Abstract
Because of their increasing prevalence, gastrointestinal (GI) cancers are regarded as an important global health challenge. Microorganisms residing in the human GI tract, termed gut microbiota, encompass a large number of living organisms. The role of the gut in the regulation of the gut-mediated immune responses, metabolism, absorption of micro- and macro-nutrients and essential vitamins, and short-chain fatty acid production, and resistance to pathogens has been extensively investigated. In the past few decades, it has been shown that microbiota imbalance is associated with the susceptibility to various chronic disorders, such as obesity, irritable bowel syndrome, inflammatory bowel disease, asthma, rheumatoid arthritis, psychiatric disorders, and various types of cancer. Emerging evidence has shown that oral administration of various strains of probiotics can protect against cancer development. Furthermore, clinical investigations suggest that probiotic administration in cancer patients decreases the incidence of postoperative inflammation. The present review addresses the efficacy and underlying mechanisms of action of probiotics against GI cancers. The safety of the most commercial probiotic strains has been confirmed, and therefore these strains can be used as adjuvant or neo-adjuvant treatments for cancer prevention and improving the efficacy of therapeutic strategies. Nevertheless, well-designed clinical studies are still needed for a better understanding of the properties and mechanisms of action of probiotic strains in mitigating GI cancer development.
Keywords: probiotic, gastrointestinal disorders, cancer, pathology, therapy
Introduction
The incidence of gastrointestinal (GI) neoplasms is rapidly increasing globally (Ashrafizadeh et al., 2020; Pourhanifeh et al., 2020; Shafabakhsh et al., 2021). GI cancers are a complex set of heterogenous diseases and disorders (Wang et al., 2021) and are classified into more frequent sporadic and rare inherited forms. Environmental and genetic risk factors can cooperatively alter normal tissue into a precursor or a premalignant injury, culminating in malignancy. While the precise genetic mechanisms are somewhat understood in a tissue-type– and cell-type–specific context, many common aspects exist between GI cancers of heterogenous origin (Wang et al., 2021). Consistent with the advances made in developing new diagnostic and therapeutic approaches for GI cancers, several probiotic strains are being used as nutritional supplements.
Probiotics are a group of viable microorganisms including bacteria and yeasts that if consumed in sufficient amounts, may afford health benefits to the host (Ganguly et al., 2011; Tamtaji et al., 2019a; Tamtaji et al., 2019b; Alipour Nosrani et al., 2021; Davoodvandi et al., 2021). The major advantage of probiotic administration is its ability to maintain gut microbial homeostasis, reduce pathogenic microorganisms in the GI tract, and restores homeostasis of intestinal microorganisms (Floch et al., 2011; Butt and Epplein, 2019). Moreover, by modulating microbiota and immune responses, decreasing bacterial translocation, promoting the function of the gut barrier, inducing anti-inflammatory properties, triggering anti-pathogenic activity, and decreasing tumor development and metastasis, probiotics might contribute to the prevention and treatment of GI cancers (Servin, 2004; Cotter et al., 2005; Javanmard et al., 2018). Considering the potential roles of Helicobacter pylori (H. pylori) in the initiation of colorectal (Teimoorian et al., 2018; Butt and Epplein, 2019) and gastric cancers (Alfarouk et al., 2019), the possible properties of probiotics against GI neoplasm in humans have been investigated in relation to their suppressive effects on H. pylori (Taremi et al., 2005; Sanders et al., 2013; Russo et al., 2014; Khoder et al., 2016; Rasouli et al., 2017). By triggering immune activity, probiotics, as functional dietary supplements, may mitigate neoplastic predisposition and development of GI cancers (Liong, 2008; Zuccotti et al., 2008; Kumar et al., 2010; De Preter et al., 2011; Zhang et al., 2011).
Clinical Overview on GI Neoplasms
Carcinogenesis is a multistage process characterized by genetic mutations (Nowell, 1976; Yuasa, 2003; Vogelstein and Kinzler, 2004). In the past, initiation and progression of tumors were considered as distinct processes. A critical observation that led to the multistage hypothesis was that neoplasm was clonal, with each neoplastic cell originating from a single progenitor (Nowell, 1976; Cahill et al., 1999). This model implied that genetic mutations required for neoplastic transformation did not occur at once, but rather progressively. With each stage in this process, the transforming cell obtained a new mutation that promoted cell survival or proliferation.
A cell clone was developed with all of the necessary aspects for neoplastic transformation through evolution or natural selection. Selection is a critical element of this process because mutations are random events; thus, only rare mutations result in activation of cell survival and growth-promoting pathways or inactivation of apoptotic pathways or tumor suppressors (Ponder, 2001). These mutations impart a selective survival and growth dominance to that cell and its progeny. This leads to the expansion of that cell into a clonal population. Further mutations that occur in cells of that clonal population provide a few rare cells with new superiority. These daughter cells are subjected to an additional round of clonal expansion. This process continues, building on round after round of clonal expansion, till a mass is generated, and neoplastic transformation has taken place (Nowell, 1976; Cahill et al., 1999).
The specific number of somatically acquired gene mutations necessary for neoplastic transformation is dependent upon which genes and tissues are targeted. In common solid tumors, such as those derived from the colon or pancreas, an average of 33–66 genes displays subtle somatic mutations that would be expected to alter their protein products. About 95% of these mutations are single-base substitutions (such as C > G), whereas the remainder are deletions or insertions of one or a few bases (such as CTT > CT). Of the base substitutions, 90.7% result in missense changes, 7.6% result in nonsense changes, and 1.7% result in alterations of splice sites or untranslated regions immediately adjacent to the start and stop codons (Vogelstein et al., 2013).
Typically, benign dysplastic intermediates develop before GI neoplasm. Indeed, they do not originate from normal tissues directly, and the dysplastic lesions are characterized by their morphology and categorized based on certain pathological indicators (Said, 2012). For example, in the colon, the adenoma–carcinoma pattern shows this promotion from normal mucosa to invasive carcinoma via dysplastic intermediates. This pattern has been well supported by many pathological and animal studies (Kim and Lance, 1997; Lynch and Hoops, 2002).
The same multistep pattern from normal tissue via dysplastic intermediates to malignancy has been shown for human pancreatic, esophageal, and gastric cancers (Hruban et al., 2001; Yuasa, 2003; Hruban et al., 2004; Lin and Beerm, 2004). Cancer always emerges in a dysplastic precursor lesion that is histologically or grossly apparent. Current models have shown that the sequence of events prior to intestinal gastric cancer is as follows: atrophic gastritis, intestinal-metaplasia, and adenomas, which develop into carcinomas (Yuasa, 2003). Precursor lesions that lead to pancreatic cancer have been formally agreed upon, and the characteristics necessary for their classification have been established (Hruban et al., 2001; Hruban et al., 2004). These criteria classify the pancreatic lesions for both scientific and clinical uses.
The concept of cancer stem cells highlighted new perspectives in understanding this disease. Although it is tempting to explain tumor formation and metastasis by the presence of stem cells, after almost a decade of intense research, it seems that cancer stem cells fail to explain how neoplasia evolves. It seems most likely that this population of cells is not a defined group of cells resting in a niche and populating the tumor with amplifying cells, but rather, that few or maybe multiple cells within the tumor can function as cancer stem cells if induced, yet also revert to the state of a “normal” cancer cell. In general, cancer stem cells resulting from mutations in stem/progenitor cells most likely undergo uncontrolled proliferation (Li and Neaves, 2006; Abdul Khalek et al., 2010; Welte et al., 2010).
Probiotic and Cancer Therapy
Advances have been made over the last century to develop anticancer drugs that lead to drastically reducing of the side effects of medications (Falzone et al., 2018). However, the beneficial effects of probiotics on metabolic profiles and biomarkers of inflammation and oxidative stress were previously reported (Asemi et al., 2012a; Asemi et al., 2012b; Tajadadi-Ebrahimi et al., 2014; Bahmani et al., 2016). Modifying the intestinal microbiome with oral probiotics has been applied to decrease side effects associated with drugs. The adverse effects caused by anticancer treatments mainly include mucositis and diarrhea. Among the advantages of probiotics are their low cost and general safety (Rondanelli et al., 2017). Probiotic application in clinical practice has displayed a wide range of advantages, such as improving antibiotics and Clostridium difficile-related diarrhea and respiratory tract infections (Rondanelli et al., 2017). Populating the gut microbiota in cancer patients with probiotics re-establishes both the functionality and quantities of commensal bacteria, which are reduced after treatments (Zitvogel et al., 2018). Nonetheless, probiotic administration in several clinical trials has been shown to re-establish healthy intestinal microbiota composition and to diminish diarrhea and other treatment-related damages to the gut, such as mucositis (Mego et al., 2013). Consistently, Lactobacillus containing probiotics prevent diarrhea and mucositis in individuals, who received chemotherapy/radiotherapy for pelvic malignancy (Gianotti et al., 2010; Lalla et al., 2014).
The specific mechanism associated with the antitumor properties of probiotics remains unclear. Gut microbiota affect a variety of pathways, which are considered to play a central role in this process. Primarily, probiotic bacteria play an essential role in the preservation of homeostasis, thus maintaining sustainable physicochemical conditions in the colon. Reduced pH causing inter alia by the excessive presence of bile acids in feces may be a direct cytotoxic factor affecting colonic epithelium leading to colon carcinogenesis. Regarding their involvement in the modulation of the pH and bile acid profile, probiotic bacteria, such as L. acidophilus and B. bifidum, have shown efficacy in cancer prevention (Biasco et al., 1991; Bernstein et al., 2005; Jia et al., 2018).
Probiotic strains are also responsible for maintaining the balance between the quantity of other participants of natural intestinal microflora and their metabolic activity. Putrefactive bacteria, such as Escherichia coli and Clostridium perfringens, naturally present in the gut, have been proven to be involved in production of carcinogenic compounds using enzymes such as β-glucuronidase, azoreductase, and nitroreductase (Górska et al., 2019).
Another cancer-preventing strategy involving probiotic bacteria, such as chiefly Lactobacillus and Bifidobacillus strains, has been linked to the binding and degradation of potential carcinogens. Mutagenic compounds associated with the increased risk of colon cancer are commonly found in unhealthy food, especially fried meat. Ingestion of the Lactobacillus strain by human volunteers alleviated the mutagenic effect of diet rich in cooked meat, which resulted in decreased urinary and fecal excretion of heterocyclic aromatic amines (HAAs) (Lidbeck et al., 1992; Hayatsu and Hayatsu, 1993; Górska et al., 2019).
Many beneficial compounds produced and metabolized by gut microbiota have been demonstrated to play an essential role in maintaining homeostasis and suppressing carcinogenesis. A specific population of gut microbiota is dedicated to the production of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate as a result of the fermentation of fiber-rich prebiotics. Except for their principal function as an energy source, SCFAs have also been proven to act as signaling molecules affecting the immune system, cell death, and proliferation as well as intestinal hormone production and lipogenesis, which explains their crucial role in epithelial integrity maintenance (Garrett, 2015; Requena et al., 2018; Górska et al., 2019).
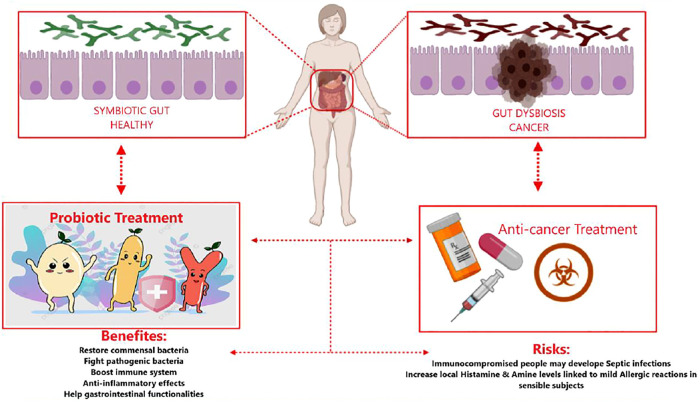
Figure 1 shows both the advantages and potential disadvantages of probiotic administration as adjuvants during cancer treatments. The figure highlights show probiotics’ regulation of the gut’s subtle equilibrium, from microbial imbalance (dysbiotic) to functional and healthy microbiota.
FIGURE 1.
Risks and benefits of probiotics associated with cancer treatment. Schematic depiction of healthy gut microbiota in humans, occupied by symbiotic bacteria (top left box) against tumor-affected microbiota and dysbiosis of the gut (top right box). Anticancer treatment may negatively influence gut microbiota, leading to dysbiotic unbalance (bottom right square). Probiotic administration may re-adjust the dysbiotic conditions mediated by tumor growth and treatment. Probiotics may improve gastrointestinal therapy–related side effects, so they re-establish the intestinal symbiosis (bottom left square). The application of probiotics in anticancer therapy has benefits and risks (central bottom box).
Effects of Probiotics on Gastrointestinal Cancer Cells
Probiotics and Gastric Cancer
H. pylori-mediated inflammation is one of the potential factors in the induction of gastric cancer in infected populations (Moss, 2017). Evidence evaluating the anti-gastric cancer effects of probiotics has focused on H. pylori-induced pathophysiology of this type of cancer. Maleki-Kakelar and others reported that by mediating numerous molecular pathways, Lactobacillus plantarum (L. plantarum) caused significant inhibitory effects on the H. pylori growth rate. Upon downregulation of the AKT gene and upregulation of the phosphatase and tensin homolog (PTEN), Bcl-2–associated X (Bax), and toll-like receptor 4 (TLR4), L. plantarum significantly inhibited the proliferation of AGS and CRL-1739 human gastric cell lines (Maleki-Kakelar et al., 2020). Interleukin-8 (IL-8) is an inflammatory chemokine that plays critical roles in inflammatory pathways (Meniailo et al., 2018). In the human gastric epithelial cell line-1 (GES-1), Lactobacillus bulgaricus (L. bulgaricus) inhibited the production of IL-8. In addition, Lactobacillus acidophilus (L. acidophilus) and L. bulgaricus inhibited adhesion of H. Pylori to GES-1 cells that attenuated inflammation in these cells (Song et al., 2019). Lin et al. reported that supplementation with Lactobacillus fermentum P2 (L. bacillus P2), L. casei L21, L. rhamnosus JB3, or their combination in H. pylori-infected mice reduced the expression level of interferon gamma (IFN-γ) along with interleukin-1 beta (IL-1β). Besides, H. pylori concentrations in the stomach of infected mice were decreased after probiotic supplementation (Lin et al., 2020). Another study demonstrated that L. acidophilus, L. plantarum, and L. rhamnosus supplementation significantly attenuated H. pylori-induced inflammation in vivo (Asgari et al., 2020). As mentioned earlier, most of the anti-gastric cancer studies have been directed at the inhibitory effects on H. pylori infection. Further experimental studies are needed for evaluating the effects of probiotic on gastric cancer inhibition mechanistically.
Ornithine decarboxylase is a crucial enzyme in the polyamine biosynthesis pathway and is responsible for catalyzing the decarboxylation of ornithine into putrescine (Svensson et al., 2008). Ornithine decarboxylase is a neovascularization agent in tumoral cells and has been overexpressed in tumors of epithelial origin including colorectal, prostate, and gastric cancers (Ma et al., 2007). Russo and others demonstrated that treatment with L. rhamnosus GG homogenate and cytoplasm extracts significantly decreased the activity of ornithine decarboxylase, reducing the polyamine content of HGC-27 human gastric cancer cells. Furthermore, in comparison with the untreated control group, probiotic treatment considerably increased the ratio of Bax/Bcl-2 (Russo et al., 2007). Xie and others reported that 8-day postoperative probiotic supplementation in gastric cancer patients significantly reduced diarrhea occurrence. Furthermore, in probiotic-induced patients, the expression level of interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α) was significantly decreased compared with that in patients in the control group (Xie et al., 2018).
The urokinase plasminogen activator (uPA) is an important serine proteinase enzyme which catalyzes the production of active protease plasmin from its proenzyme plasminogen (Pavón et al., 2016; Mahmood et al., 2018). The activation of the uPA is dependent on the expression of the uPA receptor (uPAR) in the cell surface. By increasing the activity of matrix metalloproteinases (MMPs), plasmin degrades extracellular matrix components, contributing to cancer metastasis and invasion (Beamish et al., 2019). Therefore, the activation of the uPA/uPAR system plays crucial roles in the induction of invasiveness and metastatic features in cancerous cells (Rubina et al., 2017). Rasouli et al. reported that treatment with Lactobacillus reuteri (L.reuteri), in AGS gastric cancer cells, downregulated the expression level of the uPA/uPAR gene (Rasouli et al., 2017). Table 1 provides a summary of studies on probiotics and gastric cancers. Nami et al. studied the anticancer effects of Lactobacillus plantarum species on human cancer cell lines (cervical, HeLa; gastric, AGS; colon, HT-29; and breast, MCF-7) and on a human normal cell line (HUVEC). The strain exhibited desirable probiotic properties and anticancer activity against the tested human cancer cell lines; no significant cytotoxic effects on normal cells were exhibited (Nami et al., 2014).
TABLE 1.
