Abstract
Background
We aimed to evaluate the effect of low doses (LD) bone morphogenetic protein‐2 (BMP2) and BMP4 micro‐immunotherapy (MI) in two in vitro models of periodontal wound healing/regeneration.
Methods
We first evaluated the effect of LD of BMP2 and BMP4 MI on a 2D cell culture using human gingival fibroblasts (hGF) under inflammatory conditions induced by IL1β. Biocompatibility, inflammatory response (Prostaglandin E2 (PGE2) release), collagen deposition and release of extracellular matrix (ECM) organization‐related enzymes (matrix metalloproteinase‐1 (MMP1) and metalloproteinase inhibitor 1 (TIMP1)) were evaluated after short (3 days) and long‐term (24 days) treatment with BMP2 or BMP4 MI. Then, given the results obtained in the 2D cell culture, LD BMP4 MI treatment was evaluated in a 3D cell culture model of human tissue equivalent of gingiva (GTE) under the same inflammatory stimulus, evaluating the biocompatibility, inflammatory response and effect on MMP1 and TIMP1 release.
Results
LD BMP4 was able to decrease the release of the inflammatory mediator PGE2 and completely re‐establish the impaired collagen metabolism induced by IL1β treatment. In the 3D model, LD BMP4 treatment improved tissue viability compared with the vehicle, with similar levels to 3D tissues without inflammation. No significant effects were observed on PGE2 levels nor MMP1/TIMP1 ratio after LD BMP4 treatment, although a tendency to decrease PGE2 levels was observed after 3 days.
Conclusions
LD BMP4 MI treatment shows anti‐inflammatory and regenerative properties on hGF, and improved viability of 3D gingiva under inflammatory conditions. LD BMP4 MI treatment could be used on primary prevention or maintenance care of periodontitis.
Keywords: bone morphogenetic protein 4, gingiva, in vitro technique, periodontitis, bone morphogenetic protein 2
1. INTRODUCTION
Periodontal disease is defined as a set of infectious oral inflammatory conditions that affect the supporting tissues of the teeth, including soft tissues such as gingiva and periodontal ligaments and hard tissues such as the alveolar bone. 1 Periodontitis is the most advanced, irreversible and destructive form of periodontal disease, which leads to progressive attachment loss and bone destruction due to this acute (sometimes aggressive) or chronic inflammation. 2 , 3 , 4
Pathogenesis of chronic periodontitis involves a cascade of sequential events, leading to host immunomodulatory responses which in turn activate various cytokines, chemokines and pro‐inflammatory mediators responsible for the progressive destruction of underlying gingival tissues and subsequent tooth loss. 3 , 5 Thus, enhanced periodontal tissue regeneration by suppressing the inflammatory response and stimulating regeneration of the destroyed tissues, is the ultimate goal of periodontal therapy.
Bone morphogenetic proteins (BMPs) constitute the largest subgroup of the transforming growth factor‐β (TGFβ) superfamily of cytokines, 5 , 6 which are well known for their osteoinductive potential in periodontal hard tissue regeneration. 7 , 8 Indeed, studies confirmed the specific importance of bone morphogenetic protein‐2 (BMP2) and bone morphogenetic protein‐4 (BMP4) (subfamily with an 80% of homology) as power inductors of the osteogenic activity. 9 , 10 , 11 , 12 Nevertheless, extensive research has shown that their effects can go far beyond the induction of bone formation. 6 , 13 , 14 Thus, BMPs have been shown to be biologically active in periodontal ligament cells, mesenchymal cells and in cells of the periodontal soft tissue. 15
BMP2 is currently the only Food and Drug Administration (FDA)‐approved osteoinductive growth factor used as a bone graft substitute. 16 However, with increasing clinical use of BMP2, a growing and well‐documented side effect profile has emerged, including postoperative inflammation and associated adverse effects, ectopic bone formation, osteoclast‐mediated bone resorption, and inappropriate adipogenesis. 11 Thus, to overcome safety concerns for the use of growth factors in therapeutics, an alternative would be the use of very low doses (LD). In any case, dose optimization is still needed for the use of BMPs for periodontal regeneration and repair. 9 , 10 , 11
Micro‐immunotherapy (MI), is a therapeutic approach that uses active substances such as cytokines, hormones, growth factors, neuropeptides, nucleic acids, and specific nucleic acids (SNAs) at LD and ultra‐low doses (ULD) to target the immune system and regulate immune responses in diseases, thereby reducing side effects and safety concerns. 17 , 18 , 19 , 20 , 21 This therapy is administered sublingually in the oral cavity, which could be interesting as adjunctive treatment in the maintenance phase of supportive periodontal therapy or as preventive treatment 22 , 23 , due to the proximity to gingival tissues, allowing to act both locally and systemically.
Gingival fibroblasts are the major constituents of gingiva, being responsible for the production and regeneration of the matrix constituents that support the tooth. 24 , 25 In vitro studies on gingival tissues have mainly been done in two‐dimensional cultures (2D), with monolayer cultures of epithelial or gingival fibroblasts. 26 However, monolayer models lack polarized cell phenotype and systemic components, which affect their function and response to stimuli. 27 To overcome these limitations several three‐dimensional (3D) oral mucosa models have been developed, providing a higher degree of complexity than monolayer cell cultures being closer to explant cultures, closely able to resemble the in vivo situation. 26 , 27 , 28
In the present study, we aimed at evaluating the effect of LD BMP2 and BMP4 used in MI for periodontal applications. First, a 2D cell culture set‐up using human gingival fibroblasts (hGF) was used to evaluate cytotoxicity, inflammatory response (human Prostaglandin E2 (PGE2) release), collagen deposition and release of extracellular matrix (ECM) organization‐related enzymes (matrix metalloproteinase‐1 (MMP1) and metalloproteinase inhibitor 1 (TIMP1)). Then, given the results obtained in the 2D cell culture with hGF, LD BMP4 was evaluated in a 3D cell culture model of human tissue equivalent of gingiva (GTE), evaluating biocompatibility, inflammatory response and effect on MMP1 and TIMP1 release. In both models, a periodontitis condition was simulated by using interleukin 1 beta (IL1β) as inflammatory stimulus.
