Abstract
Background
Hepatocellular carcinoma is the sixth most common cancer worldwide. Hepatic resection is regarded as the curative therapy for hepatocellular carcinoma. However, only about 20% of people with hepatocellular carcinoma are candidates for resection, which highlights the importance of effective nonsurgical therapies. Until now, transcatheter arterial chemoembolisation (TACE) is the most common palliative therapy for hepatocellular carcinoma, but its clinical benefits remain unsatisfactory. During recent years, some studies have reported that the combination of TACE plus thermal ablation can confer a more favourable prognosis than TACE alone. However, clear and compelling evidence to prove the beneficial or harmful effects of the combination of TACE and thermal ablation therapy is lacking.
Objectives
To assess the beneficial and harmful effects of the combination of thermal ablation with TACE versus TACE alone in people with hepatocellular carcinoma.
Search methods
We performed searches in the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials in the Cochrane Library, MEDLINE, Embase, LILACS, Science Citation Index Expanded, and Conference Proceedings Citation Index‐Science. We endeavoured to identify relevant randomised clinical trials also in the China National Knowledge Infrastructure (CNKI) and Wanfang databases. We searched trial registration websites for ongoing studies. We also handsearched grey literature sources. The date of last search was 22 December 2020.
Selection criteria
We planned to include all randomised clinical trials comparing the combination of TACE plus thermal ablation versus TACE alone for hepatocellular carcinoma, no matter the language, year of publication, publication status, and reported outcomes.
Data collection and analysis
We planned to use standard methodological procedures expected by Cochrane. We planned to calculate risk ratios (RRs) with the corresponding 95% confidence intervals (CIs). For time‐to‐event variables, we planned to use the methods of survival analysis and express the intervention effect as a hazard ratio (HR) with 95% Cl. If the log HR and the variance were not directly reported in reports, we planned to calculate them indirectly, following methods for incorporating summary time‐to‐event data into meta‐analysis. We planned to assess the risk of bias of the included studies using the RoB 2 tool. We planned to assess the certainty of evidence with GRADE and present the evidence in a summary of findings table.
Main results
Out of 2224 records retrieved with the searches, we considered 135 records eligible for full‐text screening. We excluded 21 of these records because the interventions used were outside the scope of our review or the studies were not randomised clinical trials. We listed the remaining 114 records, reporting on 114 studies, under studies awaiting classification because we could not be sure that these were randomised clinical trials from the information in the study paper. We could not obtain information on the registration of the study protocol for any of the 114 studies. We could not obtain information on study approval by regional research ethics committees, either from the study authors or through our own searches of trial registries. Corresponding authors did not respond to our enquiries about the design and conduct of the studies, except for one from whom we did not receive a satisfactory response. We also raised awareness of our concerns to editors of the journals that published the 114 studies, and we did not hear back with useful information. Moreover, there seemed to be inappropriate inclusion of trial participants, based on cancer stage and severity of liver disease, who should have obtained other interventions according to guidelines from learned societies.
Accordingly, we found no confirmed randomised clinical trials evaluating the combination of TACE plus thermal ablation versus TACE alone for people with hepatocellular carcinoma for inclusion in our review.
We identified five ongoing trials, by handsearching in clinical trial websites.
Authors' conclusions
We could not find for inclusion any confirmed randomised clinical trials assessing the beneficial or harmful effects of the combination of TACE plus thermal ablation versus TACE alone in people with hepatocellular carcinoma. Therefore, our results did not show or reject the efficiency of the combination of TACE plus thermal ablation versus TACE alone for people with hepatocellular carcinoma.
We need trials that compare the beneficial and harmful effects of the combination of TACE plus thermal ablation versus TACE alone in people with hepatocellular carcinoma, not eligible for treatments with curative intent (liver transplantation, ablation surgical resection) and who have sufficient liver reserve, as assessed by the Child Pugh score, and who do not have extrahepatic metastases. Therefore, future trial participants must be classified at Barcelona Clinic Liver Cancer Stage B (intermediate stage) (BCLC‐B) or an equivalent, with other staging systems.
Plain language summary
The combination of transcatheter arterial chemoembolisation and thermal ablation versus TACE alone for hepatocellular carcinoma
Background
Hepatocellular carcinoma (a common kind of liver cancer) is the sixth most common cancer in the world. Transcatheter arterial chemoembolisation (TACE) (injecting agents into the feeding vessels of the tumour to reduce the blood supply to the tumour and kill the tumour) is the most common therapy for hepatocellular carcinoma, but the clinical outcome is poor. In recent years, the combination of TACE plus thermal ablation (killing the tumour cell by producing heat or cold) has shown better efficacy than TACE alone. However, evidence to prove the beneficial or harmful effect of the combination of TACE with ablation for people with hepatocellular carcinoma is still lacking.
Aim
We aimed to assess the beneficial and harmful effects of the combination of TACE with thermal ablation versus TACE alone for hepatocellular carcinoma.
Key results
We considered 135 records eligible for full‐text screening. We excluded 21 of these records because the interventions used were outside the scope of our review or the studies were not randomised clinical trials. We listed the remaining 114 records, reporting on 114 studies, under studies awaiting classification because we could not be sure that these were randomised clinical trials from the information in the study paper. We could not obtain information on the registration of the study protocol for any of the 114 studies. We could not obtain information on study approval by regional research ethics committees, either from the study authors or through our own searches of trial registries. Corresponding authors did not respond to our enquiries about the design and conduct of the studies, except for one from whom we did not receive a satisfactory response. We also raised awareness of our concerns to editors of the journals that published the 114 studies, and we did not hear back with useful information. Moreover, there seemed to be inappropriate inclusion of trial participants, based on cancer stage and severity of liver disease, who should have obtained other interventions according to guidelines from learned societies.
We identified five ongoing trials, by handsearching in clinical trial websites.
Conclusions
We found no confirmed randomised clinical trials evaluating the combination of TACE plus thermal ablation versus TACE alone for people with hepatocellular carcinoma for inclusion in our review. Therefore, we cannot conclude anything on the treatment of hepatocellular carcinoma using TACE plus thermal ablation versus TACE alone.
We need trials that compare the beneficial and harmful effects of the combination of TACE plus thermal ablation versus TACE alone in people with hepatocellular carcinoma, not eligible for treatments with curative intent (liver transplantation, ablation surgical resection) and who have sufficient liver reserve, as assessed by the Child Pugh score, and who do not have extrahepatic metastases. Therefore, future trial participants must be classified at Barcelona Clinic Liver Cancer Stage B (intermediate stage) (BCLC‐B) or an equivalent, with other staging systems.
Background
Description of the condition
Hepatocellular carcinoma is the most predominant form of primary liver cancer, accounting for approximately 90% of occurrences, and it represents an increasing serious health problem worldwide (Mohd 2013; Laursen 2014; National Center for Health Statistics (US) 2015). The pathogenesis of hepatocellular carcinoma is a highly complex process which usually occurs in the context of liver cirrhosis, mainly involving chronic inflammation injury and the accumulation of genetic alterations (Schulze 2016). Hepatocellular carcinoma is the sixth most common cancer and the second most common cancer‐related cause of death worldwide. Around 782,000 people are diagnosed and 746,000 die from hepatocellular carcinoma every year worldwide, with China accounting for about 50% of the total number of cancers and deaths (Torre 2015; Forner 2018). The incidence of hepatocellular carcinoma varies among different global regions. Approximately 80% of hepatocellular carcinomas occur in sub‐Saharan Africa and eastern Asia, due to the high prevalence of hepatitis B virus infection and the intake of aflatoxin B1, with an incidence of over 20 per 100,000 individuals (El‐Serag 2012). An intermediate hepatocellular carcinoma burden occurs in Mediterranean countries, with an incidence of 10 to 20 per 100,000 individuals. In America, the incidence is lower than 5 per 100,000 individuals (Mittal 2013). The main causes of hepatocellular carcinoma in Europe and America is hepatitis C virus infection and alcohol abuse (Trad 2017). Hepatocellular carcinoma incidence among men is four to eight times higher than among women (Yang 2014). Most hepatocellular carcinoma patients are older than 45 years (Llovet 2016).
The most prevalent staging system for hepatocellular carcinoma is the Barcelona Clinic Liver Cancer (BCLC) system which divides hepatocellular carcinoma into five stages based on the size and number of tumours, vascular invasion, and liver function (EASL‐EORTC 2012). The main risk factors are liver cirrhosis, infection with hepatitis B virus and C virus, intake of toxic substance (alcohol and aflatoxin B1), and metabolic syndromes (diabetes, obesity, non‐alcoholic fatty liver disease, and hereditary haemochromatosis). Approximately 80% of hepatocellular carcinoma develops in people with liver cirrhosis (Kew 2014). The hepatocellular carcinoma mortality among men with a high baseline body mass index is five times higher than among men with a normal body mass index (Forner 2018). Other risk factors include age, tobacco use, and coinfection of human immunodeficiency virus (HIV). Diagnosis of hepatocellular carcinoma is confirmed by either histopathological biopsy or imaging techniques (ultrasound, contrast‐enhanced computed tomography, or contrast‐enhanced magnetic resonance imaging (MRI)) according to the current practice guideline of the American Association for the Study of Liver Diseases (AASLD) (Bruix 2011).
The treatment for hepatocellular carcinoma can be divided into curative therapies and palliative therapies. Resection, liver transplantation, and locoregional ablation are radical therapies with the curative intention of prolonging survival. However, only 20% of hepatocellular carcinoma patients, mostly diagnosed by regular screening, may gain survival benefit from resection and liver transplantation (Abdel‐Rahman 2013). Curative ablation is recommended for patients with only two or three nodules which are less than 3 cm or a single nodule. The palliative therapies mainly involve transcatheter arterial chemoembolisation (TACE), sorafenib, and systemic treatment, with no, or moderate survival benefits (Oliveri 2011; Chacko 2016).
Description of the intervention
In this review, we planned to focus on the combination of TACE with sequential thermal ablation therapy. During this combined therapy, TACE is performed firstly for all baseline tumours, followed by thermal ablation on all baseline tumours or only tumours that remain active after TACE. Baseline tumours refer to all active tumours before TACE. Active tumours are defined as 'living' tumours, which show characteristic vascular features of hepatocellular carcinoma — arterial hyper‐vascularisation with washout in the portal venous system or the late phase at contrast‐enhanced computed tomography, or contrast‐enhanced MRI.
TACE is the most common treatment for hepatocellular carcinoma, which is recommended as the first‐line treatment for intermediate stage hepatocellular carcinoma, according to the BCLC staging system (EASL‐EORTC 2012). The mechanism of TACE consists of the injection of chemotherapeutic drugs, lipiodol and vascular occlusive agents into the hepatic artery; these can inhibit tumour growth, promote cell death, and maybe prolong survival (Oliveri 2011). The rationale for TACE is based on the concept that most of the blood supply of intra‐hepatic tumours is provided by the hepatic artery, while 75% of the blood flow of the normal liver parenchyma is supplied by the portal vein (Vogl 2003). Therefore, TACE can lead to selective necrosis of the liver tumour while it hardly affects normal liver parenchyma (Jaeger 1996). Alternatively, TACE can also be used to downsize a tumour or as a bridge to liver transplantation (Martin 2015).
Thermal ablation refers to the ablation therapies that induce irreversible cellular injury of tumour cells through heat mechanisms or cold mechanisms. Most kinds of ablation therapies are performed using a percutaneous approach, under real‐time contrast‐enhanced computed tomography, dynamic MRI, or ultrasound guidance. A puncture needle is used to lead the electrode into the target. After setting appropriate output power and duration, the electrode begins to produce heat or cold to surrounding tissue to induce complete necrosis (Ahmed 2011).
There are five main thermal ablation techniques (Goldberg 2003): radiofrequency ablation (Ahmed 2011), microwave ablation (Brace 2007; Lubner 2013; Poggi 2015), laser ablation (Ahmed 2011), ultrasound ablation (Wijlemans 2012), and cryoablation (Rubinsky 1990; Ahmed 2011)
Radiofrequency ablation is the most widely used and the most well‐studied thermal ablation, and it is regarded as the standard therapy for BCLC‐A tumours which are not suitable for surgery (EASL‐EORTC 2012). It has been proved to have a therapeutic efficacy similar to that of surgical resection or liver transplantation for hepatocellular carcinoma with a diameter within 3 cm (Zhu 2016). Radiofrequency ablation can induce complete necrosis of surrounding tissue by generating heat. The radiofrequency ablation technique also serves as a model for exploring the use of thermal ablation in clinical practice.
Microwave ablation can induce tumour cell death by microwave heating, which is generated by dielectric hysteresis (Ahmed 2011). Microwave ablation can reduce tumour tissue in a more efficient way by producing faster heating and higher temperatures compared to radiofrequency ablation (Brace 2007; Yang 2007). Furthermore, microwave ablation, compared to radiofrequency ablation, has better performance on overcoming heat sink effect (Ahmed 2011). However, microwave ablation is still a novel ablation technique; more details should be explored in further clinical practice.
Laser ablation is an ablative therapy that can induce electromagnetic heating to increase tissue temperatures to lethal levels by laser beam and results in complete necrosis of surrounding tissue (Ahmed 2011).
Ultrasound ablation therapy can concentrate intersecting beams of ultrasound on a target tumour through an acoustic lens and thus induce irreversible damage (Zhu 2013a).
Cryoablation destroys cells by the application of alternating freezing and thawing to induce irreversible cellular injury (Awad 2009; Song 2016a).
How the intervention might work
TACE is a palliative therapy, with a tumour response rate of 24% to 53% (Yang 2009). Generally, several sessions of TACE are needed to achieve a high necrosis rate and local tumour control (Satake 2008). Due to high toxicity and adverse effects of chemotherapeutic agents, repeated TACE may result in liver failure (Li 2010). Besides, the incomplete necrosis of the tumour after TACE may cause intra hepatic recurrence of malignancy (Wu 2005).
Thermal ablation is a minimally invasive and curative therapy, with a complete necrosis rate of 76% to 100% for small hepatocellular carcinoma (Morimoto 2010); and 30% to 70% for larger hepatocellular carcinoma (Livraghi 2000). In patients with early‐stage hepatocellular carcinoma (BCLC 0 or A) who are not suitable for resection, ablation therapy achieved five‐year survival rates of 50% to 70% (EASL‐EORTC 2012). The main advantages of thermal ablation include effective tumour ablation, preservation of maximal normal liver parenchyma, and low rates of complications (Yang 2009). The introduction of the mechanism of five types of thermal ablation therapies is shown below.
During radiofrequency ablation, an electrical circuit is created between a radiofrequency probe, the patient, and the grounding pads (Ahmed 2011). The alternating current leads to frictional agitation at the ionic level and heat generation around the probe (Corwin 2001). Dehydration and subsequent carbonisation of surrounding tissues would occur when the temperature is above 100°C (Poggi 2015).
Microwave ablation generates heat through a process known as dielectric hysteresis, in which polar molecules in tissue (primarily water) are forced to continuously realign with the oscillating electric field (Lubner 2013). Thus, the kinetic energy of reformed molecules and the temperature of tissue increase. Microwave power can produce extremely high temperatures (> 150 °C) and induce necrosis of tissue (Brace 2007).
Laser ablation treats the tumour by irradiating it with a laser beam, which is an efficient and precise energy source for tissue heating (Ahmed 2011).
Ultrasound ablation is a non‐invasive therapy. The main mechanism of ultrasound ablation is the thermal energy deposition by a focussed ultrasound beam. The targeted tissue absorbs a significant amount of energy from a highly directional ultrasound beam, resulting in elevation of temperature (Wijlemans 2012).
Cryoablation is an ablative technique which can induce protein denaturation, cellular dehydration and subsequent tissue necrosis by the application of extreme low temperatures to tumour tissue (Rubinsky 1990; Wu 2015).
The rationale of the combination of TACE and sequential ablation is that sequential ablation therapy can remedy the limitation of TACE alone. Firstly, ablation therapy can directly destroy tumour tissue, increase complete necrosis rate and produce a favourable prognosis (Li 2010); secondly, sequential ablation therapy reduces the time needed for interventional treatment, which reduces liver damage and improves quality of life (Li 2016). In addition, the combination of TACE and sequential ablation has synergistic effects on treating liver tumours. The occlusion of hepatic arteries achieved by TACE can reduce blood flow and decrease the heat sink effect, which is helpful for enlarging the ablation zone and achieving complete necrosis (Peng 2013).
Why it is important to do this review
Hepatic surgical resection is regarded as a curative therapy for hepatocellular carcinoma. However, only about 20% of hepatocellular carcinoma patients are candidates for surgical resection, which highlights the importance of effective non‐surgical therapies (Yin 2014). Until now, TACE is the most commonly used palliative therapy for hepatocellular carcinoma, but the effect remains unsatisfactory (Oliveri 2011). In recent years, the combination of TACE plus thermal ablation has shown better survival than TACE alone for people with hepatocellular carcinoma. Some studies have reported that the combination modality can confer a more favourable prognosis than TACE alone for different stages of hepatocellular carcinoma (Yang 2009; Azuma 2016; Hyun 2016; Song 2016a). However, there is still a lack of clear and compelling evidence on the beneficial or harmful effect of the combination of TACE and thermal ablation therapy. Therefore, we embarked on this Cochrane Review hoping to provide the best available level of evidence of the role of the combination of TACE plus thermal ablation versus TACE alone for hepatocellular carcinoma.
Objectives
To assess the beneficial and harmful effects of the combination of TACE plus thermal ablation compared with TACE alone in people with hepatocellular carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all randomised clinical trials comparing the combination of TACE and thermal ablation with TACE alone for hepatocellular carcinoma, irrespective of publication status or blinding.
Types of participants
All trial participants older than 18 years, with hepatocellular carcinoma, diagnosed by either histopathological biopsy or the radiological criteria in clinical practice guidelines.
Types of interventions
Experimental intervention
A combination of TACE plus thermal ablation. Thermal ablation can be performed with any of the following techniques: radiofrequency ablation, microwave ablation, laser ablation, ultrasound ablation, and cryoablation.
Control intervention
TACE alone
For both experimental group and control groups, we planned to include all TACE treatments irrespective of dosage and types of chemotherapeutic drugs, and vascular occlusive agents (Imai 2014).
Types of outcome measures
We planned to measure the outcomes listed below. We planned to base our primary conclusions on the outcome results at the longest follow‐up. We planned to include trials regardless of whether they reported on our outcomes of interest.
Primary outcomes
All‐cause mortality.
Progression‐free survival. This is defined as the period from the date of first treatment to the date of the first documented disease progression by either radiological assessment or liver biopsy or death caused by any reason, whichever happened first.
Proportion of participants with serious adverse events. We planned to use the definition of serious adverse events in the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice (ICH‐GCP 1997): that is, any untoward medical occurrence that results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, or any medical event that might have jeopardised the patient, or required intervention to prevent it. All other adverse events were considered as non‐serious adverse events. We planned to accept all reported serious adverse events assessed at variable time points throughout the conduct of the review. If possible, we noted the period of reported serious adverse events and classified them as short‐term (primary observed period) and long‐term serious adverse events.
Secondary outcomes
-
Tumour response. We planned to evaluate the tumour response according to the Modified Response Evaluation Criteria in Solid Tumours (mRECIST) guideline (Lencioni 2010), as follows.
Complete response (CR): disappearance of any intratumoural arterial enhancement in all target lesions.
Partial response (PR): at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions.
Progressive disease (PD): an increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started.
Stable disease (SD): any cases that do not qualify for either partial response or progressive disease.
Whenever appropriate, we also planned to consider other criteria, such as World Health Organization (WHO) criteria (Kim 2015) and the Response Evaluation Criteria in Solid Tumours (RECIST) guideline (Therasse 2000). However, the mRECIST guideline was considered as the main tool.
Proportion of participants with adverse events not considered serious. We planned to accept all reported non‐serious adverse events assessed at variable time points throughout the conduct of the review. If possible, we planned to note the period of reported non‐serious adverse events and classify them as short‐term (primary observed period) and long‐term adverse events.
Health‐related quality of life as defined by the trial authors (short term: up to one year; medium term: one to five years; long term (primary time point): beyond five years).
Duration of hospital stay.
Search methods for identification of studies
Electronic searches
We performed electronic searches in the Cochrane Hepato‐Biliary Group Controlled Trials Register (searched through the Cochrane Library; December 2020), The Cochrane Central Register of Controlled Trials (CENTRAL; 2020, issue 12) in the Cochrane Library, MEDLINE (PubMed; December 2020), Embase (www.embase.com; December 2020), LILACS (Bireme; 1982 to December 2020), Science Citation Index Expanded (Web of Science; 1900 to December 20209), and Conference Proceedings Citation Index‐Science (Web of Science; 1990 to December 2020). We also endeavoured to identify relevant RCTs in the China National Knowledge Infrastructure (CNKI) and Wanfang databases. Appendix 1 shows the search strategies with the time spans of the searches.
Searching other resources
We checked the reference lists of potentially relevant articles identified in the electronic searches. We also searched trial registration resources such as ClinicalTrials.gov, Chinese Clinical Trial Register (ChiCTR), and the World Health Organisation (WHO) International Clinical Trial Registry Platform (www.who.int/ictrp) to identify study protocols of the identified studies from the electronic searches and also to identify ongoing studies. We also handsearched grey literature sources, such as meeting abstracts and internal reports. We adapted the same or similar search terms to those used in the searching of English electronic databases.
During the selection of trials, whenever we identified observational studies of interest to the topic of this review (i.e. quasi‐randomised studies, cohort studies, case‐control studies, case reports, and case series) and also reporting on harms, we planned to discuss the data on harm in the review discussion part. We also planned to create a table with the extracted data on harm. In this way, we pay attention to late‐occurring or rare events which are often underreported or overlooked by trialists (Storebø 2018).
Data collection and analysis
Selection of studies
We merged all search results and removed duplicates by using reference management software. Two review authors (BZL and WL) independently examined titles and abstracts of the electronic search output to remove obviously irrelevant publications. After the initial assessment, we retrieved the full text of all potentially eligible articles, and we linked together multiple reports of the same trial. Two review authors (BZL and WL) independently screened the full text to evaluate whether these trials met the inclusion criteria. We resolved disagreements on the eligibility of a trial by discussion. We consulted HC (the last author) or we wrote to the original trial investigators when necessary, to clarify trial eligibility. Then, we made a final decision on which trials fulfilled the inclusion criteria of our review. We did not blind our selection process regarding article information. We recorded the details of the whole screening process in a PRISMA flow chart. We also added information on the excluded studies in the 'Characteristics of excluded studies' table.
Data extraction and management
Two authors (BZL and YCZ) planned to independently extract the data from all included publications on the trials and complete the 'Characteristics of included studies' table. We planned to contact the authors of original trials whenever needed. We planned to resolve disagreement by discussion. We planned to consult HC (another review author), or we planned to write to the original trial investigators whenever needed. Two authors (HC and WL) planned to enter data into Review Manager 5. We planned to double‐check that the data had been entered correctly by comparing the data presented in the systematic review with those in the data extraction form, which we had pre‐piloted for the purpose of the review.
We planned to extract the following trial characteristics.
Source (e.g. author, year of publication, contact details, journal citation, trial registration, ethics committee approval)
Methods (e.g. trial design, total trial duration, sequence generation, allocation sequence concealment, blinding and other concerns about bias)
Participants (e.g. age, sex, country, number randomised, number lost to follow‐up/withdrawn, number analysed, inclusion criteria, exclusion criteria, diagnostic criteria)
Interventions (e.g. intervention, comparison)
Outcomes (for each outcome listed in the protocol, e.g. outcome definition and unit of measurement (if relevant), time points reported, scales, intensity)
Miscellaneous (e.g. funding for trial, a notable conflict of interests of trial authors).
Assessment of risk of bias in included studies
Two review authors (BZL and WL) planned to independently assess the risk of bias in the included studies. We planned to assess risk of bias by using the RoB 2 tool, according to Chapter 8 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a). We planned to use the following domains.
Domain 1: bias arising from the randomisation process;
Domain 2: bias due to deviations from intended interventions;
Domain 3: bias due to missing outcome data;
Domain 4: bias in measurement of the outcome;
Domain 5: bias in selection of the reported report.
For each domain, there are a series of signalling questions: 'Yes’, ‘Probably yes’, ‘Probably no’, ‘No’, and ‘No information’.
Based on the replies, we planned to reach a risk‐of‐bias judgement, and we assigned one of three levels to each domain: ‘low risk of bias’, ‘some concerns’, or ‘high risk of bias’, following the RoB 2 tool (Sterne 2019).
Overall risk of bias
The following definitions of risk of bias were considered.
Low risk of bias: the trial is judged to be at low risk of bias for all domains for this result.
Some concerns: the trial is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain.
High risk of bias: the trial is judged to be at high risk of bias in at least one domain for this result; or the trial is judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result.
We planned to present information in support of each response in the free‐text box alongside the signalling questions and judgements. Additionally, for domain 2, we planned to assess the effect of the assignment to the intervention.
In this review, we planned to assess the risk of bias in the following outcome results: all‐cause mortality; time to progression; serious adverse events; tumour response rate; and health‐related quality of life, all at the longest follow‐up.
Measures of treatment effect
For dichotomous variables, we planned to calculate the risk ratio (RR) and 95% confidence interval (CI) and Trial Sequential Analysis adjusted‐CI.
For continuous variables, we planned to use the mean difference (MD) (if all studies were made on the same scale) or the standardised mean difference (SMD) (if different scales were used) with 95% CI and Trial Sequential Analysis adjusted‐CI.
For time‐to‐event variables, we planned to use the methods of survival analysis and express the intervention effect as a hazard ratio (HR) with 95% Cl. If the logHR and their variance were not directly reported in reports, we planned to calculate them indirectly, following the methods introduced by Tierney 2007.
Unit of analysis issues
We planned to set the unit of analysis according to the methods mentioned in Chapter 6 and Chapter 23 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b; Higgins 2019c).
We planned to analyse data at the single randomised individual level (Higgins 2019b). In trials with a two‐parallel‐group design, we planned to compare the experimental intervention group versus the control group. In the trials with a parallel‐group design with more than two intervention groups, if relevant, we planned to compare separately each of the experimental groups with half of the control group if used within the same comparison (Higgins 2019c).
When only a subset of relevant participants was included in a trial, we planned to consider the trial only when the results were presented separately for the subgroup of interest for this review.
For cluster‐randomised trials, we planned to analyse data by using the average cluster size and an estimate of the intraclass correlation coefficient (ICC) and the design effect to calculate effective sample size (Higgins 2019b; Higgins 2019c).
For crossover trials, we planned to only include data from the first intervention period to avoid carry‐over effects (Higgins 2019a).
Dealing with missing data
We planned to contact the original investigators to request missing data; and we planned to extract all data for an intention‐to‐treat (ITT) analysis if data were available. Otherwise, we planned to perform available case analyses, which assume that data are missing at random. We planned to assess if this assumption was reasonable by collecting data on the number of participants excluded or lost to follow‐up, and the reasons for loss to follow‐up by treatment group, from each included study (as reported). We planned to address the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to assess clinical and methodological heterogeneity by carefully examining the characteristics and design of the included trials. We planned to assess the presence of clinical heterogeneity by comparing effect estimates in people with different BCLC stage of hepatocellular carcinoma, different Child‐Pugh Class of liver function, different criteria on assessment of tumour response and different follow‐up time. Different study designs and risk of bias may contribute to methodological heterogeneity.
We planned to explore statistical heterogeneity by the Chi² test with significance set at a P value of less than 0.10. In addition, we planned to access the degree of heterogeneity by using the I² statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error.
Interpretation of I² is listed as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity*
50% to 90%: may represent substantial heterogeneity*
75% to 100%: considerable heterogeneity*
*The importance of the observed value of I² depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity, e.g. P value from the Chi² test, or a confidence interval for I².
Assessment of reporting biases
We planned to assess reporting bias by drawing funnel plots if ten or more trials were included.
Data synthesis
Meta‐analysis
We aimed to conduct this review following the instructions stated in Chapter 10 in Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). We planned to meta‐analyse data whenever possible. Otherwise, we planned to provide a summary of the trial results in a narrative way. We planned to perform the primary analyses by pooling the results of all eligible trials, regardless of their risk of bias. We planned to analyse data using the Review Manager 5 software (Review Manager 2014) and RevMan Web provided by Cochrane (RevMan Web 2019). We aimed to perform all meta‐analyses using the random‐effect model because we expected that the included trials would be heterogeneous. We planned to present dichotomous outcomes as RR with 95% CI. We planned to present continuous outcomes as MD or SMD, with 95% CI.
Subgroup analysis and investigation of heterogeneity
We aimed to assess differences between subgroups using the formal test for subgroup differences in Review Manager Web (RevMan Web 2019). We aimed to conduct the following subgroup analyses.
Trials at low risk of bias, at some concern, and at high risk of bias
Different ablation methods
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies at high risk of bias. Additionally, if cluster‐randomised studies were found, we planned to perform sensitivity analysis to investigate possible effects of the randomisation unit. We planned to assess the intervention effect on mortality at one, three, and five years. We planned to repeat our analyses with the fixed‐effect model.
We also planned to use Trial Sequential Analysis to assess imprecision for the following outcomes: all‐cause mortality; time to progression; serious adverse events; tumour response; and quality of life (Thorlund 2011; Castellini 2018; Gartlehner 2019).
Trial Sequential Analysis
To control random errors from sparse data and repeated significance testing, we planned to apply Trial Sequential Analysis in our meta‐analysis (Thorlund 2011; TSA 2011; Wetterslev 2017). Trial Sequential Analysis is a methodology that includes a combination of techniques, providing the threshold for a statistically significant treatment effect and the threshold for futility. Conclusions conducted by Trial Sequential Analysis indicate the potential to be more reliable than those using traditional meta‐analysis techniques (Thorlund 2011; Wetterslev 2017).
