Highlights
-
•
This is the first study to explore the combined effects of grip strength and hypertension on the risk of major cardiovascular incidence, cardiovascular mortality, and all-cause mortality.
-
•
The results of the combined analyses indicate that the risk of cardiovascular incidence, cardiovascular mortality, and all-cause mortality is highest for people with hypertension and low grip strength.
-
•
Hypertensive patients with high grip strength were not at a higher risk of death than the normotensive participants.
Keywords: Cardiovascular disease, Cohort study, Grip strength, Hypertension, Mortality
Abstract
Background
Both hypertension and grip strength (GS) are predictors of mortality and cardiovascular disease (CVD), but whether these risk factors interact to affect CVD and all-cause mortality is unknown. This study sought to investigate the associations of GS with the risk of major CVD incidence, CVD mortality, and all-cause mortality in patients with hypertension.
Methods
GS was measured using a Jamar dynamometer (Sammons Preston, Bolingbrook, IL, USA) in participants aged 35–70 years from 12 provinces included in the Prospective Urban Rural Epidemiology China Study. Cox frailty proportional hazards models were used to examine the associations of GS and hypertension and the outcomes of all-cause mortality and CVD incidence/mortality.
Results
Among 39,862 participants included in this study, 15,964 reported having hypertension, and 9095 had high GS at baseline. After a median follow-up of 8.9 years (interquartile range, 6.7–9.9 years), 1822 participants developed major CVD, and 1250 deaths occurred (388 as a result of CVD). Compared with normotensive participants with high GS, hypertensive patients with high GS had a higher risk of major CVD incidence (hazard ratio (HR) = 2.39; 95% confidence interval (95%CI): 1.86–3.06; p < 0.001) or CVD mortality (HR = 3.11; 95%CI: 1.59–6.06; p < 0.001) but did not have a significantly increased risk of all-cause mortality (HR = 1.24; 95%CI: 0.92–1.68; p = 0.159). These risks were further increased if hypertensive participants whose GS level was low (major CVD incidence, HR = 3.31, 95%CI: 2.60–4.22, p < 0.001; CVD mortality, HR = 4.99, 95%CI: 2.64–9.43, p < 0.001; and all-cause mortality, HR = 1.93, 95%CI: 1.47–2.53, p < 0.001).
Conclusion
The present study demonstrates that low GS is associated with the highest risk of major CVD incidence, CVD mortality, and all-cause mortality among hypertensive patients. High levels of GS appear to mitigate long-term mortality risk among hypertensive patients.
Graphic Abstract
1. Introduction
Muscle function is important not only for physical performance but also in maintaining optimal health throughout life.1,2 Considerable evidence suggests that muscle function impacts the development of diabetes,3 lipids,4 the risk of sudden cardiac death,5 and all-cause mortality.6 However, muscle function decline is correlated with aging, and aging is getting more and more attention in modern society. According to recent data, the prevalence of sarcopenia (loss of muscle mass) in community-dwelling older adults was 11% (95% confidence interval (95%CI): 8%–13%) in men and 9% (95%CI: 7%–11%) in women.7 Decreased muscle strength reduces the quality of life and increases healthcare expenditures.8
Muscle strength measured by the grip strength (GS) test is a simple, inexpensive indicator for predicting the risk of cardiovascular disease (CVD) incidence, CVD mortality, and all-cause mortality.9 Low GS has also been reported to be associated with the risk of hypertension and arterial stiffness,10, 11, 12 but reports on the association between GS and blood pressure (BP) have been conflicting in several cross-sectional studies. Three of the studies conducted in the United States and England revealed an inverse association between GS and BP or hypertension,13, 14, 15 but a positive association between GS and BP was found in other studies.16,17 In the National Health and Nutrition Examination Survey for the years 2011–2012, GS was lower in individuals with diagnosed and undiagnosed hypertension compared with those without hypertension.13 Worldwide, BP is the most prevalent modifiable risk factor for cardiovascular morbidity and mortality and is associated not only with muscle strength but also with adverse health outcomes. We hypothesize that there may be an interaction between BP and GS for the association with CVD and all-cause mortality.
To date, there have been few studies that have investigated the associations of muscle strength with both CVD and all-cause mortality among patients with hypertension. A previous population-based cohort study observed a significant association between high levels of muscle strength and lower all-cause mortality among the subgroup of people with hypertension (n = 1506);18 however, only male participants were included and there were no comparative results with people without hypertension. Whether this relationship persists after more robust adjustment for confounding factors and whether these associations differ between people with and without hypertension remain to be established. Given these knowledge gaps in the literature, we aimed to examine the associations between hypertension and GS for the risk of major CVD incidence, CVD mortality, and all-cause mortality among 39,862 participants in the large-scale Prospective Urban Rural Epidemiology (PURE) China cohort study.
2. Methods
2.1. Study design and participants
The PURE China cohort study recruited 47,677 participants (the majority of whom were aged 35–70 years) from the general population between 2005 and 2009. Participants from 115 communities (45 urban and 70 rural) in 12 provinces completed a standardized questionnaire and underwent a physical examination, as has been previously described.9,19,20 The locations were grouped into 1 of 3 regions based on national economic criteria: Eastern regions (Beijing, Jiangsu, Shandong, and Liaoning), Central regions (Shanxi, Jiangxi, and Inner Mongolia), and Western regions (Yunnan, Qinghai, Shaanxi, Xinjiang, and Sichuan). In the PURE prospective cohort study, the major incidence and mortality of CVD (defined as myocardial infarction, stroke, and heart failure) and all-cause mortality were the main outcomes; hypertension and GS were the exposures of interest. Sociodemographic factors (age, sex, location, education, and occupation), health-related variables (anthropometric indicators, including height, weight, and waist and hip circumferences), BP medication history, prevalence of diabetes at baseline, and lifestyle factors (tobacco and alcohol use, total physical activity (PA), and dietary intake) were treated as potential confounders. Hypertension was defined as having a diagnosis of hypertension, receiving BP-lowering treatment, or having an average measured systolic BP of at least 140 mmHg or a diastolic BP of at least 90 mmHg (or both) at baseline. Only baseline BP was available for our study.
