Abstract
Background:
Identifying appropriate biomarkers for predicting type 2 diabetes (T2D) patients with increased HbA1c may prove helpful in preventing increased risk of cardiovascular disease (CVD). The present study was conducted to analyze the diagnostic performance of the atherogenic index log (TG/HDL-C) in T2D patients with increased HbA1c.
Methods:
Patients with T2D were classified into two groups according to having an HbA1c <8% or ≥8%. Atherogenic index was calculated from the logarithmic transformation of TG/HDL-C. Receiver operating characteristic curve was used to evaluate the diagnostic performance of log (TG/HDL-C). Insulin and fasting glucose concentrations were used to determine homeostasis model assessment for insulin resistance (HOMA-IR).
Results:
Compared with the patients with HbA1c <8%, log (TG/HDL-C) was significantly higher in the patients with HbA1c ≥8% (p = 0.025). The atherogenic index was a biomarker for the prediction of T2D patients with HbA1c ≥8% versus patients with HbA1c <8%, as shown in the area under the curve (AUC = 0.61, P = 0.013). The best cut-off point of log (TG/HDL-C) for the discrimination between patients with HbA1c ≥8% versus patients with HbA1c <8% determined to be 0.44. Atherogenic index was significantly and positively correlated with HOMA-IR in female patients (r = 0.313, P = 0.003) and in patients with an age ≥5o (r = 0.253, P = 0.021).
Conclusion:
The log (TG/HDL-C) in addition to its known association with enhanced CVD risk could be considered as a biomarker to predict T2D patients with poor glycemic control. Therefore, the increased ratio may provide a simple and useful way of identifying poor glycemic T2D patients who are possibly to be at elevated risk of CVD.
Keywords: Biomarker, cardiovascular disease, HbA1c, Log (TG/HDL-C), type 2 diabetes
Introduction
Diabetes mellitus is a major threat to public health worldwide and its prevalence is increasing rapidly. These conditions are likely to worsen in the future as a result of increasing lifestyle negative changes, such as obesity. Patients with type 2 diabetes (T2D) often develop dyslipidemia that can lead to the increases risk of cardiovascular disease (CVD).[1,2] Evaluation of dyslipidemia and oxidative stress markers can be useful for the assessment of cardiovascular risk in patients with T2D.[1,3] Atherogenic dyslipidemia including increased triglycerides (TGs) and low levels of high-density lipoprotein (HDL) cholesterol, often with increased apolipoprotein B and non-HDL cholesterol, is common in patients with established CVD, T2D, or metabolic syndrome and contributes vascular residual risk.[4]
Studies in prediabetic subjects have indicated that an atherogenic pattern of risk factors may contribute to the risk of macrovascular outcomes as much as the duration of clinical diabetes itself, therefore in order to reduce the cardiovascular risk in diabetic individuals it may be necessary to intervene before the onset of clinical diabetes.[5] The association of hypertriglyceridemia with impaired glucose tolerance, impaired fasting glucose, insulin resistance, and T2D shows importance of high TG in T2D.[6,7] According to recent studies, risk factors for CVD such as log (TG/HDL-C) and low-density lipoprotein (LDL)/HDL have higher predictive value than individual concentrations of lipid parameters; therefore, it could be an advantage for these atherogenic indices to be an alert to the increased cardiovascular risk.[8]
The longer periods of exposure to HbA1c levels ≥8% were associated with increasing cardiovascular complications and mortality risk.[9] The present study thus seeks to answer the question whether the atherogenic index can have the predictive role in T2D patients with poor glycemic control. The importance of the predictor role of log (TG/HDL-C) in these patients becomes more apparent when noting that this index contribute to the prediction of cardiovascular complications in patients with T2D.