For dichotomous outcomes, we aimed to calculate the required meta‐analysis information size based on the event proportion in the control group; assumption of a plausible RR reduction of 20% or the RR reduction observed in the included trials at low risk of bias; a risk of type I error of 2.5% because of our three primary outcomes and 2.0% because of four secondary outcomes (Jakobsen 2014); a risk of type II error of 10%; and the assumed diversity of the meta‐analysis (Wetterslev 2009). For continuous outcomes, we aimed to calculate the required information size based on the SD observed in the control group of trials with low risk of bias and a minimal relevant difference of 50% of this SD, an alpha of 2.5%, a beta of 10%, and the diversity suggested by the trials in the meta‐analysis.
The underlying assumption of Trial Sequential Analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We aimed to add the trials according to the year of publication. If more than one trial was published during the same year, we planned to add trials alphabetically according to the last name of the first author. We aimed to construct trial sequential monitoring boundaries on the basis of the required information size (Wetterslev 2008; Thorlund 2011; Wetterslev 2017). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that does not reach the required information size; if the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may, perhaps, have been established and further trials may be superfluous. On the other hand, if the boundaries are not surpassed, it probably is necessary to continue conducting trials in order to detect or reject a certain intervention effect. That is determined by assessing if the cumulative Z‐curve crosses the trial sequential boundaries for futility.
Summary of findings and assessment of the certainty of the evidence
We aimed to create the Summary of findings tables using GRADEpro GDT software (GRADEpro GDT). We aimed to assess all‐cause mortality, progression‐free survival, serious adverse events, tumour response rate, and health‐related quality of life. We planned to provide a range of follow‐up, and median follow‐up, for all outcomes.
We aimed to use the GRADE approach to assess the certainty of evidence based on risk of bias, indirectness of evidence (population, intervention, control, outcomes), unexplained heterogeneity, inconsistency of results (including problems with subgroup analyses), imprecision of results, and a high probability of publication bias (Atkins 2004). The details are shown as follows:
(1) Risk of bias or limitations in the detailed design and implementation: the results of assessment of risk of bias by using RoB 2 tool in included RCTs were to be fed directly into the domain of 'Risk of bias' in GRADE. In particular, ‘low’ risk of bias would indicate ‘no limitation’; ‘some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘high’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. We also planned to use our judgements to decide between alternative categories, depending on the likely magnitude of the potential biases. (2) Unexplained heterogeneity or inconsistency of results: when studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. (3) Indirectness of evidence: two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomised trials are available, but they have compared A with placebo and B with placebo. Second, a review may find randomised trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. (4) Imprecision of results: when studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. (5) High probability of publication bias: the certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non‐reporting bias).
We planned to define the levels of evidence as 'high', 'moderate', 'low', or 'very low' certainty. These grades are defined as follows.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
Appendix 1 shows the search strategies. We identified 2803 records through the electronic database search of the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 8), CENTRAL (n = 224), PubMed (n = 173), Embase (n = 825), LILACS (n = 2), Web of Science (n = 735), CNKI (n = 457), and Wanfang databases (n = 379).
After removing duplicates, we screened the titles and abstracts of 2224 records. In total, we considered 135 records eligible for full‐text screening. We excluded 21 of these records (see below). We listed the remaining 114 records, reporting on 114 studies, under studies awaiting classification because we could not be sure that these were randomised clinical trials from the information in the study paper. We could not obtain information on the registration of the study protocol for any of the 114 studies. We could not obtain information on study approval by regional research ethics committees, neither from the study authors nor through our own searches of trial registries. Corresponding authors did not respond to our enquiries about the design and conduct of the studies, except for one from whom we did not receive a satisfactory response. We also raised awareness of our concerns to editors of the journals that published the 114 studies, and we did not hear back with useful information. Moreover, there seemed to be inappropriate inclusion of trial participants based on cancer stage and severity of liver disease, who should have obtained other interventions according to guidelines from learned societies (Omata 2010; EASL‐EORTC 2012; Heimbach 2018).
We identified five ongoing trials, by hand‐searching in clinical trial websites (see Characteristics of ongoing studies).
The details of our selection are shown in the flow diagram (Figure 1).
1.
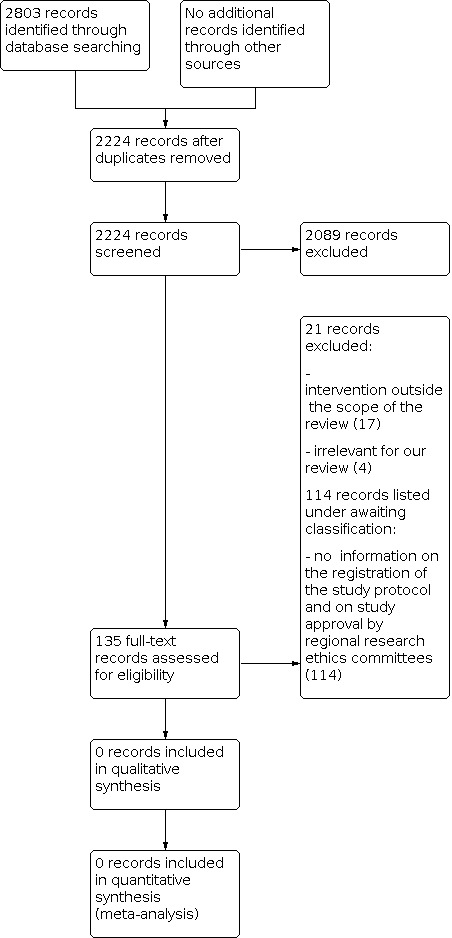
Study flow diagram Date of last search 22 December 2020
Included studies
We were unable to identify any randomised clinical trials evaluating the combination of TACE plus thermal ablation versus TACE alone for people with hepatocellular carcinoma. Please see above.
Excluded studies
The reasons for exclusion of the 21 studies are shown in Characteristics of excluded studies. The main reasons for exclusion of the studies were that studies used interventions outside the scope of our review, or studies were not randomised clinical trials.
Risk of bias in included studies
There were no trials to assess.
Effects of interventions
We could not assess the beneficial or harmful effects of the combination of TACE with ablation versus TACE alone for people with hepatocellular carcinoma, as we could find no trials for inclusion.
Discussion
Summary of main results
Although we identified 114 potentially eligible studies, claiming to assess the beneficial or harmful effects or both combination of TACE plus thermal ablation versus TACE alone for hepatocellular carcinoma, these are all listed as awaiting classification and were not analysed.
There are a number of points that deserve attention and discussion.Firstly, there was absence of evidence of randomisation in the published reports, i.e. that the participants were indeed randomised. This means that the randomisation process was not described, or that the study authors did not provide details on the randomised trial design; there was no description of the methods used for generation of the allocation sequence and allocation concealment. Secondly, study authors, except for one, did not reply to our requests for missing information. We identified no multiple publications of the studies in order to check for required information. Furthermore, we could not obtain information from the study authors on the registration of the study protocol and on study approval by regional research ethics committees. We also raised awareness of our concerns to editors of the journals that published the 114 studies, and we did not hear back with useful information. Thirdly, there was no mention of ethical approval of the studies with appropriate approval number and documentation of the ethical review board. Fourthly, none of the 113 studies conducted after 2005 or the one study from 2003 (Jin 2003) were registered at the protocol stage as per current requirement for randomised clinical trials. There was no study registration number or similar identification and no published protocol, and we were not able to find these studies in any trial registry. Fifthly, there seemed to be inappropriate inclusion of participants based on cancer stage and severity of liver disease, that contravenes guidelines from learned societies (Omata 2010; EASL‐EORTC 2012; Heimbach 2018). This could have resulted in significant harm for participants and raised important ethical issues. TACE should not be offered in patients with Child Pugh B8/B9 or Child Pugh C due to significant risk of deterioration of liver function and death (EASL‐EORTC 2012;Granito 2017). TACE should also not be first‐line treatment in patients with tumours greater than 3 cm, where therapies with curative intent such as resection, ablation, or liver transplantation are recommended. All these recommendations are clearly stated in the Asian Pacific Association for the Study of the Liver (APASL), European Association for the Study of the Liver (EASL), and AASLD guidelines (Omata 2010; EASL‐EORTC 2012; Heimbach 2018). In the studies awaiting classification, 6% to 23% of included patients had Child Pugh C cirrhosis, and there was no information on Child Pugh B8/B9 (apart from the fact that up to 50% of patients had Child Pugh B). Moreover, 25% of patients had TNM stage I (and should, therefore, have received a curative treatment, not TACE which is a palliative treatment (Sirivatanauksorn 2011)). Therefore, there are significant concerns on the selection of patients.
If our decision to list all 114 studies in studies awaiting classification is not considered appropriate in any way, we hereby invite trialists or journal editors to send us information that can prove or disprove that these studies were indeed randomised clinical trials. We wrote to the journals that published the 114 studies, and we did not hear back with useful information.
Overall completeness and applicability of evidence
We designed comprehensive and scientific search strategies. We searched in English, Spanish, and Chinese databases.
Quality of the evidence
We found no eligible randomised clinical trials evaluating the beneficial or harmful effects, or both, of the combination of TACE plus thermal ablation versus TACE alone, thus we cannot analyse the certainty (quality) of evidence.
Potential biases in the review process
The systematic review has been conducted following the corresponding protocol (Liu 2019a). The process of preparing this review was rigorous. We did a comprehensive search for eligible trials.
Agreements and disagreements with other studies or reviews
We found 15 meta‐analyses (Fan 2009; Fan 2011; Sun 2011; Lei 2013; Zhao 2013; Cao 2014; Gu 2014; Hu 2015; Wang 2016b; Katsanos 2017; Yang 2017; Zhao 2017; Liu 2018a; Xiong 2018a; Xiong 2018b) comparing the efficacy and safety of TACE plus radiofrequency ablation versus TACE alone. These 15 meta‐analyses were based on the same studies that we have identified during our trial selection. We have not included these studies in our review for the reasons listed above.
Authors' conclusions
Implications for practice.
No eligible randomised clinical trials assessing the beneficial and harmful effects of the combination of TACE plus thermal ablation versus TACE alone were included into this review. Therefore, our results did not show or reject the efficiency of any treatment strategy for hepatocellular carcinoma.
Implications for research.
Large prospectively registered trials with rigorous methods comparing the beneficial and harmful effects of the combination of TACE plus thermal ablation versus TACE alone in hepatocellular carcinoma are needed. Such randomised clinical trials should be designed according to the SPIRIT statement (Chan 2013); registered in a WHO data register; with obtained full ethical approval; and reported according to the CONSORT statement (Schulz 2010). Such trials should be conducted in people who are not eligible for treatments with curative intent (liver transplantation, ablation surgical resection), who have sufficient liver reserve as assessed by the Child Pugh score, and do not have extrahepatic metastases. Therefore, future trial participants must be classified at Barcelona Clinic Liver Cancer Stage B (intermediate stage) (BCLC‐B) or an equivalent, with other staging systems.
In view of the large number of studies we have identified as potentially problematic, there is an urgent need for validated tools to assist systematic review teams in identifying problematic studies. Our approach to assessing the 114 studies identified through electronic searching reinforces the importance of systematic review teams carefully appraising the studies they identify in order to reduce the impact of potentially problematic studies on evidence used to inform healthcare decision‐making.
History
Protocol first published: Issue 5, 2019
Acknowledgements
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, the Capital Region, Rigshospitalet, Copenhagen, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Authors' Disclaimer: The presented information within this review covers the current status of published studies on the topic, as assessed by our review team. We welcome journals or authors of the studies to help us in further classification of the studies, and on basis of this to decide whether to retract the studies or not.
Peer reviewers: Karl Heinz Weiss, Germany; Vanja Giljaca, UK Contact editors: Stefano Trastulli, Italy; Luit Penninga, Denmark Sign‐off editor: Christian Gluud, Denmark Methodological Editor: Theresa Moore, UK Methods Support Unit Lead and Statistical Editor: Kerry Dwan, UK Cochrane Abdomen and Endocrine Network Editor: Rachel Richardson, UK
Appendices
Appendix 1. Search Strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register (through the Cochrane Library) | 2020, Issue 12 | (((hepat* or liver) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or hepatocellular caricoma or HCC) AND (((thermal or (radiofrequenc* or radio‐frequenc* or radio frequenc*) or microwave or laser* or high intensity focused ultrasound or cryo*) AND (ablati* or therap* or treat* or suger* or coag*)) OR cryoablati* or cryosuger* or RFA or RFTA or RFT or RFCA or MWA or HIFU) AND (((transcatheter or transarterial) and chemoemboli*) or TACE) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2020, Issue 12 | #1 MeSH descriptor: [Carcinoma, Hepatocellular] explode all trees #2 MeSH descriptor: [Liver Neoplasms] explode all trees #3 (((hepat* or liver) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or hepatocellular caricoma or HCC) #4 #1 or #2 or #3 #5 MeSH descriptor: [Catheter Ablation] explode all trees #6 MeSH descriptor: [Ablation Techniques ] explode all trees #7 MeSH descriptor: [Cryosurgery] explode all trees #8 MeSH descriptor: [Laser Therapy ] explode all trees #9 MeSH descriptor: [High‐Intensity Focused Ultrasound Ablation] explode all trees #10 ((thermal or (radiofrequenc* or radio‐frequenc* or radio frequenc*) or microwave or laser* or high intensity focused ultrasound or cryo*) AND (ablati* or therap* or treat* or suger* or coag*)) OR cryoablati* or cryosuger* or RFA or RFTA or RFT or RFCA or MWA or HIFU #11 #5 or #6 or #7or #8 or #9 or #10 #12 MeSH descriptor [Embolization, Therapeutic] explode all trees #13 ((transcatheter or transarterial) and chemoemboli*) orTACE #14 #12 or #13 #15 #4 and #11 and #14 in trials |
| MEDLINE (PubMed) | 1946 to December 2020 | ((((hepatocellular OR hepato‐cellular OR hepatic OR liver) and (carcinom* OR cancer OR neoplasm* OR malign* OR tumor)) OR hepatocellular carcinoma OR HCC) OR Carcinoma, Hepatocellular[MeSH] OR Liver Neoplasms[MeSH]) and ((((thermal OR (radiofrequenc* OR radio‐frequenc* OR radio frequenc*) OR microwave OR laser OR high intensity focused ultrasound) AND (ablati* OR therapy OR therapies OR treat* OR suger* OR coag*)) OR cryoablati* OR RFA OR rfta OR RFT OR rfca OR MWA OR hifu) OR Catheter Ablation[MeSH] OR Ablation Techniques[MeSH] OR Cryosurgery[MeSH] OR Laser Therapy[MeSH] OR High Intensity Focused Ultrasound Ablation[MeSH]) and ((((transcatheter OR transarterial) and chemoemboli*) OR TACE) OR Chemoembolization, Therapeutic[MeSH]) and ((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR clinical trials as topic[mesh:noexp] OR randomly[tiab] OR trial[ti]) NOT (animals[mh] NOT humans[mh])) |
| Embase (www.embase.com) | 1974 to December 2020 | #1 'liver cell carcinoma'/exp #2 ((hepat* or liver) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or ‘hepatocellular caricoma’ or HCC #3 #1 or #2 #4 'radiofrequency ablation'/exp #5 'catheter ablation'/exp #6 'microwave thermotherapy'/exp #7 'cryoablation'/exp #8 'laser surgery'/exp #9 'high intensity focused ultrasound'/exp #10 thermal or (radiofrequenc* or radio‐frequenc* or radio frequenc*) or microwave or laser* or ‘high intensity focused ultrasound’ or cryo* #11 ablation* or therap* or treat* or suger* or coag* #12 #10 and #11 #13 cryoablati* or cryosuger* or RFA or RFTA or RFT or RFCA or MWA or HIFU #14 #4 or #5 or #6 or #7 or #8 or #9 or #12 or #13 #15 'chemoembolization'/exp #16 ((transcatheter or transarterial) and chemoemboli*) or TACE #17 #15 or #16 #18 #3 and #14 and #17 #19 random* or blind* or placebo* or 'meta‐analysis' #20 #18 and #19 |
| LILACS (Bireme) | 1982 to December 2020 | (((hepat$ or liver) and (carcinom$ or cancer$ or neoplasm$ or malign$ or tumo$)) or hepatocellular caricoma or HCC) AND (((thermal or (radiofrequenc$ or radio‐frequenc$ or radio frequenc$) or microwave or laser$ or high intensity focused ultrasound or cryo$) [Words] and (ablati$ or therap$ or treat$ or suger$ or coag$)) OR cryoablati$ or cryosuger$ or RFA or RFTA or RFT or RFCA or MWA or HIFU) [Words] and (((transcatheter or transarterial) and chemoemboli$) or TACE) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to December 2020 | #1 TS=(((hepat* or liver) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or hepatocellular caricoma or HCC) #2 TS=(thermal or (radiofrequenc* or radio‐frequenc* or radio frequenc*) or microwave or laser* or high intensity focused ultrasound or cryo*) #3 TS=(ablati* or therap* or treat* or suger* or coag*) #4 #2 AND #3 #5 TS=(cryoablati* or cryosuger* or RFA or RFTA or RFT or RFCA or MWA or HIFU) #6 #4 OR #5 #7 TS=(((transcatheter or transarterial) and chemoemboli*) or TACE) #8 TS=(random* OR blind* OR placebo* OR meta‐analysis) #9 #1 AND #6 AND #7 AND #8 |
| Conference Proceedings Citation Index–Science (Web of Science) |
1990 to December 2020 | #1 TS=(((hepat* or liver) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or hepatocellular caricoma or HCC) #2 TS=(thermal or (radiofrequenc* or radio‐frequenc* or radio frequenc*) or microwave or laser* or high intensity focused ultrasound or cryo*) #3 TS=(ablati* or therap* or treat* or suger* or coag*) #4 #2 AND #3 #5 TS=(cryoablati* or cryosuger* or RFA or RFTA or RFT or RFCA or MWA or HIFU) #6 #4 OR #5 #7 TS=(((transcatheter or transarterial) and chemoemboli*) or TACE) #8 TS=(random* OR blind* OR placebo* OR meta‐analysis) #9 #1 AND #6 AND #7 AND #8 |
| China National Knowledge Infrastructure | 1994 to December 2020 | TI=('TACE' + '肝动脉化疗栓塞' + '栓塞') AND TI=('热消融' + '消融' + '射频消融' + 'RFA' + '微波消融' + 'MWA' + '氩氦刀消融' + '冷冻消融' + 'HIFU' + '高强度聚焦超声' + '超声消融' + '激光消融') AND TI=('肝癌' + '肝细胞癌') AND (AB=('随机' + '随机对照') OR FT=('随机' + '随机对照')) |
| Wanfang | 1998 to December 2020 | 主题: (TACE + "肝动脉栓塞" + "肝动脉化疗栓塞") * ("热消融" + "射频消融" + RFA + "微波消融" + MWA + "氩氦刀消融" + "冷冻消融" + HIFU + "高强度聚焦超声" + "超声消融" + "激光消融") * ("肝癌" + "肝细胞癌") * ("随机" + "随机对照") |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2014 | Intervention not of interest to our review |
| Chen 2015 | Not an RCT |
| Hou 2017 | Intervention not of interest to our review |
| Hu 2010 | Intervention not of interest to our review |
| Huang 2015b | Intervention not of interest to our review |
| Kong 2015 | Intervention not of interest to our review |
| Li 2013a | Not an RCT |
| Li 2013b | Intervention not of interest to our review |
| Li 2015b | Intervention not of interest to our review |
| Liu 2007 | Intervention not of interest to our review |
| Liu 2015 | Intervention not of interest to our review |
| Liu 2016b | Intervention not of interest to our review |
| Lu 2015 | Intervention not of interest to our review |
| Sha 2012 | Intervention not of interest to our review |
| Wang 2011 | Intervention not of interest to our review |
| Wang 2016a | Intervention not of interest to our review |
| Wu 2006 | Not a RCT |
| Wu 2011a | Not an RCT |
| Wu 2017a | Intervention not of interest to our review |
| Xiang 2007 | Intervention not of interest to our review |
| Yang 2016 | Intervention not of interest to our review |
RCT: randomised clinical trial
Characteristics of studies awaiting classification [ordered by study ID]
Ai 2019.
| Methods | Study design: randomised clinical trial Study duration: July 2010 to June 2012 Setting: hospital |
| Participants | Number of participants: 70 Inclusion criteria: diagnosed as HCC; BCLC B stage Age (mean ± SD, range ): TACE + RFA: 56.97 ± 7.41 years, 21‐73 years; TACE alone: 56.58 ± 8.66 years, 21‐74 years Male (n/total): TACE + RFA: 30/35; TACE alone: 29/35 |
| Interventions | TACE + RFA group (n = 35): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin and oxaliplatin RFA: CT‐guided Control group (n = 35): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin and oxaliplatin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Survival rates: measured at 1 year after treatment Adverse events Liver function: blood testing |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
An 2017.
| Methods | Study design: randomised clinical trial Study duration: January 2013 to January 2015 Duration of follow‐up: 2 years Setting: hospital |
| Participants | Number of participants: 98 Inclusion criteria: diagnosed as HCC; single or huge tumour; no PVTT; no history of other tumours Age (mean ± SD, range): TACE + MWA: 51.3 ± 2.9 years, 24‐78 years; TACE alone: 50.3 ± 2.6 years, 23‐78 years Male (n/total): TACE + MWA: 40/49; TACE alone: 39/49 |
| Interventions | TACE + MWA group (n = 49): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: adriamycin 40 mg and fluorouracil 1 g MWA: the interval between TACE and MWA was 2 weeks. Output power of 50‐60W. Time of 10‐20 minutes per ablation application Control group (n = 49): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: adriamycin 40 mg and fluorouracil 1 g |
| Outcomes | Tumour response: measured by contrast‐enhanced CT; at 4 months after treatment 1‐year and 2‐year survival rates: measured at 1 year and 2 years after treatment Adverse events |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Bao 2020.
| Methods | Study design: randomised clinical trial Study duration: February 2018 to February 2020 Duration of follow‐up: 1 month Setting: hospital |
| Participants | Number of participants: 54 Inclusion criteria: diagnosed as HCC; intermediate‐ or advanced‐stage Age (mean ± SD, range ): TACE + RFA: 59.87 ± 5.20 years, 21‐73 years; TACE alone: 59.15 ± 6.75 years, 40‐73 years Male (n/total): TACE + RFA: 14/27; TACE alone: 15/27 |
| Interventions | TACE + RFA group (n = 27): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin 20‐30g, cisplatin 80 mg RFA: the interval between TACE and RFA was 2 weeks. Output power of 150 W Control group (n = 27): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin 20‐30g, cisplatin 80 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT according to WHO criteria Tumour diameter: measured by CT images Adverse events Liver function: measured by blood testing |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Bian 2020.
| Methods | Study design: randomised clinical trial Study duration: June 2014 to June 2017 Duration of follow‐up: 2 years Setting: hospital |
| Participants | Number of participants: 101 Inclusion criteria:diagnosed as HCC; age < 80; BCLC B or C stage; Child‐Pugh Class A or B Age (mean ± SD, range ): TACE + MWA: 56.12 ± 6.59 years; TACE alone: 57.03 ± 6.73 years Male (n/total): TACE + MWA: 38/52; TACE alone: 35/49 |
| Interventions | TACE + MWA group (n = 52) TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil 30–40 mL, cisplatin 40–60 mg MWA: The interval between TACE and MWA was 3–7 days. Output power of 60 W Control group (n = 49): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil 30–40 mL, cisplatin 40–60 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Survival rates: measured at 2 years after treatment Adverse events SSC‐Ag: blood testing |
| Notes | Country of study: China Source of funding: China National Science Funding There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Chen 2020.
| Methods | Study design: randomised clinical trial Study duration: April 2017 to April 2019 Duration of follow‐up: 6 months Setting: hospital |
| Participants | Number of participants: 80 Inclusion criteria: diagnosed as HCC; tumour diameter ≤ 3 cm Age (mean ± SD, range ): TACE + RFA: 67.25 ± 7.28 years; TACE alone: 67.98 ± 7.94 years Male (n/total): TACE + RFA: 22/40; TACE alone: 21/40 |
| Interventions | TACE + RFA group (n = 40): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin, mitomycin, and lobaplatin RFA: CT‐guided Control group (n = 40): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin, mitomycin, and lobaplatin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 6 months after treatment Adverse events Liver function: measured by blood testing |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Cui 2015.
| Methods | Study design: randomised clinical trial Study duration: September 2012 to October 2014 Duration of follow‐up: 3 years Setting: hospital |
| Participants | Inclusion criteria: diagnosed as HCC; tumour diameter of 2‐10 cm and tumour number ≤ 3; liver function of Child‐Pugh Class A or B; willing to sign a written informed consent document Age (mean ± SD, range): TACE + RFA: 45.38 ± 4.72 years, 21‐70 years; TACE alone: 45. 96 ± 5.12 years, 22‐70 years Male (n/total): TACE + RFA: 65/110; TACE alone: 66/110 With single tumour/multiple tumours (patients): TACE + RFA: 79/31; TACE alone: 75/35 Child‐Pugh Class (patients): Class A: TACE + RFA: 77; TACE alone: 66 Class B: TACE + RFA: 33; TACE alone: 44 |
| Interventions | TACE + RFA group (n = 110): TACE: Chemotherapeutic drugs: epirubicin 40 mg; oxaliplatin 100 mg. For patients with poor liver function, the dose of chemotherapeutic drugs was reduced. RFA: The interval between TACE and RFA was 2 weeks. COSMAN MEDICAL, INC, RFG‐4 system. Ablation margin of 1 cm. Ultrasound‐guided RFA TACE group (n = 110): Chemotherapeutic drugs: epirubicin 40 mg; oxaliplatin 100 mg. For patients with poor liver function, the dose of chemotherapeutic drugs was reduced. |
| Outcomes | Clinical efficacy: measured by contrast‐enhanced CT 1‐, 2‐, and 3‐year survival rates Adverse events |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Cui 2017.
| Methods | Study design: randomised clinical trial Study duration: November 2014 to November 2016 Duration of follow‐up: 2 months Setting: hospital |
| Participants | Inclusion criteria: diagnosed as HCC by pathology Exclusion criteria: intrahepatic disseminated tumours; with electrolyte imbalance; with arrhythmia; with contraindications for intervention therapy Age (mean ± SD, range): TACE + RFA: 63.1 ± 6.9 years, 51‐76 years; TACE alone: 62.3 ± 6.5 years, 50‐76 years Male (n/total): TACE + RFA: 27/43; TACE alone: 26/43 |
| Interventions | TACE + RFA group (n = 43): TACE: chemotherapeutic drugs: cisplatin 80 mg, epirubicin 30 mg, and theprubicin 30 mg RFA: The interval between TACE and RFA was two weeks. RADIONICS system. Output power of 40 W. Fifteen minutes per RFA application TACE group (n = 43): Chemotherapeutic drugs: cisplatin 80 mg, epirubicin 30 mg, and theprubicin 30 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced images at 2 months after treatment Serum level of CD3+ cell, CD4/CD8, NK cell, and TNF‐α。 Serum level of AFP, CA199, and GGT |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Dan 2014.
| Methods | Study design: randomised clinical trial Study duration: January 2010 to January 2012 Duration of follow‐up: 2 years Setting: hospital |
| Participants | Inclusion criteria: diagnosed as HCC Age (mean ± SD, range): 58.5 ± 2.4 years, 25‐74 years Male (n/total): 85/120 |
| Interventions | TACE + MWA group (n = 60): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g, cisplatin 40‐60 mg, and epirubicin 20‐40 mg MWA: the interval between TACE and MWA was 2 weeks. TACE group (n = 60): Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g, cisplatin 40‐60 mg, and epirubicin 20‐40 mg |
| Outcomes | Tumour response: measured by image examinations 2‐year survival rate Adverse events |
| Notes | Country of study: China
Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Ding 2017.
| Methods | Study design: randomised clinical trial Study duration: January 2015 to April 2017 Duration of follow‐up: not reported Setting: hospital |
| Participants | Inclusion criteria: diagnosed as middle or advanced HCC Exclusion criteria: with intrahepatic disseminated tumours; liver function of Child‐Pugh Class C; with tumour thrombus in portal vein trunk. Age (mean ± SD, range): TACE + RFA: 57. 3 ± 2. 9 years, 52‐78 years; TACE alone: 57. 6 ± 2. 1 years, 51‐76 years Male (n/total): TACE + RFA: 20/44; TACE alone: 21/44 Tumour diameter (mean ± SD, range): TACE + RFA: 6.2 ± 2.1 cm, 3‐13 cm; TACE alone: 6.2 ± 2.2 cm, 3‐13 cm With single/multiple/giant tumours (patients): TACE + RFA: 22/11/11; TACE alone: 23/10/11 |
| Interventions | TACE + RFA group (n = 44): TACE: Chemotherapeutic drugs: hydroxy camptothecin 10 mg, cisplatin 30 mg, and 5‐fluorouracil 1 g RFA: Ablation of 1‐2 cm. Twelve minutes per ablation session TACE group (n = 44): Chemotherapeutic drugs: hydroxy camptothecin 10 mg, cisplatin 30 mg, and 5‐fluorouracil 1 g |
| Outcomes | Serum level of AFP Clinical response: measured by image examinations Adverse events |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Dong 2013.
| Methods | Study design: randomised clinical trial Study duration: May 2007 to March 2011 Duration of follow‐up: 6 months Setting: hospital |
| Participants | Inclusion criteria: diagnosed as large HCC (tumour maximal diameter > 5 cm) by liver biopsy; single tumour Age (mean ± SD, range): TACE + RFA: 57.3 ± 3.2 years; TACE alone: 58.4 ± 2.9 years Male (n/total): TACE + RFA: 14/22; TACE alone: 16/22 Tumour diameter (mean ± SD): TACE + RFA: 8.7 ± 2.1 cm; TACE alone: 9.1 ± 2.5 cm Serum level of AFP: Abnormal: TACE + RFA: 21 patients; TACE alone: 19 patients Normal: TACE + RFA: 1 patients; TACE alone: 3 patients |
| Interventions | TACE + RFA group (n = 22): TACE: the combination of hepatic arterial infusion chemotherapy and hepatic artery embolisation RFA: ultrasound‐guided RFA TACE group (n = 22): The combination of hepatic arterial infusion chemotherapy and hepatic artery embolisation |
| Outcomes | Serum level of AFP Clinical efficacy: measured by image examinations at 6 months after treatment Adverse events |
| Notes | Country of study: China Source of funding: none There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Dong 2018.
