Abstract
Individuals with bipolar disorder (BP) show abnormalities in the default mode network (DMN), a brain network active at rest and during self-referential cognition. In healthy individuals, the DMN is anti-correlated (strongly negatively correlated) with the task positive network (TPN), a brain network that is active during attention demanding tasks. Mindfulness has been linked to changes in DMN connectivity. We investigated the effects of mindfulness-based cognitive therapy (MBCT) versus supportive psychotherapy (SP) on the relationship between these two networks in individuals with BP. We identified differences in BOLD resting state DMN-TPN connectivity between healthy controls (HC; n=22) and individuals with DSM-IV BP before treatment (n=22) using a seed region in the dorsolateral prefrontal cortex (DLPFC), a key TPN node. We then explored changes in DMN-TPN connectivity after 12 weeks of MBCT or SP. Before treatment, BP individuals showed positively correlated activity and the HC group showed negatively correlated activity between the DLPFC and the posterior cingulate cortex (PCC). After treatment, BP individuals who received MBCT showed negatively correlated DLPFC-PCC activity. BP individuals who received SP did not show a significant change. Mindfulness-based cognitive therapy can restore the anti-correlation between the DMN and TPN in individuals with BP.
Keywords: Mindfulness, posterior cingulate cortex, dorsolateral prefrontal cortex, attention
1. Introduction
The default mode network (DMN) is a network of structurally and functionally connected brain regions that was first identified during “passive” states (Gusnard and Raichle, 2001; Raichle et al., 2001). Since its initial discovery, our conceptualization of the DMN has evolved over time; the DMN has now been linked with a range of higher-order cognitive processes such as thinking about oneself in the past and future (e.g., Buckner and Carroll, 2007; Schacter et al., 2008) and thinking about others (Amodio and Frith, 2006; Saxe et al., 2004). DMN activity has also been linked to ongoing spontaneous cognition or mind-wandering in general (Andrews-Hanna et al., 2010; Christoff et al., 2009; Mason et al., 2007).
In healthy individuals, the DMN has also been found to be anti-correlated or strongly negatively correlated with a task positive network (TPN), a network of brain regions involved in attention demanding tasks (Buckner et al., 2008; Fox et al., 2005). That is, during tasks requiring high cognitive demand, the TPN typically increases its activity while the DMN decreases its activity. Conversely, during tasks requiring low cognitive demand, the DMN typically increases its activity while the TPN decreases its activity. Therefore, in healthy individuals, this DMN-TPN anti-correlation appears to support the ability to flexibly shift attention from internal processes to the external environment for cognitively demanding tasks (Buckner et al., 2008; Fox et al., 2005).
Individuals with bipolar disorder (BP), a mood disorder characterized by depressive episodes and/or mania, show resting state DMN hyperactivity (e.g., Gong et al., 2019; Liu et al., 2012). During attention demanding tasks, individuals with BP have also demonstrated persistent DMN hyperactivation and TPN hypoactivation (e.g., Fernández-Corcuera et al., 2013). In other words, individuals with BP do not show the typical DMN-TPN anti-correlation seen in healthy individuals.
Consistent with the fact that the DMN is involved in mind-wandering, interventions targeting mind-wandering behavior, such as mindfulness, have been linked with changes in DMN activation and connectivity (Brewer et al., 2011; Doll et al., 2015; Hölzel et al., 2011). During mindfulness practice, individuals purposely redirect their attention to the present moment in a non-judgmental way. Since DMN-TPN dynamics are involved in the flexible shift of attention, it is possible that mindfulness interventions can also change DMN-TPN connectivity. In this study, we sought to investigate the effects of mindfulness-based cognitive therapy (MBCT) versus supportive psychotherapy (SP) on the relationship between the DMN and TPN in individuals with BP. We hypothesized that: 1) individuals with BP would not show the typical anti-correlation between the DMN and the TPN compared to a healthy control sample, 2) BP individuals who received MBCT would show a restoration of the DMN-TPN anti-correlation, and 3) changes in DMN-TPN connectivity would be associated with changes in mindfulness.
2. Methods
2.1. Sample
This research study was approved by Massachusetts General Hospital’s Institutional Review Board, the Mass General Brigham Human Research Committee (IRB# 2009p001987) and conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants provided written informed consent prior to participation. Participants who completed baseline MRI scans (described below) were 22 adults (age M = 37.5 years old, SD = 12.60 years, 8 females) who met DSM-IV criteria for bipolar disorder. Participants with BP had an average Young Mania Rating Scale (Young et al., 1978) score of 6.60 (SD = 5.91), suggesting low levels of residual manic symptoms, and an average Hamilton Depression Rating Scale (Hamilton, 1960) score of 10.60 (SD = 6.57) suggesting mild levels of depression. The average age of onset for bipolar disorder was 18.09 years old (SD = 8.25 years). Participants took mood-stabilizing medications including lithium (n = 7), lamotrigine (n = 6), valproate (n = 1), antipsychotics including quetiapine (n = 3), aripiprazole (n = 4), risperidone (n = 1), selective serotonin reuptake inhibitors including citalopram (n = 2), sertraline (n = 1), escitalopram (n = 1), paroxetine, (n = 1), and anxiolytics including clonazepam (n = 2), lorazepam (n = 2). The individuals with BP were compared with 22 healthy controls (age M = 33.32 years old, SD = 8.93 years, 8 females) who had MRI scans with the same acquisition parameters. The two groups did not significantly differ in age (t(42) = −1.27, p = 0.21) or years of education (t(41) = 1.11, p = 0.27).
