Abstract
Background:
Migraine is a prevalent health condition associated with significant pain and disability. Neurogenic inflammation has a key role in migraine pathophysiology. Curcumin is a well-known herb compound with anti-inflammatory function. This study was aimed to evaluate the effects of curcumin supplementation on clinical features, as well as on serum levels of calcitonine gene-related peptide (CGRP) and interleukin-6 (IL-6).
Methods:
This randomized double-blind placebo-controlled clinical trial was carried out on 44 women with migraine, receiving either 500 mg curcumin twice a day or placebo supplements for 8 weeks. Serum CGRP and IL-6 concentration, and clinical symptoms including headache severity, duration and frequency were measured at the baseline and end of study.
Results:
After 8-week intervention, compared with placebo, curcumin supplementation led to significand reduction in CGRP (P < 0.001), IL-6 (P = 0.041), severity (P = 0.001), and duration of headache (P = 0.007). Headache frequency showed marginal improvement in curcumin group, compared to controls (P = 0.052). Within-analysis indicated significant decrease in CGRP and severity (P < 0.001), frequency (P = 0.014) and duration (P = 0.003) and no significant decrease in IL-6 (P = 0.454), compared to baseline in curcumin group. There were no significant changes in body mass index (BMI), weight, percent body fat (PBF), and percent body muscle (PBM) between the two groups.
Conclusions:
Curcumin supplementation improved the pro-inflammatory markers and clinical features of migraine headaches and that could be contributed to could be to its anti-inflammatory properties.
Keywords: Calcitonin gene-related peptide, curcumin, inflammation, interleukin-6, migraine disorders, randomized controlled trial
Introduction
Migraine is one of the most common primary headaches, affecting one in nine adults worldwide.[1] Migraine is characterized by recurrent episodes of incapacitating and intense headaches, often unilateral and pulsatile. Symptoms of migraine include moderate to severe pain, hyper-sensitivity to light and noise, nausea or vomiting, worsened with routine activity.[2] It has been reported that the incidence of migraine is about 12% of the general population, with a higher incidence in women than in men (18% vs. 6%).[3,4]
Multiple pathological changes occur to precipitate a migraine attack. Evidence suggests that elevated neuro-inflammation in the intracranial meninges may play the main role, resulting in the sensitizing of trigeminal meningeal nociceptors in migraine. Peptides such as calcitonin gene related protein (CGRP), may lead to vasodilation effects, binding to receptors on smooth muscle cells (including CLR (calcitonin receptor-like receptor), RAMP1 (receptor activity modifying protein type-1) and RCP (receptor component protein)).[5] Moreover, mitochondrial dysfunction, oxidative stress, and increased level of glutamate, along with changes in other inflammatory factors such as interleukin-6 (IL-6), CRP, and TNFα have been indicated in triggering migraine attacks.[6]
Despite an abundance of treatment options for migraine prevention and pain management, all these therapies have, often, an insufficient efficacy and are accompanied with a numerous side effects.[7] Therefore, within the integrative health studies growing interest pertaining to dietary interventions, the number of studies concerning effects of diet on headache/migraine is not considerable.[8] Moreover, many people may prefer traditional medical practice including herbal therapy because of its cultural acceptability, easy accessibility, and affordability.[7,9]
Curcumin is an active component and natural polyphenol compound that is found in an herb called CURCUMA LONGA (turmeric). It is a non-toxic and highly promising natural compound with a long history of use without any side effects.[10] A review article in 2012 showed that curcumin modulates multiple molecular targets and exerts multifaceted pharmacological activities, including anti-inflammation effects for the treatment and prevention of chronic inflammatory diseases.[11] Also, it is found that curcumin relieves neurogenic pain by down-regulating inflammatory mediator expression.[12] Moreover, experimental studies show that curcumin can be considered as a new promising target in migraine prevention through antioxidative, anti-inflammatory, and analgesic effects.[13,14] In humans, the beneficial effects of curcumin, alone, or in combination with other dietary factors, have been also confirmed.[15,16,17,18] However, studies on humans are scarce and further studies are warranted to discover its mechanism of action and appropriate dosage. Therefore, the present study was aimed to evaluate the effects of curcumin supplementation on migraine symptoms. Besides, since several documents confirm the integral role of neuropeptide calcitonin gene-related peptide (CGRP) and pro-inflammatory cytokine IL-6 in the pathophysiology of migraine[15,19] and considering that few studies, if any, have examined the effect of curcumin on these variables in migraine patients, the effect of curcumin was also investigated in relation to these factors.
