Abstract
The checkpoint kinase Chk2 has a key role in delaying cell cycle progression in response to DNA damage. Upon activation by low-dose ionizing radiation (IR), which occurs in an ataxia telangiectasia mutated (ATM)-dependent manner, Chk2 can phosphorylate the mitosis-inducing phosphatase Cdc25C on an inhibitory site, blocking entry into mitosis, and p53 on a regulatory site, causing G1 arrest. Here we show that the ATM-dependent activation of Chk2 by γ- radiation requires Nbs1, the gene product involved in the Nijmegen breakage syndrome (NBS), a disorder that shares with AT a variety of phenotypic defects including chromosome fragility, radiosensitivity, and radioresistant DNA synthesis. Thus, whereas in normal cells Chk2 undergoes a time-dependent increased phosphorylation and induction of catalytic activity against Cdc25C, in NBS cells null for Nbs1 protein, Chk2 phosphorylation and activation are both defective. Importantly, these defects in NBS cells can be complemented by reintroduction of wild-type Nbs1, but neither by a carboxy-terminal deletion mutant of Nbs1 at amino acid 590, unable to form a complex with and to transport Mre11 and Rad50 in the nucleus, nor by an Nbs1 mutated at Ser343 (S343A), the ATM phosphorylation site. Chk2 nuclear expression is unaffected in NBS cells, hence excluding a mislocalization as the cause of failed Chk2 activation in Nbs1-null cells. Interestingly, the impaired Chk2 function in NBS cells correlates with the inability, unlike normal cells, to stop entry into mitosis immediately after irradiation, a checkpoint abnormality that can be corrected by introduction of the wild-type but not the S343A mutant form of Nbs1. Altogether, these findings underscore the crucial role of a functional Nbs1 complex in Chk2 activation and suggest that checkpoint defects in NBS cells may result from the inability to activate Chk2.
The integrity of genetic information is essential for the life and survival of cells. Genomic lesions arising spontaneously during DNA replication or in response to oxidative metabolism or exposure to radiation or chemical mutagens need to be recognized and repaired. Delay of cell cycle progression at specific checkpoints provides the time necessary to prevent replication and segregation of damaged DNA and to process lesions (reviewed in references 52 and 57). A defective or incorrect activation of the surveillance and repair systems can lead to increased mutagenesis, genomic instability, and ultimately cancer (for a review, see reference 13).
The Nijmegen breakage syndrome (NBS) and ataxia telangiectasia (AT) are rare human autosomal recessive diseases (22, 51) exhibiting hypersensitivity to ionizing radiation (IR), immunodeficiency, and increased predisposition to develop cancer. NBS patients, however, do not manifest the hallmarks of AT, i.e., cerebellar ataxia and oculocutaneous telangiectasia. At the cellular level, NBS and AT patients show chromosome instability, hypersensitivity to genotoxic agents, and cell cycle checkpoints defects (1, 29, 30). These similarities suggest that ATM (AT mutated) (39) and Nbs1 (38, 51), the gene products defective in AT and NBS, are involved in maintaining genomic integrity, possibly by operating through a common pathway.
Nbs1, a protein with a forkhead-associated domain and a carboxy-terminal repeat frequently found in cell cycle regulatory and DNA repair proteins (11, 49), is essential for the formation of radiation-induced nuclear foci, probably at the sites of DNA breaks, together with Mre11 and Rad50 proteins (6, 26). Biochemical evidence (33) and the homology with the yeast Xrs2-Mre11-Rad50 complex (48) suggest a major role for the human Nbs1-Mre11-Rad50 complex in double-strand break repair and also in sensing DNA damage and recruiting proteins with kinase activity (36, 52).
ATM is a protein kinase that shares similarities with the phosphatidylinositol 3-kinases (39) involved in signaling pathways that regulate genome stability and cell cycle checkpoint arrest after DNA damage or incomplete DNA replication. The ATM kinase is critical for the regulation of G1, S and G2/M checkpoints in response to genotoxic agents (21), as its activation by DNA damage leads to phosphorylation of targets proteins that mediate cell cycle arrest. The phosphorylation of p53 at serine 15 by ATM, in particular, appears relevant for the G1-phase arrest, as this event contributes to p53 protein stabilization (41) and enhanced transcriptional activation of the cyclin-dependent kinase inhibitor p21waf1 (32). A role for Nbs1 in G1 checkpoint is controversial, since the extents of p53 accumulation and G1 arrest after radiation differ substantially among NBS cell lines (1, 17, 29, 43, 55). ATM interacts with and phosphorylates Nbs1 on Ser343 after IR (12, 23, 53, 56). This event, though not involved in Nbs1, complex and focus formation, is functionally relevant because a serine-to-alanine change at amino acid (aa) 343 of Nbs1 only partially complements radiosensitivity and S-phase checkpoint deficiency in NBS cells, while in normal cells it acts as dominant negative in the activation of the S-phase checkpoint by IR.
Chk2, the mammalian homolog of Saccharomyces cerevisae Rad53 and Schizosaccharomyces pombe Cds1, is a kinase whose activation by DNA damage prevents entry into mitosis (27) and into S phase (9, 14, 40). Chk2 kinase is activated by phosphorylation in ATM-dependent manner (5, 8, 27, 28) on threonine 68 (31). In vitro, Chk2 is capable of phosphorylating all members of the Cdc25 family, Cdc25A, -B, and -C (27). Cdc25C, in particular, is phosphorylated by Chk2 on Ser216, the consensus binding site for the 14-3-3 family of proteins that regulate biochemical activities by binding to and sequestering phosphorylated proteins (54). In homology with S. pombe, the Cdc25C–14-3-3 interaction may prevent mitosis by sequestering Cdc25C in the cytoplasm (25, 37), where it cannot dephosphorylate and activate Cdc2-cyclin B nuclear complexes (35). After DNA damage, Chk2 phosphorylates p53 on Ser20 (9, 14, 40), attenuating the binding of p53 to Mdm2, a protein that targets p53 for degradation, and allowing accumulation and subsequent activation of p21waf1 and G1 arrest. Noteworthy, inherited mutations of CHK2, like those of ATM and NBS1 genes, confer tumor susceptibility, according to recent findings showing germ line CHK2 mutations in a subset of cancer-prone Li-Fraumeni cases wild type for p53 (3), further underscoring the function of cell cycle checkpoints in preventing genetic instability and cancer.
In this work, we have assessed the role of Nbs1 in the phosphorylation and activation of Chk2 kinase by using different primary and immortalized NBS cell lines with undetectable levels of Nbs1 protein. We show that DNA damage induces a time-dependent increase in Chk2 phosphorylation and activation in normal but not in NBS cells. Moreover, we demonstrate that this defect can be complemented by a functional Nbs1 nuclear complex.
MATERIALS AND METHODS
Cell lines and irradiation.