Probiotics and gastric cancer.
| Cancer cell line | Probiotic agent | Probiotic concentration | Duration of the study | Effect (s) | Model | Sample (n) | Ref. |
|---|---|---|---|---|---|---|---|
| AGS | Lactobacillus reuteri | 1.5 × 108 CFU/ml | 24, 48, and 72 h | Inhibited cell proliferation and decreased uPA and uPAR | In vitro | NA | Rasouli et al. (2017) |
| HGC-27 | Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG | 1 × 108 CFU/ml | 24 or 48 h | Induced apoptosis and inhibited tumor growth | In vitro | NA | Orlando et al. (2012) |
| NCI-N87 and AGS | Lactobacillus acidophilus 74-2 and Bifidobacterium lactis 420 | 8.24 × 107 and 2.20 × 108 CFU, respectively | NA | Upregulated the expression of COX-1 | In vitro | NA | Mahkonen et al. (2008) |
| HGC-27 | Lactobacillus rhamnosus GG (ATCC 53103) | 1 × 108 CFU/ml | 24 and 48 h | Reduced the polyamine content and neoplastic proliferation | In vitro | NA | Linsalata et al. (2010) |
| HGT-1 | Propionibacterium freudenreichii ITG P9 | 9 × 1012 CFU/ml | 24, 48, or 72 h | Induced caspase activation and cytochrome c release | In vitro | NA | Cousin et al. (2012) |
| AGS | Lactobacillus fermentum UCO-979C and Lactobacillus casei Shirota | 1.5 × 109 CFU/ml | 0–48 h | Inhibited urease activity of H. pylori | In vitro | NA | Salas-Jara et al. (2016) |
| AGS | Lactobacillus plantarum 5BL | NA | 12, 24, and 48 h | Induced anti-proliferative effects and apoptosis | In vitro | NA | Nami et al. (2014) |
| Postoperative patients with gastric cancer | NA | NA | 7–8 days | Decreased the expression of IL-6, IL-8, and TNF-α | Human | 70 | Xie et al. (2018) |
| Gastric cancer patients | Bifidobacterium | NA | 4 weeks | Decreased SIBO and symptoms of gastric cancer in the intervention group | Human | 112 | Liang et al. (2016) |
uPA, urokinase-type plasminogen activator; uPAR, urokinase-type plasminogen activator receptor; COX-1, cyclooxygenase 1; H. pylori, Helicobacter pylori; IL-6, interleukin 6; IL-8, interleukin 8; TNF-α, tumor necrosis factor alpha; SIBO, small intestine bacterial overgrowth.
Probiotics and Colon Cancer
Probiotics and Colon Cancer in Human Studies
One of the important goals in treating colorectal cancer patients is improving their quality of life. The role of probiotics in decreasing the symptoms and improving the quality of life in colorectal cancer patients has been evaluated at different stages of the disease. Lacidofil supplementation for 12 weeks in patients with colorectal cancer reduced the frequency of bowel symptoms while promoted functional well-being scores compared with those of patients in the placebo group (Lee et al., 2014). Zonulin (haptoglobin 2 precursor) is a regulator of tight junctions and intestinal permeability in the wall of the digestive tract (Sturgeon and Fasano, 2016). The increased serum level of zonulin was associated with autoimmunity, inflammatory diseases, and gastrointestinal cancers (Mörkl et al., 2018). Supplementation for 16 days (6 days preoperatively and 10 days postoperatively) with an admixture of L. plantarum, L. acidophilus-11, and B. longum-88 in colorectal cancer patients caused significant reduction in serum concentrations of zonulin as well as the duration of postoperative pyrexia, antibiotic therapy, and infectious complications in comparison with those in the placebo group. In addition, probiotic intervention inhibited the p38 mitogen-activated protein kinase signaling pathway (Liu et al., 2012). Yang and others reported that probiotic intervention with an admixture of B. longum, L. acidophilus, and Enterococcus faecalis (E. faecalis) for 12 days (5 days preoperatively and 7 days postoperatively) reduced the number of days to first defecation, days to first flatus, and diarrhea in the probiotic-treated group (Yang et al., 2016). 5-Fluorouracil (5-FU) is one of the most effective drugs for chemotherapy in colorectal cancer patients (Fu et al., 2019). However, its use is associated with diarrhea (Cheng et al., 2020). In a recent study, 24-week supplementation with L. rhamnosus GG in colorectal cancer patients who received 5-FU, the frequency of diarrhea was significantly decreased (Osterlund et al., 2007). Aisu et al. reported that supplementation with a probiotic mixture containing Enterococcus faecalis T110, Clostridium butyricum TO-A, and Bacillus mesentericus TO-A in colorectal cancer patients (n = 75) significantly diminished the occurrence of superficial incisional infection compared with that in untreated patients (Aisu et al., 2015). Fusobacterium is an important bacterial pathogen, which causes overexpression of E-cadherin/β-catenin and subsequent colorectal cancer proliferation (Zhou et al., 2018). Using an admixture of B. longum, L. acidophilus, and Enterococcus faecalis (E. faecalis) in colorectal cancer patients (n = 11) for 5 days significantly altered mucosa-associated microbiota of the intestine. Furthermore, probiotic intervention reduced the secretion of taxon assigned to the Fusobacterium (Gao et al., 2015). The treatment of colorectal cancer patients (n = 84) with a combination of probiotics, which consisted of L. acidophilus, L. plantarum, B. lactis, and Saccharomyces boulardii (1 day preoperatively and 15 days postoperatively), significantly decreased pneumonia, surgical site infections, anastomosis leakage, and need for mechanical ventilation compared with those who did not receive probiotic supplementation (Kotzampassi et al., 2015). Other studies evaluating the properties of probiotics in colorectal cancer patients are summarized in Table 2.
TABLE 2.
Probiotics and colon cancer in human studies.
| Subject | Probiotic agent | Probiotic concentration | Duration of the study | Effect (s) | Sample (n) | Ref. |
|---|---|---|---|---|---|---|
| Postoperative patients with colorectal cancer | Lactobacillus acidophilus LA-5, Lactobacillus plantarum, Bifidobacterium lactis BB-12, and Saccharomyces boulardii | 1.75 × 109, 0.5 × 109,1.75 × 109, and 1.75 × 109 CFU per capsule, respectively | 16 days (1 day prior to operation and 15 days after operation) | Modulated the gene expression of SOCS3 and significantly decreased postoperative complications including mechanical ventilation, infections, and anastomotic leakage | 84 | Kotzampassi et al. (2015) |
| Colorectal cancer | Bifidobacterium lactis | 1 × 109 CFU/gr | 4 weeks | The amounts of IL-1β, IL-2, IL-12, and hs-CRP in the probiotic group was significantly lower than those in symbiotic and prebiotic intervention groups | 19 | Worthley et al. (2009) |
| Perioperative patients with colorectal cancer | Bifidobacterium longum (BB536) and Lactobacillus johnsonii (La1) | 2 × 107 CFU/d and 2 × 109 CFU/d (two separate doses) | 8 days (3 days before operation and 5 days after operation) | The count of CD3, CD4, and CD8 in both of the intervention groups was greater than that in the placebo group | 11 and 10 (two groups) | Gianotti et al. (2010) |
| Perioperative patients with colon cancer | Bifidobacterium bifidum | 1 × 1010 CFU | 17 days (7 days before operation and 10 days after operation) | Surgical site infection in the probiotic group significantly decreased compared to that in the antibiotic group | 100 | Sadahiro et al. (2014) |
| Colorectal cancer | Bifidobacterium | NA | 4 weeks | Decreased the symptoms of colorectal cancer in the intervention group | 88 | Liang et al. (2016) |
| Colorectal cancer | Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052 | 2 × 109 CFU | 12 weeks | Attenuated bowel symptoms and improved quality of life in colorectal cancer subjects | 28 | Lee et al. (2014) |
| Perioperative patients with colorectal and colon cancer | Bacillus natto and Lactobacillus acidophilus | NA | 3 months | In the colonic group, defecation frequency, anal pain, and the Wexner score were significantly better than those in patients in the rectal cancer group | 77 | Ohigashi et al. (2011) |
| Perioperative patients with colorectal cancer | Enterococcus faecalis T110, Clostridium butyricum TO-A, and Bacillus mesentericus TO-A | 2 mg, 2 mg, and 10 mg, respectively, per each tablet | 6–30 days (3–15 days prior to and after the surgery) | Enhanced the immune responses and improved the intestinal microbial environment in the probiotic group | 75 | Aisu et al. (2015) |
| Healthy subjects | Bifidobacterium longum (BB536-y) | NA | 5 weeks | Inhibited colorectal carcinogenesis | 14 | Ohara and Suzutani, (2018) |
| Colorectal cancer | Lactobacillus acidophilus and Lactobacillus plantarum | NA | NA | Reduced the severity of colorectal cancer | 25 | Zinatizadeh et al. (2018) |
| Perioperative patients with colorectal cancer | Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis | 0.21 gr (1 × 108 CFU/gr) in each capsule | 3 days before operation | Promoted the expression levels of IgG and sIgA, while diminished the IL-6 and CRP serum in the intervention group | 30 | Zhang et al. (2012) |
| Perioperative patients with colorectal cancer | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium longum, and Bifidobacterium infantis | 3 × 1010 CFU | 7 days before operation | Hospital stay duration in the probiotic-administrated patients was shorter than that of the patients in the placebo group | 20 | Tan et al. (2016) |
| Colorectal cancer | Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis | 6 × 107 CFU | 5 days | Probiotic treatment altered the mucosal microbial flora | 11 | Gao et al. (2015) |
| Perioperative patients with colorectal cancer | Lactobacillus plantarum, Lactobacillus acidophilus, and Bifidobacterium longum | 2 g⁄day in a concentration of 2.6 × 1014 CFU | 16 days (6 days preoperatively and 10 days postoperatively) | Probiotic treatment upregulated the mucosal tight junction protein expression | 50 | Liu et al. (2011a) |
| Patients with colorectal tumors | Lactobacillus casei Shirota | 1 × 1010 CFU/gr | 4 years | Occurrence of tumors much significantly decreased in probiotic-administrated subjects compared with that in other groups | 99 | Ishikawa et al. (2005) |
| Perioperative patients with colorectal cancer | Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis | ≥3 × 107 CFU/gr | 12 days (5 days preoperatively and 7 days postoperatively) | The incidence of diarrhea in the probiotic group was lower than that in the placebo group | 30 | Yang et al. (2016) |
| Perioperative patients with colorectal cancer | Lactobacillus plantarum, Lactobacillus acidophilus 11,and Bifidobacterium longum 88 | 2.6 × 1014 CFU | 16 days (6 days preoperatively and 10 days postoperatively) | Treatment with the probiotic decreased the infection rate, serum zonulin concentration, and duration of antibiotic therapy | 75 | Liu et al. (2012) |
| Healthy subjects | Lactobacillus rhamnosus LC705 and Propionibacterium freudenreichii ssp. shermanii JS | 4 × 1010 CFU (2 × 1010 CFU of each strain per day) | 4 weeks | Probiotic supplementation decreased the activity of β-glucosidase | 37 | Hatakka et al. (2008) |
SOCS3 suppressor of cytokine signaling 3; IL-1β, interleukin 1 beta; IL-2, interleukin 2; IL-12, interleukin 12; hs-CRP, high-sensitivity C-reactive protein; IgG, immunoglobulin G; sIgA, sensitive immunoglobulin A; CRP, C-reactive protein.
Probiotics and Colon Cancer in Animal Studies
Probiotics exert their effects via activation or inhibition of cellular and molecular pathways (Figure 2). TNF-α is a pro-inflammatory cytokine which is produced by macrophages and T-cells and has numerous immunological roles in the regulation of inflammation (Farajzadeh et al., 2017). Mi et al. reported that chemotherapy induced significant increases in the levels of interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and TNF-α expression in rats. In turn, treatment with Bifidobacterium infantis (B. infantis) decreased the level of the aforementioned cytokines. Furthermore, probiotic treatment reduced the expression of cytokines related to Th17 and Th1 cells, and these changes led to decreased chemotherapy-induced mucositis (Mi et al., 2017). Ras-p21 is an oncoprotein and plays critical roles in the induction of different cancers (Banys-Paluchowski et al., 2018). In rats with azoxymethane-induced colorectal cancer, Bifidobacterium longum (B. longum) administration significantly suppressed the tumor volume, tumor incidence, cell proliferation, and the expression of ras-p21 (Singh et al., 1997). Administration of L. plantarum and L. rhamnosus promoted the expression of anti-oxidant enzymes such as glutathione, superoxide dismutase, catalase, glutathione reductase, glutathione peroxidase, and glutathione-S-transferase in rats with 1,2-dimethylhydrazine-induced colorectal cancer. Furthermore, the treatment increased the concentrations of pro-apoptotic agents, such as p53, B-cell lymphoma 2 (Bcl-2), BCL2-associated X (Bax), caspase-9, and caspase-3, which are involved in the p53-mediated apoptotic pathway (Walia et al., 2018). Walia and others demonstrated that 16-week supplementation with L. plantarum and L. rhamnosus decreased the expression of cyclooxygenase-2 (COX-2). Therefore, it appears that suppressing COX-2 is a potential protective mechanism against colon cancer development, leading to decreased tumor volume and incidence (Walia et al., 2015). Ki-67 is a tumor proliferative marker that is associated with the upper proliferation rate in various types of cancers (Sun and Kaufman, 2018). An admixture of L. fermentum and L. acidophilus in the mouse model of colorectal cancer reduced tumor growth, survival, and proliferation and decreased the expression of Ki-67 compared with those of the placebo group. Concomitantly, probiotic supplementation had no significant effects on the expression of cleaved caspase-3, E-cadherin, and β-catenin in comparison with that of the other group (Kahouli et al., 2017). In the dimethylhydrazine-induced colon cancer model, the probiotic strain L. rhamnosus GG suppressed the expression of β-catenin, COX-2, and TNF-α. Moreover, probiotic supplementation upregulated the expression of pro-apoptotic proteins Bax, p53, and caspase 3 and downregulated the expression of Bcl-2 as an anti-apoptotic agent (Kumar et al., 2012). Agah and others compared the efficacy of L. acidophilus and B. bifidum probiotic strains against the azoxymethane-induced mouse model of colon cancer. The results showed that the colonic lesions incidence was decreased after probiotic intervention compared with that of the control group, and these effects were more potent for L. acidophilus than for B. bifidum. Serum concentrations of tumor markers CEA and CA19-9 were reduced after treatment with probiotics, while the expression of interferon gamma (IFN-γ), interleukin-10(IL-10), and the count of CD4+ and CD8+ cells were upregulated upon intervention (Agah et al., 2019a). Wang et al. evaluated the efficacy of 12-week probiotic VSL#3 supplementation on azoxymethane/dextran sulfate sodium-induced colitis-associated carcinogenesis (1.5 × 109 CFU). Compared with that of the untreated group, probiotic supplementation downregulated the expression level of IL-6 and TNF-α in a considerable manner. Furthermore, probiotic intervention decreased the Oscillibacter and Lachnoclostridium genera, coupled with increased presence of Bacillus and Lactococcus genera in the fecal microbial composition of mice samples (Wang et al., 2018a). The c-Jun NH2-terminal kinase (JNK) is a major protein kinase which belongs to the MAPK signaling pathway and plays pivotal functions in the regulation of cell proliferation, cell death, apoptosis, and other features of cancerous cells (Wu et al., 2019). Considering its interfering role in different molecular pathways including NF-kB, JNK has binary roles in cancer development/progression (Tournier, 2013). By inhibiting the phosphorylation of glycogen synthase kinase 3 beta (GSK3β), JNK has suppressive effects on the expression of β-catenin (Hu et al., 2009). Ali et al. reported that L. casei probiotic supplementation in mice with 1,2-dimethylhydrazine-induced colon cancer significantly reduced the number of aberrant crypt foci compared with that in untreated animals. Furthermore, by upregulating the expression of phosphorylated JNK-1, L. casei regulated the expression of β-catenin and phosphorylated GSK3β, leading to significant protective effects against colon cancer (Ali et al., 2019). Sakatani and others have demonstrated that a L. brevis-derived polyphosphate significantly promoted the activation of the ERK signaling pathway, expression of cleaved PARP, and the ratio of cleaved PARP/PARP in SW620 colon cancer cells and mice bearing SW620 tumor xenografts. These changes led to increased apoptosis and inhibition of colon cancer growth (Sakatani et al., 2016a). By increasing the level of various inflammatory cytokines including IL-18, TNF-α, and TGF-β1, the NLR family pyrin domain–containing 3 (NLRP3) inflammasome can trigger metastasis in colon and colorectal cancer samples (Shaima’a Hamarsheh, 2020). The results of a recent study demonstrated that probiotic supplementation with the E. faecalis strain caused inhibitory effects on the activation of caspase-1 and maturation of IL-1β in vivo. Furthermore, E. faecalis suppressed the activation of NLRP3 inflammasome, and thereby protected animals from intestinal inflammation in dextran sodium sulfate-induced colitis-associated colorectal cancer (Chung et al., 2019a). Two-week intervention with L. casei in 1,2-dimethylhydrazine dihydrochloride-induced colon cancer in mice reduced the occurrence of chemical-induced aberrant crypt foci and the activity of ornithine decarboxylase. As noted previously, by promoting the polyamine metabolism in tumoral cells, ornithine decarboxylase has a pivotal function in the induction of cell proliferation. Hence, suppression of this enzyme in vivo diminished colon cancer growth and proliferation (Irecta-Nájera et al., 2017a). Numerous investigations have demonstrated that the expression level of insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) in colorectal cancer patients is associated with poor prognosis, chemoresistance, and increased invasiveness features (Shiratsuchi et al., 2011; Vigneri et al., 2015). Valadez-Bustos and others demonstrated that probiotic intervention with B. longum BAA-999 in the colorectal murine model reduced the expression level and activity of IGF-1/IGF-1R in a considerable manner. Furthermore, after probiotic supplementation, the expression level of insulin-like growth factor-binding protein-3 (IGFBP3) was normalized. Overall, the noted alterations led to reduction in the tumor volume and size (Valadez-Bustos et al., 2019). In a comprehensive in vivo investigation, Jacouton and others found that by decreasing the expression grade of IL-22 as a pro-inflammatory cytokine and upregulating the expression of caspase-7, caspase-9, and Bik, probiotic treatment with L. casei BL23 had significant anti-proliferative effects in the azoxymethane-induced colorectal cancer model (O'Mahony et al., 2001). Table 3 provides a summary of in vivo investigations on the efficacy of probiotics in colorectal cancers.
FIGURE 2.