Out of the 22 individuals with BP, n = 15 also completed MRI scans after either 12 weeks of mindfulness-based cognitive therapy (MBCT; n = 10) or supportive psychotherapy (SP; n = 5). There were no post-treatment MRI scans for n = 7 individuals due to participants either withdrawing from treatment and therefore being ineligible for the post-treatment MRI scans (2 patients in the MBCT group and 2 patients in the SP group), or due to the fact that the MRI scans were optional (2 patients in the MBCT group and 1 patient in the SP group completed treatment but declined participating in the post-treatment MRI scan). Therefore, 82% of patients completed treatment, which is comparable to other studies of MBCT in individuals with bipolar disorder (e.g. average attrition of 16% in a review by Bojic and Becerra, 2017). Of note, there have been attrition rates as high as 42% in individuals with bipolar disorder (Miklowitz et al., 2015). Due to the final sample size being small for this set of analyses, the analyses comparing the two interventions should be considered exploratory.
The MBCT and SP groups did not significantly differ in years of education (MBCT group M = 15.5 years, SD = 1.58, SP group M = 15.6 years, SD = 0.89 years; t(13) = −0.13, p = 0.90). However, the individuals in the MBCT group were slightly older (M = 45.9 years old, SD = 10.13) than individuals in the SP group (M = 33.4 years old, SD = 14.45; t(13) = 1.96, p = 0.07). Therefore, as a conservative measure, subsequent analyses with these groups included age as a covariate.
2.2. Interventions
The present study was a secondary analysis of data collected during the main clinical trial that is reported in Deckersbach et al. (2012). The MBCT intervention is described in detail in the main outcomes paper. Patients in the MBCT and SP groups participated in 12 weekly 120-minute group sessions facilitated by two doctoral-level psychologists. For the MBCT group, early sessions focused on psychoeducation, mood monitoring, and identifying risk factors for relapse such as irregular activity patterns, lack of sleep and stress. Participants were next introduced to the concept of mindfulness and began short body scans, mindful movement exercises, and breath awareness sitting meditations. They also began practicing mindfulness during routine daily activities. Later sessions included mindfulness to difficult emotions and thoughts, self-compassion activities, and loving kindness meditations. Problem solving was included throughout the sessions to address treatment obstacles and stressors that may have led to treatment withdrawal.
For the SP group, the general treatment techniques used are described in detail by Pinsker and colleagues (Pinsker and Rosenthal, 1988, Pinsker, 1997). SP is a common mode of treatment (often in conjunction with psychopharmacology) for many patients with psychiatric or chronic medical problems in the community. The focus of SP was on reflecting and expressing feelings about current life issues. Participants received support and comfort when coping with difficult situations, depression, mood swings, or anger. For this study, SP also included psychoeducation and mood charting to make it more similar to MBCT. Therefore, SP provided nonspecific treatment factors (empathy, support, etc.) but, unlike MBCT, it did not provide systematic training in cognitive, behavioral, or mindfulness skills. In this sense, SP could be considered an active control intervention group.
2.3. Five Facet Mindfulness Questionnaire
Multiple questionnaires were administered before and after treatment in the main clinical trial as reported in Deckersbach et al. (2012). Since we were interested in mindfulness-related changes, for the present study, we focused on the mindfulness outcome measure. Participants completed the Five Facet Mindfulness Questionnaire (FFMQ; Baer et al., 2006) before and after MBCT or SP. The FFMQ is a 39-item self-report scale on five factors of mindfulness: observing (“When I’m walking, I deliberately notice the sensations of my body moving”), describing (“I’m good at finding words to describe my feelings”), acting with awareness (“When I do things, my mind wanders off and I’m easily distracted”), non-judgment of inner experience (“I criticize myself for having irrational or inappropriate emotions”), and non-reactivity to inner experience (“I perceive my feelings and emotions without having to react to them”). Items are rated on a 1–5 scale with 1 = never or very rarely true and 5 = very often or always true. Of note, all the items for the acting with awareness and non-judgment of inner experience factors are reverse coded, such that lower scores indicate higher levels of acting with awareness and non-judgment.
2.4. MRI Data Acquisition
For the present study, we analyzed previously unpublished blood oxygen level dependent (BOLD) resting state scan sequence data. Functional magnetic resonance imaging (fMRI) data during a cognitive task in these patients was included in Ellard et al. (2019). Participants were scanned using a 3.0 Tesla Siemens Trio “Tim” system whole body high-speed imaging device equipped for echo planar imaging (EPI; Siemens Medical Systems, Iselin, NJ) at Massachusetts General Hospital’s Athinoula A. Martinos Center for Biomedical Imaging. After automated scout and shimming procedures to optimize field homogeneity (Reese et al., 1995), a high-resolution 3D MPRAGE sequence (TR/TE/flip angle = 2530ms/3.39ms/7°) with an in-plane resolution and slice thickness of 1.3 mm, was collected to be used for co-registration with fMRI data. BOLD resting state images were acquired (TR/TE/flip angle = 3000ms/30ms/85°) with a slice thickness of 3 mm.
