Abstract
The current COVID-19 pandemic caused by constantly emerging SARS-CoV-2 variants still poses a threat to public health worldwide. Effective next-generation vaccines and optimized booster vaccination strategies are urgently needed. Here, we sequentially immunized mice with a SARS-CoV-2 wild-type inactivated vaccine and a heterologous mutant RBD vaccine, and then evaluated their neutralizing antibody responses against variants including Beta, Delta, Alpha, Iota, Kappa, and A.23.1. These data showed that a third booster dose of heterologous RBD vaccine especially after two doses of inactivated vaccines significantly enhanced the GMTs of nAbs against all SARS-CoV-2 variants we tested. In addition, the WT and variants all displayed good cross-immunogenicity and might be applied in the design of booster vaccines to induce broadly neutralizing antibodies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-021-01737-3.
Keywords: SARS-CoV-2 variant, Inactivated vaccine, Mutant RBD vaccine, Sequential immunization, Broadly neutralizing antibody
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to more than 200 million infections and 4 million deaths worldwide since the outbreak began. To date, approximately 18 kinds of SARS-CoV-2 vaccines, including inactivated, mRNA, DNA, adenovirus vector, and recombinant subunit vaccines, have been developed to control the pandemic, some of which have received a wide range of approvals for use [1]. However, with the emergence of various SARS-CoV-2 variants, the protection afforded by current vaccines has decreased significantly, which has been proven for several waves of the COVID-19 pandemic caused by Alpha, Beta, Iota, and Kappa variants. Especially, the Delta variant has spread rapidly to many countries and become the predominant variant worldwide. These SARS-CoV-2 variants continuously threaten global public health. Therefore, it is critical to develop more efficient vaccines and immunization strategies for controlling the COVID-19 pandemic and preventing breakthrough infection of variants after vaccination.
A third booster dose based on conventional two-dose vaccination is usually considered as a direct and effective strategy to enhance the immune responses such as the titer of neutralizing antibodies (nAbs). Recently, clinical data have shown that a third dose of homologous mRNA vaccine could substantially reduce the new viral infection and the risk of severe illness [2]. As compared with homologous boosters, the heterologous prime-boost strategy may improve the levels of nAbs more effectively [3]. However, it is unclear whether the sequential immunization with vaccines based on different platforms could increase the antibody response to SARS-CoV-2 variants. Two recent studies have made important strides toward enhancing broadly neutralizing activities against SARS-CoV-2 variants by heterologous ChadOx1/BNT162b2 vaccination, a combination of adenovirus-vectored vaccine and mRNA vaccine [4, 5]. However, the immunogenicity and efficacy of other vaccine combinations have not been comprehensively analyzed, especially using the SARS-CoV-2 variant-based vaccine. Two doses of inactivated vaccines are widely used vaccines but which type of booster vaccines have not been reported.
In this study, we sequential immunized mice with a wild-type (WT) inactivated vaccine and a mutant receptor-binding domain (RBD) vaccine, and evaluated the neutralizing antibody response to SARS-CoV-2 WT strain or variants including Beta, Delta, Alpha, Iota, Kappa, or A.23.1, which will offer more options choices in vaccination strategies to control the current COVID-19 pandemic.
Materials and methods
Animals, immunization and serum sample collection
To assess the immunogenicity of the SARS-CoV-2 vaccines, 80 specific pathogen-free (SPF) female BALB/c mice were randomly divided into 8 groups (n = 10) and then immunized intraperitoneally with the SARS-CoV-2 inactivated vaccine or RBD subunit vaccines at an interval of 2 weeks in different procedures or immunized with aluminum adjuvant, which served as a control. No adverse events were observed throughout the experiment. The serum samples were collected 14 days after the last dose and heat inactivated at 56 °C for 30 min before use.
SARS-CoV-2 pseudovirus-based neutralization assay
SARS-CoV-2 pseudovirus was generated by cotransfection of HEK-293 T cells with a SARS-CoV-2 spike-expressing plasmid and an env-deficient HIV-1 backbone vector (pNL4-3.Luc.R-E-). Two days post-transfection, the culture supernatant was harvested, clarified by centrifugation, filtered and stored at − 80 °C. To determine the neutralizing activity, serially diluted serum samples were incubated with an equal volume of SARS-CoV-2 pseudovirus at 37 °C for 1 h. HEK-293 T-hACE2 cells were subsequently added to the plates. After a 48 h incubation, the culture medium was removed, and 100 μL of Bright-Lite Luciferase reagent (Vazyme Biotech) was added to the cells. After a 2-min incubation at RT, 90 μL of cell lysate was transferred to 96-well white solid plates to measure luminescence using a Varioskan™ LUX multimode microplate reader (Thermo Fisher Scientific). The 50% inhibitory dilution (ID50) was calculated using GraphPad Prism 8.0 software by the log (inhibitor) versus normalized response—variable slope (four parameters) model.