The lymphoblastoid cell lines (LCL) derived by Epstein-Barr immortalization were LCL-N, LCL-N1, and LCL-N2 from healthy individuals, AT52RM from an AT patient, 524RM and 227RM from two AT heterozygotes, 1548 from an Italian NBS patient (47), and NBS02LA, NBS03LA, and NBS04LA from three unrelated NBS patients (12). The lymphoblastoid cells GM07078 (NBS patient) and GM08036 and GM08037 (NBS-heterozygous parents) were obtained from the Coriell Cell Repository (Camden, N.J.). FB-N and 18ATRM are established fibroblasts from a healthy individual and an AT patient, respectively. GM07166 fibroblasts are established from the same NBS patient as GM07078; ILB1 are simian virus 40-immortalized NBS fibroblasts, GM07166/NBS1 are fibroblasts stably transfected with the full-length NBS1 cDNA, GM07166/s590 are stably transfected cells with the NBS1 cDNA truncated at codon 590, and ILB1/S343A cells are stably transfected with Nbs1 with a serine-to-alanine change at aa 343. The LCL were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, Md.) supplemented with 15% heat-inactivated fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml), while the fibroblasts were maintained in Dulbecco modified Eagle medium with the same serum and antibiotic concentrations. Stable cells transfectants were selected in medium containing hygromycin B (Boehringer, Mannheim, Germany) at 200μg/ml and maintained in the presence of the drug at 100 μg/ml. Cells were γ irradiated in an IBL437CO instrument with a 137 Ce source emitting a dose rate of 8 Gy/min.
Immunoblot analysis.
Untreated or treated cells were washed with phosphate-buffered saline plus 0.1 mM Na3VO4 (Sigma), pelleted, and lysed in Laemmli buffer (0.125 M Tris-HCl [pH 6.8], 5% sodium dodecyl sulfate [SDS]) containing as inhibitors 1 mM phenylmethylsulfonyl fluoride (PMSF), pepstatin (10 μg/ml), aprotinin (100 KIU/ml), leupeptin (10 μg/ml) (all from Calbiochem, San Diego, Calif.) and 1 mM Na3VO4. Lysates were boiled for 2 min, sonicated, and quantitated by the micro-bicinchoninic acid method (Pierce, Rockford, Ill.). Aliquots containing proteins (10 to 50 μg/ml) plus 5% β-mercaptoethanol were size fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) 7 to 10% gels and electroblotted onto polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). After blocking with 5% nonfat dried milk in phosphate-buffered saline plus 0.1% Tween (Sigma), the membranes were incubated with rabbit antibodies specific for Chk2 (46) and Nbs1 (Novus Biologicals, Littleton, Cols.) and subsequently with peroxidase-conjugated secondary antibodies. The immunoreactive bands were visualized by ECL Super Signal (Pierce) on autoradiographic films. Autoradiographic bands were analyzed by optical densitometry using a DuoScan system (Agfa, Mortsel, Belgium) and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.).
IF.
Cytospin preparations were stained by indirect immunofluorescence (IF) as reported previously (26), using 1:50 to 1:100 dilutions of rabbit antibodies specific for Nbs1 and Mre11 (both from Novus Biologicals). IF for Chk2 was performed on cytospins fixed for 10 min in 2% paraformaldehyde, washed in Tris-buffered saline, permeabilized with 0.1% Triton X-100, and incubated with 1:50 dilution of a rabbit anti-Chk2 antibody (Santa Cruz Biotech, Santa Cruz, Calif.). The specificity of this antibody was verified in IF analysis by competition with a recombinant full-length Chk2 protein. A normal rabbit serum was used as a negative control. Binding of primary antibodies was revealed with a fluorescein isothiocyanate-labeled F(ab)2 goat anti-rabbit antibody (1:50 dilution). Coverslips were mounted with an antifade solution containing the DNA counterstain 4′,6-diamidino-2-phenylindole (DAPI). Images were collected with a Zeiss Axioskop (Germany) fluorescence microscope and digital imaging.
Immunoprecipitations and kinase reaction.
Cells were lysed for 30 min in ice-cold buffer containing 50 mM Tris-HCl (pH 7.4), 0.2% Triton X-100, 0.3% NP-40, 150 mM NaCl, 1 mM PMSF, pepstatin (1 μg/ml), leupeptin (2 μg/ml), aprotinin (2 μg/ml), 25 mM NaF, 1 mM EDTA, and 1 mM Na3VO4. The clarified lysates were precleared with 10 μl of immobilized protein A (Sigma) for 10 min at 4°C and immunoprecipitated with 5 μg of anti-Chk2 antibody and 10 μl of immobilized protein A at 4°C for 2 h. Chk2 activity was assayed at 30°C for 30 min in a 20-μl reaction mixture containing 50 mM HEPES (pH 8.0), 10 mM MgCl2, 2.5 mM EDTA, 1 mM dithiothreitol, 10 μM β-glycerophosphate, 1 mM NaF, 0.1 mM Na3VO4, 0.1 mM PMSF, 10 μM ATP, and 30 μCi of [γ-32P]ATP and, when necessary, using a glutathione S-transferase (GST)–Cdc25C fragment as a substrate (46). The reaction products were separated by SDS-PAGE, autoradiographed, and Western blotted for Ckh2 to verify the amount of immunoprecipitated Chk2 per sample.
Mitotic index.
The analysis were performed as described elsewhere (2). Briefly, lymphoblastoid cells, unirradiated or irradiated with 1.5 Gy, were collected at hourly intervals, washed with 0.075 M KCl, and after cytocentrifugation onto glass slides, fixed in methanol-acetic acid (3:1) and stained for 5 min with diluted Giemsa stain (BDH, Poole, England). Fibroblasts were plated on sterile coverslips contained in petri dishes 2 days prior to irradiation and then processed as described above. In all cases, no mitotic-phase-arresting agents were used. Nuclei were counted by light microscope from at least 1,000 cells from each slide preparation. For each time point, the fraction of mitotic cells present in the unirradiated sample were normalized to 100%; the mitotic index indicates the variation (in percentage) of mitotic figures present in the irradiated sample counterpart.
RESULTS
ATM-dependent Chk2 phosphorylation in normal cell lines.