2. MATERIALS AND METHODS
2.1. Tested micro‐immunotherapy medicine (MIM)
The MIM were manufactured by Labo'Life Belgium and are notified to the Belgian Federal Agency for Medicines and Health Products (FAMHP) under notification number 1507 UH 106 F33 (BMP2 5CH) and 1507 UH 107 F33 (BMP4 5CH). The active substances present in the tested MIM composition are BMP2 and BMP4. Each active substance goes through a specific GMP manufacturing process allowing to reach LD or ULD, following a “Serial Kinetic Process” (SKP). The SKP consists of a 1/100 dilution process followed by vertical shaking. SKP is reproduced a defined number of times depending on the LD to be reached; and that is reported in the composition of the MIM as the number of Centesimal Hahnemannian dilutions (CH). 19 The excipient consists of lactose‐sucrose pillules which were impregnated with the ethanolic preparation of BMP2 or BMP4 at 5CH (4.67 × 10–13 µg/capsule). The vehicle, used as controls, consists of lactose‐sucrose pillules impregnated with the ethanolic preparation only, without active substance.
2.2. 2D model
2.2.1. hGF cell culture
Primary hGF§ (27 years, Caucasian, female, lot number 313 × 100401) were used. All donors provided written informed consent, and the investigators were not able to ascertain the identity of the donors. The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. hGF cells were routinely cultured at 37°C and 5% CO2 in DMEM low glucose**, supplemented with 10%(v/v) fetal calf serum††, 100 µg/mL penicillin and 100 µg/mL streptomycin‡‡ and 50 µg/mL ascorbic acid§§, refreshed in each media change. Cells were seeded in 48‐well plates at a density of 2.0 × 104 cells/well. At confluence, cells were treated as detailed above. The experiment was run in six sample replicates (n = 6) for each group.
2.2.2. Treatment with the MIM
A blind study was performed using the vehicle and two treatments containing LD BMP2 and BMP4. In addition, uninflamed non‐treated cells were also tested in parallel. In order to establish inflammatory conditions, after seeding, cells were incubated with 1 ng/mL IL1β*** except the uninflamed non‐treated control. To treat the cells, all the pillules contained in 1 capsule (380 mg) were dissolved in 50 mL of media (1X concentration). At confluence, hGF cells were treated every other day for 24 days using freshly prepared treatments as previously described.
2.2.3. Cell cytotoxicity
Lactate dehydrogenase (LDH) activity in the culture media was used as an index of cell death. LDH activity was determined from culture media after 3 days of treatment, following the manufacturer's instructions†††. Results are presented relative to the LDH activity in the medium of non‐treated cells cultured in tissue culture plastic (negative control, 0% of cell death) and of cells growing on tissue culture plastic treated with 1% non‐ionic surfactant‡‡‡ (positive control, 100% of cell death), using the equation: Cytotoxicity(%) = (exp.value–negative control)/(positive control–negative control) × 100. The experiment was run in six sample replicates (n = 6) for each group.
2.2.4. Collagen deposition
Collagen deposition was evaluated as a marker of differentiation of cultured hGF. Ascorbic acid was used to aid collagen deposition. After 24 days of cell culture, cells were washed with PBS, dried for 1 hour at RT, followed by an incubation for 1 hour at −80°C. Then, cells were dried overnight at 37°C in a humidified atmosphere, followed by 24 hours at 37°C in a dry atmosphere. Collagen was stained with 0.1% Sirius Red F3BA§§§ in saturated picric acid for 1 hour at RT. Unbounded dye was removed with 10 mM HCl washes, and the dye was solubilized with 0.1 M NaOH. Absorbance was measured at 540 nm. Readings were compared with control untreated cells which were set to 100%. The experiment was run in six sample replicates (n = 6) for each group.
2.2.5. Enzyme‐linked immunosorbent assays
PGE2****, MMP1†††† and TIMP1‡‡‡‡ were evaluated from cell culture media at days 3 and 24 of treatment (n = 6) following the manufacturer's instructions.
2.3. 3D Model
2.3.1. Cell culture
Immortalized Human Gingival Fibroblasts‐hTERT (iHGF)§§§§ were grown at 37°C in an atmosphere of 5% CO2 using fibroblast medium that consisted in Dulbecco's modified Eagle's medium (DMEM) low glucose /Ham's F12 (3/1), supplemented with 10%(v/v) fetal bovin serum embryonic stem cells tested (FBS) and 100 µg/mL penicillin, and 100 µg/mL streptomycin***** . The culture media was renewed twice per week.
Immortalized Human Gingival Keratinocytes (iHGK)††††† were routinely cultured on tissue culture flasks for sensitive adherence cells‡‡‡‡‡ at 37°C and 5% CO2 cultured in keratinocyte medium, that consisted in DMEM without magnesium and calcium§§§§§/Ham's F12 (3/1), supplemented with 0.01 mg/mL insulin; 0.4 ng/mL hydrocortisone; 6.7 ng/mL selenium******; 0.01 µg/mL Human Epithelial Growth factor††††††, 1 M HEPES‐buffer, 5.5 µg/mL transferrin; 10−10 M cholera toxin; 2 mM L‐glutamine‡‡‡‡‡‡; 5%(v/v) FBS and 100 µg/mL penicillin and 100 µg/mL streptomycin. The culture media was renewed twice per week. Cultures at 70% to 80% confluency were used for the construction of Gingival Tissue (GTE) as described above.
2.3.2. Engineering 3D Gingival Tissue Equivalent (GTE)
The GTE was constructed according to the technique described by Dongari Bagtzoglou and Kashleva 29 with some modifications (M. Munar‐Bestard; et al, article in preparation, 2020). In short, a rat tail type I collagen solution§§§§§§ (2.2 mg/mL) was mixed with iHGF (1 × 105cells/mL) and pipetted into a 24‐well transwell insert with 0.4 mm pores. The fibroblast‐embedded collagen was cultured for 7 days submerged in fibroblast medium. iHGK (2.2 × 105cells/well) were then seeded on top and GTE were cultured submerged in Keratinocyte medium for 3 days. GTE were then lifted to the air–liquid interface and cultured for 15–17 days in differentiation medium consisting of DMEM low glucose/Ham's F12 (3/1), supplemented with 5 µg/mL insulin; 0.4 µg/mL hydrocortisone; 2 × 10−11M 3,3′, 5‐triiodo‐L‐thyronine (T3); 1.8 × 10−4M adenine; 5 µg/mL transferrin;10−10M cholera toxin; 2mM L‐glutamine; 5%(v/v) FBS; 100 µg/mL penicillin and 100 µg/mL streptomycin.
