Highlights
-
•
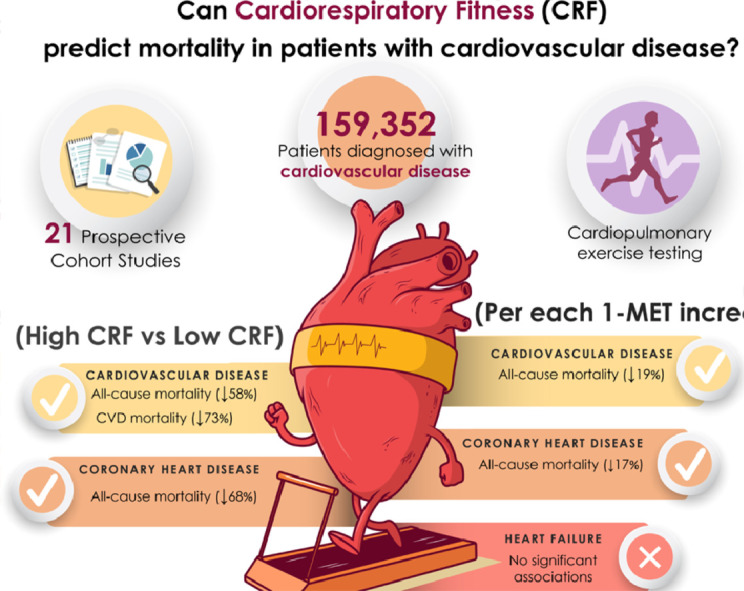
High cardiorespiratory fitness (CRF) in patients with cardiovascular disease (CVD) is associated with 58% lower all-cause mortality risk and 73% lower cardiovascular mortality risk compared to unfit counterparts.
-
•
Each 1 metabolic equivalent (1-MET) increase in CRF is associated with a 19% lower CVD mortality risk among patients with CVD.
-
•
Coronary artery disease patients with high CRF have a 68% lower all-cause mortality risk than their unfit counterparts.
-
•
Each 1-MET increase in CRF is associated with a 17% lower all-cause mortality risk among patients with coronary artery disease.
-
•
No significant associations were found between increments of 1-MET and lower mortality risk among heart failure patients.
Keywords: Cardiopulmonary fitness, Coronary artery disease, Exercise capacity, Heart failure, Survival
Abstract
Background
Cardiorespiratory fitness (CRF) is inversely associated with mortality in apparently healthy subjects and in some clinical populations, but evidence for the association between CRF and all-cause and/or cardiovascular disease (CVD) mortality in patients with established CVD is lacking. This study aimed to quantify this association.
Methods
We searched for prospective cohort studies that measured CRF with cardiopulmonary exercise testing in patients with CVD and that examined all-cause and CVD mortality with at least 6 months of follow-up. Pooled hazard ratios (HRs) were calculated using random-effect inverse-variance analyses.
Results
Data were obtained from 21 studies and included 159,352 patients diagnosed with CVD (38.1% female). Pooled HRs for all-cause and CVD mortality comparing the highest vs. lowest category of CRF were 0.42 (95% confidence interval (95%CI): 0.28–0.61) and 0.27 (95%CI: 0.16–0.48), respectively. Pooled HRs per 1 metabolic equivalent (1-MET) increment were significant for all-cause mortality (HR = 0.81; 95%CI: 0.74–0.88) but not for CVD mortality (HR = 0.75; 95%CI: 0.48–1.18). Coronary artery disease patients with high CRF had a lower risk of all-cause mortality (HR = 0.32; 95%CI: 0.26–0.41) than did their unfit counterparts. Each 1-MET increase was associated with lower all-cause mortality risk among coronary artery disease patients (HR = 0.83; 95%CI: 0.76–0.91) but not lower among those with heart failure (HR = 0.69; 95%CI: 0.36–1.32).
Conclusion
A better CRF was associated with lower risk of all-cause mortality and CVD. This study supports the use of CRF as a powerful predictor of mortality in this population.
Graphical abstract
1. Introduction
Despite noteworthy advances in management and treatment over the last decades, cardiovascular disease (CVD) remains the leading cause of mortality worldwide.1 The early identification of modifiable risk factors for CVD can improve long-term survival,2 which places emphasis on the importance of developing biomarkers that can predict lifetime CVD risk. Regarding risk prediction, a recent scientific update from the American Heart Association has stated that cardiorespiratory fitness (CRF) may provide additional prognostic value for CVD and associated mortality risk beyond traditional cardiovascular risk factors, such as hypertension, smoking, obesity, hyperlipidemia, and type 2 diabetes mellitus.3
A wealth of evidence from many large (retrospective and prospective) epidemiological cohort studies underpins the link between CRF and health outcomes, including the risk of all-cause, CVD, and cancer mortality in apparently healthy and clinical populations, which includes patients with diabetes,4 those who are overweight,5 or who have hypertension.6 Nes et al.7 found that in healthy men (n = 18,348) and women (n = 18,764) <60 years of age, each 1 metabolic equivalent (1-MET) increase in exercise capacity from baseline reduced the risk of CVD mortality by 21%. Additionally, higher levels of CRF in midlife have been associated with lower risk of heart failure, myocardial infarction, and stroke.8 To date, however, little is known about the predictive value of CRF levels in adults with CVD.
It is well-established that exercise—through increases in CRF—has quantifiable biological effects on the cardiovascular system in terms of structure and function, and it is considered a cornerstone of cardiac rehabilitation programs.9 Increases in CRF have been shown to provide substantial health benefits in patients with CVD, including reduced risk of heart failure-related hospitalization in later life,10 improved short-term mortality after coronary artery bypass grafting,11 lower rates of recurrent myocardial infarction, and decreases in both CVD and all-cause mortality.12,13
Despite the recognition of the utility of CRF measurements in many CVD management guidelines,3,14 these assessments are not frequently included in routine clinical health screenings and, when performed, are rarely used for risk stratification.15,16 This lack of use might be explained by the paucity of information endorsing the role of CRF in predicting long-term mortality in adults with CVD. It has been proposed that the use of CRF as a biomarker should be included as a standard part of clinical encounters (e.g., an accepted “vital sign”).3,14 Understanding the associations between CRF and cardiovascular health could help identify individuals at high risk, improve care delivery, and promote policy recommendations for physical fitness and exercise. Thus, the present study aimed to quantify the association between CRF in adults with established CVD and the risk of all-cause and CVD mortality.