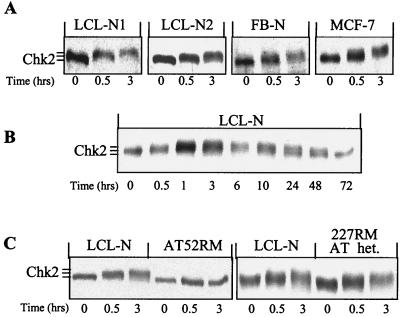
Shifts in Chk2 electrophoretic mobility reflect modifications of its phosphorylation status (5, 27). We performed time course Western analysis on extracts from exponentially growing cell lines to monitor the phosphorylation of Chk2 after 4 Gy of IR. In four different cell lines, two normal LCL (LCL-N1 and LCL-N2), a normal fibroblast (FB-N), and a breast cancer with apparently normal ATM-dependent radiation response (MCF-7), Chk2 showed an electrophoretic mobility delay at 30 min, which increased further at 3 h post-IR, compared to unirradiated controls (Fig. 1A). In a more detailed time course analysis on LCL-N cells, Chk2 showed a maximum migration delay between 1 and 3 h post-IR; 10 h later it started migrating faster, reverting to the basal level at 72 h (Fig. 1B). As the electrophoretic retardation of Chk2 is due to phosphorylation since it can be eliminated by treatment with phosphatases (reference 27 and our data not shown), these results indicate that the full phosphorylation of Chk2 occurs within 1 h of IR and appears to involve most, if not all, Chk2 molecules. Of note, the Chk2 phosphorylation changes seen in the early hours post-IR were not due to a cell cycle phase redistribution, according to flow cytofluorimetric analysis (data not shown).
FIG. 1.
Time course analysis of Chk2 phosphorylation following exposure to low-dose IR. Western blotting was performed on exponentially growing LCL-N, LCL-N1, LCL-N2, and FB-N normal cells and MCF7 breast cancer cells (A) and (B) and on the AT and AT-heterozygous (het.) cell lines AT52RM and 277RM (C). Cells were harvested before or at various time points after 4 Gy of IR. Note the progressive increase in Chk2 retardation in the early hours post-IR in normal but not AT cells. This retardation is no longer seen in normal cells at 72 h post-IR.
As in budding and fission yeast, the activation of Rad53 and Cds1 is dependent on the ATM homologs Mec1 and Rad3, respectively, and the same is true in mammalian cells (5, 8, 27) we analyzed the regulation of Chk2 in the AT-derived cell line AT52RM, negative for ATM protein expression (10). In this AT cell line, and in contrast to LCL-N, no Chk2 phosphorylation was seen at any time point post-IR (Fig. 1C), thus confirming previous results and validating that the experimental conditions used here allow to detect ATM-dependent Chk2 phosphorylation changes. To assess whether a reduced expression of ATM affects Chk2, we analyzed the AT-heterozygous cell line 227RM, which exhibits 30% of the normal levels of ATM protein (10). The IR-induced phosphorylation of Chk2 in this cell line was normal (Fig. 1C). Altogether, these findings demonstrate that Chk2 is rapidly phosphorylated after low-dose DNA damage in an ATM-dependent manner and even in an ATM-haploinsufficient background.
Nbs1-dependent Chk2 phosphorylation.
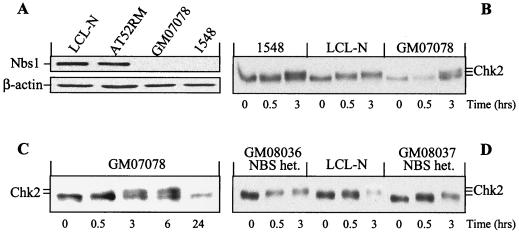
The overlapping cellular abnormalities between AT and NBS prompted us to determine the impact of Nbs1 deficiency on Chk2 regulation. The NBS-derived LCL 1548 and GM07078A, homozygous for truncation mutations of the NBS1 gene (835de14 and 657de15, respectively) (7, 47, 49) were, unlike normal and AT cells, negative for Nbs1 protein (Fig. 2A). In these NBS cells, and in contrast to normal cells, Chk2 failed to show any electrophoretic mobility shift at 30 min after IR (Fig. 2B and C), whereas at 3 h it evidenced a modest shift which suggested that a small fraction of Chk2 molecules may have undergone phosphorylation at this time point. Collectively, these findings demonstrate a marked impairment in NBS cells of IR-induced Chk2 phosphorylation and lend support to the role of Nbs1 in the rapid and sustained phosphorylation of this kinase. Of note, Chk2 phosphorylation after IR was normal in two NBS-heterozygous carriers (Fig. 2D) expressing >45% of the normal Nbs1 levels (data not shown), indicating that like the case for ATM, NBS1 haploinsufficiency has no effect on Chk2 responses.
FIG. 2.
Nbs1 protein expression and time course analysis of Chk2 phosphorylation in NBS cells. Western blotting was performed on normal (LCL-N), AT (AT52RM), NBS (1548 and GM07078), and NBS-heterozygous (het.; GM08036 and GM08037) cells harvested before or at various times after 4-Gy IR. (A) Samples tested for Nbs1 and normalized for β-actin. Note the absence of Nbs1 protein in the two NBS cell lines. (B to D) Samples tested for Chk2. Note the absence of mobility shift 30 min post-IR in NBS cells but not in NBS-heterozygous cells.
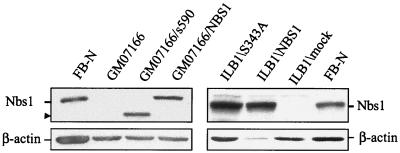
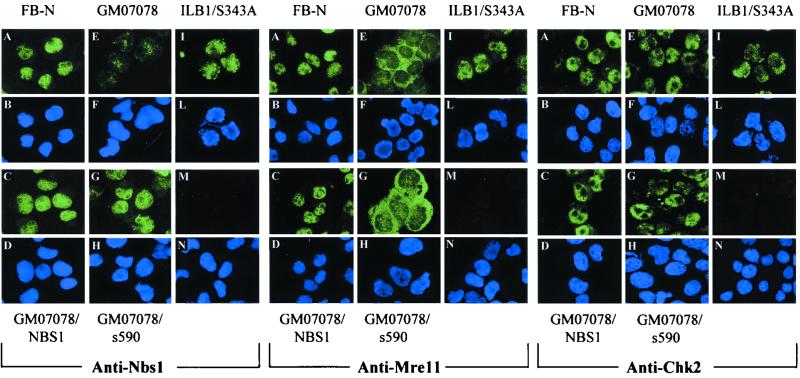
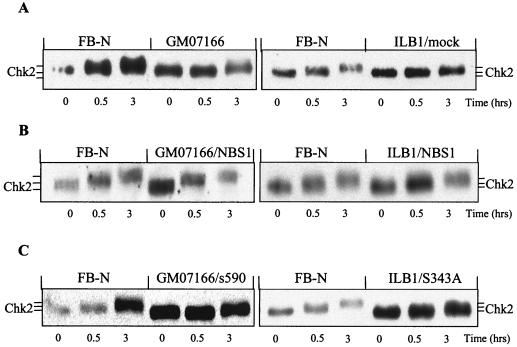
To verify that the defective Chk2 regulation in NBS cells was entirely due to Nbs1 protein deficiency rather than to other genetic defects, we analyzed Chk2 in NBS cells complemented with wild-type NBS1. The ectopic expression of NBS1 in GM07166/NBS1 and ILB1/NBS1 cells was confirmed by immunoblotting (Fig. 3). To further define the role of NBS1 in this response, we analyzed NBS cells transfected with an NBS1 cDNA encoding a protein truncated at aa 590 (GM07166/s590) that deletes the carboxy-terminus region containing the Mre11 binding domain (45). We also analyzed an Nbs1 mutant containing a serine-to-alanine change at position 343 (ILB1/S343A). Nbs1 Ser343 is directly phosphorylated by ATM immediately after DNA damage, and mutations of this residue only partially complement the S-phase checkpoint in NBS cells (12, 24). These mutants, which demonstrated on immunoblots Nbs1 proteins of the expected molecular size (Fig. 3), were also characterized for Nbs1, Mre11, and Chk2 expression by IF of cytospin preparations to determine the relationship between NBS1 status, Nbs1 complex formation, and Chk2 localization. Nbs1 protein, undetectable in NBS cells (Fig. 4, left panel, E and F), demonstrated a nuclear fluorescence both in normal cells (A and B) and in NBS cells ectopically expressing wild-type (C and D) or mutant s590 (G and H) and S343A (I and L) Nbs1 proteins. Mre11 (Fig. 4, middle panel) was nuclear in normal cells (A and B) and NBS cells ectopically expressing wild-type (C and D) or S343A (I and L) Nbs1, whereas it showed a diffuse and cytoplasmic fluorescence in NBS and (E and F) and in NBS/s590 (G and H) cells, the latter finding demonstrating that the C-terminal region of Nbs1 is necessary for the nuclear localization of the Nbs1-Mre11-Rad50 complex (45). The nuclear Chk2 expression found in normal cells was retained in NBS cells, whether or not transfected with the various Nbs1 mutants (Fig. 4, right panels), and even 8 h after IR (not shown). These findings would thus exclude a defect in Chk2 localization as the cause of its failed phosphorylation in NBS cells.