Main text
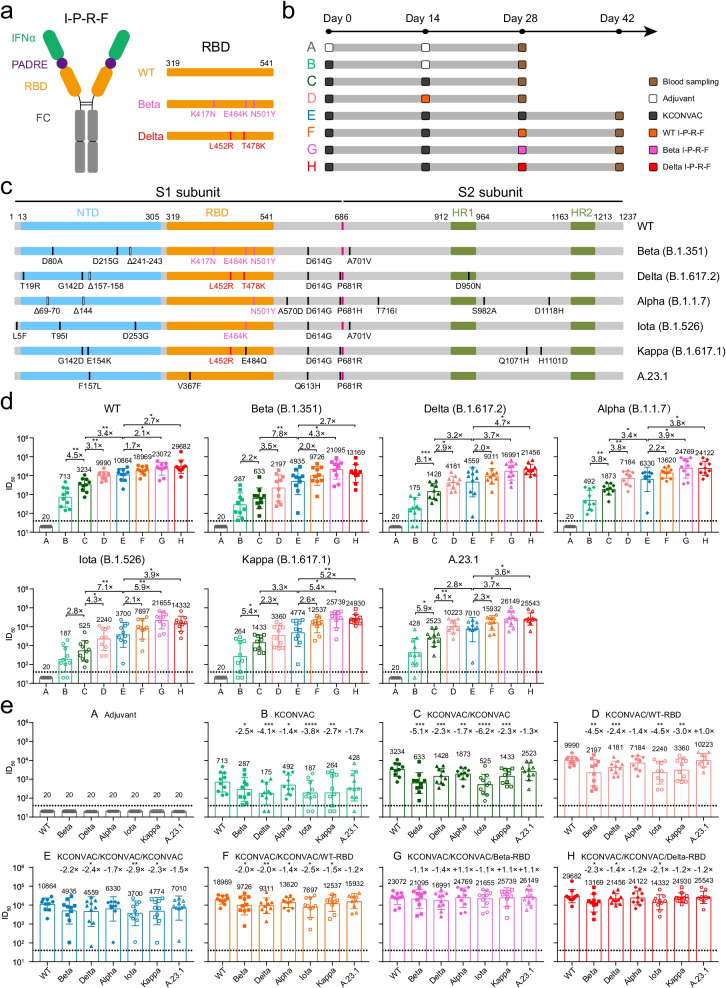
Previously, Sun et al. constructed an interferon-armed RBD dimer vaccine inducing the production of high titers of long-lasting nAbs in mice and rhesus macaques [6]. Here, we acquired three kinds of RBD dimer vaccines carrying WT, Beta, and Delta RBD proteins from the Peng group (Fig. 1a) and obtained a WT inactivated vaccine from Shenzhen Kangtai Biological Products Co., Ltd. [7]. To be paralleled with the clinical trials, we immunized mice with a human IFN-Pan-RBD-human Fc containing one mutation in IFN for cross-binding to the mouse IFN receptor (named I-P-R-F, or V-01). Although V-01 binds to the mouse IFN receptor with very low affinity and mediates weaker immune response than V-01 in human, we still observed significant booster effect of V-01 for the inactivated vaccine. As shown in the schematic (Fig. 1b), 80 female mice were randomly divided into 8 groups. In Group A, 10 mice received two doses of aluminum adjuvant and served as a negative control. In Groups B, C, and E, 30 mice were injected with one, two, or three doses of 1 μg of the inactivated vaccine, respectively. The rest of the mice were sequentially immunized with same doses of 1 μg of the inactivated vaccine and RBD vaccines: one dose of inactivated vaccine and a WT RBD booster in Group D and two doses of inactivated vaccine followed by a WT, Beta, or Delta RBD booster in Groups F, G, and H, respectively.
Fig. 1.
