Abstract
Per- and polyfluoroalkyl substances (PFAS) are persistent organic pollutants of concern because of their ubiquitous presence in surface and ground water; analytical methods that can be used for rapid comprehensive exposure assessment and fingerprinting of PFAS are needed. Following the fires at the Intercontinental Terminals Company (ITC) in Deer Park, TX in 2019, very large quantities of PFAS-containing firefighting foams were deployed. The release of these substances into the Houston Ship Channel/Galveston Bay (HSC/GB) prompted concerns over the extent and level of PFAS contamination. A targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based study of temporal and spatial patterns of PFAS associated with this incident revealed presence of 7 species; their levels gradually decreased over a 6-month period. Because the targeted LC-MS/MS analysis was focused on about 30 PFAS molecules, it may have missed other PFAS compounds present in firefighting foams. Therefore, we utilized untargeted LC-ion mobility spectrometry-mass spectrometry (LC-IMS-MS)-based analytical approach for a more comprehensive characterization of PFAS in these water samples. We analyzed 31 samples from 9 sites in the HSC/GB that were collected over 5 months after the incident. Our data showed that additional 19 PFAS were detected in surface water of HSC/GB, most of them decreased gradually after the incident. PFAS features detected by LC-MS/MS correlated well in abundance with LC-IMS-MS data; however, LC-IMS-MS identified a number of additional PFAS, many known to be components of firefighting foams. These findings therefore illustrate that untargeted LC-IMS-MS improved our understanding of PFAS presence in complex environmental samples.
Keywords: Emergency, Environmental Disaster, PFAS
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of synthetic environmental contaminants that have been manufactured for over 60 years (Domingo and Nadal, 2019). These compounds are characterized by extensive fluorination along carbon chains which make them chemically inert and thermally stable (Buck et al., 2011). The unique physicochemical properties of PFAS make them desirable components in a variety of industrial and consumer products such as cookware, oil and water-resistant fabrics, carpets, and firefighting foams (ATSDR, 2018; Hekster et al., 2003; Sinclair et al., 2020). Currently, there are well over 5,000 compounds classified as PFAS (OECD, 2020).
PFAS have become a major concern to human and environmental health due to their ubiquitous presence, high stability, and studies suggesting they may pose harm to humans and animals (ATSDR, 2018; Sinclair et al., 2020). To better characterize potential exposure of PFAS to humans, advancements in analytical chemistry have been made to detect and quantify PFAS in various environmental media including air, water, soil, and food. Targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS) assessment is the analytical method of choice, typically used for detection and quantification of PFAS (Anumol et al., 2018; Rodriguez et al., 2020). Although targeted LC-MS/MS is a widely used and reliable technique for quantification of PFAS, the methods are typically limited to the well-studied “legacy” compounds (AWWA, 2019). For example, in 20 previous studies that tested for PFAS in various matrices, including surface water, using LC-MS/MS (SI Table 1), the number of PFAS analytes that were assayed for ranged from 8 to 61 with an average of 24 analytes across these studies. To improve exposure assessment of additional PFAS in the environment, untargeted analyses are therefore needed (National Academies of Sciences Engineering and Medicine, 2021).
One promising method for untargeted analysis of PFAS is coupling ion mobility spectrometry (IMS) with LC-MS measurements (Dodds et al., 2020; Luo et al., 2020). IMS is a rapid analytical separation technique typically performed in milliseconds with capabilities of distinguishing analytes by their size, shape, and charge state (Viehland and Mason, 1975). IMS can be coupled to LC to assist in resolving homologous species, and to high-resolution mass spectrometry (HRMS) for simultaneous mass analyses. IMS is also able to generate collision cross sections (CCS) for individual molecules (Dodds and Baker, 2019), which further enhances confidence in feature identification in this untargeted approach and is particularly beneficial for PFAS because they are highly homologous molecules (Dodds et al., 2020). Feature matching to a library of CCS values for PFAS standards is possible and increases confidence in feature identification in the multi-dimensional data generated by LC-IMS-MS analyses (Dodds et al., 2020).
Large-scale environmental releases of PFAS are often the result of their presence in aqueous film forming foams (AFFF), substances that are commonly used for fire suppression (ATSDR, 2018; Munoz et al., 2017; NIEHS, 2020; OECD, 2020). The identity of PFAS present in various AFFF is typically not disclosed due to proprietary nature of the formulations (Luo et al., 2020; McCord et al., 2018; Mejia-Avendano et al., 2017). AFFF are extensively used during emergency response, firefighter training, and on-board ships to contain fuel fires (ATSDR, 2018; Coggan et al., 2019). One recent example of a large-scale release of AFFF into the environment is the petrochemical fire that took place in March 2019 in the Houston, Texas area at the Intercontinental Terminals Company (ITC). The fire lasted for several days and millions of liters of AFFF were released (Thomas, 2019). While containment of the chemical and AFFF release into the Houston Ship Channel/Galveston Bay (HSC/GB) was attempted, a breach occurred along the dike wall surrounding the site that subsequently caused foams to infiltrate the HSC/GB and spread both down- and up-stream in this estuary (Aly et al., 2020). The presence of AFFF in the HSC/GB waters prompted concerns over the nature and extent of PFAS contamination and potential exposures.
A targeted study of HSC/GB contamination by PFAS after the ITC incident was conducted using an LC-MS/MS method that evaluated 27 compounds in surface water samples collected from 9 locations in the HSC/GB; only 7 PFAS were consistently detected at quantifiable levels between March and August 2019 (Aly et al., 2020). This study was informative with respect to both spatial and temporal trends in PFAS contaminant levels; however, the narrow range of the analytes evaluated by the LC-MS/MS method limited the ability to assess exposure to a diverse number of PFAS species that could be present due to the AFFF release. Therefore, we hypothesized that an untargeted analysis would reveal the presence of many more PFAS in the HSC/GB waters. We tested this hypothesis by using an untargeted LC-IMS-MS analysis of the environmental samples, enabling a more comprehensive characterization of the PFAS contaminants. Specifically, 31 surface water samples that represented both temporal and spatial dimensions of this incident were tested by LC-IMS-MS. This study provides a direct comparison between targeted and untargeted analyses of PFAS. In addition, we demonstrate the application of LC-IMS-MS for comprehensive exposure assessment of PFAS in an emergency situation.
1. Materials and Methods
1.1. Incident Description.
The fire incident at the petrochemical storage facility of ITC in Deer Park, TX occurred in March 2019 and involved eight 80,000-barrel (12.7 million liter) above-ground storage tanks containing naphtha. During this incident, undetermined naphtha and AFFF chemicals were released into the Tucker Bayou, a tributary to HSC/GB. Recovery of the spilled chemical waste yielded over 28,000 barrels (1.2 million gallons) of oily water mix from the waterways surrounding ITC and over 35,000 barrels (approximately 1.5 million gallons) of product mixed with water and AFFF from the tank farm (Deer Park Emergency Services, 2019).