| Methods | Study design: Randomised clinical trial Study duration: July 2014 to June 2015 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology; the total tumour volume < 70% of volume of whole liver; without complete obstruction of portal vein; life expectancy > 3 months Exclusion criteria: With serious systematic disease; with obvious fistula Age (mean ± SD, range): TACE + RFA: 42.52 ± 8.50 years, 29‐68 years; TACE alone: 43.03 ± 7.66 years, 27‐66 years Male (n/total): TACE + RFA: 22/31; TACE alone: 21/31 |
| Interventions | TACE + RFA group (n = 31): TACE: Chemotherapeutic drugs: mitomycin 2‐6 mg, hydroxy camptothecin 16‐20 mg, and epirubicin 30‐60 mg RFA: Ultrasound‐guided RFA. The number of patients treated with one session and 2‐3 sessions of TACE were 7 and 24, respectively. The number of patients treated with one session and 2‐4 sessions of RFA were 9 and 22, respectively. TACE group (n = 31): Chemotherapeutic drugs: mitomycin 2‐6 mg, hydroxy camptothecin 16‐20 mg, and epirubicin 30‐60 mg. The number of patients treated with one sessions and 2‐3 sessions of TACE were 5 and 26, respectively. |
| Outcomes | Clinical efficacy: measured by image examinations and serum level of AFP at 1 month after treatment 1‐ and 2‐year mortality Adverse events |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Du 2017.
| Methods | Study design: Randomised clinical trial Study duration: December 2012 to December 2015 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by liver biopsy; the maximal tumour diameter ≥ 5 cm; single tumour Exclusion criteria: Portal vein was completely obstructed; serious portal hypertension Age (mean ± SD, range): TACE + RFA: 57.14 ± 5.27 years, 28‐73 years; TACE alone: 57.48 ± 3.71 years, 26‐71 years Male (n/total): TACE + RFA: 28/40; TACE alone: 27/40 Tumour diameter (mean ± SD): TACE + RFA: 8.27 ± 2.35 cm; TACE alone: 8.80 ± 2.57 cm Serum level of AFP: Abnormal: TACE + RFA: 38 patients; TACE alone: 36 patients Normal: TACE + RFA: 2 patients; TACE alone: 4 patients |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: epirubicin 50‐60 mg RFA: Output power of 70‐90 W. Multiple sessions of RFA were performed with an interval of 1‐2 weeks. For patients with the tumour maximal diameter less than 10 cm, the total time per RFA treatment was 30‐60 minutes. For patients with the tumour maximal diameter ≥ 10 cm, the total time per RFA treatment was 80‐100 minutes. TACE group (n = 40): Chemotherapeutic drugs: epirubicin 50‐60 mg |
| Outcomes | Tumour response: measured by image examinations at 6 months after treatment Clinical efficacy (serum level of ALT, TBIL, AFP, and tumour diameter) Adverse events |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Fan 2016.
| Methods | Study design: Randomised clinical trial Study duration: 2013 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD, range ): TACE + RFA: 60. 4 ± 11. 2 years, 46‐72 years; TACE alone: 60. 4 ± 11. 2 years, 45‐73 years Male (n/total): TACE + RFA: 20/33; TACE alone: 20/32 Child‐Pugh Class (patients): Class A: TACE + RFA: 22; TACE alone: 23 Class B: TACE + RFA: 10; TACE alone: 10 |
| Interventions | TACE + RFA group (n = 33): TACE: Chemotherapeutic drugs: oxaliplatin 200 mg and fluorouracil 15 g RFA: The interval between TACE and RFA was 2 weeks. Output power of 60 W. TACE group (n = 32): Chemotherapeutic drugs: oxaliplatin 200 mg and fluorouracil 15 g |
| Outcomes | Tumour response: measured by image examinations Serum level of AFP Survival rates (free tumour) |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Fang 2016.
| Methods | Study design: Randomised clinical trial Study duration: February 2014 to February 2015 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by liver biopsy; tumour maximal diameter ≥ 5 cm Age (mean ± SD, range): TACE + RFA: 63.4 ± 5.6 years, 37‐79 years; TACE alone: 62.8 ± 5.2 years, 35‐81 years Male (n/total): TACE + RFA: 16/25; TACE alone: 14/25 Child‐Pugh Class (patients): Class A: 44; Class B: 6 |
| Interventions | TACE + RFA group (n = 25): TACE: Chemotherapeutic drugs: epirubicin, oxaliplatin, and hydroxy camptothecin RFA: Ultrasound‐guided RFA TACE group (n = 25): Chemotherapeutic drugs: epirubicin, oxaliplatin, and hydroxy camptothecin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT or MRI at 3 days after treatment |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Fang 2020.
| Methods | Study design: Randomised clinical trial Study duration: August 2017 to November 2019 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Number of participants: 50 Inclusion criteria: Diagnosed as HCC; Child‐Pugh Class A or B; large or huge tumour Age (mean ± SD, range ): TACE + MWA: 55.10 ± 5.32 years; TACE alone: 54.26 ± 5.09 years Male (n/total): TACE + MWA: 15/25; TACE alone: 16/25 |
| Interventions | TACE + MWA group (n = 25): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: epirubicin 20–50 mg and oxaliplatin 50–150 mg MWA: The interval between TACE and MWA was 14 days. Control group (n = 25): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: epirubicin 20–50 mg and oxaliplatin 50–150 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 6 months after treatment Survival rates: measured at 1 and 2 years after treatment Adverse events |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Geng 2020.
| Methods | Study design: Randomised clinical trial Study duration: August 2016 to March 2020 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Number of participants: 32 Inclusion criteria: Diagnosed as HCC; age < 75; tumour number ≤ 3; tumour diameter of 5–10 cm; TNM Ⅲ‐Ⅳ Age (mean ± SD, range ): TACE + MWA: 57.51 ± 4.66 years; TACE alone: 59.51 ± 4.83 years Male (n/total): TACE + MWA: 10/16; TACE alone: 11/16 |
| Interventions | TACE + MWA group (n = 16): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: oxaliplatin 50–100 mg and theprubicin 20 mg MWA: ultrasound‐guided; MTI‐5DT Control group (n = 16): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: oxaliplatin 50–100 mg and theprubicin 20 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment The level of AFP and GGT: measured by blood testing |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Gong 2019.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to June 2016 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Number of participants: 98 Inclusion criteria: Diagnosed as HCC; TNM Ⅲ‐Ⅳ stage; tumour diameter > 5 cm Age (mean ± SD, range ): TACE + MWA: 58.1 ± 7.9 years; TACE alone: 57.5 ± 7.1 years Male (n/total): TACE + MWA: 29/49; TACE alone: 28/49 |
| Interventions | TACE + RFA group (n = 49): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin, fluorouracil, and oxaliplatin MWA: The interval between TACE and MWA was 14 days. Output power of 50‐70 W Control group (n = 49): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin, fluorouracil, and oxaliplatin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Immunological function: measured by blood testing The level of AFP: measured by blood testing |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Guo 2015c.
| Methods | Study design: Randomised clinical trial Study duration: February 2011 to January 2013 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Age (mean ± SD): 51.8 ± 9.6 years Male (n/total): 47/72 Tumour diameter: 6.3 ± 2.5 cm Child‐Pugh Class: Class A: 9; Class B: 46; Class C: 17 |
| Interventions | TACE + ultrasound ablation group (n = 36): TACE: Chemotherapeutic drugs: 10‐40 mg epirubicin Ultrasound ablation frequency 0.96 MHz, focal length 134 mm TACE group (n = 36): Chemotherapeutic drugs: 10‐40 mg epirubicin |
| Outcomes | Tumour response: measured by image examinations at 1 month after treatment Clinical efficacy Serum level of AFP 1‐year survival rate Adverse events |
| Notes | Country of study: China Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
He 2016.
| Methods | Study design: Randomised clinical trial Study duration: March 2015 to December 2015 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology or imaging examination; AFP > 400 μg/L; liver function of Child‐Pugh Class A or B; the number of tumours ≤ 2 Exclusion criteria: With portal vein tumour thrombus and extrahepatic metastasis Age (mean): TACE + MWA: 52.6 years; TACE alone: 52.9 years Male (n/total): TACE + MWA: 25/32; TACE alone: 22/28 Tumour diameter (mean): .TACE + MWA: 8.2 cm; TACE alone: 7.9 cm Child‐Pugh Class (patients): Class A: TACE + MWA: 13; TACE alone: 11 Class B: TACE + MWA: 19; TACE alone: 17 |
| Interventions | TACE + MWA group (n = 32): TACE: Chemotherapeutic drugs: epirubicin 30‐50 mg and oxaliplatin 200‐150 mg MWA: CT‐guided MWA. Output power of 55‐60 W. The ablation time of per application was 5‐10 minutes. TACE group (n = 28): Chemotherapeutic drugs: epirubicin 30‐50 mg and oxaliplatin 200‐150 mg |
| Outcomes | Serum level of AFP Tumour response: measured by contrast‐enhanced CT or MRI at 1 month after treatment Adverse events 1‐ and 2‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
He 2020.
| Methods | Study design: Randomised clinical trial Study duration: January 2017 to December 2018 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Number of participants: 72 Inclusion criteria: Diagnosed as HCC; age > 30 Age (mean ± SD, range ): TACE + RFA: 52.41 ± 5.85 years; TACE alone: 52.39 ± 5.97 years Male (n/total): TACE + RFA: 22/36; TACE alone: 20/36 |
| Interventions | TACE + RFA group (n = 36): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil 500 mg and oxaliplatin 50 mg RFA: US‐guided Control group (n = 36): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil 500 mg and oxaliplatin 50 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 2 weeks after treatment Survival rates: measured at 2 years after treatment Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Hou 2013.
| Methods | Study design: Randomised clinical trial Study duration: January 2008 to December 2012 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by images or liver biopsy; with liver function of Child‐Pugh Class A or B; adequate renal function; expected survival > 3 months Age (mean, range): 52.3 years, 32‐72 years Male (n/total): 34/42 Child‐Pugh Class A‐B: 42 patients |
| Interventions | TACE + cryoablation group (n = 22): TACE: Chemotherapeutic drugs: theprubicin 20‐40 mg, 5‐fluorouracil 1‐1.5 g, and cisplatin 60‐80 mg. TACE treatment was performed 2‐4 times with an interval of 4 weeks. Cryoablation: The temperature was 40℃ or ‐150℃. Cryoablation was performed 1‐2 times. TACE group (n = 20): Chemotherapeutic drugs: theprubicin 20‐40 mg, 5‐fluorouracil 1‐1.5 g, and cisplatin 60‐80 mg TACE was performed at 2‐4 weeks. |
| Outcomes | Tumour response: measured by image examinations at 4 weeks after treatment Serum level of AFP Immune function |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Hua 2020.
| Methods | Study design: Randomised clinical trial Study duration: December 2017 to January 2019 Setting: Hospital |
| Participants | Number of participants: 86 Age (mean ± SD, range ): TACE + RFA: 55.4 ± 4.57 years; TACE alone: 55.45 ± 4.25 years Male (n/total): TACE + RFA: 24/43; TACE alone: 26/43 |
| Interventions | TACE + RFA group (n = 43): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: cisplatin 40–80 mg and epirubicin 30–50 mg RFA: The interval between TACE and RFA was 2‐4 weeks. Control group (n = 43): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: cisplatin 40–80 mg and epirubicin 30–50 mg |
| Outcomes | Liver function, AFP: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Huang 2013.
| Methods | Study design: Randomised clinical trial Study duration: January 2009 to November 2011 Duration of follow‐up: 38 months Setting: Hospital |
| Participants | Inclusion criteria: The number of tumours was 1‐3; diagnosed as HCC by liver biopsy. Age (mean ± SD): TACE + RFA: 37.1 ± 5.9 years; TACE alone: 35.7 ± 6.2 years Male (n/total): TACE + RFA: 24/33; TACE alone: 26/33 Tumour diameter (mean ± SD): TACE + RFA: 5.41 ± 0.25 cm; TACE alone: 5.33 ± 0.31 cm |
| Interventions | TACE + RFA group (n = 33): TACE: Chemotherapeutic drugs: epirubicin 40‐60 mg RFA: Hispeed x/i CT‐guided or Acuson X300‐guided RFA. Output power of 25‐90 W. 10‐15 minutes per RFA application TACE group (n = 33): Chemotherapeutic drugs: epirubicin 40‐60 mg |
| Outcomes | Tumour response: measured by image examinations 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Huang 2015a.
| Methods | Study design: Randomised clinical trial Study duration: August 2008 to January 2011 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology; with history of liver resection before tumour recurrence; rejection of the secondary liver resection Age (range): TACE + RFA: 65‐78 years; TACE alone: 66‐77 years Male (n/total): TACE + RFA: 21/38; TACE alone: 19/30 Child‐Pugh Class (patients): Class A: TACE + RFA: 26; TACE alone: 20 Class B: TACE + RFA: 12; TACE alone: 10 |
| Interventions | TACE + RFA group (n = 38): TACE: Chemotherapeutic drugs: theprubicin 20 mg and carboplatin 1000 mg RFA: The interval between TACE and RFA was 2 weeks. The ablation time of per RFA application was 5‐12 minutes. TACE group (n = 30): Chemotherapeutic drugs: theprubicin 20 mg and carboplatin 1000 mg |
| Outcomes | Serum level of AFP Tumour response: measured by contrast‐enhanced CT at 1 month after treatment 1‐, 2‐, and 3‐year survival rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Huang 2016.
| Methods | Study design: Randomised clinical trial Study duration: February 2013 to September 2014 Duration of follow‐up: 15 months Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as middle or advanced HCC Age (mean ± SD): TACE + RFA: 42.6 ± 4.8 years; TACE alone: 41.3 ± 4.4 years Male (n/total): TACE + RFA: 52/90; TACE alone: 49/90 |
| Interventions | TACE + RFA group (n = 90): TACE: Chemotherapeutic drugs: theprubicin 450 mg RFA: The interval between TACE and RFA was 3‐5 weeks. CT‐guided RFA TACE group (n = 90): Chemotherapeutic drugs: theprubicin 450 mg |
| Outcomes | Serum level of AFP and CA‐199 Survival rate at 5, 10, and 15 months after treatment Recurrence rate at 15 months after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Huang 2017a.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to December 2016 Duration of follow‐up: 1 week Setting: Hospital |
| Participants | Age (mean ± SD): TACE + ultrasound ablation: 50.2 ± 6.3 years; TACE alone: 50.5 ± 6.1 years Male (n/total): TACE + ultrasound ablation: 22/42; TACE alone: 24/42 Single/multiple tumours (patients): TACE + ultrasound ablation: 27/15; TACE: 25/17 |
| Interventions | TACE + ultrasound ablation group (n = 42): TACE: Chemotherapeutic drugs: mitomycin 10 mg, 5‐fluorouracil 500 mg, and adriamycin 40 mg. Ultrasound ablation: Frequency 0.8 MHz, focal length 135 mm, and treatment duration 3000‐14000 s TACE group (n = 42): Chemotherapeutic drugs: mitomycin 10 mg, 5‐fluorouracil 500 mg, and adriamycin 40 mg |
| Outcomes | Tumour response: measured by contrasted‐enhanced CT and liver function at 1 week after treatment Liver function at 1 week after treatment Quality of life at 1 week after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Huang 2017b.
| Methods | Study design: Randomised clinical trial Study duration: April 2013 to April 2015 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 51.2 ± 3.6 years; TACE alone: 51.1 ± years Male (n/total): TACE + RFA: 23/40; TACE alone: 24/40 |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: fluorouracil, mitomycin, and cisplatin RFA: The interval between TACE and RFA was 2 weeks. TACE group (n = 40): Chemotherapeutic drugs: fluorouracil, mitomycin, and cisplatin |
| Outcomes | Tumour response: measured by image examinations at 12 months after treatment Adverse events Survival rate at 6 and 12 months after treatment Recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Huang 2019.
| Methods | Study design: Randomised clinical trial Study duration: January 2016 to June 2018 Follow‐up: 1 month Setting: Hospital |
| Participants | Number of participants: 72 |
| Interventions | TACE + RFA group (n = 36) Control group (n = 36) |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment TGF‐β1, CD4+, CD8+, CD4+/CD8+: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Jiang 2015a.
| Methods | Study design: Randomised clinical trial Study duration: October 2011 to May 2012 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: diagnosed as HCC by pathology and images; the number of tumours ≤ 4 cm; tumour maximal diameter of 5‐9 cm Exclusion criteria: With contraindications for intervention therapies Age (mean ± SD, range): TACE + RFA: 5 0 ± 9 years, 36‐72 years; TACE alone: 52 ± 10 years, 32‐78 years Male (n/total): TACE + RFA: 12/21; TACE alone: 14/21 Single/multiple tumour (patients): TACE + RFA: 13/8; TACE: 11/10 |
| Interventions | TACE + RFA group (n = 21): TACE: Chemotherapeutic drugs: epirubicin 55 mg, cisplatin 50 mg, and mitomycin 8 mg RFA: The interval between TACE and RFA was 2 weeks. RANDIONICS system. Output power of 200 W and 12 minutes per RFA session TACE group (n = 21): Chemotherapeutic drugs: epirubicin 55 mg, cisplatin 50 mg, and mitomycin 8 mg |
| Outcomes | Tumour response: measured by CT, PET, or DSA 6‐month, 1‐year, and 2‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Jiang 2015b.
| Methods | Study design: Randomised clinical trial Study duration: Not reported Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 63.65 ± 9.31 years, 41‐82 years; TACE alone: 62.18 ± 9.07 years, 43‐79 years Male (n/total): TACE + RFA: 17/30; TACE alone: 16/30 Tumour diameter (mean ± SD, range): TACE + RFA: 5.07 ± 1.13 cm, 3‐10.5 cm, TACE: 5.12 ± 1.04 cm, 2.8‐10.6 cm TNM stage (patients): Stage Ⅰ: TACE + RFA: 10; TACE: 9 Stage Ⅱ: TACE + RFA: 12; TACE: 14 Stage Ⅲ: TACE + RFA: 7; TACE: 7 Child‐Pugh Class (patients): Class A: TACE + RFA: 25; TACE alone: 23 Class B: TACE + RFA: 5; TACE alone: 7 |
| Interventions | TACE + RFA group (n = 30): TACE: Chemotherapeutic drugs: oxaliplatin 100‐150 mg and theprubicin 40‐60 mg. Two sessions of TACE were performed, with an interval of one month. RFA: The interval between TACE and RFA was 2 weeks. RANDIONICS system. Output power of 0‐200 W and frequency of 480 kHz TACE group (n = 30): Chemotherapeutic drugs: oxaliplatin 100‐150 mg and theprubicin 40‐60 mg. Two sessions of TACE were performed, with an interval of one month. |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment 1‐ and 2‐year survival rates Progression‐free survival |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Jiang 2017.
| Methods | Study design: Randomised clinical trial Study duration: June 2012 to June 2014 Duration of follow‐up: 2 weeks Setting: Hospital |
| Participants | All patients were confirmed with hepatic cell carcinoma through biopsy pathology, except for patients who could not receive surgical resection of liver cancer. Age (mean ± SD, range): TACE + RFA: 63 ± 7 years, 53‐76 years; TACE alone: 63 ± 6 years, 53‐74 years Male (n/total): TACE + RFA: 30/53; TACE alone: 31/53 Child‐Pugh Class (patients): Class A: TACE + RFA: 29; TACE: 28 Class B: TACE + RFA: 21; TACE: 20 Class C: TACE + RFA: 3; TACE: 5 |
| Interventions | TACE + RFA group (n = 53): The same TACE method was performed as that in control group. After completion, RFA was conducted, the patient’s posture was set according to the puncture path under CT guidance, CT scan locating was performed, and a 15 G RFA needle and RITA 1500 RF emitting device was used to treat the liver cancer lesions of patients. Lesion ablation temperature was set to 105 ◦C, the diameter of the ablation range might reach 5 cm, and single point puncture and ablation range superposition in different directions were adopted. TACE group (n = 53): After ensuring that the catheter was in position, 40–80 mg of cisplatin and 30–50 mg of epirubicin was injected. Then, the emulsion of 20 mg of pirarubicin + 5–20 mL of iodised oil mixture was injected. |
| Outcomes | Clinical efficacy assessment: measured at one and two weeks after treatment Tumour activity‐related indicators: measured at two weeks after treatment Tumour recurrence‐related indicators: measured at two weeks after treatment Serum indicators for inflammation and oxidative stress |
| Notes | Country of study: China
Source of funding: National Natural Science Foundation of China (No.30670612); China International Medical Foundation (No.Z‐2014‐06‐15322); Navy Logistics Department (No.HJHQ‐20130987) There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Jiang 2018.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to June 2016 Duration of follow‐up: 6 weeks Setting: Hospital |
| Participants | Inclusion criteria: Willing to sign a written informed consent document; diagnosed as HCC; Karnofsky score ≥ 60; life expectancy > 3 months; no history of interventional treatment; no obvious fistula Exclusion criteria: not suitable for TACE or MWA; with hepatic metastasis; with serious injuries of other organs; with abnormal haematopoietic function; with mental disorders; with concurrent other tumours Age (mean ± SD, range): TACE + MWA: 56.87 ± 12.70 years, 38‐71 years; TACE alone: 57.98 ± 13.05 years, 32‐74 years Male (n/total): TACE + MWA: 31/46; TACE alone: 28/46 Tumour diameter (mean ± SD, range): TACE + MWA: 6.97 ± 3.82 cm, 2. 50‐13. 27 cm; TACE: 7.32 ± 4.05 cm, 2. 58‐14. 19 cm. TNM stage (patients): Stage Ⅰ: TACE + MWA: 8; TACE: 7 Stage Ⅱ: TACE + MWA: 28; TACE: 26 Stage Ⅲ: TACE + MWA: 10; TACE: 13 Child‐Pugh Class (patients): Class A: TACE + MWA: 20; TACE: 22 Class B: TACE + MWA: 26; TACE: 24 |
| Interventions | TACE + MWA group (n = 46): TACE: Chemotherapeutic drugs: oxaliplatin 130 mg/m2 and epirubicin 35 mg/m2. MWA: Ultrasound‐guided MWA. KV2000 system. Output power of 55 W and 6 minutes per MWA application TACE group (n = 46): Chemotherapeutic drugs: oxaliplatin 130 mg/m2 andepirubicin 35 mg/m2 |
| Outcomes | Serum level of AFP: measured at 3 and 6 weeks after treatment; Serum level of VEGF: measured at 3 and 6 weeks after treatment; Immune function: measured at 3 and 6 weeks after treatment; |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Jin 2003.
| Methods | Study design: Randomised clinical trial Study duration: November 1998 to May 2000 Duration of follow‐up: 25 months Setting: Hospital |
| Participants | Age (mean ± SD): TACE + ultrasound ablation: 47 ± 12.6 years; TACE: 44.5 ± 8.4 years Male (n/total): TACE + ultrasound ablation: 15/24; TACE alone: 21/26 Single/multiple tumours: TACE + ultrasound ablation: 6/18; TACE: 9/17 TNM stage: Ⅳ Child‐Pugh Class (patients): Class A: TACE + ultrasound ablation: 24; TACE: 24 Class B: TACE + ultrasound ablation: 0; TACE: 2 |
| Interventions | TACE + ultrasound ablation group (n = 24): TACE: Chemotherapeutic drugs: adriamycin 40‐60 mg and cisplatin 80‐120 mg Ultrasound ablation: frequency 0.8 MHz, focal length 135 mm TACE group (n = 26): Chemotherapeutic drugs: adriamycin 40‐60 mg and cisplatin 80‐120 mg |
| Outcomes | Survival rates: measured at 6 months and 1 year after treatment |
| Notes | Country of study: China
Source of funding: National Natural Science Foundation of China There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Lai 2019.
| Methods | Study design: Randomised clinical trial Study duration: March 2017 to March 2018 Duration of follow‐up: 3 weeks Setting: Hospital |
| Participants | Number of participants: 32 Inclusion criteria: Diagnosed as HCC; expected survival time ≥ 3 months |
| Interventions | TACE + MWA group (n = 24): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: carboplatin 300 mg, and epirubicin 30 mg MWA: The interval between TACE and MWA was 1–3 weeks. Control group (n = 8): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: carboplatin 300 mg, and epirubicin 30 mg |
| Outcomes | Immunological function (CD3, CD4, CD8, and NK cell): measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2012.
| Methods | Study design: Randomised clinical trial Study duration: July 2006 to September 2010. Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: diagnosed as HCC by biopsy and images Age (mean, range): TACE + ultrasound ablation: 55 years, 31‐77 years; TACE: 53 years, 28‐69 years Male (n/total): TACE + ultrasound ablation: 24/33; TACE: 23/33 TNM stage (patients): TNM Ⅱ: TACE + ultrasound ablation: 10; TACE: 9 TNM III: TACE + ultrasound ablation: 15; TACE: 16 TNM IV: TACE + ultrasound ablation: 8; TACE: 7 |
| Interventions | TACE + ultrasound ablation group (n = 33): TACE: TACE was performed 1‐2 times. The average sessions of TACE treatment was 1.5 sessions. Ultrasound ablation: The interval between TACE and ultrasound ablation was 2‐3 weeks. Frequency of 0.8 or 1.6 MHz, output power of 250‐400 W, focal length of 135 mm and treatment duration of 436‐8955 s TACE group (n = 33): Chemotherapeutic drugs: pingyangmycin 8 mg. TACE was performed 1‐3 times, with an interval of 3‐4 weeks. The average sessions of TACE treatment was 2.3 sessions. |
| Outcomes | Serum level of AFP Liver function Survival rate: at 6 months and 1 year after treatment |
| Notes | Country of study: China
Source of funding: National important basic project (2011CB707905) There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2015.
| Methods | Study design: Randomised clinical trial Study duration: August 2013 to August 2014 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed by Standardisation of Diagnosis and Treatment for Hepatocellular Carcinoma (2011 edition); unsuitable for resection, unwilling to accept surgery or with tumour recurrence after hepatectomy; single tumour with diameter ≤ 5 cm or multiple tumours (number of tumours < 4 and tumour diameter ≤ 3 cm); no tumour thrombus in hepatic veins or portal veins; with liver function of Child‐Pugh Class A or B; no extrahepatic metastasis; no antivascular drugs use history within 6 months; expected life time > 3 months Age (mean ± SD): TACE + cryoablation: 52.2 ± 3.2 years; TACE alone: 49.8 ± 3.2 years Male (n/total): TACE + cryoablation: 40/50; TACE alone: 36/50 |
| Interventions | TACE + cryoablation group (n = 50): TACE: Chemotherapeutic drugs: lobaplatin, 50 mg; epirubicin, 50 mg. 1‐month treatment interval. Cryoablation: CYROCARE‐TM‐24 system (Endocare) TACE group (n = 50): Chemotherapeutic drugs: lobaplatin, 50 mg; epirubicin, 50 mg. 1‐month treatment interval |
| Outcomes | Serum level of vascular endothelial growth factor: by using ELISA |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2016a.
| Methods | Study design: Randomised clinical trial Study duration: April 2014 to April 2015 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 46.38 ± 11.54 years, 35‐81 years; TACE alone: 46.56 ± 11.48 years, 36‐81 years Male (n/total): TACE + RFA: 28/43; TACE alone: 29/42 Child‐Pugh Class (patients): Class A: TACE + RFA: 18; TACE: 19 Class B: TACE + RFA: 15; TACE: 16 Class C: TACE + RFA: 10; TACE: 7 |
| Interventions | TACE + RFA group (n = 43): TACE: Chemotherapeutic drugs: mitomycin 15 mg and hydroxy camptothecin 15 mg/m2 RFA: The interval between TACE and RFA was 3‐5 weeks. TACE group (n = 42): Chemotherapeutic drugs: mitomycin 15 mg and hydroxy camptothecin 15 mg/m2 |
| Outcomes | Serum level of AFP Liver function Tumour diameter Adverse events Recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2016b.
| Methods | Study design: Randomised clinical trial Study duration: January 2013 to December 2015 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 58.7 ± 6.3 years; TACE alone: 58.5 ± 6.2 years Male (n/total): TACE + RFA: 27/40; TACE alone: 26/40 Tumour diameter (mean ± SD): TACE + RFA: 5.1 ± 1.4 cm; TACE:5.0 ± 1.3 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 29; TACE: 28 Class B: TACE + RFA: 11; TACE: 12 |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: oxaliplatin 200 mg and 5‐fluorouracil 2 g RFA: The interval between TACE and RFA was 2 weeks. Output power of 60 W TACE group (n = 40): Chemotherapeutic drugs: oxaliplatin 200 mg and 5‐fluorouracil 2 g |
| Outcomes | Tumour response: measured by image examinations Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2017a.
| Methods | Study design: Randomised clinical trial Study duration: May 2012 to December 2014 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as middle or advanced HCC by pathology Age (mean ± SD): 48.7 ± 6.3 years Male (n/total): 82/120 Tumour diameter (mean ± SD, range): 8.4 ± 3.5 cm, 5.5‐14.2 cm |
| Interventions | TACE + cryoablation group (n = 60): TACE: Chemotherapeutic drugs: epirubicin 40‐60 mg, fluorouracil 0.5‐1 g, and cisplatin 60‐120 mg Cryoablation: The interval between TACE and cryoablation was 2 weeks. TACE group (n = 60): Chemotherapeutic drugs: epirubicin 40‐60 mg, fluorouracil 0.5‐1 g, and cisplatin 60‐120 mg |
| Outcomes | Serum level of AFP: measured at 2 weeks, 1 month, 3 months, and 6 months after treatment Tumour response: measured by contrast‐enhanced CT and DSA at 1 month after treatment Survival rate: at 1 year after treatment Recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2017b.