2.3.3. Treatment with the MIM
A blind study was performed using the vehicle and the treatment containing BMP4 that was selected according to the previous 2D‐study results. Non‐treated tissues were also tested in parallel. In order to establish inflammatory conditions, air‐lifted GTEs were treated on top of the tissue with 30 µl of 1 ng/mL IL1β the first day of treatment except for the non‐treated control. To treat the GTEs, all the pillules contained in 1 capsule (380 mg) were dissolved in 25 mL of PBS (2X concentration). Air‐lifted tissues were treated daily with 30 µl on top of the tissues for 10 days using freshly prepared treatments as previously described. The experiment was run in six sample replicates (n = 6) for each group.
2.3.4. MTT test
At day 11 after culture, the tissues were rinsed with PBS and placed on 400 µl of 0.5 mg/mL MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide)*******. After 3 hours of incubation at 37°C and 5% CO2, cultures were placed in 2 mL of isopropanol overnight at RT and absorbance was measured at 570 nm. Positive control was obtained from tissues treated with PBS and was set at 100%. Negative control was obtained from tissues treatment with 5% Sodium dodecyl sulfate (SDS) diluted in PBS (1:1). Results are expressed as percentage of viability compared with negative control. The experiment was run in two sample replicates (n = 2) for each group.
2.3.5. Enzyme‐linked immunosorbent assays
Cell culture supernatants after 3 and 11 days of treatment were used to determine PGE2, MMP1, and TIMP1 as detailed in section 2.2.5.
2.3.6. Histology and immunohistochemistry
The GTEs were fixed in 4% formaldehyde and paraffin embeded. Paraffin sections (6 µm) were cut and stained with Hematoxylin and Eosin (H&E) for histological examination or processed for immunohistochemistry (IHC). The sections were dewaxed in xylene (30 minutes) and rehydrated through 100% and 70% alcohol series for 5 minutes each. The antigens were retrieved with 0.01M citrate buffer (pH 6.0) in a microwave (900 W) for 20 minutes and cooled to RT for at least 1 hour. Endogenous peroxidase was quenched with 5% hydrogen peroxide in H2O2 for 10 minutes and washed in PBS. Unspecific proteins were blocked with Normal Goat Serum (NGS)††††††† for 20 minutes. The sections were incubated overnight at 4°C with mouse monoclonal primary antibodies (Table 1); treated with biotinylated anti‐mouse secondary antibody‡‡‡‡‡‡‡ for 30 minutes at RT and followed by incubation in avidin‐biotinylated peroxidase complex for 30 minutes at RT. An endogenous tissue background control was used, using a section from GTE to which the primary antibody was not applied. The reactions were visualized using diaminobenzidine (DAB) for 5 minutes. The sections were counterstained with Hematoxylin for light‐microscopy (LM) examination. The experiment was run in four sample replicates (n = 4) for each group.
TABLE 1.
Primary antibodies used for immunohistochemical staining
| Antibody | Dilution | Clone | Isotype |
|---|---|---|---|
| Keratin 17 a | 1:50 | E‐4 | IgG1 |
| Keratin 19 a | 1:50 | A‐3 | IgG1 |
| Involucrin a | 1:20 | SY5 | IgG1 |
| Vimentin a | 1:1000 | E‐5 | IgG1 |
Santa Cruz Biotechnology Inc., Santa Cruz, CA.
2.4. Statistical analysis
All data are presented as mean values ± standard error of the mean (SEM). The Shapiro‐Wilk test was done to assume parametric or non‐parametric distribution for the normality tests. Differences between groups were assessed, depending on their normal distribution, by the Mann−Whitney U test or the two‐way analysis of variance (ANOVA) test, followed by post‐hoc pairwise comparisons using the LSD test. A specific computer program§§§§§§§ was used. The results were considered statistically significant at P values < 0.05. All the results were analysed before the experiment was unblinded.
3. RESULTS
3.1. 2D model
To ensure that neither the vehicle composed of lactose‐sucrose pillules impregnated with ethanolic solution nor the MI treatments were cytotoxic at the tested concentration, LDH activity was measured in the culture media from hGF treated for 3 days. As seen in Figure 1A, none of the treatments tested were cytotoxic for the cells since values for all the groups were < 10%.
FIGURE 1.
