Abstract
Background
Alcohol and hepatotoxic viruses cause the majority of liver diseases. Randomised clinical trials have assessed whether extracts of milk thistle, Silybum marianum (L) Gaertneri, have any effect in patients with alcoholic and/or hepatitis B or C virus liver diseases.
Objectives
To assess the beneficial and harmful effects of milk thistle or milk thistle constituents versus placebo or no intervention in patients with alcoholic liver disease and/or viral liver diseases (hepatitis B and hepatitis C).
Search methods
TheCochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and full text searches were combined (December 2003). Manufacturers and researchers in the field were contacted.
Selection criteria
Only randomised clinical trials in patients with alcoholic and/or hepatitis B or C virus liver diseases (acute and chronic) were included. Interventions encompassed milk thistle at any dose or duration versus placebo or no intervention. The trials could be double blind, single blind, or unblinded. The trials could be unpublished or published and no language limitations were applied.
Data collection and analysis
The primary outcome measure was mortality. Binary outcomes are reported as relative risks (RR) with 95% confidence interval (CI). Subgroup analyses were performed with regard to methodological quality.
Main results
Thirteen randomised clinical trials assessed milk thistle in 915 patients with alcoholic and/or hepatitis B or C virus liver diseases. The methodological quality was low: only 23% of the trials reported adequate allocation concealment and only 46% were considered adequately double‐blinded. Milk thistle versus placebo or no intervention had no significant effect on mortality (RR 0.78, 95% CI 0.53 to 1.15), complications of liver disease (RR 0.95, 95% CI 0.83 to 1.09), or liver histology. Liver‐related mortality was significantly reduced by milk thistle in all trials (RR 0.50, 95% CI 0.29 to 0.88), but not in high‐quality trials (RR 0.57, 95% CI 0.28 to 1.19). Milk thistle was not associated with a significantly increased risk of adverse events (RR 0.83, 95% CI 0.46 to 1.50).
Authors' conclusions
Our results question the beneficial effects of milk thistle for patients with alcoholic and/or hepatitis B or C virus liver diseases and highlight the lack of high‐quality evidence to support this intervention. Adequately conducted and reported randomised clinical trials on milk thistle versus placebo are needed.
Keywords: Humans; Hepatitis B; Hepatitis B/drug therapy; Hepatitis B/mortality; Hepatitis C; Hepatitis C/drug therapy; Hepatitis C/mortality; Liver Cirrhosis; Liver Cirrhosis/drug therapy; Liver Cirrhosis/mortality; Liver Cirrhosis, Alcoholic; Liver Cirrhosis, Alcoholic/drug therapy; Liver Cirrhosis, Alcoholic/mortality; Phytotherapy; Phytotherapy/methods; Randomized Controlled Trials as Topic
Plain language summary
No evidence supporting or refuting milk thistle for alcoholic and/or hepatitis B or C virus liver diseases
Milk thistle (Silybum marianum (L) Gaertneri) extracts have been used as medical remedies since the time of ancient Greece. Alcohol and hepatotoxic viruses are the major causes of liver diseases. Several trials have studied the effects of milk thistle for patients with liver diseases. This systematic review could not demonstrate significant effects of milk thistle on mortality or complications of liver diseases in patients with alcoholic and/or hepatitis B or C liver diseases combining all trials or high‐quality trials. Low‐quality trials suggested beneficial effects. High‐quality randomised clinical trials on milk thistle versus placebo are needed.
Background
Liver fibrosis and the subsequent development of liver cirrhosis are common reactions to a number of hepatotoxic substances, hepatotropic viruses, autoimmune liver diseases, metabolic liver diseases, etc. Alcohol and hepatotropic viruses cause the majority of liver fibrosis and cirrhosis in the Western World. The attributable risk for symptomatic liver cirrhosis in Italy explained by alcohol consumption, hepatitis B virus, and hepatitis C virus was 98 per cent in men and 67 per cent in women (Corrao 1998a).
Alcohol is the major hepatotoxin (Morgan 1999). Alcohol leads to fatty liver (Rubin 1968) and alcoholic hepatitis, fibrosis, and cirrhosis (Sørensen 1984; Marbet 1987; Morgan 1999). Five‐year survival rates in patients with alcoholic cirrhosis who stop drinking are in the order of 50 to 75 per cent; whereas survival rates in patients who continue to drink rarely exceed 40 per cent (Powell 1968). There is no universally accepted therapy for alcoholic liver disease. Meta‐analyses and randomised clinical trials have been unable to demonstrate significant effects on mortality of glucocorticosteroids (Christensen 1995; Gluud 2001), anabolic‐androgenic steroids (Gluud 1988; Rambaldi 2002a), colchicine (Rambaldi 2001a), propylthiouracil (Rambaldi 2002b), insulin/glucagon (Trinchet 1992), parenteral amino acid supplementation (Mezey 1991), or polyenylphosphatidylcholine (Lieber 2001; Lieber 2003b). S‐adenosyl‐L‐methionine may seem a promising medical intervention for alcoholic liver disease (Mato 1999), but more randomised clinical trials are needed before this treatment can be recommended (Rambaldi 2001b). Liver transplantation may be considered in patients with advanced alcoholic liver disease (Poynard 1994; Lieber 2000).
The progression of liver fibrosis and cirrhosis in alcoholics is enhanced by the presence of hepatitis B and hepatitis C virus (Chang 1994; Corrao 1998b). Interferon or lamivudine are presently the recommended therapy for hepatitis B (Main 1998; Zavaglia 2000; Lok 2001). Ribavirin plus interferon combination therapy is the recommended therapy for chronic hepatitis C whether interferon naive, relapsers, or non‐responders (Main 1998; Pianko 2000; Zavaglia 2000; Brok 2005a). These therapies have shown significant benefit in terms of increased survival (Brok 2005a). They are associated with frequent adverse events (De Franceschi 2000; Russo 2000) and are too expensive to be widely used in low‐income countries.
Many patients have turned to alternative medicines in hope of identifying substances with less toxicity and better effectiveness. The extracts of milk thistle, Silybum marianum (L) Gaertneri, have been used as medical remedies since the time of ancient Greece and the extracts are now widely used as an alternative medication (Flora 1998; Luper 1998; Saller 2001). Silymarin is the collective name for the flavonolignans (silybin or silibinin, silydianin, silychristin) extracted from milk thistle (Luper 1998). These extracts have been shown to protect animals against various hepatotoxins including acetaminophen (Campos 1989; Muriel 1992), radiation (Hakova 1993), iron overload (Szilard 1988), phalloidin (Floersheim 1978; Tuchweber 1979), carbon tetrachloride (Rauen 1971; Rauen 1973; Halim 1997), and thioacetamide (Schriewer 1973). The 'hepatoprotective' actions of milk thistle may include inhibition of lipid peroxide formation, scavenging of free radicals, and changing of the physical properties of cell membranes (Ramellini 1974; Bindoli 1977; Valenzuela 1985; Flora 1998). Milk thistle may also reduce liver fibrogenesis (Boigk 1997; Lieber 2003a).
Based on a questionnaire survey among European hospital‐based specialists in gastroenterology/hepatology in 1992, 13 to 18 per cent of the specialists considered using milk thistle for patients with alcoholic fatty liver, alcoholic fibrosis, alcoholic hepatitis, or alcoholic cirrhosis (Gluud 1993). There were significant regional differences; milk thistle was being considered a treatment for alcoholic liver disease mostly in Eastern Europe (Gluud 1993). According to a recent meta‐analysis on milk thistle for patients with liver diseases no significant reduction in mortality or improvements in liver histology, or liver function could be demonstrated, but data were too limited to exclude a substantial benefit or harm of milk thistle on mortality (Lawrence 2000; Jacobs 2002). Accordingly, there is insufficient evidence to support or refute recommending this herbal compound to patients for the treatment of liver diseases (Lawrence 2000; Jacobs 2002). However, another meta‐analysis (Saller 2001) demonstrated significant effects of milk thistle on some outcomes like liver‐related mortality, but data were not conclusive.
This systematic review summarised the data from randomised clinical trials to examine the beneficial and harmful effects of milk thistle or its constituents for alcoholic and/or hepatitis B or C liver diseases. The reasons for including these different aetiologies are the following. First, many trials conducted before the 1980s did not exclude hepatitis B virus and many trials conducted before the 1990s did not exclude hepatitis C virus as an aetiology. Second, alcoholic and viral liver diseases often coexist. Third, alcohol and hepatitis B and/or C constitute the major aetiologies of chronic liver diseases in the Western World (Corrao 1998a).
Objectives
The objectives were to assess the beneficial and harmful effects of milk thistle or milk thistle constituents versus placebo or no intervention in patients with alcoholic liver disease and/or viral liver diseases (hepatitis B and hepatitis C) based on the results of randomised clinical trials, irrespective of blinding, publication status, or language.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised clinical trials were included. The randomised clinical trials should have used a proper method of randomisation, ie, central randomisation; serially numbered opaque, sealed envelopes; or other description that contains elements convincing of adequate allocation concealment. Trials using quasi‐randomisation were excluded. Randomised clinical trials could be double blind, single blind, or unblinded. The randomised clinical trials could be unpublished or published as an article, an abstract, or a letter. No language limitations were applied.
Types of participants
Patients with alcoholic liver cirrhosis, liver fibrosis, hepatitis and/or steatosis as well as patients with viral induced liver disease (hepatitis B and/or hepatitis C) according to the diagnostic work‐up used in the individual trial were included. Both acute and chronic liver disease were included. However, patients with rarer specific forms of liver disease (such as primary biliary cirrhosis, drug induced liver diseases, etc.) were not included as these diseases have different pathogenic mechanisms. Further, we excluded trials on prevention of liver disease, eg, prior to toxic exposure, as well as patients with liver disease of unknown aetiology. The individual patient groups were considered separately as well as collectively in order to estimate the efficacy of milk thistle and milk thistle constituents in specific diagnostic groups and in all groups.
Types of interventions
Administration of milk thistle or any milk thistle constituent at any dose or duration versus placebo or no intervention. The efficacy of milk thistle and milk thistle constituents were evaluated separately as well as collectively. Additional interventions were allowed, as long as both intervention groups received the additional intervention(s).
Types of outcome measures
The following outcome measures were assessed: (1) Number of patients dying (total number of death and liver‐related death) (primary outcome measures). (2) Development of clinical symptoms and complications (ie, ascites, variceal bleeding, hepatic encephalopathy, etc.), analysed separately and combined. (3) Liver biochemistry and function. (4) Liver biopsy findings. (5) Number and type of adverse event. Adverse event was defined as any untoward medical occurrence that did not have a causal relationship with the treatment. Severe adverse event was defined according to the ICH guidelines (ICH‐GCP 1997) as any event that increase mortality; was life‐threatening; required inpatient hospitalisation; resulted in a persistent or significant disability; or any important medical event, which might have jeopardised the patient or required intervention to prevent it.
In addition, any data on quality of life and health economics (eg, costs or length of hospitalisation) were compared.
Search methods for identification of studies
Searches in The Cochrane Hepato‐Biliary Group Controlled Trials Register (December 2003), The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 4 2003), MEDLINE (1966 to December 2003), and EMBASE (1974 to December 2003) were done entering the search terms 'milk thistle' or 'silymarin' or 'silybin' or 'silibinin' or 'silydianin' or 'silychristin' or commercial names (Legalon®, Silipide®, Realsil®, Carsil®, Siliphos®) and 'liver disease' or 'alcoholic liver disease' or 'viral liver disease' or 'hepatitis B or hepatitis C' (see Table 1).
1. Database searches.
| Database | Date of search | Search strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | December 2003 | ('milk thistle' OR silymarin OR silybin OR silibinin OR silydianin OR silychristin OR Legalon OR Silipide OR Realsil OR Carcil OR Siliphos) AND ('liver disease' OR 'hepatitis B' OR 'hepatitis C') |
| The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | Issue 4, 2003 | #1 MILK THISTLE explode all trees (MeSH) #2 SILYMARIN explode all trees (MeSH) #3 ((milk next thistle) or silymarin or silimarin or silybin or silibin or silybinin or silibinin or silydianin or silidianin or silychristin or silichristin or legalon or silipide or realsil or carsil or siliphos) #4 (#1 or #2 or #3) #5 LIVER DISEASES explode all trees (MeSH) #6 LIVER DISEASES ALCOHOLIC explode all trees (MeSH) #7 HEPATITIS B explode all trees (MeSH) #8 HEPATITIS C explode all trees (MeSH) #9 ((liver next disease) or (alcoholic next liver next disease) or (viral next liver next disease) or (hepatitis next b) or (hepatitis next c)) #10 (#5 or #6 or #7 or #8 or #9) #11 (#4 and #10) |
| MEDLINE | 1966 to December 2003 | #1 explode "Milk‐Thistle"/ all subheadings #2 explode "Silymarin"/ all subheadings #3 milk thistle or sil*marin or sil*bin* or sil*dianin or sil*christin or legalon or silipide or realsil or Carsil or siliphos #4 #1 or #2 or #3 #5 explode "Liver‐Diseases"/ all subheadings #6 explode "Liver‐Diseases‐Alcoholic"/ all subheadings #7 explode "Hepatitis‐B"/ all subheadings #8 explode "Hepatitis‐C"/ all subheadings #9 liver disease or alcoholic liver disease or viral liver disease or hepatitis B or hepatitis C #10 #5 or #6 or #7 or #8 or #9 #11 #4 and #10 #12 random* or blind* or placebo or meta‐analysis #13 #11 and #12 |
| EMBASE | 1974 to December 2003 | #1 explode "Silybum‐marianum"/ all subheadings #2 explode "silymarin"/ all subheadings #3 explode "silibinin"/ all subheadings #4 explode "silidianin"/ all subheadings #5 explode "silicristin"/ all subheadings #6 explode "silibinin‐phosphatidylcholine‐complex"/ all subheadings #7 milk thistle or sil*marin or silbi* or sil*dianin or sil*christin or legalon or silipide or realsil or carsil or siliphos #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 explode "liver‐disease"/ all subheadings #10 explode "alcohol‐liver‐disease"/ all subheadings #11 explode "virus‐hepatitis"/ all subheadings #12 explode "hepatitis‐B"/ all subheadings #13 explode "hepatitis‐C"/ all subheadings #14 liver disease or alcoholic liver disease or viral liver disease or hepatitis B or hepatitis C #15 #9 or #10 or #11 or #12 or #13 or #14 #16 #8 and #15 #17 random* or blind* or placebo or meta‐analysis #18 #16 and #17 |
The MEDLINE search was combined with the search strategy of The Cochrane Hepato‐Biliary Group (Gluud 2003).
Further trials were identified by reading the reference lists of the identified studies.
The principal authors of the identified trials were approached and inquired about additional randomised clinical trials they might know of.
Pharmaceutical companies involved in the production of milk thistle products were contacted in order to obtain unidentified published or unpublished randomised clinical trials.
Data collection and analysis
The meta‐analysis was conducted according to our protocol for the review (Rambaldi 2003) following the recommendations given by The Cochrane Collaboration (Higgins 2005).