2.5. Data Analysis
2.5.1. MRI data analyses.
SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) was used for temporal and spatial preprocessing of the BOLD resting state scan images. For each subject, fMRI images were corrected for differences in slice acquisition times, realigned to a reference image using a 6-parameter rigid body spatial transformation and a least squares approach, co-registered to high-resolution structural images, segmented into spatially normalized tissue maps, stereotactically normalized to the standardized space established by the Montreal Neurological Institute (MNI; http://www.bic.mni.mcgill.ca), and smoothed/convolved with a three-dimensional Gaussian filter of 6 mm full-width at half maximum (FWHM).
CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012; www.nitrc.org/projects/conn) was next used to create functional connectivity maps representing time-series correlations between BOLD signal from a left dorsolateral prefrontal cortex (DLPFC) seed and every voxel in the brain. BOLD signal from white matter and cerebrospinal fluid, and realignment parameters were identified as confounders and their effects were removed. We then extracted Fisher-transformed correlation coefficient values reflecting the correlation between the DLPFC seed region and the posterior cingulate cortex (PCC).
2.5.2. Mindfulness measure analyses.
We used SPSS Version 24 to conduct exploratory repeated measures ANOVAs to investigate the effects of MBCT and SP on scores from the self-report measure of mindfulness, the FFMQ. We also ran Pearson’s r bivariate correlations between the Fisher-transformed correlation coefficient values and scores on the FFMQ to explore if changes in mindfulness were associated with changes in functional connectivity.
3. Results
3.1. Changes in Mindfulness
For the FFMQ, there was a significant treatment group (MBCT versus SP) by time (pre versus post treatment) interaction effect (F(1,13) = 9.084, p = 0.01, partial eta2 = 0.41). Follow-up independent samples t-tests revealed that individuals with BP who received MBCT had a significant increase pre to post treatment in acting with awareness (t(14) = −2.18, p = 0.047) and non-judgment of inner experience (t(15) = −2.24, p = 0.041) factors of mindfulness relative to individuals with BP who received SP (as previously described in the Methods section, items on these scales were reverse coded such that lower scores or decreases in scores signified increased acting with awareness and non-judgment). Individuals with BP who received MBCT also showed a trending-level of significance increase in the observing factor of mindfulness relative to individuals who received SP (t(15) = 2.04, p = 0.059). Finally, individuals with BP who received MBCT did not significantly differ from those who received SP pre to post treatment in the describing or non-reactivity to inner experience factors of mindfulness (all ps > 0.06).