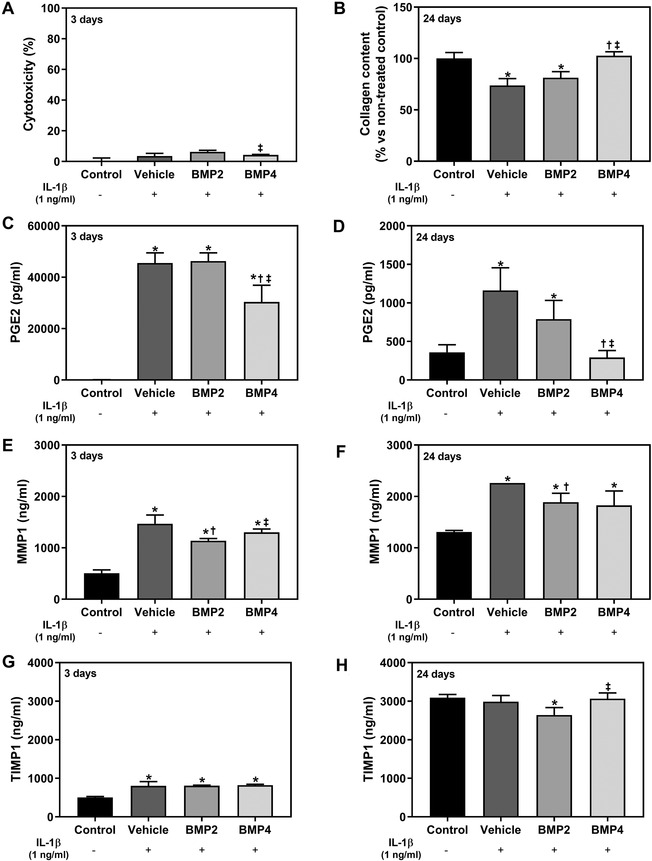
Effect after 3 and 24 days of treatment of human gingival fibroblasts (hGF) cells. The study was performed using the vehicle, bone morphogenetic protein‐2 (BMP2) and BMP4 MI treatments at a treatment concentration of 1X. After seeding cells were treated with IL1β to mimic inflammation for all the groups but not for the non‐treated control. Uninflamed non‐treated cells are represented as control. (A) Lactate dehydrogenase (LDH) activity, an indicator of cytotoxicity, measured in culture media after 3 days of treatment. Results are presented relative to the LDH activity in the media of cells cultured in tissue culture plastic (negative control was set to 0% of cell death) and of cells growing on tissue culture plastic treated with 1% triton X‐100 (positive control that was set to 100% of cell death). (B) Collagen deposition in hGF cells after 24 days of treatment. (C) and (D) PGE2 release of hGF cells after 3 days (C) and 24 days (D) of treatment. (E) and (F) MMP1 protein released to cell culture media after 3 days (E) and 24 days (F) of treatment. (G) and (H) TIMP1 protein released to cell culture media after 3 days (G) and 24 days (H) of treatment. Data represents the media ± SEM of six sample replicates (n = 6) for each group. Results from (A, C, D, and F) were compared by Kruskal‐Wallis test, while (B and E) were compared by ANOVA and LSD as post hoc. Statistically significant differences were considered for P < 0.05 and represented with * compared with control, † compared with vehicle, and ‡ compared with LD BMP2
Collagen deposition on the cell monolayer 24 days after treatment (Figure 1B) was significantly higher for LD BMP4 compared with both, LD BMP2 and vehicle, reaching the same collagen levels than uninflamed non‐treated cells (control). In contrast, LD BMP2 and Vehicle could not reach it.
The inflammatory stimulus was confirmed by PGE2 levels at day 3 (Figure 1C), showing significantly increased levels for all the groups but not for the uninflamed non‐treated cells (up to about 300‐fold) that were decreased after 24 days of treatment (to about two‐fold levels). Interestingly, lower PGE2 levels were observed for the LD BMP4 treated group compared with both, LD BMP2 and Vehicle groups after 3 days of treatment, and also after 24 days of treatment. Remarkably, the group treated with LD BMP4 showed similar PGE2 levels than the uninflamed non‐treated group after 24 days of treatment.
The inflammatory stimulus also induced higher MMP1 released to cell culture media after 3 days and 24 days of treatment as shown in Figure 1E and 1F compared with uninflamed non‐treated cells. Moreover, LD BMP2 treatment decreased the release of MMP1 compared with control and LD BMP4 after 3 days of treatment and to control after 24 days of treatment. On the other hand, the inhibitor TIMP1 was increased by IL1‐β in all groups after 3 days (Figure 1G), but after 24 days of treatment and removal of inflammatory stimulus, TIMP1 levels declined significantly to the levels of uninflamed control group, except for BMP2 that showed lower levels (Figure 1H).
3.2. 3D model
The histological observation of GTEs with Hematoxylin and Eosin staining (Figure 2A) revealed for LD BMP4, vehicle and non‐treated uninflamed tissue (control), a good multilayer epithelial structure, organized similar to the cells in the native oral mucosa, with fibroblasts embedded in the collagen matrix.
FIGURE 2.
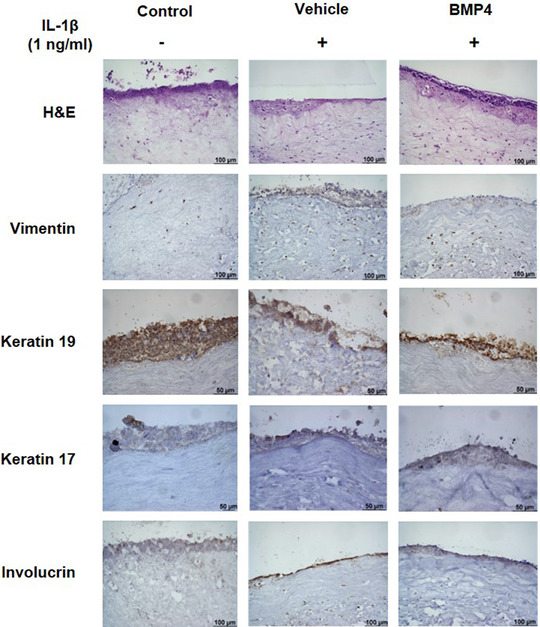
Histologic characterization of GTE. Representative images for each group are shown. (A) Hematoxylin and Eosin (H&E) staining of GTE 200×; (B) expression of Vimentin (fibroblasts marker) 200× (C) expression of Keratin 19 (epithelial differentiation marker) 400×; (D) expression of Keratin 17 (epithelial differentiation marker) 400×; E) expression of Involucrin (epithelial differentiation marker) 200×
Immunohistochemistry was done to confirm differentiation of GTEs. Images in Figure 2B, showed expression of Vimentin differentiation marker for fibroblast at all groups. To confirm differentiation of the multilayer epithelial structure of keratinocytes different markers were studied too. Expression of Keratin 19 (Figure 2C) and Keratin 17 (Figure 2D) was confirmed for keratinocytes at multilayer, with a good staining for all groups, although staining was weaker for k17. Also, expression of Involucrin present in keratinocytes was confirmed (Figure 2E), with differences between groups on the strength of staining. All in all, a multilayer epithelial structure in which layers are organized similar to the cells in the native oral mucosa could be confirmed. Histological differences were not observed either by treatment with LD BMP4 compared withvehicle nor by the inflammatory conditions compared with the control uninflamed conditions.