1.2. Sample Collection
Surface (at a depth of approximately 30 cm) water samples were collected from 9 locations throughout HSC/GB (Fig. 1a) as previously described (Aly et al., 2020) following the general guidance on sampling for PFAS (Shoemaker and Tettenhorst, 2018). In brief, water samples (700–1,000 mL each) were collected from either open water (from a boat) or from several shore-accessible locations between March 21, 2019 and August 2, 2019 in HSC/GB. The details on the sampling dates and exact locations are included in SI Table 2. In this study, the samples collected in April, June, July, and August 2019 (n=31) were used for analysis via LC-IMS-MS. Sample extraction, processing and storage after extraction followed guidance of the EPA Method 537.1.A (Shoemaker and Tettenhorst, 2018).
Figure 1. Map of sampling locations in the Houston Ship Channel/Galveston bay (HSC/GB) near the ITC fire site and example two-dimensional IMS-MS spectra from surface water samples collected April 2019.

(a) Area of detail magnified in the map is indicated in the inset (bottom left panel). A series of surface water samples (blue pins) were collected between April 12 and August 2, 2019 from shore-access locations or open water (SCW). The number in brackets beneath each sampling location indicates the distance (negative values represent up-stream locations) from ITC fire location (indicated by a fire symbol). Background map and inset map are from ESRI/OpenStreetMap. (b) The summed IMS-MS nested spectra for a procedural blank (left) and the two representative water samples collected April 2019 from various locations (as shown in the title of each plot) with m/z shown on the x-axis and IMS drift time shown on the y-axis.
1.3. Sample Preparation
Sample extraction was previously detailed in (Aly et al., 2020). Specifically, surface water samples were extracted following the procedure outlined in Waters Perfluorinated Compound Analysis manual (Silcock et al., 2020). A 50 mL aliquot of each water sample was used for April samples while a 250 mL aliquot of each water sample was used for extraction in June, July, and August 2019 because lower PFAS concentrations were expected. Water samples were placed in HDPE bottles (Cat No: 414004–113, VWR, USA) and extraction standards were added. Next, samples were extracted using Oasis weak anion exchange (WAX) cartridges (Cat No.: 186003519, Waters, USA) in a vacuum manifold. Next, samples were washed with 100% methanol (Cat No.: A456–500, Fisher Scientific, USA). In order to determine if PFAS analytes were lost during sample washes, LC-MS/MS analysis was performed on the collected 100% methanol wash from samples and no detectable PFAS was found (data not shown). Lastly, samples were eluted with NH4OH (Cat No.: A470–500, Fisher Scientific, USA) to further remove salts and prevent interference from matrices present in complex environmental samples. After extraction, samples were stored in −20°C until analysis.
1.4. Instrumental Analysis
Liquid Chromatography-Ion-Mobility Spectrometry-Mass Spectrometry (LC-IMS-MS) analysis was performed using 1260 Infinity II LC system (Agilent, USA) coupled with a 6560A drift tube IMS-QTOF mass spectrometer (Agilent, USA). In all analyses, the IMS drift tube utilized nitrogen gas and prior to sample analysis, the IMS-QTOF mass spectrometer was calibrated using Agilent protocols. Specifically, electron spray ionization (ESI) Tuning Mix solution (Cat No.: #G2421–60001, Agilent, USA) was directly injected into the instrument and a 50–1700 m/z tune was performed in both positive and negative modes. LC conditions were adapted from (Anumol et al., 2018) as detailed in (Aly et al., 2020). In brief, the chromatographic separation was performed using a ZORBAX SB C-18, 2.1×50 mm, 1.8 μm C18 column (Agilent, USA) with a flow rate of 0.4 mL/min and injection volume of 20 μL. Mobile phases consisted of 5 mM ammonium acetate in water (A) and 5 mM ammonium acetate in 95% methanol (B). The gradient was as follows: 10% B held for 0.5 min, B increased to 30% by 2 min, B increased to 95% by 14 min and 100% by 14.5 min, 100% B was held for 2 min then switched back to 10% B and held for 6 min to equilibrate stationary phase. Blank samples were injected at the start and end of each analysis to ensure that no carryover occurred between samples. IMS-MS analyses were performed using an ESI source operated in negative or positive mode. Instrument parameters are listed in SI Table 3.
1.5. Data Analysis
Data from the LC-IMS-MS measurements were processed using Skyline software (ver. 20.2.0.343; MacCoss Lab Software, University of Washington, Seattle, WA). First, in order to identify PFAS compounds among the features from each individual sample, a library of PFAS compounds with corresponding CCS values was used (Baker, 2021). The library was generated by running PFAS standards in triplicate to obtain their CCS values. Extracted ion chromatograms were evaluated for each PFAS within the individual samples. Compounds with chromatograms of poor quality (inadequate peak shape or inconsistent retention time) were deemed invalid features and excluded from final analysis. The abundance of each PFAS as reported by Skyline analysis was normalized to the abundance of an isotope-labeled extraction standard (13C2PFDoA) to account for loss during sample preparation and instrumental variability. Each feature in the procedural blank samples was also subtracted prior to normalization to the isotope-labeled extraction standard.
Features were also extracted from individual data files using Agilent Mass Profiler software (ver. B.08.01) and aligned across all surface water samples (SI Table 4) to facilitate the identification of homologous series present in samples through Kendrick Mass Defect (KMD)-based data analysis workflow (Luo et al., 2020). During feature extraction, thresholds for identification were set by applying a Q-score (Agilent MassHunter peak quality metric that ranges from 0 to 100 which is an algorithmic estimate of how likely a feature is an actual compound) >80 for each individual feature and average feature abundance among all samples >500. Ions of 1− and 1+ charge states were the only ones evaluated to facilitate proper alignment of features. Similar to analysis by Skyline, the abundance of each feature present in the blank samples was subtracted from the experimental data. Features were matched by m/z values to a PFAS library made exclusively for Mass Profiler in negative and positive mode (SI Tables 5 and 6). Next, mass defect was calculated for each retained feature using CF2 scale (49.996806). Homologous series were identified based on similarity of mass defects and patterns in CCS values. Mass defect was determined through the following equation:
Tolerance of mass defect for features within a homologous series was set at 2.5 parts per thousand (ppt) based on the standard error in the calibration data from ESI tuning mix. The tolerance for difference in CCS between subsequent compounds in a series was set at 6–11 Å2. Features identified to be in homologous series that were not included in the PFAS library for Skyline analysis were subsequently evaluated in Skyline to validate the chromatograms of each feature.
IMS-MS arrival time distributions were produced using R statistical environment (ver. 3.6.3). Heatmap visualization was generated using R packages “gplots” (ver. 3.0.4) and “RColorBrewer” (ver. 1.1–2). Correlation plots, XY graphs, and stacked bar graphs were created using GraphPad Prism software (ver. 9.0.0; USA).
