Abstract
High carbon dioxide tensions (hypercapnia) are toxic to mammals by both pH-dependent and pH-independent mechanisms that remain partially understood. Relevantly, carbon dioxide reacts with biologically ubiquitous oxygen metabolites such as peroxynitrite and hydrogen peroxide to produce carbonate radical and peroxymonocarbonate, respectively. These metabolites are redox active making it timely to discuss the potential role of carbon dioxide redox metabolites in oxidative eustress and oxidative distress conditions.
Life adaptation to molecular oxygen allowed evolution of complex life forms but came with a cost because oxygen is prone to one-electron transfers, producing radical and oxidant metabolites that are toxic to cells. To cope with these metabolites during evolution, aerobic organisms developed antioxidant defenses (enzymatic and not) and learned to use radicals and oxidants in processes essential to them. Although oxygen and its metabolites imprinted the evolution of complex life forms, the cell damaging mechanisms of these metabolites received most of the attention up to the 1990s. More recently, the participation of radicals and oxidants in both physiological and pathological processes became consensual, and the classical concepts of homeostasis and oxidative stress are being replaced by oxidative eustress and oxidative distress, respectively (Sies and Jones 2020).
In this context, it is timely to discuss the potential role of carbon dioxide (CO2) redox metabolites in oxidative eustress and oxidative distress conditions. CO2 levels are increasing in the atmosphere and inside modern refrigerated buildings. This gas is a normal constituent of the human body that produces about 1 kg of CO2/day through respiration and relies in the CO2/HCO3─ pair (bicarbonate buffer) as the main physiological buffer. High CO2 tensions (hypercapnia) are toxic to mammals by both pH-dependent and pH-independent mechanisms that remain partially understood. Relevantly, CO2 reacts with biologically ubiquitous oxygen metabolites such as peroxynitrite (ONOOH/ONOO─) (Ferrer-Sueta et al. 2018) and hydrogen peroxide (H2O2) (Bakhmutova-Albert et al. 2010) to produce carbonate radical (CO3●─) and peroxymonocarbonate (HCO4─), respectively. These metabolites are redox active. The CO3●─ is a strong one-electron oxidant that oxidizes biomolecules to radicals likely resulting in oxidative damage (Augusto and Miyamoto 2012). In contrast, HCO4─ is a two-electron oxidant more reactive than H2O2 towards thiol proteins and may be involved in cellular signaling (Truzzi and Augusto 2018). In the presence of transition metal ions, HCO4─ produces the CO3●─.
Several lines of evidence indicate the participation of CO3●─ in situations of oxidative damage and distress associated with nitric oxide overproduction (Ferrer-Sueta et al. 2018), hypercapnia (Dean 2010), and related clinical conditions. Conversely, HCO4─ received limited attention in the literature. Nevertheless, its formation explains the accelerating effects of the CO2/HCO3─ pair on H2O2-mediated oxidation of thiol proteins that are confirmed players in cellular redox signaling, such as protein tyrosine phosphatases (Zhou et al. 2011) and 2-Cys peroxiredoxins (Truzzi et al. 2019; Peskin et al. 2019). Additionally, the requirement of HCO3─ for protein-tyrosine phosphatase 1B oxidation and cellular signaling through epidermal growth factor-triggered phosphorylation cascades in adenocarcinoma cells was attributed to HCO4─ formation (Dagnell et al. 2019). These recent studies support a role for HCO4─ in H2O2-mediated cellular signaling.
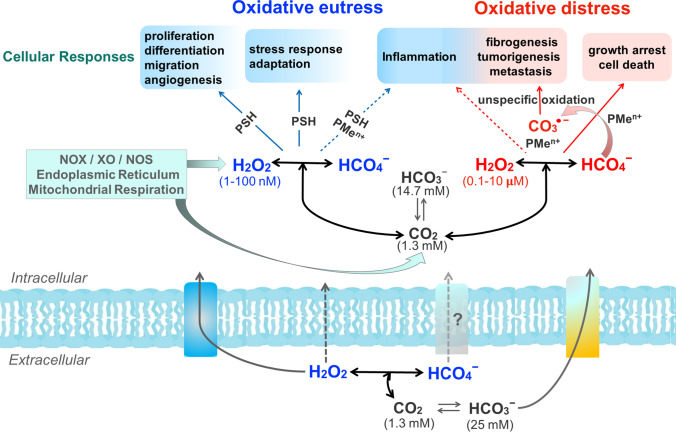
The accumulated knowledge on CO2 redox metabolites, taken together with the estimated ranges of H2O2 concentration associated with different cellular responses (Sies and Jones 2020), permit hypothesizing the influence of HCO4─ and CO3●─ on them (Fig. 1). The first likely increases H2O2-mediated physiological responses whereas the CO3●─ likely increases the pathophysiological ones.