Methods
Study population
This study conducted on 133 patients with T2D. All patients were diagnosed according to the diagnostic criteria as recommended by the WHO incorporating both fasting glucose and a 2-h oral glucose tolerance test. Based on the recommendations of the American Diabetes Association Standards of Medical Care in Diabetes (2020), HbA1C goals <8% may be appropriate for patients with an advanced microvascular or macrovascular complications.[10] Furthermore, poor glycemic control has been defined as HbA1c values ≥8% in some other studies.[11,12] Therefore, the study patients with T2D were classified according to having an HbA1c <8% (n = 69) or ≥8% (n = 64). Information about demographic parameters, medical history, personal habits, and medication use was obtained using a questionnaire. Criteria for exclusion in the study were type 1 diabetes, history of myocardial infarction or stroke, previous history of renal failure, liver diseases, cancer, and pregnancy. The patients were taking medications, including metformin, a sulfonylurea and a statin. The study protocol was planned based on the ethical criteria detailed in the Declaration of Helsinki and was approved by the university local ethics committee. Written informed consent was received from all participants.
Assay of laboratory parameters
Insulin levels were measured using ELISA kit (Diametra Corporation, Milan, Italy). TG, HDL-C, and fasting blood glucose were measured by commercial kits (Greiner Diagnostics GmbH, Bahlingen, Germany). Atherogenic index was calculated from the logarithmic transformation of TG/HDL-C. HbA1c was measured using a commercially available immunoturbidimetric assay kit (Pars Azmoon Co, Tehran, Iran). Insulin and fasting glucose concentrations were used to determine HOMA-IR using the following equation[13]:
HOMA-IR = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5
Statistical analyses
Comparisons between groups were performed using t-test for parametric variables and Mann–Whitney U test for nonparametric variables. Receiver operating characteristic (ROC) analyses were performed to analyze the predictive performance of atherogenic index in the study groups. The area under the curve (AUC) was determined in order to estimate the overall discriminative capacity of the variables. Pearson correlation coefficient was used to analyze the association between study variables. SPSS (version 16.0) and R (version 3.0.0) software were used for statistical analyses. Two-tailed P value less than 0.05 was considered to be significant.
Results
Table 1 shows the comparison of values of the study parameters when HbA1c was categorized as <8% or ≥8%. There also was no statistically significant difference between patients with HbA1c ≥8% and patients with <8% with respect to medications, including metformin, a sulfonylurea and a statin. As shown in Figure 1, compared to the patients with HbA1c <8%, atherogenic index was significantly higher in the patients with HbA1c ≥8% (p = 0.025).
Table 1.
Clinical and biochemical characteristics of patients with type 2 diabetes according to HbA1c
| Parameter | HbA1c <8% (n=69) | HbA1c ≥8% (n=64) | P |
|---|---|---|---|
| Age (year) | 52.35±5.99 | 51.29±6.56 | 0.334 |
| Women (%) | 76.8 | 60.9 | 0.059 |
| BMI (kg/m2) | 29.95±4.35 | 30.06±4.94 | 0.998 |
| Smoking (%) | 4.35 | 7.81 | 0.466 |
| Triglyceride (mmol/L) | 2.03±1.26 | 2.42±1.34 | 0.096 |
| HDL-C (mmol/L) | 1.21±0.31 | 1.13±0.27 | 0.099 |
| HOMA-IR | 3.89±3.45 | 6.59±4.7 | <0.001 |
| Medications (%) | |||
| Metformin | 71.1 | 81.2 | 0.216 |
| Sulfonylurea | 14.5 | 26.6 | 0.084 |
| Statins | 32.8 | 35.6 | 0.853 |
Values are presented as percent or mean±SD.
Figure 1.
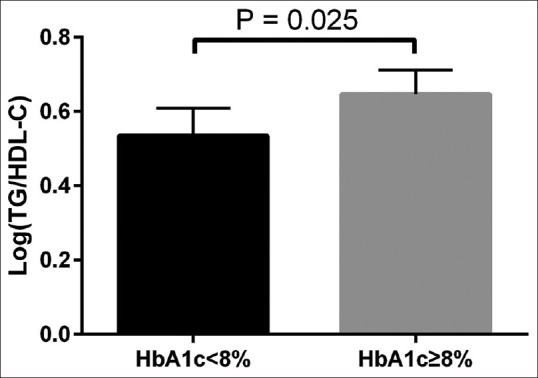
Comparison of atherogenic index in type 2 diabetes patients with HbA1c ≥8% and patients with HbA1c <8%.