Selection of trials for inclusion Two reviewers (AR and GI) independently selected the trials to be included in the Review according to the prespecified selection criteria. A third opinion plus discussion resolved any disagreement.
Patient characteristics, diagnosis, and treatments The following items were recorded from the included trials: mean (or median) age, sex ratio, form of liver disease according to the aetiology (acute viral hepatitis B and/or C; chronic viral hepatitis B and/or C; alcoholic liver disease (alcoholic steatosis; alcoholic hepatitis; alcoholic fibrosis; alcoholic cirrhosis; mixed), duration of liver disease, severity of liver disease at entry, alcohol consumption at entry and during the follow‐up, type and dose of milk thistle‐intervention (route of administration, formulation, frequency, and duration of dosing), type of intervention in the control group as well as any co‐interventions. The diagnostic work‐up before entry was registered, specifically if hepatitis markers were evaluated and the types of liver diseases that were excluded from the randomised clinical trials. Development of clinical symptoms and complications, liver biochemistry (serum (s)‐bilirubin, prothrombin time (PT), s‐albumin, s‐aspartate aminotransferase (AST), s‐alanine aminotransferase (ALT), s‐alkaline phosphatases (AP), s‐gamma‐glutamyl transferase (GGT), liver biopsy findings, alcohol consumption, quality of life, health economics (eg, length of hospital stay, cost of medication, and cost of additional follow‐up weighted against any gains in health), and adverse events during follow‐up were registered.
Selection and data extraction All randomised clinical trials considered for inclusion were analysed at least by two authors. All randomised clinical trials had the pertinent data extracted by two authors, who conferred with the reviewers in case disagreements could not be solved. All identified trials were listed and trials excluded from the meta‐analysis of the review were identified with the reason for exclusion.
Assessment of methodological quality The methodological quality of the randomised clinical trials was assessed using individual components of methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001):
Generation of the allocation sequence The procedure used to create a random sequence ensuring that each participant has a known, unpredictable, and usually equal chance of being assigned to intervention groups. The allocation sequence generation can be classified as (1) Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice may also be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure. (2) Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described. (3) Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients. Such studies are known as quasi‐randomised studies and were excluded from the review due to the risk of bias. Allocation concealment The procedure used to conceal the allocation sequence from the investigators who assign participants to the intervention groups. The allocation concealment can be classified as (1) Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes. Envelopes should be serially numbered, sealed, and opaque. However, this information is rarely provided, indicating an increased risk of bias. In that case, sealed envelopes may constitute an intermediate category between adequate and unclear. (2) Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described. (3) Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding Blinding was classified as (1) Adequate, if the trial was described as double blind and the method of blinding involved identical placebo and active drugs. (2) Unclear, if the trial was described as double blind, but the method of blinding was not described. (3) Not performed, if the trial was not double blind.
Follow‐up The reported follow‐up was classified as (1) Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals. (2) Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. (3) Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Intention‐to‐treat analysis We registered whether the randomised clinical trial reported or not on the use of intention‐to‐treat analysis (Gluud 2001).
Data on the number of patients with each outcome event by allocated treatment group, irrespective of compliance of follow‐up, were sought to allow an intention‐to‐treat analysis. If the above data were not available in the trial reports, further information was sought by correspondence with the principal investigators.
Statistical methods All analyses were performed according to the intention‐to‐treat method, that is, all randomised patients were included. We conducted analyses counting outcomes as reported in the individual trials. Further for patients without clear description of the outcome, we conducted a 'worst‐case scenario' analysis regarding dichotomous outcome measures considering patients dropped out or withdrawn as having the outcome (eg, died).
The statistical package (RevMan Analyses 1.0.2) provided by The Cochrane Collaboration was used. We examined all outcomes with both the random‐effects model and the fixed‐effect model. In case both models reached the same conclusion regarding intervention effect (ie, both non‐significant or both significant), only the fixed‐effect model results were reported. In case both models reached different conclusions regarding intervention effect (ie, one model found no significant difference and the other a significant difference), the results of both analyses were reported (with fixed or random appended) (DerSimonian 1986; Demets 1987).
Dichotomous data were analysed by calculating the relative risk (RR) and continuous outcomes as weighed mean difference (WMD), both with 95% confidence intervals (CI).
Heterogeneity and funnel plot asymmetry Heterogeneity in the results of the trials was initially assessed by inspection of graphical presentations and by calculating tests of heterogeneity (chi square and I2) (Higgins 2003; Alderson 2004). Potential causes for heterogeneity were explored by performing subgroup analyses. The review performed subgroup analyses with regard to the stage (aetiology, acuity) of liver disease, methodological quality of included randomised clinical trials (analysing separately randomised clinical trials with adequate quality components and inadequate quality components (generation of the allocation sequence, allocation concealment, blinding, and follow‐up) (Kjaergard 2001), and way (frequency) of administration of milk thistle or milk thistle constituents as well as preparation (formulation), dose and duration of milk thistle or milk thistle constituent treatment. In addition to the assessment of the potential impact of the individual quality components, we stratified the analyses of interventions effects on the major outcome measures into trials having all components adequate, trials with only some of the components adequate, and trials without any of the components adequate.
Due to the risk of chance, statistical findings among the secondary outcome measures were interpreted conservatively.
Funnel plots to identify publication bias and other biases were analysed by regression analyses (Egger 1997; Vickers 1998).
Results
Description of studies
Search results We identified 1,831 references through electronic searches of The Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 40), The Cochrane Library (n = 75), and MEDLINE and EMBASE (n = 1,716). Of these, 1,622 were in vitro studies, animal studies, studies unrelated to liver disease, or duplicate reports. Therefore, these studies were excluded.
Among the remaining 209 publications, 67 publications were on patients with alcoholic and/or hepatitis B or C liver diseases treated with milk thistle. Of these 67 publications, 43 had to be excluded for various reasons (see Characteristics of excluded studies). Accordingly, 24 publications could be included. Reading bibliographies identified two further publications, not identified by the electronic searches, on two trials (Salvagnini 1985; Buzzelli 1994), which could be included.
Included trials Accordingly, 26 publications fulfilled our inclusion criteria. The 26 publications described 13 trials randomising patients with alcoholic and/or hepatitis B or C liver diseases to milk thistle versus placebo or no intervention. The individual randomised clinical trials are described in the table 'Characteristics of included studies'.
Eleven randomised clinical trials were described in full paper articles (Magliulo 1978; Fehér 1988; Ferenci 1989; Trinchet 1989; Láng 1990; Bunout 1992; Buzzelli 1993; Velussi 1997; Pár 2000; Lirussi 2002; Lucena 2002) and two randomised clinical trials in abstracts (Salvagnini 1985; Buzzelli 1994).
The experimental treatment consisted of silymarin orally in 10 randomised clinical trials (Magliulo 1978; Salvagnini 1985; Fehér 1988; Ferenci 1989; Trinchet 1989; Láng 1990; Bunout 1992; Velussi 1997; Parés 1998; Lucena 2002); IdB1016 orally in two randomised clinical trials (Buzzelli 1993; Buzzelli 1994) (IdB1016 is a lipophilic complex with silybin and phosphatidylcholine in a molar ratio of 1:1); and silybin‐beta‐cyclodextrin, a new formulation containing silybin, in one randomised clinical trial (Lirussi 2002).
The entry criteria in the randomised clinical trials varied, but the inclusion criteria were generally of good quality making it highly likely that all patients did have alcoholic and/or hepatitis B or C virus liver diseases. The randomised clinical trials could be divided into four groups according to etiology: ‐ 657 patients with alcoholic liver disease, the majority having cirrhosis (Salvagnini 1985; Fehér 1989; Ferenci 1989; Trinchet 1989; Láng 1990; Bunout 1992; Velussi 1997; Lirussi 2002; Lucena 2002); ‐ 28 patients with acute hepatitis B (Magliulo 1978); ‐ 10 patients with chronic hepatitis C (Buzzelli 1994); ‐ patients with alcoholic and/or hepatitis B or C liver diseases (Buzzelli 1993; Par), of which one trial included 20 patients with hepatitis B and hepatitis C (Buzzelli 1993) and the other included 200 patients with alcoholic liver disease with or without HCV antibody positivity (Parés 1998). Anti‐HCV antibodies were positive in 29 (13 receiving milk thistle and 16 receiving placebo) of the 75 patients for whom stored sera were available after completion of the trial.
Out of the 915 patients randomised, 364 patients were males (Fehér 1989; Trinchet 1989; Láng 1990; Buzzelli 1993; Buzzelli 1994; Parés 1998; Lucena 2002), while 118 patients were females (Fehér 1989; Trinchet 1989; Láng 1990; Buzzelli 1993; Buzzelli 1994; Parés 1998; Lucena 2002). The sex of the patients was not given for 433 patients (Magliulo 1978; Salvagnini 1985; Ferenci 1989; Bunout 1992; Velussi 1997; Lirussi 2002).
All the randomised clinical trials compared milk thistle versus placebo, except one that compared milk thistle versus no intervention (Velussi 1997). The median duration of treatment was six months and varied from seven days (Buzzelli 1993) to 41 months (Ferenci 1989).
Excluded studies A total of 33 studies, described in 43 publications, were excluded mainly because they were observational studies or case series (Characteristics of excluded studies).
Two of the excluded studies were randomised clinical trials on milk thistle (Fintelmann 1980; Salmi 1982). The aetiology was toxic liver disease in the Fintelmann trial (Fintelmann 1980) mostly due to alcoholic liver disease. The aetiology in the Salmi trial (Salmi 1982) was also mostly due to alcohol problems (the majority 81% in the milk thistle arm and 76% in the placebo arm admitted previous alcohol consumption). These trials were excluded in the main analyses, but included in an explorative sensitivity analysis.
We were unable to obtain three studies, which were excluded (Berenguer 1977; Dittrich 1980; Conti 1989) .
Risk of bias in included studies
Only one (Trinchet 1989) of the 13 randomised clinical trials provided a sample size estimation, which was based on liver histology.
The method to generate the allocation sequence was considered adequate in six (46.2%) of the trials (Ferenci 1989; Trinchet 1989; Bunout 1992; Parés 1998; Lirussi 2002; Lucena 2002).
Only three (23.1%) of the trials described adequate allocation concealment (Trinchet 1989; Parés 1998; Lucena 2002).
All but one (7.7%) of the trials (Velussi 1997) were described as double blinded. However, only six (46.2%) trials (Ferenci 1989; Trinchet 1989; Buzzelli 1993; Parés 1998; Lirussi 2002; Lucena 2002) described the use of placebo with identical presentation in the control arm.
There was a fair description of follow‐up and withdrawals/drop‐outs in 12 (92.3%) trials (Magliulo 1978; Fehér 1989; Ferenci 1989; Trinchet 1989; Láng 1990; Bunout 1992; Buzzelli 1993; Buzzelli 1994; Velussi 1997; Parés 1998; Lirussi 2002; Lucena 2002).
None of the randomised clinical trials stated that they used an intention‐to‐treat method to evaluate their data. All the randomised clinical trials but three (Bunout 1992; Lirussi 2002; Lucena 2002) presumably used intention‐to‐treat analysis.
Effects of interventions
Mortality Combining the results of the 13 randomised clinical trials demonstrated no significant effect of milk thistle versus placebo/no intervention on mortality (RR 0.78, 95% confidence interval (CI) 0.53 to 1.15). There was no significant heterogeneity. In the milk thistle group 36/456 (7.9%) patients died versus 45/459 (9.8%) patients in the control group (Comparison 01‐01).
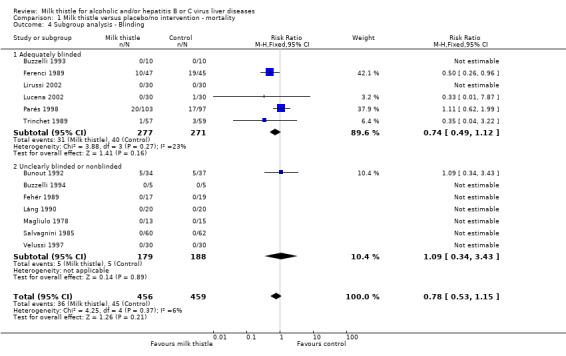
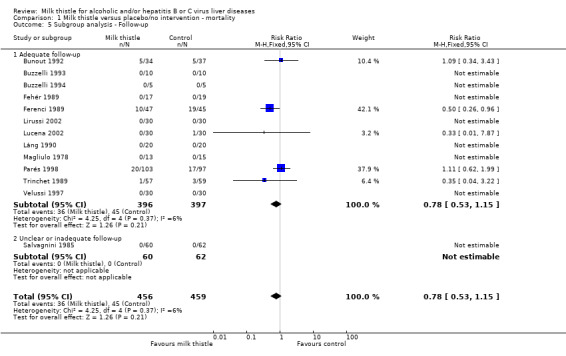
Subgroup analyses stratifying the trials according to the single methodological quality components (generation of the allocation sequence, allocation concealment, blinding, and follow‐up) did not demonstrate significant differences regarding the effect of milk thistle on mortality between trials with and without adequate methodology components (Comparison 01‐02, 01‐03, 01‐04, 01‐05).
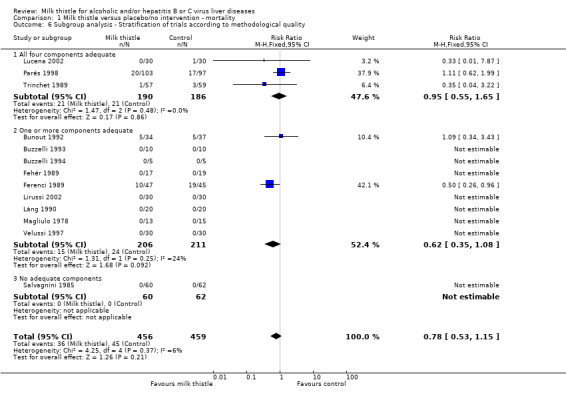
Subgroup analysis stratifying the trials into trials having all methodological components adequate, trials with some of the components adequate, and trials without any of the components adequate did not demonstrate significant differences regarding the effect of milk thistle on mortality: trials having all components adequate (RR 0.95, 95% CI 0.55 to 1.65); trials having some components adequate (RR 0.62, 95% CI 0.35 to 1.08) (test of interaction z = ‐1.06; P = 0.29); trial without any of components adequate (not estimable since no events happened in this group) (Comparison 01‐06).
Milk thistle did not significantly influence mortality in the trials with a treatment duration less than six months (RR 0.35; 95% CI 0.04 to 3.22) or in the trials with a duration of treatment of at least six months (RR 0.81, 95% CI 0.54 to 1.20) (Comparison 01‐07).