Fig.1.
Potential effects of CO2 redox metabolites (HCO4─ and CO3●─) on physiological and pathological responses mediated by H2O2 and associated with oxidative eustress and oxidative distress conditions, respectively. The used ranges of H2O2 concentration physiological (blue) and supraphysiological (red) were those from Sies and Jones, 2020. Based on the estimated value of the K (0.33 M−1) of the equilibrium H2O2 plus HCO3─ with HCO4─, the range of HCO4─ concentration corresponds to approximately 1% of the H2O2 range (Bakhmutova-Albert et al. 2010). CO2 is membrane permeable, H2O2 has limited permeability to membranes, but its transport is facilitated by aquaporins, and HCO3─ can be exchanged by Cl─ through electroneutral Na+ and HCO3─ cotransporters; nothing is known about HCO4─ exchange. The reactions are not balanced. NOX, XO, NOS, PSH, and PMen+ represent NAPDH oxidase, xanthine oxidase, nitric oxide synthase, a generic thiol protein, and a generic protein containing metal center, respectively
Most of these hypothetical increases remain to be experimentally, proved but the scenario displayed in Fig. 1 emphasizes the likely relevance of CO2 redox metabolites in health and disease.
Funding
The authors acknowledge the support of the São Paulo Research Foundation (FAPESP, Grants 2013/07937-8 (to OA) and 2019/17483-0) (to DRT) and the National Council for Scientific and Technological Development (CNPq, Grant 300465/2009-2 (to OA)).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Augusto O, Miyamoto S. Principles of Free Radical Biomedicine. New York: Nova Science Publishers; 2012. Oxygen radicals and related species; pp. 19–41. [Google Scholar]
- Bakhmutova-Albert EV, Yao H, Denevan DE, Richardson DE. Kinetics and mechanism of peroxymonocarbonate formation. Inorg Chem. 2010;49(24):11287–11296. doi: 10.1021/ic1007389. [DOI] [PubMed] [Google Scholar]
- Dagnell M, Cheng Q, Rizvi SHM, Pace PE, Boivin B, Winterbourn CC, Arnér ESJ. Bicarbonate is essential for protein-tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades. J Biol Chem. 2019;294(33):12330–12338. doi: 10.1074/jbc.RA119.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JB. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: implications for CO2 chemoreceptor function and dysfunction. J Appl Physiol. 2010;108(6):1786–1795. doi: 10.1152/japplphysiol.01337.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Campolo N, Trujillo M, Bartesaghi S, Carballal S, Romero N, Alvarez B, Radi R. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem Rev. 2018;118(3):1338–1408. doi: 10.1021/acs.chemrev.7b00568. [DOI] [PubMed] [Google Scholar]
- Peskin AV, Pace PE, Winterbourn CC. Enhanced hyperoxidation of peroxiredoxin 2 and peroxiredoxin 3 in the presence of bicarbonate/CO2. Free Radic Biol Med. 2019;145:1–7. doi: 10.1016/j.freeradbiomed.2019.09.010. [DOI] [PubMed] [Google Scholar]
- Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- Truzzi DR, Augusto O. Influence of CO2 on hydroperoxide metabolism. In: Vissers MCM, Hampton M, Kettle AJ, editors. Hydrogen peroxide metabolism in health and disease. Boca Raton: Taylor & Francis/CRC Press; 2018. pp. 83–101. [Google Scholar]
- Truzzi DR, Coelho FR, Paviani V, Alves SV, Netto LES, Augusto O. The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1. J Biol Chem. 2019;294(38):14055–14067. doi: 10.1074/jbc.RA119.008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Singh H, Parsons ZD, Lewis SM, Bhattacharya S, Seiner DR, LaButti JN, Reilly TJ, Tanner JJ, Gates KS. The biological buffer bicarbonate/CO2 potentiates H2O2-mediated inactivation of protein tyrosine phosphatases. J Am Chem Soc. 2011;133(40):15803–15805. doi: 10.1021/ja2077137. [DOI] [PMC free article] [PubMed] [Google Scholar]