3.2. MRI Data
3.2.1. Pre-treatment individuals with bipolar disorder versus healthy controls.
Before treatment, there was a significant difference between the BP and HC group in DMN-TPN connectivity (t(42) = 4.604, p < 0.001). Individuals with BP showed positively correlated activity between the DLPFC and the PCC (MNI coordinates = −10, −44, 38, k = 153 voxels, Z-score = 4.31). The HC group, in contrast, showed negatively correlated activity between these regions (Figure 1).
Figure 1. Individuals with BP Pre Treatment > HC Resting State DMN-TPN Functional Connectivity.
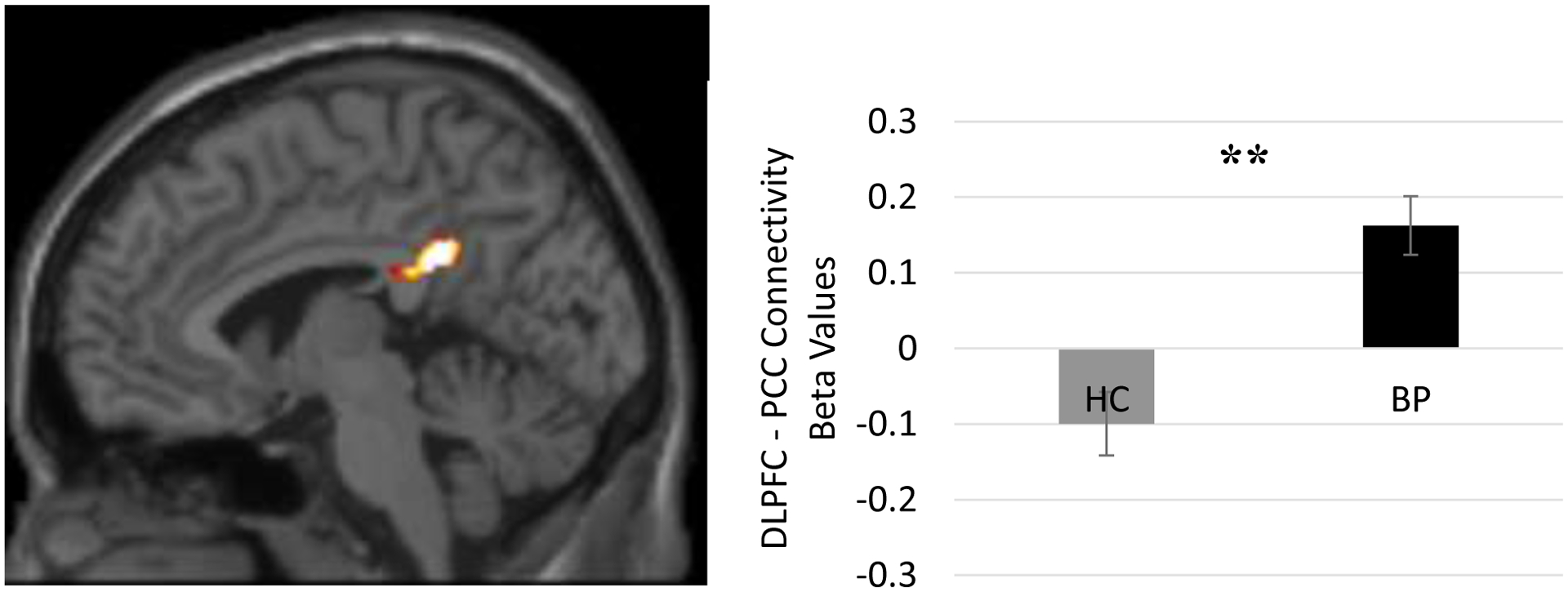
Before treatment, there was a significant difference between the bipolar (BP) and healthy control (HC) group in resting state functional connectivity between core regions of the Task Positive Network (dorsolateral prefrontal cortex [DLPFC]) and the Default Mode Network (posterior cingulate cortex [PCC]; t(42) = 4.604, p < 0.001). Individuals with BP showed positively correlated activity between the DLPFC and the PCC (MNI coordinates = −10, −44, 38, k = 153 voxels, Z-score = 4.31). The HC group, in contrast, showed negatively correlated activity between these regions. Note: **p < 0.001
3.2.2. MBCT versus SP.
There was a significant interaction between treatment group (MBCT versus SP) and time (pre versus post treatment; F(1,12) = 7.04, p = 0.021, partial eta2 = 0.37) in DLPFC-PCC connectivity. Follow-up paired t-tests revealed a significant change from pre to post treatment for the MBCT treatment group (paired t(9) = 4.345, p = 0.002, Cohen’s d = 1.374). That is, individuals with BP who received MBCT showed a significant change and now showed negatively correlated DLPFC-PCC activity (Figure 2). Importantly, individuals with BP who received SP did not show a significant change.
Figure 2. MBCT > SP Pre Minus Post Treatment DMN-TPN Functional Connectivity.
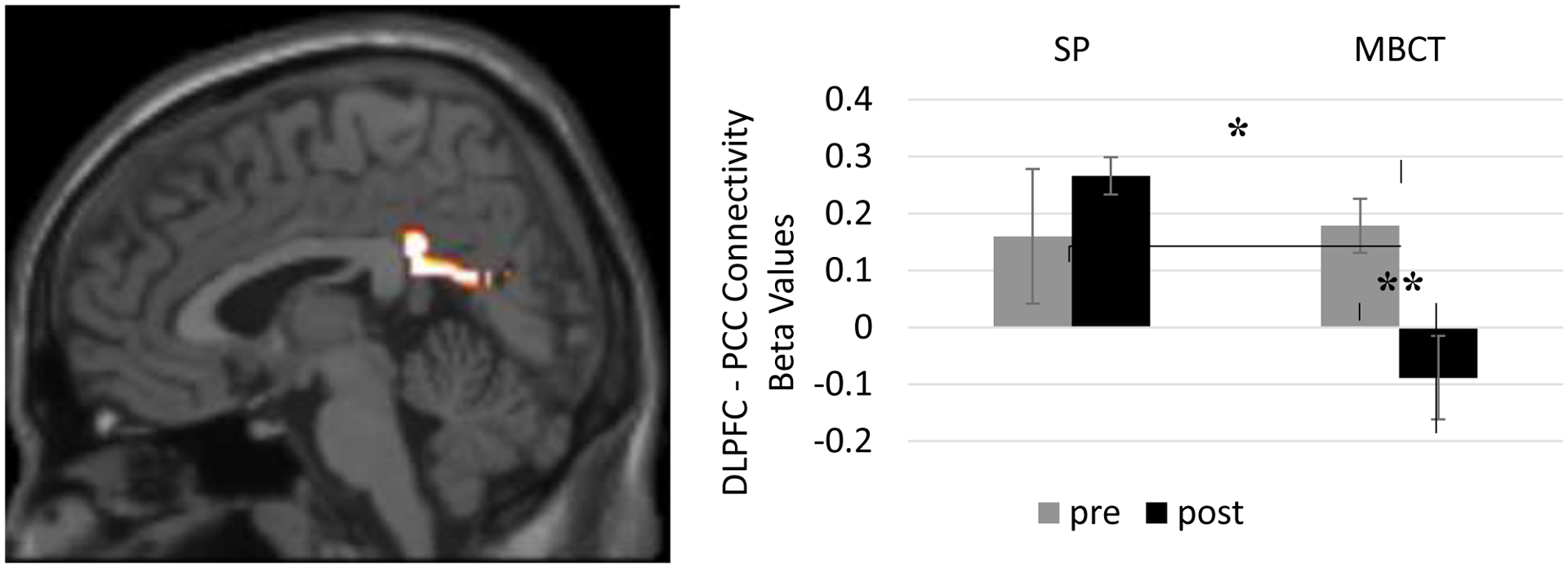
Individuals with bipolar disorder (BP) who received Mindfulness-Based Cognitive Therapy (MBCT) showed a significant change in resting state functional connectivity between core regions of the Task Positive Network (dorsolateral prefrontal cortex [DLPFC]) and the Default Mode Network (posterior cingulate cortex [PCC]) following treatment (paired t(9) = 4.345, p = 0.002, Cohen’s d = 1.374). Individuals with bipolar disorder who received Supportive Psychotherapy did not show a significant change in functional DLPFC-PCC connectivity. Note: *p < 0.05, **p < 0.005
Post-hoc whole-brain analyses investigating the wider effects of MBCT and SP can be found in Table 1.