2. Methods
The present systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and recommendations.17 Patient consent and ethical committee approval were not required to conduct the present study. Neither patients nor the public were involved in the design, conduct, reporting, or dissemination plans of our research. The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42020203619).
2.1. Search strategy
The search was conducted independently by 2 investigators (YE and AGH) using MEDLINE, Embase, and SPORTDiscus electronic databases, from inception to May 2021. Additionally, a gray literature search of abstracts and conference proceedings from the European Society of Cardiology, American Heart Association, American College of Sports Medicine, and the American College of Cardiology conventions from the last 5 years was undertaken. The following string of medical subject headings terms was used to identify all possible studies investigating the predictive value of CRF on mortality in patients with CVD: peak oxygen, oxygen consumption, aerobic capacity, exercise capacity, aerobic fitness, cardiopulmonary fitness, cardiovascular fitness, cardiac rehabilitation, major adverse cardiovascular events, mortality, death rate, prospective, longitudinal, follow-up, heart failure, CVD, peripheral artery disease, coronary heart disease, myocardial infarction, coronary artery disease, cardiomyopathy, and acute coronary syndrome (Supplementary File 1). Searching was restricted to published articles in the English and Spanish languages. A medical librarian was consulted to audit the quality of the search strategy. Reference lists of eligible articles were manually scrutinized for further identification of relevant articles. Any disagreement was resolved by consensus with a third author (RRV).
2.2. Selection criteria
After reviewing the title and abstract, 2 investigators (YE and AGH) systematically assessed the full text of identified articles for eligibility. To be eligible for inclusion in the meta-analysis, studies needed to meet the following criteria: (a) exposure: CRF assessed directly from expired gas analysis or estimated through various cardiopulmonary maximal or submaximal exercise tests using a treadmill or cycle ergometer; (b) participants: patients with any type of CVD (i.e., heart failure, coronary artery disease, peripheral arterial disease, or cardiomyopathy); (c) outcomes analyzed: all-cause mortality, and/or overall survival, and/or CVD mortality; and (d) study design: prospective cohort studies with at least 6 months of follow-up. Studies were excluded if they: (a) did not report data regarding the variables of interest; (b) reported estimated CRF through non-exercise prediction algorithms; (c) did not assess CRF directly from a cardiopulmonary exercise test using a treadmill or cycle ergometer; or (d) reported insufficient information for calculating hazard ratios (HRs) and 95% confidence intervals (95%CIs). In the case of duplicate studies, the most recent version was included. Any disagreement was resolved by consensus with a third author (RRV).
2.3. Data collection process and data items
Data collection was conducted independently by 2 investigators (YE and AGH), using a Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA, USA) specifically designed for the present study. The following information was extracted from each study that met the selection criteria: (a) study characteristics (first author's name, publication year, study location, sample size, number of females, study design, follow-up duration, and cut-offs defining CRF categories); (b) participants’ information (sex, age, and number of death events); (c) assessment details (measurement of peak oxygen uptake, maximal oxygen consumption (VO2max), and estimation of METs); and (d) statistical analysis and study results (confounding factors, outcome of interest, and main results). Missing data from the studies were requested from the corresponding authors of the original published papers.
2.4. Risk of bias in individual studies
The Quality Assessment Tool for Observational Cohort and Cross-sectional Studies was used to determine the risk of bias of each study. The checklist comprised 14 items for longitudinal studies. Each item of methodological quality was classified as “yes”, “no”, or “not reported”.
2.5. Summary measures
The a priori plan was to conduct a one-step individual participant data meta-analysis. All analyses were carried out using STATA (Version 16.1; STATA Corp., College Station, TX, USA). Meta-analysis was performed when at least 3 studies provided data. HRs with associated 95%CIs were extracted from studies for mortality (all-cause and CVD), and pooled HRs were then calculated using the random-effects inverse-variance model with the Hartung-Knapp-Sidik-Jonkman adjustment.18 HRs were pooled, comparing the highest vs. the lowest CRF category in relation to all-cause and CVD mortality. Hazard estimates that were provided in units other than per 1-MET increments (e.g., per 1-mL/kg/min) were converted into 1-MET increments using exponential functions. When studies presented several statistical risk-adjustment models, only HRs associated with the statistical models that contained the highest number of additional covariates were considered.
2.6. Synthesis of results
The percentage of variation across studies was estimated using the inconsistency index (I2) derived from the Cochran Q statistic,19 considering I2 values of 25%, 50%, and 75% as low, moderate, and high inconsistency, respectively.19
2.7. Risk of bias across studies
Small-study effects and publication bias were examined using the Doi plot and Luis Furuya-Kanamori (LFK) index, both of which have been shown to be superior to the traditional funnel plot and Egger's regression intercept test.19 Values of –1, between –1 and –2, and >–2, are considered to represent no, minor, and major asymmetry, respectively.20
2.8. Additional analysis
A sensitivity analysis was conducted to assess the robustness of the summary estimates, i.e., to determine whether or not a particular study accounted for the heterogeneity. Thus, to examine the effects of every individual result from every individual study on the overall findings, results were analyzed with each study deleted from the model once.
A subgroup analysis according to CVD type (i.e., coronary artery disease and heart failure) was conducted.
Finally, random-effects meta-regression analyses using the Hartung-Knapp-Sidik-Jonkman adjustment18 were used to independently evaluate whether or not results differed according to the length of follow-up (in months).