In our analyses, we included all outcome events known until September 2017. A total of 45,249 participants had at least one follow-up. Among the remaining individuals, 44,336 (98.0%) provided GS and hypertension information at baseline. We also excluded 21 participants (0.1%) who did not have complete data on age and gender, 440 participants (1.0%) who were less than 35 or more than 70 years of age, and 4013 participants (8.9%) who had pre-existing CVDs, cancers, human immunodeficiency virus infection, acquired immune deficiency syndrome, or chronic obstructive pulmonary disease at baseline. A total of 39,862 individuals who were free of CVD at baseline were included in our analysis. Participants were categorized according to their hypertension status (yes/no) and GS status (low/moderate/high).
2.2. Procedures
Information on outcome events (death, myocardial infarction, stroke, heart failure) was obtained from participants or their family members (if the individual had died). This information was adjudicated centrally by trained physicians using standardized definitions.21 Event documentation was based on information mainly from household interviews and medical records, death certificates, and other sources.
GS (kg) was measured 3 times for each hand using a Jamar dynamometer (Sammons Preston, Bolingbrook, IL, USA). The arm was positioned at the side of the body and the dynamometer held with the elbow flexed to 90°. The participant was asked to squeeze the device as hard as possible for 3 s. The measurement was repeated twice more at intervals of at least 30 s. GS was calculated by taking the mean of the maximal values of non-dominant and dominant GS. Because GS varies greatly between sexes and age groups,22 we categorized gender- and age-specific GS into low (<25th percentile), moderate (25th–75th percentile), and high (>75th percentile) strength subgroups using their respective 25th and 75th percentile values as a cutoff point (Supplementary Table 1).
PA was evaluated using the long-form International Physical Activity Questionnaire, in which 4 main types of PA—occupation, housework, transportation, and recreation—were recorded in minutes per week. The total PA was computed as the sum of these 4 PA types in metabolic equivalents (METs) × minutes per week. According to the intensities of different types of PA, the following equation was also applied to calculate the total PA:23 minutes per week = 0.825 × walking minutes + 1 × moderate minutes + 1.375 × garden minutes + 1.5 × cycling minutes + 2 × vigorous minutes.24 Total PA was classified as low (<600 MET × min/week or <150 min/week of moderate-intensity PA), moderate (600–3000 MET × min or 150–750 min/week), or high (>3000 MET × min or >750 min/week).
Dietary information was recorded by interview using a validated Food Frequency Questionnaire.25 The Food Frequency Questionnaire contains a list of food items (including staple foods, unprocessed/processed meats, aquatic products, dairy, eggs, legumes, fruits, vegetables, snacks and nuts, cooking oil, condiments, and so on) commonly consumed over the previous year, with open-ended frequencies of consumption and the average amount of consumption. To compute the daily nutrient intake, the reported frequency of consumption for each food item was converted to daily intake and then was multiplied by the nutrient contents obtained from the Chinese Food Composition Tables. On the basis of responses to the Food Frequency Questionnaire, we estimated daily calorie intake and the proportion of calorie intake from protein.
Sociodemographic information, such as occupation (professionals/managers, skilled workers, unskilled workers, and homemakers) and education (primary education level or less, secondary school education, and college/trade school/university education), was self-reported at baseline. Using a standard questionnaire, information on smoking and alcohol status was collected, and participants were categorized into never, former (quit smoking and drinking for the previous 12 months), or current. Height (without shoes) was measured to the nearest 0.1 cm using a tape measure (Version 3021060; H.A. Kidd & Company, Shanghai, China). Weight was measured to the nearest 0.1 kg using a digital scale (Version 701-BC554; Tanita Corp., Shanghai, China), with participants being lightly clothed and wearing no shoes. Waist and hip circumferences were measured unclothed to the nearest 0.1 cm using a tape measure. Waist circumference was measured at the midpoint between the lowest rib margin and the level of the anterior superior iliac crest. Hip circumference was measured at the level of the greater trochanters. BP (mmHg) was read using an automated sphygmomanometer (Omron HEM-757; Omron, Kyoto, Japan). Body mass index (kg/m2) was calculated as weight divided by height squared. Waist to hip ratio was calculated as waist circumference divided by hip circumference. The follow-ups for each participant occurred at least every 3 years after the baseline recruitment via either telephone or face-to-face interviews performed by the local research team.
The study was approved by the institutional review board at Fuwai Hospital of the Chinese Academy of Medical Sciences and Beijing Hypertension League Institute. All participants read and signed the informed written consent form before participating in the study.
2.3. Statistical analysis
Baseline characteristics for the groups of the participants were described using frequencies with percentages for categorical variables and means with standard deviations for continuous variables. Linearity was explored with multivariable cubic regression splines between GS and adverse outcomes, with no evidence of deviation from linearity. The unadjusted Kaplan–Meier curve was used to visualize the adverse event risk trajectories between hypertensive/non-hypertensive patients with different levels of GS. Time-to-event analyses were examined using Cox frailty proportional hazards models with random intercepts for regions in the absence of a violation of the proportional hazard assumption. The proportional hazards assumption was checked by Schoenfeld residuals tests. The results were reported as hazard ratios (HRs) together with 95%CI. To investigate whether levels of GS modified the associations between hypertension and adverse outcomes, multiplicative interaction between hypertension and age- and sex-specific categories of GS were assessed in the Cox model by including a product term hypertension × GS. We took high strength as the reference group for GS status. When examining the association of the combination of GS status and hypertension with outcomes, the combination of high strength and non-hypertension was used as the reference group.