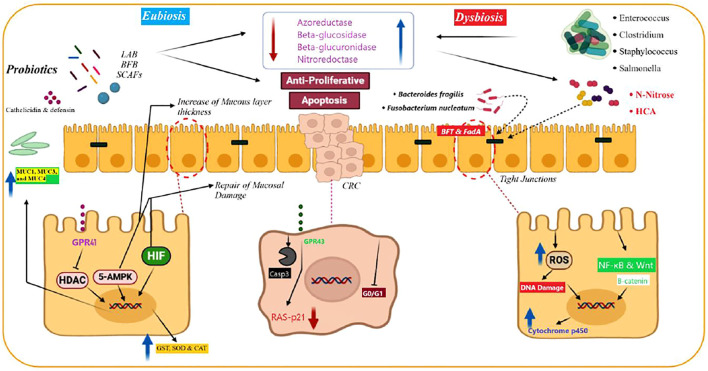
Physiological nonspecific mechanisms of probiotics for preventing and treating colorectal cancer (CRC). Probiotics produce short‐chain fatty acid (SCFA) and mediate apoptotic and anti-proliferative reactions in CRC cells. Produced SCAFs by probiotics protect the intestinal tract by preventing the histone deacetylases (HDACs) and overexpression of mucins, including MUC1, MUC3, and MUC4. SCFAs activate 5′‐adenosine monophosphate‐activated protein kinase. This is a critical factor in keeping the hypoxia‐inducible factor via SCAFs, which improves the epithelial duct’s survival and function. Probiotics elevate antimicrobial peptides, including defensin and (LL‐37) cathelicidin, from the intestinal mucosal layer. These peptides protect them against bacterial inoculation and invasion. Probiotics inhibit enzymatic activity of pathogenic bacteria, including enzymes such as nitroreductase, β‐glucuronidase, azoreductase, and β‐glucosidase. They also decrease the production of carcinogenic agents. Probiotics inhibit carcinogenic agents (N‐nitrous and heterocyclic aromatic amines [HCA]) by two mechanisms (deactivation and binding). They are potent mutagens and result in carcinogenic mutations in intestinal cells. Moreover, probiotics increase the antioxidant enzyme production and inactivate carcinogen‐deactivating agents, including glutathione reductase, glutathione‐S‐transferase (GST), superoxide dismutase (SOD), glutathione peroxidase, and catalase (CAT), and decrease their adverse effects. Besides, probiotics eliminate the risk of CRC development due to metabolites that have effects on the cytochrome p450. This figure is adapted from Eslami et al., (2019).
TABLE 3.
Probiotics and colon cancer in animal studies.
| Probiotic agent | Probiotic concentration | Duration of the study | Effect (s) | Ref. |
|---|---|---|---|---|
| Bifidobacterium longum BAA-999 | 8.992 × 1010 CFU/ml | 16 weeks | Regulated IGF-1, IGF-1R, and IGFBP3 protein expressions | Valadez-Bustos et al. (2019) |
| VSL#3 | 1.5 × 109 CFU | 3 months (5 days weekly) | The level of TNF-α and IL-6 was reduced in colon tissue and tumor load after probiotic intervention | Wang et al. (2018b) |
| VSL#3 | 109 CFU daily | 18 weeks | Altered the microbial composition | Arthur et al. (2013) |
| Lactobacillus plantarum | 1 × 109 CFU/ml | 8 months | Reduced β-galactosidase and β-glucuronidase activities. Besides, reduced the number of total coliforms | Čokášová et al. (2012) |
| Lactobacillus casei strain Shirota | 2.1 × 1010 | 8, 12, and 25 weeks | Significantly inhibited aberrant crypt foci and colon carcinogenesis | Yamazaki et al. (2000) |
| Lactobacillus fermentum and Lactobacillus plantarum | 2 × 108 CFU/g and 2 × 108 CFU/g | 21 days | Decreased the number of crypts in the mice and the activities of β-galactosidase and β-glucuronidase | Asha and Gayathri, (2012) |
| VSL#3 | 1.3 × 106 CFU | 44 days | Protected against carcinogenesis through regulating the IL-6/STAT3 signaling pathway | Do et al. (2016) |
| Saccharomyces boulardii | 3 × 108 CFU/ml and 6 × 108 CFU/ml | 9 weeks | Suppressed HER-2, HER-3, IGF-1R, EGFR-Erk, and EGFR-Akt expression levels and intestinal tumor growth | Chen et al. (2009) |
| Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus | less than 1 × 102 CFU/ml | 5 months | Reduced β-glucuronidase and nitroreductase activity | de Moreno de LeBlanc and Perdigón, (2005) |
| Lactobacillus casei ATCC 393 | 106 CFU | 2 weeks | Showed protective effects against ornithine decarboxylase activities | Irecta-Nájera et al. (2017b) |
| Lactobacillus acidophilus and Lactobacillus rhamnosus GG | 1 × 109 lactobacilli/0.1 ml | 18 weeks | Caused decrease in Bcl-2 and K-ras and increase in Bax and p53 expression levels. Promoted Bax-mediated apoptosis in colon carcinogenesis | Sharaf et al. (2018) |
| Lactobacillus rhamnosus GG MTCC #1408, Lactobacillus casei MTCC #1423, and Lactobacillus plantarum MTCC #1407 | 1 × 109 CFU/0.1 ml | 7 weeks | Probiotic administration decreased the activity of β-glucosidase | Verma and Shukla, (2013) |
| Lactobacillus casei BL23 | 5 × 109 CFU/ml | 53 days | Decreased the expression of IL-22 while increased the expression of caspase-7, -9, and Bik | Jacouton et al. (2017) |
| Lactobacillus salivarius ssp. salivarius UCC118 | NA | 16 weeks | Reduced the number of fecal coliform and enterococci levels | O'Mahony et al. (2001) |
| Enterococcus faecium CRL 183 | NA | 42 weeks | Increased the immune response by promoting the expression of NO, IL-4, IFN-γ, and TNF-α | Sivieri et al. (2008) |
| Lactobacillus acidophilus LaVK2 and Bifidobacterium bifidum BbVK3 | 2 × 109 CFU/g of each strain (20 g) | 32 weeks | Probiotics decreased the pre-neoplastic lesions and PCNA expression level | Mohania et al. (2014) |
| VSL#3 | 333 × 109 CFU/g | 115 days | Promoted angiostatin, VDR, and alkaline sphingomyelinase expression | Appleyard et al. (2011) |
| Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis | 1 × 107 CFU of each | 9 weeks | Alleviated colitis through regulating CXCR2 signaling | Song et al. (2018) |
| Enterococcus faecalis KH2 | 17 mg/kg | 2 weeks | Modulated the activity of the NLRP3 inflammasome and ameliorated colitis-associated colorectal cancer | Chung et al. (2019b) |
| B. bifidum (Bla/016P/M) and Lactobacillus acidophilus | 1 × 109 CFU/g of each strain | 10 days before tumor induction and 5 months after it | IFN-γ and IL-10 serum levels and the number of CD4+ and CD8+ cells were decreased after probiotic administration | Agah et al. (2019b) |
| Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium bifidum | 0.6 × 106 CFU of each strain | 1 week | Reduced the expression of RANTES, eotaxin, p-IKK, and TNF-α while increased IL-10 expression | Mendes et al. (2018) |
| Lactobacillus salivarius Ren | 5×108 and 1 × 1010 CFU/kg | 2 weeks | Prevented carcinogenesis by regulating the intestinal microflora | Zhu et al. (2014) |
| Lactobacillus rhamnosus GG CGMCC 1.2134 | 1 × 109 CFU/1 ml | 25 weeks | β-catenin, Bcl-2, NFkB-p65, COX-2, and TNF-α expression levels were decreased after probiotic intervention | Gamallat et al. (2016) |
| Lactobacillus plantarum AS1 | 109 CFU/ml | 26 weeks | Had antioxidant-induced prevention of colon carcinogenesis | Kumar et al. (2012) |
| Lactobacillus casei Zhang | 4 × 109 CFU | NA | Suppressed tumorigenesis through modulating various adiponectin-elevated signaling pathways | Zhang et al. (2017) |
| Lactobacillus casei BL23 and Lactococcus lactis MG1363 | 1 ± 0.4 × 109 CFU/mouse | 6 months | Along with the modulation of regulatory T-cells, promoted the expression of IL-6, IL-17, IL-10, and TGF-β | Lenoir et al. (2016) |
| Bacillus subtilis-SKm (KFCC11520P) and Lactococcus lactis-GAm (KFCC11510P) | 106 CFU/g of Bacillus subtilis-SKm and 106 CFU/g of Lactococcus lactis-GAm | 4 weeks | Probiotics decreased the expression of iNOS, COX-2, and Bcl-2 while increased Bax, p21, and p53 expression levels | Jeong et al. (2012) |
| VSL#3 | 333 × 109 CFU/g | 2 weeks | Reduced the expression of TNF-α, IL-1β, IL-6, and COX-2 while increased IL-10 expression | Talero et al. (2015) |
| Propionibacterium freudenreichii TL133 | 2 × 1010 CFU/ml | 18 days | Increased the induction of apoptosis | Lan et al. (2008) |
| VSL#3 | 1.2 × 109 bacteria per day | 32 days | Increased the expression of TNF-α, angiostatin, IL-17, and PPAR-γ | Bassaganya-Riera et al. (2012) |
| Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis and both of them | 5 × 107 CFU/g and 5 × 107 CFU/g and both strains (2.5 × 107 CFU/g for each strain) | 10 weeks | Increased the expression of caspase-3 and decreased the expression of Bcl-2 | Lin et al. (2019) |
| Lactobacillus acidophilus | 1010 CFU/ml | 12 weeks | Adenomas have been reported to be decreased after probiotic administration | Urbanska et al. (2009) |
| Streptococcus thermophilus CRL807 and Lactobacillus delbrueckii subsp. bulgaricus CRL864 | NA | 5 days | Prevented colitis and carcinogenesis via modulating anti-inflammatory responses | Del Carmen et al. (2016) |
| Lactobacillus plantarum (AdF10) and Lactobacillus rhamnosus GG (LGG) | 1 × 1010 CFU | 16 weeks | Regulated COX-2 expression | Walia et al. (2015) |
| VSL#3 | 1.3×106 bacteria | 8 weeks | Diminished the severity of colitis and tumor growth | Chung et al. (2017) |
| Lactobacillus acidophilus | 2 × 108 CFU/ml | 1 month | Attenuated COX‐2, iNOS, and c‐Myc expression levels | Deol et al. (2018) |
| Lactobacillus plantarum (AdF10) and Lactobacillus rhamnosus GG (LGG) | 1010 CFU/ml | 16 weeks | Had chemopreventive effects | Walia et al. (2018) |
| Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 | At least 50 × 109 CFU/g of strains | 12 weeks | Decreased the activity of β-glucosidase and β-glucuronidase along with the reduction in aberrant crypt foci counts | Desrouillères et al. (2015) |
| Lactobacillus plantarum A and Lactobacillus rhamnosus b | 1 × 108 CFU for 14 consecutive days, then 1 × 109 CFU for 3 weeks | 5 weeks | Increased production of IFN-γ and promoted Th1-type CD4+ T differentiation | Hu et al. (2015a) |
| Streptococcus thermophilus CRL807, Streptococcus thermophilus CRL807, Streptococcus thermophilus CRL807, Lactococcus lactis subsp. cremoris MG1363, Lactococcus lactis subsp. cremoris MG1363, and Lactococcus lactis subsp. cremoris MG1363 | 1 × 1010 CFU/ml | 6 months | Exerted anti-tumorigenic properties via increasing antioxidant enzymes and IL-10 expression level | Del Carmen et al. (2017) |
| Lactobacillus acidophilus (NCK 2025) | 5 × 108 CFU | 4 weeks | Regulated inflammation and prevented colonic polyposis | Khazaie et al. (2012) |
| Lactobacillus acidophilus (Delvo Pro LA-1), Lactobacillus rhamnosus (GG), Bifidobacterium animalis (CSCC 1941), and Streptococcus thermophilus (DD145) | 1010 CFU/g | 4 weeks | Suppressed DMH-induced colon cancer in rats | McIntosh et al. (1999) |
| Bifidobacterium longum | NA | NA | Exerted anti-proliferative and anti-oxidative properties | Allen et al. (2015) |
| Bifidobacterium adolescentis SPM0212 | 1 × 108 CFU | 3 weeks | Inhibited activity of harmful enzymes and proliferation | Kim et al. (2008) |
IGF-1, insulin-like growth factor 1; IGF-1R, insulin-like growth factor 1 receptor; IGFBP3, insulin-like growth factor-binding protein 3; TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6; STAT3, signal transducer and activator of transcription 3; HER-2, human epidermal growth factor receptor 2; HER-3, human epidermal growth factor receptor 3; EGFR, epidermal growth factor receptor; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2–ssociated X; IL-22, interleukin 22; Bik, Bcl-2–interacting killer; IL-4, interleukin 4; IFN-γ, interferon gamma; PCNA, proliferating cell nuclear antigen; CXCR2, CXC chemokine receptor 2; NLRP3, NLR family pyrin domain–containing 3; RANTES, regulated upon activation, normal T cell expressed, and presumably secreted; IL-10, interleukin 10; NF-κB, nuclear factor kappa B; COX-2, cyclooxygenase 2; IL-17, interleukin 17; TGF-β, transforming growth factor beta; iNOS, inducible nitric oxide synthase; IL-1β, interleukin 1 beta; PPAR-γ, peroxisome proliferator-activated receptor gamma.
Probiotics and Colon Cancer in In Vitro Studies
As mentioned earlier, caspase-3 is a pro-apoptotic factor and its decreased levels are associated with the shortened survival time in various types of cancers (Vince et al., 2018). Bacillus coagulans (B. coagulans) Unique IS2 exerted anti-proliferative and pro-apoptotic properties in the COLO 205 human colon cancer cell line. By activating the p-53-mediated apoptotic pathway, treatment with probiotics increased the expression of BAX, activation of caspase-3, cleavage of poly (ADP-ribose) polymerase, and release of cytochrome C. Furthermore, B. coagulans reduced the mitochondrial membrane potential and Bcl2 expression level (Madempudi and Kalle, 2017). Orlando and others reported that L. rhamnosus GG intervention in Caco-2, HT-29, and SW480 colon cancer cell lines upregulated the Bax/Bcl-2 ratio, increasing apoptosis in these cells (Orlando et al., 2016). The cyclin family is a group of cell cycle regulators. Their aberrant expression is associated with tumorigenesis (Wood and Endicott, 2018). Intervention with L. paracasei subsp. paracasei reduced the expressions of cyclin D1 and cyclin E1 X12 in HT-29 colon cancer cells. In addition, probiotic intervention upregulated the expression of p27 as a cyclin-dependent kinase (CDK) inhibitor (Huang et al., 2016). CKD inhibition represents a potential mechanism for suppressing over proliferation of cancer cells induced by aberrant regulation of the cyclin family (Sánchez-Martínez et al., 2019). PTEN (phosphatase and tensin homolog) has been demonstrated to be a prominent tumor suppressor gene, which plays critical roles in the dephosphorylation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) (Di Cristofano et al., 1998). The additional evidence indicated that downregulation of PTEN is associated with increased tumor growth and survival. Therefore, targeting PTEN inhibitors is one of the most effective means for decreasing the tumor incidence, tumor volume, and tumor growth rate (Lee et al., 2019a). Sambrani et al. demonstrated, in HT-29 colon cancer cells, that treatment with Saccharomyces cerevisiae (S. cerevisiae) caused a significant upregulation in the expression of PTEN and caspase-3, while the expression levels of Bcl-xL and RelA were markedly decreased after probiotic intervention (Sambrani et al., 2019). Pichia kudriavzevii AS-12 treatment showed considerable cytotoxic properties in HT-29 and Caco-2 cells compared with those in normal control cells. In addition, Pichia kudriavzevii upregulated the expression of pro-apoptotic agents including Fas-R, caspase-3, -8, and -9, and BAD protein, while the expression of anti-apoptotic Bcl-2 was decreased after yeast probiotic treatment in mentioned cell lines (Saber et al., 2017). Bacillus polyfermenticus treatment reduced ErbB2, ErbB3, cyclin D1, and E2F-1 transcription factor in HT-29, DLD-1, and Caco-2 colon cancer cells. These changes led to the suppression of over proliferation of cancerous cells (Ma et al., 2010). In another study, Lee et al. investigated the anti-cancer effects of the B. adolescentis-derived butanol extract in Caco-2, HT-29, and SW480 colorectal cell lines. The results showed that the butanol extract significantly promoted the activation of macrophages and upregulated the production of TNF-α and nitric oxide in tumor cells. These changes led to the induction of cytotoxic and anti-proliferative properties against colorectal cancer (Lee et al., 2008). Survivin is an anti-apoptotic agent, which has been reported to be a crucial agent in the inhibition of apoptosis and subsequent tumor growth, proliferation, metastases, and invasiveness in various types of cancer, especially in colorectal cancer (Hernandez et al., 2011). Tiptiri-Kourpeti et al. demonstrated that L. casei ATCC 393 administration (109 CFU/ml) in CT26 and HT29 colon carcinoma cells upregulated the expression of the ligand TRAIL, which was induced by TNF-mediated apoptosis. Furthermore, L. casei declined the level of survivin expression (Tiptiri-Kourpeti et al., 2016). By significantly decreasing the expression of Bcl-2 and remarkable up-regulation in the expression grade of pro-apoptotic agents Bak and Bax, probiotic intervention with L. paracasei K5 showed anti-proliferative effects in Caco-2 cells (Chondrou et al., 2018). In another investigation, Chen et al. reported that various strains of Lactobacillus genera in HT-29 colon cancer cells promoted the expression level of the Bax protein, while decreasing the expression of Bcl-2, leading to a notable increase in the Bax/Bcl-2 ratio. Furthermore, the increased lactate dehydrogenase activity and the ensuing degradation of the cell membrane of tumor cells were observed (Chen et al., 2017). A summary of mechanistic in vitro investigations on probiotics and colon cancer is summarized in Table 4.
TABLE 4.
