Abstract
G protein-coupled receptors (GPCRs) are the largest family of transmembrane proteins that relay extracellular signals across the plasma membrane and elicit an intricate cascade of cellular signaling events. A significantly large fraction of available drugs target GPCRs in order to exert fine control over functional outcomes from these receptors in pathological conditions. In this context, endocytosis and intracellular trafficking of GPCRs stringently regulate signaling outcomes from GPCRs within physiologically relevant spatiotemporal regimes. The membrane microenvironment around GPCRs has recently emerged as a key player in receptor function. Cholesterol is the single most abundant lipid in the eukaryotic plasma membrane and plays a central role in membrane organization and dynamics, with far-reaching functional implications in cellular physiology. In this review, we discuss current excitements in GPCR endocytosis and trafficking, with an emphasis on the role of membrane cholesterol. We envision that a detailed understanding of the contribution of membrane lipids such as cholesterol in spatiotemporal regulation of GPCR signaling would enable the development of therapeutic interventions fine-tuned to receptors residing in specific membrane microenvironments.
Keywords: Cholesterol, GPCRs, Endocytosis, Intracellular Trafficking, Therapeutics
GPCRs: stringently regulated signaling nanomachines in a lipid microenvironment
G protein-coupled receptors (GPCRs) are membrane-resident signal transducers that enable the recognition of chemically and physiologically diverse extracellular cues essential for a repertoire of cellular functions (Chattopadhyay 2014; Pierce et al. 2002; Rosenbaum et al. 2009; Weis and Kobilka 2018). Signaling by GPCRs has been implicated in diverse cellular physiology, ranging from growth and development, to immune responses and pathogen uptake (Gutierrez and McDonald 2018; Hameid et al. 2021; Lämmermann and Kastenmüller 2019; Palczewski and Orban 2013). It is therefore not surprising that GPCRs account for more than one-third of the current drug targets (Chan et al. 2019; Hauser et al. 2017; Sriram and Insel 2018). However, these receptors constitute only ~ 10% of GPCRs encoded in the human genome, leaving a major fraction of druggable targets untapped (Cooke et al. 2015; Jacobson 2015; Stockert and Devi 2015). In addition, the GPCRs that are targeted by currently available drugs are primarily aminergic and opioid receptors (Hauser et al. 2017). These statistics suggest that GPCRs offer a largely unexplored, yet potentially rewarding pool of drug targets (Foster et al. 2019; Huang et al. 2017; Sloop et al. 2018).
Owing to the diverse array of intracellular signaling processes elicited by GPCRs, regulation of their activity within physiological regimes and/or toward specific downstream responses assumes relevance. Such regulatory features could intervene at various stages of signal transduction via GPCRs—from exposure to ligands to coupling of effector proteins to receptors (Gurevich and Gurevich 2019; Lane et al. 2013; Magalhaes et al. 2012; Morris and Malbon 1999). Apart from their role in modulating the extent of signaling (desensitization and resensitization), regulatory features of GPCR signaling also contribute to spatiotemporal control over downstream effects emanating from these receptors (Eichel and von Zastrow 2018; Ferguson 2001; Weinberg and Puthenveedu 2019).
As a direct consequence of their seven transmembrane domain architecture, GPCRs are predisposed to considerable interaction with their membrane lipid microenvironment (Chattopadhyay 2014; Oates and Watts 2011; Sengupta et al. 2018). Several examples of these interactions have been captured in GPCR structures displaying lipids bound to the receptors (Jafurulla et al. 2019; Sarkar and Chattopadhyay 2021a, b; Sejdiu and Tieleman 2020). Importantly, such interactions have been found to be correlated with lipid-sensitive functional readouts in case of several GPCRs (Jafurulla et al. 2019; Sarkar and Chattopadhyay 2021b). Although the molecular underpinnings of signal transduction by GPCRs have been a subject of intense exploration, our understanding of the mechanistic details underlying lipid-mediated modulation of GPCR function is relatively nascent (Kumar et al. 2021). In this review, we highlight the role of the membrane microenvironment in GPCR endocytosis, an important regulatory feature of receptor function, with an emphasis on membrane cholesterol as a modulator of GPCR endocytosis. Using our work on the serotonin1A receptor as an example, we allude to the relevance of the interplay between cholesterol and GPCR endocytosis and its implications in pathophysiology and development of fine-tuned therapeutic interventions.
Endocytosis of GPCRs: desensitization and beyond
Endocytosis is a major regulatory mechanism employed by GPCRs to sustain their downstream signaling within physiological levels under a stringent spatiotemporal regime (Ferguson 2001; Hanyaloglu and von Zastrow 2008; Kunselman et al. 2021b). Endocytosis offers an effective means to decouple a GPCR from its pool of extracellular ligands by sequestering the receptor into intracellular locations. The first observation on internalization of GPCRs dates back to the late 1970s when ligand-induced desensitization of the β-adrenergic receptor was correlated to internalization of a fraction of the plasma membrane–associated receptor pool in frog erythrocytes (Chuang and Costa 1979). Subsequent work led to the identification of regulatory proteins such as G protein-coupled receptor kinases (GRKs) and arrestins that predispose GPCRs to endocytosis as a means of desensitization (Benovic et al. 1987; Ferguson et al. 1996; Lohse et al. 1990). It was further discovered that β-arrestin could act as an adaptor for the assembly of clathrin coats for endocytosis of the β2-adrenergic receptor (Goodman et al. 1996). The advent of molecular biology and the ability to express fluorescently tagged receptors led to the discovery that phosphorylation of key residues in the intracellular loops of the receptor acts as ‘barcodes’ (identifiers) for the recruitment of endocytic machinery for GPCR internalization and trafficking (Bahouth and Nooh 2017; Liggett 2011; Yang et al. 2017).
Although GPCR endocytosis was initially identified as a mode of ligand-induced desensitization of receptor-mediated signaling, several other regulatory features associated with their internalization and trafficking have emerged more recently (see Fig. 1). One of the earliest evidence of the role of GPCR endocytosis beyond desensitization implicated β-arrestin–mediated internalization of the β2-adrenergic receptor as a prerequisite for the activation of MAP kinase signaling (Daaka et al. 1998; Luttrell et al. 1999). A fascinating feature of GPCR endocytosis that has emerged through research over the recent past is the ability of these receptors to signal from intracellular compartments (Crilly and Puthenveedu 2021; Irannejad and von Zastrow, 2014; Jong et al. 2018; Sposini and Hanyaloglu 2017). For example, signaling responses from internalized GPCRs have been reported for the thyroid-stimulating hormone receptor (Calebiro et al. 2009), sphingosine-1-phosphate receptor (Mullershausen et al. 2009), parathyroid hormone receptor (Ferrandon et al. 2009), vasopressin receptor type 2 (Feinstein et al. 2013), and leutinizing hormone receptor (Lyga et al. 2016). More importantly, direct evidences from conformation-specific nanobodies that recognize specific activated receptor states have revealed signaling-competent conformations for internalized receptors with distinct cellular signaling consequences (Irannejad et al. 2013, 2017; Kunselman et al. 2021a; Stoeber et al. 2018; Tsvetanova and von Zastrow 2014).
Fig. 1.