| Methods | Study design: Randomised clinical trial Study duration: February 2014 to February 2017 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology Exclusion criteria: Liver function of Child‐Pugh Class C; history of gastrointestinal bleeding within 3 months; with extrahepatic metastasis; abnormal haematopoietic function; serious infection; inadequate lung, renal, and cardiac function Age (mean ± SD): TACE + MWA: 54.37 ± 12.94 years; TACE alone: 54.68 ± 12.1 3 years Male (n/total): TACE + MWA: 26/36; TACE alone: 27/36 Tumour diameter (mean ± SD, range): TACE + MWA: 3. 74 ± 1. 10 cm, 1.12‐7.45 cm; TACE: 3.26 ± 1.31 cm, 1.30‐7.39 cm. Child‐Pugh Class (patients): Class A: TACE + MWA: 28; TACE: 29 Class B: TACE + MWA: 8; TACE: 7 |
| Interventions | TACE + MWA group (n = 36): TACE: Chemotherapeutic drugs: oxaliplatin 10 mL MWA: CT‐guided MWA TACE group (n = 36): Chemotherapeutic drugs: oxaliplatin 10 mL |
| Outcomes | Tumour response: Classified as complete necrosis and progression; measured at 6 months after treatment Adverse events Survival rate Recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2017c.
| Methods | Study design: Randomised clinical trial Study duration: January 2010 to January 2015 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Age (mean ± SD): 61.3 ± 2.5 years Male (n/total): 100/186 Tumour diameter (mean ± SD, range): 5.2 ± 1.4 cm, 4.5‐8.7 cm |
| Interventions | TACE + RFA group (n = 93) TACE: Chemotherapeutic drugs: cisplatin 50 mg, epirubicin 40 mg, and hydroxy camptothecin 30 mg RFA: The interval between TACE and RFA was 1 week. TACE group (n = 93): TACE: Chemotherapeutic drugs: cisplatin 50 mg, epirubicin 40 mg, and hydroxy camptothecin 30 mg |
| Outcomes | Tumour response: Classified as obvious effect, moderate effect, and no effect; measured at 12 months after treatment Adverse events Quality of life: assessed by QOL questionnaire; assessed at 1 year after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2018.
| Methods | Study design: Randomised clinical trial Study duration: January 2016 to December 2016 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC; no serious hepatic disease; no history of liver resection Exclusion criteria: Be allergic to chemotherapeutic drugs or lipiodol oil Age (mean ± SD, range): TACE + RFA: 51.7 ± 1.1 years, 39‐67 years; TACE: 51.8 ± 1.2 years, 38‐69 years Male (n/total): TACE + RFA: 15/30; TACE alone: 14/30 |
| Interventions | TACE + RFA group (n = 30): Chemotherapeutic drugs: mitomycin 5 mg and theprubicin 20 mg RFA: The interval between TACE and RFA was 2 weeks. CT‐guided RFA TACE group (n = 30): Chemotherapeutic drugs: mitomycin 5 mg and theprubicin 20 mg |
| Outcomes | Tumour response: measured by area of tumour; assessed at 1 month after treatment Immune function |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2019a.
| Methods | Study design: Randomised clinical trial Study duration: June 2012 to June 2015 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD, range ): TACE + RFA: 51.22 ± 2.98 years, 31‐72 years; TACE: 53.46 ± 3.0 years, 29‐69 years Male (n/total): TACE + RFA: 27/41; TACE alone: 31/41 Child‐Pugh Class (patients): Class A: TACE + RFA: 20; TACE: 21 Class B: TACE + RFA: 18; TACE: 15 Class C: TACE + RFA: 3; TACE: 5 |
| Interventions | TACE + RFA group (n = 41): TACE: A total of 1‐3 sessions of TACE, with an interval of 3‐4 weeks RFA: The interval between TACE and RFA was 1 week. Ultrasound‐guided RFA TACE group (n = 41): A total of 1‐3 sessions of TACE, with an interval of 3‐4 weeks |
| Outcomes | Serum level of AFP Liver function Survival rate Tumour response: based on mRECIST criteria; measured by images |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Li 2020.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to January 2020 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Number of participants: 66 Inclusion criteria: Diagnosed as HCC; number of tumours < 3; tumour diameter of 5–10 cm; unresectable tumour Age (mean ± SD): TACE + MWA: 56.12 ± 6.59 years; TACE alone: 57.03 ± 6.73 years Male (n/total): TACE + MWA: 18/33; TACE alone: 19/33 |
| Interventions | TACE + MWA group (n = 33): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: mitomycin 4‐10 mg, epirubicin 10–50 mg, and cisplatin 30–60 mg MWA: Output power of 40–60 Hz, 6‐9 minutes per session Control group (n = 33): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: mitomycin 4‐10 mg, epirubicin 10–50 mg, and cisplatin 30–60 mg |
| Outcomes | Survival rates: measured at 1 and 3 years after treatment AFP: blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Lin 2017.
| Methods | Study design: Randomised clinical trial Study duration: February 2016 to January 2017 Duration of follow‐up: 3 months Setting: Hospital |
| Participants | Inclusion criteria: No serious diseases in other organs; tumour numbers < 3; no tumour thrombus in inferior vena cava or portal vein; no extrahepatic metastasis Age (mean ± SD, range): TACE + RFA: 50.1 ± 2.5 years, 34‐68 years; TACE: 49.2 ± 2.3 years, 33‐67 years Male (n/total): TACE + RFA: 22/30; TACE alone: 21/30 Child‐Pugh Class (patients): Class A: TACE + RFA: 22; TACE: 21 Class B: TACE + RFA: 5; TACE: 6 Class C: TACE + RFA: 3; TACE: 3 |
| Interventions | TACE + RFA group (n = 30): TACE: Chemotherapeutic drugs: theprubicin 50 mg, mitomycin 10 mg, and cisplatin 50 mg RFA: The interval between TACE and RFA was 2 weeks. Output power of 200 W. Total treatment time of 10‐30 minutes TACE group (n = 30): Chemotherapeutic drugs: theprubicin 50 mg, mitomycin 10 mg, and cisplatin 50 mg |
| Outcomes | Tumour necrosis: measured by DSA or CT Tumour recurrence |
| Notes | Funding source: None
Conducted in China There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2016a.
| Methods | Study design: Randomised clinical trial Study duration: January 2012 to September 2013 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Tumour diameter > 5 cm; tumour numbers ≤ 4; liver function of Child‐Pugh Class A and B; willing to sign a written informed consent document Exclusion criteria: Not suitable for TACE or MWA; with history of liver function or liver transplantation Age (mean ± SD, range): TACE + MWA: 58.74 ± 7.06 years, 35‐81 years; TACE alone: 58.26 ± 7.31 years, 34‐79 years Male (n/total): TACE + MWA: 43/62; TACE alone: 45/62 |
| Interventions | TACE + MWA group (n = 62): TACE: Chemotherapeutic drugs: cisplatin 40‐60 mg and 5‐fluorouracil 0.75‐1 g MWA: The interval between TACE and RFA was 2‐4 weeks. Multiple sessions of MWA were performed with an interval of 2 weeks. TACE group (n = 62): Chemotherapeutic drugs: cisplatin 40‐60 mg and 5‐fluorouracil 0.75‐1 g |
| Outcomes | Tumour response: based on WHO criteria; measured at 4 weeks after treatment Survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2017.
| Methods | Study design: Randomised clinical trial Study duration: March 2013 to March 2014 Duration of follow‐up: 6 months Setting: Hospital |
| Participants | Age (mean ± SD, range): 52.8 ± 7.2 years, 36‐68 years Male (n/total): 53/72 Tumour diameter (mean ± SD, range): 6.8 ± 2.4 cm, 3‐10.6 cm BCLC stage (patients): Stage A: 27; Stage B: 30; Stage C: 15 Child‐Pugh Class (patients): Stage A: 40; Stage B: 32 |
| Interventions | TACE + MWA group (n = 36): TACE: Chemotherapeutic drugs: cisplatin 100 mg, epirubicin 100 mg, mitomycin 20 mg MWA: The interval between TACE and RFA was 1‐3 weeks. Output power of 55‐70 W, 6‐12 minutes per MWA application TACE group (n = 36): Chemotherapeutic drugs: cisplatin 100 mg, epirubicin 100 mg, mitomycin 20 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT Serum level of AFP Survival rate Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2018b.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to Novermber 2016 Duration of follow‐up: 3 months Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + MWA: 54.3 ± 3.64 years, 31‐81 years; TACE alone: 54.32 ± 3.68 years, 30‐82 years Male (n/total): TACE + MWA: 28/40; TACE alone: 26/40 |
| Interventions | TACE + MWA group (n = 40): TACE: Chemotherapeutic drugs: epirubicin MWA: The interval between TACE and MWA was 2 weeks. Output power of 40‐70 W, 6‐8 minutes per MWA session TACE group (n = 40): Chemotherapeutic drugs: epirubicin |
| Outcomes | Serum level of AFP Quality of life: measured by KPS score; measured at 3 months after treatment Serum level of VEGF Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2019b.
| Methods | Study design: Randomised clinical trial Study duration: January 2014 to December 2018 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Number of participants: 100 Inclusion criteria: Diagnosed as HCC Age (mean ± SD, range ): TACE + RFA: 43.6 ± 3.7 years; TACE alone: 42.8 ± 3.2 years Male (n/total): TACE + RFA: 26/50; TACE alone: 29/50 |
| Interventions | TACE + RFA group (n = 50): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil 40–60 mg, cisplatin 40–60 mg, and epirubicin 10–30 mg RFA: CT‐guided Control group (n = 50): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil 30–40 mL, cisplatin 40–60 mg, and epirubicin 10–30 mg |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2019c.
| Methods | Study design: Randomised clinical trial Study duration: January 2012 to December 2013 Duration of follow‐up: 5 years Setting: Hospital |
| Participants | Number of participants: 83 Inclusion criteria: Diagnosed as HCC Age (mean ± SD, range ): TACE + RFA: 57.1 ± 7.5 years; TACE alone: 55.3 ± 7.1 years Male (n/total): TACE + RFA: 25/41; TACE alone: 25/42 |
| Interventions | TACE + RFA group (n = 41) Control group (n = 42) |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Survival rates: measured at 1, 3, and 5 years after treatment Liver function and the level of AFP: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2019d.
| Methods | Study design: Randomised clinical trial Study duration: January 2016 to January 2018 Duration of follow‐up: 3 months Setting: Hospital |
| Participants | Number of participants: 76 Inclusion criteria: Diagnosed as HCC; advanced tumour; Child‐Pugh Class A or B Age (mean ± SD, range ): TACE + MWA: 58.4 ± 4.5 years; TACE alone: 58.2 ± 4.4 years Male (n/total): TACE + MWA: 27/38; TACE alone: 26/38 |
| Interventions | TACE + MWA group (n = 38) Control group (n = 38) |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 4 and 12 weeks after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Liu 2020.
| Methods | Study design: Randomised clinical trial Study duration: January 2018 to April 2019 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Number of participants: 72 Inclusion criteria: Diagnosed as HCC; expected survival time > 6 months; Child‐Pugh Class A or B Age (mean ± SD, range ): TACE + MWA: 52.61 ± 10.04 years; TACE alone: 51.49 ± 10.14 years Male (n/total): TACE + MWA: 29/36; TACE alone: 27/36 |
| Interventions | TACE + MWA group (n = 36) Control group (n = 36) |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Immunological function: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Lu 2018.
| Methods | Study design: Randomised clinical trial Study duration: October 2014 to October 2017 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD, range ): TACE + RFA: 51.85 ± 2.47 years, 46‐72 years; TACE alone: 50.63 ± 2.86 years, 45‐70 years Male (n/total): TACE + RFA: 24/40; TACE alone: 26/40 |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: mitomycin 10‐20 mg, cisplatin 40‐80 mg, and 5‐fluorouracil 0.5‐1 g RFA: The interval between TACE and RFA was 2 weeks. Total treatment time of 30 minutes TACE group (n = 40): Chemotherapeutic drugs: mitomycin 10‐20 mg, cisplatin 40‐80 mg, and 5‐fluorouracil 0.5‐1 g |
| Outcomes | Tumour response: Classified as obvious effect, moderate effect, and no effect Duration of hospital stay Quality of life |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Luo 2019.
| Methods | Study design: Randomised clinical trial Study duration: March 2015 to March 2017 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria were in line with the diagnostic criteria of middle and advanced liver cancer, diagnosed as liver cancer by pathology, impossibility in radical resection, no combined distant metastasis; stable vital signs, normal coagulation mechanism, normal liver and kidney functions, complete clinical data, completing one year's postoperative follow‐up Exclusion criteria: with combined severe mental illness, severe heart, lung, kidney disease, accompanied with other malignancies and haematological diseases, pregnant and lactating women with abnormal coagulation mechanisms, with possibility of radical resection, poor compliance and failure to complete follow‐up Age (mean ± SD, range): 58.34 ± 2.95 years, 35‐73 years Male (n/total): 52/90 |
| Interventions | TACE + HIFU group (n = 45): HIFU was performed 2‐4 weeks after TACE treatment. The parameters of HIFU tumour treatment system were: frequency 0.8 MHz, focal length 150 mm and treatment duration 4946‐16223 s. The therapeutic range and therapeutic dose were adjusted by monitoring B‐mode ultrasound images during the treatment. Forty‐five patients were treated with HIFU for at least 2 times and up to 6 times, with an average of 3.16 times. TACE group (n = 45): The chemotherapy drugs included 5‐fluorouracil 1.0 g and epirubicin 30 mg (10 mg of which was mixed with an appropriate amount of ultra‐liquid iodised oil to form emulsion). The dose of lipiodol emulsion varied depending on the size of the lesion and intraoperative tolerability of the patient, with an average dose at 10.5 mL. TACE treatment was performed 1‐2 times depending on the patient's tolerability, with intervals of 3‐4 weeks. |
| Outcomes | Tumour response: After six months of treatment, the efficacy was evaluated according to WHO criteria. Liver function Adverse events Recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Lv 2016.
| Methods | Study design: Randomised clinical trial Study duration: January 2013 to January 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 52.25 ± 2.12 years, 34‐76 years; TACE alone: 52.61 ± 2.13 years, 34‐77 years Male (n/total): TACE + RFA: 27/30; TACE: 25/30 |
| Interventions | TACE + RFA group (n = 30): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g, hydroxy camptothecin 10 g, and epirubicin 10‐40 mg. Total sessions of TACE ranged between 1‐3, with an interval of 4‐5 weeks. RFA: The interval between TACE and RFA was 3‐4 weeks. CT‐guided RFA TACE group (n = 30): Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g, hydroxy camptothecin 10 g, and epirubicin 10‐40 mg. Total sessions of TACE ranged between 1‐3, with an interval of 4‐5 weeks. |
| Outcomes | Serum level of AFP Tumour response, classified as complete response, partial response, stable, and progression 2‐year survival rate Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Ma 2017.
| Methods | Study design: Randomised clinical trial Study duration: May 2014 to June 2016 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Age (mean ± SD, range ): TACE + RFA: 55.04 ± 20.13 years, 35‐75 years; TACE: 54.56 ± 19.49 years, 35‐74 years Male (n/total): TACE + RFA: 21/35; TACE alone: 22/35 Tumour diameter (mean ± SD, range): TACE + RFA: 3.78 ± 2.25 cm, 1.5‐6.0 cm; TACE: 3.81 ± 2.21 cm, 1.6‐6.0 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 18; TACE: 20 Class B: TACE + RFA: 17; TACE: 15 |
| Interventions | TACE + RFA group (n = 35): TACE: Chemotherapeutic drugs: oxaliplatin and epirubicin RFA: The interval between TACE and RFA was 2‐3 weeks. RITA 1500 X system TACE group (n = 35): Chemotherapeutic drugs: oxaliplatin and epirubicin |
| Outcomes | Tumour response, according to WHO criteria, measured at 4 weeks after treatment Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Ma 2019a.
| Methods | Study design: Randomised clinical trial Study duration: April 2017 to February 2018 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 65.1 ± 5.9 years, 52‐77 years; TACE: 64.7 ± 5.8 years, 53‐76 years Male (n/total): TACE + RFA: 32/50; TACE alone: 34/50 Tumour diameter (mean ± SD, range): TACE + RFA: 4.1 ± 1.6 cm, 2‐7 cm; TACE alone:4.0 ± 1.3 cm, 2‐8 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 29; TACE: 31 Class B: TACE + RFA: 21; TACE: 19 |
| Interventions | TACE + RFA group (n = 50): TACE: Chemotherapeutic drugs: cisplatin 40‐80 mg, epirubicin 30‐50 mg, and theprubicin 20 mg RFA: CT‐guided RFA. RITA 1500 system TACE group (n = 50): Chemotherapeutic drugs: cisplatin 40‐80 mg, epirubicin 30‐50 mg, and theprubicin 20 mg |
| Outcomes | Tumour response, based on RECIST criteria Liver function Tumour diameter Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Ma 2019b.
| Methods | Study design: Randomised clinical trial Study duration: January 2013 to February 2016 Duration of follow‐up: 2 years |
| Participants | Age (mean ± SD): TACE + MWA: 53.72 ± 8.72 years; TACE alone: 53.24 ± 8.45 years Male (n/total): TACE + MWA: 26/41; TACE alone: 24/41 Tumour diameter (mean ± SD): TACE + MWA: 49.82 ± 11.27 mm; TACE: 50.15 ± 11.62 mm |
| Interventions | TACE + MWA group: TACE: Chemotherapeutic drugs: oxaliplatin 100 mg. 1‐2 sessions of TACE, with an interval of 4 weeks RFA: The interval between TACE and RFA was 1 week. CT‐guided RFA TACE group: Chemotherapeutic drugs: oxaliplatin 100 mg. 1‐2 sessions of TACE, with an interval of 4 weeks |
| Outcomes | Tumour response: measured by CT image Survival rate Recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Ma 2020.
| Methods | Study design: Randomised clinical trial Study duration: January 2017 to December 2019 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Number of participants: 86 Inclusion criteria: Diagnosed as HCC; no metastasis; no history of other anti‐cancer treatments Age (mean ± SD): TACE + MWA: 58.75 ± 7.65 years; TACE alone: 55.65 ± 8.17 years Male (n/total): TACE + MWA: 30/43; TACE alone: 28/43 |
| Interventions | TACE + MWA group (n = 43): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin MWA: The interval between TACE and MWA was 2 weeks. Output power of 40–60 W Control group (n = 43): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: theprubicin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Adverse events: measured at 1 month after treatment TBIL, DBIL, and ALT: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Mo 2017.
| Methods | Study design: Randomised clinical trial Study duration: May 2011 to May 2013 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as primary liver cancer; aged ≥ 18 years; with liver function of Child‐Pugh A or B; unsuitable for resection or unwilling to accept surgery; abnormal haematopoietic function; no portal vein tumour thrombus; no extrahepatic metastasis. Exclusion criteria: Intrahepatic dissemination; huge liver cancer with intrahepatic or extrahepatic metastasis; with liver, cardiac, or kidney function damage; with the liver function of Child‐Pugh Class C; inadequate haematopoietic function; with systematic infection; allergic to contrast agent Male (n/total): TACE + RFA: 39/61; TACE alone: 35/56 Child‐Pugh Class (patients): Class A: TACE + RFA: 28; TACE: 20 Class B: TACE + RFA: 33; TACE: 36 |
| Interventions | TACE + RFA group (n = 61): TACE: Chemotherapeutic drugs: theprubicin, 5‐fluorouracil, and oxaliplatin RFA: The interval between TACE and RFA was 2‐4 weeks. CT/Ultrasound‐guided RFA, with the ablation margin of 5‐10 mm TACE group (n = 56): Chemotherapeutic drugs: theprubicin, 5‐fluorouracil, and oxaliplatin. A total of 1‐3 sessions of TACE, with an interval of 1‐2 months |
| Outcomes | Serum level of AFP, VEGF, and CA‐199 Tumour response, according to the mRECIST criteria 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Pan 2016.
| Methods | Study design: Randomised clinical trial Study duration: February 2012 to February 2013 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 55.4 ± 11.7 years, 29‐75 years; TACE alone: 53.3 ± 10.8 years, 28‐53 years Male (n/total): TACE + RFA: 29/56; TACE alone: 32/56 Tumour diameter: TACE + RFA: 2.5‐11 cm; TACE: 2‐10 cm |
| Interventions | TACE + RFA group (n = 56): TACE: Chemotherapeutic drugs: epirubicin 60 mg and carboplatin 300 mg RFA: The interval between TACE and RFA was 1 week. LDRF‐12S system. CT‐guided RFA TACE group (n = 56): Chemotherapeutic drugs: epirubicin 60 mg and carboplatin 300 mg |
| Outcomes | Tumour response, according to WHO criteria 6‐month recurrence rate 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Peng 2017.
| Methods | Study design: Randomised clinical trial Study duration: December 2013 to June 26 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Age (mean ± SD, range): 65.5 ± 3.5 years, 21‐80 years Male (n/total): 34/64 |
| Interventions | TACE + MWA group (n = 32): TACE: Chemotherapeutic drugs: cisplatin 30‐60 mg, mitomycin 4‐10 mg, and adriamycin 10‐50 mg. MWA: DSA/CT‐guided MWA. Frequency of 40‐60 Hz, 6‐10 minutes per MWA session TACE group (n = 32): Chemotherapeutic drugs: cisplatin 30‐60 mg, mitomycin 4‐10 mg, and adriamycin 10‐50 mg |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression, measured at 4 weeks after treatment Adverse events Liver function 2‐year survival rate and metastasis rate |
| Notes | Country of study: China
Source of funding: Maoming Medical Project There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Qu 2014.
| Methods | Study design: Randomised clinical trial Study duration: January 2009 to January 2013 Duration of follow‐up: 6 months Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by biopsy or images Exclusion criteria: Inadequate haematopoietic function; with history of other tumours; abnormal immune function; platelet less than 5 × 109/L Age (mean ± SD, range ): 58.2 ± 6.2 years, 47‐75 years Tumour diameter (range): 5.1‐7.3 cm |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg RFA: The interval between TACE and RFA was 2 weeks. Output power of 60 W, ablation margin of 1 cm TACE group (n = 40): Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg |
| Outcomes | Serum level of AFP and CEA, measured at 3 days, 7 days, and 21 days after treatment Tumour response, measured at 6 months after treatment Recurrence rate Survival rate, measured at 6 months after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Qu 2015.
| Methods | Study design: Randomised clinical trial Study duration: April 2012 to April 2014 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Age (mean ± SD, range ): TACE + RFA: 58.8 ± 13.4 years, 45‐77 years; TACE: 59.3 ± 15.7 years, 47‐75 years Male (n/total): TACE + RFA: 26/50; TACE alone: 28/50 Tumour diameter (mean ± SD): TACE + RFA: 3.9 ± 1.7 cm; TACE: 3.6 ± 1.9 cm |
| Interventions | TACE + RFA group (n = 50): TACE: Chemotherapeutic drugs: 5‐fluorouracil 1 g and oxaliplatin 100 mg RFA: The interval between TACE and RFA was 6 weeks. Output power of 50‐120 W, 12‐16 minutes per RFA session TACE group (n = 50): Chemotherapeutic drugs: 5‐fluorouracil 1 g and oxaliplatin 100 mg |
| Outcomes | Serum level of brain‐derived neurotrophic factor Tumour response, according to the RECIST criteria, measured at 4 weeks after treatment Adverse events |
| Notes | Country of study: China
Source of funding: National Natural Science Foundation of China, No. 81172365 There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Shen 2015.
| Methods | Study design: Randomised clinical trial Study duration: January 2012 to November 2013 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Age (mean ± SD, range): 40.23 ± 8.23 years, 32‐59 years Male (n/total): 66/96 Tumour diameter (mean ± SD, range): 6.23 ± 2.45 cm, 2.5‐11.4 cm Child‐Pugh Class (patients): Child‐A 50, Child‐B 40, Child‐C 6 |
| Interventions | TACE + RFA group (n = 48): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation RFA: The interval between TACE and RFA was 2‐4 weeks. In total of 20‐30 minutes TACE group (n = 48): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation |
| Outcomes | Tumour response, according to RECIST criteria, measured at 6 months 1‐ and 2‐ year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Shen 2020.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to February 2017 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Number of participants: 67 Inclusion criteria: Diagnosed as HCC Age (mean ± SD): TACE + RFA: 59.62 ± 5.05 years; TACE alone: 59.63 ± 5.02 years Male (n/total): TACE + MWA: 21/33; TACE alone: 24/34 |
| Interventions | TACE + RFA group (n = 33) Control group (n = 34) |
| Outcomes | Sleep: measured by a score system for sleeping Survival rates and recurrence: measured at 2 years after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Song 2016b.
| Methods | Study design: Randomised clinical trial Study duration: January 2008 to June 2012 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by images; no history of liver resection, liver transplantation, and ablation; with liver function of Child‐Pugh Class A or B; maximal tumour diameter > 5 cm; tumour number < 5; willing to sign a written informed consent document Exclusion criteria: With history of upper gastrointestinal bleeding; with abnormal haematopoietic function; with liver function of Child‐Pugh C; with liver abscess or biliary system infection Age (mean ± SD, range): TACE + MWA: 56.7 ± 8.5 years, 32‐86 years; TACE: 57.6 ± 7.6 years, 33‐85 years Male (n/total): TACE + MWA: 32/50; TACE alone: 33/50 Tumour diameter (mean ± SD, range): TACE + MWA: 8.45 ± 2.34 cm, 5‐15 cm; TACE: 9.25 ± 3.15 cm, 5.2‐16.3 cm Child‐Pugh Class (patients): Class A: TACE + MWA: 34; TACE: 35 Class B: TACE + MWA: 16; TACE: 15 |
| Interventions | TACE + MWA group (n = 50): TACE: Chemotherapeutic drugs: 5‐fluorouracil 1 mg and oxaliplatin 120 mg MWA: The interval between TACE and RFA was 2‐4 weeks. Ultrasound‐guided MWA. In total of 6‐10 minutes per MWA session, with the ablation margin of 1 cm TACE group (n = 50): Chemotherapeutic drugs: 5‐fluorouracil 1 mg and oxaliplatin 120 mg |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression Adverse events 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Song 2018.
| Methods | Study design: Randomised clinical trial Study duration: January 2016 to January 2017 Duration of follow‐up: 6 months Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + MWA: 53.01 ± 4.54 years; TACE alone: 53.64 ± 3.65 years Male (n/total): TACE + MWA: 19/24; TACE alone: 20/24 |
| Interventions | TACE + MWA group (n = 24): TACE: Chemotherapeutic drugs: epirubicin 20‐80 mg and oxaliplatin 100‐150 mg. One to three sessions of TACE were performed for each patient. MWA: The interval between TACE and MWA was 2‐4 weeks. Output power of 40‐60 W, 15‐20 minutes per MWA session TACE group (n = 24): Chemotherapeutic drugs: epirubicin 20‐80 mg and oxaliplatin 100‐150 mg. One to three sessions of TACE were performed for each patient. |
| Outcomes | Tumour response Survival rate at 0.5 year after treatment Serum level of AFP |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Song 2019.
| Methods | Study design: Randomised clinical trial Study duration: March 2015 to February 2016 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by images; be willing to sign a written informed consent document Age (mean ± SD, range): TACE + RFA: 57.86 ± 5.42 years, 25‐70 years; TACE alone: 57.96 ± 5.85 years, 26‐71 years Male (n/total): TACE + RFA: 19/32; TACE alone: 20/32 |
| Interventions | TACE + RFA group (n = 32): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation RFA: CT‐guided RFA. Multiple RFA treatments were performed for each patient, with an interval of 1‐2 weeks TACE group (n = 32): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation |
| Outcomes | Serum level of AFP Tumour diameter 1‐ and 2‐year survival rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Tong 2018.
| Methods | Study design: Randomised clinical trial Study duration: July 2014 to July 2016 Duration of follow‐up: 2 weeks Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC, at TNM Ⅱ‐Ⅲ stage; with no history of chemotherapy or radiotherapy; be willing to sign a written informed consent document Exclusion criteria: With serious disease of other organs; with mental disease; abnormal haematopoietic or immune function Age (mean ± SD, range): TACE + RFA: 62.3 4 ± 7.4 years, 52‐75 years; TACE: 62.1 4 ± 7.5 years, 53‐75 years Male (n/total): TACE + RFA: 33/54; TACE alone: 32/54 TNM stage (patients): TNMⅡ: TACE + RFA: 38; TACE: 37 TNMⅢ: TACE + RFA:16, TACE:17 Child‐Pugh Class (patients): Class A: TACE + RFA: 34; TACE: 35 Class B: TACE + RFA: 20; TACE: 19 |
| Interventions | TACE + RFA group (n = 54): TACE: Chemotherapeutic drugs: cisplatin 40‐80 mg, theprubicin 20 mg, and epirubicin 30‐50 mg RFA: The interval between TACE and RFA was 2‐4 weeks. CT‐guided RFA. RITA 1550 system TACE group (n = 54): Chemotherapeutic drugs: cisplatin 40‐80 mg, theprubicin 20 mg, and epirubicin 30‐50 mg |
| Outcomes | Serum level of VEGF and MMP Serum level of AFP, CA‐199, and GGT Tumour response, according to RECIST criteria, measured at 2 weeks after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2013.
| Methods | Study design: Randomised clinical trial Study duration: March 2008 to March 2010 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Unresectable HCC; tumour number ≤ 4; tumour maximal diameter ≤ 10 cm; Karnofsky score ≥ 70; with liver function of Child‐Pugh Class A or B; without inferior vena cava or portal vein tumour thrombus; with no extrahepatic metastasis Child‐Pugh Class (patients): Class A: 41, Class B: 23 |
| Interventions | TACE + RFA group (n = 35): TACE: Chemotherapeutic drugs: mitomycin 4‐10 mg, carboplatin 25‐100 mg, and adriamycin 10‐30 mg. In total, 2‐3 sessions of TACE were performed for each patient, with an interval of 3‐4 weeks. RFA: The interval between TACE and RFA was 2 weeks. 10‐15 minutes per RFA session TACE group (n = 29): Chemotherapeutic drugs: mitomycin 4‐10 mg, carboplatin 25‐100 mg, and adriamycin 10‐30 mg. In total, 2‐3 sessions of TACE were performed for each patient, with an interval of 3‐4 weeks. |
| Outcomes | Tumour response, according to WHO criteria 1‐, 2‐, and 3‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2015a.