Table 1.
Post-Hoc Whole Brain Results
| Mindfulness Based Cognitive Therapy > Supportive Psychotherapy | Montreal Neurological Institute Coordinates (x, y, z) | Cluster Size (k; voxels) | Z-Score |
|---|---|---|---|
| Superior Temporal Gyrus | 66, −6, 0 | 941 | 3.65 |
| Precuneus | 18, −50, 38 | 1275 | 3.27 |
| Superior Temporal Gyrus | −52, 16, −16 | 768 | 3.10 |
| Hippocampus | −30, −10, −12 | 142 | 3.05 |
| Lentiform Nucleus | 22, −6, −8 | 275 | 3.04 |
| Anterior Cingulate | 2, 4, −12 | 182 | 2.89 |
| Hippocampus | −20, −38, 2 | 60 | 2.87 |
| Middle Temporal Gyrus | 52, 12, −28 | 61 | 2.84 |
| Inferior Parietal Lobule | 44, −44, 28 | 69 | 2.84 |
| Thalamus | 2, −20, 4 | 90 | 2.78 |
| Superior Temporal Gyrus | 68, −24, 10 | 97 | 2.67 |
| Inferior Temporal Gyrus | 54, 2, −44 | 51 | 2.61 |
| Superior Frontal Gyrus | 18, 60, 14 | 79 | 2.56 |
| Middle Occipital Gyrus | 50, −72, 4 | 66 | 2.47 |
| Hippocampus | 28, −32, −4 | 50 | 2.23 |
| Supramarginal Gyrus | 52, −58, 32 | 116 | 2.12 |
| Supportive Psychotherapy > Mindfulness Based Cognitive Therapy | Montreal Neurological Institute Coordinates (x, y, z) | Cluster Size (k; voxels) | Z-Score |
| Cerebellum | −50, −66, −24 | 1507 | 4.03 |
| Cerebellar Tonsil | 32, −44, −42 | 371 | 3.48 |
| Superior Frontal Gyrus | 20, 10, 62 | 208 | 3.37 |
| Brainstem | 6, −4, −32 | 143 | 3.28 |
| Medulla | 6, −42, −48 | 107 | 2.93 |
| Fusiform Gyrus | −42, −32, −26 | 55 | 2.72 |
| Superior Frontal Gyrus | −30, −2, 70 | 182 | 2.68 |
| Middle Occipital Gyrus | −26, −78, 4 | 82 | 2.66 |
| Cerebellar Tonsil | −10, −50, −48 | 94 | 2.56 |
| Uncus | 34, −8, −36 | 125 | 2.55 |
| Precuneus | −12, −64, 46 | 60 | 2.53 |
| Superior Frontal Gyrus | 18, 50, −22 | 72 | 2.49 |
| Cerebellum | −22, −48, −38 | 178 | 2.40 |
Note. Top: Regions that showed functional connectivity changes linked to mindfulness based cognitive therapy. Bottom: Regions that showed functional connectivity changes linked to supportive psychotherapy. Post-hoc analyses were conducted as significance level p < 0.05 and a cluster threshold of k ≥ 50 voxels.
3.2.3. Correlations between connectivity and mindfulness.
There was a trending-level correlation between changes in DLPFC-PCC connectivity and change in the acting with awareness subscale of the FFMQ (r(13) = −0.542, p = 0.056; Figure 3). As previously mentioned in the Methods section, all the items for the acting with awareness subscale are reverse coded, such that lower scores indicate higher levels of acting with awareness. In other words, the negative correlation coefficient meant that the greater the change in DMN-TPN connectivity, the greater the increase in acting with awareness. There was no significant correlation between changes in DLPFC-PCC connectivity and change in non-judgment of inner experience or observing factors of mindfulness (all ps > 0.06).
Figure 3. Correlation Between Change in FFMQ and Change in DMN-TPN Functional Connectivity.