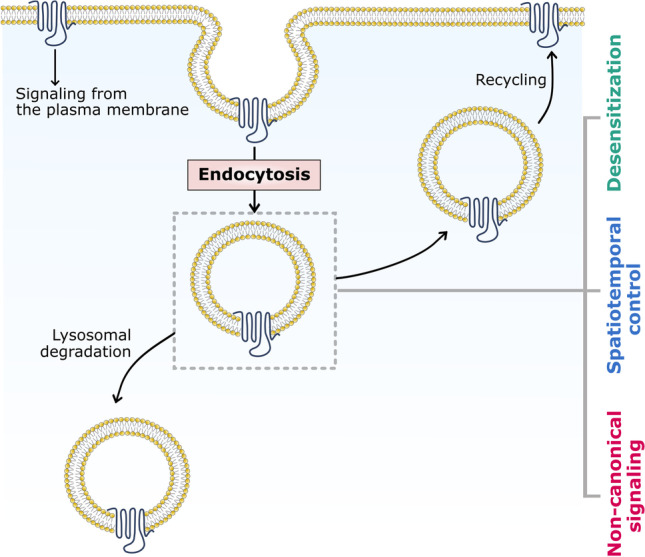
Regulation of GPCR signaling by endocytosis. A schematic representation depicting various regulatory features associated with GPCR endocytosis. Endocytosis allows regulation of GPCR signaling by spatially decoupling the binding of an extracellular ligand to the receptor (desensitization). In general, upon endocytosis, GPCRs could either recycle back to the plasma membrane or undergo lysosomal degradation within the cell. Such movements are regulated by intricately coordinated intracellular trafficking pathways within cells. Several other regulatory features associated with GPCR internalization and intracellular trafficking have emerged recently. Endocytosis and intracellular trafficking of GPCRs are known to confer spatiotemporal control to receptor-mediated signaling, which is manifested as distinct signals emerging from the plasma membrane and endosomes in case of several receptors
Endocytosis of membrane receptors is a highly complex and stringently regulated phenomenon that involves a wide variety of membrane-associated and cytoplasmic protein machinery that orchestrate the process in a concerted fashion. In this context, several mechanisms facilitating the internalization of membrane receptors have been studied (Doherty and McMahon 2009; Kunselman et al. 2021b). Although a majority of GPCRs have been shown to utilize clathrin-mediated endocytic machinery for internalization (Hanyaloglu and von Zastrow 2008; Wolfe and Trejo 2007), caveolar localization (Bhatnagar et al. 2004; Ostrom and Insel 2004) and caveolin-mediated internalization (Cho et al. 2012; Janoshazi et al. 2007) of GPCRs have also been reported. In addition, multiple possibilities exist in terms of intracellular trafficking routes available for GPCRs upon internalization. Broadly, receptors could either recycle back to the plasma membrane, or they could be sorted to the lysosomal system for degradation (Fig. 1; Hanyaloglu and von Zastrow 2008; Kunselman et al. 2021b; Marchese et al. 2008).
Although much has been explored in terms of the functional consequences of GPCR endocytosis, our mechanistic understanding of this process primarily comprises insights into the protein machinery that orchestrate the GPCR endocytic framework, and details about the role of membrane lipids in this process are rather limited. This is in spite of the fact that GPCRs are polytopic membrane proteins and the polypeptide chain of the receptor crosses the membrane several times. The following sections highlight the importance of cholesterol in various facets of GPCR function. Using examples from the limited body of literature and recent work from our group, we make a case for exploring the role of membrane lipids (particularly cholesterol) in the endocytosis and trafficking of GPCRs.
Cholesterol and GPCRs: an intimate association
Cholesterol is an essential lipid in higher eukaryotic cell membranes that assumes a unique functional role in cellular physiology. It is the single most abundant lipid in the eukaryotic plasma membrane accounting for ~ 30–50% of the total lipid content (Maxfield and van Meer 2010; Mouritsen 2005). Cholesterol assumes a critical role in regulating membrane organization and dynamics, and associated cellular functions such as signaling, sorting, trafficking, and pathogen entry (Kumar and Chattopadhyay 2016; Kumar et al. 2016; Lippincott-Schwartz and Phair 2010; Mouritsen and Zuckermann, 2004; Simons and Ikonen 2000). The unique physicochemical properties of cholesterol that have been fine-tuned over long time scales of evolution are manifested in terms of its nonrandom distribution in the plasma membrane and the ability to interact with membrane lipids and proteins. From a structural standpoint, cholesterol comprises a polar 3β-hydroxyl group, a tetracyclic-fused steroid ring, and a flexible isooctyl chain (see Fig. 2). Each of these features has been suggested to confer distinct interaction profiles to the molecular structure of cholesterol (Chaudhuri and Chattopadhyay 2011; Fantini and Barrantes 2013; Fantini et al. 2019; Paila and Chattopadhyay 2010; Sarkar and Chattopadhyay 2020). The 3β-hydroxyl group allows cholesterol to orient and anchor in the membrane (Villalaín 1996), and facilitates electrostatic interaction with polar residues such as lysine and arginine of membrane proteins. The methyl groups in the α-face of cholesterol have been proposed to exhibit CH-π stacking interactions with aromatic amino acids such as tyrosine and phenylalanine, and the isooctyl chain is believed to intercalate with the side chains of branched amino acids such as valine, leucine, and isoleucine via van der Waals interactions. In addition, cholesterol is a key modulator of membrane physical properties such as fluidity, viscosity, curvature, thickness, and dipole potential, which constitute important features of the membrane microenvironment that influence membrane function (Arora et al. 2004; Bacia et al. 2005; Chen and Rand 1997; Haldar et al. 2012; Nezil and Bloom 1992; Pal et al. 2016; Simon et al. 1982).
Fig. 2.