2. Results
Extensive water quality monitoring in HSC/GB after the ITC incident was conducted by the Texas Commission on Environmental Quality (TCEQ), Environmental Protection Agency (EPA), Coast Guard, and ITC contractors to determine which chemicals were released and their amounts. Even though the chemical characterization of the water samples extended over 2 months and included hundreds of analytes, only a few of the tested chemicals (benzene, toluene, ethylbenzene, xylenes, oil & grease, suspended solids, and total petroleum hydrocarbons) exhibited high concentrations in the locations near the ITC site, and only during the first week after the incident, before decreasing rapidly to levels generally below US EPA water quality criteria (Jang et al., 2021). Large quantities of chemically-diverse AFFF were used in the ITC response (Aly et al., 2020); however, the government agencies-led exposure assessment included PFAS analysis only for the surface water samples collected on March 21, 2019 (U.S. EPA, 2019). Indeed, both wide-spread and persistent (over months after the incident) contamination of HSC/GB with PFAS was observed (Aly et al., 2020) in connection to the ITC incident, but most of the PFAS detected and quantified were undetectable as they are not present in AFFF. Therefore, to enable a more comprehensive characterization of PFAS contamination in HSC/GB and to trace their dispersion, we analyzed surface water samples from 5 open water and 4 shore-accessible locations in HSC/GB (Fig. 1a and SI Table 2). Water samples were obtained upstream (n=2), downstream (n=6), and adjacent to the ITC incident site (n=1). Samples collected over the course of 5 months (April – August 2019) were analyzed to identify PFAS present in the water and to determine temporal trends in their fate. Even though the information on the types of AFFF that were used to extinguish the fire was publicly available (Aly et al., 2020), the amount of each product that was used, and the samples of these AFFF for chemical analyses were not available. Therefore, water samples collected near ITC after the incident were the best available samples we could use to investigate PFAS contamination from AFFF use.
2.1. Variability in PFAS Detected in HSC/GB
Surface water samples were initially analyzed with a targeted LC-MS/MS method for 27 PFAS as reported in (Aly et al., 2020). Of the 27 target PFAS, only 7 compounds were consistently detected at quantifiable amounts – perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorohexane-sulfonic acid (PFHxS), perfluorooctane sulfonate (PFOS), and 6:2 fluorotelomer sulfonate (FTS). Following targeted screening, we performed an untargeted LC-IMS-MS analysis of the samples studied in (Aly et al., 2020). Due to differences in sample extraction, only samples from April through August (n=31) were available for untargeted screening.
Analysis with LC-IMS-MS revealed variability in features detected across different types of samples collected in April (Fig. 1b). We evaluated the compositional similarity in the water samples across all sample locations and time points. Fig. 2 shows a heatmap and an unsupervised hierarchical clustering dendrogram based on the data from the top 50 most abundant LC-IMS-MS features (SI Table 7). Overall, sampling locations from the same time period showed high similarity (as evidenced by close clustering) in abundance among these 50 features. It is noteworthy that seven PFAS were identified within the top fifty features. The intensity in abundance for a majority of the 50 features markedly declined from April to August 2019, indicating a time-dependent trend.
Figure 2. Hierarchical clustering of top 50 most abundant features detected by LC-IMS-MS.

Heatmap shows the relative abundance of the top 50 features obtained from Mass Profiler (ver. B.08.01) for all open water (SCW1–5) and shore-access (Baytown Nature Center, BNC; Lynchburg Ferry, LF; Morgan’s Point, MP; River Terrace Park, RTP) surface water samples across all time points (April–August 2019). See SI Table 2 for sample location information. Open water samples were not collected in August. Seven PFAS species (labeled above map) were identified within the top 50 features. See SI Table 7 for data used to create this heatmap.
2.2. Concordance of Targeted LC-MS/MS and Untargeted LC-IMS-MS Analyses of PFAS
All 7 major PFAS identified by LC-MS/MS (Aly et al., 2020) were also detected by LC-IMS-MS. We compared normalized LC-IMS-MS-derived feature abundances of the 7 compounds across all samples to absolute concentrations reported in (Aly et al., 2020) (Fig. 3). Because of the differences in data scales and semi-quantitative nature of untargeted LC-IMS-MS analysis, we used rank correlation for these comparisons. Of the 7 PFAS, 6 showed significant concordance between LC-MS/MS and LC-IMS-MS data among samples. Levels of PFOA were negatively correlated between the two analyses (Spearman ρ = −0.38, data not shown). The lack of concordance for PFOA between targeted and untargeted analyses is likely due to the lack of sufficient precision in estimating abundances of this analyte that was detected at very low concentrations by both methods. Indeed, among these 7 PFAS, we noticed more variation in PFAS that had lower abundance as compared to those detected at higher levels.
Figure 3. Correlations of PFAS detected by LC-MS/MS and LC-IMS-MS.

Pair-wise correlation (Spearman rank correlation) plots of PFAS from each sample location and time point (n=31). For LC-MS/MS data, each PFAS value was ranked from highest (1) to lowest (31) in terms of absolute concentration. For LC-IMS-MS data, each PFAS value was ranked from highest (1) to lowest (31) in terms of relative normalized abundance. Spearman (ρ) correlation values are shown in each graph together with a corresponding p-value. Red dotted line is a unit line.
2.3. PFAS Feature Validation and Kendrick Mass Defect (KMD) Analysis
In order to determine what other PFAS species were present in the surface water samples, LC-IMS-MS data were analyzed via Skyline and KMD approaches. Features present in samples were matched to a library of 76 PFAS containing their corresponding m/z and CCS values. All data files were imported simultaneously to Skyline and validated through inspection of chromatography for each individual sample (Fig. 4a and 4b). Chromatograms were inspected for peak shape regularity and the drift time spectra were assessed for the alignment of the features to the expected drift time window and presence of the isotopic patterns. As an example of feature validation, spectra for 6:2 fluorotelomer sulfonamide alkylbetaine (FTAB) (Fig. 4a) and 6:2 FTS (Fig. 4b) are shown to demonstrate that both low and high abundance compounds, respectively, have excellent chromatography and expected CCS and isotopic patterns. Following analysis in Skyline, the final list of validated PFAS present in surface water samples contained 26 compounds (SI Table 8). KMD analysis was performed using CF2 scale on the 26 PFAS to determine which species belong to a homologous series (Fig. 4C). Although there were 26 PFAS present in surface water samples analyzed in this study, only 24 of those are plotted on Fig. 4C because two compounds are isomers of PFOS and thus cannot be separated by the KMD vs m/z analyses. While about one half of all PFAS detected belong to one of three homologous series, many compounds were detected as individual features based on the PFAS library matches (Fig. 4C).
Figure 4. Sample LC-IMS-MS chromatograms and spectra of two PFAS features with drift time spectra and KMD plot for PFAS features identified in this study.

(a) Chromatogram and drift spectra for 6:2 FTAB. Top is the LC chromatogram for the precursor ion. Bottom, IMS-MS spectra indicating the precursor ion and its isotopic patterns with the shaded area indicating drift time tolerance for confident identification. Colors indicate feature abundance. (b) Chromatogram and drift time spectra for 6:2 FTS. (c) KMD CF2 scale plot versus m/z for all PFAS features identified in surface water samples from HSC/GB. Open symbols indicate features that belong to a homologous series. Shaded symbols indicate features that do not belong to a homologous series.