FIG. 3.
Nbs1 expression in NBS-transfected cells. Lysates from the NBS fibroblast cell lines GM07166/NBS1, ILB1/NBS1, GM07166/s590, and ILB1/S343A were Western blotted for Nbs1 to verify ectopic expression of the NBS1 cDNA constructs. Blots were reprobed for β-actin to normalize lanes for protein content.
FIG. 4.
Nbs1, Mre11, and Chk2 localization in NBS-transfected cells. Normal fibroblasts (A and B), NBS cells (E and F), and NBS cells stably expressing full-length Nbs1 (C and D), carboxy-truncated Nbs1(s590) (G and H), or S343A Nbs1 (I and L) were analyzed. The negative control for each antibody, tested on normal fibroblasts, is shown (M and N). Green and blue color images represent IF labeling and DAPI nuclear DNA staining, respectively.
In contrast to parental or mock-transfected cells, NBS cells ectopically expressing wild-type NBS1 (GM07166/NBS1 and ILB1/NBS1) showed a restoration of Chk2 phosphorylation in response to 4 Gy of IR (Fig. 5A and B). Neither s590 nor S343A Nbs1 mutants were able to complement the Chk2 phosphorylation defect (Fig. 5C).
FIG. 5.
Chk2 mobility shifts in transfected NBS cells. Exponentially growing normal (FB-N) and NBS (GM07166 and ILB1/mock) fibroblasts (A) and NBS fibroblasts ectopically expressing either full-length NBS1 cDNA (GM07166/NBS1 and ILB1/NBS1) (B), a C-terminal deletion (GM07166/s590), or an S343A mutation (ILB1/S343A) (C) were harvested before or at the indicated time points after 4 Gy of IR and examined on Western blots for Chk2.
Collectively these data demonstrate that Chk2 phosphorylation is Nbs1 dependent, since in Nbs1-null NBS cells this event is largely impaired but can be complemented with wild-type Nbs1. Our data additionally indicate that the phosphorylation of Chk2 requires the expression of a functional, ATM-phosphorylatable Nbs1 protein and the nuclear localization of the Nbs1 complex.
Nbs1-dependent Chk2 kinase activity.
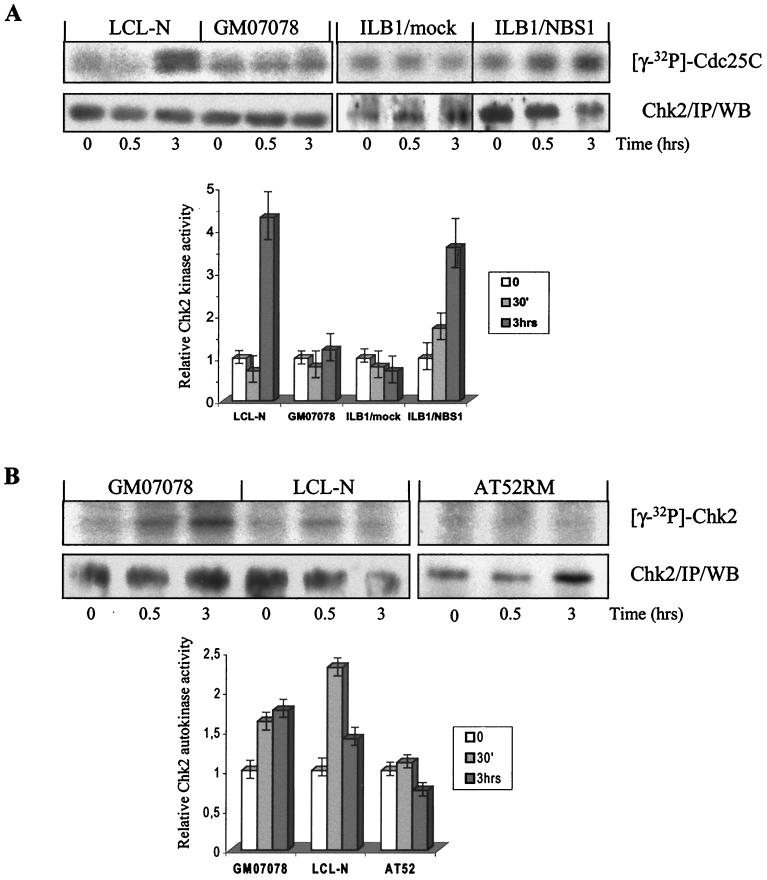
The catalytic activity of Chk2 in relation to its phosphorylation status was examined in vitro on Chk2 immunoprecipitates extracted from normal and NBS cells before, 30 min after, and 3 h after 4 Gy of IR and assayed for phosphorylation of the target residue Ser216 of Cdc25C. In normal cells, the basal Chk2 kinase activity increased up to ∼5-fold at 3 h but not at 30 min after IR treatment (Fig. 6A). Conversely, in NBS cells Chk2 kinase activity did not increase at any time point after IR. Western blots of the kinase reactions verified the presence of similar amounts of Chk2 protein (Fig. 6A). Importantly, the ectopic expression in NBS cells of wild-type Nbs1 restored not only the phosphorylation (as shown above) but also the catalytic activity of Chk2 to almost the same extent as in normal cells (Fig. 6A), suggesting that Nbs1 is necessary for the phosphorylative activation of Chk2 by IR. Moreover, the finding that Chk2 kinase activity does not increase in normal cells at 30 min and in NBS cells at 3 h post-IR, when Chk2 shows a modest phosphorylation, (Fig. 2B) suggests that the activation of Chk2 depends on multiple phosphorylation steps.