The MTT assay was used as a marker of cell viability, as it measures the MTT reduction by mitochondrial reductase enzymes. As shown in Figure 3A, the inflammatory stimulus reduced tissue viability after complete treatment of 11 days in the vehicle group. This reduction was statistically different compared with LD BMP4 treatment and uninflamed non‐treated tissues (control).
FIGURE 3.
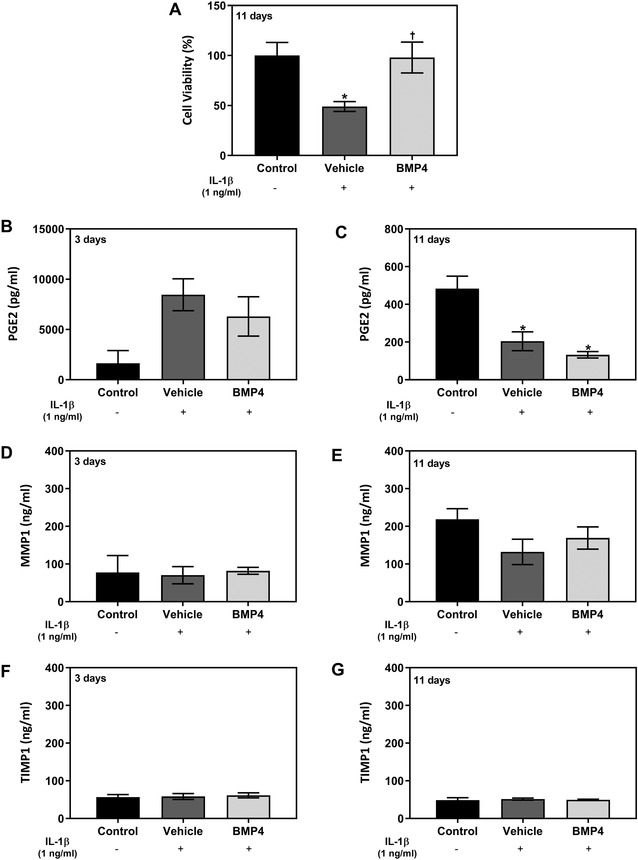
Effect after 3 and 11 days of treatment of GTEs 3D culture model. The study was performed using the vehicle and BMP4 MI treatment at a concentration of 2×. Tissues were cultured with IL1β to mimic inflammation at first day of treatment for all the groups but not for the non‐treated tissue control. Non‐treated uninflamed tissues are represented as control. (A) Tissue viability was measured with an MTT test after 11 days of treatment. Positive control was obtained from tissues treated with PBS and was set at 100% of viability. Data represents the media ± SEM of two sample replicates (n = 2) for each group. (B) and (C) PGE2 release of GTEs media culture after 3 days (B) and 11 days (C) of treatment. (D) and (E) Effect of treatment on the MMP1 protein release after 3 days (D) and 11 days (E) of GTEs culture. (F) and (G) Effect of treatment on the TIMP1 protein release after 3 days (F) and 11 days (G) of GTEs culture. Data represents the media ± SEM of six sample replicates (n = 6) for each group. Results were compared by ANOVA and LSD as post hoc. Statistically significant differences were considered for P < 0.05 and represented with * compared with control, and † compared with vehicle
The total amount of PGE2 at 3 days and 11 days produced by GTE tissues are shown in Figure 3B and 3C. After 3 days of culture, release of PGE2 to the media increased for LD BMP4 and vehicle treatments compared with uninflamed non‐treated tissues. Moreover, there was a tendency for BMP4 MI treatment to decrease PGE2 release compared with the vehicle, although the results did not reach statistically differences. After 11 days, PGE2 levels in all groups were significantly lower than the level of PGE2 released by non‐treated tissues without inflammatory conditions.
In relation to ECM organization‐related enzymes, MMP1 (Figure 3D and 3E), and TIMP1 (Figure 3F and 3G), release levels at 3 days and 11 days of culture showed no important changes between treatments nor time.
4. DISCUSSION
In this study we have demonstrated the anti‐inflammatory properties and the regenerative effect on collagen metabolism of a LD BMP4 MI treatment on hGF, and the improved tissue viability on engineered 3D gingiva under inflammatory conditions. Thus, LD BMP4 MI treatment could be considered as a new approach for preventing the progression and recurrence of periodontal disease in patients, included in the maintenance phase of supportive periodontal therapy or as preventive treatment of the disease.
First, for the evaluation of LD BMP2 and LD BMP4 in the 2D‐cell culture model, tested concentration was selected since it was below the lactose‐sucrose concentration that leads to autophagy and osmotic stress in mammalian cells. 30 , 31 , 32 , 33 This treatment had no impact on cell viability for any of the active MI treatments nor for the vehicle one, all of them containing the lactose‐sucrose excipients of the pillules, which validated our in vitro experimental MI treatment. We also needed to set up the inflammatory conditions with IL1β to examine anti‐inflammatory effects of these MI treatments. IL1β is broadly used to induce experimental inflammation and to enhance the proinflammatory response, imitating the inflammatory pathways activated in response to oral pathogens in periodontitis. 34 , 35 PGE2 is a potent lipid mediator produced by the metabolism of arachidonic acid via the cyclooxygenase (COX) pathway. PGE2 production is highly related to periodontal disease, where a first requisite to achieve a complete periodontal regeneration is to reduce inflammation. 36 We must take in account that the presence of IL1β and the detection of the inflammatory mediator PGE2, is a simple in vitro approximation of a more complex system of inflammatory responses involved in the periodontal in vivo situation.
Our results revealed the potential of LD BMP4 (9.35 × 10−15 µg/mL) to effectively inhibit PGE2 release in hGF under inflammatory stimulus. Importantly, other MIM containing IL1β (17CH), TNF‐α (17CH) and IL2 (10CH) 19 or Progesterone (10 pg/mL) and IL10 (10 fg/mL) 37 have shown in vitro beneficial anti‐inflammatory effects in other inflammatory related diseases. Moreover, in some recent works, the use of BMP7 as a direct anti‐inflammatory agent resulted in the inhibition of cardiac pathophysiology. 6 , 38 However, this is the first time that an effect of BMP4 on inflammation using such a small dose in hGF is reported.