Methods
Participants
This randomized, double-blinded, placebo-controlled clinical trial was performed at Clinic, affiliated with the University of Medical Sciences. The study protocol was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and the approval of the University Medical Ethics Committee was granted. All participants provided written informed consent.
Inclusion criteria were: Women who had episodic migraine without aura (according to the International Headache Society (IHS) diagnostic criteria diagnosed by a neurologist (FK), using the International Classification of Headache Disorders, version 3 (ICHD-3) criteria[20]); within the age range of 20–50 years.
Exclusion criteria included: Any symptoms of allergic reactions to the components of curcumin supplements; patients who were unwilling to cooperate, any drastic changes in lifestyle, such as starting a new diet, or changes in type and/or dosage of prescribed medications; pregnancy and/or lactation; and post-menopausal. Additional exclusion criteria included: Use of vitamins, minerals and antioxidant supplements for at least 3 months prior to and during the intervention; suffering from major depression, tension and cluster headaches, cancers, or hepatitis; any metabolic, inflammatory or kidney diseases; and history of heart disease or stroke.
Study design
Forty-four women with migraine who met the inclusion criteria were enrolled and randomly allocated into two groups to receive either curcumin pellets (n = 22) or a placebo (n = 22) for 8 weeks. To calculate sample size, data was utilized from a study conducted evaluating the effect of curcumin supplementation on serum CGRP in patients with sulfur mustard intoxication.[21] This used a standard formula suggested for clinical trials, considering a type one error (α) of 0.05 and a type two error (ß) of 0.20 (power = 80%) with standard deviation of 3.92 for curcumin group and 4.52 for placebo group and d = 3.8. A sample size of 20 in each group was calculated, then, predicting a 10% dropout rate, the final number was increased to 22 per group. Curcumin was administered at a daily dose of 1 g (500 mg b.i.d.), a dose that was found to be effective and safe in previous trials.[22,23,24] Since curcumin is a fat-soluble component, and also displays higher bioavailability in acidic pH, participants were asked to take capsules after meals (lunch and dinner). The shape and size of the supplements and placebo capsules were identical, with all tablets manufactured by KAREN Company (Yazd, Iran). Curcumin supplements contained a standardized 95% turmeric extract in pellet form (475 mg curcuminoids, covering 70-80% curcumin, 15-20% dimethoxy curcumin and 2.5-6.5% bis-demethoxy curcumin). In considering ethical issues, all 44 participants took prophylactic medication, including an anticonvulsant (topiramate 50), during the 8 weeks of intervention. Also, rizatriptan was used for migraine attacks- a Selective serotonin 5-hydroxy tryptamine (5-HT) 1B/1D agonist with biological half-life of 2-3 hour without any persistent effect on inflammation.[25] In order to prevent medication overdose, patients were allowed to take up to 6 tablets per month (5 mg each) only for acute migraine management[26] and if any of patients changed their drug or dose, they were excluded from the study.
All participants were advised to maintain their routine habits without any significant changes to their lifestyle and dietary patterns. None of the patients in two groups of the study reported any side effects.
Random allocation was performed using computer-generated random numbers. Randomization was carried out in blocks of four, and random assignment was undertaken by a separate technician. The investigators, subjects, and laboratory technicians were all blinded to the randomization procedure during the trial.
To evaluate treatment adherence, participants visited the clinic one month after the onset of the study and again at the end of the study period. Each visit lasted for about 15-20 minutes and empty containers were gathered. In addition, they received text messages reminding them to take the supplements once a week.
Dietary and nutrient assessment
All women completed 3-day food intake records (two weekdays and one weekend day) at baseline and post-intervention. The portion sizes reported in the records were converted to grams using household measures. All intake data were entered into customized Nutritionist IV software (First Databank Inc., Hearst Corp., San Bruno, CA) to analyze nutrient intakes.
Since tyramine and phenylethylamine may affect migraine onset, participants in both groups were given a list of tyramine- and phenylethylamine-containing foods and were asked not to consume any of these during the study. In addition, they were advised to avoid fasting and/or skipping meals to prevent migraine attacks.[27]
Anthropometric measurements
At baseline and post-intervention, all participants underwent anthropometric measurements, including height, weight, body mass index (BMI), percent of body fat mass (PBF), and percent body muscle mass (PBM). Measurement of height was undertaken in a standing position without shoes and with relaxed shoulders, using a non-stretchable tape. PBF, PBM and weight were evaluated by bio-impedance analysis (Omron, BF-511, Japan). BMI was calculated as weight in kg divided by height in meters squared.