3. Results
3.1. Study selection
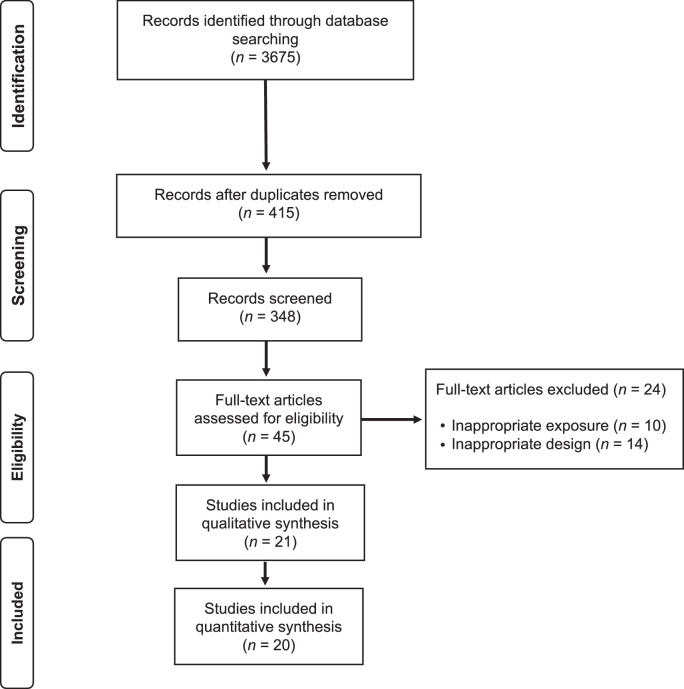
The electronic search strategy retrieved 3675 studies. After removing duplicates and screening titles and abstracts, 348 studies were assessed for eligibility based on their full text. A reference list of excluded articles and the reasons for their exclusion based on the full text is reported in Supplementary File 2. The PRISMA flow diagram illustrating the number of studies excluded at each stage of the systematic review and meta-analysis is shown in Fig. 1.
Fig 1.
PRISMA flow diagram of literature search and study selection. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
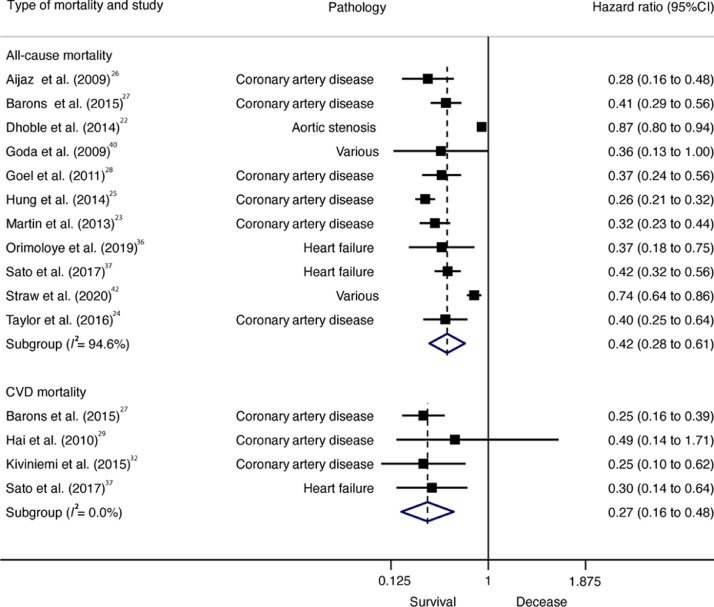
Fig 2.
Forest plot showing the hazard ratios of all-cause mortality in patients with CVD, comparing high vs. low CRF. Weights are from random-effects model. 95%CI = 95% confidence interval; CRF = High cardiorespiratory fitness; CVD = cardiovascular disease.
3.2. Study characteristics
Twenty-one prospective cohort studies were included in the qualitative synthesis, although only 20 were analyzed in the quantitative synthesis (1 study21 was excluded because participants were not exclusively patients with established CVD). Our study comprised a total of 156,371 patients diagnosed with CVD (38.1% female), with a mean age of 61.4 years. Sample sizes across studies ranged from 15522 to 122,00721 patients. The follow-up length ranged from 1223 to 16824 months. The most common CVDs were coronary artery disease23, 24, 25, 26, 27, 28, 29, 30, 31, 32 and heart failure,33, 34, 35, 36, 37 and other studies included patients diagnosed with aortic stenosis,22 hypertrophic cardiomyopathy,38 peripheral artery disease,39 and a combination of various pathologies.21,40, 41, 42 General characteristics of the 21 included studies are summarized in Table 1.
Table 1.
General characteristics of the prospective cohort studies included.