As can be seen in Figure 2, log (TG/HDL-C) was an appropriate biomarker for the prediction of T2D patients with HbA1c ≥8% versus patients with HbA1c <8%, as shown in the AUC (AUC = 0.61, P = 0.013). The ratio log (TG/HDL-C) remained a significant predictor for patients with HbA1c ≥8% after multivariate adjustment for age, sex, and body mass index. Based on Youden's index, the best cut-off point of log (TG/HDL-C) for the discrimination between patients with HbA1c ≥8% versus patients with HbA1c <8% was 0.44, with a specificity of 48% and a sensitivity of 81%.
Figure 2.
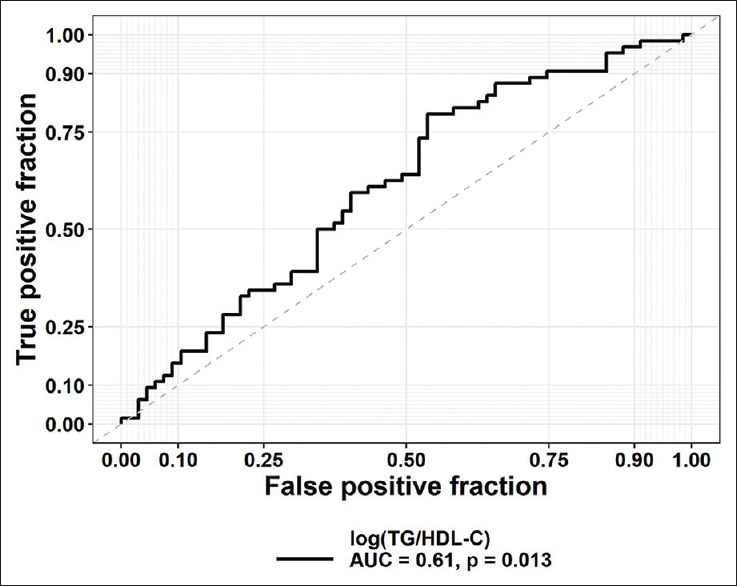
The receiver operating characteristics (ROC) curves for atherogenic index for discriminating patients with HbA1c ≥8% versus patients with HbA1c <8%. The corresponding value of the area under the curve (AUC) and P value are reported
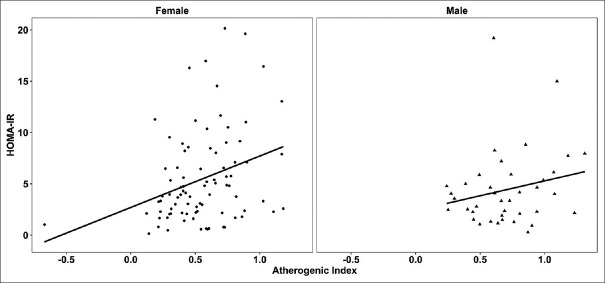
As indicated in Figure 3, our findings showed that atherogenic index was significantly and positively correlated with HOMA-IR (r = 0.313, P = 0.003) in female patients. This index was also found to be positively correlated with HOMA-IR in male patients; however, the correlation was not statistically significant (r = 0.146, P = 0.375). When patients were classified according to age, atherogenic index was found to be positively correlated with HOMA-IR in both patients with an age <50 and age ≥5o; however, only the correlation in the age ≥5o group was statistically significant (r = 0.253, P = 0.021).
Figure 3.