All analyses were presented as the 3 models that included an increasing number of covariates. Model 1 was adjusted for age, sex, and location. Model 2 included variables of Model 1 plus education, occupation, body mass index, waist to hip ratio, smoking, alcohol, baseline prevalence of diabetes, and history of medication for hypertension. Model 3 included variables of Model 2 plus daily dietary energy intake, the proportion of calorie intake from protein, and PA. All analyses were performed by using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A 2-sided p value of less than 0.05 was considered significant.
3. Results
Of the 47,677 participants recruited for the PURE China study, 39,862 participants were included in our study, of which 15,964 reported having hypertension. During a median follow-up of 8.9 years (interquartile range, 6.7–9.9 years), 1822 participants developed major CVD, and 1250 deaths occurred (388 as a result of CVD).
Table 1 shows the baseline characteristics of the participants based on hypertension status and the level of GS. The mean age of the overall population was 51 years, 23,223 (58.26%) of the 39,862 participants were women, and 20,722 (51.98%) participants were from rural areas. In both the non-hypertension group and the hypertension group, the proportion of participants who reported current tobacco or alcohol use, post-secondary school education, and non-diabetes was highest in the high strength group, followed by the moderate strength group, and low strength group. The mean energy intake and body mass index were lowest in the low strength group, intermediate in the moderate strength group, and highest in the high strength group, regardless of hypertension status. For hypertensive patients, the proportion of participants performing high-level PA was highest in the high strength group, whereas for non-hypertension the proportion of participants performing high-level PA was highest in moderate strength group. For hypertension patients, the proportion of patients with antihypertensive medications was highest in the high strength group (1179, 30.70%).
Table 1.
Participants’ baseline characteristics by grip strength and hypertension strata.
| Non-hypertension (n = 23,898) |
Hypertension (n = 15,964) |
||||||
|---|---|---|---|---|---|---|---|
| Overall | Low strength | Moderate strength | High strength | Low strength | Moderate strength | High strength | |
| Total participants | 39,862 | 5831 | 12,813 | 5254 | 3835 | 8288 | 3841 |
| Women | 23,223 (58.26) | 3428 (58.79) | 7833 (61.13) | 3127 (59.52) | 2233 (58.23) | 4540 (54.78) | 2062 (53.68) |
| Median age (year) | 51 (42–58) | 48 (40–56) | 47 (41–55) | 47 (41–55) | 55 (49–61) | 54 (47–61) | 54 (47–61) |
| Living in rural areas | 20,722 (51.98) | 3783 (64.88) | 6303 (49.19) | 2236 (42.56) | 2623 (68.40) | 4190 (50.56) | 1587 (41.32) |
| Education | |||||||
| Pre-secondary school | 13,305 (33.46) | 2282 (39.22) | 3539 (27.70) | 1241 (23.68) | 1990 (52.04) | 3074 (37.18) | 1179 (30.78) |
| Secondary school | 20,737 (52.16) | 2780 (47.77) | 7193 (56.30) | 3153 (60.16) | 1489 (38.94) | 4055 (49.04) | 2067 (53.95) |
| Post-secondary school | 5718 (14.38) | 757 (13.01) | 2045 (16.01) | 847 (16.16) | 345 (9.02) | 1139 (13.78) | 585 (15.27) |
| Occupation | |||||||
| Professional/managers | 4933 (12.40) | 597 (10.25) | 1666 (13.03) | 732 (13.96) | 307 (8.03) | 1062 (12.85) | 569 (14.84) |
| Skilled workers | 13,928 (35.02) | 1551 (26.64) | 4799 (37.54) | 2187 (41.71) | 923 (24.14) | 2903 (35.13) | 1565 (40.83) |
| Unskilled workers | 14,143 (35.56) | 2621 (45.01) | 4276 (33.46) | 1538 (29.33) | 1768 (46.25) | 2877 (34.82) | 1063 (27.73) |
| Homemakers | 6764 (17.01) | 1054 (18.10) | 2042 (15.97) | 786 (14.99) | 825 (21.58) | 1421 (17.20) | 636 (16.59) |
| Physical activity level | |||||||
| Low | 6038 (15.36) | 936 (16.37) | 1866 (14.78) | 761 (14.67) | 659 (17.42) | 1256 (15.32) | 560 (14.73) |
| Moderate | 16,602 (42.23) | 2411 (42.17) | 5240 (41.50) | 2191 (42.24) | 1733 (45.81) | 3456 (42.17) | 1571 (41.32) |
| High | 16,671 (42.41) | 2371 (41.47) | 5519 (43.71) | 2235 (43.09) | 1391 (36.77) | 3484 (42.51) | 1671 (43.95) |
| Tobacco use | |||||||
| Former | 1709 (4.33) | 223 (3.86) | 446 (3.52) | 212 (4.08) | 173 (4.55) | 430 (5.25) | 225 (5.92) |