2.4. Temporal Analysis of the PFAS
As previously noted, the LC-IMS-MS analysis allowed us to detect 19 additional PFAS species as compared to the LC-MS/MS targeted analysis (Aly et al., 2020). Temporal patterns in the relative abundance of the PFAS identified in each sample are plotted in Fig. 5. Relative feature abundances were derived by normalizing the response of each individual PFAS feature to the response of the isotope-labeled internal standard, 13C2PFDoA, that was added during sample extraction. A time-dependent decline in PFAS abundance was a common pattern observed at all locations, regardless whether the location was up-stream or down-stream from the incident. The reason for detection of PFAS in both directions was explained by hydrodynamic modeling of water movements in HSC/GB. It was previously shown that water movement at the location of the incident is influenced not only by the elevations, but also by the waves, tides and winds; indeed, we showed bi-directional transport of the contaminants near ITC location in both up-stream and down-stream directions (Aly et al., 2020). Therefore, it is not surprising that residue concentrations of PFAS display similar time-dependent decline patterns at various locations in HSC/GC.
Figure 5. Comparison of relative feature abundance among PFAS detected by both LC-MS/MS and LC-IMS-MS (PFPeA, PFOA, PFHxA, PFHpA, PFHxS, PFOS, and 6:2 FTS) and PFAS features only detected by LC-IMS-MS.

Temporal trends (months of sampling in 2019 are shown on the x-axis) are shown for PFAS in HSC/GB surface water post-ITC fire incident. Individual species detected by both LC-MS/MS and LC-IMS-MS are shown individually (see color legend), while species only detected by LC-IMS-MS (Untargeted) are all combined. The abundance of each PFAS feature was normalized to the response of the isotope labeled internal standard 13C2PFDoA that was added during sample extraction. The number in brackets beneath each sampling location ID represents distance from ITC fire location. Positive values denote down-stream and negative values denote up-stream. Red box indicates the sampling site (SCW2) that was closest to the ITC fire location.
Importantly, this study shows that the 7 previously identified PFAS account for less than 50% of the total abundance of all PFAS detected by LC-IMS-MS, regardless of sampling site and time point. Also, we found that on average, samples from the open water sampling locations showed less abundance of PFAS compared to shore-accessible sites. The trend was consistent for all time points. Among the 7 PFAS identified through previous targeted analyses, 6:2 FTS and PFOS, known components of some AFFF formulations (Luo et al., 2020; Rotander et al., 2015), had the highest relative abundance across all samples.
From the 26 total PFAS detected by LC-IMS-MS, a majority of species belonged to several distinct subclasses of PFAS including perfluorinated carboxylic acids (PFCA) and perfluorosulfonic acids (PFSA). Therefore, we analyzed the time trends in abundance of the PFAS by subclass (Fig. 6). To facilitate time-trend analysis, the total abundance across time points was normalized to the class abundance in April 2019 and plotted for each individual location. The abundance of each class was expected to be highest in April 2019 because it was the closest time point to the date of the incident (March 19, 2019). Overall, a majority of classes showed a gradual reduction in abundance over time. Open water samples had an average reduction of 58% across all PFAS classes compared to April 2019, whereas shore-accessible samples showed an average reduction of 71%. The abundance of PFAS in subclass of PFCA remained at a constant level throughout sampling time. It is noteworthy that many PFCA species are legacy PFAS (e.g., PFOA) that are abundant in the environment and the fact that their amounts were invariable over the time of this experiment suggests that they were not released during firefighting at the ITC incident. Additionally, the subclass of perfluoroether carboxylic acids (PFECA) increased from the initial abundance in April 2019; however, it is important to note that PFECA subclass only contained one compound. Temporal trends in the relative feature abundance of each PFAS subclass showed a decrease when all subclasses were combined (SI Fig. 1).
Figure 6. Time trends in the abundance of different PFAS subclasses detected by LC-IMS-MS across all sampling locations and timepoints (April-August 2019).

Line graphs show normalized feature abundances of each subclass (n=8) of PFAS species detected by LC-IMS-MS. The total abundance within each subclass of PFAS features from different time points were normalized to the total abundance in April. The number of PFAS features within each subclass is indicated by the value next to the subclass name. The number in brackets beneath each sampling location represents distance from ITC fire location. Positive values denote down-stream and negative values denote up-stream. Red box indicates the sampling site (SCW2) that was closest to the ITC fire location. Fluorotelomer sulfonic acids (FTSA), perfluoroalkane sulfonamide (FASA), perfluorophosphonic acids (PFPA); additional abbreaviations for PFAS classess can be found in text.
2.5. Comparison of the PFAS Profiles in HSC/GB and in the Cape Fear River Watershed of North Carolina
We detected 26 PFAS in surface water samples collected in HSC/GB following the ITC fire incident. A number of previous studies have analyzed PFAS in surface water samples where contamination may have been likely from an industrial PFAS manufacturing instead of release of AFFF. A comparison between PFAS detected in our study and those detected downstream of a PFAS manufacturing site located in the Cape Fear River Watershed of North Carolina (Sun et al., 2016) shows a clear difference in compositional profiles of surface water samples (Fig. 7). Between the 26 PFAS detected in our study and the 17 PFAS detected in (Sun et al., 2016) there was an overlap of 9 PFAS. Most of the overlapping PFAS belong to the PFCA subclass. It is important to note that several PFAS detected in North Carolina were emerging PFAS rather than legacy compounds. Individual PFAS profiles for all 9 locations in HSC/GB are detailed in SI Table 9.
Figure 7. Comparison of PFAS compositional profiles between HSC/GB after the ITC incident and PFAS detected in Cape Fear River Watershed in North Carolina.