Potent and broad neutralization against SARS-CoV-2 variants elicited by sequential immunization with a wild-type inactivated vaccine and a mutant RBD subunit vaccine in mice. a Schematic diagram of the RBD dimer vaccine named I-P-R-F [6]. The structural elements show IFNα, Pan-DR epitope (PADRE), RBD, and immunoglobulin Fc fragment. The RBD regions of three kinds of I-P-R-F, including WT and Beta and Delta variants, are shown on the right, and mutations are labeled as sticks. b Schematic representation of the immunization schedule and blood sample collection of mice in 8 groups (Groups A-H, n = 10 in each group). c Schematic representation of mutations located in viral spike proteins of the Beta, Delta, Alpha, Iota, Kappa, and A.23.1 variants. d Neutralization titers against SARS-CoV-2 WT and variants of vaccine-elicited mouse serum. The data are shown for different pseudoviruses. e Neutralization titers against SARS-CoV-2 WT and variants of vaccine-elicited mouse serum. The data are shown for different groups and all comparisons are made to the WT readings. Geometric mean titers, in ID50 values, were calculated and are written in each column. The error bars are standard deviations, and the symbols represent individual mice. The dotted line indicates the limit of detection (1:40 dilution) for the assay. Non-neutralizing serum was assigned a value of one-half of the limit of detection for visualization (1:20 dilution). Statistical analysis was performed with paired or unpaired t tests using GraphPad Prism 8.0 software. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Based on the HIV-1 backbone, we constructed a series of SARS-CoV-2 pseudoviruses bearing spike proteins of the WT strain or Beta, Delta, Alpha, Iota, Kappa, or A.23.1 variant (Fig. 1c). A substitution at the E484 residue was found in the RBD region of the Beta, Iota, and Kappa variants. The Beta and Alpha variants shared a common mutation of N501Y. Both the Delta and Kappa variants carried the L452R mutation. This phenomenon of sharing common mutations suggests that viral infection or vaccination might induce cross neutralization against SARS-CoV-2 variants. Two weeks after the last vaccination, all serum samples were collected and then tested by a pseudovirus neutralizing assay (Fig. 1d, Additional file 1: Fig. S1, and Additional file 1: Table S1). In Groups B, C, and E, the geometric mean titers (GMTs) of nAbs in vaccinated mice gradually increased with the increase in the number of immunizations.
It is widely known that SARS-CoV-2 uses its RBD to recognize the host cell receptor (angiotensin-converting enzyme 2, ACE2). Therefore, the RBD should be a good immunogen and could trigger an effective antibody response [8, 9]. Compared with the homologous booster, sequential immunization with inactivated vaccine and RBD vaccine indeed induced more potent neutralization against SARS-CoV-2 WT and mutant pseudoviruses (Group D vs. Group C, Group F vs. Group E). Considering that the Beta variant exhibited the greatest reduction in neutralizing activity thus far and that the Delta variant dominated the current wave of the COVID-19 pandemic worldwide, we also evaluated the neutralizing antibody responses elicited by a third booster of the Beta-RBD vaccine or Delta-RBD vaccine. As shown in Fig. 1d, the GMTs of serum nAbs against WT SARS-CoV-2 and variants in Group G or Group H were the highest among all immunization strategies, suggesting that mutant RBD vaccines following two doses of inactivated vaccines could induce more broadly neutralizing antibody responses against variants.
To further analyze the spectrum of serum nAbs elicited by all of the aforementioned immunization strategies, we adjusted these neutralization results by different groups to compare their neutralizing activities against SARS-CoV-2 variants. As shown in Fig. 1e, serum nAb levels of mice that received either one or two doses of vaccines (Groups B, C, and D) significantly declined against variants including Beta, Delta, Iota, and Kappa. Similar to previous reports [10, 11], the L452R and E484K/Q mutations in the RBD contributed to the significant decline in neutralizing activities. In contrast, the Alpha or A.23.1 variant carrying a single mutation (N501Y or V367F, respectively) in the RBD slightly escaped the neutralization of vaccine-induced serum, which was also consistent with other studies [12, 13]. Encouragingly, the serum of mice that received three doses of vaccines (Groups E, F, G, and H) maintained quite a high level of nAbs against all tested SARS-CoV-2 variants, with degrees of neutralization that declined less than threefold compared to that against WT. Notably, two doses of WT inactivated vaccines followed by a third booster of Beta-RBD vaccine elicited the most broadly neutralizing antibody response in mice of Group G. All SARS-CoV-2 variants we tested did not escape from the neutralization of serum elicited by inactivated/inactivated/Beta-RBD vaccines. Overall, in this study, although the viral clearance from various organs, histopathology studies, and T-cell responses are not investigated due to the limitation of live virus and animal model, the data based on the neutralizing antibody responses are still certainly to prove that either WT or mutant RBD might be a suitable candidate for the design of a next-generation broad vaccine. There may be no need to generate each SARS-CoV-2 variant, which will save resources and time that require to develop each variants.