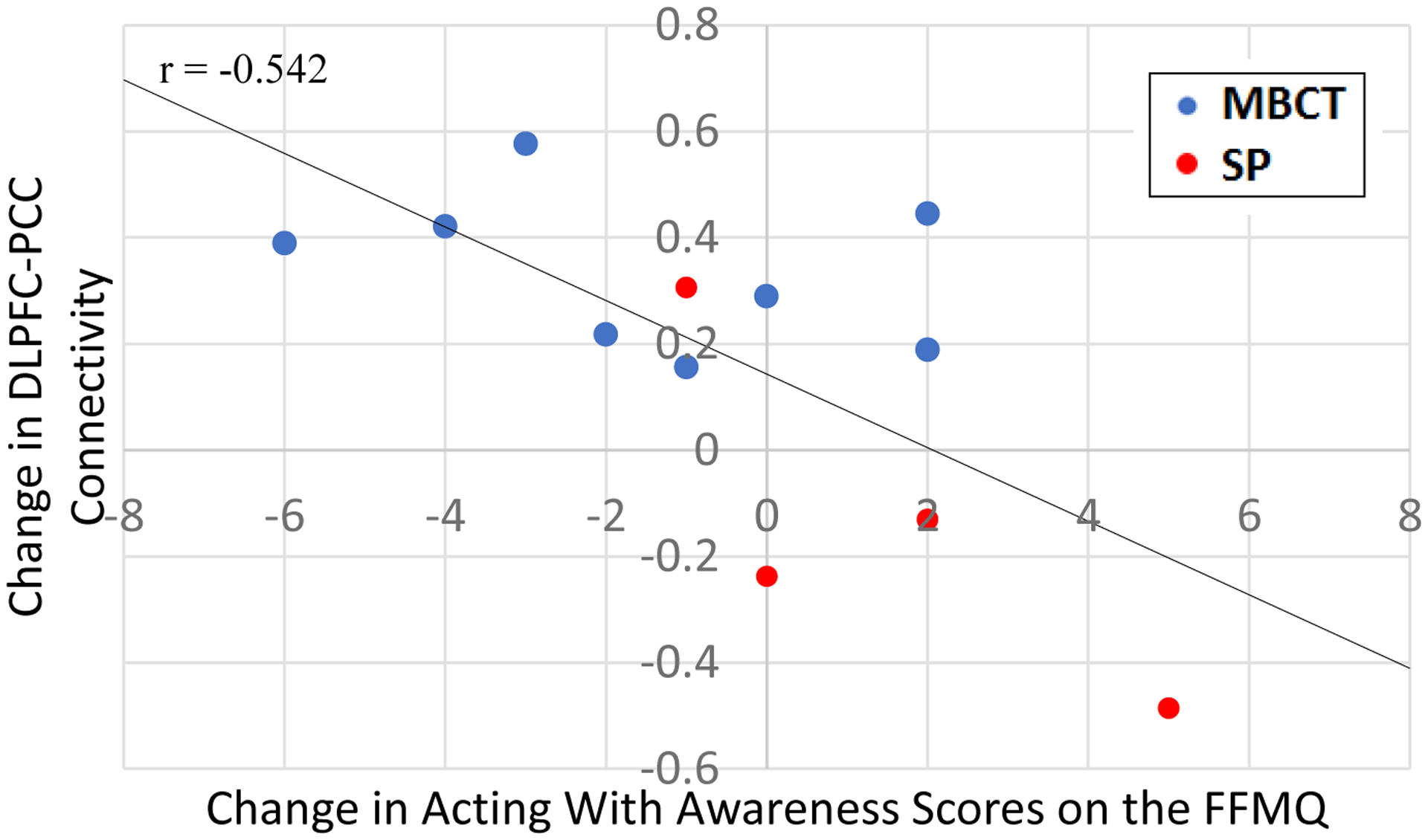
There was a correlation of trending-level of significance (r(13) = −0.542, p = 0.056) between the change in connectivity between the dorsolateral prefrontal cortex (DLPFC) and the posterior cingulate cortex (PCC) and the change in the Acting with Awareness scale of the Five Facet Mindfulness Questionnaire (FFMQ). All items on the Acting with Awareness scale are reverse coded, such that lower scores indicate higher levels of acting with awareness. In other words, the negative correlation coefficient means that the greater the change in DMN-TPN connectivity, the greater the increase in acting with awareness. MBCT = Mindfulness-Based Cognitive Therapy group, SP = Supportive Psychotherapy Group
4. Discussion
In this study, we sought to investigate the effects of mindfulness-based cognitive therapy (MBCT) versus supportive psychotherapy (SP) on the relationship between the DMN and the TPN in individuals with BP. Consistent with prior literature (Fernández-Corcuera et al., 2013; Gong et al., 2019; Liu et al., 2012), we first found that, prior to treatment, our sample of individuals with BP showed positively correlated activity between the DLPFC, a key node of the TPN, and the PCC, a key node of the DMN (Figure 1). This was relative to a sample of healthy controls who showed the typical negatively correlated or anti-correlated activity between the DMN and TPN.