Biochemical assessment
Pain and inflammation status were assessed through serum markers of CGRP and IL-6 levels, respectively. At the baseline and end of intervention, 10 ml blood samples were collected at the reference laboratory. They were immediately centrifuged to collect the serums, then maintained at -80 degrees Celsius for further tests. Serum CGRP and IL-6 levels were measured by EASTBIOPHARM human kits (Hangzhou, China). The procedures for CGRP and IL-6 kits were similar. These kits use a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). Tests were carried out in the faculty laboratory by a technician and the absorbance was read at 630 nm with BioTek instruments (Highland Park, Winooski, USA). Inter and intra assay CVs for all biochemical indicators were <12% and <10%, respectively.
Migraine assessment
Characteristics of migraine, including headache severity, frequency and duration of attacks, were evaluated directly from the patients using a questionnaire.[28,29]
Headache intensity was measured using a visual analogue scale (VAS) questionnaire scored between 0 to 10, ranging from non-pain at a score of 0 to the highest possible level, scored as 10. The validity of this test has previously been confirmed in other trials.[30] The frequency and duration of migraine attacks were evaluated as the number of attacks per month and duration of attacks, respectively.
Statistical analysis
Data were expressed as mean ± SE.[21] The Shapiro-Wilk test was used to check the normal distribution of variables, and non-normality distributed data were subjected to natural logarithmic transformation. Independent sample t-test was used to show the basic differences between the two groups. For comparisons within and between the group values, paired t-test and MANCOVA test were utilized, respectively. MANCOVA test was used to eliminate the effect of confounding variables. Adjustment for the baseline values of clinical characteristics was performed. P < 0.05 was considered statistically significant. All statistical analyses were carried out using the Statistical Package for Social Sciences, version 16 (SPSS, Chicago, Illinois, USA).
Results
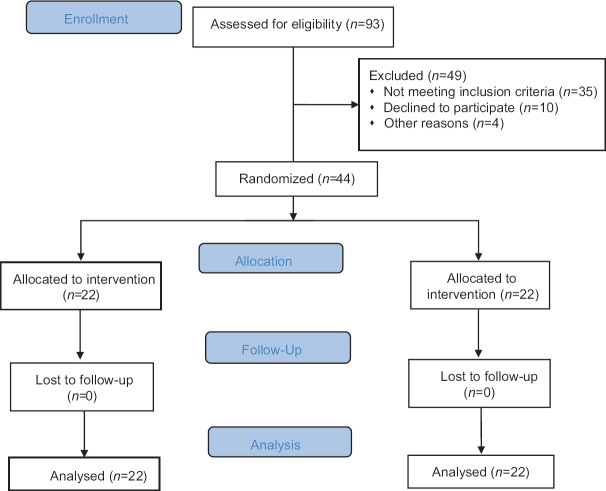
In total, 44 participants out of 93 individuals who met the eligibility criteria completed the trial [Figure 1]. Mean ± SE ages of participants in the intervention and control groups were 37 ± 1.7 vs 37 ± 1.5 years. Also, there were no remarkable differences in the baseline characteristics, including weight, BMI, PBF, and PBM between groups [Table 1]. In addition, no significant differences were observed in terms of energy, as well as macro- and micro-nutrients intake between the groups [Table 2].
Figure 1.
Flow chart of the study
Table 1.
Effect of curcumin supplementation on anthropometric measures
| Variables | Measurement period | Curcumin group n=22 | Placebo group n=22 | P 1 |
|---|---|---|---|---|
| Weight (kg) | Before | 72.14±3.1 | 68.87±1.83 | 0.37b |
| After | 72.59±3.37 | 69.76±1.89 | 0.47c | |
| P | 0.422a | 0.144a | - | |
| BMI (kg/m2) | Before | 27.50±1.3 | 25.8±0.78 | 0.276b |
| After | 28.22±1.3 | 26.48±0.81 | 0.28 | |
| - | 0.013a | 0.018a | P | |
| PBF (%) | Before | 38.84±2.09 | 35.39±1.34 | 0.172b |
| After | 39.34±2.09 | 35.58±1.39 | 0.14c | |
| P | 0.271a | 0.657a | - | |
| PBM (%) | Before | 25.82±0.58 | 25.27±0.59 | 0.510b |
| After | 25.99±0.53 | 25.64±0.49 | 0.63c | |
| P | 0.480a | 0.078a | - |
Data are presented as means±SE; Pa Result from paired samples t-test; Pb Result from independent t-test; Pc Result from Multivariate Analysis of Covariance (MANCOVA); Adjustment was made for pre-intervention values and energy intake. BMI: Body Mass Index; PBF: Percent Body Fat; PBM: Percent body Muscle
Table 2.