Primary hGF were used in this study since they have the capacity to produce and secrete new ECM components and are responsible for the constant adaptation of the tissue. 39 Among the different ECM components, collagen is the most abundant. 24 It builds up the periodontal ligament and its fibers, to secure the attachment of root cement to alveolar bone and allowing regeneration of the periodontal ligament that occurs upon injury. 40 , 41 Our results show the capacity of a LD BMP4 treatment to promote collagen synthesis and deposition in the 2D‐cell culture set‐up under inflammatory conditions, reaching levels similar to the control cells that were not submitted to inflammatory stimulus. Thus, we show that MI treatment with LD BMP4 was able to completely re‐establish the impaired collagen metabolism induced by IL1β treatment in hGF.
ECM undergoes a constant turnover of its components, where matrix metalloproteinases (MMPs) enzymes degrade fibrillary collagens and their activity is inhibited mainly by metallopeptidase inhibitors (TIMPs). Excess MMPs production and activity leads to an accelerated ECM degradation associated with periodontitis. 42 , 43 The gingival fibroblast is the most predominant cell in the GTE, and participates in the local inflammatory response by its ability to produce MMP1. 44 In the present 2D study, treatment with both LD BMPs decreased MMP1 levels, but only the LD BMP2 was significant against the control. As TIMP1 secretion levels did not show important differences against the control, the differences found in the increased collagen content after the LD BMP4 treatment might be explained by an increased de novo production of collagen rather than a decreased degradation of collagen caused by a lower MMP1/TIMP1 ratio.
From the 2D study we selected LD BMP4 as the most promising MI treatment to go further into a more developed engineered 3D gingival in vitro tissue model to mimic the oral mucosa. Immunostaining results of the histological sections for the 3D study confirmed a good multilayer epithelial structure, with similarities to cells in native oral mucosa and more importantly with expression of different typical markers of an oral mucosa. Growth and development of human fibroblasts were allowed, as proved by the expression of Vimentin. 45 Moreover, a well differentiated epidermis was confirmed by the expression of Keratin 17 and Involucrin, two markers of differentiated keratinocytes that are expressed in the basal layer of complex epithelia. 46 , 47 , 48 Furthermore, the proliferative capacity of keratinocytes was confirmed by the expression of Keratin 19, marker of early differentiation. 29 , 49 Qualitatively, we observed no differences among the groups studied, suggesting no major pathological changes in the of human tissue equivalent of gingiva.
However, in contrast with the 2D model, tissue viability under inflammatory conditions was reduced in the vehicle group. On one hand, the inflammatory stimuli might have induced such reduction, but also, we should take into consideration the composition of the vehicle, being lactose‐sucrose, which might have caused a stress on the tissue. 33 , 50 However, the reduced tissue viability induced by the inflammatory stimulus in the vehicle group was overcome in the LD BMP4 group, with similar biocompatibility to the uninflamed control tissue, emphasizing the effect of LD BMP4 treatment to protect and prevent the tissue from the inflammatory stimuli. The potential of LD BMP4 to effectively inhibit PGE2 release could not be confirmed in this 3D study, although, the results revealed a tendency to decrease inflammation as shown by the measured by PGE2 levels at 11 days. Also, no effect was observed after stimulation of inflammation and LD BMP4 treatment on the MMP1 or TIMP1 release after 3 or 11 days, showing that MMP1 or TIMP1 production did not play an important role in this 3D GTE after inducing an inflammatory status. The differences on the effects of LD BMP4 between the 2D hGF monolayer and the 3D gingiva culture model emphasize the utility of the use of 3D culture models to study the effects of specific molecules, since they are closer to the in vivo situation.
In summary, LD BMP4 treatment decreases the release of the inflammatory mediator PGE2 and counteracted the collagenolytic metabolism induced by IL1β stimulation of primary hGF. In a 3D situation of gingival mucosa, LD BMP4 treatment shows beneficial effects on the viability of the tissue under inflammation. All in all, this study suggests that LD BMP4 could contribute to protect and recover the integrity of gingival tissues in a situation of periodontitis, with significant potential for stimulating periodontal regeneration. Thus, although further studies should confirm the effects in an in vivo periodontitis model, we believe that BMP4 5CH could be a potential MI treatment for primary prevention of periodontitis or maintenance care in patients suffering this disease.
5. CONCLUSIONS
The present manuscript reports for the first time a beneficial effect of LD BMP4 MI in two in vitro models of periodontitis. LD BMP4 MI treatments presented anti‐inflammatory properties and beneficial effects on collagen metabolism on hGF culture. Moreover, in a more complex 3D model, LD BMP4 recovered tissue viability under inflammatory conditions. Thus, in this study, we verified the in vitro efficacy of LD BMP4 treatment as a new and promising approach for periodontitis treatment.
CONFLICTS OF INTEREST
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Maria del Mar Ferrà‐Cañellas and Laura Garcia‐Sureda works for Labo'Life España, the company that co‐funded this research; Beatrice Lejeune works for Labo'Life France, the company service provider of Labo'Life, specialized in pre‐clinical, clinical development, and regulatory affairs. This professional relationship does not imply any misconduct on the part of the authors.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to conception and design of the study. Maria del Mar Ferrà‐Cañellas and Marta Munar‐Bestard have been involved in data collection and data analysis. Maria del Mar Ferrà‐Cañellas, Joana Maria Ramis and Marta Monjo have been involved in data interpretation and drafting first version of the article. Joana Maria Ramis, Marta Monjo, Laura Garcia‐Sureda and Beatrice Lejeune have been involved in revising the article critically and have given final approval of the version to be published.
ACKNOWLEDGMENTS
The project (CONCE 2019 1179) was supported by the Vice Presidency and Ministry of Innovation, Research and Tourism, General Directorate of Innovation and Research, from the Balearic Government (Balearic Islands, Spain), co‐founded with ERDF Euroauthor pean Regional Development Fund. Contract to Joana Maria Ramis (MS16/00124) was funded by the Instituto de Salud Carlos III (Madrid, Spain), co‐funded with ESF European Social Fund. The authors thank E. Ceresi (University of Balearic Islands, UIB) for the help of histologic characterization, and I. Floris (Labo'Life France, Nantes, France) for the revision of the manuscript.