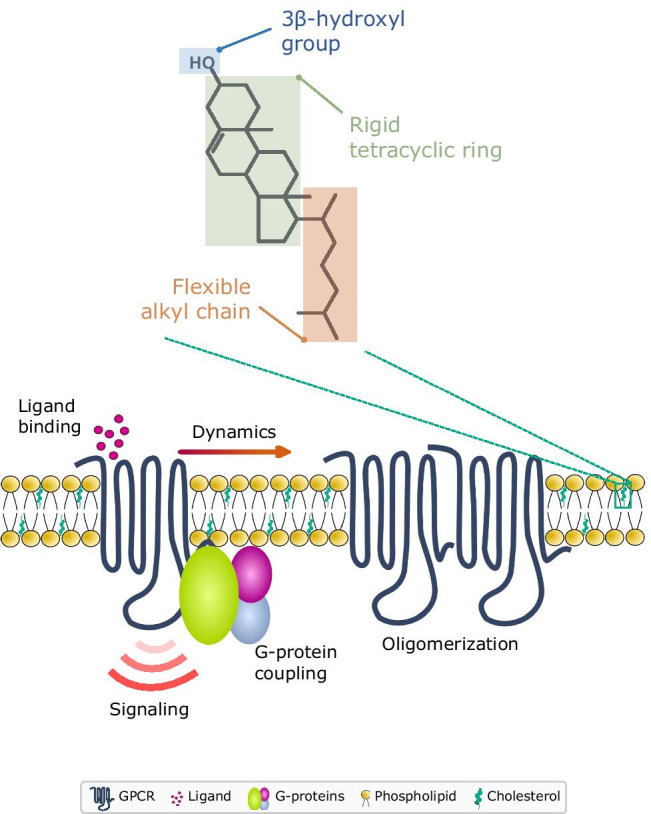
Multifaceted regulation of GPCR function by cholesterol. The interplay between GPCRs and cholesterol in the membrane milieu has emerged as a major thrust area with far-reaching implications in the pathophysiology mediated by GPCRs. The unique physicochemical properties of cholesterol (encoded in its chemical structure comprising of a polar 3β-OH group, a rigid tetracyclic ring and a flexible isooctyl chain) coupled with its relative abundance in the plasma membrane contribute to the crucial modulatory effects of cholesterol on GPCRs. Extensive work using biochemical, biophysical, and computational approaches has shown that cholesterol affects various features of GPCRs such as ligand binding, G-protein coupling, downstream signaling, dynamics, and oligomerization
The interplay between membrane cholesterol and GPCRs has emerged as a major theme in GPCR biology. The interaction of cholesterol with GPCRs is multifaceted, and has been shown to be implicated in ligand binding, G-protein coupling, signaling, lateral dynamics, and oligomerization of receptors (see Fig. 2; Chattopadhyay 2014; Gimpl 2016; Jafurulla and Chattopadhyay 2013; Jafurulla et al. 2019; Oates and Watts 2011; Pucadyil and Chattopadhyay 2006; Paila and Chattopadhyay 2010; Sengupta and Chattopadhyay 2015; Sengupta et al. 2017, 2018). A careful dissection of the literature on GPCR-cholesterol interaction suggests that the mechanism underlying the cholesterol sensitivity observed by GPCRs is most likely a combination of the direct interaction of cholesterol with GPCRs and its modulatory effects on the membrane microenvironment that houses these receptors (Jafurulla et al. 2019). GPCR structures with cholesterol bound to receptors could suggest direct interaction of cholesterol with GPCRs, although there are some caveats (see Sarkar and Chattopadhyay 2021a, b). In fact, analysis of several GPCR sequences and structures has revealed specific sites for these interactions which have been proposed as cholesterol interaction motifs such as the cholesterol recognition/interaction consensus (CRAC), inverse-CRAC (CARC) and cholesterol consensus motifs (CCM) (recently reviewed in Sarkar and Chattopadhyay 2020). Very recent work from our group has shown that a lysine residue in a CRAC motif in transmembrane helix 2 of the serotonin1A receptor acts as a molecular sensor for changes in membrane cholesterol levels and imparts cholesterol sensitivity to cAMP signaling by the receptor (Kumar et al. 2021). Although the interaction of cholesterol with GPCRs has been shown to be weak and transient (~ μs time scale), such interactions appear to act as functional switches between receptor conformations (Sengupta and Chattopadhyay 2015). On the other hand, the effects of cholesterol on membrane physicochemical properties (such as membrane fluidity, viscosity, hydrophobic mismatch and membrane dipole potential) could modulate receptor conformation(s) facilitating specific functional states.
Cholesterol in endocytosis: why do we care and what do we know?
The motivation behind exploring the role of membrane cholesterol in endocytosis stems from the ability of cholesterol to modulate membrane physical properties and participate in functional interactions with membrane protein cargo as well as the cellular endocytic machinery. The process of endocytosis involves several remodeling events at the plasma membrane that include generation of membrane curvature, recognition of cargo and translocation of cargo into endocytic structures on the membrane. In this context, cholesterol has been shown to impart negative curvature in membranes (Chen and Rand 1997) and accumulate in highly curved regions of bilayers (Wang et al. 2007). The scaffolding protein caveolin-1, involved in caveolin-mediated endocytosis, segregates into cholesterol-rich domains in the membrane (Örtegren et al. 2004; Smart and Anderson 2002). Notably, caveolin-1 contains a stretch of amino acids constituting the CRAC motif (Epand et al. 2005), and a peptide fragment from caveolin-1 containing this motif has been shown to drive the formation of cholesterol-rich domains (Epand et al 2003). In addition, it was recently demonstrated that caveolin-1 can induce higher membrane curvature in cholesterol-rich membranes (Krishna and Sengupta 2019).
One of the earliest studies probing the role of membrane cholesterol on “cellular uptake” reported that metabolic inhibition of cholesterol biosynthesis in mouse fibroblasts using oxygenated derivatives of cholesterol such as 25-hydroxycholesterol and 7-ketocholesterol resulted in reduced internalization of the soluble enzyme horseradish peroxidase (Heinger et al. 1976). Initial insights into the role of cholesterol in the endocytosis of a membrane protein came from studies on transferrin, which demonstrated that acute cholesterol depletion using methyl-β-cyclodextrin (MβCD) significantly reduced the rate of transferrin receptor internalization without affecting recycling. Ultrastructural studies using electron microscopy showed that this was accompanied by accumulation of flat clathrin-coated membranes and decrease in deep-coated pits (Subtil et al. 1999). In another report published around the same time, MβCD was shown to strongly inhibit the endocytosis of transferrin and epidermal growth factor, but not of the general membrane marker, ricin. This effect could be reversed upon replenishment of cholesterol (Rodal et al. 1999). Contrary to this, depletion of cholesterol using MβCD was reported to enhance the rate of internalization of nicotinic acetylcholine receptors (Borroni et al. 2007) via a pathway involving the small GTPase Arf6 (Borroni and Barrantes 2011). Metabolic depletion of cholesterol using mevalonin, an inhibitor of cholesterol biosynthesis, resulted in reduction in nicotinic acetylcholine receptors in the plasma membrane which accumulated in the intracellular trans-Golgi network (Pediconi et al. 2004).
Cholesterol in GPCR endocytosis: an evolving story
Depletion of membrane cholesterol has been shown to inhibit agonist-induced internalization of GPCRs such as the lysophosphatidic acid receptor 1 (LPA1; Urs et al. 2005), the δ-opioid receptor (Brejchova et al. 2016), and the formyl peptide receptor 1 (Wang et al. 2019). However, cholesterol depletion did not affect the agonist-induced endocytosis of the M1 muscarinic acetylcholine receptor (Urs et al. 2005) and the cholecystokinin receptor (Harikumar et al. 2005). In case of LPA1, cholesterol was shown to be essential for the interaction of the receptor with β-arrestin leading to its internalization via clathrin-mediated endocytosis. On the other hand, the β2-adrenergic receptor did not exhibit cholesterol dependence in its interaction with β-arrestin (Urs et al. 2005). Constitutive endocytosis and recycling are characteristic features of the melanocortin-4 receptor. Cholesterol depletion from immortalized neuronal cells exogenously expressing these receptors inhibited the constitutive endocytosis of the receptor, resulting in a loss of agonist-induced cAMP generation over time (McDaniel et al. 2012). Such differences in cholesterol-dependent receptor-effector interactions could arise due to partitioning of GPCRs and their endocytic effectors (β-arrestins) into distinct membrane microdomains, and the dynamics of these molecules under various states of receptor activation. For example, although both β1- and the β2-adrenergic receptors associate with caveolae under basal conditions, β2- (but not β1)-adrenergic receptors were shown to dissociate from these regions upon agonist stimulation (Rybin et al. 2000). The molecular mechanism underlying the interaction of specific GPCRs with the associated endocytic machinery in membrane microdomains with varying cholesterol content is still an important unanswered question.