Associations between atherogenic index and HOMA-IR in patients with type 2 diabetes according to gender (r = 0.313, P = 0.003 for females and r = 0.146, P = 0.375 for males). HOMA-IR (homeostasis model assessment for insulin resistance)
Discussion
In the lipid and lipoprotein therapy strategies, after the introduction of statins, clinical priority first focused on LDL cholesterol-lowering, then raising HDL cholesterol, with less focus on lowering TG. However, it has now generated renewed interest in lowering TGs because recent studies are showing that TG levels and TG-rich lipoproteins are in the causal pathway of CVD.[14] According to standards of medical care in diabetes, high serum levels of TG and low HDL cholesterol are associated with pre-diabetes and diabetes in asymptomatic adult subjects.[15] Identifying appropriate biomarkers can help develop therapeutic strategies to improve the survival of patients.[16]
Our results indicated that the log (TG/HDL-C) in addition to its known link to enhanced CVD risk is a significant predictor of T2D patients with poor glycemic control. In other words, the elevated ratio may provide a simple and useful approach to identify poor glycemic T2D patients who are possibly to be at increased risk of CVD. The following studies support the present results. The ratio of TG/HDL-C was found to be the predictor of insulin resistance and LDL particle diameter.[17] Log (TG)/HDL-C was a predictor of progression speed of islet beta cell dysfunction in Chinese T2D patients.[18] Intriguingly, in a recent community-based Korean cohort over 12 years, Lim et al.[19] found that high TG/HDL-C ratio at baseline may be a useful indicator of future incident T2D. The importance of the predictor role of log (TG/HDL-C) in T2D patients becomes more apparent when noting that this atherogenic index may be a warning for the increased cardiovascular risk in individuals who do not yet have extremely altered lipid levels.[20] Therefore, the log (TG/HDL-C) may be considered as an important biomarker in the monitoring of cardiovascular complications in T2D.
In our findings, the TG/HDL-C ratio as an atherogenic index was positively associated with HOMA-IR which is consistent with previous studies.[21] However, when the analyses were performed with regard to gender and age, there were significant correlations between the atherogenic index and HOMA-IR only in the female patients with T2D as well as patients with an age ≥50. This shows that in this group of patients, the higher the insulin resistance, the greater the likelihood of atherogenicity. In other words, it appears that these patients will need more medical care.
Noteworthy, antidiabetic drugs, which are also able to reduce the atherogenic ratios in addition to reducing blood glucose, will likely have positive effects on cardiovascular complications in patients with T2D. In this context, Tan et al.[22] showed that pioglitazone monotherapy or in combination therapy with a sulfonylurea, metformin, or insulin reduced the atherogenic ratio log (TG/HDL-C). In addition, fibrates, niacin, and omega-3 fatty acids that are more efficacious in reducing TGs and/or increasing HDL cholesterol should be considered in combinations with statins in order to reducing cardiovascular risk in patients with T2D or metabolic syndrome.[1]
Conclusions
The log (TG/HDL-C) in addition to its known association with enhanced CVD risk could be considered as a biomarker to predict T2D patients with poor glycemic control. Therefore, the increased ratio may provide a simple and useful way of identifying poor glycemic T2D patients who are possibly to be at elevated risk of CVD. The association between log (TG/HDL-C) and insulin resistance differs according to gender and age.
Financial support and sponsorship
This study was supported by the Iran National Science Foundation (INSF) (Project No. 97013130). We would also like to thank the Mazandaran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study received funding support from Mazandaran University of Medical Sciences under the Grant No. 10375.
The manuscript has been read and approved by all the authors and each author believes that the manuscript represents honest work.