Probiotics and colon cancer (in vitro).
| Cancer cell line | Probiotic agent | Probiotic concentration | Effect (s) | Ref. |
|---|---|---|---|---|
| SW620 | Lactobacillus brevis SBL8803 | NA | Via activating the Erk pathway and inhibiting tumor growth | Sakatani et al. (2016b) |
| SW620 | Lactobacillus delbrueckii | NA | Through triggering the caspase 3-mediated pathway and decreasing Bcl-2 and caused apoptosis. Besides, MMP-9 was decreased after intervention | Zhou et al. (2014) |
| SW742 | Bifidobacterium | NA | Inhibited the growth of cancer cells | Otte et al. (2008) |
| SW742 | Bifidobacterium and Lactobacillus | NA | Prevented the development of colorectal cancer | Bahmani et al. (2019) |
| Colo320 and SW480 | Lactobacillus acidophilus, Escherichia coli Nissle 1917, and the probiotic mixture VSL#3 | 1 × 106 CFU/ml | Regulated the expression of COX-2 | Otte et al. (2008) |
| SW480 and HCT-116 | Lactococcus lactis | NA | Induced apoptosis in human colon cancer cells and increased the ratio of f Bax/Bcl2 | Bohlul et al. (2019) |
| HCT-116 | Lactobacillus fermentum | NA | Lactobacillus cell-free supernatant activated the intrinsic apoptosis pathway | Lee et al. (2019b) |
| HCT-116 | Lactobacillus plantarum 27 (NCDC 012), Lactobacillus casei (NCDC 297), and Lactobacillus brevis (NCDC 021) | NA | Exerted anti-proliferative activities. Inhibited activity of α-glucosidase and α-amylase | Mushtaq et al. (2019) |
| HCT-116 | Lactobacillus sp., Lactobacillus casei, and Lactobacillus rhamnosus GG | 109–1011 CFU/ml | Decreased the expression of MMP-9 and increased protein levels of ZO-1 | Escamilla et al. (2012) |
| HCT-116 | Pediococcus pentosaceus GS4 | 1.1 × 109 CFU/ml | Downregulated NF-κB and p-Akt signaling pathways | Dubey et al. (2016) |
| HCT-116, AGS, A549, MCF-7, and HepG2 | Aspergillus sp | NA | Exhibited anti-tumor properties | Choi et al. (2011) |
| HT-29, HCT-116, and Caco-2 | Bifidobacterium bifidum BGN4 | NA | Inhibited the growth of cancer cell lines | You et al. (2004) |
| HT-29 | Lactobacillus casei K11, Lactobacillus casei M5, Lactobacillus casei SB27, and Lactobacillus casei × 12 | NA | Cell cycle arrest induced at the G0/G1 phase | Di et al. (2018) |
| HT-29 | Lactobacillus kefiri (SGL 13) | 5 × 108 CFU/ml | Increased Bax expression and decreased the caspase 3, mutant p53, and IL-8 expression | Brandi et al. (2019) |
| HT-29 | Enterococcus faecium YF5 | 1 × 1011 CFU | Inhibited foodborne pathogens | Tan et al. (2013) |
| HT-29 | Lactobacillus acidophilus 145 and Bifidobacterium longum 913 | 106–108 and 105 CFU/g | Increased oxidative-induced damage | Oberreuther-Moschner et al. (2004) |
| Caco-2 and HT-29 | Lactobacillus rhamnosus MD 14 | NA | Showed anti-genotoxic and cytotoxic properties against colon cancer | Sharma et al. (2019) |
| HT-29 | Lactobacillus casei 01 | 109 CFU/ml | Exerted cytotoxic effects | Liu et al. (2011b) |
| HT-29 | Lactobacillus casei ATCC 393, Lactobacillus plantarum ATCC 14917,and Lactobacillus paracasei K5 | 109 CFU/ml | Caused a significant decrease in proliferation of cancer cells in a time- and dose-dependent manner | Mantzourani et al. (2019) |
| HT-29 and Caco-2 | VSL3(Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus plantarum, Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, and Streptococcus thermophilus) | NA | Increased the expression of PPARγ | Ewaschuk et al. (2006) |
| HT-29 and L-929 | Lactobacillus paracasei and Lactobacillus brevis | NA | Induced apoptosis in cancer cells | Mojibi et al. (2019) |
| HT-29 | Lactobacillus acidophilus 606 | NA | Exerted anti-tumorigenic properties by inducing the expression of Beclin-1, GRP78, Bcl-2, and Bak | Kim et al. (2010) |
| HT-29 and HCT-116 | Lactobacillus plantarum | NA | Increased the activity of caspase-3 and suppressed the Wnt/β-catenin signaling pathway. Therefore, reversed chemoresistance and enhanced the therapeutic effect of 5-FU in colon cancer | Mirzaei et al. (2016) |
| HT-29 and HCT-116 | Lactobacillus spp | 3 × 108 CFU/ml | Down-regulated expression of IL-1β and TNF-α.cfos and cjun transcripts were significantly upregulated after probiotic intervention | Shyu et al. (2014) |
| HT-29 | Lactobacillus paracasei subsp. paracasei M5L | 109 CFU/ml | Via generating ROS production, inducing cell cycle arrest, and calreticulin translocation | Hu et al. (2015b) |
| HT-29 | Leuconostoc mesenteroides | NA | By regulating MAPK1, Bax, and caspase 3 and downregulation of Akt, NF-Kb, and Bcl-XL promoted apoptosis. Besides, suppressed the expression of miRNA-21 and miRNA-200b | Zununi Vahed et al. (2017) |
| HT-29, Caco2, and HeLa | Propionibacterium acidipropionici strain CNRZ80, Propionibacterium freudenreichii subsp. freudenreichii strain ITG18, and Propionibacterium freudenreichii subsp. shermanii strain SI41 | NA | Via short-chain fatty acids acting on the mitochondria, caused apoptosis in cancer cells | Jan et al. (2002) |
| HT-29 and HCT-116 | Propionibacterium freudenreichii | NA | Induced apoptosis by increasing pro-apoptotic gene expression (TRAIL-R2/DR5) and decreasing FLIP and XIAP. | Cousin et al. (2016) |
| Caco-2 | Bifidobacterium animalis subsp. lactis DSM10140, Bifidobacterium longum subsp. longum DSM20097, and Bifidobacterium breve DSM20213 | >5.0 logs CFU/g | Caused remarkable cytotoxic activities | Ayyash et al. (2018) |
| Caco-2 | Lactobacillus rhamnosus and Bifidobacterium lactis | 108 CFU/ml | Induced FAS-independent apoptosis and increased BAX translocation and release of cytochrome c and cleavage of caspase-3 and -9 | Altonsy et al. (2010) |
| Caco-2 and HT-29 | Lactobacillus plantarum A7 and Lactobacillus rhamnosus GG | NA | Decreased the growth rate of cancer cells | Sadeghi-Aliabadi et al. (2014) |
| Caco-2 | Escherichia coli Nissle 1917 | 25 × 107 CFU | Decreased ROS generation | Wang et al. (2015) |
| Caco-2 | Lactobacillus plantarum | NA | Upregulated the mRNA expression of HBD-2 and modulated the TLR-2 and IL-23 expression | Paolillo et al. (2009) |
| Caco-2 | Lactobacillus paracasei | 108 CFU/ml | Inhibited the mRNA expressions of CXCR4 | Nozari et al. (2019) |
| Caco-2 | Pediococcus pentosaceus FP3, Lactobacillus salivarius FP25, Lactobacillus salivarius FP35, and Enterococcus faecium FP51 | NA | Triggered the biosynthesis of short-chain fatty acids | Thirabunyanon and Hongwittayakorn, (2013) |
| Caco-2 and CLS | Enterococcus faecium RM11 and Lactobacillus fermentum RM28 | NA | Triggered anti-proliferative activities in colon cancer cells | Thirabunyanon et al. (2009) |
| Caco2, SKCO-1, SW620, and IEC-18 | Lactobacillus casei ATCC334 | NA | Suppressed colon cancer progression via affecting the JNK pathway | Konishi et al. (2016) |
| DLD-1 | Lactobacillus rhamnosus strain GG | 108 CFU/ml | Exerted anti-proliferative effects | Orlando et al. (2009) |
| DLD-1 | Lactobacillus rhamnosus (LR) KCTC 12202BP | NA | Inhibited cell proliferation through affecting the p53-p21-cyclin B1/Cdk1 signaling pathway | An et al. (2019) |
| TC-1 | Lactobacillus casei BL23, Lactococcus lactis MG1363, and Lactococcus lactis NZ9000 | 1 × 109 CFU of each strain or recombinant | Probiotic strain Lactobacillus casei BL23 caused IL-2-mediated anti-tumoral properties | Jacouton et al. (2018) |
| CT-26 | Lactobacillus casei variety rhamnosus (Lcr35) | 1 × 103–7 CFU of the probiotics | Downregulated the expression of TNF-α and IL-6 | Chang et al. (2018) |
| CT-26 | Lactobacillus acidophilus NCFM | 1 × 108 CFU | Suppressed tumor growth in intestinal tissue | Chen et al. (2012) |
| MCF-7, HT-29, HeLa, HepG2, HL60, K562, and MCF-10A | Lactobacillus plantarum strains | NA | Caused anti-proliferative and pro-apoptotic effects against malignant cancer cells | Chuah et al. (2019) |
| LS513 | Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R | 108 CFU/ml | Via upregulating the caspase-3 protein and enhanced the pro-apoptotic capacity of the 5-FU. | Baldwin et al. (2010) |
Probiotics and Other Gastrointestinal Cancer
The mitogen-activated protein kinase (MAPK) signaling pathway has crucial roles in the induction of intracellular responses from extracellular signals in cells. Aberrant regulation of this pathway leads to numerous homeostatic and pathologic sequels, such as cancer (Chapnick et al., 2011). In the KB oral cancer cell line, probiotic intervention with L. plantarum reduced the expression of MAPK and caused significant upregulation in the expression of PTEN signaling transduction (Asoudeh-Fard et al., 2017). Zhang and others evaluated the properties of Lactobacillus salivarius (L. salivarius) REN supplementation in an animal model of 4-nitroquinoline-1-oxide-induced oral cancer. By decreasing the expression level of COX-2 and proliferating cell nuclear antigen (PCNA), L. salivarius intervention had significant inhibitory effects on tumor growth of oral cancer (Zhang et al., 2013). Another study demonstrated that Acetobacter syzygii and L. acidophilus (PTCC 1643) probiotic strains caused significant cytotoxicity and inhibitory effects against the KB cancer cell line (Aghazadeh et al., 2017). Barrett’s esophagus is a pathological condition in which the lining of the distal esophagus is damaged due to the exposure of the esophagus to stomach acid. In this situation, squamous epithelium of the esophagus is replaced by columnar epithelium (Spechler, 2013). Barrett’s esophagus plays a critical role in the induction of esophageal cancer and acts as an important risk factor for development of esophageal cancer (Conteduca et al., 2012). Mozaffari Namin et al. reported that B. longum and L. acidophilus treatment of Barrett’s esophagus cell lines downregulated the expression of CDX1 (caudal type homeobox 1), COX-2, TNF-α, and p53, while the expression level of IL-18 was enhanced after intervention of both probiotic strains (Mozaffari Namin et al., 2015). Table 5 provides a summary on the effectiveness of probiotics for oral, esophageal, and pancreatic cancer.
TABLE 5.
Probiotics and other gastrointestinal cancers.
| Cancer | Probiotic agent | Probiotic concentration | Duration of the study | Effect (s) | Model | Sample (n) | Ref. |
|---|---|---|---|---|---|---|---|
| Oral cancer | Lactobacillus plantarum | NA | 2, 6, and 24 h | Displayed apoptosis effects via upregulating PTEN and downregulating MAPK signaling pathways | In vitro | NA | Asoudeh-Fard et al. (2017) |
| Oral cancer | Lactobacillus salivarius REN | 5 × 1010 CFU/kg per day | 32 weeks | Inhibited rat oral cancer progression through regulating the expression of COX-2 and PCNA. | In vivo | NA | Zhang et al. (2013) |
| Oral cancer | Acetobacter syzygii and Lactobacillus acidophilus (PTCC 1643) | Acetobacter syzygii: 60 μg/ml and Lactobacillus acidophilus: 10 μg/ml | 24 h | Exhibited cytotoxicity against cancer cell lines | In vitro | NA | Aghazadeh et al. (2017) |
| Barrett’s esophagus | Bifidobacterium longum and Lactobacillus acidophilus | 3 × 107 bacteria or microbes/ml | 1, 3, 5, and 7 h | Increased the expression of IL-18 while decreased the expression of CDX1, COX-2, and TNF-α | In vitro | NA | Mozaffari Namin et al. (2015) |
PTEN, phosphatase and tensin homolog; MAPK, mitogen-activated protein kinase; COX-2, cyclooxygenase 2; PCNA, proliferating cell nuclear antigen; IL-18, interleukin 18; CDX1, caudal type homeobox 1; COX-2, cyclooxygenase 2; TNF-α, tumor necrosis factor alpha.
Conclusion
Owing to their effects on different aspects of host health, probiotics have been demonstrated to be important tools in clinical medicine. Various investigations using a plethora of experimental models, including in vitro, animal models, and human clinical studies, have shown that by inducing anti-carcinogenic properties, anti-mutagenic effects, producing short-chain fatty acids, activating the immune system of the hosts, inhibiting the bacteria-induced conversion of pro-carcinogens to carcinogens, and reducing intestinal pH (which results in reduced microbial activity), probiotics can assist in the prevention and treatment of gastrointestinal cancers. Nonetheless, to date, the benefits of probiotic strains as bio-therapeutic agents have not been adequately investigated against GI cancers. Moreover, the clinical efficacy of probiotics, especially on mortality, remains largely unexplored. Hence, more clinical studies with adequate follow-up durations are needed to obtain a clearer understanding on the potential utility of various strains and optimal doses for the administration of probiotics as pharmacological tools to combat GI cancers.