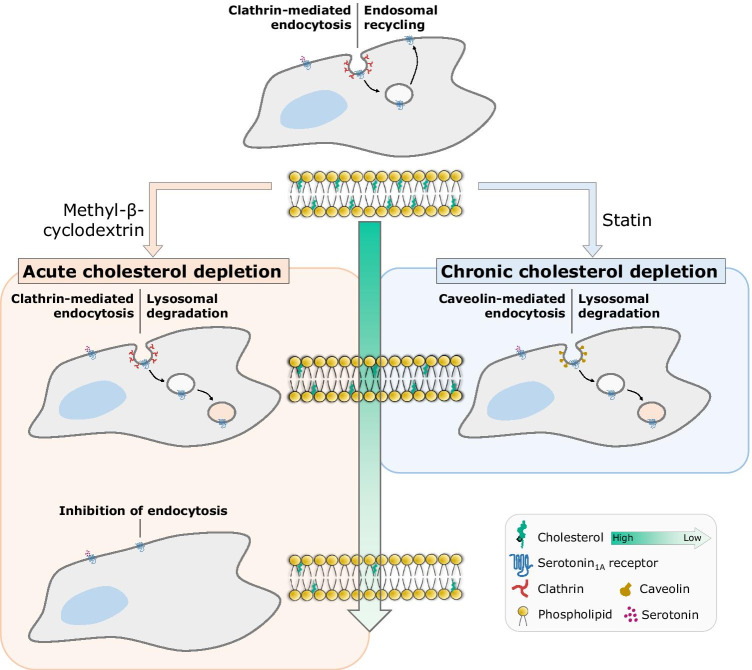
Extensive work from our group has demonstrated the prominent role of membrane cholesterol in the organization, function, and dynamics of the serotonin1A receptor (Chakraborty et al. 2018; Ganguly and Chattopadhyay 2010; Ganguly et al. 2011; Jafurulla et al. 2014; Paila et al. 2011; Prasanna et al. 2016; Pucadyil and Chattopadhyay 2004, 2007; Sarkar et al. 2020; Shrivastava et al. 2010; Saxena and Chattopadhyay 2012). We recently explored the role of cholesterol in the endocytosis and intracellular trafficking of the serotonin1A receptor using acute and chronic methods of cholesterol depletion (Fig. 3). We observed that under normal conditions, the serotonin1A receptor undergoes agonist-induced clathrin-mediated endocytosis and traffics along the endosomal recycling pathway back to the plasma membrane (Kumar et al. 2019). Acute cholesterol depletion using MβCD resulted in a concentration-dependent inhibition of receptor endocytosis, which could be restored by replenishment of cholesterol (Kumar and Chattopadhyay 2021). Notably, under mild acute cholesterol depletion conditions achieved using a lower concentration of MβCD, the serotonin1A receptor continued to internalize via clathrin-mediated endocytosis, but exhibited a switch in its intracellular trafficking itinerary from recycling to lysosomal degradation (Kumar and Chattopadhyay 2021). Interestingly, when a similar extent of cholesterol was depleted using a chronic approach by treating cells with statin (an inhibitor of the rate limiting step in cellular cholesterol biosynthesis (Istvan and Deisenhofer 2001; Nes 2011)), the serotonin1A receptor exhibited a switch in the mechanism of internalization from clathrin- to caveolin-mediated endocytosis, along with re-routing of intracellular traffic to lysosomes instead of the plasma membrane (Kumar and Chattopadhyay 2020). A similar effect was previously reported for the endothelin receptor type A where oxidation of cholesterol using cholesterol oxidase resulted in a switch in the mechanism of receptor internalization from caveolin- to clathrin-mediated endocytosis (Okamoto et al. 2000). The differential effects of acute and chronic methods of cholesterol depletion on serotonin1A receptor endocytosis point toward the relevance of the actual method used to deplete cholesterol, and not just the extent of cholesterol depletion. The fundamental differences in these methods could arise due to their varied effects on intracellular distribution of cholesterol (and other lipids), physical properties of membranes along intracellular trafficking pathways, function of the protein machinery involved in cargo sorting and trafficking, and other pleiotropic effects of such treatments, all of which constitute essential factors contributing to endocytosis and trafficking in cells (Breusegem et al. 2005; Goodwin et al. 2005; Hilgemann et al. 2020; Liao and Laufs 2005; Sahu et al. 2019; Sarkar et al. 2017; Shvartsman et al. 2006).
Fig. 3.
Cholesterol-induced switch in the endocytosis and intracellular trafficking of the neurotransmitter serotonin1A receptor. A schematic representing the effects of acute and chronic cholesterol depletion on the endocytosis and intracellular trafficking of the serotonin1A receptor. The serotonin1A receptor undergoes agonist (serotonin)-induced internalization via clathrin-mediated endocytosis, and subsequently recycles back to the plasma membrane as its preferred mode of intracellular trafficking. The role of cholesterol in this process was probed using acute (treatment with the soluble sterol-carrier methyl-β-cyclodextrin, MβCD) and chronic (inhibition of cellular cholesterol biosynthesis using statin) approaches of cholesterol depletion. Mild (acute) cholesterol depletion using a lower concentration of MβCD resulted in re-routing of receptors internalized via clathrin-mediated endocytosis toward lysosomal degradation. In addition to an altered intracellular trafficking itinerary, similar extent of cholesterol depletion using statin induced an additional switch in the mechanism of internalization from clathrin- to caveolin-mediated endocytosis. Depletion of higher amounts of cholesterol using MβCD led to complete inhibition in the endocytosis of the serotonin1A receptor. These observations highlight the nuanced role of cholesterol in endocytosis and intracellular trafficking of the serotonin1A receptor and the importance of taking into consideration the approach used to explore such phenomena in interpreting the results. The nuclei are shown in blue
Cholesterol in GPCR endocytosis: implications in pathophysiology and therapeutics
Impaired trafficking of GPCRs has been shown to be associated with pathophysiological conditions such as nephrogenic diabetes insipidus (Bernier et al. 2004), retinitis pigmentosa (Hollingsworth and Gross 2012), and cancer (Dorsam and Gutkind 2007). Furthermore, dysfunctional GPCR trafficking could be mapped to defects associated with intracellular trafficking machinery and sorting proteins in disease conditions (Chandra et al. 2021; Wang et al. 2013). Where does cholesterol feature in this scenario? As discussed in the previous section, changes in membrane cholesterol levels appear to modulate endocytic and intracellular trafficking pathways for some GPCRs. Notably, membrane cholesterol levels and its biosynthetic pathway exhibit differences across cell and tissue types, and vary with age and development (Dietschy and Turley 2004; Karnell et al. 2005; Mitsche et al. 2015). At subcellular scales, cholesterol content varies across membranes of intracellular organelles (Ikonen 2008), and this distribution exhibits dynamic regulation (Mesmin and Maxfield 2009). In addition, defective cholesterol biosynthesis and metabolism are hallmarks of several pathophysiological conditions (Meng et al. 2020; Platt et al. 2014). These aspects raise the interesting possibility of cholesterol-dependent regulation in trafficking of receptors with consequences in their role in cellular physiology. As an example, recent work from our group has shown that the serotonin1A receptor exhibits late endosomal/lysosomal accumulation accompanied by reduction in the plasma membrane receptor pool in a cellular model of Smith-Lemli-Opitz syndrome, a congenital developmental defect characterized by defective cholesterol biosynthesis (Sharma et al. 2021).