FIG. 6.
In vitro Chk2 kinase activity and autophosphorylation. Chk2 was immunoprecipitated (IP) from normal cells, NBS cells (GM07078 and ILB1/mock), and NBS cells ectopically expressing wild-type Nbs1 (ILB1/NBS1) exposed or not to 4 Gy of IR. Kinase reactions were assayed on GST-Cdc25C substrate, separated by gel electrophoresis, autoradiographed, and then Western blotted (WB) for Chk2 to verify the amount of immunoprecipitated protein per sample (A). Chk2 autophosphorylation was examined in kinase assays performed without target substrate (B). The graphs were obtained by the densitometric analysis of autoradiographic bands, as described in Materials and Methods, from three independent experiments. The reported kinase values were normalized for immunoprecipitated Chk2 content in each lane.
In S. cerevisiae, the activation of the Chk2 homolog Rad53 by DNA damage involves an initial phosphorylation by Mec1 (the homolog of human ATM) and autophosphorylation thereafter (34). Like Rad53, human Chk2 undergoes autophosphorylation in vitro after DNA damage (5, 27, 46). To establish whether this event occurs in NBS cells, Chk2 immunoprecipitates from unirradiated and 4-Gy-irradiated cells were kinase assayed in the absence of target substrate. Under these conditions, both normal and NBS cells exhibited 30 min after IR a Chk2 autophosphorylation signal whose intensity at 3 h declined in the former cells but persisted in the latter (Fig. 6B). Conversely, in AT cells no Chk2 autophosphorylation signal was seen after IR (Fig. 6B), thus verifying the ATM dependence of this event. These results indicate that Nbs1 deficiency does not impair the initial steps of Chk2 activation leading to its autophosphorylation and suggest that Nbs1 could regulate subsequent phosphorylation events necessary for Chk2 to gain in trans activity.
Chk2 phosphorylation after high-dose IR.
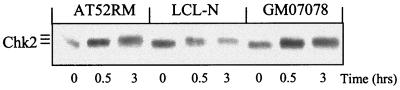
Whereas low doses of IR activate Chk2 in a ATM-dependent manner, high doses of IR activate Chk2 independently of ATM (5, 8, 27). Therefore, we analyzed Chk2 phosphorylation in NBS cells exposed to 50 Gy of IR to determine Nbs1 dependence of this modification. In contrast to the findings observed after 4 Gy of IR, the time-dependent phosphorylation of Chk2 in NBS cells progressed as in normal LCL-N and AT cells (Fig. 7), indicating that Chk2 activation becomes NBS independent (and ATM independent) following large-scale DNA damage.
FIG. 7.
Chk2 mobility shifts in response to high doses of IR. Normal, AT, and NBS cells were harvested before or after exposure to 50 Gy of IR and Western blotted for Chk2. Note the similar IR-induced Chk2 gel retardation in all cell lines, indicating an ATM- and NBS-independent Chk2 phosphorylation event.
The G2/M checkpoint is defective in NBS cells.
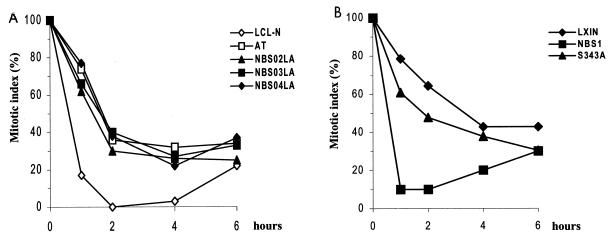
Upon activation by DNA damage, Chk2 phosphorylates Ccd25C, which interferes with Cdc25C's ability to dephosphorylate and activate the mitotic kinase Cdc2 (19, 25, 58). This event is thought to block the progression from G2 to M phase, perhaps in cooperation with other molecules signaling along the same checkpoint pathway (e.g., Chk1) (55). On the basis of these findings, we measured the time-dependent delay in progression to mitosis after low-dose (1.5-Gy) IR to determine the possible correlation in NBS cells between defective Chk2 activation and alterations of the G2/M checkpoint. In normal cells, within 1 h of IR, the mitotic index came down to less than 20%, compared to 100% before irradiation, whereas in NBS cells, as in AT cells, this value was around 70 to 80% (Fig. 8A). Within 2 h of IR, the mitotic index in normal cells dropped to 0% but in NBS and AT cells was significantly higher (30 to 40%), indicating that like AT, NBS cells do not stop their entry into mitosis immediately after irradiation. The failure of AT cells to suppress the mitotic index after IR is concordant with previous results (2). Importantly, the G2/M transition defect in NBS could be complemented by wild-type Nbs1 but only slightly by the S343A Nbs1 mutant (Fig. 8B), underscoring the dependence of this event on Nbs1.
FIG. 8.
Mitotic index delay in irradiated NBS cells. Cells, treated or not with 1.5 Gy of IR, were collected at hourly intervals, fixed, and stained as detailed in Materials and Methods. No mitotic-phase-arresting agents were used. Mitotic nuclei were counted by microsopy from at least 1,000 cells from each sample. For each time point, the fraction of mitotic cells present in the unirradiated sample was normalized to 100%, and the mitotic index indicates the percentage change in mitotic figures present in the irradiated sample counterpart.
Collectively, these findings highlight a defective G2/M transition in NBS cells. Given that ectopically expressed Nbs1 rescues this defect as well as the function of Chk2 (as shown above), these results establish a link between Nbs1 and Chk2 in G2/M checkpoint control.
DISCUSSION
Eukaryotic cells respond to DNA damage by delaying cell cycle progression through the activation of checkpoint pathways, in order to assess and repair the genetic lesions and to prevent replication of cells which have lost their genome integrity (52, 57). In this study, we have examined the ATM-dependent DNA damage response pathway in relation to the role of Nbs1 in the activation of Chk2, a protein kinase that regulates cell cycle checkpoints. ATM and NBS1 are the genes responsible for AT and NBS, respectively, two disorders that share hypersensitivity to genotoxic agents, immunodeficiency, and increased predisposition to cancer. ATM is a protein kinase which upon activation by double-strand breaks (16) phosphorylates several target proteins (e.g., p53, Chk2, MDM2, RPA, BRCA1, and c-Ab1) (20) that are crucial for the regulation of checkpoint arrest and DNA repair (22). Nbs1 is a component of the multifunctional protein complex containing Mre11 and Rad50, involved in recognition and processing of DNA lesions (33, 36). Several lines of evidence suggest a functional link between these proteins, including the common G2/M checkpoint defect exhibited by the associated diseases (2, 15, 44), the DNA damage-induced phosphorylation of Nbs1 by ATM (12, 24, 53, 56), and their physical association in the BRCA1-associated supercomplex (50).