| Current | 9118 (23.13) | 1297 (22.45) | 2923 (23.09) | 1256 (24.16) | 813 (21.39) | 1900 (23.18) | 929 (24.46) |
| Never | 28,601 (72.54) | 4256 (73.68) | 9291 (73.39) | 3730 (71.76) | 2814 (74.05) | 5866 (71.57) | 2644 (69.62) |
| Alcohol use | |||||||
| Former | 1123 (2.83) | 175 (3.02) | 305 (2.40) | 118 (2.26) | 130 (3.40) | 275 (3.33) | 122 (3.19) |
| Current | 8661 (21.86) | 1102 (19.01) | 2622 (20.61) | 1210 (23.17) | 805 (20.04) | 1946 (23.57) | 982 (25.69) |
| Never | 29,837 (75.31) | 4520 (77.97) | 9798 (77.00) | 3894 (74.57) | 2891 (75.56) | 6036 (73.10) | 2718 (71.11) |
| Energy (kcal/day) | 1904 (1504–2385) | 1833 (1416–2338) | 1926 (1533–2406) | 1951 (1561–2415) | 1805 (1406–2274) | 1906 (1509–2387) | 1949 (1556–2428) |
| Protein/energy (%) | 15.25 ± 2.92 | 14.89 ± 2.91 | 15.38 ± 2.93 | 15.32 ± 2.91 | 14.90 ± 2.86 | 15.37 ± 2.92 | 15.41 ± 2.86 |
| Diabetes | 1513 (3.80) | 167 (2.86) | 326 (2.55) | 104 (1.98) | 229 (5.98) | 492 (5.94) | 195 (5.08) |
| Height (cm) | 160.88 ± 8.23 | 158.63 ± 8.02 | 160.75 ± 7.82 | 163.55 ± 8.08 | 158.12 ± 7.99 | 160.79 ± 8.16 | 164.00 ± 8.41 |
| Weight (kg) | 63.77 ± 12.32 | 58.52 ± 10.78 | 61.76 ± 11.49 | 66.19 ± 11.86 | 62.54 ± 12.44 | 66.17 ± 12.27 | 71.17 ± 12.42 |
| Waist circumference (cm) | 80.83 ± 10.48 | 76.82 ± 9.94 | 78.42 ± 9.81 | 81.10 ± 9.58 | 82.07 ± 11.10 | 83.96 ± 10.07 | 86.59 ± 10.02 |
| Hip circumference (cm) | 94.05 ± 8.04 | 90.83 ± 7.90 | 92.72 ± 7.46 | 95.02 ± 7.00 | 94.09 ± 8.94 | 95.81 ± 7.96 | 98.18 ± 7.80 |
| BMI (kg/m2) | 24.50 ± 3.63 | 23.17 ± 3.34 | 23.76 ± 3.36 | 24.61 ± 3.36 | 24.88 ± 3.97 | 25.45 ± 3.61 | 26.37 ± 3.59 |
| WHR | 0.86 ± 0.07 | 0.85 ± 0.07 | 0.84 ± 0.07 | 0.85 ± 0.07 | 0.87 ± 0.07 | 0.87 ± 0.07 | 0.88 ± 0.06 |
| SBP (mmHg) | 132.97 ± 22.23 | 119.53 ± 11.67 | 119.32 ± 11.38 | 120.34 ± 10.97 | 155.00 ± 20.54 | 152.82 ± 19.03 | 151.25 ± 18.37 |
| DBP (mmHg) | 82.63 ± 13.12 | 75.53 ± 7.80 | 75.78 ± 7.53 | 76.25 ± 7.48 | 93.37 ± 17.84 | 92.65 ± 11.37 | 92.58 ± 10.53 |
| Antihypertensive medications | 4478 (11.23) | 0 (0) | 0 (0) | 0 (0) | 1041 (27.14) | 2258 (27.24) | 1179 (30.70) |
| Grip strength (kg) | 32.14 ± 10.27 | 23.48 ± 7.23 | 32.46 ± 7.83 | 41.29 ± 9.56 | 22.42 ± 6.95 | 32.17 ± 7.94 | 41.31 ± 9.75 |
Notes: Data are expressed as numbers mean ± SD, median (25th percentile, 75th percentile), or number (%), unless otherwise stated. Some values shown in the table may not add up to 100% owing to weighting or rounding.
Abbreviations: BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure; WHR = waist to hip ratio.
Classified by the level of GS and hypertension status, all-cause death, CVD death, and major CVD events are summarized in Table 2. Regardless of the hypertension status, as the level of GS decreased, the event rates of all-cause death, CVD death, and major CVD events increased. All event rates were higher among hypertensive participants compared to those without hypertension in each stratum of GS. The lowest event rates of all-cause death (1.48%), CVD death (0.23%), and major CVD events (1.79%) occurred in the group without hypertension and high GS. The adverse event risk trajectories between hypertensive/non-hypertensive patients with different levels of GS are shown in the Supplementary Figs. 1–3.
Table 2.
Number of adverse health events by grip strength and hypertension strata.
| Non-hypertension |
Hypertension |
Total |
||||
|---|---|---|---|---|---|---|
| Grip strength | Participants | Eventsa | Participants | Eventsa | Participants | Eventsa |
| All-cause death | ||||||
| Higher | 5254 | 78 (1.48) | 3841 | 116 (3.02) | 9095 | 194 (2.13) |
| Moderate | 12,813 | 254 (1.98) | 8288 | 360 (4.34) | 21,101 | 614 (2.91) |
| Lower | 5831 | 183 (3.14) | 3835 | 259 (6.75) | 9666 | 442 (4.57) |
| CVD death | ||||||
| Higher | 5254 | 12 (0.23) | 3841 | 46 (1.20) | 9095 | 58 (0.64) |
| Moderate | 12,813 | 53 (0.41) | 8288 | 124 (1.50) | 21,101 | 177 (0.84) |
| Lower | 5831 | 43 (0.74) | 3835 | 110 (2.87) | 9666 | 153 (1.58) |
| Major CVD events | ||||||
| Higher | 5254 | 94 (1.79) | 3841 | 255 (6.64) | 9095 | 349 (3.84) |
| Moderate | 12,813 | 297 (2.32) | 8288 | 636 (7.67) | 21,101 | 933 (4.42) |
| Lower | 5831 | 175 (3.00) | 3835 | 365 (9.52) | 9666 | 540 (5.59) |
aData are expressed as number (%), unless otherwise stated.
Abbreviation: CVD = cardiovascular disease.