| Study | Follow-up (month) | Sample size (women/men) | Mean age (year) | Disease condition | Exposure measurement | Outcome | High vs. low definition | Per increment definition | Number of deaths | Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Aijaz et al. (2009)26 | 120.0 | 282 (48/234) | 61.0 | Coronary heart disease | Cardiopulmonary treadmill testing (symptom-limited graded exercise testing) | All-cause mortality | Low: VO2peak <19 mL/kg/min (men) and <15 mL/kg/min (women) | 55 | Age and gender | |
| Barons et al. (2015)27 | 136.0 | 2714 (547/2167) | 61.3 | Coronary heart disease | Exercise test using the Bruce protocol or the modified Bruce protocol | All-cause mortality and CVD mortality | VO2peak ≥ 22 mL/kg/min (men), VO2peak ≥ 19 mL/kg/min (women, high) vs. VO2peak <15 mL/kg/min (women, low) |
385 (all-cause) and 192 (CVD) | NR | |
| Coats et al. (2015)38 | 67.2 | 1898 (626/1272) | 46.0 | Hypertrophic cardiomyopathy | Maximal exercise test | All-cause mortality, or CVD mortality, sudden cardiac death, and heart failure death | Per 1 mL/kg/min increase in VO2peak | 156 (all cause) and 117 (CVD) | Age, sex, and all exercise parameters | |
| Dhoble et al. (2014)22 | 61.2 | 155 (16/139) | 69.6 | Aortic stenosis | Maximal exercise test | All-cause mortality | VO2peak ≥ 19 mL/kg/min (men) or ≥ 15 mL/kg/min (women), or ≥ 80% (high) vs. <80% (low) for men and women | 41 | Age, gender, symptom status, Charlson index, and AV area | |
| Goda et al. (2009)40 | 45.6 | 549 (112/437) | 63.4 | Various (ischemic heart disease, valvular heart disease, dilated cardiomyopathy, hypertrophic cardiomyopathy, and other CVD) | Incremental symptom-limited exercise test | CVD mortality | >14.5 mL/min/kg (high) vs. <14.5 mL/min/kg (low) | Per 1-SD mL/kg/min change in VO2 | 29 (all-cause) and 28 (CVD) | NR |
| Goel et al. (2011)28 | 24.0 | 855 (170/685) | 62.4 | CAD | Cardiopulmonary exercise testing | All-cause mortality | High vs. <21.5 mL kg/1/min (men) or <16.8 mL kg/1/min (women) | Per 1 mL/kg/min in VO2peak | 159 | Age, smoking, systolic blood pressure, diastolic blood pressure, myocardial infarction, heart failure, diabetes, COPD, renal disease, and BMI |
| Hai et al. (2010)29 | 78.5 | 386 (89/297) | 64.0 | Myocardial infarction | Cardiopulmonary exercise testing | CVD mortality | For men and women, >4 METs (high) vs. <4 METs (low) | 40 (CVD) | Diabetes, LVEF, acute pulmonary edema, revascularizations, angiotensin-converting enzyme inhibitor, statin, exercise training | |
| Hung et al. (2014)30 | 72.6 | 9852 (3016/6836) | 61.0 | CAD | Cardiopulmonary exercise testing | All-cause mortality | For men and women, >12 METs (high) vs. <6 METs (low) | Per 1-MET increase | 3824 | Age; sex; race; obesity; resting heart rate and systolic and diastolic blood pressures; history of hypertension, dyslipidemia, smoking, diabetes mellitus, atrial fibrillation, or heart failure; indication for stress testing; medications used to treat COPD, hypertension, or hyperlipidemia; aspirin; clopidogrel; and β-adrenergic blocking agents |
| Kato et al. (2018)33 | 48.0 | 1501 (400/1111) | 61.0 | Heart failure | Cardiopulmonary exercise testing | Heart failure events (including death) | Per 1 mL/kg/min increase in VO2peak | 141 | Estimated glomerular filtration rate, peak oxygen consumption, atrial fibrillation, and brain natriuretic peptide | |
| Keteyian et al. (2008)31 | 59.0 | 2812 (794/2018) | 61.0 | Coronary heart disease | Cardiopulmonary exercise testing | All-cause and CVD mortality | For men, VO2peak ≥ 22.8 (high) vs. VO2peak <14.9 (low) For women, VO2peak ≥ 16.6 (high) vs. VO2peak <11.9 (low) |
Per 1-MET increase | 280 (103 CVD) | NR |
| Keteyian et al. (2018)34 | 93.6 | 707 (287/420) | 59.0 | Heart failure | Cardiopulmonary exercise testing | All-cause mortality | Per 1-MET increase | 242 | Age, sex, hypertension, diabetes, history of ischemic heart disease, year of cardiac rehabilitation, Charlson comorbidity index, β-adrenergic blockade, angiotensin-converting enzyme inhibitor or angiotensin receptor blockade, and diuretic use | |
| Kiviniemi et al. (2015)32 | 24.0 | 1531 (477/1054) | 67.0 | Stable CAD | Cardiopulmonary exercise testing | CVD deaths and hospitalization | For men and women, ≥73% exercise capacity (high) vs. <73% (low) | 39 | Age, sex, diabetes, smoking, history of acute myocardial infarction, history of coronary intervention, BMI, Canadian Cardiovascular Society grading of angina pectoris, LVEF, mass index, and resting heart rate | |
| Leeper et al. (2013)39 | 135.6 | 725 (17/708) | 62.0 | Peripheral arterial disease | Cardiopulmonary exercise testing | All-cause and CVD mortality | Per 1-MET increase | 364 | Age, pack-years, and heart failure history | |
| Mandsager et al. (2018)21 | 100.8 | 122,007 (49,834/72,173) | 53.4 | CAD, diabetes, hypertension, hyperlipidemia, ESRD, and current/prior smoker | Cardiopulmonary exercise testing | All-cause mortality | For men and women, >97.7th percentile (high) vs. <25th percentile (low) | 13,637 | Age, sex, BMI, history of CAD, hyperlipidemia, hypertension, diabetes, smoking, ESRD, year of testing, and current use of aspirin, β-blockers, or statins | |
| Martin et al. (2013)23 | 12.0 | 5641 (1359/4282) | 60.0 | CAD | Cardiopulmonary exercise testing | All-cause mortality | For men and women, >8 METs (high) vs. <5 METs (low) | Per 1-MET increase | NR | Age, sex, and comorbid conditions |
| Mikkelsen et al. (2020)41 | 72.0 | 1561 (406/1155) | 63.6 | CAD, chronic heart failure, and heart valve replacement | Cardiopulmonary exercise testing | All-cause mortality | Per 1 mL/kg/min increase in VO2peak at baseline | 52 | Age, sex, working status (employed, unemployed, retired, and being on disability pension), education, index diagnosis, medication, tobacco use, COPD, diabetes, kidney disease, and peripheral artery disease. | |
| Nadruz et al. (2017)35 | 50.4 | 969 (315/654) | 55.0 | Heart failure | Cardiopulmonary exercise testing | All-cause mortality | Per 1 mL/kg/min increase in VO2peak | 164 | Age, sex, LVEF, chronic kidney disease, resting heart rate, resting systolic blood pressure, and CAD | |
| Orimoloye et al. (2019)36 | 116.4 | 167 (91/76) | 63.9 | Heart failure with preserved ejection fraction | Cardiopulmonary exercise testing | All-cause mortality | ≥7 METs (high) vs. 1–4 For men and women, METs (low) | Per 1-MET increase | 103 | Age, sex, race, modifiable risk factors (hypertension, diabetes mellitus, smoking status, weight), medication use including β-blockers, angiotensin receptor blockers, medications for chronic lung disease, and history of CAD |
| Sato et al. (2017)37 | 90.0 | 1190 (221/969) | 61.0 | Heart failure | Cardiopulmonary exercise testing | All-cause mortality and cardiac death | 248 (173 cardiac deaths) | Age, gender, BMI, presence of anemia and COPD | ||
| Straw et al. (2020)42 | 48.0 | 199 (27/172) | 76.0 | Various (before endovascular aneurysm repair) | Cardiopulmonary exercise testing | All-cause mortality | For men and women, >15 mL/kg/min vs. <15 mL/kg/min (low) in VO2peak | 98 | Age and sex | |
| Taylor et al. (2016)24 | 168.0 | 670 (136/534) | 60.0 | Coronary heart disease | Cardiopulmonary exercise testing | All-cause mortality | ≥7 METs for women and ≥8 METs for men (high) vs. <5 METs for women and <6 METs for men (low) | Per 1-MET increase | 206 | CAD diagnosis, CVD and other comorbidities, exercise test abnormalities, secondary prevention medications, and self-report physical activity |
Abbreviations: AV = aortic valve; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease; ESRD = end-stage renal disease; LVEF = left ventricular ejection fraction; METs = metabolic equivalents; NR = not reported; SD = standard deviation; VO2 = oxygen consumption; VO2peak = peak oxygen consumption.