| Methods | Study design: Randomised clinical trial Study duration: January 2008 to June 2011 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 52.97 ± 9.23 years; TACE alone: 51.68 ± 9.87 years Male (n/total): TACE + RFA: 36/45; TACE alone: 32/45 Child‐Pugh Class (patients): Class A: TACE + RFA: 26; TACE: 29 Class B: TACE + RFA: 19; TACE: 16 |
| Interventions | TACE + RFA group (n = 45): TACE: Chemotherapeutic drugs: 5‐fluorouracil 1‐1.5 g, oxaliplatin 80‐100 mg, and adriamycin 10‐20 mg. In total, 3 sessions of TACE were performed, with an interval of 4 weeks. RFA: The interval between TACE and RFA was 2 weeks. Output power of 100‐150 W, 30‐60 minutes per RFA treatment TACE group (n = 45): Chemotherapeutic drugs: 5‐fluorouracil 1‐1.5 g, oxaliplatin 80‐100 mg, and adriamycin 10‐20 mg. In total, 3 sessions of TACE were performed, with an interval of 4 weeks. |
| Outcomes | Tumour response, according to WHO criteria, measured at 4 months after treatment Serum level of ALT, AST, TBIL, and γGT Serum level of AFP, CEA, CA‐199, and VEGF 1‐year and 3‐year survival rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2015b.
| Methods | Study design: Randomised clinical trial Study duration: January 2011 to September 2014 Duration of follow‐up: 6 months Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by images and pathology Exclusion criteria: Abnormal haematopoietic and immune function; with other tumours Age (mean ± SD): TACE + RFA: 58.6 ± 6.2 years; TACE alone: 58.3 ± 6.5 years Male (n/total): TACE + RFA: 32/50; TACE alone: 31/50 Tumour diameter (mean ± SD): TACE + RFA: 5.1 ± 1.2 cm, TACE: 5.0 ± 1.3 cm TNM stage (patients): StageⅠ: TACE + RFA: 13; TACE: 13 StageⅡ: TACE + RFA: 31; TACE: 30 StageⅢ: TACE + RFA: 6; TACE: 7 Child‐Pugh Class (patients): Class A: TACE + RFA: 36; TACE: 35 Class B: TACE + RFA: 14; TACE: 15 |
| Interventions | TACE + RFA group (n = 50): TACE: Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg RFA: The interval between TACE and RFA was 2 weeks. Output power of 60 W, ablation margin of 1 cm TACE group (n = 50): Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression 6‐month survival rate 6‐month recurrence rate Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2017a.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to December 2015 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology; expected survival > 3 months; Karnofsky score ≥ 70 Exclusion criteria: With cognitive disorder; with abnormal liver, renal, or cardiac function Age (mean ± SD, range): TACE + RFA: 53.42 ± 4.1 years, 39‐79 years; TACE alone: 53.25 ± 4.09 years, 44‐78 years Male (n/total): TACE + RFA: 28/42; TACE alone: 26/42 |
| Interventions | TACE + RFA group (n = 42): TACE: Chemotherapeutic drugs: cisplatin 50 mg, epirubicin 30 mg, and mitomycin 10 mg RFA: Radio Therapeutics TMRF 2000 system TACE group (n = 42): Chemotherapeutic drugs: cisplatin 50 mg, epirubicin 30 mg, and mitomycin 10 mg |
| Outcomes | Serum level of ALT Serum level of AFP Tumour response, classified as complete response, partial response, stable disease, and progression Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2017b.
| Methods | Study design: Randomised clinical trial Study duration: October 2014 to October 2016 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 52.07 ± 2.12 years, 34‐75 years; TACE alone: 52.13 ± 2.16 years, 35‐76 years Male (n/total): TACE + RFA: 21/36; TACE alone: 21/36 |
| Interventions | TACE + RFA group (n = 36): TACE: Chemotherapeutic drugs: theprubicin 20 mg, oxaliplatin 150 mg, and mitomycin 8 mg RFA: Ultrasould‐guided RFA. TACE group (n = 36): Chemotherapeutic drugs: theprubicin 20 mg, oxaliplatin 150 mg, and mitomycin 8 mg |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2017c.
| Methods | Study design: Randomised clinical trial Study duration: May 2012 to September 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology and images; single tumour, with tumour diameter ≤ 5 cm or multiple tumours (≤ 3) with tumour diameter ≤ 3 cm; AFP > 200 μg/L; with liver function of Child‐Pugh Class A or B; unwilling to receive liver resection Exclusion criteria: With the tumour near major vessels or organs; serious hypertension; tumour diameter ≥ 5 cm; with disseminated metastasis; abnormal haematopoietic function; with liver function of Child‐Pugh Class C Age (mean ± SD, range): TACE + RFA: 50.13 ± 8.75 years, 28‐65 years; TACE: 49.47 ± 9.13 years, 31‐66 years Male (n/total): TACE + RFA: 28/36; TACE alone: 26/36 |
| Interventions | TACE + RFA group (n = 36): TACE: Chemotherapeutic drugs: 5‐fluorouracil 1‐1.5 g, oxaliplatin 50‐100 mg, and adriamycin 20‐40 mg RFA: The interval between TACE and RFA was 4 weeks. Ablation margin of 5‐10 mm TACE group (n = 36): Chemotherapeutic drugs: 5‐fluorouracil 1‐1.5 g, oxaliplatin 50‐100 mg, and adriamycin 20‐40 mg |
| Outcomes | Serum level of AFP 1‐ and 2‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2017d.
| Methods | Study design: Randomised clinical trial Study duration: October 2012 to July 2016 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + RFA: 55.9 ± 5.72 years, 43‐70 years; TACE: 56.2 ± 5.02 years, 45‐68 years Male (n/total): TACE + RFA: 13/20; TACE alone: 11/20 |
| Interventions | TACE + RFA group (n = 20): TACE: Chemotherapeutic drugs: cisplatin 50 mg, mitomycin 8 mg, and adriamycin 10 mg RFA: The interval between TACE and RFA was 2 weeks. 15‐20 minutes per RFA session TACE group (n = 20): Chemotherapeutic drugs: cisplatin 50 mg, mitomycin 8 mg, and adriamycin 10 mg |
| Outcomes | Serum level of AFP Tumour response, classified as complete response, partial response, stable disease, and progression |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2018a.
| Methods | Study design: Randomised clinical trial Study duration: January 2013 to June 2016 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by biopsy and images; unsuitable for resection; with liver function of Child‐Pugh Class A or B; no history of radiotherapy or chemotherapy Exclusion criteria: Abnormal haematopoietic function; with serious immune, digestive or nervous system diseases; with no history of other tumours Age (mean ± SD, range): TACE + RFA: 56. 97 ± 7.49 years, 31‐69 years; TACE: 55.26 ± 7.82 years, 34‐68 years Male (n/total): TACE + RFA: 29/45; TACE alone: 30/45 Tumour diameter (mean ± SD, range): TACE + RFA: 5.17 ± 1.12 cm; TACE: 5.03 ± 1.21 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 32; TACE: 30 Class B: TACE + RFA: 13; TACE: 15 |
| Interventions | TACE + RFA group (n = 45): TACE: Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg. Multiple sessions of TACE were performed, with an interval of 1‐1.5 months. RFA: The interval between TACE and RFA was 2 weeks. Output power of 60 w, ablation margin of 1 cm TACE group (n = 45): Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg. Multiple sessions of TACE were performed, with an interval of 1‐1.5 months |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression, measured at 4 weeks after treatment 1‐, 2‐, and 3‐year survival rates Recurrence rate |
| Notes | Country of study: China
Source of funding: Luohe City Medical Project (1400134) There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2018b.
| Methods | Study design: Randomised clinical trial Study duration: January 2013 to January 2017 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by biopsy and images; with liver function of Child‐Pugh Class A or B; adequate haematopoietic function; tumour number ≤ 3; no extrahepatic metastasis Exclusion criteria: With hepatic arterio‐venous fistula; abnormal renal or cardiac function Age (mean ± SD, range): TACE + RFA: 43.02 ± 7.14 years, 41‐63 years; TACE: 40.48 ± 7.26 years, 37‐59 years Male (n/total): TACE + RFA: 14/27; TACE alone: 15/27 Child‐Pugh Class (patients): Class A: TACE + RFA: 18; TACE: 15 Class B: TACE + RFA: 9; TACE: 12 |
| Interventions | TACE + RFA group (n = 27): TACE: Chemotherapeutic drugs: oxaliplatin and 5‐fluorouracil RFA: The interval between TACE and RFA was 1 week. TACE group (n = 27): Chemotherapeutic drugs: oxaliplatin and 5‐fluorouracil |
| Outcomes | Serum level of AFP Liver function Tumour response, classified as complete response, partial response, stable disease, and progression 1‐year survival, recurrence, and metastasis rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wang 2018c.
| Methods | Study design: Randomised clinical trial Study duration: January 2016 to January 2017 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Tumour number ≤ 3; tumour diameter of 5‐9 cm Exclusion criteria: With mental diseases; abnormal cardiac function Age (mean ± SD, range): TACE + RFA: 47.6 ± 6.8 years, 23‐66 years; TACE alone: 48.7 ± 6.5 years, 22‐68 years Male (n/total): TACE + RFA: 40/50; TACE alone: 36/50 Child‐Pugh Class (patients): Class A: TACE + RFA: 11; TACE: 10 Class B: TACE + RFA: 4; TACE: 5 |
| Interventions | TACE + RFA group (n = 50): TACE: Chemotherapeutic drugs: oxaliplatin, mitomycin, and adriamycin RFA: Ultrasound‐guided RFA TACE group (n = 50): Chemotherapeutic drugs: oxaliplatin, mitomycin, and adriamycin |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression Quality of life 1‐year survival rate Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wen 2018.
| Methods | Study design: Randomised clinical trial Study duration: March 2013 to March 2015 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology; huge HCC; tumour volume < 70% of whole liver volume; with liver function of Child‐Pugh Class A or B; ECOG ≤ 2 Exclusion criteria: With liver function of Child‐Pugh Class C; abnormal haematopoietic function; life expectancy < 6 months; with serious disease of other organs Age (mean ± SD): TACE + cryoablation: 53. 2 ± 12. 3 years; TACE alone: 54.8 ± 10.2 years Male (n/total): TACE + cryoablation: 35/43; TACE alone: 37/43 Vascular invasion (patients): TACE + cryoablation: 6; TACE: 4 |
| Interventions | TACE + cryoablation group (n = 43): TACE: Chemotherapeutic drugs: lobaplatin Cryoablation: The interval between TACE and RFA was 1 week. Ultrasound‐guided cryoablation. TACE group (n = 43): Chemotherapeutic drugs: lobaplatin |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression Adverse events 1‐ and 2‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wu 2011b.
| Methods | Study design: Randomised clinical trial Study duration: Not reported Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD): 50.6 ± 12.3 years Male (n/total): 76/90 Tumour diameter > 3 cm |
| Interventions | TACE + cryoablation group (n = 45): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g and epirubicin 10‐40 mg Cryoablation: The interval between TACE and RFA was 1 week. TACE group (n = 45): Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g and epirubicin 10‐40 mg |
| Outcomes | Serum level of AFP Tumour diameter 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wu 2017b.
| Methods | Study design: Randomised clinical trial Study duration: February 2013 to May 2015 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology and images; with normal renal and haematopoietic function; tumour number ≤ 5; no extrahepatic metastasis Exclusion criteria: With other serious diseases; with mental disease Age (mean ± SD, range): TACE + MWA: 42.1 ± 1.5 years, 25‐60 years; TACE alone: 43.1 ± 1.3 years, 26‐60 years Male (n/total): TACE + MWA: 20/35; TACE alone: 21/35 |
| Interventions | TACE + MWA group (n = 35): TACE: Chemotherapeutic drugs: cisplatin and epirubicin. Three sessions of TACE treatment were performed for each patient, with an interval of 4 weeks. MWA: CT‐guided MWA. Output power of 50 W TACE group (n = 35): Chemotherapeutic drugs: cisplatin and epirubicin. Three sessions of TACE treatment were performed for each patient, with an interval of 4 weeks. |
| Outcomes | Recurrence rate, classified as complete response, partial response, stable disease, and progression Tumour response rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Wu 2020.
| Methods | Study design: Randomised clinical trial Study duration: June 2014 to June 2018 Duration of follow‐up: 1 month Setting: Hospital |
| Participants | Number of participants: 90 Inclusion criteria: Diagnosed as HCC; 18–75 years old Male (n/total): TACE + MWA: 22/45; TACE alone: 24/45 |
| Interventions | TACE + MWA group (n = 45): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: epirubicin MWA: The interval between TACE and MWA was 2 weeks. Output power of 40–80 W Control group (n = 45): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: epirubicin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Physical health: measured by survey at 1 month after treatment Adverse events: measured by follow‐up calls at 1 month after treatment |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Xie 2017.
| Methods | Study design: Randomised clinical trial Study duration: February 2012 to February 2014 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC; aged ≥ 50 years; willing to sign a written informed consent document Exclusion criteria: Abnormal immune and haematopoietic function; with history of organ transplantation; with serious infection Age (mean ± SD, range): TACE + HIFU: 55.0 3 ± 3.97 years, 50‐75 years; TACE: 54.85 ± 3.64 years, 50‐76 years Male (n/total): TACE + HIFU: 25/42; TACE alone: 24/42 Tumour diameter (mean ± SD, range): TACE + RFA: 8.46 ± 1.9 cm, 1.2‐13.8 cm; TACE: 8.52 ± 2.36 cm, 1.1‐14 cm |
| Interventions | TACE + HIFU group (n = 42): TACE: Chemotherapeutic drugs: cisplatin and theprubicin HIFU: The interval between TACE and HIFU was 1‐2 weeks. TACE group (n = 42): Chemotherapeutic drugs: cisplatin and theprubicin |
| Outcomes | Tumour volume Tumour response, according to WHO criteria Immune function 1‐, 2, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: Guangdong Province Science Project There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Xiong 2013.
| Methods | Study design: Randomised clinical trial Study duration: January 2009 to May 2011 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Inclusion criteria: Aged ≥ 60 years; diagnosed as HCC by pathology; unsuitable for resection or unwilling to accept surgery; Karnofsky score ≥ 60; life expectancy ≥ 3 months; no contraindications for TACE or HIFU; no extrahepatic metastasis; tumour volume ≤ 70% of whole liver volume Age (mean ± SD, range): TACE + RFA: 73.4 ± 4.5 years; TACE alone: 74.2 ± 5.6 years Male (n/total): TACE + RFA: 20/35; TACE alone: 21/35 Tumour diameter (mean ± SD): TACE + RFA: 6.3 ± 2.1 cm; TACE: 6.5 ± 2.0 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 27; TACE: 28 Class B: TACE + RFA: 8; TACE: 7 |
| Interventions | TACE + RFA group (n = 35): TACE: Chemotherapeutic drugs: 5‐fluorouracil, epirubicin, and mitomycin RFA: The interval between TACE and RFA was 1‐2 weeks. Ablation margin of 0.5‐1 cm TACE group (n = 35): Chemotherapeutic drugs: 5‐fluorouracil, epirubicin, and mitomycin |
| Outcomes | Serum level of AFP Tumour response, assessed at 6 weeks after treatment, measured by contrast‐enhanced CT or MRI |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Xiong 2017.
| Methods | Study design: Randomised clinical trial Study duration: February 2013 to January 2014 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: diagnosed in line with the Criteria for Clinical Diagnosis of Primary Hepatic Carcinoma; they were diagnosed as having PHC, as demonstrated by serum alpha fetoprotein (AFP), CT or MRI; the Karnofsky Performance Status score ≥ 70; the Child‐Pugh scores or TNM stages of liver function were accurate; no previous systemic chemotherapy or radiotherapy. Exclusion criteria: with obvious hepatic arteriovenous fistula; had hepatic tumour which exceeded 70% of the volume of the liver; had obvious cachexia, jaundice, ascites or distant metastasis or a contraindication to chemotherapy Age (mean ± SD): TACE + RFA: 59.45 ± 5.34 years; TACE alone: 60.06 ± 5.41 years Male (n/total): TACE + RFA: 21/37; TACE alone: 24/37 Tumour diameter (mean ± SD, range): TACE + RFA: 5.08 ± 0.84 cm; TACE: 5.09 ± 0.86cm TNM stage (patients): StageⅠ: TACE+RFA: 11; TACE: 13 StageⅡ: TACE+RFA: 18; TACE: 19 StageⅢ: TACE+RFA: 8; TACE: 5 Child‐Pugh Class (patients): Class A: TACE + RFA: 25; TACE: 23 Class B: TACE + RFA: 12; TACE: 14 |
| Interventions | TACE + RFA group (n = 37): The procedures of RFA were initiated at 15 d after TACE. The electrode was inserted into the tumour tissue under the guidance of CT, with the ablation power at 60 W for 10‐15 min. Single needle ablation was administered for 1‐3 bulbous focus, bilateral focal ablation for 4‐6 bulbous focus, and fractional ablation for patients with poor toleration. The RFA range can be extended to 1 cm inside the normal tissues to ensure full ablation. TACE group (n = 37): A catheter was inserted into the feeding artery, into which nonionic contrast agent lipiodol (5 mL), 5‐fluorouracil (2 g), and oxaliplatin (200 mg) were injected. |
| Outcomes | Serum level of GGT and AFP Clinical response, according to RECIST criteria, measured at 3 years after treatment Tumour necrosis Recurrence and survival at 3‐year Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Xu 2016.
| Methods | Study design: Randomised clinical trial Study duration: December 2010 to July 2012 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 52.9 ± 5.4 years; TACE alone: 53.4 ± 6.8 years Male (n/total): TACE + RFA: 28/38; TACE alone: 27/34 Child‐Pugh Class (patients): Class A: TACE + RFA: 20; TACE: 19 Class B: TACE + RFA: 18; TACE: 15 |
| Interventions | TACE + RFA group (n = 38): TACE: Chemotherapeutic drugs: 5‐fluorouracil, theprubicin, and epirubicin RFA: The interval between TACE and RFA was 2 weeks. Output power of 50‐100 W TACE group (n = 34): Chemotherapeutic drugs: 5‐fluorouracil, theprubicin, and epirubicin |
| Outcomes | Serum level of AFP Liver function Tumour response, measured by contrast‐enhanced CT or MRI; measured at 3 months after treatment 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: Qinhuangdao Science and Develoment Funding There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Xue 2019.
| Methods | Study design: Randomised clinical trial Study duration: February 2017 to February 2018 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Number of participants: 122 Inclusion criteria: Diagnosed as HCC; tumour diameter < 5 cm; number of tumours < 3; no metastasis Age (mean ± SD): TACE + MWA: 47.56 ± 4.71 years; TACE alone: 47.63 ± 4.62 years Male (n/total): TACE + MWA: 37/61; TACE alone: 36/61 |
| Interventions | TACE + MWA group (n = 61): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 20–30 mg theprubicin and 80 mg cisplatin MWA: The interval between TACE and MWA was 2 weeks. Output power of 60W Control group (n = 61): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 20–30 mg theprubicin and 80 mg cisplatin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Survival rates: measured at 1 and 2 years after treatment Adverse events Liver function and AFP: blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Yao 2018.
| Methods | Study design: Randomised clinical trial Study duration: April 2013 to March 2015 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology or images; willing to sign a written informed consent document Exclusion criteria: With serious diseases of other organs; abnormal haematopoietic and cardiac function Age (mean ± SD): TACE + RFA: 42.18 ± 5.31 years; TACE alone: 41.76 ± 5.62 years Male (n/total): TACE + RFA: 26/42; TACE alone: 25/42 Child‐Pugh Class (patients): Class A: TACE + RFA: 34; TACE: 33 Class B: TACE + RFA: 8; TACE: 9 |
| Interventions | TACE + RFA group: TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.75‐1 g, cisplatin 40‐60 mg, and epirubicin 10‐30 mg RFA: CT‐guided RFA TACE group: Chemotherapeutic drugs: 5‐fluorouracil 0.75‐1 g, cisplatin 40‐60 mg, and epirubicin 10‐30 mg |
| Outcomes | Serum level of AFP Tumour response Survival rate Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Yi 2015.
| Methods | Study design: Randomised clinical trial Study duration: January 2009 to January 2011 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by images; tumour number < 4; maximal tumour diameter ≤ 10 cm; Karnofsky score ≥ 70; with liver function of Child‐Pugh Class A or B Exclusion criteria: With history of other tumours; with extrahepatic metastasis; with inferior vena cava or portal vein tumour thrombus Age (mean ± SD): TACE + RFA: 52.14 ± 7.46 years; TACE alone: 51.98 ± 7.28 years Male (n/total): TACE + RFA: 35/49; TACE alone: 35/48 Child‐Pugh Class (patients): Class A: TACE + RFA: 32; TACE: 33 Class B: TACE + RFA: 17; TACE: 15 |
| Interventions | TACE + RFA group (n = 49): TACE: Chemotherapeutic drugs: mitomycin and adriamycin RFA: The interval between TACE and RFA was 2 weeks. 10‐15 minutes per RFA session, ablation margin of 1 cm TACE group (n = 48): Chemotherapeutic drugs: mitomycin and adriamycin |
| Outcomes | Tumour response, according to WHO criteria Adverse events 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Yu 2019.
| Methods | Study design: Randomised clinical trial Study duration: May 2013 to May 2016 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by biopsy or images; with liver function of Child‐Pugh Class A or B; tumour diameter ≥ 5 cm Exclusion criteria: With abnormal haematopoietic function; with portal vein tumour thrombus or extrahepatic metastasis; with abnormal renal or cardiac function Age (mean ± SD): TACE + RFA: 55.9 ± 4.3 years; TACE alone: 55.6 ± 4.1 years Male (n/total): TACE + RFA: 23/34; TACE alone: 22/34 Tumour diameter (mean ± SD): TACE + RFA: 6.23 ± 0.35 cm; TACE: 6.2 ± 0.35 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 17; TACE: 15 Class B: TACE + RFA: 17; TACE: 19 |
| Interventions | TACE + RFA group (n = 34): TACE: Chemotherapeutic drugs: theprubicin 20‐60 mg and oxaliplatin 60‐100 mg. Multiple sessions of TACE can be performed for each patient, with an interval of 4‐6 weeks RFA: The interval between TACE and RFA was 1‐2 weeks. LDRF‐20S ablation system. With tumour margin of 0.5‐1 cm TACE group (n = 34): Chemotherapeutic drugs: theprubicin 20‐60 mg and oxaliplatin 60‐100 mg Multiple sessions of TACE can be performed for each patient, with an interval of 4‐6 weeks. |
| Outcomes | Tumour response, according to mRECIST criteria Adverse events 1‐ and 2‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Yuan 2015.
| Methods | Study design: Randomised clinical trial Study duration: March 2011 to December 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Age (mean): TACE + MWA: 54 years; TACE: 53 years Male (n/total): TACE + MWA: 39/55; TACE alone: 41/55 |
| Interventions | TACE + MWA group (n = 55): TACE: Chemotherapeutic drugs: mitomycin 10 mg and adriamycin 50 mg MWA: The interval between TACE and RFA was 2 weeks. Tumour margin of 1 cm TACE group (n = 55): Chemotherapeutic drugs: mitomycin 10 mg and adriamycin 50 mg |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression Adverse events 1‐ and 2‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2013.
| Methods | Study design: Randomised clinical trial Study duration: March 2009 to September 2011 Duration of follow‐up: 35 months Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by liver biopsy; tumour maximal diameter of 5‐10 cm; with liver function of Child‐Pugh Class A or B; unsuitable for resection or unwilling to accept surgery; with no history of treatments on HCC Age (mean ± SD): TACE + MWA: 52.1 ± 1.64 years; TACE alone: 55.57 ± 1.76 years Male (n/total): TACE + MWA: 48/60; TACE alone: 33/42 Tumour diameter (mean ± SD): TACE + MWA: 7.8 ± 1.37 cm; TACE: 7.34 ± 1.23 cm Child‐Pugh Class (patients): Class A: TACE + MWA: 42; TACE: 25 Class B: TACE + MWA: 18; TACE: 17 |
| Interventions | TACE + MWA group (n = 60): TACE: Chemotherapeutic drugs: adriamycin 30‐50 mg. Multiple sessions of TACE were performed, with an interval of 3‐4 weeks. MWA: Output power of 50‐60 W; 10‐15 minutes per MWA session TACE group (n = 42): Chemotherapeutic drugs: adriamycin 30‐50 mg. Multiple sessions of TACE were performed, with an interval of 3‐4 weeks. |
| Outcomes | Serum level of AFP Tumour response, according to mRECIST criteria, measured at 2 months after treatment 1‐ and 2‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2014.
| Methods | Study design: Randomised clinical trial Study duration: May 2008 to May 2013 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Unresectable HCC; single tumour with tumour diameter < 5 cm or multiple tumours with tumour diameter ≤ 3; no extrahepatic metastasis; diagnosed as HCC by liver biopsy Age (mean): TACE + RFA: 74 years; TACE alone: 73 years Male (n/total): TACE + RFA: 32/40; TACE alone: 32/40 |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.5 g, epirubicin 0.025 g, and mitomycin 0.006 g RFA: HGCF 3000 ablation system. Ultrasound‐guided RFA TACE group (n = 40): Chemotherapeutic drugs: 5‐fluorouracil 0.5 g, epirubicin 0.025 g, and mitomycin 0.006 g |
| Outcomes | Serum level of CD3+, CD4+/CD8+, NK cell, and TNF‐ 2‐year survival and metastasis rates Quality of life |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2016.
| Methods | Study design: Randomised clinical trial Study duration: March 2011 to January 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as middle or advanced HCC Exclusion criteria: With serious infection; with metal disease; with serious systematic diseases Age (mean ± SD): TACE + cryoablation: 64.39 ± 4.25 years; TACE alone: 65.02 ± 4.72 years Male (n/total): TACE + cryoablation: 16/28; TACE alone: 17/28 |
| Interventions | TACE + cryoablation group (n = 28): TACE: Chemotherapeutic drugs: epirubicin and oxaliplatin Cryoablation: CT‐guided cryoablation. 30 minutes per ablation session TACE group (n = 28): Chemotherapeutic drugs: epirubicin and oxaliplatin |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression, measured at 4 weeks after treatment, measured by contrast‐enhanced CT or MRI; 2‐, 6‐, 9‐, 12‐, and 24‐month survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2017a.
| Methods | Study design: Randomised clinical trial Study duration: June 2011 to June 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology; no extrahepatic metastasis and tumour thrombus; unresectable for liver resection; with liver function of Child‐Pugh Class A or B; no history of radiotherapy or chemotherapy; life expectancy ≥ 3 months; willing to sign a written informed consent document Exclusion criteria: With other serious diseases; disseminated tumour; abnormal renal or cardiac function; with contraindications for TACE or ablation Age (mean ± SD): TACE + MWA: 57.4 ± 6.5 years; TACE alone: 58.2 ± 6.3 years Male (n/total): TACE + MWA: 54/117; TACE alone: 52/117 BCLC stage (patients): Stage B: TACE + MWA: 66; TACE: 64 Stage C: TACE + MWA: 33; TACE: 39 Stage D: TACE + MWA: 13; TACE: 14 |
| Interventions | TACE + MWA group (n = 117): TACE: Allura Xper FD20 guiding system. Oxaliplatin 100 mg. Multiple sessions of TACE can be performed. MWA: The interval between TACE and MWA was 2 weeks. KY‐2000 ablation system. CT‐guided MWA. Ablation margin of 0.5‐1 cm, 5‐15 minutes per ablation session TACE group (n = 117): Allura Xper FD20 guiding system. Oxaliplatin 100 mg. Multiple sessions of TACE can be performed. |
| Outcomes | Tumour response, according to mRECIST criteria, measured at 4 weeks after treatment, measured by contrast‐enhanced CT 6‐, 12‐, 18‐, and 24‐month survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2017b.
| Methods | Study design: Randomised clinical trial Study duration: April 2012 to March 2013 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Inclusion criteria: Tumour number < 4; maximal tumour diameter ≤ 15 cm; with liver function of Child‐Pugh Class A or B Exclusion criteria: With history of other tumours; with extrahepatic metastasis; with metal diseases Age (mean ± SD): 44.07 ± 7.56 years, 27‐66 years Male (n/total): 33/60 Child‐Pugh Class (patients): Class A: 44; Class B: 16 |
| Interventions | TACE + RFA group (n = 30): TACE: Chemotherapeutic drugs: mitomycin and adriamycin RFA: The interval between TACE and RFA was 2 weeks. RITA ablation system. 10‐15 minutes per ablation session, ablation margin of 1 cm TACE group (n = 30): Chemotherapeutic drugs: mitomycin and adriamycin |
| Outcomes | Tumour response, according to WHO criteria Serum level of AFP 1‐, 2‐, and 3‐year of survival rates |
| Notes | Country of study: China
Source of funding: Jinan Science Project (J115N009); Taian Science Project (2015NS1132) There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2017c.
| Methods | Study design: Randomised clinical trial Study duration: 2012 to 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by liver biopsy or images; maximal tumour diameter > 5 cm; tumour number ≤ 4; with liver function of Child‐Pugh Class A or B; willing to sign a written informed consent document Exclusion criteria: With history of liver resection or liver transplantation Age (mean ± SD): TACE + MWA: 56.9 ± 7.7 years; TACE alone: 58.2 ± 7.3 years Male (n/total): TACE + MWA: 21/30; TACE alone: 23/30 |
| Interventions | TACE + MWA group (n = 30): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.075‐1 g and cisplatin 40‐60 mg MWA: The interval between TACE and RFA was 2‐4 weeks. Multiple sessions of MWA were performed, with an interval of 2 weeks. TACE group (n = 30): Chemotherapeutic drugs: 5‐fluorouracil 0.075‐1 g and cisplatin 40‐60 mg |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression, measured at 4 weeks after treatment 1‐ and 2‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2018a.