The role of spatiotemporal regulation in GPCR signaling is increasingly recognized in the development of novel drugs targeting these receptors (Nezhady et al. 2020; Retamal et al. 2019; Thomsen et al. 2018). A comprehensive understanding of lipid-mediated effects on GPCR trafficking is therefore essential to tune drug responses based on the membrane environment at the site of drug action (Payandeh and Volgraf 2021; Wang et al. 2021). The modulatory effects of cholesterol on the endocytosis of the serotonin1A receptor (discussed above) are a relevant case in point. Endocytosis of the serotonin1A receptor has been implicated in the action of a popular class of anti-depressant drugs called selective serotonin reuptake inhibitors (SSRIs). Cohort studies have suggested that SSRIs exhibit enhanced anti-depressant activity when administered to patients as a combination with cholesterol-lowering statin therapy relative to those on SSRI treatment alone (Ghanizadeh and Hedayati 2013; Köhler et al. 2016). These observations provide a potential mechanistic basis for our data showing altered endocytic and intracellular trafficking profiles of the serotonin1A receptor upon statin treatment (Kumar and Chattopadhyay 2020). In another example, the endocytosis of a pH-sensitive peptide-drug conjugate via formyl peptide receptor 1 was insensitive to treatment with pharmacological inhibitors of clathrin- or caveolin-mediated endocytosis, yet exhibited cholesterol dependence pointing toward a crucial role for cholesterol in the drug delivery via cholesterol-sensitive internalization of the receptor (Wang et al. 2019).
Concluding thoughts and future excitements
Our evolving understanding of intracellular landscapes of GPCR signaling has opened up exciting avenues on the role of the membrane lipid milieu in enforcing spatiotemporal control over signaling through modulatory effects on intracellular trafficking and signaling. Differential enrichment of lipids such as cholesterol in various membrane-bound intracellular organelles and endosomal compartments could offer unique microenvironments with distinct effector partners for differential signaling outcomes. We envision that a systematic understanding of lipid dynamics in the spatiotemporal regulation of GPCR signaling would enable the development of therapeutic interventions fine-tuned to precisely target receptors in specific microenvironments.
Acknowledgements
Work in A.C.’s laboratory was generously funded by Department of Biotechnology, Govt. of India, Council of Scientific and Industrial Research, Indo-French Centre for the Promotion of Advanced Research, and Science & Engineering Research Board, Department of Science and Technology, Govt. of India. A.C. gratefully acknowledges SERB Distinguished Fellowship (SERB, DST, Govt. of India). G.A.K. was supported as a Senior Project Associate by a CSIR FBR grant to A.C. (MLP 0146). We thank Ashwani Sharma for help during preparation of the manuscript and members of the Chattopadhyay laboratory for critically reading the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arora A, Raghuraman H, Chattopadhyay A. Influence of cholesterol and ergosterol on membrane dynamics: a fluorescence approach. Biochem Biophys Res Commun. 2004;318:920–926. doi: 10.1016/j.bbrc.2004.04.118. [DOI] [PubMed] [Google Scholar]
- Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc Natl Acad Sci USA. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahouth SW, Nooh MM. Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell Signal. 2017;36:42–55. doi: 10.1016/j.cellsig.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, Kühn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated β-adrenergic receptor by the β-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier V, Lagacé M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Gαq-coupled protein receptors. J Biol Chem. 2004;279:34614–346123. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Borroni V, Barrantes FJ. Cholesterol modulates the rate and mechanism of acetylcholine receptor internalization. J Biol Chem. 2011;286:17122–17132. doi: 10.1074/jbc.M110.211870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni V, Borroni V, Baier CJ, Lang T, Bonini I, White MM, Garbus I, Barrantes FJ. Cholesterol depletion activates rapid internalization of submicron-sized acetylcholine receptor domains at the cell membrane. Mol Membr Biol. 2007;24:1–15. doi: 10.1080/09687860600903387. [DOI] [PubMed] [Google Scholar]
- Brejchova J, Vosahlikova M, Roubalova L, Parenti M, Mauri M, Chernyavskiy O, Svoboda P. Plasma membrane cholesterol level and agonist-induced internalization of δ-opioid receptors; colocalization study with intracellular membrane markers of Rab family. J Bioenerg Biomembr. 2016;48:375–396. doi: 10.1007/s10863-016-9667-7. [DOI] [PubMed] [Google Scholar]
- Breusegem SY, Halaihel N, Inoue M, Zajicek H, Lederer E, Barry NP, Sorribas V, Levi M. Acute and chronic changes in cholesterol modulate Na-Pi cotransport activity in OK cells. Am J Physiol Renal Physiol. 2005;289:F154–F165. doi: 10.1152/ajprenal.00331.2004. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein–coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty H, Jafurulla M, Clayton AHA, Chattopadhyay A. Exploring oligomeric state of the serotonin1A receptor utilizing photobleaching image correlation spectroscopy: implications for receptor function. Faraday Discuss. 2018;207:409–421. doi: 10.1039/C7FD00192D. [DOI] [PubMed] [Google Scholar]
- Chan HCS, Li Y, Dahoun T, Vogel H, Yuan S. New binding sites, new opportunities for GPCR drug discovery. Trends Biochem Sci. 2019;44:312–330. doi: 10.1016/j.tibs.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Chandra M, Kendall AK, Jackson LP. Toward understanding the molecular role of SNX27/retromer in human health and disease. Front Cell Dev Biol. 2021;9:642378. doi: 10.3389/fcell.2021.642378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A. GPCRs: lipid-dependent membrane receptors that act as drug targets. Adv Biol. 2014;2014:143023. doi: 10.1155/2014/143023. [DOI] [Google Scholar]
- Chaudhuri A, Chattopadhyay A. Transbilayer organization of membrane cholesterol at low concentrations: implications in health and disease. Biochim Biophys Acta. 2011;1808:19–25. doi: 10.1016/j.bbamem.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Chen Z, Rand RP. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DJ, Min C, Jung KS, Cheong SY, Zheng M, Cheong SJ, Lee BK, Kim KM. The N-terminal region of the dopamine D2 receptor, a rhodopsin-like GPCR, regulates correct integration into the plasma membrane and endocytic routes. Br J Pharmacol. 2012;166:659–675. doi: 10.1111/j.1476-5381.2011.01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D-M, Costa E. Evidence for internalization of the recognition site of β-adrenergic receptors during receptor subsensitivity induced by (-)-isoproterenol. Proc Natl Acad Sci USA. 1979;76:3024–3028. doi: 10.1073/pnas.76.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RM, Brown AJH, Marshall FH, Mason JS. Structures of G protein-coupled receptors reveal new opportunities for drug discovery. Drug Discov Today. 2015;20:1355–1364. doi: 10.1016/j.drudis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Crilly SE, Puthenveedu MA. Compartmentalized GPCR signaling from intracellular membranes. J Membr Biol. 2021;254:259–271. doi: 10.1007/s00232-020-00158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SSG, Caron MG, Lekfowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Eichel K, von Zastrow M. Subcellular organization of GPCR signaling. Trends Pharmacol Sci. 2018;39:200–208. doi: 10.1016/j.tips.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand RM, Sayer BG, Epand RF. Peptide-induced formation of cholesterol-rich domains. Biochemistry. 2003;42:14677–14689. doi: 10.1021/bi035587j. [DOI] [PubMed] [Google Scholar]