The protein kinase Chk2 is phosphorylated and activated in response to IR (27), following which it can phosphorylate the mitotic phosphatase Cdc25C on an inhibitory site, thereby preventing mitotic entry (37). Chk2 can also phosphorylate the p53 oncosuppressor protein on Ser20 regulatory site, thus causing G1 arrest (8, 14, 40). Furthermore, p53 can in turn negatively regulate the transcription of Chk2 (46). Interestingly, the oncosuppressor protein BRCA1, the core of the BRCA1-associated supercomplex, physically interacts with Chk2 protein and is also a putative target of Chk2 activity (23).
In this study we have demonstrated, on the basis of the electrophoretic mobility changes on gels, a progressive time-dependent phosphorylation of Chk2 after IR that becomes maximal between 1 and 3 h and persists for up to 10 h. As Chk2 contains seven putative major phosphorylation sites, considered responsible for electrophoretic mobility delay after IR (28, 30), these findings suggest that these residues are not simultaneously modified.
The typical Chk2 phosphorylation after 4-Gy IR was ATM and NBS1 dependent, as it was found to be defective in both AT and NBS cells. It should be noted, however, that in NBS cells Chk2 showed a minor mobility shift at 3 h post-IR, suggesting that at this time point a limited and transient phosphorylation may occur in a fraction of the Chk2 pool of molecules. As Chk2 revealed the same nuclear immunostaining pattern in both normal and NBS cells, we can exclude a mislocalization phenomenon as the cause for defective Chk2 phosphorylation in Nbs1-deficient cells. We have also shown that decreased expression of ATM or Nbs1 protein does not impair the phosphorylation of Chk2, according to the analysis of AT- or NBS-heterozygous cells. The normal behavior of AT-heterozygous cells is intriguing since quite often other ATM-dependent events, such as apoptosis or spindle checkpoint control, are compromised in these cells (42).
As Chk2 phosphorylation leads to its enzymatic activation (28), we assayed Chk2 kinase in vitro at different time points after IR. In these experiments, only the hyperphosphorylated form of Chk2 present in normal cells between 1 and 3 h showed enhanced kinase activity toward Cdc25C substrate, whereas the phosphorylated forms of Chk2 present in normal cells at 30 min and in NBS cells at 3 h showed basal activity only. Therefore, the radiation-induced Chk2 activity in NBS cells is, as in AT cells, completely defective.
To provide evidence for a direct relationship between impaired Chk2 activity and Nbs1 deficiency, we complemented NBS cells with wild-type NBS1 and demonstrated that Chk2 phosphorylation and kinase activity are both restored after IR. Noteworthy, Chk2 phosphorylation was restored neither by an Nbs1 mutant protein truncated at a 590, which deletes the Mre11 binding domain (45), nor by a mutant in Ser343 (S343A), the ATM phosphorylation site. Mutants in Ser343 only partially complement S-phase checkpoint in NBS cells (24). Together with the other results, these findings indicate that radiation-induced activation Chk2 requires a correctly localized and phosphorylation-activated Nbs1 complex.
In S. cerevisiae, the activation of the Chk2 homolog Rad53 occurrs by two interdependent steps, the first involving phosphorylation by Mec1 (the homolog of human ATM) and the second involving an autophosphorylation process (34). Evidence for the autophosphorylation of Chk2 in vitro in response to IR has been recently provided (5, 27, 46). Here we have shown that, unlike in AT cells, Chk2 undergoes autophosphorylation in normal and NBS cells, even at the time point when it appears modestly phosphorylated and catalytic inactive against Cdc25C substrate (e.g., at 30 min and 3 h post-IR in normal and NBS cells, respectively). Hence, in light of these findings, it is conceivable that low doses of IR elicit an in trans phosphorylation of Chk2 by ATM followed by autophosphorylation and thereafter by other phosphorylation events sustained by Nbs1. These data, additionally, seem to temporally separate the in trans and in cis activities of Chk2. However, we cannot exclude the participation of another kinase that may potentially cooperate with ATM in Chk2 phosphorylation.
To better characterize the NBS1- and ATM-dependent Chk2 response in relation to the amount DNA damage, we examined this kinase in AT and NBS cells exposed to high doses of γ radiation. We showed that under these conditions Chk2 undergoes phosphorylation in an NBS- and ATM-independent manner. Whether this arises from the activity of ATR, an ATM-related protein kinase whose function partly overlaps with that of ATM, cannot be excluded. In this regard, it is worth noting that the closest human homolog of S. pombe Rad3, the modulator of the Chk2 homolog Cds1, is ATR (4). In any case, the finding that ATM and NBS1 are either involved or not in the activation of Chk2, depending on the extent of DNA damage, agrees with a cooperative role of Nbs1 and ATM in a DNA damage signal transduction pathway.
Data in the literature indicate that changes in p53 protein levels in NBS-derived lymphoblastoid and fibroblast cell lines exposed to low-dose IR are significantly reduced and delayed relative to normal cells (1, 17, 55). Given that Chk2 phosphorylates p53 on Ser20, an event that contributes to disrupt the interaction of p53 with MDM2, thereby allowing the p53 protein to accumulate, these observations suggest a relationship in NBS cells between defective accumulation of p53 and impaired activation of Chk2.
The arrest of mammalian cells at the G2/M checkpoint involves inactivation of Cdc25C by phosphorylation on Ser216, which intereferes with Cdc25C's ability to activate the mitotic kinase Cdc2. As both Chk1 and Chk2 can phosphorylate Cdc25C on Ser216 (18, 27), the contribution of each of these kinases in G2-to-M-phase transition arrest is unclear. Nevertheless, Chk2 appears to have a role, based on the findings that inhibition of the ATM-Chk2 pathway by caffeine abolishes the G2/M DNA damage checkpoint (58) and that Chk2−/− embryonic stem cells fail to maintain IR-induced arrest in G2 phase of the cell cycle (14). These findings led us to consider the possibility that the G2/M checkpoint in NBS cells might be impaired because of the defective Chk2 activation. We have shown that in contrast to normal cells, NBS cells fail to stop entry into mitosis immediately after irradiation, strongly suggesting that Nbs1 deficiency disrupts the G2/M checkpoint. In this respect, NBS appear to behave like AT cells null for ATM (2). Importantly, the G2/M checkpoint defect in NBS cells can be rescued by reintroduction of wild-type Nbs1 but only slightly by S343A, a mutant in the ATM phosphorylation site which fails to restore the S-phase checkpoint in NBS cells (12, 24). These findings, and the fact that ectopic Nbs1 expression compensates for both Chk2 and mitotic arrest defects, establish a link between these two molecules in G2/M checkpoint control.