Ferrà‐Cañellas MdM, Munar‐Bestard M, Garcia‐Sureda L, Lejeune B, Ramis JM, Monjo M. BMP4 micro‐immunotherapy increases collagen deposition and reduces PGE2 release in human gingival fibroblasts and increases tissue viability of engineered 3D gingiva under inflammatory conditions. J Periodontol. 2021;92:1448–1459. 10.1002/JPER.20-0552
[Correction added on Dec 31 2021, after first online publication: The copyright line was changed.]
Footnotes
Provitro GmbH, Berlin, Germany
Life Technologies, Carlsbad, CA
Biosera, Boussens, France
Biowest, Nuaille, France
Sigma‐Aldrich, St. Louis, MO
Sigma‐Aldrich
Roche Diagnostics, Mannheim, Germany
Triton X‐100, Sigma Aldrich
Sigma Aldrich
EHPGE2, Thermo Fisher Scientific, Waltham, MA
RAB0361, Sigma Aldrich
RAB0466, Sigma Aldrich
Applied Biological Materials Inc., Richmond, BC, Canada
Biowest
Gie‐No3B11, Applied Biological Materials Inc
Sarstedt, Nümbrecht, Germany
Gibco, Grand Island, NY
Sigma‐Aldrich
ThermoFisher Scientific, Waltham MA
Sigma‐Aldrich
ThermoFisher Scientific
ThermoFisher Scientific
Vector Laboratories, Burlingame, CA
Vector Laboratories
SPSS for Windows, v.24.0, IBM, Chicago, IL
REFERENCES
- 1. Kinane D, Stathopoulou PG, Papapanou PN, Periodontal diseases. Nat Rev Dis Prim. 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 2. Slots J. Periodontitis: facts, fallacies and the future. Periodontol. 2000;75:7‐23. [DOI] [PubMed] [Google Scholar]
- 3. Shazam H, Shaikh F, Hussain Z. Bone turnover markers in chronic periodontitis: a literature review. Cureus. 2020. 10.7759/cureus.6699. Published online 19 January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Cruz L. Periodontitis—a silent risk that has become louder. Prim Dent J. 2019;8:62‐66. [DOI] [PubMed] [Google Scholar]
- 5. Fawzy El‐Sayed KM, Dörfer CE. Animal models for periodontal tissue engineering: a knowledge‐generating process. Tissue Eng Part C Methods. 2017;23:900‐925. [DOI] [PubMed] [Google Scholar]
- 6. Boon MR, van der Horst G, van der Pluijm G, Tamsma JT, Smit JWA, Rensen PCN. Bone morphogenetic protein 7: a broad‐spectrum growth factor with multiple target therapeutic potency. Cytokine Growth Factor Rev. 2011;22:221‐229. [DOI] [PubMed] [Google Scholar]
- 7. Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Notodihardjo FZ, Kakudo N, Kushida S, Suzuki K, Kusumoto K. Bone regeneration with BMP‐2 and hydroxyapatite in critical‐size calvarial defects in rats. J Cranio‐Maxillofacial Surg. 2012;40:287‐291. [DOI] [PubMed] [Google Scholar]
- 9. Decambron A, Devriendt N, Larochette N, et al. Effect of the bone morphogenetic protein‐2 doses on the osteogenic potential of human multipotent stromal cells‐containing tissue engineered constructs. Tissue Eng Part A. 2019;25:642‐651. [DOI] [PubMed] [Google Scholar]
- 10. Lee K‐B, Taghavi CE, Song K‐J, et al. Inflammatory characteristics of rhBMP‐2 in vitro and in an in vivo rodent model. Spine (Phila Pa 1976). 2011;36:E149‐E154. [DOI] [PubMed] [Google Scholar]
- 11. James AW, LaChaud G, Shen J, et al. A review of the clinical side effects of bone morphogenetic protein‐2. Tissue Eng Part B Rev. 2016;22:284‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zara JN, Siu RK, Zhang X, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao Y‐T, Xiang L‐X, Shao J‐Z. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362:550‐553. [DOI] [PubMed] [Google Scholar]
- 14. Sharma A, Sharma H. Bone Morphogenetic proteins: an overview. Ann Appl Bio Sci. 2017;4:R35‐R37. [Google Scholar]
- 15. Kaur S, Grover V, Kaur H, Malhotra R. Evaluation of bone morphogenic proteins in periodontal practice. Indian J Dent. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP‐2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337‐349. [DOI] [PubMed] [Google Scholar]
- 17. Floris I, Rose T, Rojas JAC, Appel K, Roesch C, Lejeune B. Pro‐inflammatory cytokines at ultra‐low dose exert anti‐inflammatory effect in vitro: a possible mode of action involving sub‐micron particles?. Dose‐Response. 2020;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Floris I, García‐González V, Palomares B, Appel K, Lejeune B. The micro‐immunotherapy medicine 2LARTH® reduces inflammation and symptoms of rheumatoid arthritis in vivo. Int J Rheumatol. 2020;2020:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Floris I, Appel K, Rose T, Lejeune B. 2LARTH®, a micro‐immunotherapy medicine, exerts anti‐inflammatory effects in vitro and reduces TNF‐α and IL‐1β secretion. J Inflamm Res. 2018;11:397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Floris I, Chenuet P, Togbe D, Volteau C, Lejeune B. Potential role of the micro‐immunotherapy medicine 2LALERG in the treatment of pollen‐induced allergic inflammation. Dose‐Response. 2020;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lilli NL, Révy D, Robelet S, Lejeune B. Effect of the micro‐immunotherapy medicine 2LPARK® on rat primary dopaminergic neurons after 6‐OHDA injury: oxidative stress and survival evaluation in an in vitro model of Parkinson's disease. Degener Neurol Neuromuscul Dis. 2019;9:79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaur S, Grover V, Kaur H, Malhotra R. Evaluation of bone morphogenic proteins in periodontal practice. Indian J Dent. 2016;7:28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapple ILC, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42:S71‐S76. [DOI] [PubMed] [Google Scholar]