Author Contributions
HM and AS involved in conception, design, statistical analysis and drafting of the manuscript. AD, FF, ZB, HF, MA-K, and MA contributed in data collection and manuscript drafting. All authors approved the final version for submission. MT, MG, ORT, and VT, critically revised the manuscript. All authors approved the new authorship changes.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdul Khalek F. J., Gallicano G. I., Mishra L. (2010). Colon Cancer Stem Cells. Gastrointest. Cancer Res. (Suppl. 1), S16–S23. [PMC free article] [PubMed] [Google Scholar]
- Agah S., Alizadeh A. M., Mosavi M., Ranji P., Khavari-Daneshvar H., Ghasemian F., et al. (2019). More protection of Lactobacillus Acidophilus Than Bifidobacterium Bifidum Probiotics on Azoxymethane-Induced Mouse colon Cancer. Probiotics Antimicrob. Proteins 11 (3), 857–864. 10.1007/s12602-018-9425-8 [DOI] [PubMed] [Google Scholar]
- Agah S., Alizadeh A. M., Mosavi M., Ranji P., Khavari-Daneshvar H., Ghasemian F., et al. (2019). More Protection of Lactobacillus Acidophilus Than Bifidobacterium Bifidum Probiotics on Azoxymethane-Induced Mouse Colon Cancer. Probiotics Antimicrob. Proteins 11 (3), 857–864. 10.1007/s12602-018-9425-8 [DOI] [PubMed] [Google Scholar]
- Aghazadeh Z., Pouralibaba F., Yari Khosroushahi A. (2017). The Prophylactic Effect of Acetobacter Syzygii Probiotic Species against Squamous Cell Carcinoma. J. Dent Res. Dent Clin. Dent Prospects 11 (4), 208–214. 10.15171/joddd.2017.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisu N., Tanimura S., Yamashita Y., Yamashita K., Maki K., Yoshida Y., et al. (2015). Impact of Perioperative Probiotic Treatment for Surgical Site Infections in Patients with Colorectal Cancer. Exp. Ther. Med. 10 (3), 966–972. 10.3892/etm.2015.2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarouk K. O., Bashir A. H. H., Aljarbou A. N., Ramadan A. M., Muddathir A. K., AlHoufie S. T. S., et al. (2019). The Possible Role of Helicobacter pylori in Gastric Cancer and its Management. Front. Oncol. 9 (75), 75. 10.3389/fonc.2019.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. S., Hussein R. M., Gaber Y., Hammam O. A., Kandeil M. A. (2019). Modulation of JNK-1/β-catenin Signaling byLactobacillus Casei, Inulin and Their Combination in 1,2-Dimethylhydrazine-Induced colon Cancer in Mice. RSC Adv. 9 (50), 29368–29383. 10.1039/c9ra04388h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour Nosrani E., Tamtaji O. R., Alibolandi Z., Sarkar P., Ghazanfari M., Azami Tameh A., et al. (2021). Neuroprotective Effects of Probiotics Bacteria on Animal Model of Parkinson's Disease Induced by 6-hydroxydopamine: A Behavioral, Biochemical, and Histological Study. J. Immunoassay Immunochemistry 42 (2), 106–120. 10.1080/15321819.2020.1833917 [DOI] [PubMed] [Google Scholar]
- Allen J. L., Verghese M., Shackelfor L., Boateng J., Walker L. T. (2015). Chemopreventive Potential of Soy Flour, Flaxseed Meal and a Probiotic in a Rat Model. Int. J. Cancer Res. 11 (2), 67–79. 10.3923/ijcr.2015.67.79 [DOI] [Google Scholar]
- Altonsy M. O., Andrews S. C., Tuohy K. M. (2010). Differential Induction of Apoptosis in Human Colonic Carcinoma Cells (Caco-2) by Atopobium, and Commensal, Probiotic and Enteropathogenic Bacteria: Mediation by the Mitochondrial Pathway. Int. J. Food Microbiol. 137 (2-3), 190–203. 10.1016/j.ijfoodmicro.2009.11.015 [DOI] [PubMed] [Google Scholar]
- An B. C., Hong S., Park H. J., Kim B. K., Ahn J. Y., Ryu Y., et al. (2019). Anti-Colorectal Cancer Effects of Probiotic-Derived P8 Protein. Genes (Basel) 10 (8), 624. 10.3390/genes10080624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard C. B., Cruz M. L., Isidro A. A., Arthur J. C., Jobin C., De Simone C. (2011). Pretreatment with the Probiotic VSL#3 Delays Transition from Inflammation to Dysplasia in a Rat Model of Colitis-Associated Cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 301 (6), G1004–G1013. 10.1152/ajpgi.00167.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. C., Gharaibeh R. Z., Uronis J. M., Perez-Chanona E., Sha W., Tomkovich S., et al. (2013). VSL#3 Probiotic Modifies Mucosal Microbial Composition but Does Not Reduce Colitis-Associated Colorectal Cancer. Sci. Rep. 3, 2868. 10.1038/srep02868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemi Z., Jazayeri S., Najafi M., Samimi M., Mofid V., Shidfar F., et al. (2012). Effect of Daily Consumption of Probiotic Yogurt on Oxidative Stress in Pregnant Women: a Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 60 (1), 62–68. 10.1159/000335468 [DOI] [PubMed] [Google Scholar]
- Asemi Z., Samimi M., Tabasi Z., Talebian P., Azarbad Z., Hydarzadeh Z., et al. (2012). Effect of Daily Consumption of Probiotic Yoghurt on Lipid Profiles in Pregnant Women: a Randomized Controlled Clinical Trial. J. Matern. Fetal Neonatal. Med. 25 (9), 1552–1556. 10.3109/14767058.2011.640372 [DOI] [PubMed] [Google Scholar]
- Asgari B., Kermanian F., Hedayat Yaghoobi M., Vaezi A., Soleimanifar F., Yaslianifard S. (2020). The Anti-Helicobacter pylori Effects of Lactobacillus Acidophilus, L. Plantarum, and L. Rhamnosus in Stomach Tissue of C57BL/6 Mice. Visc. Med. 36 (2), 137–143. 10.1159/000500616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha D., Gayathri D. (2012). Synergistic Impact of Lactobacillus Fermentum, Lactobacillus Plantarum and Vincristine on 1,2-Dimethylhydrazine-Induced Colorectal Carcinogenesis in Mice. Exp. Ther. Med. 3 (6), 1049–1054. 10.3892/etm.2012.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M., Zarrabi A., Hashemipour M., Vosough M., Najafi M., Shahinozzaman M., et al. (2020). Sensing the Scent of Death: Modulation of microRNAs by Curcumin in Gastrointestinal Cancers. Pharmacol. Res. 160, 105199. 10.1016/j.phrs.2020.105199 [DOI] [PubMed] [Google Scholar]
- Asoudeh-Fard A., Barzegari A., Dehnad A., Bastani S., Golchin A., Omidi Y. (2017). Lactobacillus Plantarum Induces Apoptosis in Oral Cancer KB Cells through Upregulation of PTEN and Downregulation of MAPK Signalling Pathways. Bioimpacts 7 (3), 193–198. 10.15171/bi.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyash M., Johnson S. K., Liu S.-Q., Al-Mheiri A., Abushelaibi A. (2018). Cytotoxicity, Antihypertensive, Antidiabetic and Antioxidant Activities of Solid-State Fermented Lupin, Quinoa and Wheat by Bifidobacterium Species: In-Vitro Investigations. LWT 95, 295–302. 10.1016/j.lwt.2018.04.099 [DOI] [Google Scholar]
- Bahmani F., Tajadadi-Ebrahimi M., Kolahdooz F., Mazouchi M., Hadaegh H., Jamal A. S., et al. (2016). The Consumption of Synbiotic Bread Containing Lactobacillus Sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 35 (6), 506–513. 10.1080/07315724.2015.1032443 [DOI] [PubMed] [Google Scholar]
- Bahmani S., Azarpira N., Moazamian E. (2019). Anti-colon Cancer Activity of Bifidobacterium Metabolites on colon Cancer Cell Line SW742. Turk J. Gastroenterol. 30 (9), 835–842. 10.5152/tjg.2019.18451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C., Millette M., Oth D., Ruiz M. T., Luquet F. M., Lacroix M. (2010). Probiotic Lactobacillus Acidophilus and L. Casei Mix Sensitize Colorectal Tumoral Cells to 5-Fluorouracil-Induced Apoptosis. Nutr. Cancer 62 (3), 371–378. 10.1080/01635580903407197 [DOI] [PubMed] [Google Scholar]
- Banys-Paluchowski M., Fehm T., Janni W., Aktas B., Fasching P. A., Kasimir-Bauer S., et al. (2018). Elevated Serum RAS P21 Is an Independent Prognostic Factor in Metastatic Breast Cancer. BMC cancer 18 (1), 541. 10.1186/s12885-018-4282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassaganya-Riera J., Viladomiu M., Pedragosa M., De Simone C., Hontecillas R. (2012). Immunoregulatory Mechanisms Underlying Prevention of Colitis-Associated Colorectal Cancer by Probiotic Bacteria. PLoS One 7 (4), e34676. 10.1371/journal.pone.0034676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish J. A., Juliar B. A., Cleveland D. S., Busch M. E., Nimmagadda L., Putnam A. J. (2019). Deciphering the Relative Roles of Matrix Metalloproteinase- and Plasmin-Mediated Matrix Degradation during Capillary Morphogenesis Using Engineered Hydrogels. J. Biomed. Mater. Res. B Appl. Biomater. 107 (8), 2507–2516. 10.1002/jbm.b.34341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H., Bernstein C., Payne C. M., Dvorakova K., Garewal H. (2005). Bile Acids as Carcinogens in Human Gastrointestinal Cancers. Mutat. Res. 589 (1), 47–65. 10.1016/j.mrrev.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Biasco G., Paganelli G. M., Brandi G., Brillanti S., Lami F., Callegari C., et al. (1991). Effect of Lactobacillus Acidophilus and Bifidobacterium Bifidum on Rectal Cell Kinetics and Fecal pH. Ital. J. Gastroenterol. 23 (3), 142. [PubMed] [Google Scholar]
- Bohlul E., Hasanlou F., Taromchi A. H., Nadri S. (2019). TRAIL-expressing Recombinant Lactococcus Lactis Induces Apoptosis in Human colon Adenocarcinoma SW480 and HCT116 Cells. J. Appl. Microbiol. 126 (5), 1558–1567. 10.1111/jam.14237 [DOI] [PubMed] [Google Scholar]
- Brandi J., Di Carlo C., Manfredi M., Federici F., Bazaj A., Rizzi E., et al. (2019). Investigating the Proteomic Profile of HT-29 Colon Cancer Cells after Lactobacillus Kefiri SGL 13 Exposure Using the SWATH Method. J. Am. Soc. Mass. Spectrom. 30 (9), 1690–1699. 10.1007/s13361-019-02268-6 [DOI] [PubMed] [Google Scholar]
- Butt J., Epplein M. (2019). Helicobacter pylori and Colorectal Cancer-A Bacterium Going Abroad? Plos Pathog. 15 (8), e1007861–e. 10.1371/journal.ppat.1007861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill D. P., Kinzler K. W., Vogelstein B., Lengauer C. (1999). Genetic Instability and Darwinian Selection in Tumours. Trends Cel Biol 9 (12), M57–M60. 10.1016/s0962-8924(99)01661-x [DOI] [PubMed] [Google Scholar]
- Chang C. W., Liu C. Y., Lee H. C., Huang Y. H., Li L. H., Chiau J. C., et al. (2018). Lactobacillus Casei Variety Rhamnosus Probiotic Preventively Attenuates 5-Fluorouracil/Oxaliplatin-Induced Intestinal Injury in a Syngeneic Colorectal Cancer Model. Front. Microbiol. 9, 983. 10.3389/fmicb.2018.00983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapnick D. A., Warner L., Bernet J., Rao T., Liu X. (2011). Partners in Crime: the TGFβ and MAPK Pathways in Cancer Progression. Cell Biosci 1 (1), 42–48. 10.1186/2045-3701-1-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Lin W. C., Kong M. S., Shi H. N., Walker W. A., Lin C. Y., et al. (2012). Oral Inoculation of Probiotics Lactobacillus Acidophilus NCFM Suppresses Tumour Growth Both in Segmental Orthotopic colon Cancer and Extra-intestinal Tissue. Br. J. Nutr. 107 (11), 1623–1634. 10.1017/S0007114511004934 [DOI] [PubMed] [Google Scholar]
- Chen X., Fruehauf J., Goldsmith J. D., Xu H., Katchar K. K., Koon H. W., et al. (2009). Saccharomyces Boulardii Inhibits EGF Receptor Signaling and Intestinal Tumor Growth in Apc(min) Mice. Gastroenterology 137 (3), 914–923. 10.1053/j.gastro.2009.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Y., Hsieh Y. M., Huang C. C., Tsai C. C. (2017). Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules 22 (1), 107. 10.3390/molecules22010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Zhao L., Ju Z., Wang F., Qin M., Mao H., et al. (2020). Effects of 10.6-μm Laser Moxibustion and Electroacupuncture at ST36 in a 5-Fu-Induced Diarrhea Rat Model. Support Care Cancer, 1–9. 10.1007/s00520-020-05788-0 [DOI] [PubMed] [Google Scholar]
- Choi E. J., Park J. S., Kim Y. J., Jung J. H., Lee J. K., Kwon H. C., et al. (2011). Apoptosis-inducing Effect of Diketopiperazine Disulfides Produced by Aspergillus Sp. KMD 901 Isolated from marine Sediment on HCT116 colon Cancer Cell Lines. J. Appl. Microbiol. 110 (1), 304–313. 10.1111/j.1365-2672.2010.04885.x [DOI] [PubMed] [Google Scholar]
- Chondrou P., Karapetsas A., Kiousi D. E., Tsela D., Tiptiri-Kourpeti A., Anestopoulos I., et al. (2018). Lactobacillus Paracasei K5 Displays Adhesion, Anti-proliferative Activity and Apoptotic Effects in Human colon Cancer Cells. Benef Microbes 9 (6), 975–983. 10.3920/BM2017.0183 [DOI] [PubMed] [Google Scholar]
- Chuah L. O., Foo H. L., Loh T. C., Mohammed Alitheen N. B., Yeap S. K., Abdul Mutalib N. E., et al. (2019). Postbiotic Metabolites Produced by Lactobacillus Plantarum Strains Exert Selective Cytotoxicity Effects on Cancer Cells. BMC Complement. Altern. Med. 19 (1), 114. 10.1186/s12906-019-2528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E. J., Do E. J., Kim S. Y., Cho E. A., Kim D. H., Pak S., et al. (2017). Combination of Metformin and VSL#3 Additively Suppresses Western-Style Diet Induced colon Cancer in Mice. Eur. J. Pharmacol. 794, 1–7. 10.1016/j.ejphar.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Chung I. C., OuYang C. N., Yuan S. N., Lin H. C., Huang K. Y., Wu P. S., et al. (2019). Pretreatment with a Heat-Killed Probiotic Modulates the NLRP3 Inflammasome and Attenuates Colitis-Associated Colorectal Cancer in Mice. Nutrients 11 (3), 516. 10.3390/nu11030516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I. C., OuYang C. N., Yuan S. N., Lin H. C., Huang K. Y., Wu P. S., et al. (2019). Pretreatment with a Heat-Killed Probiotic Modulates the NLRP3 Inflammasome and Attenuates Colitis-Associated Colorectal Cancer in Mice. Nutrients 11 (3). 10.3390/nu11030516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čokášová D., Bomba A., Strojný L., Pramuková B., Szabadosová V., Salaj R., et al. (2012). The Effect of New Probiotic Strain Lactobacillus Plantarum on Counts of Coliforms, Lactobacilli and Bacterial Enzyme Activities in Rats Exposed to N, N-Dimethylhydrazine (Chemical Carcinogen). Acta Veterinaria Brno 81 (2), 189–194. [Google Scholar]
- Conteduca V., Sansonno D., Ingravallo G., Marangi S., Russi S., Lauletta G., et al. (2012). Barrett's Esophagus and Esophageal Cancer: an Overview. Int. J. Oncol. 41 (2), 414–424. 10.3892/ijo.2012.1481 [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Hill C., Ross R. P. (2005). Bacteriocins: Developing Innate Immunity for Food. Nat. Rev. Microbiol. 3 (10), 777–788. 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- Cousin F. J., Jouan-Lanhouet S., Dimanche-Boitrel M. T., Corcos L., Jan G. (2012). Milk Fermented by Propionibacterium Freudenreichii Induces Apoptosis of HGT-1 Human Gastric Cancer Cells. PLoS One 7 (3), e31892. 10.1371/journal.pone.0031892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin F. J., Jouan-Lanhouet S., Théret N., Brenner C., Jouan E., Le Moigne-Muller G., et al. (2016). The Probiotic Propionibacterium Freudenreichii as a New Adjuvant for TRAIL-Based Therapy in Colorectal Cancer. Oncotarget 7 (6), 7161–7178. 10.18632/oncotarget.6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodvandi A., Marzban H., Goleij P., Sahebkar A., Morshedi K., Rezaei S., et al. (2021). Effects of Therapeutic Probiotics on Modulation of microRNAs. Cell Commun Signal 19 (1), 4. 10.1186/s12964-020-00668-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moreno de LeBlanc A., Perdigón G. (2005). Reduction of Beta-Glucuronidase and Nitroreductase Activity by Yoghurt in a Murine colon Cancer Model. Biocell 29 (1), 15–24. [PubMed] [Google Scholar]
- De Preter V., Hamer H. M., Windey K., Verbeke K. (2011). The Impact of Pre- And/or Probiotics on Human Colonic Metabolism: Does it Affect Human Health? Mol. Nutr. Food Res. 55 (1), 46–57. 10.1002/mnfr.201000451 [DOI] [PubMed] [Google Scholar]
- Del Carmen S., de Moreno de LeBlanc A., LeBlanc J. G. (2016). Development of a Potential Probiotic Yoghurt Using Selected Anti-inflammatory Lactic Acid Bacteria for Prevention of Colitis and Carcinogenesis in Mice. J. Appl. Microbiol. 121 (3), 821–830. 10.1111/jam.13213 [DOI] [PubMed] [Google Scholar]
- Del Carmen S., de Moreno de LeBlanc A., Levit R., Azevedo V., Langella P., Bermúdez-Humarán L. G., et al. (2017). Anti-cancer Effect of Lactic Acid Bacteria Expressing Antioxidant Enzymes or IL-10 in a Colorectal Cancer Mouse Model. Int. Immunopharmacol 42, 122–129. 10.1016/j.intimp.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Deol P. K., Khare P., Bishnoi M., Kondepudi K. K., Kaur I. P. (2018). Coadministration of Ginger Extract-Lactobacillus Acidophilus (Cobiotic) Reduces Gut Inflammation and Oxidative Stress via Downregulation of COX-2, I-NOS, and C-Myc. Phytother Res. 32 (10), 1950–1956. 10.1002/ptr.6121 [DOI] [PubMed] [Google Scholar]