However, after receiving a mindfulness-based intervention, MBCT, these individuals with BP demonstrated a restoration of the typical anti-correlated pattern of DMN-TPN connectivity; that is, they now showed negatively correlated activity between the DLPFC and the PCC (Figure 2). Importantly, individuals with BP who received a control treatment condition, SP, did not show a significant change in DMN-TPN connectivity, and continued to show positively correlated activity between the DLPFC and PCC.
Our findings expand upon a previous study investigating the neural effects of MBCT on bipolar disorder. In a study by Ives-Deliperi et al. (2013), individuals with BP who received MBCT showed increased BOLD activation in the PCC during an instructed mindfulness task. Our findings suggest that MBCT can also affect PCC functional connectivity, and during resting state (i.e., when participants are not being instructed to engage in a specific type of cognition). Further, our study included an active control supportive psychotherapy intervention rather than a wait-list, no intervention, control group, thus showing that MBCT’s effects on the PCC are not necessarily due to receiving any kind of intervention or psychoeducation in general.
Our results also lend support to the idea that MBCT can affect a specific type of attention regulation (e.g., Hölzel et al., 2011; Marchand, 2014). In our study, MBCT appears to restore the typical anti-correlation between the DMN and TPN, which, in healthy individuals, supports the ability to flexibly redirect attention from internal processes to the external environment for cognitively demanding tasks (Buckner et al., 2008; Fox et al., 2005). Since previous studies have shown that individuals with BP continue to have DMN hyperactivation and TPN hypoactivation during attention demanding tasks (e.g., Fernández-Corcuera et al., 2013), future studies should investigate whether MBCT would facilitate the ability to freely engage the TPN during an effortful task while disengaging the DMN in individuals with BP.
Although limited by our small sample sizes, our exploratory correlational analyses further support that MBCT and DMN-TPN connectivity could be linked to changes in a specific form of attention in our BP sample. The change in DMN-TPN connectivity had a medium effect size (r(13) = −0.542) relationship specifically with changes in the FFMQ acting with awareness factor. Items on this subscale such as “I am easily distracted” and “It seems I am running on ‘automatic’ without much awareness of what I’m doing” demonstrate that this facet of mindfulness, in particular, relates to the ability to exert control over the focus of attention. MBCT-related changes in other facets of mindfulness, such as non-judgment of inner experience or observing factors of mindfulness, were not correlated with changes in DMN-TPN connectivity. In order to explore whether MBCT-related changes were due to overall improvement in general attention, a post-hoc analysis was conducted with a measure of inattention that was collected as part of the larger clinical trial (the Adult ADHD Self-Report Scale [ASRS]; Deckersbach et al., 2012). Specifically, we conducted a post-hoc repeated measures ANOVA with ASRS inattention scores as a covariate. The interaction effect between treatment type (MBCT vs. SP) and time (pre vs. post) on DMN-TPN connectivity remained significant, even after controlling for changes in general inattention (F(1,10) = 7.653, p = 0.02, partial eta2 = 0.43). This suggests that the effects of MBCT were not due to general improvements in attention. Future investigation of the neural correlates of specific aspects of mindfulness may help further elucidate MBCT’s different and specific mechanisms of action. This line of research has been identified as necessary for the future of mindfulness research (e.g., Hölzel et al., 2011; Tang et al., 2015).
4.1. Limitations
There are several limitations with this study. As previously mentioned, due to our small sample sizes, our findings are exploratory and should be replicated in a future study with larger sample sizes. Although this study benefited from having an active control intervention group (SP) rather than a waitlist control group, future studies would benefit from the inclusion of different kinds of active intervention control groups to better identify specific mechanisms of action associated with different facets of mindfulness. Neuromodulation studies can also be designed to target different DMN and TPN regions to investigate their roles in specific types of mindfulness and mind-wandering behavior. For example, transcranial Direct Current Stimulation of a different DMN node, the inferior parietal lobule, has been specifically linked to decreasing negative, past-oriented mind-wandering thoughts (Chou et al., 2020). Finally, future studies may also include other patient populations to understand the specificity of the present findings for specific disorders (e.g., if individuals with major depressive disorder show a similar pattern of pre to post resting state DMN-TPN functional connectivity).
Highlights.