Collectively, our results support a model whereby the cooperation between ATM and Nbs1 is an essential condition for the rapid and sustained activation of Chk2 checkpoint kinase in response to DNA damage. Although the mechanism for the dependence of Chk2 phosphorylation on Nbs1 remains unclear, our biochemical studies provide as yet no evidence for a physical association between these molecules (data not shown), hence suggesting a more complex regulatory interaction. Finally, our study provides insight for the better understanding of checkpoint defects in NBS cells.
ACKNOWLEDGMENTS
This work was financially supported by Italian Telethon grant E764 and by the Italian Association for Cancer Research (AIRC). G.B. and L.Z. are recipients of fellowships from the Italian Foundation for Cancer Research (FIRC), and C.S. is the recipient of a Telethon fellowship.
P. Maraschio kindly provided the 1548 cell line. Enrico Fontanella provided invaluable technical support.
REFERENCES
- 1.Antoccia A, Stumm M, Saar K, Ricordy R, Maraschio P, Tanzarella C. Impaired p53-mediated DNA damage respones, cell-cycle disturbance and chromosome aberrations in Nijmegen breakage syndrome lymphoblastoidcell lines. Int J Radiat Biol. 1999;75:583–591. doi: 10.1080/095530099140221. [DOI] [PubMed] [Google Scholar]
- 2.Beamish H, Williams R, Chen P, Lavin M F. Defects in multiple cell cycle checkpoints in ataxia telangiectasia postirradiation. J Biol Chem. 1996;271:20486–20493. doi: 10.1074/jbc.271.34.20486. [DOI] [PubMed] [Google Scholar]
- 3.Bell D W, Varley J M, Szydlo T E, Kang D H, Wahrer D C R, Shannon K E, Lubratovich M, Verselis S J, Isselbacher K J, Fraumeni J F, Birch J M, Li F P, Garber J E, Haber D A. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286:2528–2531. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- 4.Bentley N J, Holtzman D A, Flaggs G, Keegan K S, DeMaggio A, Ford J C, Hoekstra M, Carr A M. The Schizosaccharomyces pombe Rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A L, Lee C H, Schwarz J K, Mitiku N, Piwnica-Worms H, Chung J H. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney J P, Maser R S, Olivares H, Davis E M, Le Beau M, Yates III J R, Hays L, Morgan W F, Petrini J H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 7.Cerosaletti K M, Lange E, Stringham H M, Weemaes C M, Smeets D, Solder B, Belohradsky B H, Taylor A M, Karnes P, Elliott A, Komatsu K, Gatti R A, Bochnke M, Concannon P. Fine localization of the Nijmegen breakage syndrome gene to 8q21: evidence for a common founder haplotype. Am J Hum Genet. 1998;63:125–134. doi: 10.1086/301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi P, Eng W K, Zhu Y, Mattern M R, Mishra R, Hurle M R, Zhang X, Annan R S, Lu Q, Faucette L F, Scott G F, Li X, Carr S A, Johnson R K, Winkler J D, Zhou B S. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 9.Chehab N H, Malikzay A, Appel M, Halazonetis T D. Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 10.Delia D, Mizutani S, Panigone S, Tagliabue E, Fontanella E, Asada M, Yamada T, Taya Y, Prudente S, Saviozzi S, Frati L, Pierotti M A, Chessa L. ATM protein and p53-serine 15 phosphorylation in ataxia-telangiectasia (AT) patients and AT heterozygotes. Br J Cancer. 2000;82:1938–1945. doi: 10.1054/bjoc.2000.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durocher D, Henckel J, Fersht A R, Jackson S P. The FHA domain is a modular phosphopeptide recognition motif. Mol Cell. 1999;4:387–394. doi: 10.1016/s1097-2765(00)80340-8. [DOI] [PubMed] [Google Scholar]
- 12.Gatei M, Young D, Cerosaletti K M, Desai-Mehta A, Spring K, Kozlov S, Lavin M F, Gatti R A, Concannon P, Khanna K K. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat Genet. 2000;25:115–119. doi: 10.1038/75508. [DOI] [PubMed] [Google Scholar]
- 13.Hartwell L H, Kastan M B. Cell cycle controls and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 14.Hirao A, Kong Y Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge S J, Mak T W. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 15.Ito A, Tauchi H, Kobayashi J, Morishima K, Nakamura A, Hirokawa Y, Matsuura S, Ito K, Komatsu K. Expression of full-length NBS1 protein restores normal radiation responses in cells from Nijmegen breakage syndrome patients. Biochem Biophys Res Commun. 1999;265:716–721. doi: 10.1006/bbrc.1999.1737. [DOI] [PubMed] [Google Scholar]
- 16.Jeggo P A, Carr A M, Lehmann A R. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 1998;14:312–316. doi: 10.1016/s0168-9525(98)01511-x. [DOI] [PubMed] [Google Scholar]
- 17.Jongmans W, Vuillaume M, Chrzanowska K, Smeets D, Sperling K, Hall J. Nijmegen breakage syndrome cells fail to induce the p53-mediated DNA damage response following exposure to ionizing radiation. Mol Cell Biol. 1997;17:5016–5022. doi: 10.1128/mcb.17.9.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko Y, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Toresawa M, Tachibana A, Ikeda K, Nakanishi M. Cell cycle-dependent and ATM-independent expression of human Chk 1 kinase. Oncogene. 1999;18:3673–3681. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 19.Kastan M B, Lim D S. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 20.Kim S T, Lim D S, Canman C E, Kastan M B. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 21.Lavin M F, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 22.Lavin M F, Khanna K K. ATM: the protein encoded by the gene mutated in the radiosensitive syndrome ataxia telangiectasia. Int J Radiat Biol. 1999;75:1201–1214. doi: 10.1080/095530099139359. [DOI] [PubMed] [Google Scholar]
- 23.Lee J S, Collins K M, Brown A L, Lee C H, Chung J H. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 24.Lim D S, Kim S T, Xu B, Maser R S, Lin J, Petrini J H J, Kastan M B. ATM phosphorylate p95/Nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Girona A, Furnari B, Modesert O, Russel P. Nuclear localization of Cdc25C is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 26.Maser R S, Monsen K J, Nelms B E, Petrini J H J. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol Cell Biol. 1997;17:6087–6096. doi: 10.1128/mcb.17.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge S J. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura K, Balmukhanov T, Tauchi H, Weemaes C, Smeets D, Chrzanowska K, Endou S, Matsuura S, Komatsu K. Radiation induction of p53 in cells from Nijmegen breakage syndrome is defective but not similar to ataxia-telangiectasia. Biochem Biophys Res Commun. 1998;242:602–607. doi: 10.1006/bbrc.1997.7924. [DOI] [PubMed] [Google Scholar]