Dietary intake of participants
| Variables | Curcumin group (n=22) | Placebo group (n=22) | P* |
|---|---|---|---|
| Energy (kcal) | 2015±81 | 1991±56 | 0.81 |
| Carbohydrate (gr) | 275.35±14 | 260.5±10 | 0.78 |
| Protein (gr) | 75±7.1 | 70±3.7 | 0.392 |
| Fat (gr) | 68±5.4 | 66±3.9 | 0.68 |
| ß-carotene (µg) | 391.4±183 | 271.66±71.9 | 0.546 |
| Vitamin C (mg) | 95.129±14.3 | 86.22±15.6 | 0.677 |
| Vitamin E (mg) | 11.74±4.1 | 15.72±2.7 | 0.431 |
| Zinc (mg) | 7.38±0.61 | 7.7±0.68 | 0.729 |
| Selenium (µg) | 56.12±0.16 | 50.05±0.9 | 0.463 |
Data are expressed as mean±SE; *Result from independent t-test
Between-group analyses based on MANCOVA showed a significant reduction in serum CGRP levels in the curcumin group in comparison to the controls after intervention (P < 0.001). Moreover, there was significant reduction in serum IL-6 levels in curcumin group in comparison to the placebo group after intervention (P = 0.041). Furthermore, migraine symptoms, including severity and duration, showed significant reductions in the curcumin group in comparison to the control group after intervention, based on MANCOVA (P = 0.001, P = 0.007), respectively, while frequency of attacks showed a marginal decrease (P = 0.052) [Table 3].
Table 3.
Effect of curcumin supplementation on migraine symptoms and inflammatory markers
| Variables | Measurement Period | Curcumin group n=22 | Placebo group n=22 | P |
|---|---|---|---|---|
| IL-6 (ng/dL) | Before | 79.69±28 | 64.92±23.8 | 0.69b |
| after | 73.98±27 | 64.91±23.6 | 0.041c | |
| P a | 0.454a | 0.99a | - | |
| CGRP (ng/dL) | Before | 194.72±28 | 146±18 | 0.162b |
| after | 150±22 | 154±17 | <0.001c | |
| P a | <0.001a | 0.139a | - | |
| SEVERITY (VAS score) | Before | 7.59±0.19 | 7.18±0.2 | 0.153b |
| after | 3.6±0.34 | 6.27±0.34 | 0.001c | |
| P a | <0.001a | 0.002a | - | |
| FREQUENCY (per month) | Before | 11.3±1.4 | 8±0.96 | 0.075b |
| after | 6.6±1.1 | 7.2±0.88 | 0.052c | |
| P a | 0.014a | 0.051a | - | |
| DURATION (hours) | Before | 21.8±4.07 | 24.45±3.8 | 0.639b |
| after | 11.54±2.2 | 21.9±3.8 | 0.007c | |
| P a | 0.003a | 0.022a | - |
Data are presented as means±SE; Pa Result from paired samples t-test; Pb Result from independent t-test; Pc Result from Multivariate Analysis of Covariance (MANCOVA); Adjustment was made for pre-intervention values and energy intake. IL-6: Interleukin-6; CGRP: Calcitonin gene relating peptide; VAS: Visual Analogue Scale
Within-group analysis based on Paired sample t-test in curcumin group showed a significant reduction in CGRP level (P < 0.001), headache frequency (P = 0.014) and headache duration (P = 0.003) in curcumin group in comparison to the baseline, the reduction of IL-6 level was not significant (P = 0.454). Moreover, this analysis indicated no significant changes in placebo group in IL-6 (P = 0.99), CGRP (P = 0.139), headache frequency (P = 0.051) in comparison to pre-intervention. However, this change was only significant for headache severity and duration (P = 0.002, P = 0.022) respectively [Table 3].