- Desrouillères K., Millette M., Vu K. D., Touja R., Lacroix M. (2015). Cancer Preventive Effects of a Specific Probiotic Fermented Milk Containing Lactobacillus Acidophilus CL1285, L. Casei LBC80R and L. Rhamnosus CLR2 on Male F344 Rats Treated with 1, 2-dimethylhydrazine. J. Funct. Foods 17, 816–827. [Google Scholar]
- Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P. P. (1998). Pten Is Essential for Embryonic Development and Tumour Suppression. Nat. Genet. 19 (4), 348–355. 10.1038/1235 [DOI] [PubMed] [Google Scholar]
- Di W., Zhang L., Yi H., Han X., Zhang Y., Xin L. (2018). Exopolysaccharides Produced by Lactobacillus Strains Suppress HT-29 Cell Growth via Induction of G0/G1 Cell Cycle Arrest and Apoptosis. Oncol. Lett. 16 (3), 3577–3586. 10.3892/ol.2018.9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do E. J., Hwang S. W., Kim S. Y., Ryu Y. M., Cho E. A., Chung E. J., et al. (2016). Suppression of Colitis-Associated Carcinogenesis through Modulation of IL-6/STAT3 Pathway by Balsalazide and VSL#3. J. Gastroenterol. Hepatol. 31 (8), 1453–1461. 10.1111/jgh.13280 [DOI] [PubMed] [Google Scholar]
- Dubey V., Ghosh A. R., Bishayee K., Khuda-Bukhsh A. R. (2016). Appraisal of the Anti-cancer Potential of Probiotic Pediococcus Pentosaceus GS4 against colon Cancer: In Vitro and In Vivo Approaches. J. Funct. Foods 23, 66–79. 10.1016/j.jff.2016.02.032 [DOI] [Google Scholar]
- Escamilla J., Lane M. A., Maitin V. (2012). Cell-free Supernatants from Probiotic Lactobacillus Casei and Lactobacillus Rhamnosus GG Decrease colon Cancer Cell Invasion In Vitro . Nutr. Cancer 64 (6), 871–878. 10.1080/01635581.2012.700758 [DOI] [PubMed] [Google Scholar]
- Eslami M., Yousefi B., Kokhaei P., Hemati M., Nejad Z. R., Arabkari V., et al. (2019). Importance of Probiotics in the Prevention and Treatment of Colorectal Cancer. J. Cel Physiol 234 (10), 17127–17143. 10.1002/jcp.28473 [DOI] [PubMed] [Google Scholar]
- Ewaschuk J. B., Walker J. W., Diaz H., Madsen K. L. (2006). Bioproduction of Conjugated Linoleic Acid by Probiotic Bacteria Occurs In Vitro and In Vivo in Mice. J. Nutr. 136 (6), 1483–1487. 10.1093/jn/136.6.1483 [DOI] [PubMed] [Google Scholar]
- Falzone L., Salomone S., Libra M. (2018). Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 9, 1300. 10.3389/fphar.2018.01300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh D., Karimi-Gharigh S., Dastmalchi S. (2017). Tumor Necrosis Factor-Alpha and its Inhibition Strategies. Tehran Univ. Med. J. 75 (3), 159–171. [Google Scholar]
- Floch M. H., Walker W. A., Madsen K., Sanders M. E., Macfarlane G. T., Flint H. J., et al. (2011). Recommendations for Probiotic Use-2011 Update. J. Clin. Gastroenterol. 45, S168–S171. 10.1097/MCG.0b013e318230928b [DOI] [PubMed] [Google Scholar]
- Fu J., Xu Y., Yang Y., Liu Y., Ma L., Zhang Y. (2019). Aspirin Suppresses Chemoresistance and Enhances Antitumor Activity of 5-Fu in 5-Fu-Resistant Colorectal Cancer by Abolishing 5-Fu-Induced NF-Κb Activation. Sci. Rep. 9 (1), 16937. 10.1038/s41598-019-53276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallat Y., Meyiah A., Kuugbee E. D., Hago A. M., Chiwala G., Awadasseid A., et al. (2016). Lactobacillus Rhamnosus Induced Epithelial Cell Apoptosis, Ameliorates Inflammation and Prevents colon Cancer Development in an Animal Model. Biomed. Pharmacother. 83, 536–541. 10.1016/j.biopha.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Ganguly N., Bhattacharya S., Sesikeran B., Nair G., Ramakrishna B., Sachdev H., et al. (2011). ICMR-DBT Guidelines for Evaluation of Probiotics in Food. Indian J. Med. Res. 134 (1), 22–25. [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Guo B., Gao R., Zhu Q., Wu W., Qin H. (2015). Probiotics Modify Human Intestinal Mucosa-Associated Microbiota in Patients with Colorectal Cancer. Mol. Med. Rep. 12 (4), 6119–6127. 10.3892/mmr.2015.4124 [DOI] [PubMed] [Google Scholar]
- Garrett W. S. (2015). Cancer and the Microbiota. Science 348 (6230), 80–86. 10.1126/science.aaa4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti L., Morelli L., Galbiati F., Rocchetti S., Coppola S., Beneduce A., et al. (2010). A Randomized Double-Blind Trial on Perioperative Administration of Probiotics in Colorectal Cancer Patients. World J. Gastroenterol. 16 (2), 167–175. 10.3748/wjg.v16.i2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górska A., Przystupski D., Niemczura M. J., Kulbacka J. (2019). Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 76 (8), 939–949. 10.1007/s00284-019-01679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakka K., Mutanen M., Holma R., Saxelin M., Korpela R. (2008). Lactobacillus Rhamnosus LC705 Together with Propionibacterium Freudenreichii Ssp Shermanii JS Administered in Capsules Is Ineffective in Lowering Serum Lipids. J. Am. Coll. Nutr. 27 (4), 441–447. 10.1080/07315724.2008.10719723 [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Hayatsu T. (1993). Suppressing Effect of Lactobacillus Casei Administration on the Urinary Mutagenicity Arising from Ingestion of Fried Ground Beef in the Human. Cancer Lett. 73 (2-3), 173–179. 10.1016/0304-3835(93)90261-7 [DOI] [PubMed] [Google Scholar]
- Hernandez J. M., Farma J. M., Coppola D., Hakam A., Fulp W. J., Chen D. T., et al. (2011). Expression of the Antiapoptotic Protein Survivin in colon Cancer. Clin. Colorectal Cancer 10 (3), 188–193. 10.1016/j.clcc.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban R. H., Adsay N. V., Albores-Saavedra J., Compton C., Garrett E. S., Goodman S. N., et al. (2001). Pancreatic Intraepithelial Neoplasia: a New Nomenclature and Classification System for Pancreatic Duct Lesions. Am. J. Surg. Pathol. 25 (5), 579–586. 10.1097/00000478-200105000-00003 [DOI] [PubMed] [Google Scholar]
- Hruban R. H., Takaori K., Klimstra D. S., Adsay N. V., Albores-Saavedra J., Biankin A. V., et al. (2004). An Illustrated Consensus on the Classification of Pancreatic Intraepithelial Neoplasia and Intraductal Papillary Mucinous Neoplasms. Am. J. Surg. Pathol. 28 (8), 977–987. 10.1097/01.pas.0000126675.59108.80 [DOI] [PubMed] [Google Scholar]
- Hu D., Bi X., Fang W., Han A., Yang W. (2009). GSK3beta Is Involved in JNK2-Mediated Beta-Catenin Inhibition. PLoS One 4 (8), e6640. 10.1371/journal.pone.0006640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Wang C., Ye L., Yang W., Huang H., Meng F., et al. (2015). Anti-tumour Immune Effect of Oral Administration of Lactobacillus Plantarum to CT26 Tumour-Bearing Mice. J. Biosci. 40 (2), 269–279. 10.1007/s12038-015-9518-4 [DOI] [PubMed] [Google Scholar]
- Hu P., Song W., Shan Y., Du M., Huang M., Song C., et al. (2015). Lactobacillus Paracasei Subsp. Paracasei M5L Induces Cell Cycle Arrest and Calreticulin Translocation via the Generation of Reactive Oxygen Species in HT-29 Cell Apoptosis. Food Funct. 6 (7), 2257–2265. 10.1039/c5fo00248f [DOI] [PubMed] [Google Scholar]
- Huang L., Shan Y.-J., He C.-X., Ren M.-H., Tian P.-J., Song W. (2016). Effects of L. Paracasei Subp. Paracasei X12 on Cell Cycle of colon Cancer HT-29 Cells and Regulation of mTOR Signalling Pathway. J. Funct. Foods 21, 431–439. 10.1016/j.jff.2015.12.024 [DOI] [Google Scholar]
- Irecta-Nájera C. A., del Rosario Huizar-López M., Casas-Solís J., Castro-Félix P., Santerre A. (2017). Protective Effect of Lactobacillus Casei on DMH-Induced colon Carcinogenesis in Mice. Probiotics Antimicrob. Proteins 9 (2), 163–171. [DOI] [PubMed] [Google Scholar]
- Irecta-Nájera C. A., Del Rosario Huizar-López M., Casas-Solís J., Castro-Félix P., Santerre A. (2017). Protective Effect of Lactobacillus Casei on DMH-Induced Colon Carcinogenesis in Mice. Probiotics Antimicrob. Proteins 9 (2), 163–171. 10.1007/s12602-017-9253-2 [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Akedo I., Otani T., Suzuki T., Nakamura T., Takeyama I., et al. (2005). Randomized Trial of Dietary Fiber and Lactobacillus Casei Administration for Prevention of Colorectal Tumors. Int. J. Cancer 116 (5), 762–767. 10.1002/ijc.21115 [DOI] [PubMed] [Google Scholar]
- Jacouton E., Chain F., Sokol H., Langella P., Bermúdez-Humarán L. G. (2017). Probiotic Strain Lactobacillus Casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front. Immunol. 8, 1553. 10.3389/fimmu.2017.01553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacouton E., Michel M. L., Torres-Maravilla E., Chain F., Langella P., Bermúdez-Humarán L. G. (2018). Elucidating the Immune-Related Mechanisms by Which Probiotic Strain Lactobacillus Casei BL23 Displays Anti-tumoral Properties. Front. Microbiol. 9, 3281. 10.3389/fmicb.2018.03281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan G., Belzacq A. S., Haouzi D., Rouault A., Métivier D., Kroemer G., et al. (2002). Propionibacteria Induce Apoptosis of Colorectal Carcinoma Cells via Short-Chain Fatty Acids Acting on Mitochondria. Cell Death Differ 9 (2), 179–188. 10.1038/sj.cdd.4400935 [DOI] [PubMed] [Google Scholar]
- Javanmard A., Ashtari S., Sabet B., Davoodi S. H., Rostami-Nejad M., Esmaeil Akbari M., et al. (2018). Probiotics and Their Role in Gastrointestinal Cancers Prevention and Treatment; an Overview. Gastroenterol. Hepatol. Bed Bench 11 (4), 284–295. [PMC free article] [PubMed] [Google Scholar]
- Jeong J. K., Chang H. K., Park K. Y. (2012). Inhibitory Effects of Meju Prepared with Mixed Starter Cultures on Azoxymethane and Dextran Sulfate Sodium-Induced colon Carcinogenesis in Mice. J. Carcinog 11, 13. 10.4103/1477-3163.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Xie G., Jia W. (2018). Bile Acid-Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15 (2), 111–128. 10.1038/nrgastro.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahouli I., Malhotra M., Westfall S., Alaoui-Jamali M. A., Prakash S. (2017). Design and Validation of an Orally Administrated Active L. Fermentum-L. Acidophilus Probiotic Formulation Using Colorectal Cancer Apc Min/+ Mouse Model. Appl. Microbiol. Biotechnol. 101 (5), 1999–2019. 10.1007/s00253-016-7885-x [DOI] [PubMed] [Google Scholar]
- Khazaie K., Zadeh M., Khan M. W., Bere P., Gounari F., Dennis K., et al. (2012). Abating colon Cancer Polyposis by Lactobacillus Acidophilus Deficient in Lipoteichoic Acid. Proc. Natl. Acad. Sci. U S A. 109 (26), 10462–10467. 10.1073/pnas.1207230109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoder G., Al-Menhali A. A., Al-Yassir F., Karam S. M. (2016). Potential Role of Probiotics in the Management of Gastric Ulcer. Exp. Ther. Med. 12 (1), 3–17. 10.3892/etm.2016.3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. C., Lance P. (1997). Colorectal Polyps and Their Relationship to Cancer. Gastroenterol. Clin. North. Am. 26 (1), 1–17. 10.1016/s0889-8553(05)70280-6 [DOI] [PubMed] [Google Scholar]
- Kim Y., Lee D., Kim D., Cho J., Yang J., Chung M., et al. (2008). Inhibition of Proliferation in colon Cancer Cell Lines and Harmful Enzyme Activity of colon Bacteria by Bifidobacterium Adolescentis SPM0212. Arch. Pharm. Res. 31 (4), 468–473. 10.1007/s12272-001-1180-y [DOI] [PubMed] [Google Scholar]
- Kim Y., Oh S., Yun H. S., Oh S., Kim S. H. (2010). Cell-bound Exopolysaccharide from Probiotic Bacteria Induces Autophagic Cell Death of Tumour Cells. Lett. Appl. Microbiol. 51 (2), 123–130. 10.1111/j.1472-765X.2010.02859.x [DOI] [PubMed] [Google Scholar]
- Konishi H., Fujiya M., Tanaka H., Ueno N., Moriichi K., Sasajima J., et al. (2016). Probiotic-derived Ferrichrome Inhibits colon Cancer Progression via JNK-Mediated Apoptosis. Nat. Commun. 7, 12365. 10.1038/ncomms12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzampassi K., Stavrou G., Damoraki G., Georgitsi M., Basdanis G., Tsaousi G., et al. (2015). A Four-Probiotics Regimen Reduces Postoperative Complications after Colorectal Surgery: a Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 39 (11), 2776–2783. 10.1007/s00268-015-3071-z [DOI] [PubMed] [Google Scholar]
- Kumar M., Kumar A., Nagpal R., Mohania D., Behare P., Verma V., et al. (2010). Cancer-preventing Attributes of Probiotics: an Update. Int. J. Food Sci. Nutr. 61 (5), 473–496. 10.3109/09637480903455971 [DOI] [PubMed] [Google Scholar]
- Kumar R. S., Kanmani P., Yuvaraj N., Paari K. A., Pattukumar V., Thirunavukkarasu C., et al. (2012). Lactobacillus Plantarum AS1 Isolated from South Indian Fermented Food Kallappam Suppress 1,2-dimethyl Hydrazine (DMH)-induced Colorectal Cancer in Male Wistar Rats. Appl. Biochem. Biotechnol. 166 (3), 620–631. 10.1007/s12010-011-9453-2 [DOI] [PubMed] [Google Scholar]
- Lalla R. V., Bowen J., Barasch A., Elting L., Epstein J., Keefe D. M., et al. (2014). MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 120 (10), 1453–1461. 10.1002/cncr.28592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan A., Bruneau A., Bensaada M., Philippe C., Bellaud P., Rabot S., et al. (2008). Increased Induction of Apoptosis by Propionibacterium Freudenreichii TL133 in Colonic Mucosal Crypts of Human Microbiota-Associated Rats Treated with 1,2-dimethylhydrazine. Br. J. Nutr. 100 (6), 1251–1259. 10.1017/S0007114508978284 [DOI] [PubMed] [Google Scholar]
- Lee D. K., Jang S., Kim M. J., Kim J. H., Chung M. J., Kim K. J., et al. (2008). Anti-proliferative Effects of Bifidobacterium Adolescentis SPM0212 Extract on Human colon Cancer Cell Lines. BMC cancer 8 (1), 310. 10.1186/1471-2407-8-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., Lee J., Kim J. H., Cho N., Lee S. H., Park S. B., et al. (2019). Characterization of the Anti-cancer Activity of the Probiotic Bacterium Lactobacillus Fermentum Using 2D vs. 3D Culture in Colorectal Cancer Cells. Biomolecules 9 (10), 557. 10.3390/biom9100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Chu S. H., Jeon J. Y., Lee M. K., Park J. H., Lee D. C., et al. (2014). Effects of 12 Weeks of Probiotic Supplementation on Quality of Life in Colorectal Cancer Survivors: a Double-Blind, Randomized, Placebo-Controlled Trial. Dig. Liver Dis. 46 (12), 1126–1132. 10.1016/j.dld.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Lee Y. R., Chen M., Lee J. D., Zhang J., Lin S. Y., Fu T. M., et al. (2019). Reactivation of PTEN Tumor Suppressor for Cancer Treatment through Inhibition of a MYC-WWP1 Inhibitory Pathway. Science 364 (6441). 10.1126/science.aau0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M., Del Carmen S., Cortes-Perez N. G., Lozano-Ojalvo D., Muñoz-Provencio D., Chain F., et al. (2016). Lactobacillus Casei BL23 Regulates Treg and Th17 T-Cell Populations and Reduces DMH-Associated Colorectal Cancer. J. Gastroenterol. 51 (9), 862–873. 10.1007/s00535-015-1158-9 [DOI] [PubMed] [Google Scholar]
- Li L., Neaves W. B. (2006). Normal Stem Cells and Cancer Stem Cells: the Niche Matters. Cancer Res. 66 (9), 4553–4557. 10.1158/0008-5472.CAN-05-3986 [DOI] [PubMed] [Google Scholar]
- Liang S., Xu L., Zhang D., Wu Z. (2016). Effect of Probiotics on Small Intestinal Bacterial Overgrowth in Patients with Gastric and Colorectal Cancer. Turk J. Gastroenterol. 27 (3), 227–232. 10.5152/tjg.2016.15375 [DOI] [PubMed] [Google Scholar]
- Lidbeck A., Övervik E., Rafter J., Nord C. E., Gustafsson J.-A. (1992). Effect of Lactobacillus Acidophilus Supplements on Mutagen Excretion in Faeces and Urine in Humans. Microb. Ecol. Health Dis. 5 (1), 59–67. 10.3109/08910609209141305 [DOI] [Google Scholar]
- Lin C. C., Huang W. C., Su C. H., Lin W. D., Wu W. T., Yu B., et al. (2020). Effects of Multi-Strain Probiotics on Immune Responses and Metabolic Balance in Helicobacter Pylori-Infected Mice. Nutrients 12 (8), 2476. 10.3390/nu12082476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Beerm D. G. (2004). Molecular Biology of Upper Gastrointestinal Malignancies. Semin. Oncol. 31 (4), 476–486. 10.1053/j.seminoncol.2004.04.019 [DOI] [PubMed] [Google Scholar]