- 30.McKinnon P J. Ataxia-telangiectasia: an inherited disorder of ionizing-radiation sensitivity in man. Progress in the elucidation of the underlying biochemical defect. Hum Genet. 1987;75:197–208. doi: 10.1007/BF00281059. [DOI] [PubMed] [Google Scholar]
- 31.Melchionna R, Chen X, Blasina A, McGowan C H. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Natl Cell Biol. 2000;2:762–765. doi: 10.1038/35036406. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y. ATM: the booster. Nat Med. 1998;4:1231–1232. doi: 10.1038/3207. [DOI] [PubMed] [Google Scholar]
- 33.Paull T T, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;15:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelliccioli A, Lucca C, Liberi G, Marini F, Lopes M, Plevani P, Romano A, Di Fiore P, Foiani M. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 1999;15:6561–6572. doi: 10.1093/emboj/18.22.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 36.Petrini J H J. The mammalian Mre11-Rad50-Nbs1 protein complex: integration of functions in the cellular DNA-damage response. Am J Hum Genet. 1999;64:1264–1269. doi: 10.1086/302391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pines J. Checkpoint on the nuclear frontier. Nature. 1999;397:104–105. doi: 10.1038/16344. [DOI] [PubMed] [Google Scholar]
- 38.Saar K, Chrzanowska K H, Stumm M, Jung M, Nurnberg G, Wienker T, Seemanova E, Wegner R D, Reis A, Sperling K. The gene for the ataxia-telangectasia variant, Nijmegen breakage syndrome, maps to a 1-cM interval on chromosome 8q21. Am J Hum Genet. 1997;60:605–610. [PMC free article] [PubMed] [Google Scholar]
- 39.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle D A, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali S R, Simmons A, Clines G A, Sartiel A, Gatti R A, Chessa L, Sanal O, Lavin M F, Jaspers N G J, Taylor A M R, Arlett C F, Miki T, Weismann S M, Lovett M, Collins F S, Shiloh Y. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 40.Shieh S Y, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 41.Shieh S Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 42.Shigeta T, Takagi M, Delia D, Chessa L, Iwata S, Kanke Y, Asada M, Eguchi M, Mizutani S. Defective control of apoptosis and mitotic spindle checkpoint in heterozygous carriers of ATM mutations. Cancer Res. 1999;59:2602–2607. [PubMed] [Google Scholar]
- 43.Sullivan K E, Veksler E, Lederman H, Lees-Miller S P. Cell cycle checkpoints and DNA repair in Nijmegen breakage syndrome. Clin Immunol Immunopathol. 1997;82:43–48. doi: 10.1006/clin.1996.4275. [DOI] [PubMed] [Google Scholar]
- 44.Takagi M, Delia D, Chessa L, Iwata S, Shigeta T, Kanke Y, Goi K, Asada M, Eguchi M, Kodama C, Mizutani S. Defective control of apoptosis, radiosensitivity, and spindle checkpoint in ataxia telangiectasia. Cancer Res. 1998;58:4923–4929. [PubMed] [Google Scholar]
- 45.Tauchi H, Kobayashi J, Morishima K, Matsuura S, Nakamura A, Shiraishi T, Ito E, Masnada D, Delia D, Komatsu K. The forkhead-associated domain of NBS1 is essential for nuclear foci formation after irradiation but not essential for hRAD50/hMRE11/NBS1 complex DNA repair activity. J Biol Chem. 2001;276:12–15. doi: 10.1074/jbc.C000578200. [DOI] [PubMed] [Google Scholar]
- 46.Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M. Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J Biol Chem. 1999;274:1463–1467. doi: 10.1074/jbc.274.44.31463. [DOI] [PubMed] [Google Scholar]
- 47.Tupler R, Marseglia G, Stefanini M, Prosperi E, Chessa L, Nardo T, Marchi A, Maraschio P. A Nijmegen breakage syndrome with unusual chromosome rearrangements. J Med Genet. 1997;34:196–202. doi: 10.1136/jmg.34.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 49.Varon R, Vissinga C, Platzer M, Cerosaletti K M, Chrzanowska K H, Saar K, Beckmann G, Seemanova E, Cooper P R, Nowak N J, Stumm M, Weemaes C M R, Gatti R A, Wilson R K, Digweed M, Rosenthal A, Sperling K, Concannon P, Reis A. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Cortez D, Yazdi P, Neff N, Elledge S J, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 51.Weemaes C M, Hustinx T W, Scheres J M, van Munster P J, Bakkeren J A, Taalman R D. A new chromosomal instability disorder: the Nijmegen breakage syndrome. Acta Paediatr. 1981;70:557–564. doi: 10.1111/j.1651-2227.1981.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 52.Weinert T. DNA damage checkpoints update: getting molecular. Curr Opin Genet Dev. 1998;8:185–193. doi: 10.1016/s0959-437x(98)80140-8. [DOI] [PubMed] [Google Scholar]
- 53.Wu X, Ranganathan V, Weisman D S, Heine W F, Ciccone D N, O'Neill T B, Crick K E, Pierce K A, Lane W S, Rathbun G, Livingston D M, Weaver D T. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature. 2000;405:477–482. doi: 10.1038/35013089. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe M B, Rittinger K, Volinia S, Caron P R, Aitken A, Leffers H, Gamblin S J, Smerdon S J, Cantley L C. The structural basis for 14-3-3: phosphopeptide binding specificity. Cell. 1997;26:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 55.Yamazaki V, Wegne R D, Kirchgessner C U. Characterization of cell cycle checkpoint responses after radiation in Nijmegen breakage syndrome cells. Cancer Res. 1998;58:2316–2322. [PubMed] [Google Scholar]
- 56.Zhao S, Weng Y C, Yuan S S, Lin Y T, Hsu H C, Lin S C, Gerbino E, Song M H, Zdzienicka M Z, Gatti R A, Shay J W, Ziv Y, Shiloh Y, Lee E Y. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature. 2000;405:473–477. doi: 10.1038/35013083. [DOI] [PubMed] [Google Scholar]
- 57.Zhou B B, Elledge S J. Putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 58.Zhou B B, Chaturvedi P, Spring K, Scott S P, Johanson R A, Mishra R, Mattern M R, Winkler J D, Khanna K K. Caffeine abolishes the mammalian G2/M DNA checkpoint by inhibiting ataxia-telangiectasia mutated kinase activity. J Biol Chem. 2000;275:10342–10348. doi: 10.1074/jbc.275.14.10342. [DOI] [PubMed] [Google Scholar]