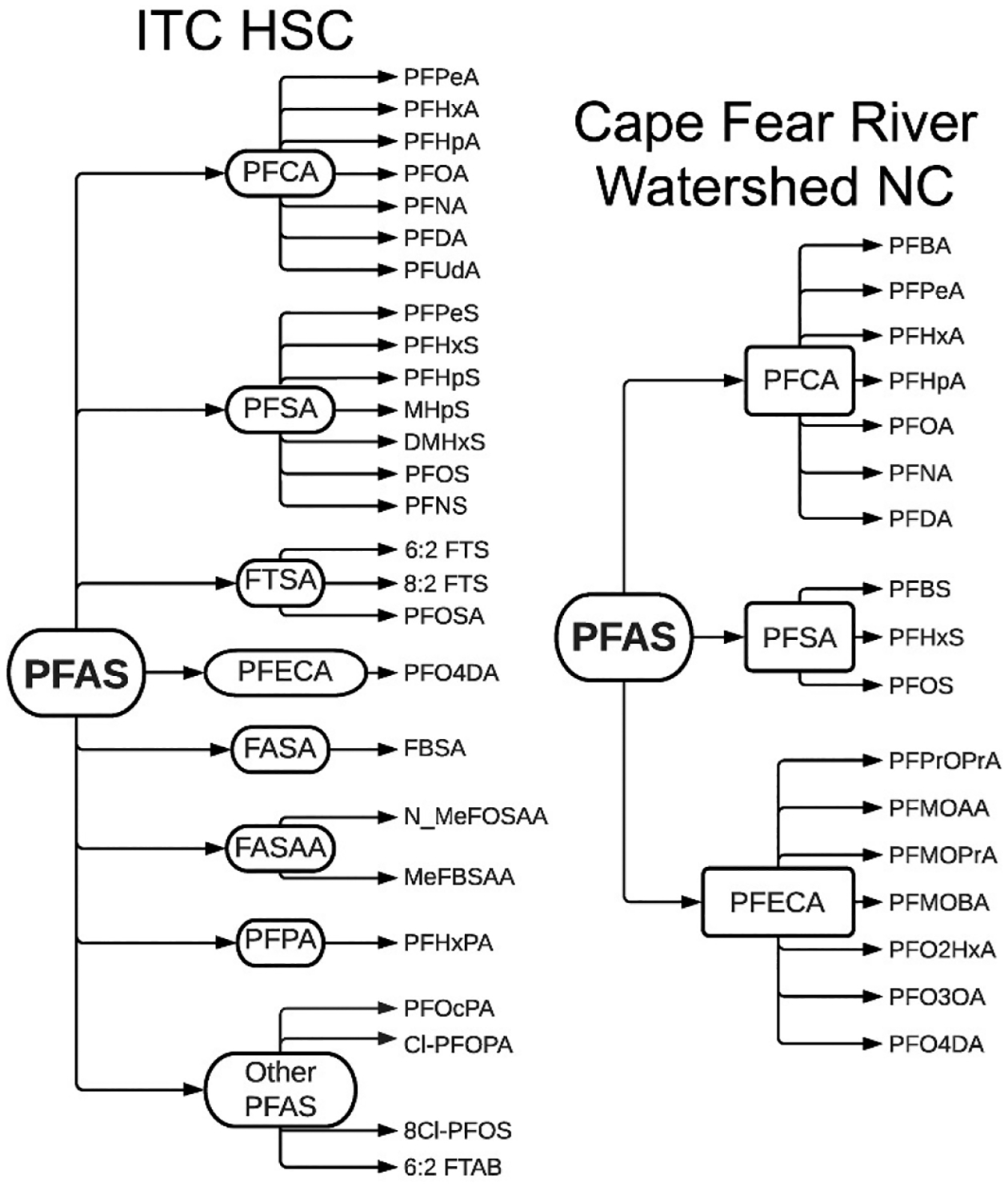
PFAS detected in surface water samples collected in HSC/GB after the ITC fire incident are shown on the left. PFAS detected in surface water samples collected at a drinking water treatment plant in the Cape Fear River watershed in North Carolina as reported in (Sun et al., 2016) are shown on the right.
3. Discussion
Traditional exposure assessment methods, including targeted analytical chemistry, have provided a wealth of knowledge about chemical concentrations in the environment (Caplin et al., 2019). However, targeted analyses are typically limited to a particular chemical or class of chemicals and may not account for the diversity of substances present in the environment (Pourchet et al., 2020; Sobus et al., 2018). Advances in HRMS have shown potential and promise to greatly expand detection of the broad spectrum of chemicals in both environmental and human exposome studies (Andra et al., 2017; David et al., 2021). HRMS can be used for both untargeted and targeted analyses because of its capability of achieving high mass accuracy with good sensitivity. Untargeted analyses facilitate rapid characterization of thousands of chemicals in various environmental and biological samples (Sobus et al., 2018). In addition, post-ionization separation techniques, such as IMS-MS further increase the peak capacity of HRMS and can be used for rapid untargeted analysis of both highly homologous and chemically diverse chemicals (Burnum-Johnson et al., 2019; Dodds et al., 2020; Monge et al., 2019). For example, recent application of LC-IMS-MS to the untargeted analysis of complex AFFF revealed 20 new PFAS-like homologous series that included a total of 124 PFAS-like features (Luo et al., 2020).
In this study, we have taken advantage of the untargeted capabilities of LC-IMS-MS and its recent successful application to the analysis of AFFF, to test a hypothesis that it will enable a more comprehensive characterization of PFAS, a growing class of persistent organic pollutants (OECD, 2020), in environmental water samples after a major industrial fire incident that was associated with a discharge of large quantities (up to 5 million liters) of AFFF into the HSC/GB watershed. Analysis was performed on 31 surface water samples collected in the HSC/GB for up to 6 months after the ITC fire incident in March 2019. Features obtained through LC-IMS-MS analysis were matched to a library of PFAS (Dodds et al., 2020) and also analyzed using KMD approach. In total, we identified 26 PFAS in the samples and these data were compared to those reported previously (Aly et al., 2020) as a direct comparison between targeted and untargeted analyses.
The use of PFAS in fire-suppressing liquids dates back to 1960s when the predominant PFAS in these formulations was PFOS, substance that was replaced starting in 1980s by a variety of fluorotelomers (ITRC, 2020). Firefighting foams are complex mixtures of proprietary composition that may contain both known and unidentified PFAS in varying proportions (Luo et al., 2020; Ruyle et al., 2021b); these substances are formulated to meet firefighting specifications, rather than to contain a specified type of PFAS (CONCAWE, 2016). Because large quantities of AFFF are typically discharged for fire suppression, surface and ground water contamination by PFAS has been attributed to their release in the industrial areas, near airports, and at the sites of large industrial fires (Houtz et al., 2018; Hu et al., 2016). Characterization of PFAS composition in AFFF and in environmental samples remains a critical data gap because the formulations are almost always proprietary (Garcia et al., 2019). Concomitantly, PFAS are chemicals of emerging concern to human and environmental health (Sunderland et al., 2019) and are subject to intense regulatory scrutiny (Hayes et al., 2019; Johnsen-Harris and Chetwynd, 2019).
A number of challenges remain for PFAS exposure assessment using either targeted analyses or untargeted HRMS. The former analytical approach provides quantitative data and is preferred by the regulatory agencies, but it is severely limited in the diversity of PFAS detected due to the need for analytical standards that are lacking for the vast majority of known PFAS (Woudneh et al., 2019). For example, targeted screening of surface water samples collected in HSC/GB following ITC fire incident revealed that only about 25% of the PFAS that were analyzed for were present in consistently quantifiable amounts, even though a number of diverse AFFF were used that likely contained many fluorotelomers (Aly et al., 2020). The latter analytical approach is also a challenge because structural elucidation of emerging PFAS and data curation required for suspect screening are time-consuming (Barzen-Hanson et al., 2017; Dauchy et al., 2017; Xiao et al., 2017). A recently proposed (Luo et al., 2020) sample analysis workflow using LC-IMS-MS may enhance exposure assessment when responding to environmental emergencies, such as the ITC fire. This method, when applied to the environmental water samples allowed us to identify additional 19 PFAS in addition to those detected by the targeted analysis (Aly et al., 2020). This finding is significant because more than half of total PFAS abundance in the samples tested in this study was contributed by these previously unidentified species.
Our study provides additional important clues with respect to the profile and fate of PFAS in the surface water of a large estuary. Our data shows that the total abundance of PFAS declined gradually over the period of months after the incident; however, the rates with which compounds in different PFAS classes were dissipating varied greatly. We found that PFCA were much more persistent than other subclasses of PFAS. This is not surprising because not only PFCA have very long half-lives in water at neutral pH (Washington and Jenkins, 2015), but they also are the products of both aerobic (Arvaniti and Stasinakis, 2015) and abiotic (Washington and Jenkins, 2015) biotransformation of other PFAS. By contrast, we found that perfluoroalkane sulfonamidoacetic acids (FASAA) were the shortest-lived species. This is also noteworthy as both methylperfluorooctanesulfonamido and perfluorobutanesulfonamido acetic acids are formed relatively fast (in weeks) from sulfonamide-based molecules that are also used in AFFF (Lange, 2018).