3.3. Summary measures (exposure)
CRF was measured using cardiopulmonary exercise testing and analyzed through the measurement of peak oxygen uptake (the value representing the highest volume of oxygen uptake attained during the test), VO2max (the value representing the point that oxygen uptake reaches a maximum beyond which no increase in effort can augment it), or estimation of METs. Cut-off points for determining CRF categories varied across studies (Table 1).
3.4. Risk of bias within studies
All studies met at least 10 out of the 14 items included in the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies and were considered to have fair-to-good methodological quality. The average score was 10.61/14.00 (Supplementary Table 1).
3.5. Synthesis of results
All-cause (HR = 0.42; 95%CI: 0.28–0.61, p < 0.001, I2 = 94.6%) and CVD (HR = 0.27; 95%CI: 0.16–0.48, p = 0.005, I2 = 0.0%) mortality was lower in individuals with high CRF than in individuals with low CRF. Cochran's Q statistic for statistical heterogeneity was 185.81 (p < 0.001) and 1.08 (p = 0.782) for all-cause and CVD mortality, respectively (Fig. 2).
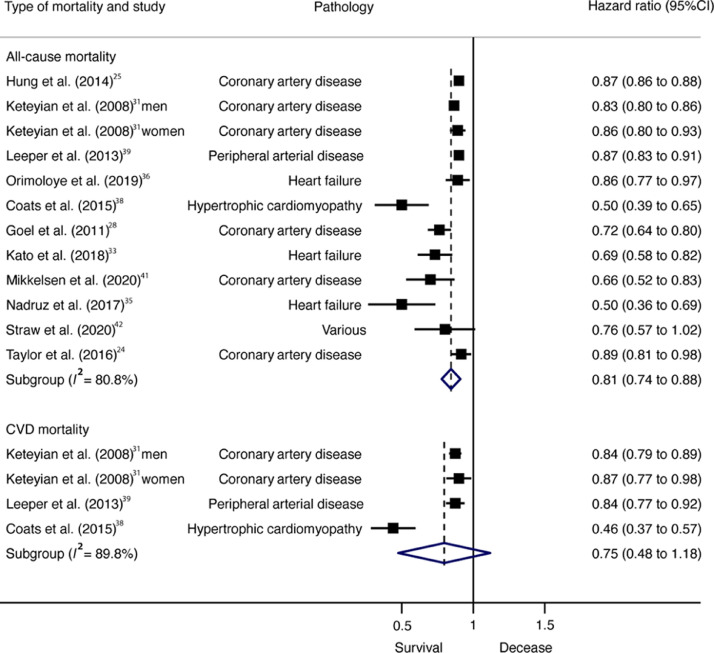
Pooled HRs for the reduction in all-cause mortality risk per 1-MET increase were also statistically significant (HR = 0.81; 95%CI: 0.74–0.88, p < 0.001, I2 = 80.8%), whereas for CVD mortality they were not (HR = 0.75; 95%CI: 0.48–1.18, p = 0.138, I2 = 89.8%) (Fig. 3). Cochran's Q statistic for statistical heterogeneity was 57.34 (p < 0.001) and 29.32 (p < 0.001) for all-cause and CVD mortality, respectively.
Fig 3.
Forest plot showing the hazard ratios of all-cause mortality in patients with CVD per each 1-MET increase in CRF. 95%CI = 95% confidence interval; CRF = High cardiorespiratory fitness; CVD = cardiovascular disease; MET = metabolic equivalent.
According to pathology, patients with coronary artery disease with high CRF had a lower risk of all-cause mortality (HR = 0.32; 95%CI: 0.26–0.41, p < 0.001, Q = 7.23, I2 = 30.9%) than did patients with low CRF. Also, higher CRF (per 1-MET increase) was associated with lower all-cause mortality risk among patients with coronary artery disease (HR = 0.83; 95%CI: 0.76–0.91, p = 0.003, Q = 21.86, I2 = 77.1%) but not among patients with heart failure (HR = 0.69; 95%CI: 0.36–1.32, p = 0.134, Q = 11.94, I2 = 83.3%).
3.6. Risk of bias across studies
Asymmetry suggestive of small-study effects was observed for all-cause mortality (LFK index = –5.07) and CVD mortality (LFK index = 3.32), taking into account high vs. low CRF categories. These results were similar for every 1-MET increase in relation to mortality (all-cause, LFK index = 1.50; CVD mortality, LFK index = –2.18) (Supplementary Fig. 1).
3.7. Additional analysis
For high vs. low CRF, there were no significant effects on mortality according to length of follow-up (all-cause mortality, β = –0.001, p = 0.665; CVD mortality, β = –0.001, p = 0.729) (Supplementary Fig. 2). In the same manner, for every 1-MET increase of CRF, the length of follow-up was not associated with all-cause mortality (β = 0.002; p = 0.053) or CVD mortality (β = 0.001, p = 0.808) (Supplementary Fig. 3).