Discussion
The results of this randomized, double-blind, placebo-controlled trial in migraine patients showed that supplementation of curcumin for 8 weeks could positively affect the migraine symptoms. To the best of our knowledge, this is the first study to evaluate the effects of curcumin on clinical features of migraine, including frequency, severity, and duration of migraine attacks. Our findings are also in agreement with the findings of earlier studies, that curcumin alone or in combination with other supplements might reduce the migraine symptoms.[16,18] In the present study, a marginally significant decrease in migraine frequency was observed which could also indicate that curcumin in combination with other compounds could exert a much greater reduction in attack frequency. Moreover, previous study used a nano-curcumin formula which is consisted of nanoparticles of curcumin that safely increases the absorption of curcumin 27 times more than that of curcumin powder.[18] This may also explain the more pronounced effect observed on attack frequency.
To assess the effect of prophylactic treatment (topiramate 50 mg) without the effects of curcumin on clinical features of migraine, we compared migraine symptoms before and after the intervention in the control group. Prophylactic treatment significantly improved migraine symptoms from baseline to the end of the study in the control group. However, the changes in the control group were lower than the test group (prophylactic treatment plus curcumin), indicating the elevated effects of curcumin in addition to prophylactic medications on migraine symptoms.
As to IL-6, within group analyses showed no significant results which could indicate that higher doses of curcumin are needed to see beneficial effects on inflammation. Also, it appears that patients with greater degrees of systemic inflammation are more likely to benefit from the anti-inflammatory effects of curcumin on IL-6.[31]
Previous studies have suggested that CGRP may be linked to the pathogenesis of migraine, and elevated levels of this marker have been observed in migraine patients.[19,32,33] CGRP, a pain mediator neuropeptide mostly produced in the trigeminal neurons of the nervous system, is a potent dilator of peripheral and cerebral blood vessels.[32] Furthermore, it stimulates inflammation via up-regulation of inflammatory cytokines.[34] Regarding the involvement of CGRP in the incidence of pain, its diminution by curcumin may represent a promising mechanism, based on the results observed in the present study. No prior studies were found that examined the effect of curcumin on CGRP in migraine patients, although different studies have revealed consistent findings on other disorders.[34] Furthermore, the present study is consistent with previous studies in which lower levels of CGRP or its antagonist could relieve migraine and improve its clinical symptoms, including severity and duration of headache.[35,36] The present findings are relatively in line with former studies conducted on nutraceuticals such as vitamin D, magnesium, CoQ10 and riboflavin.[28,30,37] It should be noted that CGRP showed no change in the controls within the 8 weeks of study despite using prophylactic agents and it rather even showed an insignificant augment. An explanation could be that blood samples may have been collected during the recurrence of migraine attacks.
Migraine has been associated with persistent vascular inflammation. Curcumin could alleviate migraine symptoms through its anti-inflammatory effects by upregulation of peroxisome proliferator-activated receptor-γ (PPAR-γ) activation.[18] On the other hand, the anti-nociceptive activity of curcumin is possible through its inhibitory action on nitro-oxidative stress, reducing NO synthesis and through its anti-inflammatory properties such as the reducing TNF-alpha release.[38]
Oxidative stress plays an important role in the pathogenesis of migraine. Curcumin is a powerful antioxidant, playing important roles in protection against reactive oxygen species injury and regeneration of other antioxidants. Furthermore, curcumin is a bifunctional antioxidant and exerts both direct and indirect antioxidant roles by scavenging reactive oxygen species and inducing the expression of antioxidant/detoxifying enzymes and scavengers such as superoxide dismutase, catalase, glutathione peroxidase, and heme oxygenase 1 in a nuclear factor E2-related factor 2 (Nrf2)-dependent pathway, respectively.[39,40]
Finally, evidence shows that the gut-brain axis may impact on migraine despite the mechanism explaining this interaction is not entirely clear. It can be hypothesized that supplementation with curcumin could lead to improvements in migraine associated features through beneficial effects on gut microbiota.[41,42]
This study has several strength points, including its randomized, double-blind placebo-controlled design; the measurement of CGRP levels as a novel biomarker in migraine management; and being one of the first attempts to examine the effects of curcumin supplementation as an stand-alone intervention for migraine therapy in humans.[43]
The study had some limitations that warrant more consideration. The most important limitations warrants its relatively short duration of study and also its small sample size. Another limitation was lack of controlling for dietary curcumin intake during the study. However, curcumin absorption from the gastrointestinal tract with normal dosage from capsules, tablets, and powders are very low due to its water insolubility. Finally, the absence of biochemical measures to evaluate participant adherence should be considered when interpreting the results.