- Lin P. Y., Li S. C., Lin H. P., Shih C. K. (2019). Germinated Brown rice Combined with Lactobacillus Acidophilus and Bifidobacterium Animalis Subsp. Lactis Inhibits Colorectal Carcinogenesis in Rats. Food Sci. Nutr. 7 (1), 216–224. 10.1002/fsn3.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsalata M., Cavallini A., Messa C., Orlando A., Refolo M. G., Russo F. (2010). Lactobacillus Rhamnosus GG Influences Polyamine Metabolism in HGC-27 Gastric Cancer Cell Line: a Strategy toward Nutritional Approach to Chemoprevention of Gastric Cance. Curr. Pharm. Des. 16 (7), 847–853. 10.2174/138161210790883598 [DOI] [PubMed] [Google Scholar]
- Liong M. T. (2008). Roles of Probiotics and Prebiotics in colon Cancer Prevention: Postulated Mechanisms and In-Vivo Evidence. Int. J. Mol. Sci. 9 (5), 854–863. 10.3390/ijms9050854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. T., Chu F. J., Chou C. C., Yu R. C. (2011). Antiproliferative and Anticytotoxic Effects of Cell Fractions and Exopolysaccharides from Lactobacillus Casei 01. Mutat. Res. 721 (2), 157–162. 10.1016/j.mrgentox.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Liu Z., Qin H., Yang Z., Xia Y., Liu W., Yang J., et al. (2011). Randomised Clinical Trial: the Effects of Perioperative Probiotic Treatment on Barrier Function and post-operative Infectious Complications in Colorectal Cancer Surgery - a Double-Blind Study. Aliment. Pharmacol. Ther. 33 (1), 50–63. 10.1111/j.1365-2036.2010.04492.x [DOI] [PubMed] [Google Scholar]
- Liu Z. H., Huang M. J., Zhang X. W., Wang L., Huang N. Q., Peng H., et al. (2012). The Effects of Perioperative Probiotic Treatment on Serum Zonulin Concentration and Subsequent Postoperative Infectious Complications after Colorectal Cancer Surgery: a Double-center and Double-Blind Randomized Clinical Trial. Am. J. Clin. Nutr. 97 (1), 117–126. 10.3945/ajcn.112.040949 [DOI] [PubMed] [Google Scholar]
- Lynch J. P., Hoops T. C. (2002). The Genetic Pathogenesis of Colorectal Cancer. Hematol. Oncol. Clin. North. Am. 16 (4), 775–810. 10.1016/s0889-8588(02)00029-1 [DOI] [PubMed] [Google Scholar]
- Ma E. L., Choi Y. J., Choi J., Pothoulakis C., Rhee S. H., Im E. (2010). The Anticancer Effect of Probiotic Bacillus Polyfermenticus on Human colon Cancer Cells Is Mediated through ErbB2 and ErbB3 Inhibition. Int. J. Cancer 127 (4), 780–790. 10.1002/ijc.25011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Wang Y., Gao X., Ma Z., Song Z. (2007). L-arginine Reduces Cell Proliferation and Ornithine Decarboxylase Activity in Patients with Colorectal Adenoma and Adenocarcinoma. Clin. Cancer Res. 13 (24), 7407–7412. 10.1158/1078-0432.CCR-07-0751 [DOI] [PubMed] [Google Scholar]
- Madempudi R. S., Kalle A. M. (2017). Antiproliferative Effects of Bacillus Coagulans Unique IS2 in colon Cancer Cells. Nutr. Cancer 69 (7), 1062–1068. 10.1080/01635581.2017.1359317 [DOI] [PubMed] [Google Scholar]
- Mahkonen A., Putaala H., Mustonen H., Rautonen N., Puolakkainen P. (2008). Lactobacillus Acidophilus 74-2 and Butyrate Induce Cyclooxygenase (COX)-1 Expression in Gastric Cancer Cells. Immunopharmacol Immunotoxicol 30 (3), 503–518. 10.1080/08923970802135229 [DOI] [PubMed] [Google Scholar]
- Mahmood N., Mihalcioiu C., Rabbani S. A. (2018). Multifaceted Role of the Urokinase-type Plasminogen Activator (uPA) and its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 8, 24. 10.3389/fonc.2018.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki-Kakelar H., Dehghani J., Barzegari A., Barar J., Shirmohamadi M., Sadeghi J., et al. (2020). Lactobacillus Plantarum Induces Apoptosis in Gastric Cancer Cells via Modulation of Signaling Pathways in Helicobacter pylori . Bioimpacts 10 (2), 65–72. 10.34172/bi.2020.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani I., Chondrou P., Bontsidis C., Karolidou K., Terpou A., Alexopoulos A., et al. (2019). Assessment of the Probiotic Potential of Lactic Acid Bacteria Isolated from Kefir Grains: Evaluation of Adhesion and Antiproliferative Properties in In Vitro Experimental Systems. Ann. Microbiol. 69 (7), 751–763. 10.1007/s13213-019-01467-6 [DOI] [Google Scholar]
- McIntosh G. H., Royle P. J., Playne M. J. (1999). A Probiotic Strain of L. Acidophilus Reduces DMH-Induced Large Intestinal Tumors in Male Sprague-Dawley Rats. Nutr. Cancer 35 (2), 153–159. 10.1207/S15327914NC352_9 [DOI] [PubMed] [Google Scholar]
- Mego M., Holec V., Drgona L., Hainova K., Ciernikova S., Zajac V. (2013). Probiotic Bacteria in Cancer Patients Undergoing Chemotherapy and Radiation Therapy. Complement. Ther. Med. 21 (6), 712–723. 10.1016/j.ctim.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Mendes M. C. S., Paulino D. S., Brambilla S. R., Camargo J. A., Persinoti G. F., Carvalheira J. B. C. (2018). Microbiota Modification by Probiotic Supplementation Reduces Colitis Associated colon Cancer in Mice. World J. Gastroenterol. 24 (18), 1995–2008. 10.3748/wjg.v24.i18.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meniailo M. E., Malashchenko V. V., Shmarov V. A., Gazatova N. D., Melashchenko O. B., Goncharov A. G., et al. (2018). Interleukin-8 Favors Pro-inflammatory Activity of Human Monocytes/macrophages. Int. Immunopharmacol 56, 217–221. 10.1016/j.intimp.2018.01.036 [DOI] [PubMed] [Google Scholar]
- Mi H., Dong Y., Zhang B., Wang H., Peter C. C. K., Gao P., et al. (2017). Bifidobacterium Infantis Ameliorates Chemotherapy-Induced Intestinal Mucositis via Regulating T Cell Immunity in Colorectal Cancer Rats. Cell Physiol Biochem 42 (6), 2330–2341. 10.1159/000480005 [DOI] [PubMed] [Google Scholar]
- Mirzaei H., Khataminfar S., Mohammadparast S., Sales S. S., Maftouh M., Mohammadi M., et al. (2016). Circulating microRNAs as Potential Diagnostic Biomarkers and Therapeutic Targets in Gastric Cancer: Current Status and Future Perspectives. Curr. Med. Chem. 23 (36), 4135–4150. 10.2174/0929867323666160818093854 [DOI] [PubMed] [Google Scholar]
- Mohania D., Kansal V. K., Kruzliak P., Kumari A. (2014). Probiotic Dahi Containing Lactobacillus Acidophilus and Bifidobacterium Bifidum Modulates the Formation of Aberrant Crypt Foci, Mucin-Depleted Foci, and Cell Proliferation on 1,2-Dimethylhydrazine-Induced Colorectal Carcinogenesis in Wistar Rats. Rejuvenation Res. 17 (4), 325–333. 10.1089/rej.2013.1537 [DOI] [PubMed] [Google Scholar]
- Mojibi P., Tafvizi F., Bikhof Torbati M. (2019). Cell-bound Exopolysaccharide Extract from Indigenous Probiotic Bacteria Induce Apoptosis in HT-29 Cell-Line. Iran J. Pathol. 14 (1), 41–51. 10.30699/IJP.14.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörkl S., Lackner S., Meinitzer A., Mangge H., Lehofer M., Halwachs B., et al. (2018). Gut Microbiota, Dietary Intakes and Intestinal Permeability Reflected by Serum Zonulin in Women. Eur. J. Nutr. 57 (8), 2985–2997. 10.1007/s00394-018-1784-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S. F. (2017). The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol Gastroenterol Hepatol 3 (2), 183–191. 10.1016/j.jcmgh.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari Namin B., Daryani N. E., Mirshafiey A., Yazdi M. K. S., Dallal M. M. S. (2015). Effect of Probiotics on the Expression of Barrett's Oesophagus Biomarkers. J. Med. Microbiol. 64 (4), 348–354. 10.1099/jmm.0.000039 [DOI] [PubMed] [Google Scholar]
- Mushtaq M., Gani A., Masoodi F. A. (2019). Himalayan Cheese (Kalari/Kradi) Fermented with Different Probiotic Strains: In Vitro Investigation of Nutraceutical Properties. LWT 104, 53–60. 10.1016/j.lwt.2019.01.024 [DOI] [Google Scholar]
- Nami Y., Abdullah N., Haghshenas B., Radiah D., Rosli R., Khosroushahi A. Y. (2014). Assessment of Probiotic Potential and Anticancer Activity of Newly Isolated Vaginal Bacterium Lactobacillus Plantarum 5BL. Microbiol. Immunol. 58 (9), 492–502. 10.1111/1348-0421.12175 [DOI] [PubMed] [Google Scholar]
- Nowell P. C. (1976). The Clonal Evolution of Tumor Cell Populations. Science 194 (4260), 23–28. 10.1126/science.959840 [DOI] [PubMed] [Google Scholar]
- Nozari S., Faridvand Y., Etesami A., Ahmad Khan Beiki M., Miresmaeili Mazrakhondi S. A., Abdolalizadeh J. (2019). Potential Anticancer Effects of Cell wall Protein Fractions from Lactobacillus Paracasei on Human Intestinal Caco-2 Cell Line. Lett. Appl. Microbiol. 69 (3), 148–154. 10.1111/lam.13198 [DOI] [PubMed] [Google Scholar]
- O'Mahony L., Feeney M., O'Halloran S., Murphy L., Kiely B., Fitzgibbon J., et al. (2001). Probiotic Impact on Microbial flora, Inflammation and Tumour Development in IL-10 Knockout Mice. Aliment. Pharmacol. Ther. 15 (8), 1219–1225. 10.1046/j.1365-2036.2001.01027.x [DOI] [PubMed] [Google Scholar]
- Oberreuther-Moschner D. L., Jahreis G., Rechkemmer G., Pool-Zobel B. L. (2004). Dietary Intervention with the Probiotics Lactobacillus Acidophilus 145 and Bifidobacterium Longum 913 Modulates the Potential of Human Faecal Water to Induce Damage in HT29clone19A Cells. Br. J. Nutr. 91 (6), 925–932. 10.1079/BJN20041108 [DOI] [PubMed] [Google Scholar]
- Ohara T., Suzutani T. (2018). Intake of Bifidobacterium Longum and Fructo-Oligosaccharides Prevents Colorectal Carcinogenesis. Euroasian J. Hepatogastroenterol 8 (1), 11–17. 10.5005/jp-journals-10018-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohigashi S., Hoshino Y., Ohde S., Onodera H. (2011). Functional Outcome, Quality of Life, and Efficacy of Probiotics in Postoperative Patients with Colorectal Cancer. Surg. Today 41 (9), 1200–1206. 10.1007/s00595-010-4450-6 [DOI] [PubMed] [Google Scholar]
- Orlando A., Linsalata M., Russo F. (2016). Antiproliferative Effects on colon Adenocarcinoma Cells Induced by Co-administration of Vitamin K1 and Lactobacillus Rhamnosus GG. Int. J. Oncol. 48 (6), 2629–2638. 10.3892/ijo.2016.3463 [DOI] [PubMed] [Google Scholar]
- Orlando A., Messa C., Linsalata M., Cavallini A., Russo F. (2009). Effects of Lactobacillus Rhamnosus GG on Proliferation and Polyamine Metabolism in HGC-27 Human Gastric and DLD-1 Colonic Cancer Cell Lines. Immunopharmacol Immunotoxicol 31 (1), 108–116. 10.1080/08923970802443631 [DOI] [PubMed] [Google Scholar]
- Orlando A., Refolo M. G., Messa C., Amati L., Lavermicocca P., Guerra V., et al. (2012). Antiproliferative and Proapoptotic Effects of Viable or Heat-Killed Lactobacillus Paracasei IMPC2.1 and Lactobacillus Rhamnosus GG in HGC-27 Gastric and DLD-1 colon Cell Lines. Nutr. Cancer 64 (7), 1103–1111. 10.1080/01635581.2012.717676 [DOI] [PubMed] [Google Scholar]
- Osterlund P., Ruotsalainen T., Korpela R., Saxelin M., Ollus A., Valta P., et al. (2007). Lactobacillus Supplementation for Diarrhoea Related to Chemotherapy of Colorectal Cancer: a Randomised Study. Br. J. Cancer 97 (8), 1028–1034. 10.1038/sj.bjc.6603990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte J. M., Mahjurian-Namari R., Brand S., Werner I., Schmidt W. E., Schmitz F. (2008). Probiotics Regulate the Expression of COX-2 in Intestinal Epithelial Cells. Nutr. Cancer 61 (1), 103–113. 10.1080/01635580802372625 [DOI] [PubMed] [Google Scholar]
- Paolillo R., Romano Carratelli C., Sorrentino S., Mazzola N., Rizzo A. (2009). Immunomodulatory Effects of Lactobacillus Plantarum on Human colon Cancer Cells. Int. Immunopharmacol 9 (11), 1265–1271. 10.1016/j.intimp.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Pavón M. A., Arroyo-Solera I., Céspedes M. V., Casanova I., León X., Mangues R. (2016). uPA/uPAR and SERPINE1 in Head and Neck Cancer: Role in Tumor Resistance, Metastasis, Prognosis and Therapy. Oncotarget 7 (35), 57351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A. (2001). Cancer Genetics. Nature 411 (6835), 336–341. 10.1038/35077207 [DOI] [PubMed] [Google Scholar]
- Pourhanifeh M. H., Vosough M., Mahjoubin-Tehran M., Hashemipour M., Nejati M., Abbasi-Kolli M., et al. (2020). Autophagy-related microRNAs: Possible Regulatory Roles and Therapeutic Potential in and Gastrointestinal Cancers. Pharmacol. Res. 161, 105133. 10.1016/j.phrs.2020.105133 [DOI] [PubMed] [Google Scholar]
- Rasouli B. S., Ghadimi-Darsajini A., Nekouian R., Iragian G. R. (2017). In Vitro activity of Probiotic Lactobacillus Reuteri against Gastric Cancer Progression by Downregulation of Urokinase Plasminogen Activator/urokinase Plasminogen Activator Receptor Gene Expression. J. Cancer Res. Ther. 13 (2), 246–251. 10.4103/0973-1482.204897 [DOI] [PubMed] [Google Scholar]
- Requena T., Martínez-Cuesta M. C., Peláez C. (2018). Diet and Microbiota Linked in Health and Disease. Food Funct. 9 (2), 688–704. 10.1039/c7fo01820g [DOI] [PubMed] [Google Scholar]
- Rondanelli M., Faliva M. A., Perna S., Giacosa A., Peroni G., Castellazzi A. M. (2017). Using Probiotics in Clinical Practice: Where Are We Now? A Review of Existing Meta-Analyses. Gut microbes 8 (6), 521–543. 10.1080/19490976.2017.1345414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubina K. A., Sysoeva V. Y., Zagorujko E. I., Tsokolaeva Z. I., Kurdina M. I., Parfyonova Y. V., et al. (2017). Increased Expression of uPA, uPAR, and PAI-1 in Psoriatic Skin and in Basal Cell Carcinomas. Arch. Dermatol. Res. 309 (6), 433–442. 10.1007/s00403-017-1738-z [DOI] [PubMed] [Google Scholar]
- Russo F., Linsalata M., Orlando A. (2014). Probiotics against Neoplastic Transformation of Gastric Mucosa: Effects on Cell Proliferation and Polyamine Metabolism. World J. Gastroenterol. 20 (37), 13258–13272. 10.3748/wjg.v20.i37.13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F., Orlando A., Linsalata M., Cavallini A., Messa C. (2007). Effects of Lactobacillus Rhamnosus GG on the Cell Growth and Polyamine Metabolism in HGC-27 Human Gastric Cancer Cells. Nutr. Cancer 59 (1), 106–114. 10.1080/01635580701365084 [DOI] [PubMed] [Google Scholar]
- Saber A., Alipour B., Faghfoori Z., Mousavi Jam A., Yari Khosroushahi A. (2017). Secretion Metabolites of Probiotic Yeast, Pichia Kudriavzevii AS-12, Induces Apoptosis Pathways in Human Colorectal Cancer Cell Lines. Nutr. Res. 41, 36–46. 10.1016/j.nutres.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Sadahiro S., Suzuki T., Tanaka A., Okada K., Kamata H., Ozaki T., et al. (2014). Comparison between Oral Antibiotics and Probiotics as Bowel Preparation for Elective colon Cancer Surgery to Prevent Infection: Prospective Randomized Trial. Surgery 155 (3), 493–503. 10.1016/j.surg.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Sadeghi-Aliabadi H., Mohammadi F., Fazeli H., Mirlohi M. (2014). Effects of Lactobacillus Plantarum A7 with Probiotic Potential on colon Cancer and normal Cells Proliferation in Comparison with a Commercial Strain. Iran J. Basic Med. Sci. 17 (10), 815–819. [PMC free article] [PubMed] [Google Scholar]
- Said H. M. (2012). Physiology of the Gastrointestinal Tract, Two Volume Set. Academic Press. [Google Scholar]
- Sakatani A., Fujiya M., Ueno N., Kashima S., Sasajima J., Moriichi K., et al. (2016). Polyphosphate Derived from Lactobacillus Brevis Inhibits colon Cancer Progression through Induction of Cell Apoptosis. Anticancer Res. 36 (2), 591–598. [PubMed] [Google Scholar]
- Sakatani A., Fujiya M., Ueno N., Kashima S., Sasajima J., Moriichi K., et al. (2016). Polyphosphate Derived from Lactobacillus Brevis Inhibits Colon Cancer Progression through Induction of Cell Apoptosis. Anticancer Res. 36 (2), 591–598. [PubMed] [Google Scholar]
- Salas-Jara M. J., Sanhueza E. A., Retamal-Díaz A., González C., Urrutia H., García A. (2016). Probiotic Lactobacillus Fermentum UCO-979C Biofilm Formation on AGS and Caco-2 Cells and Helicobacter pylori Inhibition. Biofouling 32 (10), 1245–1257. 10.1080/08927014.2016.1249367 [DOI] [PubMed] [Google Scholar]
- Sambrani R., Abdolalizadeh J., Kohan L., Jafari B. (2019). Saccharomyces cerevisiae Inhibits Growth and Metastasis and Stimulates Apoptosis in HT-29 Colorectal Cancer Cell Line. Comp. Clin. Pathol. 28 (4), 985–995. 10.1007/s00580-018-2855-6 [DOI] [Google Scholar]