With each study deleted from each model once, non-overlapping 95%CIs were observed across all deletions.
4. Discussion
The main finding of the present study is that adults diagnosed with CVD with high CRF have substantially reduced all-cause mortality (58%) and CVD mortality risk (73%) than do their least fit counterparts. According to the dose-response analyses, for each 1-MET increase in CRF there was a 19% reduction in risk for all-cause mortality but not CVD mortality. These results support the use of CRF measurements as a prognostic health indicator, which may help to identify individuals with CVD who are at high risk and then guide clinical decision-making to improve their CRF.
Cross-sectional population studies have suggested that higher CRF is associated with more favorable coronary or cardiovascular risk factor profiles.43,44 This accords with a previous observation showing stronger associations between CRF and all-cause mortality than between CRF and self-reported physical activity in 42,373 men and women.45 Diverse physiological mechanisms have been proposed to explain these associations. For instance, insulin sensitivity and high blood pressure are major determinants of CVD. It is well-established that CRF is associated with insulin sensitivity,46 and a significant inverse association between CRF and incident hypertension in both men and women has also been recognized.47,48 Likewise, CRF is central to the management of different cardiometabolic risk factors (i.e., glycemic control, lipid profiles, blood pressure). Improving these clustered cardiometabolic risk variables associated with increased risk of diabetes, CVD, and all-cause mortality49,50 may partly explain the survival benefits of CRF in this population.
There is increasing evidence of an inverse relationship between CRF and all-cause and cardiovascular mortality in healthy people.51 In our analysis, the survival benefit of a better CRF in patients with coronary artery disease equated to a 68% reduction in all-cause mortality risk when compared to their unfit counterparts and a 17% lower all-cause mortality risk for each 1-MET increase in CRF. Similar observations were noted in a longitudinal study of men with a median follow-up of 20 years,52 which found that for each 1-MET increase in CRF level there was a 12% reduction in all-cause mortality. This was reaffirmed in a different retrospective longitudinal study with a follow-up of 9 years,53 which found that a change in CRF from low to intermediate/high resulted in a significant reduction of death risk for black (45%) and white (59%) patients. A meta-analysis of observational studies by Kodama and colleagues51 showed that for every 1-MET increase in CRF in healthy people there was 13% reduction in all-cause mortality and a 15% reduction in CVD mortality. This finding has important clinical implications, as patients with established coronary artery disease have an increased risk of new cardiovascular events.54 It reinforces the need for risk stratification in this population and confirms the great potential for patient health gain through the promotion of CRF.
Our meta-analysis revealed a positive association between higher CRF levels in patients with heart failure and a reduction in their mortality risk. This result could be explained in part by the small number of analyzed studies (n = 3). Additionally, because heart failure is a common endpoint for various CVDs and typically appears in aged individuals, it could be argued that changes in CRF by themselves could confer the same survival benefit as they do in other forms of CVD. Similarly, other studies have reported positive findings. In a population-based follow-up study of 1873 men aged 42–61 years without heart failure or chronic respiratory disease, 152 incident heart failure events were recorded after a mean follow-up of 20.4 years. The age-adjusted heart rate per unit increase (mL/kg/min) in CRF was 21%, which was minimally attenuated to 6% after further adjustment for established heart failure risk factors (body mass index, systolic blood pressure, history of CVD, diabetes, heart rate, and left ventricular hypertrophy).55 Similarly, associations between low CRF and a substantially higher risk for heart failure hospitalization later in life have been found in healthy, middle-aged adults.8 Other cohort studies have reported similar associations between physical activity, CRF, and incident heart failure.56,57
Interestingly, patients living with CVD experience marked reductions in their CRF. Abnormal endothelial function, impaired stroke volume response, ventilatory dysfunction, chronotropic incompetence, and abnormal peripheral oxygen utilization have been highlighted as potential contributors to exercise limitation.58,59 Among the different strategies aimed to manage the deleterious effects of these disease-related changes (frequently in combination with age-related changes), increasing physical activity has shown to be one of the most effective approaches by far. This is mainly because of its ability to elicit protective benefits in the development of subclinical changes in heart structure and function in patients with CVD, including improvements in endothelial function,60,61 left ventricular distensibility,62 and diastolic function.63 Regular physical activity also promotes biological adaptations in skeletal muscle through increased size and number of skeletal muscle mitochondria64 and increased muscle capillary density,65 which collectively may confer a positive synergistic effect on survival among patients with CVD. Nevertheless, individualized exercise should be prescribed by an experienced professional and should be based on several factors determined by a patient's clinical history, exercise stress testing, or functional imaging and echocardiography.
The generalizability of these results is subject to certain limitations. First, our analysis and interpretation were limited by the availability of studies conducted in adults diagnosed with CVD, which mainly included patients with coronary heart disease and heart failure. Therefore, we could not conclusively determine whether CRF confers a survival benefit in all patients with CVD. Second, most studies measured CRF at baseline with subsequent mortality follow-up and did not address changes in individual levels of fitness over time due to changes in lifestyle habits. Third, it is possible that misclassification bias may have affected our results (e.g., in cases where we transformed the reported CRF data into MET units or where calls were made in the classification of low vs. high CRF patients, which varied from study to study based on prediction equations or standard cut-off points). Further studies should recognize and implement universal thresholds or cut-off points that distinguish low, moderate, and high CRF categories in patients with CVDs, thereby allowing comparison within studies and improving risk stratification. We believe that evaluating VO2max as a percentage of age-, body mass index-, and sex-predicted VO2max equations may infer the contribution of physical fitness in prognosis with more accuracy.66
Several studies in our analysis assessed CRF in patients undergoing an exercise-based cardiac rehabilitation program,23,24,26, 27, 28, 29, 30, 31,34,41 which may have provided additional health benefits in this population. However, whenever possible, we included data on CRF levels prior to cardiac rehabilitation programs, minimizing the potential influence of these interventions on our results. We therefore advocate caution when interpreting the present findings, as they may be affected by limitations present in the original articles. In the era of precision medicine, there is a need for more sophisticated tools, such as machine learning, modeling, and simulation solutions techniques, which could help to better predict the association between CRF and clinical outcomes.