References
- 1.Jones PH. Expert perspective: Reducing cardiovascular risk in metabolic syndrome and type 2 diabetes mellitus beyond low-density lipoprotein cholesterol lowering. Am J Cardiol. 2008;102:41L–7L. doi: 10.1016/j.amjcard.2008.09.074. [DOI] [PubMed] [Google Scholar]
- 2.Shokri F, Ghaedi H, Fard SG, Movafagh A, Abediankenari S, Mahrooz A, et al. Impact of ATM and SLC22A1 polymorphisms on therapeutic response to metformin in Iranian diabetic patients. Int J Mol Cell Med. 2016;5:1–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Zargari M, Sharafeddin F, Mahrooz A, Alizadeh A, Masoumi P. The common variant Q192R at the paraoxonase 1 (PON1) gene and its activity are responsible for a portion of the altered antioxidant status in type 2 diabetes. Exp Biol Med. 2016;241:1489–96. doi: 10.1177/1535370216641786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruchart J-C, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, et al. The residual risk reduction initiative: A call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–8. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 6.Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride–to–high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Invest Med. 2014;62:345–9. doi: 10.2310/JIM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 7.Pishva H, Mehdipour P, Eshraghian MR, Mahboob SA. Effects of eicosapentaenoic acid supplementation on lipid and lipoprotein profile in hypertriglyceridemic subjects with different proliferator-activated receptor alpha genotypes. Int J Prevent Med. 2014;5:333–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M. Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin Chem. 2004;50:2316–22. doi: 10.1373/clinchem.2004.037556. [DOI] [PubMed] [Google Scholar]
- 9.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study) Diabetes Care. 2019;42:416–26. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Association AD. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 11.Cortez-Espinosa N, García-Hernández MH, Reynaga-Hernández E, Cortés-García JD, Corral-Fernández NE, Rodríguez-Rivera JG, et al. Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (HbA1c >8%) Metabolism. 2012;61:1538–46. doi: 10.1016/j.metabol.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Mahrooz A, Hashemi-Soteh MB, Heydari M, Boorank R, Ramazani F, Mahmoudi A, et al. Paraoxonase 1 (PON1)-L55M among common variants in the coding region of the paraoxonase gene family may contribute to the glycemic control in type 2 diabetes. Clin Chim Acta. 2018;484:40–6. doi: 10.1016/j.cca.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinzadeh P, Javanbakht MH, Mostafavi S-A, Djalali M, Derakhshanian H, Hajianfar H, et al. Brewer's yeast improves glycemic indices in type 2 diabetes mellitus. Int J Prev Med. 2013;4:1131. [PMC free article] [PubMed] [Google Scholar]
- 14.Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138–45. doi: 10.1016/j.amjcard.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes. 2017;35:5–26. doi: 10.2337/cd16-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Variji A, Shokri Y, Fallahpour S, Zargari M, Bagheri B, Abediankenari S, et al. The combined utility of myeloperoxidase (MPO) and paraoxonase 1 (PON1) as two important HDL-associated enzymes in coronary artery disease: Which has a stronger predictive role? Atherosclerosis. 2019;280:7–13. doi: 10.1016/j.atherosclerosis.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 18.Zhou M, Li Z, Min R, Dong Y, Sun Q, Li Y. Log (TG)/HDL-C ratio as a predictor of decreased islet beta cell function in patients with type 2 diabetes: 6-year cohort study. J Diabetes. 2015;7:689–98. doi: 10.1111/1753-0407.12229. [DOI] [PubMed] [Google Scholar]
- 19.Lim T-K, Lee HS, Lee Y-J. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 diabetes in community dwelling adults: A longitudinal 12-year analysis of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. 2020:108150. doi: 10.1016/j.diabres.2020.108150. [DOI] [PubMed] [Google Scholar]
- 20.Viktorinova A, Svitekova K, Stecova A, Krizko M. Relationship between selected oxidative stress markers and lipid risk factors for cardiovascular disease in middle-aged adults and its possible clinical relevance. Clin Biochem. 2016;49:868–72. doi: 10.1016/j.clinbiochem.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, et al. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34:1869–74. doi: 10.2337/dc10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clin Chem. 2004;50:1184–8. doi: 10.1373/clinchem.2004.031757. [DOI] [PubMed] [Google Scholar]