Conclusions
Totally, it seems that curcumin might be considered as a complementary medicine for migraine patients. Further research is needed to extend the application and to understand the related mechanisms using the standard end points currently accepted in efficacy trials including having no headache pain and a demonstrated effect on the most bothersome migraine-associated symptom (self-reported) observed within at 2 hours post supplementation with curcumin.
This study was approved by School of Nutrition and Food Science, Isfahan University of Medical Sciences.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This work was supported by School of Nutrition and Food Science, Isfahan University of Medical Sciences (IR.MUI.REC.1396.2.086).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The study was part of Sheida Rezaie MSc thesis that was financially supported by a grant from the Vice Chancellor for research at Isfahan University of Medical Science, Isfahan, Iran [Grant No. 296086].
References
- 1.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton RB, Scher A, et al. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlovic JM, Akcali D, Bolay H, Bernstein C, Maleki N. Sex-related influences in migraine. J Neurosci Res. 2017;95:587–93. doi: 10.1002/jnr.23903. [DOI] [PubMed] [Google Scholar]
- 5.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, He Q, Ren Z, Li F, Chen W, Lin X, et al. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol Sci. 2015;36:535–40. doi: 10.1007/s10072-014-2010-3. [DOI] [PubMed] [Google Scholar]
- 7.D'Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35:135–40. doi: 10.1007/s10072-014-1757-x. [DOI] [PubMed] [Google Scholar]
- 8.Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M. Association of diet and headache. J Headache Pain. 2019;20:106. doi: 10.1186/s10194-019-1057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Hashel JY, Ahmed SF, Alshawaf FJ, Alroughani R. Use of traditional medicine for primary headache disorders in Kuwait. J Headache Pain. 2018;19:118. doi: 10.1186/s10194-018-0950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–25. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 11.Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors. 2013;39:69–77. doi: 10.1002/biof.1066. [DOI] [PubMed] [Google Scholar]
- 12.Shishodia S. Molecular mechanisms of curcumin action: Gene expression. Biofactors. 2013;39:37–55. doi: 10.1002/biof.1041. [DOI] [PubMed] [Google Scholar]
- 13.Bulboacă AE, Bolboacă SD, Stănescu IC, Sfrângeu CA, Bulboacă AC. Preemptive analgesic and antioxidative effect of curcumin for experimental migraine. BioMed Res Int. 2017:2017. doi: 10.1155/2017/4754701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulboacă AE, Bolboacă SD, Stănescu IC, Sfrângeu CA, Porfire A, Tefas L, et al. The effect of intravenous administration of liposomal curcumin in addition to sumatriptan treatment in an experimental migraine model in rats. Int J Nanomedicine. 2018;13:3093. doi: 10.2147/IJN.S162087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdolahi M, Sarraf P, Javanbakht MH, Honarvar NM, Hatami M, Soveyd N, et al. A novel combination of ω-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: A randomized clinical trial study. CNS Neurol Disord Drug Targets. 2018;17:430–8. doi: 10.2174/1871527317666180625101643. [DOI] [PubMed] [Google Scholar]
- 16.Abdolahi M, Tafakhori A, Togha M, Okhovat AA, Siassi F, Eshraghian MR, et al. The synergistic effects of ω-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-α gene expression and serum level in migraine patients. Immunogenetics. 2017;69:371–8. doi: 10.1007/s00251-017-0992-8. [DOI] [PubMed] [Google Scholar]
- 17.Abdolahi M, Honarvar NM, Tafakhori A, Sarraf P, Hatami M, Soveyd N, et al. The combined effects of Omega3 fatty acids and nano-curcumin supplementation on gene expression and serum levels of some inflammatory and endothelial factors in migraine patients: Study protocol for a randomized controlled trial. Int J Pharm Sci Invent. 2016;5:12–7. [Google Scholar]
- 18.Parohan M, Sarraf P, Javanbakht MH, Foroushani AR, Ranji-Burachaloo S, Djalali M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Nutr Neurosci. 2019:1–10. doi: 10.1080/1028415X.2019.1627770. doi: 10.1080/1028415X.2019.1627770. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1(Suppl 1)):S3–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olesen J. International classification of headache disorders. Lancet Neurol. 2018;17:396–7. doi: 10.1016/S1474-4422(18)30085-1. [DOI] [PubMed] [Google Scholar]