- Epand RM, Sayer BG, Epand RF. Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol. 2005;345:339–350. doi: 10.1016/j.jmb.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front Physiol. 2013;4:31. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Epand RM, Barrantes FJ. Cholesterol-recognition motifs in membrane proteins. Adv Exp Med Biol. 2019;1135:3–25. doi: 10.1007/978-3-030-14265-0_1. [DOI] [PubMed] [Google Scholar]
- Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Hallows KR, Brown D, Bouley R, Vilardaga JP. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288:27849–27860. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferguson SSG, Downey WE, III, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga J-P. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SR, Hauser AS, Vedel L, Strachan RT, Huang X-P, Gavin AC, Shah SD, Nayak AP, Haugaard-Kedström LM, Penn RB, Roth BL, Bräuner-Osborne H, Gloriam DE. Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell. 2019;179:895–908. doi: 10.1016/j.cell.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Clayton AHA, Chattopadhyay A. Organization of higher-order oligomers of the serotonin1A receptor explored utilizing homo-FRET in live cells. Biophys J. 2011;100:361–368. doi: 10.1016/j.bpj.2010.12.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Chattopadhyay A. Cholesterol depletion mimics the effect of cytoskeletal destabilization on membrane dynamics of the serotonin1A receptor: a zFCS study. Biophys J. 2010;99:1397–1407. doi: 10.1016/j.bpj.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A, Hedayati A. Augmentation of fluoxetine with lovastatin for treating major depressive disorder, a randomized double-blind placebo controlled-clinical trial. Depression Anxiety. 2013;30:1084–1088. doi: 10.1002/da.22195. [DOI] [PubMed] [Google Scholar]
- Gimpl G. Interaction of G protein coupled receptors and cholesterol. Chem Phys Lipids. 2016;199:61–73. doi: 10.1016/j.chemphyslip.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Drake KR, Remmert CL, Kenworthy AK. Ras diffusion is sensitive to plasma membrane viscosity. Biophys J. 2005;89:1398–1410. doi: 10.1529/biophysj.104.055640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez AN, McDonald PH. GPCRs: emerging anti-cancer drug targets. Cell Signal. 2018;41:65–74. doi: 10.1016/j.cellsig.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Haldar S, Kanaparthi RK, Samanta A, Chattopadhyay A. Differential effect of cholesterol and its biosynthetic precursors on membrane dipole potential. Biophys J. 2012;102:1561–1569. doi: 10.1016/j.bpj.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameid RA, Cormet-Boyaka E, Kuebler WM, Uddin M, Berdiev BK. SARS-CoV-2 may hijack GPCR signaling pathways to dysregulate lung ion and fluid transport. Am J Physiol Lung Cell Mol Physiol. 2021;320:L430–L435. doi: 10.1152/ajplung.00499.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Puri V, Singh RD, Hanada K, Pagano RE, Miller LJ. Differential effects of modification of membrane cholesterol and sphingolipids on the conformation, function, and trafficking of the G protein-coupled cholecystokinin receptor. J Biol Chem. 2005;280:2176–2185. doi: 10.1074/jbc.M410385200. [DOI] [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinger HJ, Kandutsch AA, Chen HW. Depletion of L-cell sterol depresses endocytosis. Nature. 1976;263:515–517. doi: 10.1038/263515a0. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Lin MJ, Fine M, Deisl C. On the existence of endocytosis driven by membrane phase separations. Biochim Biophys Acta. 2020;1862:183007. doi: 10.1016/j.bbamem.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth TJ, Gross AK. Defective trafficking of rhodopsin and its role in retinal degenerations. Int Rev Cell Mol Biol. 2012;293:1–44. doi: 10.1016/B978-0-12-394304-0.00006-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Todd N, Thathiah A. The role of GPCRs in neurodegenerative diseases: avenues for therapeutic intervention. Curr Opin Pharmacol. 2017;32:96–110. doi: 10.1016/j.coph.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M, von Zastrow M. Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol. 2017;13:799–806. doi: 10.1038/nchembio.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- Jacobson KA. New paradigms in GPCR drug discovery. Biochem Pharmacol. 2015;98:541–555. doi: 10.1016/j.bcp.2015.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafurulla M, Chattopadhyay A. Membrane lipids in the function of serotonin and adrenergic receptors. Curr Med Chem. 2013;20:47–55. doi: 10.2174/0929867311302010006. [DOI] [PubMed] [Google Scholar]
- Jafurulla M, Kumar GA, Rao BD, Chattopadhyay A. A critical analysis of molecular mechanisms underlying membrane cholesterol sensitivity of GPCRs. Adv Exp Med Biol. 2019;1115:21–52. doi: 10.1007/978-3-030-04278-3_2. [DOI] [PubMed] [Google Scholar]
- Jafurulla M, Rao BD, Sreedevi S, Ruysschaert J-M, Covey DF, Chattopadhyay A. Stereospecific requirement of cholesterol in the function of the serotonin1A receptor. Biochim Biophys Acta. 2014;1838:158–163. doi: 10.1016/j.bbamem.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay J-M, Maroteaux L. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. 2007;71:1463–1474. doi: 10.1124/mol.106.032656. [DOI] [PubMed] [Google Scholar]
- Jong YJI, Harmon SK, O’Malley KL. GPCR signalling from within the cell. Br J Pharmacol. 2018;175:4026–4035. doi: 10.1111/bph.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnell FG, Brezski RJ, King LG, Silverman MA, Monroe JG. Membrane cholesterol content accounts for developmental differences in surface B cell receptor compartmentalization and signaling. J Biol Chem. 2005;280:25621–25628. doi: 10.1074/jbc.M503162200. [DOI] [PubMed] [Google Scholar]
- Köhler O, Gasse C, Petersen L, Ingstrup KG, Nierenberg AA, Mors O, Østergaard SD. The effect of concomitant treatment with SSRIs and statins: a population-based study. Am J Psychiatry. 2016;173:807–815. doi: 10.1176/appi.ajp.2016.15040463. [DOI] [PubMed] [Google Scholar]
- Krishna A, Sengupta D. Interplay between membrane curvature and cholesterol: role of palmitoylated caveolin-1. Biophys J. 2019;116:69–78. doi: 10.1016/j.bpj.2018.11.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GA, Chattopadhyay A. Cholesterol: an evergreen molecule in biology. Biomed Spectros Imaging. 2016;5:S55–S66. doi: 10.3233/BSI-160159. [DOI] [Google Scholar]