As previously mentioned, there are well over 5,000 compounds classified as PFAS (OECD, 2020). While a majority of legacy PFAS have been phased out of production, they are routinely detected in various environmental media (National Academies of Sciences Engineering and Medicine, 2021; NIEHS, 2020). Over the past decade, fluorotelomerization-based PFAS are predominating and replace legacy electrochemical fluorination-based PFAS (National Academies of Sciences Engineering and Medicine, 2021; OECD, 2020). Some of the most common novel PFAS such as “GenX” have not been found in AFFF formulations (Luo et al., 2020; Wood Environment and Infrastructure Solutions, 2020); however, they have been detected in surface water samples collected at drinking water treatment plants located in a watershed of a PFAS manufacturing site (Sun et al., 2016). A comparison between PFAS detected in our study and those found downstream from a PFAS manufacturer revealed major differences in PFAS profiles. Various legacy compounds that belong to the PFCA and PFSA subclasses were detected at both sites. However, the following compounds were only found in our study: 6:2 FTS, 8:2 FTS, perfluorobutane sulfonamide (FBSA), N-methylperfluorooctane sulfonamidoacetic acid (N-MeFOSAA), and 6:2 FTAB. All 5 of these PFAS are known components of AFFF formulations (D’Agostino and Mabury, 2017; Dauchy et al., 2019; Gonzalez et al., 2021; Ruyle et al., 2021b; Wood Environment and Infrastructure Solutions, 2020). On the other hand, the following emerging PFAS were only detected in the Cape Fear River watershed in North Carolina downstream of the PFAS manufacturing site (Sun et al., 2016): PFPrOPrA (“GenX”), perfluoro-2-methoxyacetic acid (PFMOAA), perfluoro-3-methoxypropanoic acid (PFMOPrA), perfluoro-4-methoxybutanoic acid (PFMOBA), perfluoro-3,5-dioxahexanoic acid (PFO2HxA), and perfluoro-3,5,7-trioxaoctanoic acid (PFO3OA). These differences in PFAS profiles from our study provide strong evidence that contamination of PFAS in HSC/GB was predominantly due to AFFF use as compared to other environmental sources such as PFAS manufacturing or landfills. Also, the profile of the PFAS detected in the HSC/GB is highly consistent with observations that both modern 6:2 fluorotelomer-containing (Sunderland et al., 2019) and legacy PFSA-containing (Wang et al., 2015) AFFF were deployed in this massive industrial fire incident.
While previous geospatial time-trend analysis and hydrodynamic simulations (Aly et al., 2020) point to the ITC fire and associated use of large quantities of AFFF as the primary source for the wide-spread contamination of HSC/GB with PFAS, even in this case the potential for pre-existing background level of PFAS may warrant consideration. Indeed, our study shows that even 6 months after the incident, detectable levels of PFAS were present across the entire watershed of HSC/GB. It is known that the widespread use and discharge of PFAS-containing materials in industrial and urbanized areas often reflects contributions from multiple sources (Anderson et al., 2016; Hu et al., 2016; Ruyle et al., 2021a; Zhang et al., 2016). Therefore, source attribution for PFAS, substances with complex metabolic and degradation patterns (Buck et al., 2011), is a key part of environmental site investigations and liability assessments (Benotti et al., 2020). It is widely acknowledged that current LC-MS/MS methods for PFAS detection and quantitation are largely unsuitable for PFAS forensics because they only characterize a small fraction of the total potential PFAS signature. More powerful analytical methods, such as LC-IMS-MS and other HRMS techniques, are crucial for characterization of some of the unknown PFAS. Therefore, our study provides valuable additional evidence on the use of untargeted analysis in the area where both incidental and historical contamination may be present. The fingerprints provided herein, both using the LC-IMS-MS, and high concordance of these data to the quantitative measurements using LC-MS/MS, will serve as useful public data for future source tracking efforts in the HSC/GB area and elsewhere. The data provided herein are commensurate with the recent suggestions for improved multi-dimensional data collection necessary for PFAS source tracking and apportionment. Specifically, as suggested by others (Benotti et al., 2020; Charbonnet et al., 2021), our dataset includes digitally accessible geospatial information, quantities of all measured target PFAS (Aly et al., 2020), and also untargeted ion mobility and mass spectral data that could enable later analyses for subsequently identified PFAS and for comparison with future sampling campaigns. These data are also highly valuable for comparison to other publicly available datasets and will lead to better identification of outliers in the PFAS fingerprints, and increase precision in source apportionment.
We note several limitations in our study. First, our untargeted analysis through LC-IMS-MS provides semi-quantitative data that may be used to compare relative levels of PFAS across samples and time points, but it does not provide absolute quantification of detected features. Relative feature abundance of PFAS is not as interpretable as absolute levels; however, the data is still highly informative and provides temporal patterns of PFAS in HSC/GB that were previously undetected using a targeted approach. Additionally, these data are informative with respect to future targeted chemical analyses if quantitation is necessary. Second, feature identification in LC-IMS-MS relies on the availability of CCS data and other computational methods (e.g., mass defect). Open software tools such as Skyline facilitate feature alignment for confident molecular identification (MacLean et al., 2018); however, there is still a need for a larger library that contains not only m/z (McDonough et al., 2020), but also CCS (Dodds et al., 2020; Luo et al., 2020) information for PFAS. In order to obtain that data for a compound, additional analytical standards are needed. Follow up re-analyses of our data using CCS information on additional PFAS would be possible and highly beneficial to further characterize PFAS that were present in these and other environmental samples.
4. Conclusions.
This study used LC-IMS-MS to characterize PFAS in HSC/GB following the ITC fire incident through untargeted analysis. Our data show that this method can be successfully applied for fingerprinting and comprehensive exposure characterization of PFAS in the environment through untargeted analyses. Data generated from untargeted analyses were highly concordant with the results of the traditional LC-MS/MS analyses, but provided information on almost three times as many additional compounds that contributed to more than half of total PFAS detected in the surface waters in HSC/GB. Thus, untargeted analyses may serve as beneficial tools for rapid exposure characterization following an emergency response before using targeted methods for absolute quantification of environmental contaminants.
Supplementary Material
Acknowledgements.
This work was funded, in part, by grants P42 ES027704 and P30 ES029067 from the National Institute of Environmental Health Sciences. N.A.A., A.C., and G.C. were supported, in part, by a training grant T32 ES026568 from the National Institute of Environmental Health Sciences.
Footnotes
Declarations. The authors declare no competing financial interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at https://www.sciencedirect.com/science/article/pii/S1001074221003089#sec0019
Data Availability Statement.
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- Aly NA, Luo YS, Liu Y, Casillas G, McDonald TJ, Kaihatu JM, et al. , 2020. Temporal and spatial analysis of per and polyfluoroalkyl substances in surface waters of Houston ship channel following a large-scale industrial fire incident. Environ. Pollut 265:115009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RH, Long GC, Porter RC, Anderson JK, 2016. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 150:678–685. [DOI] [PubMed] [Google Scholar]
- Andra SS, Austin C, Patel D, Dolios G, Awawda M, Arora M, 2017. Trends in the application of high-resolution mass spectrometry for human biomonitoring: An analytical primer to studying the environmental chemical space of the human exposome. Environ. Int 100:32–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumol T, Yang DD, Sosienski T, Batoon P, 2018. Analysis of per/polyfluoroalkyl substances (PFASs) in drinking water using the Agilent Ultivo triple quadrupole LC/MS. Agilent Available: https://www.agilent.com/cs/library/applications/5991-8969EN_PFAS_Application.pdf. Accessed May 25, 2021.