| Methods | Study design: Randomised clinical trial Study duration: January 2014 to January 2017 Duration of follow‐up: 2 months Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 58.62 ± 4.19 years; TACE alone: 57.21 ± 3.98 years Male (n/total): TACE + RFA: 30/48; TACE alone: 29/48 Tumour diameter (mean ± SD, range): TACE + RFA: 6.32 ± 1.24 cm, 5‐15 cm ; TACE: 6.25 ± 1.19 cm, 4‐14 cm |
| Interventions | TACE + RFA group (n = 48): TACE: Chemotherapeutic drugs: cisplatin 80 mg, theprubicin 30 mg, and epirubicin 20 mg RFA: The interval between TACE and RFA was 2 weeks. RADION‐ICS ablation system. Output power of 40 W, 5‐10 minutes per RFA session. Multiple sessions of RFA were performed, with an interval of 1‐2 weeks. TACE group (n = 48): Chemotherapeutic drugs: cisplatin 80 mg, theprubicin 30 mg, and epirubicin 20 mg |
| Outcomes | Tumour response, according to WHO criteria Liver function Immune function |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2018b.
| Methods | Study design: Randomised clinical trial Study duration: January 2014 to December 2015 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology; tumour number ≤ 3; tumour diameter of 5‐9 cm Age (mean ± SD): TACE + RFA: 49.21 ± 3.45 years; TACE alone: 49.11 ± 3.42 years Male (n/total): TACE + RFA: 24/42; TACE alone:26/42 |
| Interventions | TACE + RFA group (n = 42): TACE: Chemotherapeutic drugs: 5‐fluorouracil, oxaliplatin, and mitomycin RFA: The interval between TACE and RFA was 2 weeks. Ultrasound‐guided RFA, 15 minutes per ablation session TACE group (n = 42): Chemotherapeutic drugs: 5‐fluorouracil, oxaliplatin, and mitomycin |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression Adverse events Quality of life, measured by self‐designed questionnaire, with the total score of 100 1‐year survival rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2018c.
| Methods | Study design: Randomised clinical trial Study duration: January 2015 to September 2016 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology or images; Karnofsky score ≥ 70; with no history of radiotherapy or chemotherapy Exclusion criteria: With obvious arteriovenous fistula; tumour volume more than 70% of whole liver volume; with extrahepatic metastasis; with contraindications for TACE Age (mean ± SD): TACE + RFA: 60 ± 5 years; TACE alone: 59 ± 5 years Male (n/total): TACE +RFA: 14/25; TACE alone: 16/25 TNM stage (patients): StageⅠ: TACE + RFA: 7; TACE: 9 StageⅡ: TACE + RFA: 12; TACE: 13 StageⅢ: TACE + RFA: 6; TACE: 2 |
| Interventions | TACE + RFA group (n = 25): TACE: Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg RFA: The interval between TACE and RFA was 15 days. Output power of 60W, 10‐15 minutes per ablation session, ablation margin of 1 cm TACE group (n = 25): Chemotherapeutic drugs: 5‐fluorouracil 2 g and oxaliplatin 200 mg |
| Outcomes | Tumour response, according to RECIST criteria, measured at 1 year after treatment 1‐year survival rate and recurrence rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2018d.
| Methods | Study design: Randomised clinical trial Study duration: July 2013 to April 2014 Duration of follow‐up: 3 months Setting: Hospital |
| Participants | Age (mean ± SD, range): 52.6 ± 7.2 years, 31‐80 years Male (n/total): 49/86 Tumour diameter (mean ± SD, range): 3.8 ± 0.6, 3‐5 cm Child‐Pugh Class (patients): Class A: 52; Class B: 34 |
| Interventions | TACE + MWA group (n = 43): TACE: Chemotherapeutic drugs: 5‐fluorouracil, theprubicin, and epirubicin. Two sessions of TACE treatment were performed for each patient, with an interval of 4 weeks. MWA: The interval between TACE and RFA was 2 weeks. Output power of 60‐90 W TACE group (n = 43): Chemotherapeutic drugs: 5‐fluorouracil, theprubicin, and epirubicin. Two sessions of TACE treatment were performed for each patient, with an interval of 4 weeks. |
| Outcomes | Serum level of AST and ALT Serum level of AFP Tumour response, measured by ultrasound or CT, assessed by the reduction of tumour diameter Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2019.
| Methods | Study design: Randomised clinical trial Study duration: May 2011 to April 2014 Duration of follow‐up: 5 years Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 55. 04 ± 20. 13 years; TACE alone: 54. 56 ± 19. 49 years Male (n/total): TACE + RFA: 18/30; TACE alone: 22/30 Tumour diameter (mean ± SD, range): TACE + RFA: 3.78 ± 2.25 cm, 1.5‐6 cm; TACE: 3.81 ± 2.21 cm, 1.6‐6 cm Child‐Pugh Class (patients): Class A: TACE + RFA: 18; TACE: 20 Class B: TACE + RFA: 12; TACE: 10 |
| Interventions | TACE + RFA group (n = 30): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation RFA: The interval between TACE and RFA was 1‐2 weeks. RITA 1500X ablation system TACE group (n = 30): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation |
| Outcomes | Tumour response, according to WHO criteria, measured at 4 weeks after treatment 1‐, 2‐, 3‐, and 5‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhang 2020.
| Methods | Study design: Randomised clinical trial Study duration: January 2017 to May 2019 Setting: Hospital |
| Participants | Number of participants: 70 Inclusion criteria: Diagnosed as HCC; not invading into portal veins Age (mean ± SD): TACE + MWA: 61.44 ± 4.33 years; TACE alone: 61.38 ± 4.39 years Male (n/total): TACE + MWA: 23/35; TACE alone: 22/35 |
| Interventions | TACE + MWA group (n = 35): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 50 mg adriamycin and 10 mg hydroxy camptothecin MWA: The interval between TACE and MWA was 4 weeks. Output power of 50W Control group (n = 35): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 50 mg adriamycin and 10 mg hydroxy camptothecin |
| Outcomes | Immunological function and alpha fetoprotein: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhao 2014b.
| Methods | Study design: Randomised clinical trial Study duration: February 2007 to October 2010 Duration of follow‐up: 5 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by liver biopsy or images Exclusion criteria: With abnormal cardiac function; with serious mental diseases Age (mean ± SD): TACE + RFA: 57.3 ± 15.2 years; TACE alone: 56.5 ± 14.9 years Male (n/total): TACE + RFA: 22/40; TACE alone: 24/40 |
| Interventions | TACE + RFA group (n = 40): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g and theprubicin 20‐40 g RFA: CT‐guided RFA, 10 minutes per ablation session TACE group (n = 40): Chemotherapeutic drugs: 5‐fluorouracil 0.5‐1 g and theprubicin 20‐40 g |
| Outcomes | Tumour necrosis, measured by CT 1‐, 3‐, and 5‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhao 2015a.
| Methods | Study design: Randomised clinical trial Study duration: July 2014 to July 2015 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (range ): 31‐64 years Male (n/total): 63/120 |
| Interventions | TACE + RFA group (n = 60): TACE: Chemotherapeutic drugs: epirubicin 40 mg and oxaliplatin 100 mg RFA: The interval between TACE and RFA was 2 weeks. Cool‐tip ablation system. Ablation margin of 1 cm TACE group (n = 60): Chemotherapeutic drugs: epirubicin 40 mg and oxaliplatin 100 mg |
| Outcomes | Tumour necrosis rate, measured at 4 weeks after treatment by contrast‐enhanced CT 1‐, 2‐, and 3‐year disease progression rate |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhao 2016.
| Methods | Study design: Randomised clinical trial Study duration: January 2010 to December 2012 Duration of follow‐up: 29‐62 months Setting: Hospital |
| Participants | Age (mean ± SD): TACE + RFA: 65.89 ± 9.3 years; TACE alone: 66.85 ± 9.35 years Male (n/total): TACE + RFA: 37/ 47; TACE alone: 34/ 47 Child‐Pugh Class (patients): Class A: TACE + RFA: 45; TACE: 44 Class B: TACE + RFA: 2; TACE: 3 |
| Interventions | TACE + RFA group (n = 47): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.25‐1 g, theprubicin 30‐50 mg, and oxaliplatin 100‐150 mg RFA: The interval between TACE and RFA was 2 weeks. CT‐guided RFA. HGCF‐3000 ablation system, output power of 60 W TACE group (n = 47): Chemotherapeutic drugs: 5‐fluorouracil 0.25‐1 g, theprubicin 30‐50 mg, and oxaliplatin 100‐150 mg |
| Outcomes | 1‐, 3, and 5‐year survival rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhao 2018.
| Methods | Study design: Randomised clinical trial Study duration: February 2017 to April 2018 Duration of follow‐up: Not reported Setting: Hospital |
| Participants | Age (mean ± SD): TACE + MWA: 55.26 ± 1.24 years; TACE alone: 55.21 ± 1.25 years Male (n/total): TACE + MWA: 18/24; TACE alone: 17/24 |
| Interventions | TACE + MWA group (n = 24): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.75‐1 g, cisplatin 40‐60 mg, and mitomycin MWA: The interval between TACE and RFA was 21 days. CT‐guided MWA TACE group (n = 24): Chemotherapeutic drugs: 5‐fluorouracil 0.75‐1 g, cisplatin 40‐60 mg, and mitomycin |
| Outcomes | Tumour response, classified as obvious efficacy, moderate efficacy, and no efficacy; assessed by the symptoms of pain and poor appetite. |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhao 2020.
| Methods | Study design: Randomised clinical trial Study duration: August 2016 to July 2018 Duration of follow‐up: 1 year Setting: Hospital |
| Participants | Number of participants: 84 Inclusion criteria: Diagnosed as HCC; Child‐Pugh Class A or B; tumour diameter > 5 cm; unresectable tumour Age (mean ± SD): TACE + MWA: 44.31 ± 6.07 years; TACE alone: 45.62 ± 5.85 years Male (n/total): TACE + MWA: 27/42; TACE alone: 29/42 |
| Interventions | TACE + MWA group (n=42): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil, oxaliplatin, and epirubicin MWA: The interval between TACE and MWA was 1–2 weeks. Output power of 50–70 W Control group (n = 42): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation Chemotherapeutic drugs: 5‐fluorouracil, oxaliplatin, and epirubicin |
| Outcomes | Tumour response: measured by contrast‐enhanced CT at 1 month after treatment Recurrence: measured by CT at 1 year after treatment AFP: measured by blood testing |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zheng 2015.
| Methods | Study design: Randomised clinical trial Study duration: April 2012 to June 2014 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Age (mean ± SD, range ): 65.26 ± 2.68 years, 45‐80 years Male (n/total): 17/30 |
| Interventions | TACE + cryoablation group (n = 15): TACE: Chemotherapeutic drugs: oxaliplatin and adriamycin Cryoablation: The interval between TACE and RFA was 2‐3 weeks. CT‐guided cryoablation TACE group (n = 15): Chemotherapeutic drugs: oxaliplatin and adriamycin |
| Outcomes | Tumour response, classified as complete response, partial response, stable disease, and progression 3‐, 6‐, 9‐, 12‐, and 24‐month survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhu 2013b.
| Methods | Study design: Randomised clinical trial Study duration: March 2008 to June 2009 Duration of follow‐up: 3 years Setting: Hospital |
| Participants | Age (mean ± SD, range): TACE + MWA: 53.6 years, 27‐80 years; TACE alone: 51.2 years, 28‐79 years Male (n/total): TACE + MWA: 12/18; TACE alone: 11/18 |
| Interventions | TACE + MWA group (n = 18): TACE: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation A total of 2‐4 sessions of TACE were performed, with an interval of 3‐4 weeks. MWA: Output power of 50‐80 W, 4‐10 minutes per ablation session TACE group (n = 18): TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation. A total of 2‐4 episodes of TACE were performed, with an interval of 3‐4 weeks. |
| Outcomes | Serum level of AFP 1‐, 2‐, and 3‐year survival rates |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhu 2015.
| Methods | Study design: Randomised clinical trial Study duration: February 2003 to July 2015 Duration of follow‐up: 6 months Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC by pathology or images; no other serious diseases; Karnofsky score ≥ 60; life expectancy ≥ 6 months Exclusion criteria: With abnormal renal or liver function; with serious mental diseases Age (mean ± SD): TACE + RFA: 51.86 ± 9.43 years; TACE alone: 51.65 ± 9.55 years Male (n/total): TACE + RFA: 20/30; TACE alone: 22/30 Child‐Pugh Class (patients): Class A: TACE + RFA: 17; TACE: 18 Class B: TACE + RFA: 11; TACE: 9 Class C: TACE + RFA: 2; TACE: 3 |
| Interventions | TACE + RFA group (n = 30): TACE: Chemotherapeutic drugs: 5‐fluorouracil 0.5 g, oxaliplatin 100‐150 mg, and adriamycin 2.5 mg RFA: The interval between TACE and RFA was 2 weeks. Ablation margin of 1 cm TACE group (n = 30): Chemotherapeutic drugs: 5‐fluorouracil 0.5 g, oxaliplatin 100‐150 mg, and adriamycin 2.5 mg |
| Outcomes | Tumour response, according to WHO criteria 6‐month survival and recurrence rates Adverse events |
| Notes | Country of study: China
Source of funding: None There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
Zhuang 2016.
| Methods | Study design: Randomised clinical trial Study duration: November 2014 to April 2016 Duration of follow‐up: 2 years Setting: Hospital |
| Participants | Inclusion criteria: Diagnosed as HCC; with liver function of Child‐Pugh Class A or B; with normal renal function; life expectancy > 3 months Age (mean, range): 63 years, 34‐80 years Male (n/total): 54/70 |
| Interventions | TACE + cryoablation group (n = 35): TACE: Chemotherapeutic drugs: cisplatin 20‐50 mg and adriamycin 10‐40 mg Cryoablation: The interval between TACE and RFA was 2‐4 weeks. Multiple sessions of cryoablation can be performed with an interval of 4‐6 weeks. TACE group (n = 35): Chemotherapeutic drugs: cisplatin 20‐50 mg and adriamycin 10‐40 mg |
| Outcomes | 1‐month, 6‐month, 1‐year, and 2‐year response rate, measured by CT or DSA (DSA: digital subtraction angiography) |
| Notes | Country of study: China
Source of funding: Guangdong Province Science Project There was insufficient information available to satisfactorily determine the method of randomisation and the study data could not be verified. We have attempted to contact the study authors for more information, but so far, we have not been successful in doing this. |
AFP: alpha foetoprotein ALT: alanine aminotransferase BCLC B: Barcelona Clinic Liver Cancer Stage B (Intermediate Stage) CA199: carbohydrate antigen 19‐9 CEA: carcinoembryonic antigen CD(3+/4+/8+): lymphocytes CD3‐CD4‐CD8 ‐ whole blood (test) CT: computed tomography DBIL: direct bilirubin DSA: digital subtraction angiography ECOG: Eastern Cooperative Oncology Group ELISA: enzyme‐linked immunosorbent assay GGT: gamma‐glutamyl transferase HCC: hepatocellular carcinoma HIFU: high‐intensity focused ultrasound KPS: Karnofsky score mRECIST: modified Response Evaluation Criteria in Solid Tumors (guideline) MMP: matrix metalloproteinase MRI: magnetic resonance imaging MTI‐5DT: a tumor microwave therapy system MWA: microwave ablation NK: natural killer (cells) PET: positron emission tomography PHC: primary hepatic carcinoma PVTT: portal vein tumour trombosis QOL: quality of life RFA: radiofrequency ablation RFG(‐4): RET fused gene (RFG) SD: standard deviation SSC(‐Ag): squamous cell carcinoma TACE: transcatheter arterial chemoembolisation TBIL: total bilirubin (test) TGF‐β1: transforming growth factor beta 1 TNF‐α: tumour necrosis factor alpha TNM: Classification of Malignant Tumors standard for classifying the extent of spread of cancer (T: size or direct extent of the primary tumour; N: degree of spread to regional lymph nodes; M: presence of distant metastasis) VEGF: vascular endothelial growth factor yGT: gamma‐glutamyl transpeptidase (y‐GT) WHO: World Health Organization
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IOR‐16007915.
| Study name | Transarterial chemoembolization (TACE) with or without sequential cryoablation in hepatocellular carcinoma of BCLC B: a multicentral randomised controlled trial |
| Methods | Interventional study |
| Participants | Inclusion criteria: 1. CT, MRI, and AFP combined with clinical or pathological diagnosis of hepatocellular carcinoma 2. Prior informed consent; be able to follow the visit/treatment rules according to the research programmes 3. Generally in good condition, adequate bone marrow, liver and renal function 4. ECOG Performance Status of 0‐2 5. Advanced stage HCC/BCLC B stage 6. Hepatitis B history or HBsAg positive 7. Patients without previous surgery, local‐regional therapies (radiofrequency ablation, etc.), or liver transplantation 8. Aged 18 to 65 years 9. Child Pugh class A/B (=< 7) 10. Baseline laboratory tests meeting the following criteria: platelet count >= 100 * 10 ^ 9/L; ALT and AST < 3 * upper limit of normal; BUN and creatinine < 1.5 * upper limit of normal; INR < 1.5, or PT < 4 seconds above control. ALB >= 30g/L; total bilirubin =< 34 mmol/L Exclusion criteria: 1. Pregnant or lactating women 2. Extrahepatic metastasis, or tumour thrombosis invasive venous system 3. Any contraindications for hepatic embolisation procedures (known renal failure/insufficiency requiring hemo‐or peritoneal dialysis) 4. Patients with prior malignancy were ineligible for the study, with the exception of those who had been disease‐free for longer than 5 years 5. Patients with liver transplantation |
| Interventions | Group A: Transarterial chemoembolisation Group B: Transarterial chemoembolisation (TACE) with sequential cryoablation |
| Outcomes | 3‐year overall survival rate Postoperative complication Time to progression Distant metastasis rate |
| Starting date | 2016‐04‐01 |
| Contact information | |
| Notes |
ChiCTR‐IOR‐17013743.
| Study name | Treatment of BCLC medium‐term hepatocellular carcinoma (HCC) of randomised controlled study of conventional TACE sequential microwave ablation (MWA) and conventional TACE alone |
| Methods | Parallel study |
| Participants | Subjects who meet the following criteria can be included in this study:
1. Gender, age 18‐70 (inclusive)
2. HCC meets the clinical diagnostic criteria of AASLD or EASL
3. Subjects were not willing to have surgical excision
4. BCLC medium liver cancer, numbers of tumours less than 5, maximum diameter is less than 10 cm
5. Good liver reserve function (Child‐Pugh A level)
6. The ECOG score is 0. Exclusion criteria: 1. Waiting for surgical resection or liver transplantation 2. Visible portal vein, hepatic vein, bile duct invasion 3. Extrahepatic metastasis 4. History of liver cancer treatment (transplantation, excision, TACE, ablation, radiotherapy) 5. Uncorrected clotting disorder (platelet count 50 x 109/L or thrombin activity < 50%) 6. Normal blood flow results in normal range 7. Unstable coronary artery disease or recent myocardial infarction (within 1 year) 8. Echocardiography of left ventricular ejection fraction < 50% 9. Active infection requiring antibiotic treatment 10. Current anticoagulation therapy or have known haemorrhagic disease 11. Renal insufficiency (serum creatinine > 176.8 mu mol/L) 12. AST and/or ALT > 3 times normal limit 13. Pregnant or lactating subjects, women of childbearing age receiving a serum pregnancy test within 72 hours of the start of treatment 14. Allergy to known iodine contrast agents |
| Interventions | Experimental group: TACE + MWA Control group: TACE |
| Outcomes | Safety and efficacy |
| Starting date | 2017‐12‐06 |
| Contact information | zhengjiasheng6@163.com |
| Notes |
NCT02301091.
| Study name | Combine TACE and RFA versus TACE alone for HCC with PVTT (CORTT) |
| Methods | Interventional (clinical trial) |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: TACE‐RFA 2 sessions of TACE first, RFA for residual viable tumours and PVTT within 1 month Active Comparator: TACE alone Repeated TACE and 1 to 2 months interval between two sessions of TACE |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | October 2014 |
| Contact information | Ming Zhao, doctor +86 020 87343272zhaoming@sysucc.org.cn |
| Notes |
NCT02435953.
| Study name | TACE + RFA versus TACE alone for intermediate‐stage hepatocellular carcinoma |
| Methods | Experimental: TACE‐RFA 1‐2 sessions of TACE treatment, then followed by RFA treatment |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: TACE‐RFA 1‐2 sessions of TACE treatment, then followed by RFA treatment Active Comparator: TACE alone TACE treatment several times until tumour progresses to advanced stage |
| Outcomes | Primary outcome measures:
Secondary outcome measures :
|
| Starting date | April 2015 |
| Contact information | Contact: Ming Zhao, doctor +86 020 87343272, zhaoming@sysucc.org.cn Contact: Tao Pan, doctor +862087343271, pantao0909@hotmail.com |
| Notes |
NCT02646137.
| Study name | Single session combined locoregional therapies for hepatocellular carcinoma |
| Methods | Allocation: Randomised Intervention model: Parallel assignment Masking: None (open‐label) |
| Participants | Inclusion Criteria: Child classification A or B Serum albumin ≥ 3 gm/L Serum bilirubin < 2.5 mg/dL Platelet count ≥ 70,000 mm3 INR ≤ 1.6 Serum creatinine < 2 mg/dL Tumour size more than 4 cm and confined to one lobe of the liver Exclusion Criteria: Patients with portal vein thrombosis A technically inaccessible hepatic artery Metastatic HCC More than three lesions Lesions in close proximity to the portal vein, inferior vena cava, or gallbladder were excluded from the study. |
| Interventions | (1) Active comparator: Transarterial chemoembolisation (TACE) (2) Experimental: Radiofrequency ablation with TACE (3) Experimental: Microwave ablation combined with TACE |
| Outcomes | Number of patients with successful ablation [time frame: 3 months] |
| Starting date | January 2015 |
| Contact information | Sherief Abd‐Elsalam 00201000040794, mailto:Sherif_tropical%40yahoo.com?subject=NCT02646137, Combined TACE and RF, Single Session Combined Locoregional Therapies for Hepatocellular Carcinoma |
| Notes | None |
AASLD: American Association for the Study of Liver Diseases AFP: alpha foetoprotein ALB: albumin ALT: alanine aminotransferase AST: aspartate transaminase BCLC B: Barcelona Clinic Liver Cancer Stage B (Intermediate Stage) BUN: blood area nitrogen CORTT: abbreviation in study name not written out anywhere in the text record CT: computer tomography EASL: European Association for the Study of the Liver ECOG: Eastern Cooperative Oncology Group HBsAg: hepatitis B surface antigen HCC: hepatocellular carcinoma INR: international normalised ratio IVC: inferior vena cava MRI: magnetic resonance imaging MWA: microwave ablation PT: prothrombin time PV: portal vein PVTT: portal vein tumour thrombosis RFA: radiofrequency ablation TACE: transarterial chemoembolisation
Differences between protocol and review
Background We added a sentence describing the five main thermal ablation techniques to help the reader understand the experimental intervention of this review.
Methods We increased the clarity of the Methods section by rewriting existent information and adding further information in conformity with the latest Cochrane Handbook (Higgins 2021). The main changes are as follows.
In Outcomes, we added a sentence: “We planned to base our primary conclusions on the outcome results at the longest follow‐up. We planned to include trials regardless of whether they reported on our outcomes of interest.”
We moved the planned exploratory analysis on mortality regarding the intervention effect at one, three, and five (primary time point) years, to Sensitivity analysis.
We merged the exploratory outcome 'Duration of hospital stay' with the secondary outcomes, as exploratory outcomes are no longer encouraged by the Cochrane Handbook.
We replaced the text on RoB1 with RoB2, as the RoB2 tool was recommended at the time we started our work on the review (Higgins 2019a).
We extracted data on trial registration and ethics committee approval.
We updated the text in Unit of analysis issues, adding further details.
We added that we planned to repeat our analyses with the fixed‐effect model.
We moved the text on Trial sequential Analysis to Sensitivity analysis, and we planned to use it for assessment of imprecision.
We reduced the number of planned subgroup analyses from six to two, following comments from the network editor. The argument was that subgroup analyses are observational in nature and, therefore, they should be justified carefully, and their number limited. We have carefully discussed this issue, and we re‐evaluated the significance of the original six subgroup analyses, listed in the published protocol. We reached consensus that the significance of performing subgroup analyses based on the risk of bias and the ablation method was the most important.
We elaborated text in Summary of findings tables and assessment of the certainty of evidence to ease the readers' understanding.
Contributions of authors
Formulated the research question: WL Drafted the protocol: BZL and WL
Drafted the full text of this review: BZL, WL, YCZ, and ET Provision of statistical expert opinion: HC
All authors approved the publication of the current review version.
Sources of support
Internal sources
-
Capital's Funds for Health Improvement and Research, China
ID: CFH 2020‐2‐2175
External sources
-
The Cochrane Hepato‐Biliary Group, Denmark
The Cochrane Hepato‐Biliary Group (the CHBG) is one of the 53 international research publishing groups within Cochrane (formerly The Cochrane Collaboration).
Declarations of interest
BZL has no known conflicts of interest. HC has no known conflicts of interest. WL has no known conflicts of interest. ET has no known conflicts of interest.
New
References
References to studies excluded from this review
Chen 2014 {published data only}
- Chen L, Li D, Huang J, Li W, Gao Z. Comparative study of radiofrequency ablation and transcatheter arterial chemoembolization in hepatocellular carcinoma [超声引导单极冷循环射频消融联合TACE治疗肝癌的对比研究]. Zhong Guo Chao Sheng Yi Xue Za Zhi [Chinese Journal of Ultrasound in Medicine] 2014;30(3):238-42. [Google Scholar]
Chen 2015 {published data only}
- Chen W. The combination of transcatheter arterial chemoembolisation and ultrasound ablation for primary liver cancer [高强度聚焦超声联合动脉栓塞化疗治疗中晚期原发性肝癌]. In: Di Si Jie Quan Guo Xiao Hua Dao E Xing Bing Bian Yan Tao Hui Lun Wen Ji [Biomedical Engineering Meeting in China]. Vol. 735. 2015:91-2.
Hou 2017 {published data only}
- Hou P, Chen Y, Liu W, Jiao L, Yang J. Clinical effects of ultrasound-guided percutaneous microwave ablation and transcatheter arterial chemoembolization for primary liver cancer [超声引导下经皮微波消融与肝动脉化疗栓塞治疗原发性肝癌的临床效果.]. Shi Yong Ai Zheng Za Zhi [Practical Journal of Cancer] 2017;32(10):1667-70. [Google Scholar]
Hu 2010 {published data only}
- Hu Z, Peng Y, Li Y, Chen S, Zeng X, Huang Q, et al. Therapeutic effect of radio-frequency ablation and transcatheter arterial chemoembolization for the treatment of liver cancer [射频消融与肝动脉栓塞化疗对肝癌疗效的临床分析]. Zhong Hua Lin Chuang Xin Yi Xue [Chinese Journal of New Clinical Medicine] 2010;3(9):823-6. [Google Scholar]
Huang 2015b {published data only}
- Huang S, Chen S, Zhang T, Li J. The huge liver cancer treated by the combination of TACE and microwave ablation [巨大肝癌肝动脉栓塞化疗联合微波消融的临床分析]. Chong Qing Yi Xue [Chongqing Medicine] 2015;44(36):5149-52. [Google Scholar]
Kong 2015 {published data only}
- Kong Y. The short-term efficacy of the combination of transcatheter arterial chemoembolisation with radiofrequency ablation [肝癌患者行TACE联合RFA治疗的近期疗效及其对肿瘤局部复发的影响.]. Zhong Guo Shi Yong Yi Yao [China Practical Medical] 2015;19:170-1. [Google Scholar]
Li 2013a {published data only}
- Li P. Clinical study of TACE combined with high-intensity focused ultrasound in the treatment of stage Ⅲ and Ⅳ liver cancer [TACE联合高强度聚焦超声治疗Ⅲ、Ⅳ期肝癌的临床研究]. cdmd.cnki.com.cn/Article/CDMD-10114-1013223852.htm (date accessed prior to 2 Dec 2021).
Li 2013b {published data only}
- Li P, Zhou D, Lv W, Zhao Q, Li C. Clinical study of TACE in combination with high intensity focused ultrasound therapy for advanced stage hepatocellular carcinoma [TACE联合高强度聚焦超声治疗中晚期肝癌的临床研究]. Zhong Guo Xian Dai Pu Tong Wai Ke Jin Zhan [Chinese Journal of Current Advances in General Surgery] 2013;16(11):870-4. [Google Scholar]
Li 2015b {published data only}
- Li Y, Ao G, Ma W, Wang H, An W. Transarterial chemoembolization plus radiofrequency ablation for middle- and advanced- stage hepatocellular carcinoma [肝动脉栓塞联合射频消融治疗中晚期肝癌效果观察]. Ren Min Jun Yi [People's Military Surgeon] 2015;58(1):60-2. [Google Scholar]
Liu 2007 {published data only}
- Liu Y, Zheng X, Wei Y, Lin S. Transarterial chemoembolization plus cryoablation for liver cancer [TACE联合氩氦刀冷冻治疗HCC疗效分析]. Shan Dong Yi Yao [Shandong Medical Journal] 2007;47(20):36-7. [Google Scholar]
Liu 2015 {published data only}
- Liu J, Pan W, Chen C. The efficacy of the radiofrequency ablation plus TACE for primary liver cancer [射频消融联合经导管肝动脉栓塞化疗治疗原发性肝癌的疗效]. Chinese Journal of Modern Drug Application 2015;9:62-3. [Google Scholar]
Liu 2016b {published data only}
- Liu S. Analysis of the artery embolism chemotherapy with radiofrequency ablation treatment of primary liver cancer [动脉栓塞化疗联合射频消融治疗原发性肝癌临床疗效分析]. He Bei Yi Xue [Hebei Medicine] 2016;22(7):1109-11. [Google Scholar]
Lu 2015 {published data only}
- Lu A. Radio-interventional treatment for the improvement of qulaity of life in patients with hepatocellular carcinoma [放射介入治疗对中晚期肝癌患者生活质量的改善作用研究]. Yi Yao Qian Yan [Yiayao Qianyan] 2015;5(21):156-8. [Google Scholar]
Sha 2012 {published data only}
- Sha Q. The clinical efficacy of transcatheter arterial chemoembolisation plus radiofrequency ablation for liver cancer [肝动脉化疗栓塞联合射频消融治疗中晚期肝癌的临床疗效]. Zhong Liu Ji Chu Yu Lic Chuang [Journal of Basic and Clinical Oncology] 2012;6:498-500. [Google Scholar]
Wang 2011 {published data only}
- Wang X, Fu S. The combination of radiofrequency ablation with transarterial chemoembolization for liver cancer [射频消融联合肝动脉栓塞术治疗中晚期肝癌的疗效观察]. Qi Qi Ha Er Yi Xue Bao [Journal of Qiqihar University of Medicine] 2011;32(18):2957-8. [Google Scholar]
Wang 2016a {published data only}
- Wang H. The combination of transarterial chemoembolization with cryoablation in 33 patients with liver cancer [TACE 联合氩氦刀冷冻消融治疗中晚期原发性肝癌 33 例临床效果评价]. She Qu Yi Xue Za Zhi [Journal of Community Medicine] 2016;14(22):68-9. [Google Scholar]
Wu 2006 {published data only}
- Wu P. The efficacy of the combination of CT-guided ablation with transcatheter arterial chemoembolization, presented at the 1st Chinese Symposium on Minimally Iinvasive Cancer Therapy and the Founding Meeting of Minimally Invasive Therapy Committee of Chinese Anti-Cancer Association. April 2005. In: The 1st Interventional Treatment Meeting in Henan. Guangzhou, 2006.