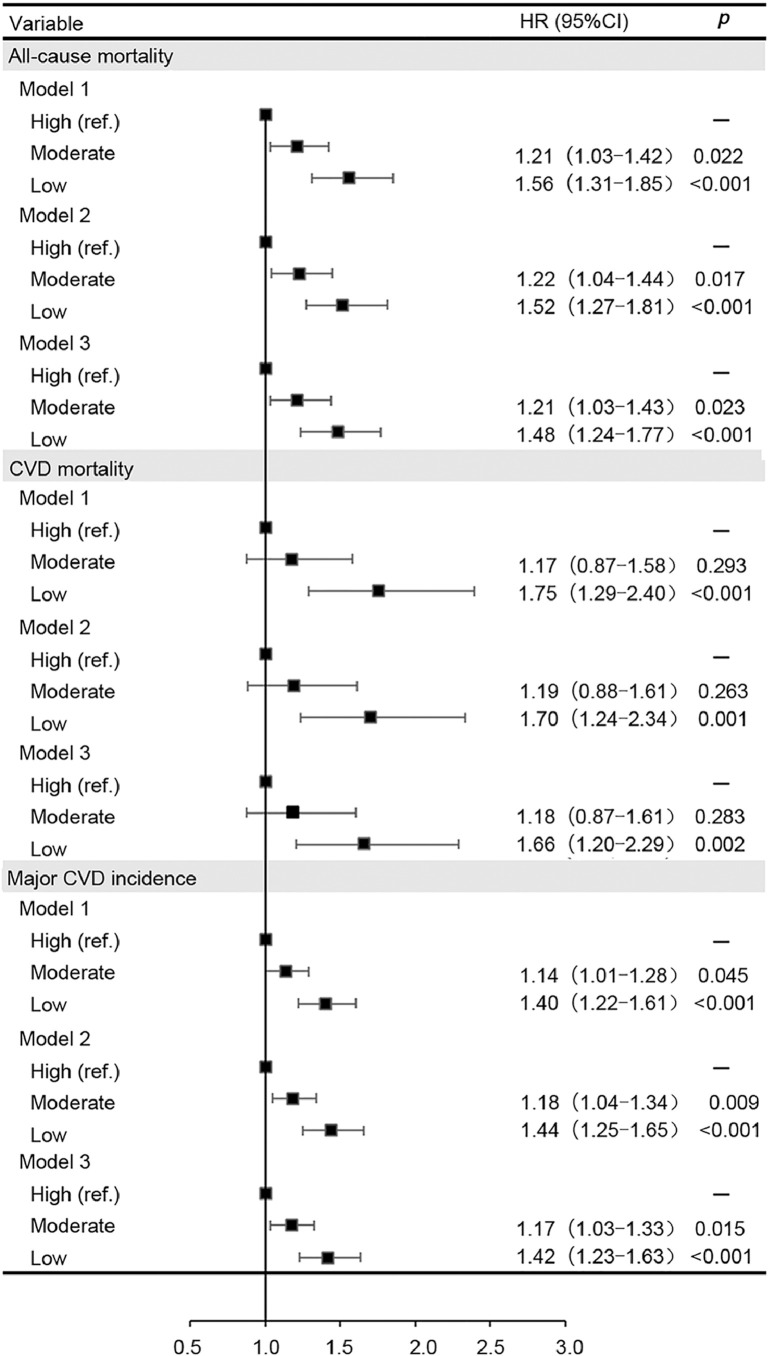
Fig. 1 shows that the HRs for major CVD incidence (1.40; 95%CI: 1.22–1.61; p < 0.001), CVD mortality (1.75; 95%CI: 1.29–2.40; p < 0.001), and all-cause mortality (1.56; 95%CI: 1.31–1.85; p < 0.001) were significantly higher in participants with low GS than in individuals with high GS in the models adjusted for age, sex, and location. After adjustment for further confounding factors, the association was slightly attenuated but remained significant. Those participants in the lower GS group had a 42%, 66%, and 48% significantly higher risk of major CVD incidence, CVD mortality, and all-cause mortality, respectively, compared with those in the higher group (Model 3). The HRs for major CVD incidence, CVD mortality, and all-cause mortality by GS and hypertension strata are summarized in Supplementary Table 2. The interactions between hypertension and GS for major CVD incidence (p = 0.567), CVD mortality (p = 0.441), and all-cause mortality (p = 0.792) were not statistically significant in Model 3.
Fig. 1.
Cox proportional hazards models for all-cause mortality and CVD incidence and mortality in participants by grip strength category. Model 1 was adjusted for age, sex, and location. Model 2 was adjusted for Model 1 plus education, occupation, BMI, WHR, smoking, alcohol, baseline prevalence of diabetes, and history of medication for hypertension. Model 3 was adjusted for Model 2 plus daily dietary energy intake, the proportion of calorie intake from protein, and physical activities. BMI = body mass index; 95%CI = 95% confidence interval; CVD = cardiovascular disease; HR = hazard ratio; ref. = reference group; WHR = waist to hip ratio.
