E et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007: 175:1304–1345. [DOI] [PubMed] [Google Scholar]
- 18.Calogero C, Simpson SJ, Lombardi E et al. Respiratory impedance and bronchodilator responsiveness in healthy children aged 2–13 years. Pediatr Pulmonol 2013: 48:707–715. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V et al. Standardisation of spirometry. Eur Respir J 2005: 26:319–338. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012: 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivappa N, Steck SE, Hurley TG et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014: 17:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavicchia PP, Steck SE, Hurley TG et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 2009: 139:2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hébert JR, Shivappa N, Wirth MD et al. Perspective: the Dietary Inflammatory Index (DII)—lessons learned, improvements made, and future directions. Adv Nutr 2019: 10:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu AH, Zeiger R, Sorkness C et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007: 119:817–825. [DOI] [PubMed] [Google Scholar]
- 25.Ricci G, Dondi A, Baldi E et al. Use of the Italian version of the Pediatric Asthma Quality of Life Questionnaire in the daily practice: Results of a prospective study. BMC Pediatr 2009: 9. doi: 10.1186/1471-2431-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989: 28:193–213. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer RM, Redd A, Carroll RJ. On the impact of model selection on predictor identification and parameter inference. Comput Stat 2017: 32:667–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cilluffo G, Sottile G, La Grutta S et al. The Induced Smoothed lasso: A practical framework for hypothesis testing in high dimensional regression. Stat Methods Med Res 2020: 29:765–777. [DOI] [PubMed] [Google Scholar]
- 29.Sottile G, Cilluffo G, Muggeo VM. The R package islasso: estimation and hypothesis testing in lasso regression. 2019. doi: 10.13140/RG.2.2.16360.11521 [DOI] [Google Scholar]
- 30.Wood LG, Shivappa N, Berthon BS et al. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy 2015: 45:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MR, Leo HL, Baptist AP et al. Asthma outcomes in children and adolescents with multiple morbidities: findings from the National Health Interview Survey. J Allergy Clin Immunol 2015: 135:1444–1449. [DOI] [PubMed] [Google Scholar]
- 32.Montalbano L, Ferrante G, Montella S et al. Relationship between quality of life and behavioural disorders in children with persistent asthma: a Multiple indicators Multiple causes (MiMic) model. Sci Rep 2020: 10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montalbano L, Cilluffo G, Gentile M et al. Development of a nomogram to estimate the quality of life in asthmatic children using the Childhood Asthma Control Test. Pediatr Allergy Immunol 2016: 27:514–520. [DOI] [PubMed] [Google Scholar]
- 34.Yuksel H, Sogut A, Yilmaz O et al. Evaluation of sleep quality and anxiety–depression parameters in asthmatic children and their mothers. Respir Med 2007: 101:2550–2554. [DOI] [PubMed] [Google Scholar]
- 35.Han Y-Y, Forno E, Alvarez M et al. Diet, lung function, and asthma exacerbations in Puerto Rican children. Pediatr Allergy Immunol Pulmonol 2017: 30:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Özbey Ü, Uçar A, Shivappa N et al. The Relationship between Dietary Inflammatory Index, Pulmonary Functions and Asthma Control in Asthmatics. Iran J Allergy Asthma Immunol 2019: :605–614. [DOI] [PubMed] [Google Scholar]
- 37.Tabung FK, Steck SE, Zhang J et al. Longitudinal changes in the dietary inflammatory index: an assessment of the inflammatory potential of diet over time in postmenopausal women. Eur J Clin Nutr 2016: 70:1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrows TL, Warren JM, Colyvas K et al. Validation of overweight children’s fruit and vegetable intake using plasma carotenoids. Obesity 2009: 17:162–168. [DOI] [PubMed] [Google Scholar]
- 39.Bonanno A, Gangemi S, La Grutta S et al. 25-Hydroxyvitamin D, IL-31, and IL-33 in children with allergic disease of the airways. Mediators Inflamm 2014: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathieson K Making sense of biostatistics: types of nonprobability sampling. J Clin Res Best Pr 2014: 10:1–2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