- Sánchez-Martínez C., Lallena M. J., Sanfeliciano S. G., de Dios A. (2019). Cyclin Dependent Kinase (CDK) Inhibitors as Anticancer Drugs: Recent Advances (2015-2019). Bioorg. Med. Chem. Lett. 29 (20), 126637. 10.1016/j.bmcl.2019.126637 [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Guarner F., Guerrant R., Holt P. R., Quigley E. M., Sartor R. B., et al. (2013). An Update on the Use and Investigation of Probiotics in Health and Disease. Gut 62 (5), 787–796. 10.1136/gutjnl-2012-302504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin A. L. (2004). Antagonistic Activities of Lactobacilli and Bifidobacteria against Microbial Pathogens. FEMS Microbiol. Rev. 28 (4), 405–440. 10.1016/j.femsre.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Shafabakhsh R., Arianfar F., Vosough M., Mirzaei H. R., Mahjoubin-Tehran M., Khanbabaei H., et al. (2021). Autophagy and Gastrointestinal Cancers: The behind the Scenes Role of Long Non-coding RNAs in Initiation, Progression, and Treatment Resistance. Cancer Gene Ther. 28 (12), 1229–1255. [DOI] [PubMed] [Google Scholar]
- Shaima'a Hamarsheh R. Z. (2020). NLRP3 Inflammasome Activation in Cancer: a Double-Edged Sword. Front. Immunol. 11. 10.3389/fimmu.2020.01444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf L. K., Sharma M., Chandel D., Shukla G. (2018). Prophylactic Intervention of Probiotics (L.Acidophilus, L.Rhamnosus GG) and Celecoxib Modulate Bax-Mediated Apoptosis in 1,2-Dimethylhydrazine-Induced Experimental colon Carcinogenesis. BMC Cancer 18 (1), 1111. 10.1186/s12885-018-4999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Chandel D., Shukla G. (2019). Antigenotoxicity and Cytotoxic Potentials of Metabiotics Extracted from Isolated Probiotic, Lactobacillus Rhamnosus MD 14 on Caco-2 and HT-29 Human Colon Cancer Cells. Nutr. Cancer, 1–10. 10.1080/01635581.2019.1615514 [DOI] [PubMed] [Google Scholar]
- Shiratsuchi I., Akagi Y., Kawahara A., Kinugasa T., Romeo K., Yoshida T., et al. (2011). Expression of IGF-1 and IGF-1R and Their Relation to Clinicopathological Factors in Colorectal Cancer. Anticancer Res. 31 (7), 2541–2545. [PubMed] [Google Scholar]
- Shyu P. T., Oyong G. G., Cabrera E. C. (2014). Cytotoxicity of Probiotics from Philippine Commercial Dairy Products on Cancer Cells and the Effect on Expression of Cfos and Cjun Early Apoptotic-Promoting Genes and Interleukin-1 β and Tumor Necrosis Factor-α Proinflammatory Cytokine Genes. Biomed. Res. Int. 2014, 491740. 10.1155/2014/491740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Rivenson A., Tomita M., Shimamura S., Ishibashi N., Reddy B. S. (1997). Bifidobacterium Longum, a Lactic Acid-Producing Intestinal Bacterium Inhibits colon Cancer and Modulates the Intermediate Biomarkers of colon Carcinogenesis. Carcinogenesis 18 (4), 833–841. 10.1093/carcin/18.4.833 [DOI] [PubMed] [Google Scholar]
- Sivieri K., Spinardi-Barbisan A. L. T., Barbisan L. F., Bedani R., Pauly N. D., Carlos I. Z., et al. (2008). Probiotic Enterococcus Faecium CRL 183 Inhibit Chemically Induced colon Cancer in Male Wistar Rats. Eur. Food Res. Technol. 228 (2), 231–237. 10.1007/s00217-008-0927-6 [DOI] [Google Scholar]
- Song H., Wang W., Shen B., Jia H., Hou Z., Chen P., et al. (2018). Pretreatment with Probiotic Bifico Ameliorates Colitis-Associated Cancer in Mice: Transcriptome and Gut flora Profiling. Cancer Sci. 109 (3), 666–677. 10.1111/cas.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Zhou L., Liu D., Ge L., Li Y. (2019). Probiotic Effect on Helicobacter pylori Attachment and Inhibition of Inflammation in Human Gastric Epithelial Cells. Exp. Ther. Med. 18 (3), 1551–1562. 10.3892/etm.2019.7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spechler S. J. (2013). Barrett’s Esophagus. Principles of Deglutition. Springer, 723–738. 10.1007/978-1-4614-3794-9_49 [DOI] [Google Scholar]
- Sturgeon C., Fasano A. (2016). Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and its Involvement in Chronic Inflammatory Diseases. Tissue barriers 4 (4), e1251384. 10.1080/21688370.2016.1251384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Kaufman P. D. (2018). Ki-67: More Than a Proliferation Marker. Chromosoma 127 (2), 175–186. 10.1007/s00412-018-0659-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K. J., Welch J. E., Kucharzewska P., Bengtson P., Bjurberg M., Påhlman S., et al. (2008). Hypoxia-mediated Induction of the Polyamine System Provides Opportunities for Tumor Growth Inhibition by Combined Targeting of Vascular Endothelial Growth Factor and Ornithine Decarboxylase. Cancer Res. 68 (22), 9291–9301. 10.1158/0008-5472.CAN-08-2340 [DOI] [PubMed] [Google Scholar]
- Tajadadi-Ebrahimi M., Bahmani F., Shakeri H., Hadaegh H., Hijijafari M., Abedi F., et al. (2014). Effects of Daily Consumption of Synbiotic Bread on Insulin Metabolism and Serum High-Sensitivity C-Reactive Protein Among Diabetic Patients: a Double-Blind, Randomized, Controlled Clinical Trial. Ann. Nutr. Metab. 65 (1), 34–41. 10.1159/000365153 [DOI] [PubMed] [Google Scholar]
- Talero E., Bolivar S., Ávila-Román J., Alcaide A., Fiorucci S., Motilva V. (2015). Inhibition of Chronic Ulcerative Colitis-Associated Adenocarcinoma Development in Mice by VSL#3. Inflamm. Bowel Dis. 21 (5), 1027–1037. 10.1097/MIB.0000000000000346 [DOI] [PubMed] [Google Scholar]
- Tamtaji O. R., Heidari-Soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F., Aghadavod E., et al. (2019). Probiotic and Selenium Co-supplementation, and the Effects on Clinical, Metabolic and Genetic Status in Alzheimer's Disease: A Randomized, Double-Blind, Controlled Trial. Clin. Nutr. 38 (6), 2569–2575. 10.1016/j.clnu.2018.11.034 [DOI] [PubMed] [Google Scholar]
- Tamtaji O. R., Taghizadeh M., Daneshvar Kakhaki R., Kouchaki E., Bahmani F., Borzabadi S., et al. (2019). Clinical and Metabolic Response to Probiotic Administration in People with Parkinson's Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 38 (3), 1031–1035. 10.1016/j.clnu.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Tan C. K., Said S., Rajandram R., Wang Z., Roslani A. C., Chin K. F. (2016). Pre-surgical Administration of Microbial Cell Preparation in Colorectal Cancer Patients: a Randomized Controlled Trial. World J. Surg. 40 (8), 1985–1992. 10.1007/s00268-016-3499-9 [DOI] [PubMed] [Google Scholar]
- Tan Q., Xu H., Aguilar Z. P., Peng S., Dong S., Wang B., et al. (2013). Safety Assessment and Probiotic Evaluation of Enterococcus Faecium YF5 Isolated from Sourdough. J. Food Sci. 78 (4), M587–M593. 10.1111/1750-3841.12079 [DOI] [PubMed] [Google Scholar]
- Taremi M., Khoshbaten M., Gachkar L., EhsaniArdakani M., Zali M. (2005). Hepatitis E Virus Infection in Hemodialysis Patients: a Seroepidemiological Survey in Iran. BMC Infect. Dis. 5 (1), 36. 10.1186/1471-2334-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teimoorian F., Ranaei M., Hajian Tilaki K., Shokri Shirvani J., Vosough Z. (2018). Association of Helicobacter pylori Infection with Colon Cancer and Adenomatous Polyps. Iran J. Pathol. 13 (3), 325–332. [PMC free article] [PubMed] [Google Scholar]
- Thirabunyanon M., Boonprasom P., Niamsup P. (2009). Probiotic Potential of Lactic Acid Bacteria Isolated from Fermented Dairy Milks on Antiproliferation of colon Cancer Cells. Biotechnol. Lett. 31 (4), 571–576. 10.1007/s10529-008-9902-3 [DOI] [PubMed] [Google Scholar]
- Thirabunyanon M., Hongwittayakorn P. (2013). Potential Probiotic Lactic Acid Bacteria of Human Origin Induce Antiproliferation of colon Cancer Cells via Synergic Actions in Adhesion to Cancer Cells and Short-Chain Fatty Acid Bioproduction. Appl. Biochem. Biotechnol. 169 (2), 511–525. 10.1007/s12010-012-9995-y [DOI] [PubMed] [Google Scholar]
- Tiptiri-Kourpeti A., Spyridopoulou K., Santarmaki V., Aindelis G., Tompoulidou E., Lamprianidou E. E., et al. (2016). Lactobacillus Casei Exerts Anti-proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in colon Carcinoma Cells. PloS one 11 (2), e0147960. 10.1371/journal.pone.0147960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C. (2013). The 2 Faces of JNK Signaling in Cancer. Genes & cancer 4 (9-10), 397–400. 10.1177/1947601913486349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska A. M., Bhathena J., Martoni C., Prakash S. (2009). Estimation of the Potential Antitumor Activity of Microencapsulated Lactobacillus Acidophilus Yogurt Formulation in the Attenuation of Tumorigenesis in Apc(Min/+) Mice. Dig. Dis. Sci. 54 (2), 264–273. 10.1007/s10620-008-0363-2 [DOI] [PubMed] [Google Scholar]
- Valadez-Bustos N., Escamilla-Silva E. M., García-Vázquez F. J., Gallegos-Corona M. A., Amaya-Llano S. L., Ramos-Gómez M. (2019). Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model. Int. J. Mol. Sci. 20 (17), 4275. 10.3390/ijms20174275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Shukla G. (2013). Probiotics Lactobacillus Rhamnosus GG, Lactobacillus Acidophilus Suppresses DMH-Induced Procarcinogenic Fecal Enzymes and Preneoplastic Aberrant Crypt Foci in Early colon Carcinogenesis in Sprague Dawley Rats. Nutr. Cancer 65 (1), 84–91. 10.1080/01635581.2013.741746 [DOI] [PubMed] [Google Scholar]
- Vigneri P. G., Tirrò E., Pennisi M. S., Massimino M., Stella S., Romano C., et al. (2015). The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 5, 230. 10.3389/fonc.2015.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince J. E., De Nardo D., Gao W., Vince A. J., Hall C., McArthur K., et al. (2018). The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep 25 (9), 2339–e4. e4. 10.1016/j.celrep.2018.10.103 [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. (2004). Cancer Genes and the Pathways They Control. Nat. Med. 10 (8), 789–799. 10.1038/nm1087 [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Papadopoulos N., Velculescu V. E., Zhou S., Diaz L. A., Jr., Kinzler K. W. (2013). Cancer Genome Landscapes. Science 339 (6127), 1546–1558. 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S., Kamal R., Dhawan D. K., Kanwar S. S. (2018). Chemoprevention by Probiotics during 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis in Rats. Dig. Dis. Sci. 63 (4), 900–909. 10.1007/s10620-018-4949-z [DOI] [PubMed] [Google Scholar]
- Walia S., Kamal R., Kanwar S. S., Dhawan D. K. (2015). Cyclooxygenase as a Target in Chemoprevention by Probiotics during 1,2-dimethylhydrazine Induced colon Carcinogenesis in Rats. Nutr. Cancer 67 (4), 603–611. 10.1080/01635581.2015.1011788 [DOI] [PubMed] [Google Scholar]
- Wang C. S., Li W. B., Wang H. Y., Ma Y. M., Zhao X. H., Yang H., et al. (2018). VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 24 (37), 4254–4262. 10.3748/wjg.v24.i37.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. S., Li W. B., Wang H. Y., Ma Y. M., Zhao X. H., Yang H., et al. (2018). VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 24 (37), 4254–4262. 10.3748/wjg.v24.i37.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Song M., Lu X., Zhu X., Deng J. (2021). Gut Microbes in Gastrointestinal Cancers. Seminars in Cancer Biology. [DOI] [PubMed] [Google Scholar]
- Wang H., Bastian S. E., Lawrence A., Howarth G. S. (2015). Factors Derived from Escherichia coli Nissle 1917, Grown in Different Growth media, Enhance Cell Death in a Model of 5-Fluorouracil-Induced Caco-2 Intestinal Epithelial Cell Damage. Nutr. Cancer 67 (2), 316–326. 10.1080/01635581.2015.990570 [DOI] [PubMed] [Google Scholar]
- Welte Y., Adjaye J., Lehrach H. R., Regenbrecht C. R. (2010). Cancer Stem Cells in Solid Tumors: Elusive or Illusive? Cel Commun Signal 8 (1), 6–10. 10.1186/1478-811X-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. J., Endicott J. A. (2018). Structural Insights into the Functional Diversity of the CDK-Cyclin Family. Open Biol. 8 (9), 180112. 10.1098/rsob.180112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley D. L., Le Leu R. K., Whitehall V. L., Conlon M., Christophersen C., Belobrajdic D., et al. (2009). A Human, Double-Blind, Placebo-Controlled, Crossover Trial of Prebiotic, Probiotic, and Synbiotic Supplementation: Effects on Luminal, Inflammatory, Epigenetic, and Epithelial Biomarkers of Colorectal Cancer. Am. J. Clin. Nutr. 90 (3), 578–586. 10.3945/ajcn.2009.28106 [DOI] [PubMed] [Google Scholar]
- Wu Q., Wu W., Fu B., Shi L., Wang X., Kuca K. (2019). JNK Signaling in Cancer Cell Survival. Med. Res. Rev. 39 (6), 2082–2104. 10.1002/med.21574 [DOI] [PubMed] [Google Scholar]
- Xie H., Lu Q., Wang H., Zhu X., Guan Z. (2018). Effects of Probiotics Combined with Enteral Nutrition on Im-Mune Function and Inflammatory Response in Postoperative Patients with Gastric Cancer. [PubMed] [Google Scholar]
- Yamazaki K., Tsunoda A., Sibusawa M., Tsunoda Y., Kusano M., Fukuchi K., et al. (2000). The Effect of an Oral Administration of Lactobacillus Casei Strain Shirota on Azoxymethane-Induced Colonic Aberrant Crypt Foci and colon Cancer in the Rat. Oncol. Rep. 7 (5), 977–982. 10.3892/or.7.5.977 [DOI] [PubMed] [Google Scholar]
- Yang Y., Xia Y., Chen H., Hong L., Feng J., Yang J., et al. (2016). The Effect of Perioperative Probiotics Treatment for Colorectal Cancer: Short-Term Outcomes of a Randomized Controlled Trial. Oncotarget 7 (7), 8432–8440. 10.18632/oncotarget.7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H. J., Oh D. K., Ji G. E. (2004). Anticancerogenic Effect of a Novel Chiroinositol-Containing Polysaccharide from Bifidobacterium Bifidum BGN4. FEMS Microbiol. Lett. 240 (2), 131–136. 10.1016/j.femsle.2004.09.020 [DOI] [PubMed] [Google Scholar]
- Yuasa Y. (2003). Control of Gut Differentiation and Intestinal-type Gastric Carcinogenesis. Nat. Rev. Cancer 3 (8), 592–600. 10.1038/nrc1141 [DOI] [PubMed] [Google Scholar]
- Zhang J. W., Du P., Gao J., Yang B. R., Fang W. J., Ying C. M. (2012). Preoperative Probiotics Decrease Postoperative Infectious Complications of Colorectal Cancer. Am. J. Med. Sci. 343 (3), 199–205. 10.1097/MAJ.0b013e31823aace6 [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang F., Jiang L., Liu R., Zhang L., Lei X., et al. (2013). Lactobacillus Salivarius REN Inhibits Rat Oral Cancer Induced by 4-nitroquioline 1-oxide. Cancer Prev. Res. (Phila) 6 (7), 686–694. 10.1158/1940-6207.CAPR-12-0427 [DOI] [PubMed] [Google Scholar]
- Zhang M. M., Cheng J. Q., Xia L., Lu Y. R., Wu X. T. (2011). Monitoring Intestinal Microbiota Profile: a Promising Method for the Ultraearly Detection of Colorectal Cancer. Med. Hypotheses 76 (5), 670–672. 10.1016/j.mehy.2011.01.028 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma C., Zhao J., Xu H., Hou Q., Zhang H. (2017). Lactobacillus Casei Zhang and Vitamin K2 Prevent Intestinal Tumorigenesis in Mice via Adiponectin-Elevated Different Signaling Pathways. Oncotarget 8 (15), 24719–24727. 10.18632/oncotarget.15791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. Y., Ding N., Du Z. Y., Cui X. X., Wang H., Wei X. C., et al. (2014). Curcumin Analogues with High Activity for Inhibiting Human Prostate Cancer Cell Growth and Androgen Receptor Activation. Mol. Med. Rep. 10 (3), 1315–1322. 10.3892/mmr.2014.2380 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Chen J., Yao H., Hu H. (2018). Fusobacterium and Colorectal Cancer. Front. Oncol. 8, 371. 10.3389/fonc.2018.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhu C., Ge S., Zhang M., Jiang L., Cui J., et al. (2014). Lactobacillus Salivarius Ren Prevent the Early Colorectal Carcinogenesis in 1, 2-Dimethylhydrazine-Induced Rat Model. J. Appl. Microbiol. 117 (1), 208–216. 10.1111/jam.12499 [DOI] [PubMed] [Google Scholar]
- Zinatizadeh N., Khalili F., Fallah P., Farid M., Geravand M., Yaslianifard S. (2018). Potential Preventive Effect of Lactobacillus Acidophilus and Lactobacillus Plantarum in Patients with Polyps or Colorectal Câncer. Arq Gastroenterol. 55 (4), 407–411. 10.1590/S0004-2803.201800000-87 [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Ma Y., Raoult D., Kroemer G., Gajewski T. F. (2018). The Microbiome in Cancer Immunotherapy: Diagnostic Tools and Therapeutic Strategies. Science 359 (6382), 1366–1370. 10.1126/science.aar6918 [DOI] [PubMed] [Google Scholar]
- Zuccotti G. V., Meneghin F., Raimondi C., Dilillo D., Agostoni C., Riva E., et al. (2008). Probiotics in Clinical Practice: an Overview. J. Int. Med. Res. 36 (1_Suppl. l), 1A–53A. 10.1177/14732300080360S101 [DOI] [PubMed] [Google Scholar]
- Zununi Vahed S., Barzegari A., Rahbar Saadat Y., Goreyshi A., Omidi Y. (2017). Leuconostoc Mesenteroides-Derived Anticancer Pharmaceuticals Hinder Inflammation and Cell Survival in colon Cancer Cells by Modulating NF-κB/AKT/PTEN/MAPK Pathways. Biomed. Pharmacother. 94, 1094–1100. 10.1016/j.biopha.2017.08.033 [DOI] [PubMed] [Google Scholar]