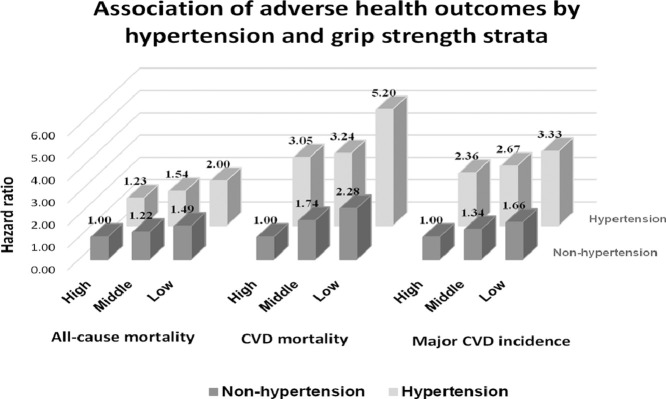
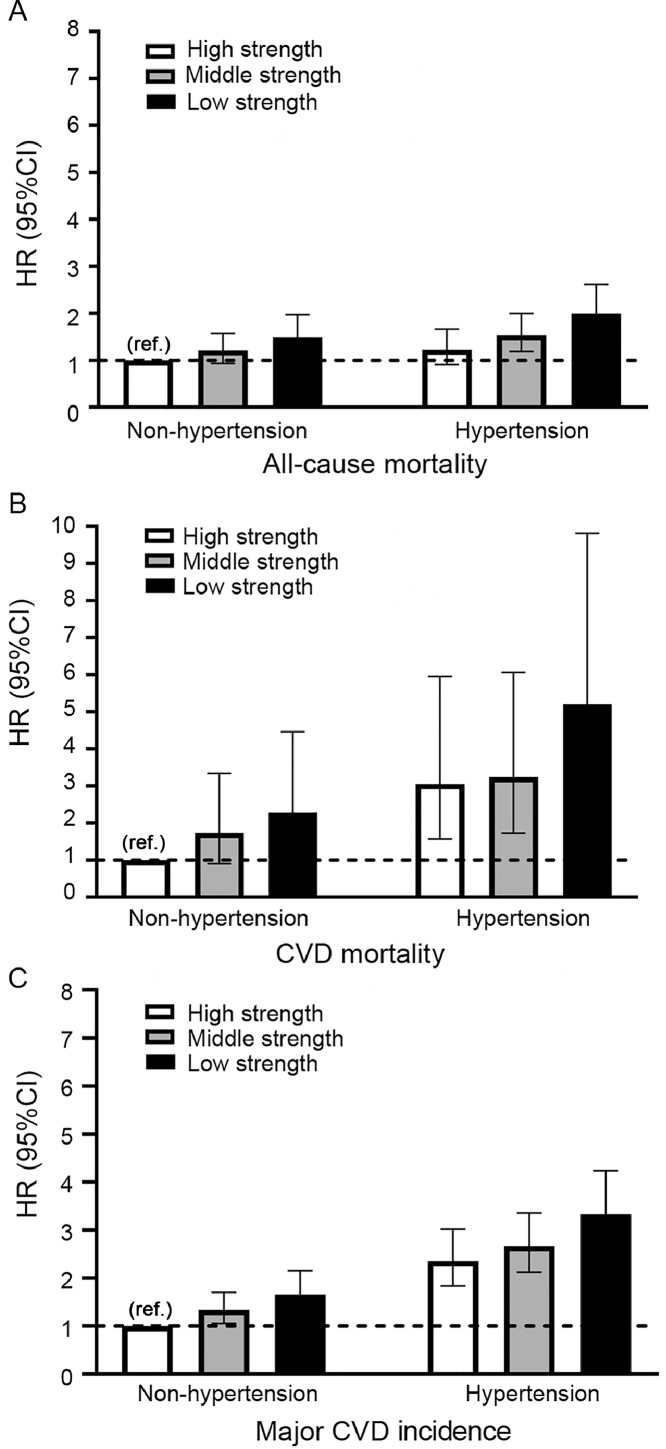
Fig. 2 shows major CVD incidence, CVD mortality, and all-cause mortality risks across combined categories of GS and hypertension status. Compared with normotensive patients with high GS, hypertensive patients with low GS had the highest risk of major CVD incidence (HR = 3.31; 95%CI: 2.60–4.22; p < 0.001), CVD mortality (HR = 4.99; 95%CI: 2.64–9.43; p < 0.001), and all-cause mortality (HR = 1.93; 95%CI: 1.47–2.53; p < 0.001) in Model 3. In contrast, hypertensive patients with high GS were at higher risk of major CVD incidence (HR = 2.39; 95%CI: 1.86–3.06; p < 0.001) or CVD mortality (HR = 3.11; 95%CI: 1.59–6.06; p < 0.001), but did not have a significantly increased risk of all-cause mortality (HR = 1.24; 95%CI: 0.92–1.68; p = 0.159).
Fig. 2.
Association of adverse health outcomes by hypertension and grip strength strata (A) all-course mortality, (B) CVD mortality, and (C) major CVD incidence. The model was adjusted for age, sex, location, education, occupation, BMI, WHR, smoking, alcohol, baseline prevalence of diabetes, history of medication for hypertension, daily dietary energy intake, the proportion of calorie intake from protein, and physical activities. BMI = body mass index; 95%CI = 95% confidence interval; CVD = cardiovascular disease; HR = hazard ratio; ref. = reference group; WHR = waist to hip ratio.
4. Discussion
Our study demonstrates that the effect of muscle strength as measured by GS on CVD does not seem to be mediated by BP. However, compared with normotensive participants with high GS, hypertensive patients with low GS in our study had the highest risk of major CVD incidence, CVD mortality, and all-cause mortality; nevertheless, hypertensive patients with high GS were not significantly associated with increased risk of all-cause mortality.
As we did in our previous study,22 we categorized participants in this study into low, moderate, and high GS groups using gender- and age-specific GS cutoffs, and the GS values followed the normal distribution in each stratum of age and gender. We found that low GS was associated with a higher risk of major CVD incidence, CVD mortality, and all-cause mortality. These findings were in line with previous studies, which have suggested that muscle strength was a risk factor for health outcomes and can predict the risk of death in people who developed either cardiovascular or non-CVD.26, 27, 28 Since skeletal muscle is essential not only for optimal physical performance, but also as the primary protein store and site of glucose disposal,29,30 it may be an important contributing factor in maintaining optimal health throughout life. Our data have implications for primary health care policies because the data indicate that GS might be used to screen patients during clinic visits. Primary care physicians could stratify people based on the GS test and prescribe PA to improve overall muscle mass of people with low GS, which may play a pivotal role in the prevention and management of CVD. Thus, the GS test should receive greater recognition as a useful clinical screening tool in the health-monitoring systems.