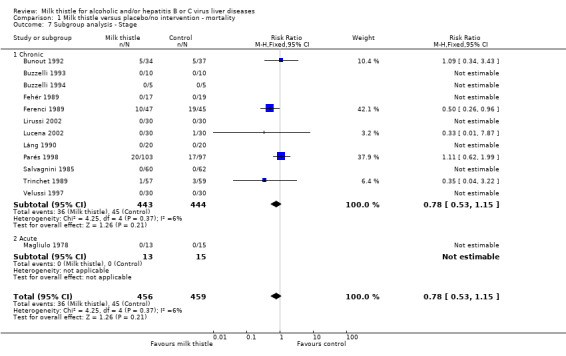
Milk thistle did not significantly influence mortality in the trials including patients with chronic liver disease (RR 0.72, 95% CI 0.48 to 1.09); the RR in the trials including patients with acute liver disease was not estimable since no events occurred in this group (Comparison 01‐08).
The RR of death of the randomised clinical trials evaluating silymarin (Legalon®) was 0.72 (95% CI 0.48 to 1.09); the RRs of the randomised clinical trials evaluating Silipide® or silybin‐beta cyclo‐dextrin were not estimable since no events occurred in these groups (Comparison 01‐09).
A worst‐case scenario analysis (all patients who dropped‐out or were withdrawn were considered dead) did not change the estimate of no significant effect of milk thistle on mortality (RR 1.09; 95% CI 0.75 to 1.58) (Comparison 01‐10).
In patients with alcoholic liver disease (Salvagnini 1985; Fehér 1989; Ferenci 1989; Trinchet 1989; Bunout 1992; Láng 1990; Velussi 1997; Lirussi 2002; Lucena 2002), a significant effect of milk thistle on mortality was demonstrated (RR 0.58, 95% CI 0.34 to 0.98; P = 0.04). There was no significant heterogeneity. In the milk thistle group 16/325 (4.9%) patients died versus 28/332 (8.4%) in the control group (Comparison 01‐01). However, focusing only on high‐quality trials (Trinchet 1989; Lucena 2002) milk thistle had no significant effect on mortality (RR 0.34, 95% CI 0.06 to 2.11) (Comparison 01‐11).
In patients with alcoholic liver disease including patients with HCV antibody positivity (Parés 1998) milk thistle demonstrated no significant effect on mortality RR (1.11, 95% CI 0.62 to 1.99). In the milk thistle group 20/103 (19.4%) patients died versus 17/97 (17.5%) in the control group (Comparison 01‐01).
In a worst‐case scenario analysis in patients with alcoholic liver disease, milk thistle was without significant effect on mortality (RR 1.09, 95% CI 0.75 to 1.58) (Comparison 01‐10).
In patients with hepatitis B (Magliulo 1978) none of the patients died out of the 13 in the milk thistle and 15 in the control group (Comparison 01‐01).
In patients with hepatitis C (Buzzelli 1994) none of the patients died out of the five in the milk thistle and five in the control group (Comparison 01‐01).
In patients with hepatitis B and hepatitis C (Buzzelli 1993) none of the patients died out of the 10 in the milk thistle and 10 in the control group (Comparison 01‐01).
Exploratory analysis adding two excluded randomised clinical trials (Fintelmann 1980; Salmi 1982) because they treated patients with other liver diseases than alcoholic liver disease, hepatitis B, and/or hepatitis C did not change the estimate significantly (RR 0.78, 95% CI 0.53 to 1.15) (Comparison 01‐12). In the milk thistle group 36/540 (6.7%) patients died versus 45/545 (8.3%) patients in the control group as no deaths occurred in the two added trials.
Liver‐related mortality Among the 13 trials, only four reported liver‐related mortality (Ferenci 1989; Trinchet 1989; Parés 1998; Lucena 2002). Three of the trials included patients with alcoholic liver disease and the Pares trial included patients with alcoholic liver disease or alcoholic liver disease with HCV antibody positivity. These trials found a significant effect of milk thistle on liver‐related mortality (RR 0.50, 95% CI 0.29 to 0.88; P = 0.02). There was no significant heterogeneity. In the milk thistle group, 16/422 (3.8%) patients died versus 31/422 (7.3%) patients in the control group (Comparison 02‐01).
Subgroup analysis demonstrated no significant effect of milk thistle on liver‐related mortality in the trials having all four methodological components adequate (RR 0.57, 95% CI 0.28 to 1.19) whereas milk thistle significantly decreased mortality in the trials having only one or more components adequate (RR 0.41, 95% CI 0.17 to 0.97). This effect was based on only one trial with less than 100 patients randomised (Ferenci 1989). There was no significant difference between the two estimates (z = 0.57). The effect of milk thistle on liver‐related deaths in the trials with no adequate methodological component was not estimable due to no deaths (Comparison 02‐02).
A worst‐case scenario analysis of patients with alcoholic liver disease (all patients who dropped‐out or were withdrawn were considered dead) changed the estimate to no significant effect of milk thistle on liver‐related mortality (RR 0.81, 95% CI 0.58 to 1.13) (Comparison 02‐03).
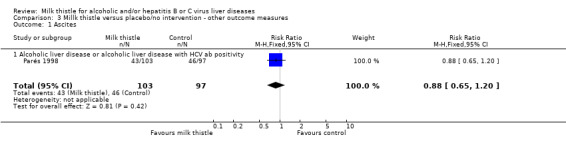
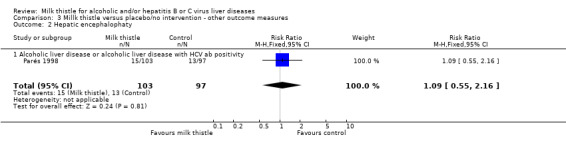
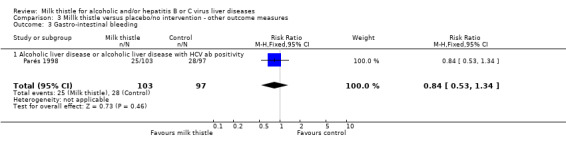
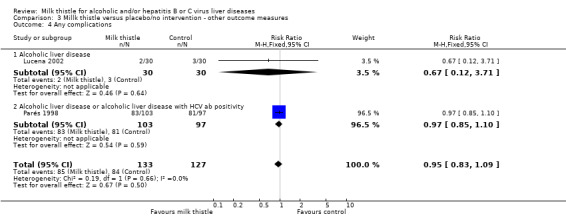
Liver‐related complications Milk thistle did not significantly affect the incidence of patients with ascites (RR 0.88, 95% CI 0.65 to 1.20), hepatic encephalopathy (RR 1.09, 95% CI 0.55 to 2.16), or gastro‐intestinal bleeding (RR 0.84, 95% CI 0.53 to 1.34) (Parés 1998) (Comparison 03‐01, 03‐02, 03‐02). None of the randomised clinical trials reported hepato‐renal syndrome as an outcome measure. Combining the results of two trials (Parés 1998; Lucena 2002) demonstrated no significant effect of milk thistle on the combined complications (RR 0.95, 95% CI 0.83 to 1.09). In the milk thistle group the total number of complications were 85/133 (63.3%) versus 84/127 (66.1%) in the control group (Comparison 03‐04).
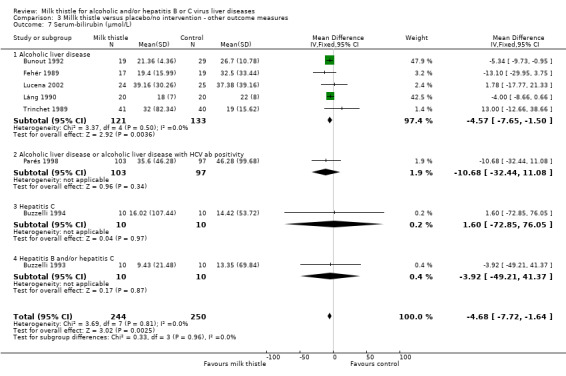
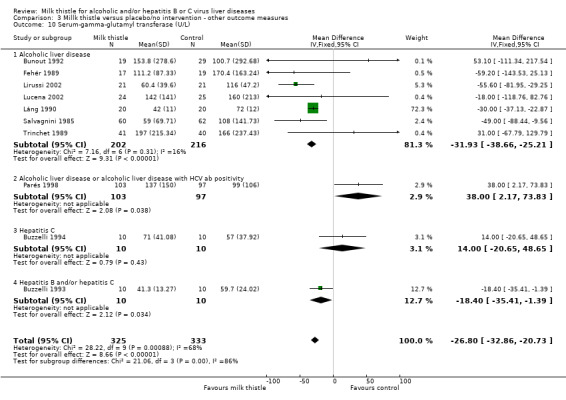
Liver biochemistry Milk thistle significantly decreased s‐bilirubin concentration and GGT activity in both fixed effect and random effects analyses when all trials are considered: ‐ s‐bilirubin (µmol/L): WMD ‐4.68 (95% CI ‐7.72 to ‐1.64; P < 0.05) (fixed effect model). There was no significant heterogeneity (Comparison 03‐07); ‐ GGT (U/L): WMD ‐26.80 (95% CI ‐32.86 to ‐20.73; P < 0.05) (fixed effect model). There was significant heterogeneity (I2 = 68%) (Comparison 03‐10). When focusing on high‐quality trials only, no significant beneficial effects of milk thistle on s‐bilirubin or GGT activity were found (data not shown).
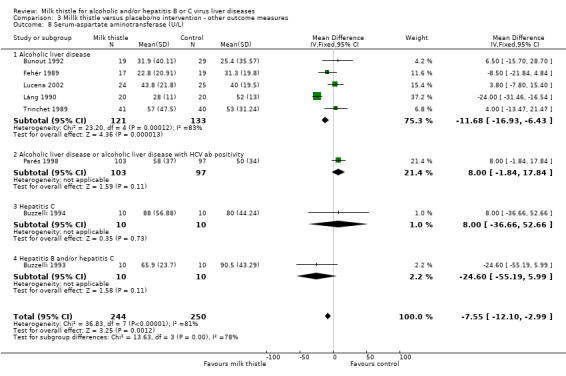
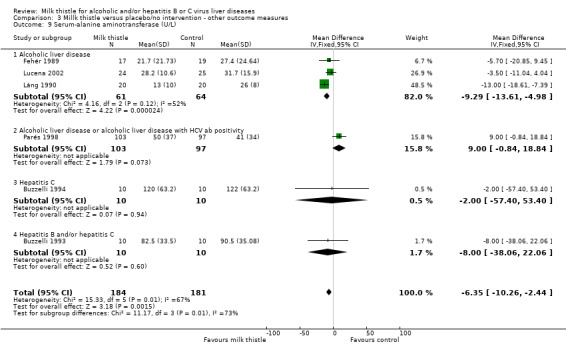
Milk thistle also showed a significant beneficial effect on some of the other biochemical measures when analysed by the fixed effect model, but not by the random effects model: ‐ AST (U/I): WMD ‐7.55 (95% CI ‐12.10 to ‐2.99; P < 0.05) (fixed effect model). There was significant heterogeneity (I2 = 81%) (Comparison 03‐08); ‐ AST (U/I): WMD ‐3.78 (95% CI ‐15.76 to 8.20) (random effects model) (Comparison 03‐08); ‐ ALT (U/L): WMD ‐6.35 (95% CI ‐10.26 to ‐2.44; P < 0.05) (fixed effect model). There was significant heterogeneity (I2 = 67%) (Comparison 03‐09); ‐ ALT (U/L): WMD ‐3.96 (95% CI ‐12.59 to 4.68) (random effects model) (Comparison 03‐09). When focusing on high‐quality trials only, no significant beneficial effects of milk thistle on AST or ALT activity were found (data not shown).
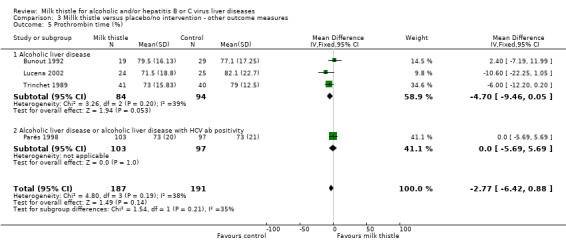
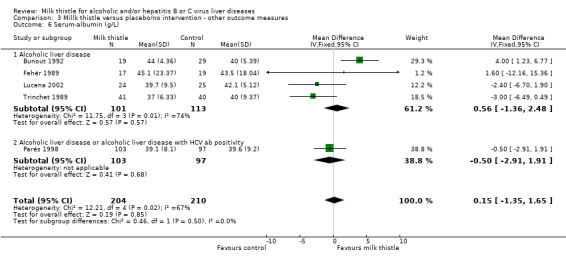
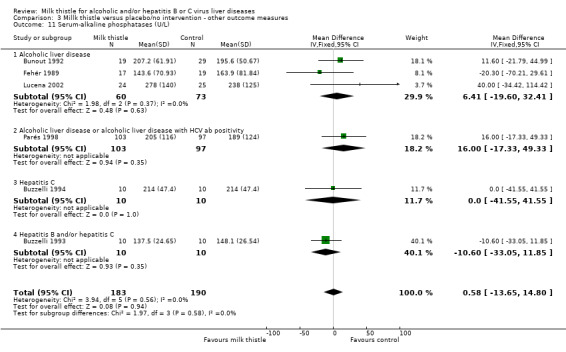
Milk thistle did not significantly influence: ‐ prothrombin time (%): WMD ‐2.77 (95% CI ‐6.42 to 0.88) (fixed effect model). There was no significant heterogeneity (Comparison 03‐05); ‐ s‐albumin (g/L): WMD 0.15 (95% CI ‐1.35 to 1.65) (fixed effect model). There was significant heterogeneity (I2 = 67%) (Comparison 03‐06); ‐ AP (U/l): WMD 0.58 (95% CI ‐13.65 to 14.80) (fixed effect model). There was no significant heterogeneity (Comparison 03‐11).
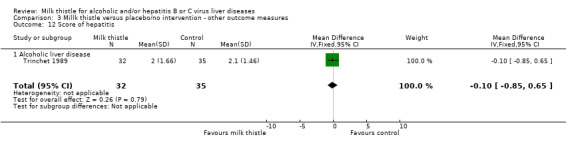
Liver histology There were no significant effects of milk thistle on hepatitis or fibrosis of liver biopsy findings in the only trial reporting this outcome (Trinchet 1989): ‐ liver biopsy change (hepatitis): WMD ‐0.10 (95% CI ‐0.85 to 0.65) (Comparison 03‐12); ‐ liver biopsy change (fibrosis): WMD 0.00 (95% CI ‐13.65 to 14.80) (Comparison 03‐13).
Adverse events In the milk thistle group 0/456 patients had serious adverse events versus 0/459 patient in the control group (Comparison 04‐01). Milk thistle had no significant effect on the occurrence of non‐serious adverse events (RR 0.83, 95% CI 0.46 to 1.50). In the milk thistle group, 16/456 (3.5%) patients had non‐serious adverse events versus 20/459 (4.4%) patients in the control group (Comparison 04‐02). The adverse events observed in the milk thistle group encompassed impotence (one patient), pruritus (four patients), cephalea (three patients), and nausea and epigastric discomfort (one patient). The authors did not report the type of adverse event in seven patients. The adverse events observed in the control group encompassed pruritus (11 patients), cephalea (four patients), and nausea and epigastric discomfort (one patient). The authors did not report the type of adverse events in four patients.
Quality of life and health economics None of the randomised clinical trials reported quality of life or health economics outcomes.