- Arvaniti OS, Stasinakis AS, 2015. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci. Total Environ 524–525:81–92. [DOI] [PubMed] [Google Scholar]
- ATSDR, 2018. Toxicological Profile for Perfluoroalkyls, in: Services U S D o H a H (Ed.). Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- AWWA, 2019. Per- and Polyfluoroalkyl Substances (PFAS) Monitoring, Sampling, and Analysis American Water Works Association. Available: https://www.awwa.org/LinkClick.aspx?fileticket=X3NIs3-j1sc%3D&portalid=0. Accessed May 22, 2021. [Google Scholar]
- Baker ES, 2021. Collision Cross Section database Available: https://brcwebportal.cos.ncsu.edu/baker/. Accessed July 12, 2021.
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N,et al. , 2017. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol 51:2047–2057. [DOI] [PubMed] [Google Scholar]
- Benotti MJ, Fernandez LA, Peaslee GF, Douglas GS, Uhler AD, Emsbo-Mattingly S, 2020. A forensic approach for distinguishing PFAS materials. Environ. Forensics 21:319–333. [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. , 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag 7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnum-Johnson KE, Zheng X, Dodds JN, Ash J, Fourches D, Nicora CD, et al. , 2019. Ion Mobility Spectrometry and the Omics: Distinguishing Isomers, Molecular Classes and Contaminant Ions in Complex Samples. Trends Analyt. Chem 116:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplin A, Ghandehari M, Lim C, Glimcher P, Thurston G, 2019. Advancing environmental exposure assessment science to benefit society. Nat. Commun 10:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnet JA, Rodowa AE, Joseph NT, Guelfo JL, Field JA, Jones GD, et al. , 2021. Environmental Source Tracking of Per- and Polyfluoroalkyl Substances within a Forensic Context: Current and Future Techniques. Environ. Sci. Technol 55:7237–7245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan TL, Moodie D, Kolobaric A, Szabo D, Shimeta J, Crosbie ND, et al. , 2019. An investigation into per- and polyfluoroalkyl substances (PFAS) in nineteen Australian wastewater treatment plants (WWTPs). Heliyon 5:e02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONCAWE, 2016. Environmental fate and effects of polyand perfluoroalkyl substances (PFAS) European Petroleum Refiners Association, Brussels, Belgium. [Google Scholar]
- D’Agostino LA, Mabury SA, 2017. Certain Perfluoroalkyl and Polyfluoroalkyl Substances Associated with Aqueous Film Forming Foam Are Widespread in Canadian Surface Waters. Environ. Sci Technol 51:13603–13613. [DOI] [PubMed] [Google Scholar]
- Dauchy X, Boiteux V, Bach C, Rosin C, Munoz JF, 2017. Per- and polyfluoroalkyl substances in firefighting foam concentrates and water samples collected near sites impacted by the use of these foams. Chemosphere 183:53–61. [DOI] [PubMed] [Google Scholar]
- Dauchy X, Boiteux V, Colin A, Hémard J, Bach C, Rosin C, et al. , 2019. Deep seepage of per- and polyfluoroalkyl substances through the soil of a firefighter training site and subsequent groundwater contamination. Chemosphere 214:729–737. [DOI] [PubMed] [Google Scholar]
- David A, Chaker J, Price EJ, Bessonneau V, Chetwynd AJ, Vitale CM, et al. , 2021. Towards a comprehensive characterisation of the human internal chemical exposome: Challenges and perspectives. Environ. Int 156:106630. [DOI] [PubMed] [Google Scholar]
- Deer Park Emergency Services, 2019. ITC Fire Updates Available: https://www.deerparktx.gov/1778/ITC-Fire. Accessed May 25, 2021.
- Dodds JN, Baker ES, 2019. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass Spectrom 30:2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds JN, Hopkins ZR, Knappe DRU, Baker ES, 2020. Rapid Characterization of Per- and Polyfluoroalkyl Substances (PFAS) by Ion Mobility Spectrometry-Mass Spectrometry (IMS-MS). Anal. Chem 92:4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Nadal M, 2019. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res 177:108648. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Chiaia-Hernandez AC, Lara-Martin PA, Loos M, Hollender J, Oetjen K, et al. , 2019. Suspect Screening of Hydrocarbon Surfactants in AFFFs and AFFF-Contaminated Groundwater by High-Resolution Mass Spectrometry. Environ. Sci. Technol 53:8068–8077. [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Thompson K, Quinones O, Dickenson E, Bott C, 2021. Assessment of PFAS fate, transport, and treatment inhibition associated with a simulated AFFF release within a WASTEWATER treatment plant. Chemosphere 262:127900. [DOI] [PubMed] [Google Scholar]
- Hayes J, Faber S, Andrews D, Lothspelch A, 2019. PFAS Nation: Toxic Discharges Suspected From Almost 500 Industrial Facilities Across U.S. Environmental Working Group Available: https://www.ewg.org/news-insights/news/pfas-nation-toxic-discharges-suspected-almost-500-industrial-facilities-across. Accessed May 25, 2021.