To the best of our knowledge, this is the first study to explore the combined effects of GS and hypertension on the risk of major CVD incidence, CVD mortality, and all-cause mortality. In our study, hypertension status and GS status were measured at baseline, while major CVD and death occurred during follow-up, which is consistent with the temporal association. The current data demonstrate that the risk of major CVD incidence, CVD mortality, and all-cause mortality is 3-fold, 5-fold, and 2-fold higher, respectively, in hypertensive patients with low GS compared to those with high GS without hypertension. The trends demonstrated in Fig. 2 clearly illustrate that for both hypertensive and non-hypertensive populations, there is a strong dose-related relationship between GS and the risk of major CVD incidence, CVD mortality, and all-cause mortality, whereas among those with hypertension, the estimates of the magnitude of associations are much stronger. Several possible mechanisms may explain how decreased muscle function could contribute to a greater risk of developing comorbidities and mortality in people with hypertension. Muscle function decline may cause a reduction in muscle contraction-inducing factors with anti-inflammatory effects,31 also known as myokines, which may increase the risk of hypertension and its serious complications.32,33 Furthermore, inflammation may act as a potential link that adversely affects both muscle function and vascular health.34,35 Our findings agree with a prospective study by Artero et al.,18 which provides supportive evidence that high levels of muscle strength appear to protect hypertensive men against all-cause mortality. We were able to extend Artero et al.’s previous work18 to women and to a population without hypertension. These findings are relevant for secondary prevention in patients with hypertension, particularly those with low GS. This population may gain the most benefits from interventions to improve muscle strength.
Hypertension is an established risk factor for the increased risk of premature deaths; however, our results interestingly showed that hypertensive patients with high GS were not at a higher risk of death than normotensive patients, which provides some potential evidence for developing nonpharmacologic therapies for hypertension. Whereas compliance to anti-hypertension medication, a modified diet, and increased PA are frontline components of BP management,36 fewer than 50% of patients adequately adhere to their hypertension medication regimen19,37 for a myriad of reasons, including sociocultural and economic considerations. Our results might be of great interest from a clinical perspective because muscle strength is a modifiable risk factor, and muscle-strengthening activities are currently included in most of the institutional recommendations of exercise for maintaining and improving overall health.2,38 Increasing GS by means of physical exercise training may benefit hypertensive patients who have a prognosis of adverse health outcomes. Additional well-designed randomized controlled trials are warranted to confirm our findings.
One strength of our study is that it was conducted using a relatively large cohort of participants from high-income (eastern), middle-income (central), and low-income (western) regions, as well as from urban and rural communities in China. The study also included extensive follow-up. Thus, our findings might guide national and regional policy-making in primary care and disease prevention. Our study also used high-quality data obtained using a systematic and standardized approach. The data accounted for a large number of potential covariates, thus avoiding potential biases related to the collection of data only from patients who visit clinics or hospitals.
Our study also has several limitations. First, the use of data from an observational prospective study cannot fully offer strong conclusions on the causal role of muscular strength in death or CVD. Second, we only examined GS as a measure of muscle strength. Although our study did not allow for more thorough measures of muscle mass or muscle strength, we focused on GS because other underlying causes germane to hypertension (e.g., neuropathy) may be manifested in this measure13 and it is verified to be a satisfactory indicator of muscle strength in epidemiological studies.26,27,39 In addition, because we only had baseline data on muscular strength and BP, we did not know whether changes in any of these variables occurred in the time before follow-up and how this might have influenced the results. It is possible that many people with hypertension were treated at some point in the follow-up interval, and others may have experienced increases or decreases in these exposures. Also, although we have adjusted many covariates and the estimates of the HR did not change appreciably from Model 1 to Model 3, the use of self-reported data for some of the covariates may have increased the risk of residual confounding. Finally, our study did not include cardiorespiratory fitness,40,41 an important covariate that is a stronger predictor of CVD compared with PA alone.42
5. Conclusion
Overall, lower GS is associated with a higher risk of major CVD incidence, CVD mortality, and all-cause mortality, independent of hypertension. In our combined analyses, the risk of CVD incidence, CVD mortality, and all-cause mortality was highest for people with hypertension and low GS. More important, hypertensive patients with high GS had similar or lower mortality risks compared with the normotensive participants. These findings suggest that GS has clinical utility in identifying hypertensive patients at risk for poor health outcomes.
Acknowledgments
The authors are grateful for the contributions of all the participants of the PURE China study as well as the local medical personnel for data collection. A list of the PURE China Investigators is provided in Supplementary List 1.
The PURE study is an investigator-initiated study that is funded by the Population Health Research Institute (PHRI), Hamilton Health Sciences Research Institute (HHSRI), the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario. Through unrestricted grants from several pharmaceutical companies and additional contributions from various national or local organizations in participating countries. PURE China study is partly funded by the National Center for Cardiovascular Diseases and ThinkTank Research Center for Health Development.
The external funders and sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Authors' contributions
WDL designed the present study, performed its statistical analysis, and had primary responsibility for writing the manuscript; SY and WL conceived and initiated the Prospective Urban Rural Epidemiology PURE China study, supervised its conduct and data analysis, and provided critical comments on all drafts of the manuscript; SR coordinated worldwide study; DPL and LAT reviewed and commented on the drafts. All other authors coordinated the study and collected the data. All authors have read and approved the final manuscript, and agree with the order of the presentation of authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jshs.2020.10.005.