Funnel plot asymmetry Additional Figure 1 shows a funnel plot of the five trials reporting on mortality. From a visual inspection one gets the impression that the smaller the trial the larger the intervention effect. Due to the paucity of mortality in a number of the randomised clinical trials we did not try to analyse for funnel plot asymmetry.
1.
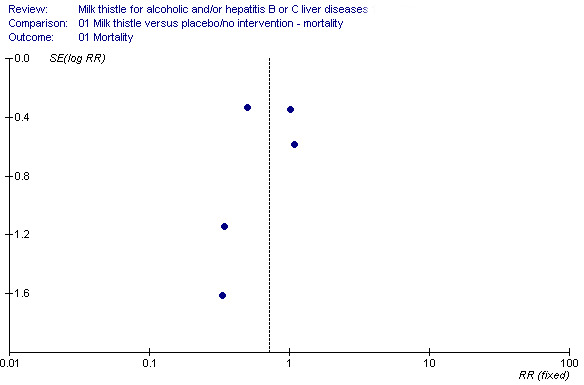
Funnel plot of five trials on milk thistle for liver diseases
Discussion
We found no significant effect of milk thistle on overall mortality when all trials were combined or in high‐quality trials. We observed a potential beneficial effects of milk thistle on mortality in patients with alcoholic liver disease, but this effect could not be demonstrated in high‐quality trials. We also observed a potential beneficial effect of milk thistle on liver‐related deaths, but again this effect could not be demonstrated in high‐quality trials. Further, we found benefit of milk thistle on some biochemical liver tests, but these observations could not be confirmed in high‐quality trials. As the methodological quality of the majority of the trials was low, bias and/or random errors may explain some or all of our positive findings.
Our observations mainly confirm two recent meta‐analyses on milk thistle for patients with liver disease of any cause, ie, no significant beneficial effect of milk thistle (Lawrence 2000; Jacobs 2002). This is in spite of the fact, that the present systematic review included five more randomised clinical trials (Salvagnini 1985; Buzzelli 1994; Velussi 1997; Lirussi 2002; Lucena 2002) and did not use data from quasi‐randomised clinical trials (Jacobs 2002), which may significantly bias estimates of interventions effects (Kjaergard 2003; Kunz 2002).
We observed a statistically significant reduction in mortality in the patients with alcoholic liver disease and a significant reduction in liver‐related mortality among all patients. We found no significant effect of milk thistle on mortality in patients with alcoholic liver disease or on liver‐related mortality when we focused on high‐quality trials. It has previously been demonstrated that the effects of many interventions are significantly overestimated in low‐quality trials (Schulz 1995; Moher 1998; Kjaergard 2001). Further, these effects could neither be confirmed in a subgroup analysis including patients with alcoholic liver disease coinfected by HCV nor in a worst‐case scenario analysis. Therefore our findings are not robust enough to form a fundament for therapeutic recommendations. On the positive side, milk thistle did not differ significantly from placebo/no intervention regarding adverse events.
The observation that overall mortality is no different but liver‐related mortality seems to improve improved appears to be a mutual contradiction. One of the reasons could be that some of the trials did not report liver‐related mortality.
We found a significant beneficial effect of milk thistle in improving bilirubin and s‐gamma‐glutamyl transferase. For the remainder of our analysis on liver biochemistry markers, milk thistle either had effects that were dependent on the method of meta‐analysis (fixed effect or random effects) or had no significant effects. Focusing on high‐quality trials, no significant effects could be demonstrated. In all circumstances, the effects of milk thistle were not dramatic.
This systematic review has a number of potential limitations. First, the small sample size limits the power of our meta‐analyses. The confidence interval for the pooled estimate is sufficiently wide, which means that a substantial benefit or harm cannot be excluded. Evidence shows how much effects of medical intervention may change over time. Ioannidis and Lau (Ioannidis 2001) applied 'recursive cumulative meta‐analyses' of randomised clinical trials to evaluate the relative change in the pooled treatment effect over time for 60 medical interventions within pregnancy/perinatal medicine and cardiology. With 500 accumulated patients, the pooled relative risk may change by about 0.6‐ to 1.7‐fold in the immediate future. When 2000 patients have been randomised, the pooled relative risk may change by 0.7‐ to 1.3‐fold. At present, only about 1000 patients with alcoholic liver disease and/or hepatitis B and C have been randomised to milk thistle versus placebo or no intervention. Second, we chose to include only alcoholic liver disease and viral liver disease in the review. The major reason is that viral and alcohol‐related liver disease frequently coexist in the same patient. Several trials were old and did not check for viral liver disease in patients with suspected alcoholic liver disease. Further, hepatitis B or C marker positivity was not an exclusion criterion for the entry of the patient in one of the trials on patients with alcoholic liver disease (Parés 1998). Other liver diseases like non‐alcoholic liver disease and toxic liver diseases should be considered in other reviews.
Among the randomised clinical trials reporting adverse drug events, milk thistle appeared safe and well‐tolerated. We recognise it is difficult to interpret the risk of adverse events from the literature for several reasons (Gluud 2002). Events may be missed since search terms related to adverse events are often not indexed, and causality is difficult to discern when events are published in a case report or case series. However, considering that among the excluded studies there were some randomised clinical trials considering unspecified form of liver diseases like the one of Tanasescu et al (Tanasescu 1988) with 180 patients, milk thistle seems to be well tolerated, although adverse events are reported in the literature (Geier 1990; Vailati 1993).
If milk thistle does not work for alcoholic liver diseases, which drug therapy can we offer these patients for their liver disease? Meta‐analyses and randomised clinical trials have been unable to demonstrate significant beneficial effects of colchicine (Rambaldi 2001a), anabolic‐androgenic steroids (Rambaldi 2002a), propylthiouracil (Rambaldi 2002b), glucocorticosteroids (Christensen 1995; Gluud 2001), insulin/glucagon (Trinchet 1992), parenteral amino acid supplementation (Mezey 1991), amlodipine (Bird 1998), and polyenylphosphatidylcholine (Lieber 2001; Lieber 2003b) for alcoholic liver disease. A recent trial has demonstrated that ursodeoxycholic acid is detrimental in patients with alcoholic liver disease (Pelletier 2003). At present, S‐adenosyl‐L‐methionine (Mato 1999), pentoxifylline (Akriviadis 2000), and potentially milk thistle may seem as promising interventions. However, more randomised clinical trials are needed before S‐adenosyl‐L‐methionine can be recommended (Rambaldi 2001b). This also applies to pentoxifylline, which has only been evaluated in one randomised trial including patients with severe alcoholic hepatitis, and to milk thistle, for which there is insufficient evidence. However, absence of evidence is not the same as evidence of absence of effect. Future trials on milk thistle should have adequate sample size, enrol patients with well‐defined liver disease, and devote adequate resources to monitor outcomes. At the present time, there is insufficient evidence to support or refute milk thistle for patients for the treatment of alcoholic liver diseases.
If milk thistle does not work for viral hepatitis B or C either, which drug therapy can we offer these patients for their liver diseases? Treatment decisions should be based on recent recommendations for treatment of acute and chronic hepatitis B (Pianko 2000; Zavaglia 2000; Liaw 2003) and of acute and chronic hepatitis C (Pianko 2000; Zavaglia 2000; Di Bisceglie 2002; Brok 2005a; Brok 2005b).
Authors' conclusions
Implications for practice.
We cannot recommend the use of milk thistle for acute or chronic alcoholic and/or hepatitis B or C virus liver diseases outside randomised clinical trials.
Implications for research.
Based on this review, milk thistle could potentially affect alcoholic and/or hepatitis B or C virus liver diseases. Therefore, large‐scale randomised clinical trials on milk thistle for alcoholic and/or hepatitis B or C liver diseases versus placebo are needed. Such trials ought to be performed with adequate methodologies (ie, generation of the allocation sequence; allocation concealment; blinding; intention‐to‐treat analyses). The randomised trials should consider including patients with alcoholic cirrhosis and should stratify patients at randomisation according to hepatitis B and hepatitis C status, the degree of liver injury, and the degree of alcoholism. Such trials should examine relevant outcomes. Based on this review such randomised clinical trials need to be large in order to be able to detect any effect. Finally, such trials ought to follow the Consolidated Standards for Reporting Trials (CONSORT) Statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Converted to new review format. |
Notes
We have contacted the following companies in order to obtain additional data, published or unpublished:
Istituto Biochimico Italiano Giovanni Lorenzini Spa Via Tucidide 56 Torre 6 Milano Italy
Madaus Via Galvani 33 39100 Bolzano Italy
Acknowledgements
We are indebted to Nader Salasshari for the expert technical computer assistance and to Dimitrinka Nikolova and Sarah Frederiksen for expert assistance with the retrieval of publications. Special thanks to Flavio Lirussi, M.I. Lucena, and A. Parés for providing us with more information on the trials they were involved in.
Data and analyses
Comparison 1. Milk thistle versus placebo/no intervention ‐ mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 1.1 Alcoholic liver disease | 9 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 1.2 Alcoholic liver disease or alcoholic liver disease with positive HCV antibodies | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.62, 1.99] |
| 1.3 Hepatitis B | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Hepatitis C | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Hepatitis B and/or hepatitis C | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Subgroup analysis ‐ Generation of the allocation sequence | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 2.1 Adequate generation of the allocation sequence | 6 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 2.2 Unclear generation of the allocation sequence | 7 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Subgroup analysis ‐ Allocation concealment | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 3.1 Adequate allocation concealment | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.65] |
| 3.2 Unclear allocation concealment | 10 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.08] |
| 4 Subgroup analysis ‐ Blinding | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 4.1 Adequately blinded | 6 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.12] |
| 4.2 Unclearly blinded or nonblinded | 7 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.34, 3.43] |
| 5 Subgroup analysis ‐ Follow‐up | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 5.1 Adequate follow‐up | 12 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 5.2 Unclear or inadequate follow‐up | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Subgroup analysis ‐ Stratification of trials according to methodological quality | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 6.1 All four components adequate | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.65] |
| 6.2 One or more components adequate | 9 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.08] |
| 6.3 No adequate components | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Subgroup analysis ‐ Stage | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 7.1 Chronic | 12 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 7.2 Acute | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Subgroup analysis ‐ Duration of treatment | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 8.1 Short‐term treatment (less than six months) | 6 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| 8.2 Long‐term treatment (at least six months) | 7 | 579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.20] |
| 9 Subgroup analysis ‐ Different formulation | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 9.1 Silymarin (Legalon®) | 10 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 9.2 IdB1016 (Silipide®) | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Silybin‐beta‐cyclodextrin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Subgroup analysis ‐ Worst‐case scenario analysis | 13 | 915 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.75, 1.58] |
| 11 Alcoholic liver disease ‐ high‐quality trials | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.06, 2.11] |
| 11.1 All four components adequate | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.06, 2.11] |
| 12 Explorative sensitivity analysis ‐ Addition of two excluded trials | 15 | 1085 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 12.1 Alcoholic liver disease | 9 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 12.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.62, 1.99] |
| 12.3 Hepatitis B | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Hepatitis C | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Hepatitis B and/or hepatitis C | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 Liver disease mostly due to alcoholic liver disease | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
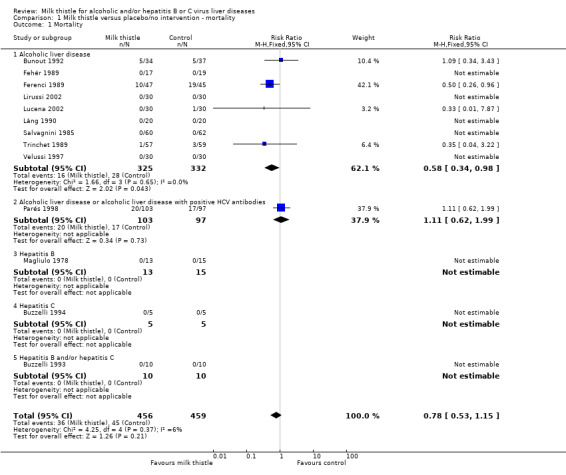
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 1 Mortality.
1.2. Analysis.
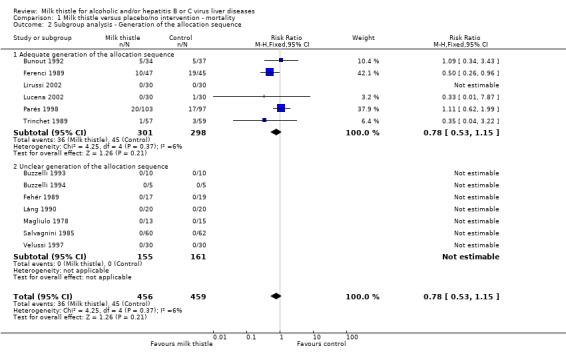
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 2 Subgroup analysis ‐ Generation of the allocation sequence.
1.3. Analysis.
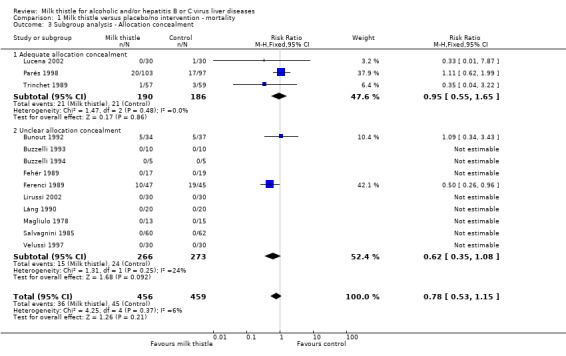
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 3 Subgroup analysis ‐ Allocation concealment.
1.4. Analysis.
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 4 Subgroup analysis ‐ Blinding.
1.5. Analysis.
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 5 Subgroup analysis ‐ Follow‐up.
1.6. Analysis.
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 6 Subgroup analysis ‐ Stratification of trials according to methodological quality.
1.7. Analysis.
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 7 Subgroup analysis ‐ Stage.
1.8. Analysis.
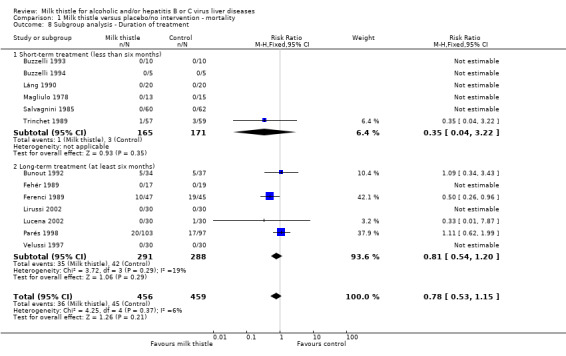
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 8 Subgroup analysis ‐ Duration of treatment.