- Hekster FM, Laane RW, de Voogt P, 2003. Environmental and toxicity effects of perfluoroalkylated substances. Rev. Environ. Contam. Toxicol 179:99–121. [DOI] [PubMed] [Google Scholar]
- Houtz E, Wang M, Park JS, 2018. Identification and Fate of Aqueous Film Forming Foam Derived Per- and Polyfluoroalkyl Substances in a Wastewater Treatment Plant. Environ. Sci. Technol 52:13212–13221. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. , 2016. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett 3:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITRC, 2020. History and Use of Per- and Polyfluoroalkyl Substances (PFAS) Interstate Technology Regulatory Council, Washington, DC. Available: https://pfas-1.itrcweb.org/fact_sheets_page/PFAS_Fact_Sheet_History_and_Use_April2020.pdf. Accessed May 25, 2021. [Google Scholar]
- Jang S, McDonald TJ, Bhandari S, Rusyn I, Chiu WA, 2021. Spatial and temporal distribution of surface water contaminants in the Houston Ship Channel after the Intercontinental Terminal Company Fire. J. Expo. Sci. Environ. Epidemiol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen-Harris B, Chetwynd J, 2019. House passes sweeping PFAS protections: 2025 ban on military use, Superfund cleanup and clean water safeguards Environment America, Denver, CO. Available: https://environmentamerica.org/news/ame/house-passes-sweeping-pfas-protections-2025-ban-military-use-superfund-cleanup-and-clean. Accessed May 25, 2021. [Google Scholar]
- Lange CC, 2018. Anaerobic biotransformation of N-methyl perfluorobutanesulfonamido ethanol and N-ethyl perfluorooctanesulfonamido ethanol. Environ. Toxicol. Chem 37:768–779. [DOI] [PubMed] [Google Scholar]
- Luo YS, Aly NA, McCord J, Strynar MJ, Chiu WA, Dodds JN, et al. , 2020. Rapid Characterization of Emerging Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foams Using Ion Mobility Spectrometry-Mass Spectrometry. Environ. Sci. Technol 54:15024–15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean BX, Pratt BS, Egertson JD, MacCoss MJ, Smith RD, Baker ES, 2018. Using Skyline to Analyze Data-Containing Liquid Chromatography, Ion Mobility Spectrometry, and Mass Spectrometry Dimensions. J. Am. Soc. Mass Spectrom 29:2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J, Newton S, Strynar M, 2018. Validation of quantitative measurements and semi-quantitative estimates of emerging perfluoroethercarboxylic acids (PFECAs) and hexfluoroprolyene oxide acids (HFPOAs). J. Chromatogr. A 1551:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough CA, Choyke S, Ferguson PL, DeWitt JC, Higgins CP, 2020. Bioaccumulation of Novel Per- and Polyfluoroalkyl Substances in Mice Dosed with an Aqueous Film-Forming Foam. Environ. Sci. Technol 54:5700–5709. [DOI] [PubMed] [Google Scholar]
- Mejia-Avendano S, Munoz G, Vo Duy S, Desrosiers M, Benoi TP, Sauve S, et al. , 2017. Novel Fluoroalkylated Surfactants in Soils Following Firefighting Foam Deployment During the Lac-Megantic Railway Accident. Environ. Sci. Technol 51:8313–8323. [DOI] [PubMed] [Google Scholar]
- Monge ME, Dodds JN, Baker ES, Edison AS, Fernandez FM, 2019. Challenges in Identifying the Dark Molecules of Life. Annu. Rev. Anal. Chem. (Palo Alto Calif) 12:177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz G, Desrosiers M, Duy SV, Labadie P, Budzinski H, Liu J, et al. , 2017. Environmental Occurrence of Perfluoroalkyl Acids and Novel Fluorotelomer Surfactants in the Freshwater Fish Catostomus commersonii and Sediments Following Firefighting Foam Deployment at the Lac-Mégantic Railway Accident. Environ. Sci. Technol 51:1231–1240. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine, 2021. Federal Government Human Health PFAS Research Workshop: Proceedings of a Workshop–in Brief, in: Johnson S, Beins K, Whitacre P (Eds.). The National Academies Press, Washington, DC, p. 12. Available: https://www.nap.edu/catalog/26054/federal-government-human-health-pfas-research-workshop-proceedings-of-a. Accessed May 25, 2021. [Google Scholar]
- NIEHS, 2020. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) National Institute of Environmental Health Sciences. Available: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm. Accessed May 25, 2021. [Google Scholar]
- OECD, 2020. Portal on Per and Poly Fluorinated Chemicals Organisation for Economic Co-operation and Development, Paris, France. Available: https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/. Accessed May 25, 2021. [Google Scholar]
- Pourchet M, Debrauwer L, Klanova J, Price EJ, Covaci A, Caballero-Casero N, et al. , 2020. Suspect and non-targeted screening of chemicals of emerging concern for human biomonitoring, environmental health studies and support to risk assessment: From promises to challenges and harmonisation issues. Environ. Int 139:105545. [DOI] [PubMed] [Google Scholar]
- Rodriguez KL, Hwang JH, Esfahani AR, Sadmani A, Lee WH, 2020. Recent Developments of PFAS-Detecting Sensors and Future Direction: A Review. Micromachines (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotander A, Toms LM, Aylward L, Kay M, Mueller JF, 2015. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environ. Int 82:28–34. [DOI] [PubMed] [Google Scholar]
- Ruyle BJ, Pickard HM, LeBlanc DR, Tokranov AK, Thackray CP, Hu XC, et al. , 2021a. Isolating the AFFF Signature in Coastal Watersheds Using Oxidizable PFAS Precursors and Unexplained Organofluorine. Environ. Sci. Technol 55:3686–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyle BJ, Thackray CP, McCord JP, Strynar MJ, Mauge-Lewis KA, Fenton SE, et al. , 2021b. Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett 8:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker J, Tettenhorst D, 2018. Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) Washington, DC. Available: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=343042&Lab=NERL. Accessed May 25, 2021. [Google Scholar]
- Sinclair GM, Long SM, Jones OAH, 2020. What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere 258:127340. [DOI] [PubMed] [Google Scholar]
- Silcock P, Karrman A, van Bavel B, 2020. Advancing perfluorinated compound analysis using simultaneous matrix monitoring. Water South Africa (Pretoria) Available: https://www.waters.com/webassets/cms/library/docs/720003162en.pdf. Accessed July 12, 2021.
- Sobus JR, Wambaugh JF, Isaacs KK, Williams AJ, McEachran AD, Richard AM, et al. , 2018. Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. J. Expo. Sci. Environ. Epidemiol 28:411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. , 2016. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Tech. Lett 3:415–419. [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG, 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol 29:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, 2019. ITC Fire Response Causes Toxic Contamination of Houston Ship Channel. Sierra Club Available: https://www.sierraclub.org/press-releases/2019/09/itc-fire-response-causes-toxic-contamination-houston-ship-channel. Accessed May 25, 2021.
- U.S. EPA, 2019. ITC Fire Response. Environmental Protection Agency Available: https://response.epa.gov/site/site_profile.aspx?site_id=14150#:~:text=At%20approximately%2010%3A20%20am,Center%20(NRC%20%231240304).&text=It%20was%20operated%20in%20the,2019%2C%20when%20it%20was%20demobilized. Accessed May 25, 2021.
- Viehland LA, Mason EA, 1975. Gaseous lon mobility in electric fields of arbitrary strength. Ann. Physics 91:499–533. [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K, 2015. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ. Int 75:172–179. [DOI] [PubMed] [Google Scholar]
- Washington JW, Jenkins TM, 2015. Abiotic Hydrolysis of Fluorotelomer-Based Polymers as a Source of Perfluorocarboxylates at the Global Scale. Environ. Sci. Technol 49:14129–14135. [DOI] [PubMed] [Google Scholar]
- Wood Environment and Infrastructure Solutions, 2020. The use of PFAS and fluorine-free alternatives in fire-fighting foams Wood Environment and Infrastructure Solutions, London, United Kingdom, p. 534. Available: https://echa.europa.eu/documents/10162/28801697/pfas_flourine-free_alternatives_fire_fighting_en.pdf/d5b24e2a-d027-0168-cdd8-f723c675fa98. Accessed May 22, 2021. [Google Scholar]
- Woudneh MB, Chandramouli B, Hamilton C, Grace R, 2019. Effect of Sample Storage on the Quantitative Determination of 29 PFAS: Observation of Analyte Interconversions during Storage. Environ. Sci. Technol 53:12576–12585. [DOI] [PubMed] [Google Scholar]
- Xiao F, Golovko SA, Golovko MY, 2017. Identification of novel non-ionic, cationic, zwitterionic, and anionic polyfluoroalkyl substances using UPLC-TOF-MS(E) high-resolution parent ion search. Anal. Chim. Acta 988:41–49. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD, et al. , 2016. Source attribution of poly- and perfluoroalkyl substances (PFASs) in surface waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett 3:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