5. Conclusion
Our findings support the relevance and use of CRF as a powerful and useful prognostic indicator for mortality in patients with CVD. More focus on physical activity in this population should be explicitly promoted with an aim to increase CRF. Further research is needed to determine whether exercise-based strategies according to risk category lead to improvements in risk of mortality; this could be achieved through the design and implementation of large-scale randomized controlled trials in different populations of patients diagnosed with CVD. This may help to determine how best to use physical activity recommendations and exercise therapy to reduce mortality risk in patients with CVD.
Acknowledgments
Data availability statement
The data that support the findings of this review are available upon reasonable request from the corresponding author (Antonio García-Hermoso).
Acknowledgments
AGH is a Miguel Servet Fellow at the Instituto de Salud Carlos III (CP18/0150). RRV is funded in part by a Postdoctoral Fellowship (Resolution ID 420/2019) from the Universidad Pública de Navarra.
Authors’ contributions
AGH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, AGH is also responsible for the data analysis; AGH and YE contributed to the conception, methodology, formal analysis, investigation, wrote the first draft of the article and reviewed and edited it, MI and RRV supervised the study data collection, contributed to data analysis, and contributed to the article preparation; JN and JC reviewed and edited the article. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.06.004.
Appendix. Supplementary materials
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 6.Church TS, Kampert JB, Gibbons LW, Barlow CE, Blair SN. Usefulness of cardiorespiratory fitness as a predictor of all-cause and cardiovascular disease mortality in men with systemic hypertension. Am J Cardiol. 2001;88:651–656. doi: 10.1016/s0002-9149(01)01808-2. [DOI] [PubMed] [Google Scholar]
- 7.Nes BM, Vatten LJ, Nauman J, Janszky I, Wisløff U. A simple nonexercise model of cardiorespiratory fitness predicts long-term mortality. Med Sci Sports Exerc. 2014;46:1159–1165. doi: 10.1249/MSS.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 8.Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price KJ, Gordon BA, Bird SR, Benson AC. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur J Prev Cardiol. 2016;23:1715–1733. doi: 10.1177/2047487316657669. [DOI] [PubMed] [Google Scholar]
- 10.Pandey A, Cornwell 3rd WK, Willis B, et al. Body mass index and cardiorespiratory fitness in mid-life and risk of heart failure hospitalization in older age: Findings from the cooper center longitudinal study. JACC Heart Fail. 2017;5:367–374. doi: 10.1016/j.jchf.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Smith JL, Verrill TA, Boura JA, Sakwa MP, Shannon FL, Franklin BA. Effect of cardiorespiratory fitness on short-term morbidity and mortality after coronary artery bypass grafting. Am J Cardiol. 2013;112:1104–1109. doi: 10.1016/j.amjcard.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 12.Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571–584. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 15.Kaminsky LA, Arena R, Ellingsen Ø, et al. Cardiorespiratory fitness and cardiovascular disease—the past, present, and future. Prog Cardiovasc Dis. 2019;62:86–93. doi: 10.1016/j.pcad.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Myers J. New American Heart Association/American College of Cardiology guidelines on cardiovascular risk: When will fitness get the recognition it deserves? Mayo Clin Proc. 2014;89:722–726. doi: 10.1016/j.mayocp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16:195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 21.Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhoble A, Enriquez-Sarano M, Kopecky SL, et al. Cardiopulmonary responses to exercise and its utility in patients with aortic stenosis. Am J Cardiol. 2014;113:1711–1716. doi: 10.1016/j.amjcard.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Martin BJ, Arena R, Haykowsky M, et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc. 2013;88:455–463. doi: 10.1016/j.mayocp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Taylor C, Tsakirides C, Moxon J, et al. Submaximal fitness and mortality risk reduction in coronary heart disease: A retrospective cohort study of community-based exercise rehabilitation. BMJ Open. 2016;6:e011125. doi: 10.1136/bmjopen-2016-011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung RK, Al-Mallah MH, McEvoy JW, et al. Prognostic value of exercise capacity in patients with coronary artery disease: The FIT (Henry Ford ExercIse Testing) project. Mayo Clin Proc. 2014;89:1644–1654. doi: 10.1016/j.mayocp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Aijaz B, Squires RW, Thomas RJ, Johnson BD, Allison TG. Predictive value of heart rate recovery and peak oxygen consumption for long-term mortality in patients with coronary heart disease. Am J Cardiol. 2009;103:1641–1646. doi: 10.1016/j.amjcard.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Barons MJ, Turner S, Parsons N, et al. Fitness predicts long-term survival after a cardiovascular event: A prospective cohort study. BMJ Open. 2015;5:e007772. doi: 10.1136/bmjopen-2015-007772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel K, Thomas RJ, Squires RW, et al. Combined effect of cardiorespiratory fitness and adiposity on mortality in patients with coronary artery disease. Am Heart J. 2011;161:590–597. doi: 10.1016/j.ahj.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Hai JJ, Siu CW, Ho HH, Li SW, Lee S, Tse HF. Relationship between changes in heart rate recovery after cardiac rehabilitation on cardiovascular mortality in patients with myocardial infarction. Heart Rhythm. 2010;7:929–936. doi: 10.1016/j.hrthm.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Hung RK, Al-Mallah MH, McEvoy JW, et al. Prognostic value of exercise capacity in patients with coronary artery disease: The FIT (Henry Ford ExercIse Testing) project. Mayo Clin Proc. 2014;89:1644–1654. doi: 10.1016/j.mayocp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156:292–300. doi: 10.1016/j.ahj.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Kiviniemi AM, Lepojärvi S, Kenttä TV, et al. Exercise capacity and heart rate responses to exercise as predictors of short-term outcome among patients with stable coronary artery disease. Am J Cardiol. 2015;116:1495–1501. doi: 10.1016/j.amjcard.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Kato Y, Suzuki S, Uejima T, et al. Relationship between the prognostic value of ventilatory efficiency and age in patients with heart failure. Eur J Prev Cardiol. 2018;25:731–739. doi: 10.1177/2047487318758775. [DOI] [PubMed] [Google Scholar]