1.9. Analysis.
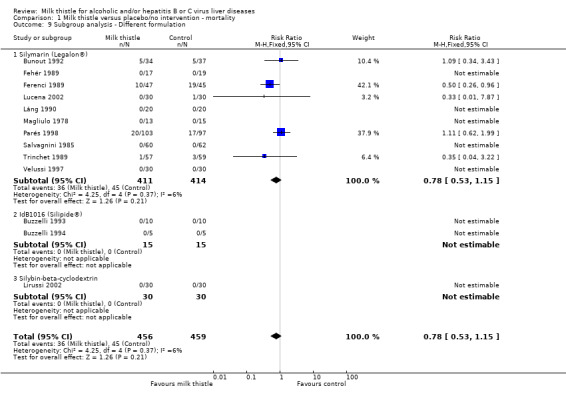
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 9 Subgroup analysis ‐ Different formulation.
1.10. Analysis.
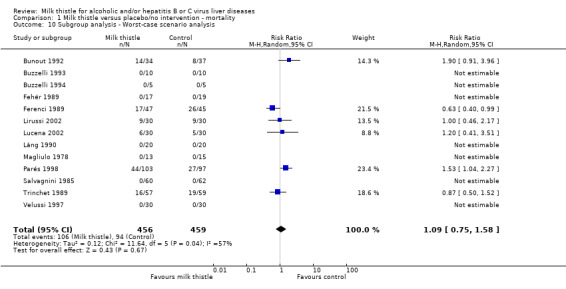
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 10 Subgroup analysis ‐ Worst‐case scenario analysis.
1.11. Analysis.
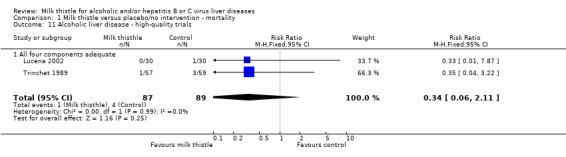
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 11 Alcoholic liver disease ‐ high‐quality trials.
1.12. Analysis.
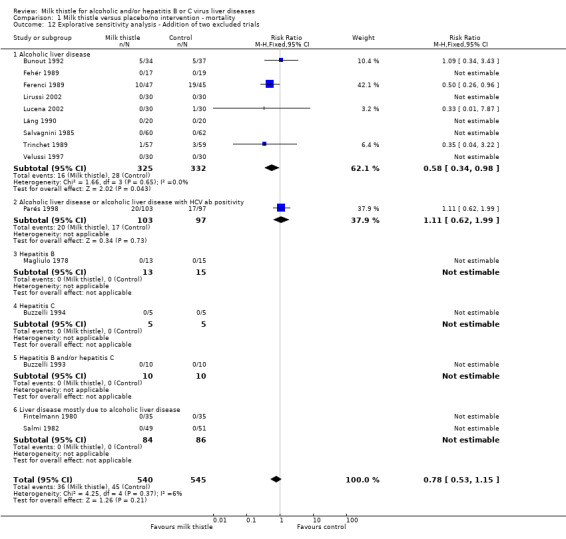
Comparison 1 Milk thistle versus placebo/no intervention ‐ mortality, Outcome 12 Explorative sensitivity analysis ‐ Addition of two excluded trials.
Comparison 2. Milk thistle versus placebo/no intervention ‐ liver‐related mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Liver‐related mortality | 12 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.88] |
| 1.1 Alcoholic liver disease | 8 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.18, 0.86] |
| 1.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
| 1.3 Hepatitis B | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Hepatitis C | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Hepatitis B and/or hepatitis C | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Subgroup analysis ‐ Stratification of trials according to methodological quality | 12 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.88] |
| 2.1 All four components adequate | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.28, 1.19] |
| 2.2 One or more components adequate | 8 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.17, 0.97] |
| 2.3 No adequate components | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Subgroup analysis ‐ Worst‐case scenario in patients with alcoholic liver disease | 8 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.13] |
2.1. Analysis.
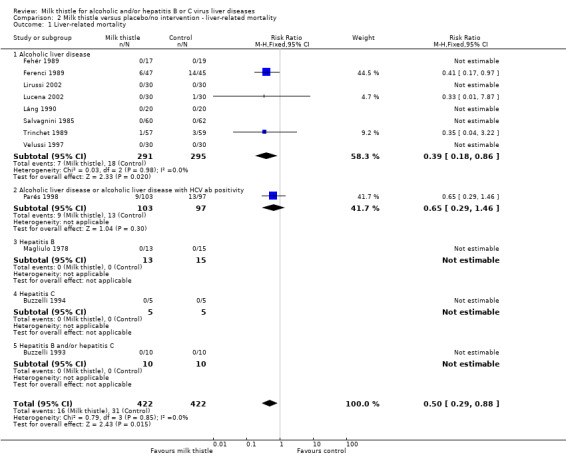
Comparison 2 Milk thistle versus placebo/no intervention ‐ liver‐related mortality, Outcome 1 Liver‐related mortality.
2.2. Analysis.
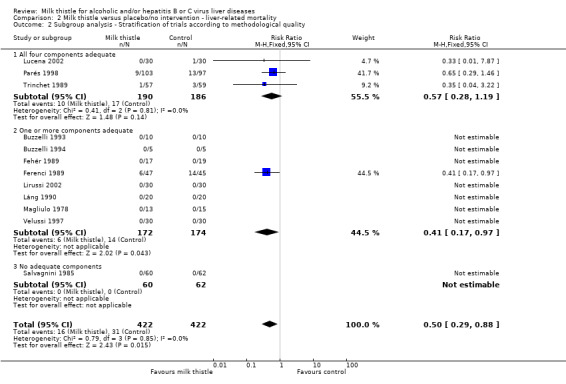
Comparison 2 Milk thistle versus placebo/no intervention ‐ liver‐related mortality, Outcome 2 Subgroup analysis ‐ Stratification of trials according to methodological quality.
2.3. Analysis.
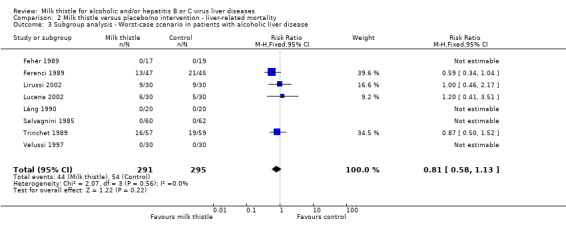
Comparison 2 Milk thistle versus placebo/no intervention ‐ liver‐related mortality, Outcome 3 Subgroup analysis ‐ Worst‐case scenario in patients with alcoholic liver disease.
Comparison 3. Millk thistle versus placebo/no intervention ‐ other outcome measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ascites | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 1.1 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 2 Hepatic encephalophaty | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.55, 2.16] |
| 2.1 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.55, 2.16] |
| 3 Gastro‐intestinal bleeding | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.34] |
| 3.1 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.34] |
| 4 Any complications | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| 4.1 Alcoholic liver disease | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.71] |
| 4.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.10] |
| 5 Prothrombin time (%) | 4 | 378 | Mean Difference (IV, Fixed, 95% CI) | ‐2.77 [‐6.42, 0.88] |
| 5.1 Alcoholic liver disease | 3 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐9.46, 0.05] |
| 5.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐5.69, 5.69] |
| 6 Serum‐albumin (g/L) | 5 | 414 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐1.35, 1.65] |
| 6.1 Alcoholic liver disease | 4 | 214 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐1.36, 2.48] |
| 6.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.91, 1.91] |
| 7 Serum‐bilirubin (µmol/L) | 8 | 494 | Mean Difference (IV, Fixed, 95% CI) | ‐4.68 [‐7.72, ‐1.64] |
| 7.1 Alcoholic liver disease | 5 | 254 | Mean Difference (IV, Fixed, 95% CI) | ‐4.57 [‐7.65, ‐1.50] |
| 7.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐10.68 [‐32.44, 11.08] |
| 7.3 Hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐72.85, 76.05] |
| 7.4 Hepatitis B and/or hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.92 [‐49.21, 41.37] |
| 8 Serum‐aspartate aminotransferase (U/L) | 8 | 494 | Mean Difference (IV, Fixed, 95% CI) | ‐7.55 [‐12.10, ‐2.99] |
| 8.1 Alcoholic liver disease | 5 | 254 | Mean Difference (IV, Fixed, 95% CI) | ‐11.68 [‐16.93, ‐6.43] |
| 8.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐1.84, 17.84] |
| 8.3 Hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐36.66, 52.66] |
| 8.4 Hepatitis B and/or hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐24.60 [‐55.19, 5.99] |
| 9 Serum‐alanine aminotransferase (U/L) | 6 | 365 | Mean Difference (IV, Fixed, 95% CI) | ‐6.35 [‐10.26, ‐2.44] |
| 9.1 Alcoholic liver disease | 3 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐9.29 [‐13.61, ‐4.98] |
| 9.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐0.84, 18.84] |
| 9.3 Hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐57.40, 53.40] |
| 9.4 Hepatitis B and/or hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐38.06, 22.06] |
| 10 Serum‐gamma‐glutamyl transferase (U/L) | 10 | 658 | Mean Difference (IV, Fixed, 95% CI) | ‐26.80 [‐32.86, ‐20.73] |
| 10.1 Alcoholic liver disease | 7 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐31.93 [‐38.66, ‐25.21] |
| 10.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 38.0 [2.17, 73.83] |
| 10.3 Hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 14.00 [‐20.65, 48.65] |
| 10.4 Hepatitis B and/or hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐18.40 [‐35.41, ‐1.39] |
| 11 Serum‐alkaline phosphatases (U/L) | 6 | 373 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐13.65, 14.80] |
| 11.1 Alcoholic liver disease | 3 | 133 | Mean Difference (IV, Fixed, 95% CI) | 6.41 [‐19.60, 32.41] |
| 11.2 Alcoholic liver disease or alcoholic liver disease with HCV ab positivity | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [‐17.33, 49.33] |
| 11.3 Hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐41.55, 41.55] |
| 11.4 Hepatitis B and/or hepatitis C | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐10.60 [‐33.05, 11.85] |
| 12 Score of hepatitis | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.85, 0.65] |
| 12.1 Alcoholic liver disease | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.85, 0.65] |
| 13 Score of fibrosis | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.55, 0.55] |
| 13.1 Alcoholic liver disease | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.55, 0.55] |
3.1. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 1 Ascites.
3.2. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 2 Hepatic encephalophaty.
3.3. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 3 Gastro‐intestinal bleeding.
3.4. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 4 Any complications.
3.5. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 5 Prothrombin time (%).
3.6. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 6 Serum‐albumin (g/L).
3.7. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 7 Serum‐bilirubin (µmol/L).
3.8. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 8 Serum‐aspartate aminotransferase (U/L).
3.9. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 9 Serum‐alanine aminotransferase (U/L).
3.10. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 10 Serum‐gamma‐glutamyl transferase (U/L).
3.11. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 11 Serum‐alkaline phosphatases (U/L).
3.12. Analysis.
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 12 Score of hepatitis.
3.13. Analysis.
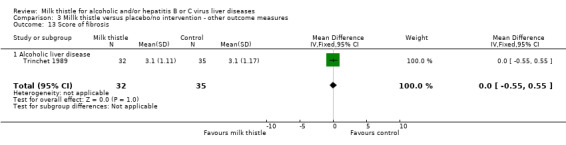
Comparison 3 Millk thistle versus placebo/no intervention ‐ other outcome measures, Outcome 13 Score of fibrosis.
Comparison 4. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Non‐serious adverse events | 13 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.50] |
4.1. Analysis.
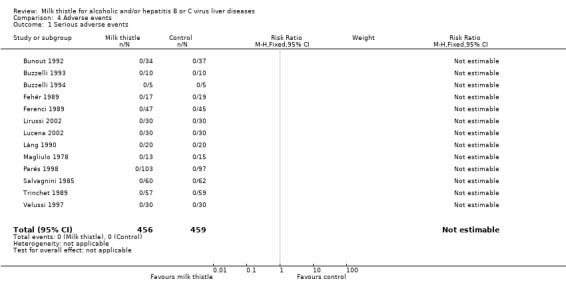
Comparison 4 Adverse events, Outcome 1 Serious adverse events.
4.2. Analysis.
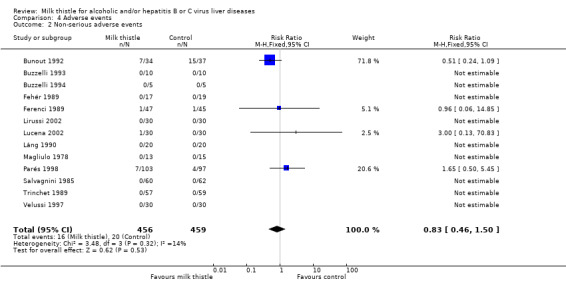
Comparison 4 Adverse events, Outcome 2 Non‐serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bunout 1992.
| Methods | Sample size: no justification. Generation of the allocation sequence: adequate, by random number tables. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: not used. |
|
| Participants | Seventy‐one patients with alcoholic liver disease. Thirty‐four patients were allocated to the silymarin while 37 to the placebo group. Chronic liver disease. Inclusion criteria: 1) alcohol intake of at least 150 gr/day; 2) at least three crisis of alcohol misuse each month; 3) advanced chronic alcoholic liver disease. Exclusion criteria: 1) HBsAg positive patients; 2) kidney failure; 3) cardiac failure; 4) end‐stage liver failure. |
|
| Interventions | MT group:
silymarin tablets 140 mg, two times daily (280 mg per day). Control group: placebo tablets, two times daily. Duration of treatment and of follow‐up: 15 months. |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | Letter to the trialist: sent (August 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Buzzelli 1993.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: adequate, double blind with identical placebo. Follow‐up: adequate, less than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Twenty patients (6 males, 14 females, mean age 53± 3.0 years, range 31‐70) with HBV and/or HCV chronic active hepatitis. Chronic liver disease. Inclusion criteria: 1) histologically chronic active hepatitis; 2) increased AST and/or ALT serum activities (twice to sixfold the upper limit of the reference range) for more than 12 months; 3) age range: 30 to 70 years. Exclusion criteria: 1) portal hypertension; 2) hepatic encephalopathy; 3) ascites; 4) hepatocellular carcinoma; 5) clinical signs and biochemical parameters of cholestasis; 6) drug addiction; 7) positive antinuclear, antimitochondrial, and antismooth muscle antibodies; 8) ethanol intake more than 30 gr per day; 9) malabsorption syndromes; 10) cardiovascular, renal or endocrine disorders; 11) pregnancy; 12) any pharmacological treatment three months before the beginning of the trial. |
|
| Interventions | MT group:
IdB1016 two capsules, twice a day (equivalent to 120 mg of silybin in each capsule) (480 mg per day).
IdB1016 is a lipophilic complex with phosphatidylcholine and silybin in a molar ratio of 1:1. Control group: placebo, 2 capsules twice a day. Duration of treatment and of follow‐up: seven days. Eight patients were also treated for two months in total. No adverse events occurred in these patients. |
|
| Outcomes | Mortality. Liver biochemistry. Adverse events. | |
| Notes | Letter to the trialist: sent (August 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Buzzelli 1994.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, less than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Ten patients (8 males and 2 females, mean age 59 years) with chronic hepatitis C. Chronic liver disease. Inclusion criteria: 1) chronic hepatitis C; 2) no significant variations of AST and ALT (non‐responders) to a previous treatment with recombinant interferon alpha 2B (3 million units thrice weekly for 6 months) (6 months withdrawal). |
|
| Interventions | MT group:
Silipide® (IdB1016) capsules 360 mg per day. Control group: placebo capsules. Duration of treatment and follow‐up: two months of treatment and one month of washout. |
|
| Outcomes | Mortality. Liver biochemistry. Adverse events. | |
| Notes | Trial characteristics:
cross over design. Patients were assigned to the Silipide® group for two months treatment, and one month washout.