Wu 2011a {published data only}
- Wu L, Li Z. TACE combined with the argon-helium cryoablation treatment of liver cancer [磁共振手术系统氩氦刀消融与TACE协同治疗无手术指证原发性肝癌的临床分析]. In: China (Seventh) Academic Conference on Minimally Invasive Tumor Therapy; 2011 Sep 23; Guangzhou, Guangdong, China. 2011:343-6.
Wu 2017a {published data only}
- Wu L, Shen F. Clinical effects of combination of microwave ablation and chemoembolization in treating hepatocellular carcinoma with portal vein tumor thrombosis [微波消融联合化疗栓塞治疗肝细胞肝癌伴门脉癌栓的临床疗效]. Zhong Guo Lin Chuang Yi Xue [Chinese Journal of Clinical Medicine] 2017;24(2):272-6. [Google Scholar]
Xiang 2007 {published data only}
- Xiang H, Long L, Zhang Z, Liu J, Fang Z, Sun L, et al. The combined interventional treatments for huge hepatocellular carcinoma [联合介入治疗大肝癌的临床疗效分析]. Nan Hua Da Xue Xue Bao [Journal Of Nanhua University] 2007;35(5):761-3. [Google Scholar]
Yang 2016 {published data only}
- Yang X, Zhang X, Liu H, Song W, Gao H. The curative effect and prognostic analysis of transcatheter arterial chemoembolization combined with radiofrequency ablation in treatment of liver cancer [肝动脉化疗栓塞联合射频消融治疗肝癌疗效及预后分析]. Zhong Guo Ji Ceng Yi Yao [China Basic Medicine] 2016;23(17):2609-12. [Google Scholar]
References to studies awaiting assessment
Ai 2019 {published data only}
- Ai Z, Cheng Y, Zhang X. A clinical study on transcatheter arterial chemoembolization combined with radiofrequency ablation in treatment of Barcelona Clinic liver cancer stage B hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融术治疗巴塞罗那B期肝癌的临床研究]. Academic Journal of Guangzhou Medical College 2019;47(4):17-9, 26. [Google Scholar]
An 2017 {published data only}
- An XY. The efficacy of the combination of TACE with microwave ablation for huge hepatocellular carcinoma [TACE联合微波消融治疗对巨大肝癌患者的疗效探究]. Lin Chuang Yi Yao Wen Xian Za Zhi [Journal of Clinical Medical Literature] 2017;4(3):420-1. [Google Scholar]
Bao 2020 {published data only}
- Bao X. The effect of transhepatic artery chemoembolization combined with radiofrequency ablation in the treatment of advanced primary liver cancer [经肝动脉化疗栓塞联合射频消融术治疗中晚期原发性肝癌的效果]. Medical Information 2020;33(19):110-2. [Google Scholar]
Bian 2020 {published data only}
- Bian J, Da T, Luo J, Zhou Q, Jiang Y, Wu T. Ultrasound-guided percutaneous microwave ablation combined with transarterial arterial chemoembolization for primary hepatocellular carcinoma [超声引导下经皮微波消融联合肝动脉化疗栓塞治疗原发性肝癌的疗效观]. Journal of Chinese Practical Diagnosis and Therapy 2020;34(5):509-12. [Google Scholar]
Chen 2020 {published data only}
- Chen J, Liang Y, Lin J, Yang J. The combination of TACE with CT-guided radiofrequency for small liver cancer [肝动脉化疗栓塞术联合CT引导下经皮射频消融治疗小肝癌的临床观察]. Modern Medical Imageology 2020;29(3):567-9. [Google Scholar]
Cui 2015 {published data only}
- Cui H, Zhao H, Wu Y, Dong Q, Zhang X. Observation of the efficacy of the combination of TACE with radiofrequency for hepatocellular carcinoma [TACE 联合 RFA 治疗原发性肝癌的临床效果观察]. Lin Chuang He Li Yong Yao [Chinese Journal of Clinical Rational Drug Use] 2015;19:130-1. [Google Scholar]
Cui 2017 {published data only}
- Cui M. Results observation of primary liver cancer treatment by transcatheter arterial chemoembolization combined radio frequency ablation [肝动脉化疗栓塞联合射频消融治疗原发性肝癌效果观察]. She Qu Yi Xue Za Zhi [Journal of Community Medicine] 2017;4:3-5. [Google Scholar]
Dan 2014 {published data only}
- Dan XG. The efficacy of intervential treatments for hepatocellular carcinoma [介入治疗原发性肝癌临床分析]. Zhong Guo Xian Dai Yao Wu Ying Yong [Chinese Journal of Modern Drug Application] 2014;8(14):27-8. [Google Scholar]
Ding 2017 {published data only}
- Ding M, Tan Y, He G, Han D, Wen W, Qiu J, et al. The efficacy of radiofrequency ablation for hepatocellular carcinoma [射频消融术在中晚期肝癌中的应用效果]. Lin Chuang He Li Yong Yao [Chinese Journal of Clinical Rational Drug Use] 2018;11(9):123-4. [Google Scholar]
Dong 2013 {published data only}
- Dong YS, Zhang Z, Yang X. The clinical efficacy of the combination of TACE with RFA for HCC [肝动脉化疗栓塞联合射频消融治疗大肝癌的临床疗效]. Pu Tong Wai Ke Jin Zhan [Chinese Journal of Current Advances in General Surgery] 2013;16(6):486-8. [Google Scholar]
Dong 2018 {published data only}
- Dong H. Clinical efficacy of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of hepatocellular carcinoma [肝动脉化疗栓塞术联合射频消融术治疗肝细胞癌患者的临床疗效观察]. Zhong Guo Shi Yong Yi Yao [China Practical Medical] 2018;13(6):25-7. [Google Scholar]
Du 2017 {published data only}
- Du X. Effect analysis of transcatheter arterial chemoembolization combined with radiofrequency catheter ablation on primary hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融术治疗原发大肝癌的疗效分析]. Lin Chuang Yi Xue [Clinical Research and Practice] 2017;2(25):15-6. [Google Scholar]
Fan 2016 {published data only}
- Fan L. The combination of transcatheter arterial chemoembolisation with radiofrequency ablation for hepatocellular carcinoma [经肝动脉化疗栓塞术联合射频消融术治疗原发性肝癌的临床疗效]. Lin Chuang He Li Yong Yao [Chinese Journal of Clinical Rational Drug Use] 2017;9(36):113-4. [Google Scholar]
Fang 2016 {published data only}
- Fang A. The clinical efficacy of the combnation of transcatheter arterial chemoembolisation with radiofrequency ablation for hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融治疗肝癌疗效的观察]. Yi Xue Li Lun Yu Shi Jian [Journal of Medical Theory and Practice] 2016;2:197-8. [Google Scholar]
Fang 2020 {published data only}
- Fang L, Ding J, Luo G, Cheng S, Zheng Y, Wang C. TACE plus microwave ablation for large and huge hepatocellular carcinoma [TACE联合微波消融同步治疗大肝癌和巨块型肝癌的临床疗效及预后分析]. World Latest Medicine Information 2020;20(19):156, 158. [Google Scholar]
Geng 2020 {published data only}
- Geng X, Li C, Xu H. Effect of TACE combined with MWA on liver cancer and its influence on serum tumour markers [TACE联合MWA治疗肝癌的效果及对血清肿瘤标志物的影响]. Clinical Research and Practice 2020;5(33):76-7. [Google Scholar]
Gong 2019 {published data only}
- Gong A, Tan Y, Luo X. The clinical efficacy of microwave ablation plus TACE on hepatocellular carcinoma [微波消融术联合肝动脉化疗栓塞术对中晚期肝癌的疗效及对免疫功能的影响]. Zhejiang Practical Medicine 2019;24(6):405-7, 430. [Google Scholar]
Guo 2015c {published data only}
- Guo J, Cao M. The assessment of efficacy and safety of the combination of transcatheter arterial chemoembolisation with high intensity focused ultrasound ablation [高强度聚焦超声在经肝动脉化疗栓塞治疗中的效果及安全性分析]. Zhong Guo Xian Dai Pu Tong Wai Ke Jin Zhan [Chinese Journal of Current Advances in General Surgery] 2015;1:76-9. [Google Scholar]
He 2016 {published data only}
- He Z, Liu Y, Wen J, Zhang Z, He W, Chen H. Clinical application of hepatic arterial chemoembolization combined with percutaneous microwave coagulation therapy for primary massive liver cancer [肝动脉化疗栓塞联合微波消融治疗原发性巨块型肝癌的疗效]. Yi Xue Lin Chuang Yan Jiu [Journal of Clinical Research] 2016;33(7):1348-50. [Google Scholar]
He 2020 {published data only}
- He X. The clincal effect of the combination of TACE with RFA for hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融治疗原发性肝癌患者的效果]. Medical Journal of Chinese People’s Health 2020;32(9):16-8. [Google Scholar]
Hou 2013 {published data only}
- Hou X, Song Q, Li L, Zhuang X. The efficacy of primary hepatic carcinoma treated by transcatheter arterial chemoembolisation combined with argon-helium cryoablation [TACE 联合氩氦冷冻消融治疗原发性肝癌效果观察]. Shi Yong Yi Yao Za Zhi [Practical Journal of Medicine & Pharmacy] 2013;30(4):293-5. [Google Scholar]
Hua 2020 {published data only}
- Hua L, Zhang T, Hua L. The observation on the clinical efficacy of the combination of TACE with radiofrequency ablation [肝动脉化疗栓塞联合射频消融术治疗原发性大肝癌的效果观察与护理]. Journal of Clinic Nursing's Practicality 2020;5(29):26, 41. [Google Scholar]
Huang 2013 {published data only}
- Huang Y, Xu Q, Shen T, Shi H. Clinical efficacy and safety of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of advanced hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融治疗中晚期肝癌的疗效评价]. Hai Nan Yi Xue [Hainan Medical Journal] 2013;24(24):3630-2. [Google Scholar]
Huang 2015a {published data only}
- Huang Q. The application value of hepatic artery interventional embolization combined with radiofrequency ablation for the recurrence of small liver cancer after surgery [动脉介入栓塞联合射频消融术在小肝癌术后复发中的应用价值]. Lin Chuang Yi Yao Shi Jian [Proceeding of Clinical Medicine] 2015;6:408-11. [Google Scholar]
Huang 2016 {published data only}
- Huang L, Shang H, Xu G. The assessment of clinical efficacy on the combination of transcatheter arterial chemoembolisation with radiofrequency ablation [肝动脉化疗栓塞和射频消融联合治疗中晚期肝癌的可行性研究]. Dang Dai Yi Xue [Contemporary Medicine] 2016;22(13):63-4. [Google Scholar]
Huang 2017a {published data only}
- Huang Q, Liu H, Xu J, Ni Y, Yang Z. The combination of high intensity focused ultrasound ablation and transcatheter arterial chemoembolisation for hepatocellular carcinoma [TACE, 热学疗法联合治疗肝癌的临床疗效分析]. Da Jia Jian Kang [For All Health] 2017;11(8):119-20. [Google Scholar]
Huang 2017b {published data only}
- Huang Y. The clinical efficacy of transcatheter arterial chemoembolisation plus radiofrequency ablation for hepatocellular carcinoma [经肝动脉化疗栓塞术与经皮穿刺射频消融治疗原发性肝癌的临床效果]. Zhong Guo Shi Yong Yi Kan [Chinese Journal of Practical Medicine] 2017;44(13):92-4. [Google Scholar]
Huang 2019 {published data only}
- Huang Y, Xu F, Zhong L. Effects of transcatheter arterial chemoembolization combined with radiofrequency ablation on cellular immune function in patients with hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融对肝癌患者细胞免疫功能的影响]. Chinese Journal of General Practice 2019;17(9):1521-3, 1614. [Google Scholar]
Jiang 2015a {published data only}
- Jiang X. The combination of transcatheter arterial chemoembolisation with radiofrequency ablation for hepatocellular carcinoma [射频消融和肝动脉栓塞化疗治疗原发性肝癌的效果比较]. Dang Dai Yi Xue [Contemporary Medicine] 2015;12:71-2. [Google Scholar]
Jiang 2015b {published data only}
- Jiang X, Cui H, Zhang J, Yang H, Zhang C. Effects of transcatheter arterial chemoembolisation combined with radiofrequency ablation for hepatocellular carcinoma and prognostic factors analysis [TACE联合RFA治疗无法手术的肝细胞癌疗效及预后分析]. Zhong Liu Yao Xue [Anti-Tumor Pharmacy] 2015;5:379-83. [Google Scholar]
Jiang 2017 {published data only}
- Jiang FQ, Lu W, Yang C, Du P, Ma JP, Yang J, et al. Curative effect of transcatheter arterial chemoembolization combined with radiofrequency ablation in treating hepatic cell carcinoma and its effect on serum markers. Cancer Biomarkers 2017;20(1):17-22. [DOI] [PubMed] [Google Scholar]
Jiang 2018 {published data only}
- Jiang B, Li H. Effects of transcatheter arterial chemoembolisation combined with MCT on vascular endothelial growth factor and T cell subsets in patients with primary liver cancer [TACE 联合 MCT 治疗对原发性肝癌患者血管内皮生长因子和T 细胞亚群的影响]. Liao Ning Yi Xue Za Zhi [Medical Journal of Liaoning] 2018;32(1):31-4. [Google Scholar]
Jin 2003 {published data only}
- Jin CB, Wu F, Wang ZB, Chen WZ, Zhu H. [High intensity focused ultrasound therapy combined with transcatheter arterial chemoembolization for advanced hepatocellular carcinoma] [高强度聚焦超声联合动脉栓塞化疗治疗晚期肝癌的初步临床研究]. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] 2003;25(4):401-3. [PMID: ] [PubMed] [Google Scholar]
Lai 2019 {published data only}
- Lai X, Ye J, Zeng J, Huang C, Wang S. The effect of the combination of TACE with MWA on the immunological function in patients with primary liver cancer [联合肝动脉栓塞化疗与微波消融对原发性肝癌患者免疫功能的影响]. Contemporary Medicine 2019;25(34):134-6. [Google Scholar]
Li 2012 {published data only}
- Li Y, Ma C, Ma W, Long J. Therapeutic efficacy of high-intensity focused ultrasound plus hepatic artery chemoembolization in patients with hepatocellular carcinoma [肝癌患者高强度聚焦超声联合肝动脉栓塞化疗的临床疗效观察]. Zhong Guo Yi Xue Zhuang Bei [China Medical Equipment] 2012;9(12):94-6. [Google Scholar]
Li 2015 {published data only}
- Li D, Xiang H, Zhang Z, Liu J, Fang Z, Long L, et al. The changes of VEGF level in patients with hepatocellular carcinoma after argon-helium cryoablation combined with transcatheter arterial chemoembolization treatment [肝细胞肝癌氩氦刀联合肝动脉介入治疗前后血清 VEGF 的变化]. Jie Ru Fang She Xue Za Zhi [Journal of Interventional Radiology] 2015;24(5):400‐3. [Google Scholar]
Li 2016a {published data only}
- Li B. The clinical effect of the combination of transcatheter arterial chemoembolisation with radiofrequency ablation for hepetocellular carcinoma [肝动脉化疗栓塞术联合射频消融治疗肝癌的临床观察]. Zhong Guo Shi Yong Yi Yao [China Practical Medical] 2016;31:88-9. [Google Scholar]
Li 2016b {published data only}
- Li X, Lv H. The efficacy observation of transcatheter arterial chemoembolization combined with radiofrequency ablation for primary liver cancer [肝动脉化疗栓塞联合射频消融治疗原发性肝癌的疗效观察]. Ji Ceng Yi Xue Lun Tan [Medical Forums in Basic] 2016;20(10):1322-3. [Google Scholar]
Li 2017a {published data only}
- Li J. The clinical effect of the combination of transcatheter arterial chemoembolisation with cryoablation for hepetocellular carcinoma [肝动脉栓塞化疗结合氩氦刀治疗中晚期肝癌的临床疗效]. Dang Dai Yi Xue [Contemporary Medicine] 2017;23:104-6. [Google Scholar]
Li 2017b {published data only}
- Li S. The effect of the combination of transcatheter arterial chemoembolisation with microwave ablation for hepatocellular carcinoma [肝动脉导管化疗栓塞联合CT引导精准微波消融治疗原发性肝癌的效果观察]. Bai Qiu En Yi Xue Za Zhi [Journal of Bethune Military Medical College] 2017;16(6):753-5. [Google Scholar]
Li 2017c {published data only}
- Li X, Zhang H. The quality of patients with hepatocellular carcinoma treated with intervention treatments [放射介入治疗对中晚期肝癌患者生活质量的改善效果]. Shan Xi Yi Xue Za Zhi [Shanxi Medical Journal] 2017;46(9):1237-8. [Google Scholar]
Li 2018 {published data only}
- Li W, Chen J, Zhao S. Improvement of the efficacy of patients with primary hepatic carcinoma by TACE combined with RFA [TACE联合RFA疗法可有效提高原发性肝癌患者的疗效]. Ji Yin Zu Xue Yu Ying Yong Sheng Wu Xue [Genomics and Applied Biology] 2018;37(1):109-14. [Google Scholar]
Li 2019a {published data only}
- Li H. Clinical study of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of hepatocellular carcinoma [肝动脉介入栓塞化疗联合射频消融治疗肝癌的临床效果]. He Ze Yi Xue [Journal of Heze Medical College] 2019;31(1):45-7, 57. [Google Scholar]
Li 2020 {published data only}
- Li X. TACE plus MWA for hepatic cancer and the level of AFP [动脉栓塞化疗联合微波消融治疗肝癌的临床疗效及对血清AFP水平的影响]. Clinical Research 2020;28(10):80-1. [Google Scholar]
Lin 2017 {published data only}
- Lin H. The efficacy of the combination of TACE with radiofrequency ablation for advanced liver cancer [肝动脉栓塞配合射频消融对中晚期肝癌的治疗作用评价]. Te Bie Jian Kang [Special Health] 2017;17:63. [Google Scholar]
Liu 2016a {published data only}
- Liu J. The efficacy and safety of the combinaiton of transcatheter arterial chemoembolisation with microwave ablation for hepatocellular carcinoma [肝动脉化疗栓塞联合微波消融治疗原发性肝癌的临床疗效观察]. Zhong Guo Gao Deng Yi Xue Jiao Yu [China Higher Medical Education] 2016;10:135-6. [Google Scholar]
Liu 2017 {published data only}
- Liu Q, Yu G, Yang G, Zhang Q, Qiu F, Li C, et al. Clinical efficacy and safety of cool-tip microwave ablation combining transcatheter arterial chemoembolization in treatment of patients with primary liver cancer [冷循环微波消融联合肝动脉灌注化疗栓塞治疗原发性肝癌患者疗效及安全性研究]. Shi Yong Gan Zang Bing Za Zhi [Journal of Practical Hepatology] 2017;20(3):316-9. [Google Scholar]
Liu 2018b {published data only}
- Liu D. The effect of the combination of transcatheter arterial chemoembolisation and microwave ablation on serum biomarkers of hepatocellular carcinoma [经动脉导管灌注化疗栓塞术联合微波消融术治疗对肝癌血清标志物的影响]. Gui Zhou Yi Yao [Guizhou Medical Journal] 2018;42(5):567-8. [Google Scholar]
Liu 2019b {published data only}
- Liu L. The analysis on the efficacy of the combination of TACE with CT-guided RFA in patients with primary liver cacner [肝动脉化疗栓塞术联合CT引导下射频消融术对原发性肝癌患者治疗效果分析]. Journal of Imaging Research and Medical Applications 2019;3(14):211-2. [Google Scholar]
Liu 2019c {published data only}
- Liu L. The analysis on the efficacy of TACE plus CT-guided ablation on primary cancer [肝动脉化疗栓塞术联合CT引导下射频消融术对原发性肝癌患者治疗效果分析]. Journal of Imaging Research and Medical Applications 2019;3(14):211-2. [Google Scholar]
Liu 2019d {published data only}
- Liu Q. The comparison on the clinical efficacy between TACE plus MWA and TACE [Tace联合微波消融与单纯tace治疗晚期肝癌的临床疗效对比]. Yiyao Qianyan 2019;9(31):82-3. [Google Scholar]
Liu 2020 {published data only}
- Liu S, Zhong Y, Peng H. The clinical efficacy of the combination of TACE with ultrasound-guided microwave ablation [肝动脉化疗栓塞TACE联合超声引导下微波消融治疗特殊部位肝癌疗效观察]. Guizhou Medical Journal 2020;44(2):212-4. [Google Scholar]
Lu 2018 {published data only}
- Lu C. The clinical effect of the combination of transcatheter arterial chemoembolisation with radiofrequency ablation in hepatocellular carcinoma [经肝动脉化疗栓塞术与经皮穿刺射频消融治疗原发性肝癌的临床效果]. Ji Lin Yi Xue [Jilin Medical Journal] 2018;39(8):1553-4. [Google Scholar]
Luo 2019 {published data only}
- Luo Y, Jiang Y. Comparison of efficiency of TACE plus HIFU and TACE alone on patients with primary liver cancer. Journal of the College of Physicians and Surgeons - Pakistan 2019;29(5):414-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lv 2016 {published data only}
- Lv S. The efficacy and safety or transcatheter arterial chemoembolisation plus radiofrequency in hepatocellular carcinma [TACE联合射频消融术治疗原发性肝癌的临床分析]. Lin Chuang Yi Yao Dian Zi Wen Xian Za Zhi [Journal of Clinical Medical Literature] 2016;3(52):10366-7. [Google Scholar]
Ma 2017 {published data only}
- Ma N. Efficacy observation of chemoembolization combined with radio-frequency ablation in the treatment of hepatocellular carcinoma [化疗栓塞术联合射频消融治疗肝癌的疗效观察]. Lin Chuang Yi Xue Yan Jiu Yu Shi Jian [Clinical Research and Practice] 2017;2(34):30-2. [Google Scholar]
Ma 2019a {published data only}
- Ma H. The clinical observation of transcatheter arterial chemoembolisation plus radiofrequency ablation [肝动脉栓塞化疗联合射频消融在原发性肝癌患者中的疗效观察]. Zhong Guo She Qu Yi Shi [Chinese Community Doctors] 2019;35(9):47, 51. [Google Scholar]
Ma 2019b {published data only}
- Ma W. Clinical effect of CT guided precise microwave ablation combined with TACE in the treatment of patients with primary liver cancer [CT引导精准微波消融术联合TACE术治疗原发性肝癌患者的临床效果]. Xian Dai Yi Yong Ying Xiang Xue [Modern Medical Imagelogy] 2019;28(2):272-3. [Google Scholar]
Ma 2020 {published data only}
- Ma Z, Yang L, Wei Q. The effect of ultrasound-guided microwave ablation on the level of TBIL, DBIL, and ALT in patients with intermediate- and advanced-liver cancer [超声引导下微波消融术对中晚期原发性肝癌患者血清TBIL、DBIL、ALT水平的影响及安全性分析]. Modern Medicine and Health Research 2020;4(18):16-8. [Google Scholar]
Mo 2017 {published data only}
- Mo Q, Liu L, Yang X, Duo Y, Yang T. Effect of TACE combined with RFA on serum tumor markers and postoperative survival in patients with primary liver cancer [肝动脉栓塞联合射频消融术治疗对原发性肝癌患者血清肿瘤标志物及术后生存的影响]. Zhong Liu Xue Za Zhi [Journal of Chinese Oncology] 2017;23(12):1142-5. [Google Scholar]
Pan 2016 {published data only}
- Pan W, Huang T, Xie W. The combination of transcatheter arterial chemoembolisation with CT-guided radiofrequency ablation for hepat cellular carcinoma [肝动脉栓塞化疗术联合 CT引导下射频消融治疗肝癌的疗效观察]. Lin Chuang He Li Yong Yao Za Zhi [Chinese Journal of Clinical Rational Drug Use] 2016;9(23):86-7. [Google Scholar]
Peng 2017 {published data only}
- Peng S, Zhu Z, Huang Q. Clinical application of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of primary liver cancer [肝动脉栓塞化疗联合微波消融术治疗原发性肝癌的临床应用]. Lin Chuang Yi Xue Gong Cheng [Clinical Medical and Engineering] 2017;24(7):923-4. [Google Scholar]
Qu 2014 {published data only}
- Qu Z, Wang W, Wan L, Cai Z. The combination of transcatheter arterial chemoembolisation with radiofrequency ablation for 40 patients with hepatocellular carcinoma [肝动脉化疗栓塞联合射频消融治疗原发性肝癌40例分析]. Chong Qing Yi Xue [Chongqing Medicine] 2014;28:3779-81. [Google Scholar]
Qu 2015 {published data only}
- Qu XY, Qin CH, Wu J, Chen ZL, Yang WJ. Transcatheter hepatic arterial chemoembolization in combination with radiofrequency ablation for treatment of patients with hepatocellular carcinoma: curative efficacy and effect on serum BDNF level [化疗栓塞联合射频消融治疗肝癌的疗效及对患者BDNF水平的影响]. Shi Jie Hua Ren Xiao Hua Za Zhi [World Chinese Journal of Digestology] 2015;23(9):1473‐8. [Google Scholar]
Shen 2015 {published data only}
- Shen J, Fu S. The efficacy of the combination of transcatheter arterial chemoembolisation with radiofrequency ablation for primary liver cancer [经导管动脉灌注栓塞化疗联合射频消融治疗原发性肝癌疗效评价]. Yi Xue Li Lun Yu Shi Jian [Journal of Medical Theory and Practice] 2015;20:2781-2. [Google Scholar]
Shen 2020 {published data only}
- Shen Q. Effect of transcatheter arterial chemoembolization combined with percutaneous radiofrequency ablation on postoperative sleep quality in patients with advanced hepatocellular carcinoma [经导管肝动脉化疗栓塞联合经皮射频消融术治疗中晚期肝癌患者术后睡眠质量的影响]. World Journal of Sleep Medicine 2020;7(2):220-1. [Google Scholar]
Song 2016b {published data only}
- Song Z, Li H, Qiang Y. To observe the therapeutic effect of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of primary hepatic carcinoma [观察肝动脉化疗栓塞术联合微波消融治疗原发性大肝癌的疗效]. Zhong Guo Ji Xu Yi Xue Jiao Yu [China Continuing Medical Education] 2016;8(23):100-2. [Google Scholar]
Song 2018 {published data only}
- Song X, Qin P, Teng X, Song R, Dong Z. The clinical efficacy of the combination of transcatheter arterial chemoembolisation with microwave ablation for middle and advanced liver cancer [肝动脉化疗栓塞联合微波消融治疗中晚期肝癌的疗效观察]. Dong Fang Shi Liao Yu Bao Jiang [Oriental Diet Therapy and Health Care] 2018;1:76. [Google Scholar]
Song 2019 {published data only}
- Song B, Jiang H. The feasibility or the combination of transcatheter arterial chemoembolisation with radiofrequency ablation for large hepatocellular carcinoma [肝动脉灌注化疗栓塞术联合射频消融对原发性大肝癌的疗效及可行性研究]. Zhong Guo Xian Dai Yao Wu Ying Yong [Chinese Journal of Modern Drug Application] 2019;13(1):43-4. [Google Scholar]
Tong 2018 {published data only}
- Tong D, Wang H, Jia J, Zhang G, Qiu H, Zhang H. Effect of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of liver cancer [肝动脉化疗栓塞术联合射频消融治疗肝癌疗效观察]. Zhong Guo Ji Ceng Yi Yao [Chinese Journal of Primary Medicine and Pharmacy] 2018;25(1):1-5. [Google Scholar]
Wang 2013 {published data only}
- Wang H, Wang Y, Hou R, Zhang M, Liu C, Xu C. The combination of TACE plus RFA for primary liver cancer [TACE 联合 RFA 治疗原发性肝癌的临床疗效]. Shan Dong Yi Yao [Shandong Medical Journal] 2013;53(29):52-4. [Google Scholar]
Wang 2015a {published data only}
- Wang W, Liu W, Duan T, Peng Z. Observation the efficacy of TACE combined with RFA for patients with primary liver cancer [TACE联合RFA治疗原发性肝癌的疗效观察]. Zhong Guo Yi Yao Zhi Nan [Guide of China Medicine] 2015;12:13-5. [Google Scholar]
Wang 2015b {published data only}
- Wang X, Sun J, Xiao B. Efficacy and safety of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of primary liver cancer [肝动脉化疗栓塞联合射频消融治疗原发性肝癌的疗效及安全性]. Zhong Guo Zhong Liu Lin Chuang Yu Kang Fu [Chinese Journal of Clinical Oncology and Rehabilitation] 2015;22(9):1068-70. [Google Scholar]
Wang 2017a {published data only}
- Wang E, Xu J. Effects of transcatheter arterial chemoembolization combined with radio frequency ablation on primary liver cancer [化疗栓塞术结合射频消融术治疗原发性肝癌的效果观察]. Zhong Guo Shi Yong Qi Kan [Chinese Journal of Practical Medicine] 2017;44(3):53-5. [Google Scholar]
Wang 2017b {published data only}
- Wang M, Yin D, Zhu X. The combination of transcatheter arterial chemoembolisation with ultrasound-guided radiofrequency ablation for liver cancer [超声引导下单极冷循环射频消融联合TACE治疗肝癌的效果观察]. Ji Ceng Yi Xue Lun Tan [The Medical Forum] 2017;21(10):1268-9. [Google Scholar]
Wang 2017c {published data only}
- Wang Y, Wang H, You L, Luo D. Clinical observation of transcatheter arterial chemoembolization combined with radio frequency ablation in the treatment of primary liver cancer [肝动脉化疗栓塞联合射频消融治疗原发性肝癌的临床观察]. Shi Yong Yi Yuan Lin Chuang Za Zhi [Practical Journal of Clinical Medicine] 2017;14(4):192-4. [Google Scholar]
Wang 2017d {published data only}
- Wang Z. The combination of transcatheter arterial chemoembolisation with radiofrequency ablation for huge liver cancer [多极射频消融联合 TACE 治疗巨块型肝癌的效果观察]. Lin Chuang He Li Yong Yao Za Zhi [Clinical Research] 2017;10(1):125-6. [Google Scholar]
Wang 2018a {published data only}
- Wang H, Chen H, Song T. Effect of radiofrequency ablation combined with transcatheter arterial chemoembolization on gene expression and survival of primary hepatocellular carcinoma [射频消融联合肝动脉化疗栓塞对原发性肝癌基因表达及生存期的影响]. Xian Dai Zhong Liu Yi Xue [Journal of Modern Oncology] 2018;26(22):3597-601. [Google Scholar]
Wang 2018b {published data only}
- Wang H, Chen H, Song T. The clinical efficacy of the combination of transcatheter arterial chemoembolisation with radiofrequency ablation for liver cancer [TACE联合射频消融术治疗巨块型肝癌临床疗效分析]. Hu Bei Ke Ji Xue Yuan Xue Bao [Journal of Hubei University of Science and Technology] 2018;32(5):390-3. [Google Scholar]
Wang 2018c {published data only}
- Wang J, Liu T, Li X. Efficacy of transcatheter arterial chemoembolization combined with ultrasound-guided radiofrequency ablation for primary carcinoma of liver [经肝动脉栓塞化疗联合超声引导下射频消融治疗原发性肝癌的效果]. Zhong Guo Shi Yong Yi Yao [China Practical Medical] 2018;13(30):22-3. [Google Scholar]
Wen 2018 {published data only}
- Wen P, Lin Z. Efficacy and safety of transcatheter arterial chemoembolization combined with cryoablation in the treatment of unresectable large hepatocellular carcinoma [经导管动脉化疗栓塞术联合冷冻消融治疗不可切除性大肝癌效果与安全性分析]. Zhong Guo Zong He Lin Chuang [Clinical Medicine of China] 2018;34(4):339-43. [Google Scholar]
Wu 2011b {published data only}
- Wu L, Li Z. The combination of cryoablation with transcatheter arterial chemoembolisation for unresectable liver cancer [氩氦刀冷冻与TACE协同治疗无手术指证肝癌的临床分析]. Zhong Wai Jian Kang Wen Zhai [World Health Digest] 2011;8(40):128-9. [Google Scholar]
Wu 2017b {published data only}
- Wu X, Cai B, Wang D. Observation on curative effect of TACE combined with cool-tip microwave ablation in treatment of primary liver cancer [TACE联合冷循环微波消融治疗原发性肝癌的疗效观察]. Zhong Guo Yi Yao Ke Xue [China Medicine and Pharmacy] 2017;7(9):214-6. [Google Scholar]
Wu 2020 {published data only}
- Wu J, Dong G, Liu T. Microwave ablation plus TACE for treating primary liver cancer [冷循环微波消融联合TACE术治疗原发性肝癌的临床研究]. Chinese Hepatology 2020;25(10):1058-61. [Google Scholar]
Xie 2017 {published data only}
- Xie H, Zhong W. Effect of TACE combined with HIFU on elderly patients with primary liver cancer and its effect on immune function [TACE联合HIFU治疗老年原发性肝癌疗效及对患者免疫功能的影响]. He Bei Yi Xue [Hebei Medicine] 2017;23(11):1761-4. [Google Scholar]
Xiong 2013 {published data only}