Conclusion
In conclusion, our results demonstrated that sequential immunization with the SARS-CoV-2 inactivated vaccine and RBD subunit vaccine indeed induced potent and broad neutralization against variants. Currently, a large number of people around the world have received two doses of inactivated vaccines but are still at the risk of breakthrough infections by various SARS-CoV-2 variants. Based on our data in mice, a third booster of a RBD vaccine following two doses of widely used WT inactivated vaccines should be a good choice and an effective strategy to combat SARS-CoV-2 variants and control the current COVID-19 pandemic.
Supplementary Information
Additional file 1. Figure S1. Neutralization curves against SARS-CoV-2 WT and variants of vaccine-elicited mice serum and a positive control mAb. All serum samples were serially 3-fold diluted from 1:40. A positive control mAb (P2C-1F11) was serially 3-fold diluted from 5 μg/ml. A 50% reduction in viral infectivity was indicated by a horizontal dashed line. Table S1. The geometric mean titers of vaccine-elicited mice serum against SARS-CoV-2 WT and variants.
Acknowledgements
We thank Professor Hua Peng in Chinese Academy of Sciences and LivzonBio, Inc. for providing the SARS-CoV-2 RBD subunit vaccines and also thank Shenzhen Kangtai Biological Products Co., Ltd. for providing the SARS-CoV-2 inactivated vaccine.
Abbreviations
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- nAb
Neutralizing antibody
- ACE2
Angiotensin-converting enzyme 2
- RBD
Receptor binding domain
- WT
Wild type
Authors' contributions
ZZ is the principal investigator of this study. ZZ, BJ, and YF conceived and designed the study. SS and BZ performed all experiments together with assistance from LC, WL, QF, and XG. ZZ, BJ, YF, and HP wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Science Fund for Distinguished Young Scholars (82025022), the National Natural Science Foundation of China (82002140, 82171752, 82101861), the Guangdong Basic and Applied Basic Research Foundation (2021B1515020034, 2019A1515011197, 2021A1515011009, 2020A1515110656), and the Shenzhen Science and Technology Program (RCYX20200714114700046, JSGG20200207155251653, JSGG20200807171401008, KQTD20200909113758004, JCYJ20190809115617365).
Availability of data and materials
We are happy to share reagents and information in this study upon request.
Declarations
Ethics approval and consent to participate
All animal experiments in mice were approved by the Ethics Committee of Shenzhen Third People’s Hospital, China. All procedures were conducted in accordance with animal ethics guidelines and approved protocols to minimize suffering during vaccination, blood collection, and euthanasia.
Consent for publication
All authors approved the submission of the manuscript for publication.
Competing interests
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuo Song and Bing Zhou contributed equally to this work
Contributor Information
Hua Peng, Email: yangxinfu@tsinghua.edu.cn.
Yang-Xin Fu, Email: yangxinfu@tsinghua.edu.cn.
Bin Ju, Email: jubin2013@163.com.
Zheng Zhang, Email: zhangzheng1975@aliyun.com.
References
- 1.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021 doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-On YM, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Q, et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect. 2021;10:629–637. doi: 10.1080/22221751.2021.1902245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozzetto B, et al. Immunogenicity and efficacy of heterologous ChadOx1/BNT162b2 vaccination. Nature. 2021 doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 5.Barros-Martins J, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun S, et al. Interferon-armed RBD dimer enhances the immunogenicity of RBD for sterilizing immunity against SARS-CoV-2. Cell Res. 2021;31:1011–1023. doi: 10.1038/s41422-021-00531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan HX, et al. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chin Med J (Engl) 2021;134:1289–1298. doi: 10.1097/CM9.0000000000001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020 doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 9.Dai L, et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell. 2020 doi: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jangra S, et al. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021;2:e283–e284. doi: 10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021 doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L, et al. Impact of the N501Y substitution of SARS-CoV-2 Spike on neutralizing monoclonal antibodies targeting diverse epitopes. Virol J. 2021;18:87. doi: 10.1186/s12985-021-01554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreno JM, et al. Evidence for retained spike-binding and neutralizing activity against emerging SARS-CoV-2 variants in serum of COVID-19 mRNA vaccine recipients. EBioMedicine. 2021;73:103626. doi: 10.1016/j.ebiom.2021.103626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1. Neutralization curves against SARS-CoV-2 WT and variants of vaccine-elicited mice serum and a positive control mAb. All serum samples were serially 3-fold diluted from 1:40. A positive control mAb (P2C-1F11) was serially 3-fold diluted from 5 μg/ml. A 50% reduction in viral infectivity was indicated by a horizontal dashed line. Table S1. The geometric mean titers of vaccine-elicited mice serum against SARS-CoV-2 WT and variants.
Data Availability Statement
We are happy to share reagents and information in this study upon request.