Results were not reported separately, we give overall results. Only published as abstract. Letter to the trialist: sent (August 2002). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fehér 1989.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, less than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Thirty‐six patients with compensated alcoholic liver cirrhosis. Of these 17 patients were allocated to the silymarin group (15 males and two females, mean age 48± 7 years), while 19 to the placebo group (12 males and seven females, mean age 44± 6 years). Chronic liver disease. |
|
| Interventions | MT group:
silymarin tablets (Legalon®) 140 mg, three times tablets daily (420 mg per day). Control group: placebo, three times daily. Patients were discouraged from consuming alcoholic beverages. Duration of treatment and follow‐up: six months. |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | Letter to the trialist: sent (August 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ferenci 1989.
| Methods | Sample size: no justification. Generation of the allocation sequence: adequate, according to a random‐number sequence. Allocation concealment: unclear, not described. Blinding: adequate, double blind with placebo of identical appearance. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Multicentre clinical trial including patients from four medical departments. Of these 92 patients 47 were allocated to silymarin group and 45 to the placebo group. Chronic liver disease. Inclusion criteria: liver cirrhosis diagnosed by biopsy in 70% of the patients. In the remaining patients no liver biopsy could be obtained due to coagulation disorders. The severity of the underlying liver disease was classified using Child‐Turcotte criteria. Exclusion criteria: 1) end‐stage liver failure; 2) known malignancies; 3) immunosuppressive treatment. The use of steroids and of D‐penicillamine was not allowed. Patients were recruited from all the patients seen at one of the four participating centres. |
|
| Interventions | MT group:
silymarin tablets (Legalon®) 140 mg, three times daily (420 mg per day). Control group: placebo tablets, three times per day. Patients were advised not to drink alcoholic beverages. Alcohol consumption was estimated and blood levels monitored. The use of steroids and of D‐penicillamine was not allowed. Mean duration of treatment and of follow‐up: 41 months (range, 2 to 6 years). |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | 170 patients with cirrhosis of the liver were included in the study originally from the authors. The data on 78 patients with liver cirrhosis of unknown etiology are not extracted in our Systematic Review. Letter to the trialist: sent (August 2002). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fintelmann 1980.
| Methods | We exclude this randomised clinical trial because the etiology is toxic liver disease. We report this randomised clinical trial, however, only to be able to include it in a exploratory analysis as the toxic liver disease was mostly due to alcoholic liver disease. Sample size: no justification. Generation of the allocation sequence: adequate, by random table. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, less than 10% of patients dropped out or were withdrawn Intention‐to‐treat analysis: not used. |
|
| Participants | Clinical trial including 70 patients; 35 were treated with silymarin while 35 received placebo. Inclusion criteria: liver biopsy proven toxic liver disease of any cause, mostly alcoholic liver disease. |
|
| Interventions | MT group:
silymarin tablets (Legalon®), no dosage was given. Control group: placebo. Collateral interventions: diet with 1000 kgcal/day Duration of treatment: 28 days. |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lirussi 2002.
| Methods | Sample size: no justification. Generation of the allocation sequence: adequate, by random table. Allocation concealment: unclear, not described. Blinding: adequate, double blind with placebo of identical appearance. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Sixty out‐patients with chronic alcoholic liver disease and non‐insulin dependent type 2 diabetes were enrolled in a three centre study.
Forty‐two out‐patients (21 in the treatment group, and 21 in the placebo group) concluded the treatment period. Chronic liver disease. Inclusion criteria: 1) more than 60 to 80 grams of daily alcohol intake for at least 5 years; 2) biochemistry and ultrasound of the liver; 3) transaminase level not more twice the upper limit of normal values and body mass index less than 31 Kg/m2. Exclusion criteria: 1) decompensated liver cirrhosis; 2) presence of antibodies to hepatitis B or hepatitis C; 3) autoimmune liver diseases; 4) Wilson's disease; 5) alfa1‐antitrypsin deficiency; 6) liver neoplasms; 7) porphyria cutanea tarda; 8) impaired renal function, 9) heart failure, 10) insulin treatment, 11) alcohol abuse. |
|
| Interventions | MT group:
Silybin‐beta‐cyclodextrin (Lorenzini, Milan, Italy) sachets three times per day ‐ 135 mg silybin per day. Control group: placebo. Duration of treatment: six months. |
|
| Outcomes | Mortality. Liver biochemistry. Adverse events. | |
| Notes | Letter to the trialist: sent December 2003. F. Lirussi answered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lucena 2002.
| Methods | Sample size: no justification. Generation of the allocation sequence: adequate, computer generated. Allocation concealment: adequate, randomisation labels were kept in sealed envelopes. Blinding: adequate, double blind with placebo of identical appearance. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: not used. |
|
| Participants | Multicentre clinical trial including 122 consecutive in‐patients from five clinical departments. Chronic liver disease. Inclusion criteria: chronic alcohol abuse and hospitalisation for liver disease. Exclusion criteria: HBsAg positivity and/or patients with decompensated liver cirrhosis. |
|
| Interventions | MT group:
silymarin MZ‐80 tablets (Legalon®) 150 mg, three times per day (450 mg per day). Control group: placebo tablets, three times per day. Patients were advised not to drink alcoholic beverages. All patients who completed the trial reported abstinence from alcohol during the study period. Duration of treatment and of follow‐up: six months. |
|
| Outcomes | Mortality.
Liver biochemistry.
Histology.
Adverse events. Alcohol consumption was estimated and blood alcohol levels monitored. |
|
| Notes | Letter to the trialist: sent (August 2002). M.Isabel Lucena answered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Láng 1990.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, less than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Forty patients with compensated alcoholic liver cirrhosis. Of these 40 patients, 20 (16 males and 4 females, mean age 46.8 years) were allocated to silymarin group and 20 (12 males and eight females, mean age 44.4 years) to the placebo group. Inclusion criteria: histological micronodular cirrhosis. The mean alcohol consumption exceed 60 gr in males and 30 gr in females. Duration of alcohol consumption was between 6‐11 years (mean 8.6 years). Chronic liver disease. Exclusion criteria: symptoms of vascular and/or parenchymal decompensation and HBsAg positivity. |
|
| Interventions | MT group:
silymarin tablets (Madaus Cerafarm, Barcelona, Spain) 140 mg, three times tablets daily (420 mg per day).
The non‐standardized silymarin MZ 80 is obtained by reformulation of non‐active plant ingredients using new excipients that enhance humectation, dissolution and availability of the main active agent (70‐80% of the silymarin complex; silibin 35.07%). Control group: placebo tablets, three times daily. Alcohol consumption was registered by careful detailed interview. Patients were discouraged from consuming alcoholic beverages. Duration of treatment and follow‐up: one month. |
|
| Outcomes | Mortality. Liver biochemistry. Adverse events. | |
| Notes | The data on the 20 patients treated with the flavonoid synthetical derivate Aica‐P are excluded. Letter to the trialist: sent (August 2002). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magliulo 1978.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Two‐centres clinical trial from Italy including fifty‐nine patients with acute viral hepatitis A or B.
Thirteen patients with acute hepatitis B were allocated to the treatment group, while the other fifteen patients were allocated to the placebo group. Acute liver disease. |
|
| Interventions | MT group:
silymarin two tablets 70 mg, three times daily (420 mg per day). Control group: placebo tablets, three times daily. Duration of treatment and of follow‐up: 28 days. |
|
| Outcomes | Mortality. Liver biochemistry. Adverse events. | |
| Notes | The data on 31 patients with acute hepatitis A are excluded. Letter to the trialist: sent (August 2002). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Parés 1998.
| Methods | Sample size: no justification. Generation of the allocation sequence: adequate, according to a random‐number sequence table. Allocation concealment: adequate, the assigned treatment was obtained from the coordinating centre by telephone (central randomisation). Blinding: adequate, double blind with placebo of identical appearance, smell and taste. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Multicentre clinical trial including 200 patients from six hospitals from Catalonia with alcoholic liver cirrhosis. 103 were allocated to silymarin and 97 to the placebo group. Chronic liver disease. Inclusion criteria: chronic alcoholism defined by a daily alcohol intake over 80 gr in men and 60 gr in women for a period longer than 5 years. Criteria for liver cirrhosis were supported by histology performed within the three months before inclusion in the trial; or by laparoscopic examination in those patients with very low prothrombin index or platelet count. Exclusion criteria: 1) previous treatment with colchicine, malotilate, penicillamine, corticosteroids; 2) life expectancy less than 6 months; 3) drug addiction; 4) pregnancy. Patients with other known etiologies for liver cirrhosis such as hepatitis B, autoimmunity, primary biliary cirrhosis or cryptogenic cirrhosis were excluded as well. All patients were advised to abstain from alcohol. Alcohol intake was estimated by questioning the patients and their relatives about the amount of alcohol consumed and by assessing alcohol in urine before and during the study. |
|
| Interventions | MT group:
silymarin tablets (Legalon®) 150 mg three times daily (450 mg per day). Control group: placebo tablets. Duration of treatment: two years. Duration of follow‐up: five years. |
|
| Outcomes | Mortality. Histology. Liver biochemistry. Adverse events. | |
| Notes | Antibodies against hepatitis C virus were assessed by third‐generation ELISA test (Ortho Diagnostics, Raritan, NJ, USA) in stored sera from 75 patients after completion of the trial. Twenty‐none of the 75 patients resulted positive (13 receiving silymarin and 16 receiving placebo). Letter to the trialist: sent (August 2002). A. Parés answered. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Salmi 1982.
| Methods | We exclude this randomised clinical trial because the etiology is mixed. We report this randomised clinical trial only to be able to include it in exploratory analysis as most of the patients had alcoholic liver disease. Sample size: no justification. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: adequate, less than 10% of patients dropped out or were withdrawn. Intention‐to‐treat analysis: not used. |
|
| Participants | One hundred and six consecutive military personnel admitted to a military hospital in Finland after serving a United Nation peace‐keeping force in Cyprus or Sinai with raised transaminases levels. The majority (81% in the experimental arm and 76% in the placebo arm admitted previous alcohol consumption). Forty‐nine patients treated with silymarin and 52 with placebo. Inclusion criteria: AST, ALT and GGT raised above the upper normal limit despite one month order to abstain from alcohol. Exclusion criteria: not mentioned. |
|
| Interventions | MT group:
silymarin tablets (Legalon®), 420 mg per day. Control group: placebo. Duration of treatment and of follow‐up: four weeks. |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Salvagnini 1985.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: unclear, described as double blind but the method to achieve this not described. Follow‐up: inadequate, not described. Intention‐to‐treat analysis: used. |
|
| Participants | Multicentre clinical trial including 122 consecutive in‐patients from five clinical departments. Chronic liver disease. Inclusion criteria: chronic alcohol abuse and hospitalisation for liver disease. Exclusion criteria: HBsAg positivity and/or patients with decompensated liver cirrhosis. |
|
| Interventions | MT group:
silymarin tablets 140 mg, three times daily (420 mg per day). Control group: placebo tablets, three times daily. All patients were requested to abstain from consuming alcohol. Duration of treatment and of follow‐up: 45 days. |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | Only published as abstract. Letter to the trialist: sent (August 2002). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trinchet 1989.