Healthy controls show anti-correlated default mode & task positive network activity
Individuals with bipolar disorder do not show this anti-correlation
This anti-correlation was not restored after supportive psychotherapy
This anti-correlation was restored after mindfulness-based cognitive therapy
Acknowledgements
This work was supported by the National Institutes of Mental Health [1K23MH074895] to Dr. Thilo Deckersbach. This work was also supported by the National Institute of Neurological Disorders and Stroke (NINDS) Training Program in Recovery and Restoration of CNS Health and Function [T32 NS100663]; and the Tiny Blue Dot Foundation to Dr. Tina Chou. The funding sources had no involvement in study design, data collection, analysis, interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Conflicts of Interest
The authors declare that there are no conflicts of interest. In the last three years, Dr. Chou’s sources of funding include NIH National Institute of Neurological Disorders and Stroke (NINDS) Training Program in Recovery and Restoration of CNS Health and Function (T32 NS100663) and Tiny Blue Dot Foundation. Dr. Dougherty’s research has been funded by NIMH, IOCDF, and Medtronic. He has received honoraria and consultation fees from Medtronic. Dr. Nierenberg’s research has been funded by Acadia, Alkermes, Assurex, Esai, FitBit, Ginger.io, J and J, Jazz, Lundbeck, Myriad, Neurocrine, Neuronetics, NeuroRx, Otsuka, Sage, Shire, Sunovion, Supernus, and Takeda. Dr. Deckersbach’s research has been funded by Assurex and Sunovion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD, 2006. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci 7(4), 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL, 2010. Evidence for the default network’s role in spontaneous cognition. J. Neurophysiol 104(1), 322–335. 10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R, Smith G, Hopkins J, Krietemeyer J, Toney L, 2006. Using self-report assessment methods to explore facets of mindfulness. Assessment 13, 27–45. 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- Bojic S, Becerra R, 2017. Mindfulness-Based Treatment for Bipolar Disorder: A systematic review of the literature. Eur. J. Psychol 13, 573–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, Kober H, 2011. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. U. S. A 108(50), 20254–20259. 10.1073/pnas.1112029108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC, 2007. Self-projection and the brain. Trends Cogn. Sci 11(2), 49–57. 10.1016/j.tics.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Chou T, Hooley JM, Camprodon JA, 2020. Transcranial direct current stimulation of default mode network parietal nodes decreases negative mind-wandering about the past. Cognit. Ther. Res 44(1), 10–20. 10.1007/s10608-019-10044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW, 2009. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U. S. A 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckersbach T, Hölzel BK, Eisner LR, Stange JP, Peckham AD, Dougherty DD, Rauch SL, Lazar S, Nierenberg AA, 2012. Mindfulness-based cognitive therapy for nonremitted patients with bipolar disorder. CNS Neurosci. Ther 18, 133–141. 10.1111/j.1755-5949.2011.00236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, Sorg C, 2015. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front. Hum. Neurosci, 9(461). 10.3389/fnhum.2015.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Gosai AK, Felicione JM, Peters AT, Shea CV, Sylvia LG, Nierenberg AA, Widge AS, Dougherty DD, Deckersbach T, 2019. Deficits in frontoparietal activation and anterior insula functional connectivity during regulation of cognitive-affective interference in bipolar disorder. Bipolar Disord., 21(3), 244–258. 10.1111/bdi.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Corcuera P, Salvador R, Monté GC, Salvador Sarró S, Goikolea JM, Amann B, Moro N, Sans-Sansa B, Ortiz-Gil J, Vieta E, Maristany T, McKenna PJ, Pomarol-Clotet E, 2013. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J. Affect. Disord 148(2–3), 170–178. 10.1016/j.jad.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Chen G, Jia Y, Zhong S, Zhao L, Luo X, Qiu S, Lai S, Qi Z, Wang Y, 2019. Disrupted functional connectivity within the default mode network and salience network in unmedicated bipolar II disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 88, 11–18. doi: 10.1016/j.pnpbp.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, 2001. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci 2(10), 685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U, 2011. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci 6(6), 537–559. 10.1177/1745691611419671 [DOI] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Howells F, Stein DJ, Meintjes EM, Horn N, 2013. The effects of mindfulness-based cognitive therapy in patients with bipolar disorder: A controlled functional MRI investigation. J. Affect. Disord 150(3), 1152–1157. 10.1016/j.jad.2013.05.074 [DOI] [PubMed] [Google Scholar]
- Liu CH, Ma X, Li F, Wang YJ, Tie CL, Li SF, Chen TL, Fan T, Zhang Y, Dong J, Yao L, Wu X, Wang CY, 2012. Regional homogeneity within the default mode network in bipolar depression: a resting-state functional magnetic resonance imaging study. PLoS One 7(11), e48181. 10.1371/journal.pone.0048181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR, 2014. Neural mechanisms of mindfulness and meditation: evidence from neuroimaging studies. World J. Radiol 6(7), 471–479. 10.4329/wjr.v6.i7.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN, 2007. Wandering minds: the default network and stimulus-independent thought. Science 315(5810), 393–395. 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Semple RJ, Hauser M, Elkun D, Weintraub MJ, Dimidjian S, 2015. Mindfulness-Based Cognitive Therapy for perinatal women with depression or bipolar spectrum disorder. Cognit. Ther. Res 39, 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H, 1997. A primer of supportive psychotherapy. The Analytic Press, New Jersey. [Google Scholar]
- Pinsker H, Rosenthal R, 1988. Beth Israel Medical Center supportive psychotherapy manual. Social and Behavior Science Documents 18, 57. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, 2001. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TG, Davis TL, Weiskoff RM, 1995. Automated shimming at 1.5 T using echoplanar image frequency maps. J Magn Reson Imaging 5, 739–745. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N, 2004. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu. Rev. Psychol 55, 87–124. 10.1146/annurev.psych.55.090902.142044 [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL, 2008. Episodic simulation of future events: concepts, data, and applications. Ann. N. Y. Acad. Sci 1124, 39–60. 10.1196/annals.1440.001 [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Hölzel BK, Posner MI, 2015. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci 16(4), 213–225. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain. Connect 2, 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: Reliability, validity, and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]