- 21.Panahi Y, Sahebkar A, Parvin S, Saadat A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann Clin Biochem. 2012;49:580–8. doi: 10.1258/acb.2012.012040. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi A, Sahebkar A, Iranshahi M, Amini M, Khojasteh R, Ghayour-Mobarhan M, et al. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother Res. 2013;27:374–9. doi: 10.1002/ptr.4715. [DOI] [PubMed] [Google Scholar]
- 23.Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendía LE, Majeed M, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016;82:578–82. doi: 10.1016/j.biopha.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Panahi Y, Sahebkar A, Amiri M, Davoudi SM, Beiraghdar F, Hoseininejad SL, et al. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: Results of a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2012;108:1272–9. doi: 10.1017/S0007114511006544. [DOI] [PubMed] [Google Scholar]
- 25.Jagdale SC, Phule PS, Chavan GJ. Formulation and evaluation of modified pulsincap drug delivery system of rizatriptan benzoate. Int J Pharm Pharm Sci. 2014;6:48–52. [Google Scholar]
- 26.Su M, Ran Y, Han X, Liu Y, Zhang X, Tan Q, et al. Rizatriptan overuse promotes hyperalgesia induced by dural inflammatory stimulation in rats by modulation of the serotonin system. Eur J Neurosci. 2016;44:2129–38. doi: 10.1111/ejn.13296. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara L. Nutrition and phytotherapy: A winning combination against headache. Int J Med Rev. 2019;6:6–13. [Google Scholar]
- 28.Parohan M, Sarraf P, Javanbakht MH, Ranji-Burachaloo S, Djalali M. Effect of coenzyme Q10 supplementation on clinical features of migraine: A systematic review and dose–response meta-analysis of randomized controlled trials. Nutr Neurosci. 2019:1–8. doi: 10.1080/1028415X.2019.1572940. doi: 10.1080/1028415X.2019.1572940. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Martami F, Togha M, Seifishahpar M, Ghorbani Z, Ansari H, Karimi T, et al. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia. 2019;39:841–53. doi: 10.1177/0333102418820102. [DOI] [PubMed] [Google Scholar]
- 30.Gaul C, Diener H-C, Danesch U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:32. doi: 10.1186/s10194-015-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derosa G, Maffioli P, Simental-Mendia LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;111:394–404. doi: 10.1016/j.phrs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Dahri M, Tarighat-Esfanjani A, Asghari-Jafarabadi M, Hashemilar M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr Neurosci. 2019;22:607–15. doi: 10.1080/1028415X.2017.1421039. [DOI] [PubMed] [Google Scholar]
- 33.Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81:1191–6. doi: 10.1212/WNL.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- 34.Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother Res. 2014;28:1461–7. doi: 10.1002/ptr.5149. [DOI] [PubMed] [Google Scholar]
- 35.Ho T, Mannix L, Fan X, Assaid C, Furtek C, Jones C, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–12. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 36.Olesen J, Diener H-C, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene–related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 37.Mottaghi T, Askari G, Khorvash F, Maracy MR. Effect of vitamin D supplementation on symptoms and C-reactive protein in migraine patients. J Res Med Sci. 2015;20:477–82. doi: 10.4103/1735-1995.163971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–61. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Hoppe U, Bergemann J, Diembeck W, Ennen J, Gohla S, Harris I, et al. Coenzyme Q10, a cutaneous antioxidant and energizer. Biofactors. 1999:9, 371-. doi: 10.1002/biof.5520090238. [DOI] [PubMed] [Google Scholar]
- 40.Tapia E, Soto V, Ortiz-Vega KM, Zarco-Márquez G, Molina-Jijón E, Cristóbal-García M, et al. Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell Longev. 2012;2012:269039. doi: 10.1155/2012/269039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arzani M, Jahromi SR, Ghorbani Z, Vahabizad F, Martelletti P, Ghaemi A, et al. Gut-brain Axis and migraine headache: A comprehensive review. J Headache Pain. 2020;21:15. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zam W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J Nutr Metab. 2018;2018:1367984. doi: 10.1155/2018/1367984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: Results from randomised multicentre DASH-Sodium clinical trial. BMJ Open. 2014;4:e006671. doi: 10.1136/bmjopen-2014-006671. [DOI] [PMC free article] [PubMed] [Google Scholar]