| Methods | Sample size: based on liver histology response. Randomisation: patients were stratified for presence or absence of liver cirrhosis in initial liver biopsy. Generation of the allocation sequence: adequate, by random tables. Allocation concealment: adequate, with sealed envelopes. Blinding: adequate, double blind with placebo of identical presentation. Follow‐up: adequate, more than 10% dropped‐out or were withdrawn. Intention‐to‐treat: used. |
|
| Participants | Multicentre clinical trial including patients from three medical departments. Chronic liver disease. Inclusion criteria: 116 patients with histologically proven alcoholic hepatitis, 58 of them with alcoholic cirrhosis. 78 were males and 38 females with a mean age of 50± 18 years in the silymarin and of 51± 11 years in the placebo group. Exclusion criteria: 1) hepatic encephalopathy; 2) resistant ascites; 3) prothrombin activity< 50%; 4) platelet count< 100 billion/L; 5) hepatocellular carcinoma; other important diseases or refusal to participate. |
|
| Interventions | MT group:
silymarin tablets 140 mg, three times daily (420 mg per day). Control group: placebo tablets, three times per day. Duration of treatment and of follow‐up: three months. |
|
| Outcomes | Mortality. Liver biochemistry. Histology. Adverse events. | |
| Notes | Letter to the trialist: sent (August 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Velussi 1997.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear. Allocation concealment: unclear, not described. Blinding: inadequate, not blinded. Follow‐up: adequate, less than 10% of the patients dropped out or were withdrawn. Intention‐to‐treat analysis: used. |
|
| Participants | Sixty insulin treated diabetic patients with alcoholic liver cirrhosis from the 7050 diabetic outpatients registered at the author's anti‐diabetes centre. Chronic liver disease. Inclusion criteria: 1) age 45 to 70 years; 2) non‐insulin‐dependent diabetes mellitus with alcoholic liver cirrhosis; 3) body mass index < 29 kg/m2; 4) ascertained diabetes for a period of at least 5 years and treated with insulin only; 5) undergoing stable insulin therapy for a period of at least two years; 6) presenting raised endogenous insulin secretion; 7) fasting insulin levels and basal and stimulated C‐peptide levels above normal range (above 15 mU/ml for insulin; above 1 ng/ml for basal C‐peptide levels); 8) negative for markers of hepatitis A, B, C; 9) not addicted to alcohol for a period of at least two years prior to the start of the study; 10) no bleeding from variceal oesophagus; 11) liver biopsy, performed no more than four years prior to enrolment, demonstrating liver cirrhosis. |
|
| Interventions | MT group:
silymarin tablets (Legalon®) 200 mg tablets, three times daily (600 mg per day). Control group: standard treatment. Duration of treatment and of follow‐up: 12 months. |
|
| Outcomes | Mortality. Liver biochemistry. Adverse events. | |
| Notes | Letter to the trialist: sent (August 2002). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benda 1973 | An observational study (case series) of patients with liver cirrhosis treated with silymarin. |
| Berenguer 1977 | We were not able to obtain this publication, but it is not likely that the data could have been used as the patients had differing etiologies or chronic hepatitis. |
| Berkson 1999 | An observational study (case series). The author describes a treatment for three patients with liver cirrhosis, portal hypertension, and oesophageal varices secondary to chronic hepatitis C. The treatment combined three antioxidants (alpha‐lipoic acid [thioctic acid], silymarin, and selenium). |
| Bode 1977 | The study is quasi‐randomised (birthday) clinical study evaluating silymarin (Legalon®) in 151 patients versus no intervention for acute viral hepatitis. |
| Canini 1985 | An observational study (case series). Ten patients with histologically confirmed alcoholic liver steatosis were treated for three months with silymarin 200 mg three times per day. |
| Cavalieri 1974 | A randomised clinical trial evaluating 20 patients treated with silymarin (Legalon®) 420 mg versus 20 patients treated with hepatoprotective drugs for acute viral hepatitis. |
| Conti 1989 | We were not able to obtain this publication (an abstract). |
| De Martiis 1984 | An observational study (case series). Seventy‐six patients were followed for chronic liver disease of mixed etiology and macrocytic anaemia and hyperhaemolysis; 27 of them were treated with silymarin for more than one year. |
| Dittrich 1980 | We were not able to obtain this publication (an abstract). |
| Fassati 1973 | An observational study (case series). Twenty‐four patients with chronic liver disease of mixed etiology were treated with silymarin (Legalon®) for six months. |
| Fehér 1988 | An observational study (case series). The effects of three hepatoprotective antioxidants (silymarin (Legalon®), cyanidol‐3 (Catergen®), and 4‐amino‐5‐imidazole‐carboxamide‐phosphate (Aica‐P®)) were compared to placebo in 40 patients. |
| Fintelmann 1970 | This is a quasi‐randomised clinical study. Fifty‐seven patients with fatty liver disease were treated with silymarin (Legalon®) compared to placebo for six weeks. |
| Fintelmann 1980ex | This is a randomised trial on milk thistle for patients with toxic liver disease: Most of the patients had toxic liver disease due to alcoholic. This trial was excluded due to the heterogeneity of the patients included, but all data were included in an explorative sensitivity analysis. |
| Flisiak 1997 | This is a quasi‐randomised clinical study. Fifty‐two male patients with acute viral hepatitis B were treated for 28 days. Of these 20 patients were allocated by alternate inclusion to the silymarin group (silymarin tablets 70 mg, three times daily (210 mg) and 12 to the control group. The remaining 20 patients were treated with misoprostol. No adverse events were reported. |
| Hammerl 1971 | An observational study (case series). Forty‐three patients with chronic liver disease were treated with silymarin (Legalon®) for more than one year. Another 90 patients were treated for six to nine months with silymarin . |
| Ippolito 2002 | An observational study (case series). Of 284 patients with chronic hepatitis C, 112 (39.4%) were using one or more herbal remedies, 52.7% of these ingesting MT. No significant effect on response rate was observed when patients using MT were compared with patients not using MT. |
| Kiesewetter 1977 | A randomised clinical trial, but the etiology of the liver disease was not given. Patients with chronic alcohol abuse and with clinical or histological signs of alcoholic liver disease were excluded. The author reports two clinical studies. The first randomises 45 patients with chronic persistent or chronic aggressive hepatitis to silymarin (Legalon®) versus placebo from six centres in Vienna. The other clinical trial used quasi‐randomisation. No adverse events were reported. |
| Kupcová 1987 | An observational study (case series). Twenty‐four patients with liver cirrhosis confirmed on laparoscopy and histology were treated with silymarin for six months. |
| Lirussi 1995 | A randomised clinical trial evaluating silymarin versus ursodeoxycholic acid in 27 patients with liver disease of mixed etiology. Patients received either ursodeoxycholic acid capsules (Deursil®, 600 mg per day) or silymarin tablets (Legalon®, 420 mg per day) for six months treatment period according to a cross‐over design. No adverse events were reported. |
| Loginov 1988 | An observational study (case series). Forty‐eight patients with chronic liver disease were administered one of the following preparations: silymarin, essentiale, trophopar, or vitamins complex for 30 days. |
| Muscher 1972 | An observational study (case series). Two‐hundred‐thirty‐seven patients with alcoholic and non‐alcoholic liver disease were treated with silymarin (Legalon®). |
| Peyton 1999 | An observational study (case series). One hundred‐seventeen patients with hepatitis C completed the survey regarding their use of MT and of other medical herbs. MT was the predominant medical herb used in 14 patients. |
| Pár 2000 | An observational study (two case series). It is a comparison of silymarin compared to no intervention for patients with alcoholic liver disease and chronic hepatitis C. |
| Realini 1975 | An observational study (case series). Twenty‐three patients with chronic liver disease of mixed etiology were treated with silymarin for 12 months. No adverse events were reported. |
| Reutter 1975 | An observational study (case series). Thirty‐four patients with chronic liver disease of mixed etiology were treated with silymarin (Legalon®) 210 mg/day. |
| Saba 1979 | An observational study (two case series) evaluating 38 patients with acute liver disease with silymarin compared to standard therapy. |
| Salmi 1982ex | This is a randomised clinical trials on milk thistle for patients with liver disease of mixed etiology, mostly due to alcohol problems (the majority 81% in the milk thistle arm and 76% in the placebo arm admitted previous alcohol consumption). This trial was excluded due to the heterogeneity of the patients included, but all data were included in an explorative sensitivity analysis. |
| Sawaryn 1977 | An observational study (two case series). A total of 46 patients with chronic hepatitis were treated with silymarin (Legalon®). |
| Schopen 1970 | An observational study (case series). It evaluates 72 patients with silymarin (Legalon®) for hepatitis of mixed etiology. |
| Schuppan 1998 | An observational study (case series). The effect of treatment with silymarin (Legalon®) 280 mg three times per day over 12 weeks in 998 patients with chronic liver disease was examined in a post‐marketing‐surveillance study. |
| Tanasescu 1988 | The study is a double blind randomised clinical trial, but the etiology of liver disease was not described. The Romanian product Silimarina® (synonym Legalon®) was administered to a group of 180 patients versus placebo. No adverse events were reported. |
| Tkacz 1983 | An observational study (two case series). The study examined 26 patients with acute viral hepatitis treated with silymarol. The results were compared with a control group of 61 patients. No adverse events were reported. |
| Vailati 1993 | A phase II randomised, open trial was performed to evaluate three doses (160 mg, 240 mg, 360 mg) of silybin and phosphatidylcholine (IdB 1016, Silipide®) in 60 patients with chronic alcoholic or viral hepatitis. A total of six adverse events was reported in the study. Three patients, treated with 160 mg, complained of nausea, heartburn and dyspepsia; one patient, treated with 240 mg, complained of dyspepsia; two patients, treated with 360 mg, complained respectively of nausea and meteorism. The treatment lasted two weeks. No placebo or no intervention group was used. |
Contributions of authors
AR drafted and revised the protocol and the review; AR coordinated the identification of trials. BPJ and GI revised the data extraction as well as the protocol and the review. CG revised the selection of trials and revised the protocol and the review.
Sources of support
Internal sources
The Copenhagen Trial Unit, Denmark.
External sources
The 1991 Pharmacy Foundation, Denmark.
The Copenhagen Hospital Corporation's Research Grant on Getting Research into Practice (GRIP), Denmark.
The Danish Medical Research Council's Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
BPJ is author of a report (Lawrence VA, Jacobs BP, Dennehy C. Milk thistle: effects on liver disease and cirrhosis and clinical adverse effects. Evidence Report/Technology Assessment No. 21 (Contract 290‐97‐0012 to the San Antonio Evidence‐based Practice Centre, based at the University of Texas Health Science Centre at San Antonio, and the Veterans Evidence‐based Research, Dissemination, and Implementation Centre, a Veterans Affairs Services Research and Development Centre of Excellence), AHRQ Publication No. 01‐E025, Agency for Healthcare Research and Quality, Rockville, MD, October 2000) and of a meta‐analysis based on this report (Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta‐analysis. American Journal of Medicine: 2002: 113(6); 506‐15) on milk thistle for liver diseases. Apart from this, no conflicts of interest are known.
Edited (no change to conclusions)
References
References to studies included in this review
Bunout 1992 {published data only}
- Bunout DB, Hirsch SB, Petermann MT, Maza MPC, Silva GP, Kelly MM, et al. Effects of silymarin on alcoholic liver disease. A controlled trial [Estudio controlado sobre el efecto de la silimarina en la enfermedad hepatica alcoholica]. Revista Médica de Chile 1992;120(12):1370‐5. [PubMed] [Google Scholar]
Buzzelli 1993 {published data only}
- Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico A. A pilot study on the liver protective effect of silybinphosphatidylcholine complex (IdB1016) in chronic active hepatitis. International Journal of Clinical Pharmacology, Therapy and Toxicology 1993;31(9):456‐60. [PubMed] [Google Scholar]
- Moscarella S, Giusti A, Marra F, Marena C, Lampertico M, Relli P, et al. Therapeutic and antilipoperoxidant effects of silybin‐phosphatidylcholine complex in chronic liver disease: preliminary results. Current Therapeutic Research Clinical and Experimental 1993;53(1):98‐102. [Google Scholar]
Buzzelli 1994 {published data only}
- Buzzelli G, Moscarella S, Barbagli S, Marena C, Gentilini P. Therapeutic effect of Silipide® in patients with chronic hepatitis C non‐responders (NRs) to interferon (IFN) treatment [abstract]. Journal of Hepatology 1994;21(Suppl 1):S100. [Google Scholar]
Fehér 1989 {published data only}
- Deák G, Müzes G, Láng I, Niederland V, Nékám K, Gonzalez‐Cabello R, et al. Immunomodulatory effects of silymarin treatment in chronic alcoholic liver disease [Silymarin kezelés immunmoduláns hatása kronikus alkoholos májbetegségben]. Orvosi Hetilap 1990;131(24):1291‐6. [PubMed] [Google Scholar]
- Fehér J, Deák G, Müzes G, Láng I, Niederland V, Nékám K, et al. Hepatoprotective activity of silymarin (Legalon®) therapy in patients with chronic alcoholic liver disease [Silymarin kezelés májvédö hatása idült alkoholos májbetegségben]. Orvosi Hetilap 1989;130:2723‐7. [PubMed] [Google Scholar]
- Fehér J, Lengyel G, Blázovics A. Oxidative stress in the liver and biliary tract diseases. Scandinavian Journal of Gastroenterology Supplements 1998;228:38‐46. [DOI] [PubMed] [Google Scholar]
- Müzes G, Deák G, Láng I, Nékám K, Niederland V, Fehér J. Effects of silymarin (Legalon®) treatment on the antioxidant defense system and lipid peroxidation in patients with chronic alcoholic liver disease. A double blind study [Silymarin (Legalon®) kezelés hatása idült alkoholos májbetegek antioxidáns védörendszerére és a lipid peroxidációra (kettös vak protocoll)]. Orvosi Hetilap 1990;131(16):863‐6. [PubMed] [Google Scholar]
Ferenci 1989 {published data only}
- Benda L, Dittrich H, Ferenci P, Frank H, Wewalka F. The influence of therapy with silymarin on the survival rate of patients with liver cirrhosis [Zur wirksamkeit von silymarin auf die überlebensrate von patienten mit leberzirrhose]. Wiener Klinische Wochenschrift 1980;92(19):678‐83. [PubMed] [Google Scholar]
- Ferenci P, Dragosics B, Csomos G, Schneider B. Treatment with silymarin in patients with cirrhosis of the liver ‐ results of a controlled prospective study and of the post study surveillance [abstract]. Hepatology 1986;6(4):790. [Google Scholar]
- Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. Journal of Hepatology 1989;9:105‐13. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Dragosics B, Frank H, Benda L, Dittrich H, Meryn S. Controlled prospective study on the effects of silymarin‐treatment on patients with cirrhosis of the liver [abstract]. Journal of Hepatology 1985;1(Suppl 2):S229. [DOI] [PubMed] [Google Scholar]
Fintelmann 1980 {published data only}
- Fintelmann V, Albert A. Proof of therapeutic effect of Legalon® for toxic liver diseases in a double blind trial [Nachweis der therapeutischen wirksamkeit von Legalon® bei toxischen lebererkrankungen im doppelblindversuch]. Therapiewoche 1980;30:5589‐94. [Google Scholar]
Lirussi 2002 {published data only}
- Lirussi F, Beccarello A, Zanette G, Monte A, Donadon V, Velussi M, et al. Silybin‐beta‐cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti‐oxidant agent. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 2002;15(4):222‐31. [MEDLINE: ; PUBMED 12416659] [PubMed] [Google Scholar]
Lucena 2002 {published data only}
- Andrade RJ, Lucena MI, Cruz JP, Blanco E, Rodriguez‐Mendizabal M, Rius F, et al. Effects of silymarin on the oxidative stress in patients with alcoholic liver cirrhosis. Results from a controlled, double blind, randomized pilot clinical trial [abstract]. Hepatology 1998;28(4):629A. [Google Scholar]
- Andrade RJ, Lucena MJ, Cruz JP, Blanco E, Garcia‐Escano MD, Alcantara R, et al. Antioxidants effects of silymarin MZ80 in patients with alcoholic liver cirrhosis: results from a randomized, placebo‐controlled, double‐blind, clinical trial [abstract]. Journal of Hepatology 1999;30(Suppl 1):150. [Google Scholar]
- Lucena MI, Andrade RJ, Cruz JP, Rodriguez‐Mendizabal M, Blanco E, Sanchez de la Cuesta F. Effects of silymarin MZ‐80 on oxidative stress in patients with alcoholic cirrhosis. Results of a randomized, double‐blind, placebo‐controlled clinical study. International Journal of Clinical Pharmacology and Therapeutics 2002;40(1):2‐8. [PMID: 11841050] [DOI] [PubMed] [Google Scholar]
Láng 1990 {published data only}
- Fehér J, Láng I, Nékám K, Gergely P, Müzes G. In vivo effect of free radical scavenger hepatoprotective agents on superoxide dismutase (SOD) activity in patients. The Tokai Journal of Experimental and Clinical Medicine 1990;15(2,3):129‐34. [PubMed] [Google Scholar]
- Láng I, Deák G, Nékám K, Müzes G, Gonzáles‐Cabello R, Gergely P, et al. Hepatoprotective and immunomodulatory effects of antioxidant therapy. Acta Medica Hungarica 1988;45(3‐4):287‐95. [PMID: 3074277] [PubMed] [Google Scholar]
- Láng I, Nekam K, González‐Cabello R, Müzes G, Gergely P, Feher J. Hepatoprotective and immunological effects of antioxidant drugs. The Tokai Journal of Experimental and Clinical Medicine 1990;15(2‐3):123‐7. [PubMed] [Google Scholar]
- Láng I, Nékám K, Deák G, Müzes G, Gonzales‐Cabello R, Gergely P, et al. Immunomodulatory and hepatoprotective effects of in vivo treatment with free radical scavengers. Italian Journal of Gastroenterology 1990;22:283‐7. [PubMed] [Google Scholar]
Magliulo 1978 {published data only}
- Magliulo E, Gagliardi B, Fiori GP. Results of a double blind study on the effect of silymarin in the treatment of acute viral hepatitis, carried out at two medical centres [Zur wirkung von silymarin bei der behandlung der akuten virushepatitis, ergebnis einer an zwei medizinischen zentren durchgeführten doppelblinstudie]. Medizinsche Klinik 1978;73(28/29):1060‐5. [PubMed] [Google Scholar]
Parés 1998 {published data only}
- Parés A, Planas R, Torres M, Caballería J, Viver JM, Acero D, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double‐blind, randomized and multicenter trial. Journal of Hepatology 1998;28(4):615‐21. [DOI] [PubMed] [Google Scholar]
Salmi 1982 {published data only}
- Salmi HA, Sarna S. Effect of silymarinon on chemical, functional, and morhological alterations of the liver: a double blind controlled study. Scandinavian Journal of Gastroenterology 1982;17:517‐21. [DOI] [PubMed] [Google Scholar]
Salvagnini 1985 {published data only}
- Salvagnini M, Martines D, Piccoli A, Sciarrone R, Rizzo A, Campesato A, et al. Silymarin treatment in alcoholic liver disease: a double blind randomized clinical trial (RCT) [abstract]. Journal of Hepatology 1985;Suppl 1:S124. [Google Scholar]
Trinchet 1989 {published data only}
- Trinchet JC, Coste T, Levy VG, Vivet F, Duchatelle V, Legendre C, et al. A randomized double blind trial of silymarin in 116 patients with alcoholic hepatitis [Traitement de l´hépatite alcoolique par la silymarine. Une étude comparative en double insu chez 116 malades]. Gastroenterologie Clinique et Biologique 1989;13(2):120‐4. [PubMed] [Google Scholar]
Velussi 1997 {published data only}
- Velussi M, Cernigoi AM, Monte A, Dapas F, Caffau C, Zilli M. Long‐term (12 months) treatment with an anti‐oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. Journal of Hepatology 1997;26(4):871‐9. [DOI] [PubMed] [Google Scholar]
- Velussi M, Cernigoi AM, Viezzoli L, Dapas F, Caffau C, Zilli M. Silymarin reduces hyperinsulinemia, malondialdehyde levels, and daily insulin need in cirrhotic diabetic patients. Current Therapeutic Research Clinical and Experimental 1993;53(5):533‐45. [Google Scholar]
References to studies excluded from this review
Benda 1973 {published data only}
- Benda L, Zenz W. Silymarin in liver cirrhosis [Silymarin bei leberzirrhose]. Wiener Medizinische Wochenschrift 1973;123(3):512‐6. [PMID: 4748151] [PubMed] [Google Scholar]
Berenguer 1977 {published data only}
- Berenguer J, Carrasco D. Double blind trial of silymarin versus placebo in the treatment of chronic hepatitis [Ensayo doble ciego de silimarina frente a placebo en el tratamiento de hepatopatias cronicas de diversa genesis]. Münchener Medizinische Wochenschrift 1977;119:240‐60. [Google Scholar]
Berkson 1999 {published data only}
- Berkson BM. A conservative triple antioxidant approach to the treatment of hepatitis C. Combination of alpha lipoic acid (thioctic acid), silymarin, and selenium: three case histories. Medizinische Klinik 1999;94(Suppl 3):84‐9. [PMID: 10554539] [DOI] [PubMed] [Google Scholar]
Bode 1977 {published data only}
- Bode JC, Schmidt U, Dürr HK. Silymarin for the treatment of acute viral hepatitis? Report of a controlled trial [Zur Behandlung der akuten virushepatitis mit silymarin]. Medizinische Klinik 1977;72(12):513‐8. [PMID: 840125] [PubMed] [Google Scholar]
- Schmidt U, Dürr HK, Bode JC. Therapy of acute virus hepatitis with silymarin. Report of a controlled study [Zur therapie der akuten virushepatitis mit silymarin. Bericht über eine kontrollierte studie]. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1977;83:563‐5. [PMID: 25535] [PubMed] [Google Scholar]
Canini 1985 {published data only}
- Canini F, Bartolucci L, Cristallini E, Gradoli C, Rossi A, Ribacchi R, et al. Use of silymarin in the treatment of alcoholic hepatic steatosis [L'impiego della silimarina nel trattamento della steatosi epatica alcoolica]. La Clinica Terapeutica 1985;114(4):307‐14. [PubMed] [Google Scholar]
Cavalieri 1974 {published data only}
- Cavalieri S. A controlled clinical trial of Legalon® in 40 patients [Valutazione clinica controllata del Legalon®]. Gazzetta Medica Italiana 1974;133:628‐35. [Google Scholar]
Conti 1989 {published data only}
- Conti M, Malandrino S, Magistretti MJ. Liver protective activity of IdB1016, a new flavanolignan complex [abstract]. 3rd International Symposium on Flavonoids in Biology and Medicine. Singapore, November 13‐17, 1989:P29.