- Xiong J, Fan G, Chen L. Therapeutic effect of transarterial chemo-embolization combined with radio frequency ablation for treatment of middle or advanced stage hepatocellular carcinoma in elderly [肝动脉化疗栓塞联合经皮射频治疗中晚期老年肝癌的临床观察.]. Zhong Guo Xian Dai Pu Tong Wai Ke Jin Zhan [Chinese Journal of Current Advances in General Surgery] 2013;16(5):355-8. [Google Scholar]
Xiong 2017 {published data only}
- Xiong L, Zhang L, Ma J, Li J. Efficacy of radiofrequency ablation plus hepatic arterial chemoembolization in primary hepatic carcinoma and its effect on serum markers. International Journal Of Clinical And Experimental Medicine 2017;10(9):14076-82. [Google Scholar]
Xu 2016 {published data only}
- Xu L, Li C, Zhang B. The combination of transcatheter arterial chemoembolisation with radiofrequency ablation for progressive liver cancer [超声引导下射频消融联合TACE治疗进展期原发性肝癌疗效及并发症分析]. Gui Zhou Yi Yao [Guizhou Medical Journal] 2016;40(3):265-7. [Google Scholar]
Xue 2019 {published data only}
- Xue J, Chen L, Liao L. The observation on the efficacy of the combination of TACE with MWA [肝动脉栓塞化疗联合冷循环微波消融治疗肝癌的临床疗效观察]. Chinese Journal of Physical Medicine and Rehabilitation 2019;41(6):458-60. [Google Scholar]
Yao 2018 {published data only}
- Yao Y. The efficacy of transcatheter arterial chemoembolisation plus radiofrequency ablation for liver cancer [肝动脉化疗栓塞术联合CT引导下射频消融术对原发性肝癌患者治疗效果]. Man Xing Bing Xue Za Zhi [Chronic Pathematology Journal] 2018;19(4):503-5. [Google Scholar]
Yi 2015 {published data only}
- Yi Y, Lv J, Wang B. The short and long term clinical effect of TACE combined with RFA for patients with primary liver cancer [TACE与RFA联合治疗原发性肝癌患者近远期疗效观察]. Zhong Guo Xian Dai Yi Sheng [China Modern Doctor] 2015;53(24):76-9. [Google Scholar]
Yu 2019 {published data only}
- Yu H. Clinical effect of trans-catheter arterial chemoembolization combined With ultrasound-guided radiofrequency ablation in the treatment of large hepatocellular carcinoma with lack of blood supply [肝动脉化疗栓塞联合超声引导下射频消融治疗乏血供大肝癌的临床效果]. Zhong Guo Dang Dai Yi Yao [China Modern Medicine] 2019;26(5):92-4. [Google Scholar]
Yuan 2015 {published data only}
- Yuan X. The combination or microwave ablation with transcatheter arterial chemoembolisation for hepatocellular carcinoma [微波消融联合血管介入治疗原发性肝癌临床研究]. Zhong Guo Yi Xue Gong Cheng [China Medical Engineering] 2015;23(12):63. [Google Scholar]
Zhang 2013 {published data only}
- Zhang SJ, Ma YL. TACE combined with percutaneous microwave ablation in treatment of large primary hepatocellular carcinoma [经导管动脉化疗栓塞术联合经皮微波消融治疗大肝癌]. Zhong Guo Jie Ru Ying Xiang Yu Zhi Liao Xue [Chinese Journal of Interventional Imaging and Therapy] 2013;10(7):397-400. [Google Scholar]
Zhang 2014 {published data only}
- Zhang S. Clinical effect of primary liver cancer treatment by transcatheter arterial chemoembolization combined radio frequency ablation [肝动脉栓塞化疗联合射频消融治疗原发性肝癌的临床观察]. Zhong Guo Zhong Liu Lin Chuang Yu Jian Kang [Chinese Journal of Clinical Oncology and Rehabilitation] 2014;21(5):576-8. [Google Scholar]
Zhang 2016 {published data only}
- Zhang Z. The combination of cryoablation with transcatheter arterial chemoembolisation for unresectable liver cancer [肝动脉化疗栓塞联合氩氦刀冷冻消融治疗对不能手术的中晚期肝癌疗效的临床分析]. Zhong Guo Xian Dai Yao Wu Fan Ying [Chinese Journal of Modern Drug Application] 2016;10(10):41-2. [Google Scholar]
Zhang 2017a {published data only}
- Zhang H, Ma X, Fu Q, Cao C, Cao L. The efficacy and safety of the combination of microwave ablation with transcatheter arterial chemoembolisation for middle and advanced hepatocellular carcinoma [经肝动脉化疗栓塞联合经皮微波消融序贯治疗中晚期肝癌的疗效及预后分析]. Gan Zang [Chinese Hepatology] 2017;22(5):431-4. [Google Scholar]
Zhang 2017b {published data only}
- Zhang W, Chen G, Sun M, An Y, Wang Y, Shang Q. Clinical efficacy of TACE and radiofrequency ablation in treatment of patients with primary liver cancer [经导管肝动脉化疗栓塞联合射频消融治疗原发性肝癌患者临床疗效评价]. Shi Yong Gan Zang Bing Za Zhi [Journal of Practical Hepatology] 2017;20(4):472-6. [Google Scholar]
Zhang 2017c {published data only}
- Zhang Y. Observation of the efficacy of the combination of microwave ablation and transcatheter arterial chemoembolisation for liver cancer [肝动脉化疗栓塞联合微波消融治疗肝癌的临床疗效探讨]. Xin Li Yi Sheng [Psychological Doctor] 2017;23(19):191-2. [Google Scholar]
Zhang 2018a {published data only}
- Zhang H, Zheng W, Ren N. Observation and nursing of effect of hepatic arterial chemoembolization combined with radiofrequency ablation in treatment of large primary hepatic carcinoma [肝动脉化疗栓塞联合射频消融术治疗原发性大肝癌的效果观察与护理]. Hu Li Shi Jian Yu Yan Jiu [Nursing Practice and Research] 2017;14(16):81-3. [Google Scholar]
Zhang 2018b {published data only}
- Zhang J. The efficacy and safety of the combination of the transcatheter arterial chemoembolisation with radiofrequency ablation for liver cancer [肝动脉化疗栓塞联合射频消融治疗原发性肝癌的疗效及安全性评价]. Lin Chuang Yi Yao Dian Zi Wen Xian Za Zhi [Journal of Clinical Medical Literature] 2018;5(3):54-5. [Google Scholar]
Zhang 2018c {published data only}
- Zhang Q, Zhang Z, Zhang W, Du X. Effect of radiofrequency ablation combined with transcatheter arterial chemoembolization on primary hepatocellular carcinoma and serum cytokines [射频消融联合肝动脉化疗栓塞对原发性肝癌的疗效及对细胞因子的影响]. Shan Xi Yi Yao Za Zhi [Shanxi Medical Journal] 2018;47(11):1244-7. [Google Scholar]
Zhang 2018d {published data only}
- Zhang J, Gao Z, Qian Y, Zhang M, Tan G, Liu Z. Ultrasound-guided microwave ablation plus transcatheter arterial chemoembolisation for primary liver cancer [超声引导下微波消融联合TACE治疗进展期原发性肝癌的疗效分析]. Zhong Guo Yi Yao Zhi Nan [Guide of China Medicine] 2018;16(4):119-20. [Google Scholar]
Zhang 2019 {published data only}
- Zhang G. The transcatheter arterial chemoembolisation plus radiofrequent ablation for hepatocellular carcinoma [肝动脉化疗栓塞术联合射频消融术治疗原发性肝癌]. Zhong Guo Lao Nian Xue Za Zhi [Chinese Journal of Gerontology] 2019;39(8):1855-7. [Google Scholar]
Zhang 2020 {published data only}
- Zhang F, Zhou H. The efficacy of the combination of TACE with MWA for primary liver cancer [肝动脉化疗栓塞联合微波消融治疗原发性肝癌的临床效果]. Henan Medical Research 2020;29(19):3524-5. [Google Scholar]
Zhao 2014b {published data only}
- Zhao Z, Wang R, Zhao F, Li J, Guo S. The transcatheter arterial chemoembolisation plus radiofrequency ablation for 40 patients with hepatocellular carcinoma [肝动脉栓塞联合射频消融治疗肝癌40例疗效评价]. Shan Xi Yi Xue Za Zhi [Shaanxi Medical Journal] 2015;4:587-8. [Google Scholar]
Zhao 2015a {published data only}
- Zhao H, Cui H. The efficacy of the combination of TACE with RFA for primary liver cancer [原发性肝癌 TACE与 RFA 联合治疗临床效果评价]. Da Jia Jian Kang [For All Health] 2015;24:119. [Google Scholar]
Zhao 2016 {published data only}
- Zhao Y, Jia B. Clinical application of hepatic arterial chemoembolization combined with CT guided radiofrequency ablation in the treatment of primary hepatic carcinoma [肝动脉栓塞化疗联合CT引导射频消融治疗原发性肝癌的临床应用]. Yi Xue Ying Xiang Xue Za Zhi [Journal of Medical Imaging] 2016;26(2):364-7. [Google Scholar]
Zhao 2018 {published data only}
- Zhao X. The efficacy of the combination of transcatheter arterial chemoembolisation with microwave ablation for large primary liver cancer [肝动脉化疗栓塞术联合微波消融治疗原发性大肝癌的临床疗效探究]. Te Bie Jian Kang [Special Health] 2018;19:64. [Google Scholar]
Zhao 2020 {published data only}
- Zhao C, Ji L. The effect of the combination of TACE and microwave ablation on the level of AFP and tumour progression [微波消融联合经肝动脉化疗栓塞术治疗肝癌的效果及对血清AFP水平的影响]. Journal of Medical Theory and Practice 2020;33(9):1454-5. [Google Scholar]
Zheng 2015 {published data only}
- Zheng Z. The transcatheter arterial chemoembolisation plus cryoablation for middle and advanced liver cancer [肝动脉化疗栓塞联合氩氦刀冷冻消融治疗中晚期肝癌患者的疗效观察]. Xian Dai Zhen Duan Yu Zhi Liao [Modern Diagnosis and Treatment] 2015;20:4728-9. [Google Scholar]
Zhu 2013b {published data only}
- Zhu L. The transcatheter arterial chemoembolisation plus microwave ablation for 36 patients with hepatocellular carcinoma [肝动脉栓塞化疗联合微波消融治疗中晚期肝癌36例]. Lin Chuang Yi Xue [Clinical Medicine] 2013;33(8):50-1. [Google Scholar]
Zhu 2015 {published data only}
- Zhu H, Chen P. Therapeutic effect of transcatheter arterial chemoembolization combined with radiofrequency ablation for primary liver cancer [经肝动脉化疗栓塞术联合射频消融对原发性肝癌的治疗效果]. Zhong Guo Ji Xu Yi Xue Jiao Yu [China Continuing Medical Education] 2015;7(33):124-6. [Google Scholar]
Zhuang 2016 {published data only}
- Zhuang W, Huang C, Ji Z, Lin S, Huang Y, Tang Y, et al. Analysis of therapeutic effectiveness of argon-helium cryoablation combined with transcatheter arterial chemoembolization for the treatment of advanced hepatocellular carcinoma [TACE 联合氩氦刀冷冻消融治疗中晚期肝癌的疗效分析]. Yi Xue Ying Xiang Za Zhi [Journal of Medical Imaging] 2016;26(12):2247-50. [Google Scholar]
References to ongoing studies
ChiCTR‐IOR‐16007915 {unpublished data only}
- ChiCTR-IOR-16007915. Transarterial chemoembolization (TACE) with or without sequential cryoablation in hepatocellular carcinoma of BCLC B: a multicentral randomized controlled trial [Transarterial chemoembolization (TACE) with or without sequential cryoablation in hepatocellular carcinoma of BCLC B: a multicentral randomized controlled trial]. chictr.org.cn/showproj.aspx?proj=13377 (first received 13 February 2016).
ChiCTR‐IOR‐17013743 {unpublished data only}
- ChiCTR-IOR-17013743. Treatment of BCLC medium-term hepatocellular carcinoma (HCC) of randomized controlled study of conventional TACE sequential microwave ablation (MWA) and conventional TACE alone [Treatment of BCLC medium-term hepatocellular carcinoma (HCC) of randomized controlled study of conventional TACE sequential microwave ablation (MWA) and conventional TACE alone]. chictr.org.cn/showproj.aspx?proj=23656 (first received 6 December 2017).
NCT02301091 {unpublished data only}
- NCT02301091. Combine TACE and RFA versus TACE alone for HCC with PVTT (CORTT) [Combine transcatheter arterial embolization and radiofrequency ablation versus transcatheter arterial embolization alone for hepatocellular carcinoma with portal vein tumor thrombus]. clinicaltrials.gov/ct2/show/NCT02301091 (first received 25 November 2014).
NCT02435953 {unpublished data only}
- NCT02435953. TACE + RFA versus TACE alone for intermediate-stage hepatocellular carcinoma [Transarterial chemoembolization plus radiofrequency ablation versus transarterial chemoembolization alone for intermediate-stage hepatocellular carcinoma]. clinicaltrials.gov/ct2/show/NCT02435953 (first received 6 May 2015).
NCT02646137 {unpublished data only}
- NCT02646137. Single session combined locoregional therapies for hepatocellular carcinoma [Comparison of single session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in treatment of hepatocellular carcinoma: a randomized controlled study]. clinicaltrials.gov/ct2/show/NCT02646137 (first received 5 January 2016).
Additional references
Abdel‐Rahman 2013
- Abdel-Rahman O, Elsayed ZA. Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature. Digestive Diseases and Sciences 2013;58(12):3389-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ahmed 2011
- Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011;258(2):351-69. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Awad 2009
- Awad T, Thorlund K, Gluud C. Cryotherapy for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD007611. [DOI: 10.1002/14651858.CD007611.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Azuma 2016
- Azuma S, Asahina Y, Nishimura-Sakurai Y, Kakinuma S, Kaneko S, Nagata H, et al. Efficacy of additional radiofrequency ablation after transcatheter arterial chemoembolization for intermediate hepatocellular carcinoma. Hepatology Research 2016;46(4):312-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brace 2007
- Brace CL, Laeseke PF, Sampson LA, Frey TM, Van der Weide DW, Lee FT Jr. Microwave ablation with a single small-gauge triaxial antenna: in vivo porcine liver model. Radiology 2007;242(2):435-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruix 2011
- Bruix J, Sherman M, American Association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md.) 2011;53(3):1020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2014
- Cao X. Meta-analysis on radiofrequency ablation in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Journal of Huazhong University of Science and Technology 2014;35(4):692-700. [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(110):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chacko 2016
- Chacko S, Samanta S. Hepatocellular carcinoma: a life-threatening disease. Biomedicine & Pharmacotherapy 2016;84:1679-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corwin 2001
- Corwin TS, Lindberg G, Traxer O, Gettman MT, Smith TG, Pearle MS, et al. Laparoscopic radiofrequency thermal ablation of renal tissue with and without hilar occlusion. Journal of Urology 2001;166(1):281-4. [PMID: ] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
EASL‐EORTC 2012
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2012;56(4):908-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
El‐Serag 2012
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142(6):1264-73.e1. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2009
- Fan W, Li J, Li H, Wang Y, Tan G, Chen Z, et al. Interventional treameant for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Contemporary Medicine 2009;15(35):677-80. [Google Scholar]
Fan 2011
- Fan W, Li J, Yang J, Wang Y, Xiang X, Li H. Interventional treatment for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Chinese Archives Of General Surgery (electronic version) 2011;5(1):62-7. [Google Scholar]
Forner 2018
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2018;391(10127):1301-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gartlehner 2019
- Gartlehner G, Nussbaumer-Streit B, Wagner G, Patel S, Swinson-Evans T, Dobrescu A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2019;106:50-9. [DOI: 10.1016/j.jclinepi.2018.10.009] [DOI] [PubMed] [Google Scholar]
Goldberg 2003
- Goldberg SN, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 2003;228(2):335-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 13 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Granito 2017
- Granito A, Bolondi L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncology 2017;18:e101-e112. [DOI: 10.1016/S1470-2045(16)30569-1] [DOI] [PubMed] [Google Scholar]
Gu 2014
- Gu L, Liu H, Fan L, Lv Y, Cui Z, Luo Y, et al. Treatment outcomes of transcatheter arterial chemoembolization combined with local ablative therapy versus monotherapy in hepatocellular carcinoma: a meta-analysis. Journal of Cancer Research and Clinical Oncology 2014;140(2):199-210. [DOI] [PubMed] [Google Scholar]
Heimbach 2018
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67(1):358-80. [DOI] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019c
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hu 2015
- Hu Yi, Xu Mingxi. A meta-analysis of the combination of transarterial chemoembolization and percutaneous radiofrequency ablation for treatment of primary liver cancer. Clinical Misdiagnosis & Mistherapy 2015;1:101-5. [Google Scholar]
Hyun 2016
- Hyun D, Cho SK, Shin SW, Park KB, Park HS, Choo SW, et al. Early stage hepatocellular carcinomas not feasible for ultrasound-guided radiofrequency ablation: comparison of transarterial chemoembolization alone and combined therapy with transarterial chemoembolization and radiofrequency ablation. Cardiovascular and Interventional Radiology 2016;39(3):417-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. Vol. 1. Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Imai 2014
- Imai N, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, et al. Transarterial chemoembolization for hepatocellular carcinoma: a review of techniques. World Journal of Hepatology 2014;6(12):844-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaeger 1996
- Jaeger HJ, Mehring UM, Castaneda F, Hasse F, Blumhardt G, Loehlein D, et al. Sequential transarterial chemoembolization for unresectable advanced hepatocellular carcinoma. Cardiovascular and Interventional Radiology 1996;19(6):388-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katsanos 2017
- Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. PLOS One 2017;12(9):e0184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2014
- Kew MC. Hepatocellular carcinoma: epidemiology and risk factors. Journal of Hepatocellular Carcinoma 2014;1:115-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015
- Kim MN, Kim BK, Han KH, Kim SU. Evolution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment. Expert Review of Gastroenterology & Hepatology 2015;9(3):335-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laursen 2014
- Laursen L. A preventable cancer. Nature 2014;516(7529):S2-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lei 2013
- Lei Y, Guo X, Wang L, Wang Y, Zhu L, Liu L. A system evaluation of radiofrequency ablation plus transcatheter hepatic arterial chemoembolization for primary hepatocellular carcinoma. Journal of Modern Oncology 2013;21(6):1285-8. [Google Scholar]
Lencioni 2010
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Seminars in Liver Disease 2010;30(1):52-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2010
- Li C, Zhang W, Zhang R, Zhang L, Wu P, Zhang F. Therapeutic effects and prognostic factors in high-intensity focused ultrasound combined with chemoembolisation for larger hepatocellular carcinoma. European Journal of Cancer 2010;46(13):2513-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2016
- Li W, Man W, Guo H, Yang P. Clinical study of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of advanced hepatocellular carcinoma. Journal of Cancer Research and Therapeutics 2016;12(Suppl):C217-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2018a
- Liu Y, Zhuo L, Zhu P, He M, Xu Y, Wang T, et al. Transcatheter arterial chemoembolization combination therapy vs transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma:a meta-analysis of survival rate. Journal of Clinical Radiology 2018;37(2):314-8. [Google Scholar]
Livraghi 2000
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000;214(3):761-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Llovet 2016
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nature Reviews. Disease Primers 2016;2:16018. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lubner 2013
- Lubner MG, Brace CL, Ziemlewicz TJ, Hinshaw JL, Lee FT Jr. Microwave ablation of hepatic malignancy. Seminars in Interventional Radiology 2013;30(1):56-66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2015
- Martin RC 2nd, Scoggins CR, Schreeder M, Rilling WS, Laing CJ, Tatum CM, et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 2015;121(20):3649-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mittal 2013
- Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. Journal of Clinical Gastroenterology 2013;47(Suppl):S2-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohd 2013
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57(4):1333-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morimoto 2010
- Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116(23):5452-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
National Center for Health Statistics (US) 2015
- National Center for Health Statistics (US). Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville (MD): National Center for Health Statistics (US); 2015 May. Available from: https://www.ncbi.nlm.nih.gov/books/NBK299348/. [PMID: ] [PubMed]
Oliveri 2011
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD004787. [DOI: 10.1002/14651858.CD004787.pub2] [DOI] [PubMed] [Google Scholar]
Omata 2010
- Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology International 2010;4(2):439-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2013
- Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. Journal of Clinical Oncology 2013;31(4):426-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Poggi 2015
- Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World Journal of Hepatology 2015;7(25):2578-89. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2019 [Computer program]
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Rubinsky 1990
- Rubinsky B, Lee CY, Bastacky J, Onik G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology 1990;27(1):85-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Satake 2008
- Satake M, Uchida H, Arai Y, Anai H, Sakaguchi H, Nagata T, et al. Transcatheter arterial chemoembolization (TACE) with lipiodol to treat hepatocellular carcinoma: survey results from the TACE study group of Japan. Cardiovascular and Interventional Radiology 2008;31(4):756-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulze 2016
- Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. Journal of Hepatology 2016;65(5):1031-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sirivatanauksorn 2011
- Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. A World Journal of Hepatic, Pancreatic & Biliary Surgery 2011;2011:818217. [DOI: 10.1155/2011/818217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2016a
- Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clinical and Molecular Hepatology 2016;22(4):509-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2011
- Sun J, He S, Shen J, Chen H, Chen Y, Jiang T. Transarterial chemoembolization in combination with radiofrequency thermal ablation in hepatocellular carcinoma: a systematic review and meta-analysis. Chinese Journal of Clinical Medicine 2011;18(6):803-8. [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al, European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute 2000;92(3):205-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/wp-content/uploads/2020/12/tsa_manual_ENG.pdf 2011 (accessed 13 May 2020).
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torre 2015
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a Cancer Journal for Clinicians 2015;65(2):87-108. [PMID: ] [DOI] [PubMed] [Google Scholar]
Trad 2017
- Trad D, Bibani N, Sabbah M, Elloumi H, Gargouri D, Ouakaa A, et al. Known, new and emerging risk factors of hepatocellular carcinoma (review). Presse Medicale 2017;46(11):1000-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- TSA - Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011. www.ctu.dk/tsa/downloads.aspx.
Vogl 2003
- Vogl TJ, Mack MG, Balzer JO, Engelmann K, Straub R, Eichler K, et al. Liver metastases: neoadjuvant downsizing with transarterial chemoembolization before laser-induced thermotherapy. Radiology 2003;229(2):457-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2016b
- Wang Y, Deng T, Zeng L, Chen W. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: a meta-analysis. Hepatology Research 2016;46(1):58-71. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Medical Research Methodology 2009;9:86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijlemans 2012
- Wijlemans JW, Bartels LW, Deckers R, Ries M, Mali WP, Moonen CT, et al. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) ablation of liver tumours. Cancer Imaging 2012;12(2):387-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2005
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235(2):659-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2015
- Wu S, Hou J, Ding Y, Wu F, Hu Y, Jiang Q, et al. Cryoablation versus radiofrequency ablation for hepatic malignancies: a systematic review and literature-based analysis. Medicine 2015;94(49):e2252. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiong 2018a
- Xiong K, Bai F, Chen D, Wei X, Xue D. Comparison of the efficacy and safety of the combined treatment of transcatheter arterial chemoembolization and radiofrequency ablation with transcatheter arterial chemoembolization or radiofrequency ablation treatment alone in patients with primary hepatic carcinoma in China. Chinese Health Resources 2018;21(3):271-4, 279. [Google Scholar]
Xiong 2018b
- Xiong K, Chen D, Bai F, Wei X, Xue D. Efficacy and safety of seven non-surgical therapies for primary hepatic carcinoma: a network meta-analysis. Chinese Health Resources 2018;21(4):307-11, 322. [Google Scholar]
Yang 2007
- Yang D, Converse MC, Mahvi DM, Webster JG. Measurement and analysis of tissue temperature during microwave liver ablation. IEEE Transactions on Bio-Medical Engineering 2007;54(1):150-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2009
- Yang W, Chen MH, Wang MQ, Cui M, Gao W, Wu W, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatology Research 2009;39(3):231-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2014
- Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a surveillance, epidemiology, and end results analysis. Cancer 2014;120(23):3707-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2017
- Yang DJ, Luo KL, Liu H, Cai B, Tao GQ, Su XF, et al. Meta-analysis of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization alone for hepatocellular carcinoma. Oncotarget 2017;8(2):2960-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yin 2014
- Yin X, Zhang L, Wang YH, Zhang BH, Gan YH, Ge NL, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer 2014;14:849. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2013
- Zhao S, Chen X, Long Q, Zhang X. Transcatheter arterial chemoembolization combined with radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta analysis. Journal of Interventional Radiology 2013;22(11):908-13. [Google Scholar]
Zhao 2017
- Zhao J, Zhang H, Wei L, Xie S, Suo Z. Comparing the long-term efficacy of standard and combined minimally invasive procedures for unresectable HCC: a mixed treatment comparison. Oncotarget 2017;8(9):15101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2013a
- Zhu J, Zhu H, Mei Z, Jin C, Ran L, Zhou K, et al. High-intensity focused ultrasound ablation for treatment of hepatocellular carcinoma and hypersplenism: preliminary study. Journal of Ultrasound in Medicine 2013;32(10):1855-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhu 2016
- Zhu ZX, Huang JW, Liao MH, Zeng Y. Treatment strategy for hepatocellular carcinoma in China: radiofrequency ablation versus liver resection. Japanese Journal of Clinical Oncology 2016;46(12):1075-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2019a
- Liu B, Chen H, Li W. The combination of transcatheter arterial chemoembolisation (TACE) and thermal ablation versus TACE alone for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013345. [DOI: 10.1002/14651858.CD013345] [DOI] [PMC free article] [PubMed] [Google Scholar]