- Kumar GA, Chattopadhyay A. Statin-induced chronic cholesterol depletion switches GPCR endocytosis and trafficking: insights from the serotonin1A receptor. ACS Chem Neurosci. 2020;11:453–465. doi: 10.1021/acschemneuro.9b00659. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Chattopadhyay A. Membrane cholesterol regulates endocytosis and trafficking of the serotonin1A receptor: insights from acute cholesterol depletion. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;2021:158882. doi: 10.1016/j.bbalip.2021.158882. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Jafurulla M, Chattopadhyay A. The membrane as the gatekeeper of infection: cholesterol in host-pathogen interaction. Chem Phys Lipids. 2016;199:179–185. doi: 10.1016/j.chemphyslip.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Sarkar P, Jafurulla M, Singh SP, Srinivas G, Pande G, Chattopadhyay A. Exploring endocytosis and intracellular trafficking of the human serotonin1A receptor. Biochemistry. 2019;58:2628–2641. doi: 10.1021/acs.biochem.9b00033. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Sarkar P, Stepniewski TM, Jafurulla M, Singh SP, Selent J, Chattopadhyay A (2021) A molecular sensor for cholesterol in the human serotonin1A receptor. Sci Adv 7:eabh2922. 10.1126/sciadv.abh2922 [DOI] [PMC free article] [PubMed]
- Kunselman JM, Gupta A, Gomes I, Devi LA, Puthenveedu MA. Compartment-specific opioid receptor signaling is selectively modulated by different dynorphin peptides. eLife. 2021;10:e60270. doi: 10.7554/eLife.60270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunselman JM, Lott J, Puthenveedu MA. Mechanisms of selective G protein-coupled receptor localization and trafficking. Curr Opin Cell Biol. 2021;71:158–165. doi: 10.1016/j.ceb.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T, Kastenmüller W. Concepts of GPCR-controlled navigation in the immune system. Immunol Rev. 2019;289:205–231. doi: 10.1111/imr.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, Abdul-Ridha A, Canals M. Regulation of G protein-coupled receptors by allosteric ligands. ACS Chem Neurosci. 2013;4:527–534. doi: 10.1021/cn400005t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett SB. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci Signal. 2011;4:pe36. doi: 10.1126/scisignal.2002331. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Phair RD. Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu Rev Biophys. 2010;39:559–578. doi: 10.1146/annurev.biophys.093008.131357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. β-Arrestin: a protein that regulates β-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SSG, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F-T, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-Arrestin-dependent formation of β2 adrenergic receptor–Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Lyga S, Volpe S, Werthmann RC, Götz K, Sungkaworn T, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology. 2016;157:1613–1621. doi: 10.1210/en.2015-1945. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Dunn H, Ferguson SSG. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins: regulation of G-protein-coupled receptor activity. Br J Pharmacol. 2012;165:1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BRS, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol. 2010;22:422–429. doi: 10.1016/j.ceb.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel FK, Molden BM, Mohammad S, Baldini G, McPike L, Narducci P, Granell S, Baldini G. Constitutive cholesterol-dependent endocytosis of melanocortin-4 receptor (MC4R) is essential to maintain receptor responsiveness to α-melanocyte-stimulating hormone (α-MSH) J Biol Chem. 2012;287:21873–21890. doi: 10.1074/jbc.M112.346890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Heybrock S, Neculai D, Saftig P. Cholesterol handling in lysosomes and beyond. Trends Cell Biol. 2020;30:452–466. doi: 10.1016/j.tcb.2020.02.007. [DOI] [PubMed] [Google Scholar]
- Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsche MA, McDonald JG, Hobbs HH, Cohen JC. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. eLife. 2015;4:e07999. doi: 10.7554/eLife.07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- Mouritsen OG (2005) Cholesterol on the scene. In: Life – as a matter of fat. Springer, Heidelberg 149–157. 10.1007/b138577
- Mouritsen OG, Zuckermann MJ. What’s so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- Nes WD. Biosynthesis of cholesterol and other sterols. Chem Rev. 2011;111:6423–6451. doi: 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhady MAM, Rivera JC, Chemtob S. Location bias as emerging paradigm in GPCR biology and drug discovery. iScience. 2020;23:101643. doi: 10.1016/j.isci.2020.101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezil FA, Bloom M. Combined influence of cholesterol and synthetic amphiphilic peptides upon bilayer thickness in model membranes. Biophys J. 1992;61:1176–1183. doi: 10.1016/S0006-3495(92)81926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates J, Watts A. Uncovering the intimate relationship between lipids, cholesterol and GPCR activation. Curr Opin Struct Biol. 2011;21:802–807. doi: 10.1016/j.sbi.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Ninomiya H, Miwa S, Masaki T. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J Biol Chem. 2000;275:6439–6446. doi: 10.1074/jbc.275.9.6439. [DOI] [PubMed] [Google Scholar]
- Örtegren U, Karlsson M, Blazic N, Blomqvist M, Nystrom FH, Gustavsson J, Fredman P, Strålfors P. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. Eur J Biochem. 2004;271:2028–2036. doi: 10.1111/j.1432-1033.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paila YD, Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell Biochem. 2010;51:439–466. doi: 10.1007/978-90-481-8622-8_16. [DOI] [PubMed] [Google Scholar]
- Paila YD, Kombrabail M, Krishnamoorthy G, Chattopadhyay A. Oligomerization of the serotonin1A receptor in live cells: a time-resolved fluorescence anisotropy approach. J Phys Chem B. 2011;115:11439–11447. doi: 10.1021/jp201458h. [DOI] [PubMed] [Google Scholar]
- Pal S, Chakraborty H, Bandari S, Yahioglu G, Suhling K, Chattopadhyay A. Molecular rheology of neuronal membranes explored using a molecular rotor: implications for receptor function. Chem Phys Lipids. 2016;196:69–75. doi: 10.1016/j.chemphyslip.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Orban T. From atomic structures to neuronal functions of G protein–coupled receptors. Annu Rev Neurosci. 2013;36:139–164. doi: 10.1146/annurev-neuro-062012-170313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Volgraf M. Ligand binding at the protein-lipid interface: strategic considerations for drug design. Nat Rev Drug Discov. 2021;20:710–722. doi: 10.1038/s41573-021-00240-2. [DOI] [PubMed] [Google Scholar]