- 24. Palaiologou AA, Yukna RA, Moses R, Lallier TE. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J Periodontol. 2001;72:798‐807. [DOI] [PubMed] [Google Scholar]
- 25. Bartold PM, Narayanan a S. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000. 2006;40:29‐49. [DOI] [PubMed] [Google Scholar]
- 26. Duval K, Grover H, Han LH, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32:266‐277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27. Ramis Morey JM. Tissue‐engineered oral mucosa constructs for in vitro research and clinical applications. Biomed J Sci Tech Res. 2018;2. [Google Scholar]
- 28. Bao K, Akguel B, Bostanci N. Establishment and characterization of immortalized gingival epithelial and fibroblastic cell lines for the development of organotypic cultures. Cells Tissues Organs. 2014;199:228‐237. [DOI] [PubMed] [Google Scholar]
- 29. Dongari‐Bagtzoglou A, Kashleva H. Development of a highly reproducible three‐dimensional organotypic model of the oral mucosa. Nat Protoc. 2006;1:2012‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higuchi T, Nishikawa J, Inoue H. Sucrose induces vesicle accumulation and autophagy. J Cell Biochem. 2015;116:609‐617. [DOI] [PubMed] [Google Scholar]
- 31. DeCourcy K, Storrie B. Osmotic swelling of endocytic compartments induced by internalized sucrose is restricted to mature lysosomes in cultured mammalian cells. Exp Cell Res. 1991;192:52‐60. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Li M, Li L, et al. Trehalose, sucrose and raffinose are novel activators of autophagy in human keratinocytes through an mTOR‐independent pathway. Sci Rep. 2016;6:28423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xing S, Zhang L, Lin H, et al. Lactose induced redox‐dependent senescence and activated Nrf2 pathway. Int J Clin Exp Pathol. 2019;12:2034‐2045. [PMC free article] [PubMed] [Google Scholar]
- 34. Ono M, Kantoh K, Ueki J, et al. Quest for anti‐inflammatory substances using IL‐1β‐stimulated gingival fibroblasts. In Vivo (Brooklyn). 2011;25:763‐768. [PubMed] [Google Scholar]
- 35. Kida Y, Kobayashi M, Suzuki T, et al. Interleukin‐1 stimulates cytokines, prostaglandin E and matrix metalloproteinase‐1 production via activation of MAPK/AP‐1 and NF‐κB in human gingival fibroblasts. Cytokine. 2005;29:159‐168. [DOI] [PubMed] [Google Scholar]
- 36. Noguchi K, Ishikawa I. The roles of cyclooxygenase‐2 and prostaglandin E2 in periodontal disease. Periodontol 2000. 2007;43:85‐101. [DOI] [PubMed] [Google Scholar]
- 37. Mancini F, Milardi D, Carfagna P, et al. Low‐dose SKA Progesterone and Interleukin‐10 modulate the inflammatory pathway in endometriotic cell lines. Int Immunopharmacol. 2018;55:223‐230. [DOI] [PubMed] [Google Scholar]
- 38. Aluganti Narasimhulu C, Singla DK. The role of Bone Morphogenetic Protein 7 (BMP‐7) in inflammation in heart diseases. Cells. 2020;9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartold PM, Walsh LJ, Narayanan AS. Molecular and cell biology of the gingiva. Periodontol 2000. 2000;24:28‐55. [DOI] [PubMed] [Google Scholar]
- 40. Jönsson D, Nebel D, Bratthall G, Nilsson B‐O. The human periodontal ligament cell: a fibroblast‐like cell acting as an immune cell. J Periodontal Res. 2011;46:153‐157. [DOI] [PubMed] [Google Scholar]
- 41. Giannopoulou C, Cimasoni G. Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res. 1996;75:895‐902. [DOI] [PubMed] [Google Scholar]
- 42. Gómez‐Florit M, Ramis JM, Monjo M. Anti‐fibrotic and anti‐inflammatory properties of melatonin on human gingival fibroblasts in vitro. Biochem Pharmacol. 2013;86:1784‐1790. [DOI] [PubMed] [Google Scholar]
- 43. Soell M, Elkaim R, Tenenbaum H. Cathepsin C, matrix metalloproteinases, and their tissue inhibitors in gingiva and gingival crevicular fluid from periodontitis‐affected patients. J Dent Res. 2002;81:174‐178. [PubMed] [Google Scholar]
- 44. Domeij H, Modeer T, Yucel‐Lindberg T. Matrix metalloproteinase‐1 and tissue inhibitor of metalloproteinase‐1 production in human gingival fibroblasts: the role of protein kinase C. J Periodontal Res. 2004;39:308‐314. [DOI] [PubMed] [Google Scholar]
- 45. Reijnders CMA, Van Lier A, Roffel S, Kramer D, Scheper RJ, Gibbs S. Development of a full‐thickness human skin equivalent in vitro model derived from TERT‐immortalized keratinocytes and fibroblasts. Tissue Eng Part A. 2015;21:2448‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Collin C, Ouhayoun JP, Grund C, Franke WW, Suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation. 1992;51:137‐148. [DOI] [PubMed] [Google Scholar]
- 47. Gröger S, Michel J, Meyle J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J Periodontal Res. 2008;43:604‐614. [DOI] [PubMed] [Google Scholar]
- 48. Watt FM. Involucrin and Other Markers of keratinocyte terminal differentiation. J Invest Dermatol. 1983;81:S100‐S103. [DOI] [PubMed] [Google Scholar]
- 49. Mujyambere B, Jayaraj R, Suja S. Cytokeratin 19 (CK19) as a marker for epithelial differentiation and malignant transformation: its clinical relevance in diagnosis, prognosis and treatment response monitoring. IRE J. 2018;2:51‐61. [Google Scholar]
- 50. Williams MA, Rhoades CJ, Provan D, Newland AC. In vitro cytotoxic effects of stabilizing sugars within human intravenous immunoglobulin preparations against the human macrophage THP‐1 cell‐line. Hematology. 2003;8:285‐294. [DOI] [PubMed] [Google Scholar]