Appendix. Supplementary materials
References
- 1.Charlier R, Knaeps S, Mertens E, et al. Age-related decline in muscle mass and muscle function in Flemish Caucasians: A 10-year follow-up. Age (Dordr) 2016;38:36. doi: 10.1007/s11357-016-9900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artero EG, Lee DC, Lavie CJ, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32:351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Lee DC, Brellenthin AG, et al. Association of muscular strength and incidence of type 2 diabetes. Mayo Clin Proc. 2019;94:643–651. doi: 10.1016/j.mayocp.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie CJ, Kachur S, Sui X. Impact of fitness and changes in fitness on lipids and survival. Prog Cardiovasc Dis. 2019;62:431–435. doi: 10.1016/j.pcad.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez-Pavón D, Brellenthin AG, Lee DC, Sui X, Blair SN, Lavie CJ. Role of muscular strength on the risk of sudden cardiac death in men. Mayo Clin Proc. 2019;94:2589–2591. doi: 10.1016/j.mayocp.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Lee DC, Li Y, et al. Associations of resistance exercise with cardiovascular disease morbidity and mortality. Med Sci Sports Exerc. 2019;51:499–508. doi: 10.1249/MSS.0000000000001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging. 2020;24:83–90. doi: 10.1007/s12603-019-1267-x. [DOI] [PubMed] [Google Scholar]
- 8.Trombetti A, Reid KF, Hars M, et al. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos Int. 2016;27:463–471. doi: 10.1007/s00198-015-3236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 10.Snijder MB, Henry RM, Visser M, et al. Regional body composition as a determinant of arterial stiffness in the elderly: The Hoorn Study. J Hypertens. 2004;22:2339–2347. doi: 10.1097/00004872-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Han K, Park YM, Kwon HS, et al. Sarcopenia as a determinant of blood pressure in older Koreans: Findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PLoS One. 2014;9:e86902. doi: 10.1371/journal.pone.0086902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SH, Park JH, Song PS, et al. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens. 2013;7:420–425. doi: 10.1016/j.jash.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Mainous AG, 3rd, Tanner RJ, Anton SD, Jo A. Grip strength as a marker of hypertension and diabetes in healthy weight adults. Am J Prev Med. 2015;49:850–858. doi: 10.1016/j.amepre.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayer AA, Syddall HE, Dennison EM, et al. Grip strength and the metabolic syndrome: Findings from the Hertfordshire Cohort Study. QJM. 2007;100:707–713. doi: 10.1093/qjmed/hcm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen DD, López-Jaramillo P, Fernández-Santos JR, Castro-Piñero J, Sandercock G. Muscle strength is associated with lower diastolic blood pressure in schoolchildren. Prev Med. 2017;95:1–6. doi: 10.1016/j.ypmed.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Demmer DL, Beilin LJ, Hands B, et al. Effects of muscle strength and endurance on blood pressure and related cardiometabolic risk factors from childhood to adolescence. J Hypertens. 2016;34:2365–2375. doi: 10.1097/HJH.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Kaji A, Sakai R, et al. Sarcopenia is associated with blood pressure variability in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr Gerontol Int. 2018;18:1345–1349. doi: 10.1111/ggi.13487. [DOI] [PubMed] [Google Scholar]
- 18.Artero EG, Lee DC, Ruiz JR, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol. 2011;57:1831–1837. doi: 10.1016/j.jacc.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Gu H, Teo KK, et al. Hypertension prevalence, awareness, treatment, and control in 115 rural and urban communities involving 47 000 people from China. J Hypertens. 2016;34:39–46. doi: 10.1097/HJH.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 20.Yan R, Li W, Yin L, Wang Y, Bo J, Investigators PURE‐China. Cardiovascular diseases and risk-factor burden in urban and rural communities in high-, middle-, and low-income regions of China: A large community-based epidemiological study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. The Lancet. 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong DP, Teo KK, Rangarajan S, et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: A prospective urban rural epidemiologic (PURE) study. J Cachexia Sarcopenia Muscle. 2016;7:535–546. doi: 10.1002/jcsm.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: The PURE study. The Lancet. 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 25.Kelemen LE, Anand SS, Vuksan V, et al. Development and evaluationof cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J Am Diet Assoc. 2003;103:1178–1184. doi: 10.1016/s0002-8223(03)00985-4. [DOI] [PubMed] [Google Scholar]
- 26.Celis-Morales CA, Welsh P, Lyall DM, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: Evidence from 498 135 UK-Biobank participants. Eur Heart J. 2017;38:116–122. doi: 10.1093/eurheartj/ehw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh CE, Celis-Morales CA, Ho FK, et al. Grip strength and walking pace and cardiovascular disease risk prediction in 406,834 UK Biobank participants. Mayo Clin Proc. 2020;95:879–888. doi: 10.1016/j.mayocp.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Burd NA, De Lisio M. Skeletal muscle remodeling: Interconnections between stem cells and protein turnover. Exerc Sport Sci Rev. 2017;45:187–191. doi: 10.1249/JES.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 30.Cokorinos EC, Delmore J, Reyes AR, et al. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metab. 2017;25:1147–1159. doi: 10.1016/j.cmet.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13–18. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 32.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the national health and nutrition examination survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin SO, Rhee SY, Chon S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One. 2013;8:e60119. doi: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanada K, Miyachi M, Tanimoto M, et al. A cross-sectional study of sarcopenia in Japanese men and women: Reference values and association with cardiovascular risk factors. Eur J Appl Physiol. 2010;110:57–65. doi: 10.1007/s00421-010-1473-z. [DOI] [PubMed] [Google Scholar]
- 35.Millar PJ, McGowan CL, Cornelissen VA, Araujo CG, Swaine IL. Evidence for the role of isometric exercise training in reducing blood pressure: Potential mechanisms and future directions. Sports Med. 2014;44:345–356. doi: 10.1007/s40279-013-0118-x. [DOI] [PubMed] [Google Scholar]
- 36.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 37.Rajpura J, Nayak R. Medication adherence in a sample of elderly suffering from hypertension: Evaluating the influence of illness perceptions, treatment beliefs, and illness burden. J Manag Care Pharm. 2014;20:58–65. doi: 10.18553/jmcp.2014.20.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavie CJ, Lee DC, Ortega FB. UK Biobank contributes to aerobic and muscle fitness research. Mayo Clin Proc. 2020;95:840–842. doi: 10.1016/j.mayocp.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Celis-Morales CA, Petermann F, Hui L, et al. Associations between diabetes and both cardiovascular disease and all-cause mortality are modified by grip strength: Evidence from UK Biobank, a prospective population-based cohort study. Diabetes Care. 2017;40:1710–1718. doi: 10.2337/dc17-0921. [DOI] [PubMed] [Google Scholar]
- 40.Ozemek C, Laddu DR, Lavie CJ, et al. An update on the role of cardiorespiratory fitness, structured exercise and lifestyle physical activity in preventing cardiovascular disease and health risk. Prog Cardiovasc Dis. 2018;61:484–490. doi: 10.1016/j.pcad.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1622–1639. doi: 10.1016/j.jacc.2018.08.2141. [DOI] [PubMed] [Google Scholar]
- 42.Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124:799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.