- 34.Keteyian SJ, Kerrigan DJ, Lewis B, Ehrman JK, Brawner CA. Exercise training workloads in cardiac rehabilitation are associated with clinical outcomes in patients with heart failure. Am Heart J. 2018;204:76–82. doi: 10.1016/j.ahj.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Nadruz W, Jr, West E, Sengeløv M, et al. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc. 2017;6:e006000. doi: 10.1161/JAHA.117.006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orimoloye OA, Kambhampati S, Hicks 3rd AJ, et al. Higher cardiorespiratory fitness predicts long-term survival in patients with heart failure and preserved ejection fraction: The Henry Ford Exercise Testing (FIT) Project. Arch Med Sci. 2019;15:350–358. doi: 10.5114/aoms.2019.83290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T, Yoshihisa A, Kanno Y, et al. Cardiopulmonary exercise testing as prognostic indicators: Comparisons among heart failure patients with reduced, mid-range and preserved ejection fraction. Eur J Prev Cardiol. 2017;24:1979–1987. doi: 10.1177/2047487317739079. [DOI] [PubMed] [Google Scholar]
- 38.Coats CJ, Rantell K, Bartnik A, et al. Cardiopulmonary exercise testing and prognosis in hypertrophic cardiomyopathy. Circ Heart Fail. 2015;8:1022–1031. doi: 10.1161/CIRCHEARTFAILURE.114.002248. [DOI] [PubMed] [Google Scholar]
- 39.Leeper NJ, Myers J, Zhou M, et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–733. doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goda A, Koike A, Iwamoto MH, et al. Prognostic value of heart rate profiles during cardiopulmonary exercise testing in patients with cardiac disease. Int Heart J. 2009;50:59–71. doi: 10.1536/ihj.50.59. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen N, Cadarso-Suárez C, Lado-Baleato O, et al. Improvement in VO2peak predicts readmissions for cardiovascular disease and mortality in patients undergoing cardiac rehabilitation. Eur J Prev Cardiol. 2020;27:811–819. doi: 10.1177/2047487319887835. [DOI] [PubMed] [Google Scholar]
- 42.Straw S, Waduud MA, Drozd M, et al. The role of cardiopulmonary exercise testing and echocardiography prior to elective endovascular aneurysm repair. Ann R Coll Surg Engl. 2020;102:383–390. doi: 10.1308/rcsann.2020.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borodulin K, Laatikainen T, Lahti-Koski M, et al. Associations between estimated aerobic fitness and cardiovascular risk factors in adults with different levels of abdominal obesity. Eur J Cardiovasc Prev Rehabil. 2005;12:126–131. doi: 10.1097/00149831-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: A population-based study in Olmsted County, Minnesota. Circulation. 1998;98:2836–2841. doi: 10.1161/01.cir.98.25.2836. [DOI] [PubMed] [Google Scholar]
- 45.Lee DC, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45:504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 46.Leite SA, Monk AM, Upham PA, Bergenstal RM. Low cardiorespiratory fitness in people at risk for type 2 diabetes: Early marker for insulin resistance. Diabetol Metab Syndr. 2009;1:8. doi: 10.1186/1758-5996-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barlow CE, LaMonte MJ, FitzGerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–150. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 48.Chase NL, Sui X, Lee DC, Blair SN. The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens. 2009;22:417–424. doi: 10.1038/ajh.2009.6. [DOI] [PubMed] [Google Scholar]
- 49.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 50.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 51.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 52.Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: A 20-year follow-up study. Circulation. 2010;122:790–797. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 53.Ehrman JK, Brawner CA, Al-Mallah MH, Qureshi WT, Blaha MJ, Keteyian SJ. Cardiorespiratory fitness change and mortality risk among black and white patients: Henry Ford Exercise Testing (FIT) project. Am J Med. 2017;130:1177–1183. doi: 10.1016/j.amjmed.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 54.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: Nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36:1163–1170. doi: 10.1093/eurheartj/ehu505. [DOI] [PubMed] [Google Scholar]
- 55.Khan H, Kunutsor S, Rauramaa R, et al. Cardiorespiratory fitness and risk of heart failure: A population-based follow-up study. Eur J Heart Fail. 2014;16:180–188. doi: 10.1111/ejhf.37. [DOI] [PubMed] [Google Scholar]
- 56.Farrell SW, Finley CE, Radford NB, Haskell WL. Cardiorespiratory fitness, body mass index, and heart failure mortality in men: Cooper Center Longitudinal Study. Circ Heart Fail. 2013;6:898–905. doi: 10.1161/CIRCHEARTFAILURE.112.000088. [DOI] [PubMed] [Google Scholar]
- 57.Koo P, Gjelsvik A, Choudhary G, et al. Prospective association of physical activity and heart failure hospitalizations among black adults with normal ejection fraction: The Jackson Heart Study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Buono MG, Arena R, Borlaug BA, et al. Exercise intolerance in patients with heart failure: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;73:2209–2225. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 59.Sharma S, Elliott P, Whyte G, et al. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol. 2000;86:162–168. doi: 10.1016/s0002-9149(00)00854-7. [DOI] [PubMed] [Google Scholar]
- 60.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int J Cardiol. 2017;231:234–243. doi: 10.1016/j.ijcard.2016.12.145. [DOI] [PubMed] [Google Scholar]
- 62.Arbab-Zadeh A, Perhonen M, Howden E, et al. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130:2152–2161. doi: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popović ZB, Prasad A, Garcia MJ, et al. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: Impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290:H1454–H1459. doi: 10.1152/ajpheart.00902.2005. [DOI] [PubMed] [Google Scholar]
- 64.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 65.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol Rev. 2017;97:495–528. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palau P, Bayés-Genís A, Núñez J. Risk stratification according to midlife physical fitness in an asymptomatic population. J Am Coll Cardiol. 2018;72:3230–3231. doi: 10.1016/j.jacc.2018.09.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this review are available upon reasonable request from the corresponding author (Antonio García-Hermoso).