- Pediconi MF, Gallegos CE, de Los Santos EB, Barrantes FJ. Metabolic cholesterol depletion hinders cell-surface trafficking of the nicotinic acetylcholine receptor. Neurosci. 2004;128:239–249. doi: 10.1016/j.neuroscience.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Platt FM, Wassif C, Colaco A, Dardis A, Lloyd-Evans E, Bembi B, Porter FD. Disorders of cholesterol metabolism and their unanticipated convergent mechanisms of disease. Annu Rev Genomics Hum Genet. 2014;15:173–194. doi: 10.1146/annurev-genom-091212-153412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna X, Sengupta D, Chattopadhyay A. Cholesterol-dependent conformational plasticity in GPCR dimers. Sci Rep. 2016;6:31858. doi: 10.1038/srep31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Cholesterol depletion induces dynamic confinement of the G-protein coupled serotonin1A receptor in the plasma membrane of living cells. Biochim Biophys Acta. 2007;1768:655–668. doi: 10.1016/j.bbamem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Retamal JS, Ramírez-García PD, Shenoy PA, Poole DP, Veldhuis NA. Internalized GPCRs as potential therapeutic targets for the management of pain. Front Mol Neurosci. 2019;12:273. doi: 10.3389/fnmol.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred Ø, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae: a mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Sahu SS, Sarkar P, Shrivastava S, Chattopadhyay A. Differential effects of simvastatin on membrane organization and dynamics in varying phases. Chem Phys Lipids. 2019;225:104831. doi: 10.1016/j.chemphyslip.2019.104831. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Chakraborty H, Chattopadhyay A. Differential membrane dipolar orientation induced by acute and chronic cholesterol depletion. Sci Rep. 2017;7:4484. doi: 10.1038/s41598-017-04769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Chattopadhyay A. Cholesterol interaction motifs in G protein-coupled receptors: slippery hot spots? Wiley Interdiscip Rev Syst Biol Med. 2020;12:e1481. doi: 10.1002/wsbm.1481. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Chattopadhyay A. Cholesterol in GPCR structures: prevalence and relevance. J Membr Biol (in Press) 2021 doi: 10.1007/s00232-021-00197-8. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Chattopadhyay A. Cholesterol footprint in high-resolution structures of serotonin receptors: where are we now and what does it mean? Chem Phys Lipids. 2021;239:105120. doi: 10.1016/j.chemphyslip.2021.105120. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Jafurulla M, Bhowmick S, Chattopadhyay A. Structural stringency and optimal nature of cholesterol requirement in the function of the serotonin1A receptor. J Membr Biol. 2020;253:445–457. doi: 10.1007/s00232-020-00138-x. [DOI] [PubMed] [Google Scholar]
- Saxena R, Chattopadhyay A. Membrane cholesterol stabilizes the human serotonin1A receptor. Biochim Biophys Acta. 2012;1818:2936–2942. doi: 10.1016/j.bbamem.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Sejdiu BI, Tieleman DP. Lipid-protein interactions are a unique property and defining feature of G protein-coupled receptors. Biophys J. 2020;118:1887–1900. doi: 10.1016/j.bpj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D, Chattopadhyay A. Molecular dynamics simulations of GPCR-cholesterol interaction: an emerging paradigm. Biochim Biophys Acta. 2015;1848:1775–1782. doi: 10.1016/j.bbamem.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Sengupta D, Kumar GA, Chattopadhyay, A. (2017) Interaction of membrane cholesterol with GPCRs: implications in receptor oligomerization. In: Giovanni G, Herrick-Davis K, Milligan, G (eds) G protein-coupled receptor dimers. Springer, Heidelberg 415–429. 10.1007/978-3-319-60174-8_16
- Sengupta D, Prasanna X, Mohole M, Chattopadhyay A. Exploring GPCR-lipid interactions by molecular dynamics simulations: excitements, challenges and the way forward. J Phys Chem B. 2018;122:5727–5737. doi: 10.1021/acs.jpcb.8b01657. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumar GA, Chattopadhyay A. Late endosomal/lysosomal accumulation of a neurotransmitter receptor in a cellular model of Smith-Lemli-Opitz syndrome. Traffic (in Press) 2021;22:332–344. doi: 10.1111/tra.12811. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin1A receptors. Biochemistry. 2010;49:5426–5435. doi: 10.1021/bi100276b. [DOI] [PubMed] [Google Scholar]
- Shvartsman DE, Gutman O, Tietz A, Henis YI. Cyclodextrins but not compactin inhibit the lateral diffusion of membrane proteins independent of cholesterol. Traffic. 2006;7:917–926. doi: 10.1111/j.1600-0854.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- Simon SA, McIntosh TJ, Latorre R. Influence of cholesterol on water penetration into bilayers. Science. 1982;216:65–67. doi: 10.1126/science.7063872. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Emmerson PJ, Statnick MA, Willard FS. The current state of GPCR-based drug discovery to treat metabolic disease. Br J Pharmacol. 2018;175:4060–4071. doi: 10.1111/bph.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Anderson RGW. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 2002;353:131–139. doi: 10.1016/S0076-6879(02)53043-3. [DOI] [PubMed] [Google Scholar]
- Sposini S, Hanyaloglu AC. Spatial encryption of G protein-coupled receptor signaling in endosomes; mechanisms and applications. Biochem Pharmacol. 2017;143:1–9. doi: 10.1016/j.bcp.2017.04.028. [DOI] [PubMed] [Google Scholar]
- Sriram K, Insel PA. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol Pharmacol. 2018;93:251–258. doi: 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert JA, Devi LA. Advancements in therapeutically targeting orphan GPCRs. Front Pharmacol. 2015;6:100. doi: 10.3389/fphar.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, Manglik A, von Zastrow M. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron. 2018;98:963–976.e5. doi: 10.1016/j.neuron.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ARB, Jensen DD, Hicks GA, Bunnett NW. Therapeutic targeting of endosomal G-protein-coupled receptors. Trends Pharmacol Sci. 2018;39:879–891. doi: 10.1016/j.tips.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol. 2014;10:1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Jones KT, Salo PD, Severin JE, Trejo J, Radhakrishna H. A requirement for membrane cholesterol in the β-arrestin- and clathrin-dependent endocytosis of LPA1 lysophosphatidic acid receptors. J Cell Sci. 2005;118:5291–5304. doi: 10.1242/jcs.02634. [DOI] [PubMed] [Google Scholar]
- Villalaín J. Location of cholesterol in model membranes by magic-angle-sample-spinning NMR. Eur J Biochem. 1996;241:586–593. doi: 10.1111/j.1432-1033.1996.00586.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen M, Li S, Ye RD. Targeted delivery of a ligand–drug conjugate via formyl peptide receptor 1 through cholesterol-dependent endocytosis. Mol Pharmaceutics. 2019;16:2636–2647. doi: 10.1021/acs.molpharmaceut.9b00188. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang L, Huang HW. Evidence of cholesterol accumulated in high curvature regions: implication to the curvature elastic energy for lipid mixtures. Biophys J. 2007;92:2819–2830. doi: 10.1529/biophysj.106.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao Y, Zhang X, Badie H, Zhou Y, Mu Y, Loo LS, Cai L, Thompson RC, Yang B, Chen Y, Johnson PF, Wu C, Bu G, Mobley WC, Zhang D, Gage FH, Ranscht B, Zhang Y, Lipton SA, Hong W, Xu H. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat Med. 2013;19:473–480. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yu Z, Xiao W, Lu S, Zhang J. Allosteric binding sites at the receptor-lipid bilayer interface: novel targets for GPCR drug discovery. Drug Discov Today. 2021;26:690–703. doi: 10.1016/j.drudis.2020.12.001. [DOI] [PubMed] [Google Scholar]
- Weinberg ZY, Puthenveedu MA. Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic. 2019;20:121–129. doi: 10.1111/tra.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang F, Zhang D, Liu Z, Lin A, Liu C, Xiao P, Yu X, Sun J-P. Phosphorylation of G protein-coupled receptors: from the barcode hypothesis to the flute model. Mol Pharmacol. 2017;92:201–210. doi: 10.1124/mol.116.107839. [DOI] [PubMed] [Google Scholar]