De Martiis 1984 {published data only}
- Martiis M, Barlattani A, Parenzi A, Sebastiani F. Relationship between changes in lipid metabolism and macrocytic anemia in chronic liver disease. Evaluation of therapy with a membranotropic drug [Rapporti tra alterazioni del metabolismo lipidico e anemia macrocitica in corso di epatopatie croniche. Valutazione di una terapia con un farmaco membranotropo]. La Clinica Terapeutica 1984;111:135‐9. [PMID: 6239735] [PubMed] [Google Scholar]
- Martiis M, Fontana M, Assogna G, D'Ottavi R, D'Ottavi O. Milk thistle (Silybin marianum) derivatives in the therapy of chronic hepatopathies [I derivati del Cardo mariano nella terapia delle epatopatie croniche]. La Clinica Terapeutica 1980;94(3):283‐315. [PMID: 7004762] [PubMed] [Google Scholar]
- Martiis M, Fontana M, Sebastiani F, Parenzi A. Silymarin, a membranotropic drug: clinical and experimental observations [La silimarina, farmaco membranotropo: osservazioni cliniche e sperimentali]. La Clinica Terapeutica 1977;81(4):333‐62. [PMID: 880785] [PubMed] [Google Scholar]
Dittrich 1980 {published data only}
- Dittrich M. Double‐blind study for the therapy of liver cirrhosis. Drug‐Symposium Legalon, XI International Congress of Gastroenterology. Hamburg 1980.
Fassati 1973 {published data only}
- Fassati P, Fassati M. Silymarin and some problems of present therapy of chronic hepatopathies [Silymarin a nekteré problémy soucasne terapie chronickych hepatopatii]. Casopis Lékasy Ceskych 1973;112:865‐9. [PMID. 4720441] [PubMed] [Google Scholar]
Fehér 1988 {published data only}
- Fehér J, Láng I, Nekam K, Csomos G, Müzes G, Deák G. Effect of silibinin on the activity and expression of superoxide dismutase in lymphocytes from patients with alcoholic liver disease. Free Radical Research Communications 1987;3(6):373‐7. [PMID: 3508451] [DOI] [PubMed] [Google Scholar]
- Fehér J, Láng I, Nekam K, Müzes GY, Deák GY. Effect of free radical scavengers on superoxide dismutase (SOD) enzyme in patients with alcoholic cirrhosis. Acta Medica Hungarica 1988;45(3‐4):265‐76. [PubMed] [Google Scholar]
Fintelmann 1970 {published data only}
- Fintelmann V. About silymarin therapy for fatty liver [Zur therapie der fettleber mit silymarin]. Therapiewoche 1970;23:1055‐62. [Google Scholar]
Fintelmann 1980ex {published data only}
- Fintelmann V, Albert A. Proof of therapeutic effect of Legalon® for toxic liver diseases in a double blind trial [Nachweis der therapeutischen wirksamkeit von Legalon® bei toxischen lebererkrankungen im doppelblindversuch]. Therapiewoche 1980;30:5589‐94. [Google Scholar]
Flisiak 1997 {published data only}
- Flisiak R, Prokopowicz D. Effect of misoprostol on the course of viral hepatitis B. Hepato‐Gastroenterology 1997;44(17):1419‐25. [PubMed] [Google Scholar]
- Flisiak R, Prokopowikz D. Effect of misoprostol on serum beta2‐microglobulin in the course of viral hepatitis B. European Journal of Gastroenterology and Hepatology 1999;11(11):1227‐30. [PMID: 10563531] [DOI] [PubMed] [Google Scholar]
- Flisiak R, Prokowicz D. One year follow‐up of patients treated with misoprostol in acute phase of viral hepatitis B. Prostaglandins and other Lipid Mediators 2000;60(4‐6):161‐5. [PMID: 10751646] [DOI] [PubMed] [Google Scholar]
Hammerl 1971 {published data only}
- Hammerl H, Pichler O, Studlar M. On the objective assessment of silymarin action in liver diseases [Über die objektivierung der silymarinwirkung bei lebererkrankungen]. Medizinsche Klinik 1971;66(36):1204‐8. [PMID: 5096207] [PubMed] [Google Scholar]
Ippolito 2002 {published data only}
- Ippolito LA, Swift JM, Hershberger JM, Schmid S, Schmidt WN, Voigt MD, et al. Possible deleterious effects of milk thistle on anti‐viral therapy for chronic hepatitis C [abstract]. Hepatology 2002;36(4 Pt2):590A. [Google Scholar]
Kiesewetter 1977 {published data only}
- Kiesewetter E, Leodolter I, Thaler H. Results of two double‐blind studies on the effect of silymarine in chronic hepatitis [Ergebnisse zweier doppelblindstudien zur wirksamkeit von silymarin bei chronischer hepatitis]. Leber Magen Darm 1977;7(5):318‐23. [PubMed] [Google Scholar]
Kupcová 1987 {published data only}
- Kupcová V, Turecký L, Holomán J, Ulicná O, Brixová E, Volmut J. Therapeutic effect of silymarin in patients with cirrhosis of the liver [Terapeuticky efekt silymarinu u pacientov s cirhozou pecene]. Casopis Lékasy Ceskych 1987;126(45):1406‐9. [PubMed] [Google Scholar]
Lirussi 1995 {published data only}
- Lirussi F, Iemmolo RM, Passera D, Okolicsanyi L. Silymarin (sily) vs ursodeoxycholic acid (UDCA) in active cirrhosis: a controlled cross‐over study [abstract]. Journal of Hepatology 1990;11(Suppl 2):S98. [Google Scholar]
- Lirussi F, Nassuato G, Orlando R, Iemmolo RM, Beccarello A, Bortolato L, et al. Treatment of active cirrhosis with ursodeoxycholic acid and a free scavenger: a two year prospective study. Medical Science Research 1995;23:31‐3. [Google Scholar]
- Lirussi F, Orlando R, Iemmolo RM, Zappalà F, Nassuato G, Okolicsanyi L. Effect of UDCA and silymarin on liver function in patients with chronic active hepatitis with cirrhosis: a prospective study [abstract]. Journal of Hepatology 1989;9(Suppl 1):S184. [Google Scholar]
- Nassuato G, Iemmolo RM, Strazzabosco M, Lirussi F, Deana R, Francesconi MA, et al. Effect of silibilin on biliary lipid composition. Experimental and clinical study. Journal of Hepatology 1991;12:290‐5. [DOI] [PubMed] [Google Scholar]
Loginov 1988 {published data only}
- Loginov AS, Matiushin BN, Sukhareva GV, Tkachev VD. Antioxidant activity of hepatotropic preparations in the treatment of chronic diseases of the liver. Terapeuticeskij Archiv 1988;60(8):74‐7. [PMID: 3227482] [PubMed] [Google Scholar]
Muscher 1972 {published data only}
- Muscher CH. Silymarin in chronic liver diseases [Silymarin bei chronischen erkrankungen der leber]. Therapeutische Erfahrungen 1972;111(12):1768‐79. [PMID: 4569030] [PubMed] [Google Scholar]
Peyton 1999 {published data only}
- Peyton BG, Spears TL, Lindsey A, Lindsey J, Sharma VK, Raufman J‐P. A survey of the use of herbal medicine in patients with hepatitis C. Hepatology 1999;30(4 Pt 2):191A. [Google Scholar]
Pár 2000 {published data only}
- Pár A, Roth E, Rumi G Jr, Kovács Z, Nemes J, Mózsik G. Oxidative stress and antioxidant defense in alcoholic liver disease and chronic hepatitis C [Oxidatív stressz és antioxidáns védelem alkoholos májbetegségben és krónikus C hepatitisben]. Orvosi Hetilap 2000;141(30):1655‐9. [PMID: 10962902] [PubMed] [Google Scholar]
Realini 1975 {published data only}
- Realini S, Gonvers JJ, Hofstetter J‐R. Clinical investigation of silymarin in chronic liver diseases [Essai clinique de la silymarine dans les affections chroniques du foie]. Schweizerische Rundschau fur Medizin Praxis 1975;64(19):595‐8. [PubMed] [Google Scholar]
Reutter 1975 {published data only}
- Reutter FW, Haase W. Clinical experience with silymarin for chronic liver disease [Klinische erfahrungen mit silymarin bei chronischen lebererkrankungen]. Schweizerische Rundschau fur Medizin Praxis 1975;64(36):1145‐51. [PMID: 1099576] [PubMed] [Google Scholar]
Saba 1979 {published data only}
- Saba P, Mignani E, Pagliai E, Guidi G, Scalabrino A, Stroppa A, et al. Efficacy of silymarin treatment for acute viral hepatitis [Efficacia del trattamento con silimarina nella epatite acuta virale]. Epatologia 1979;25(5):277‐81. [Google Scholar]
Salmi 1982ex {published data only}
- Salmi HA, Sarna S. Effect of silymarinon on chemical, functional, and morphological alterations of the liver: a double blind controlled study. Scandinavian Journal of Gastroenterology 1982;17:517‐21. [DOI] [PubMed] [Google Scholar]
- Varis K, Salmi HA, Siurala M. Therapy of liver diseases with Legalon®: a controlled double‐blind study [Die therapie der lebererkrankung mit Legalon®; eine kontrollierte doppelblindstudie]. Aktuelle Hepatologie. 3rd International Symposium, Cologne. Lübek: Hanseatisches Verlagskontor, November 15‐17, 1978:42‐3.
Sawaryn 1977 {published data only}
- Sawaryn T, Szymonski K, Machalska M. Sylimarine in the treatment of chronic hepatitis [Sylimaryna w leczeniu przewleklych zapalen watroby]. Przegla epidemiologiczny 1977;31(4):445‐50. [PubMed] [Google Scholar]
Schopen 1970 {published data only}
- Schopen RD, Lange OK. Further observations on the therapy of hepatoses. Therapeutic use of silymarin [Beitrag zur therapie der hepatosen. Weitere beobachtungen zur therapeutischen anwendbarkeit von silymarin]. Die Medizinische Welt 1970;15:691‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Schuppan 1998 {published data only}
- Schuppan D, Strosser W, Burkard G, Walosek G. Influence of Legalon® 140 on the metabolism of collagen in patients with chronic liver disease ‐ review by measurement of PIIINP‐values [Verminderung der fibrosierungs‐aktivitat durch Legalon® bei chronischen lebererkrankungen]. Zeitschrift für Allgemeinmedizin 1998;74:577‐84. [Google Scholar]
Tanasescu 1988 {published data only}
- Tanasescu C, Petrea S, Baldescu R, Macarie E, Chiriloiu C, Purice S. Use of the Romanian product Silimarina® in the treatment of chronic liver diseases. Medecine Interne 1988;26(4):311‐22. [PMID: 3072661] [PubMed] [Google Scholar]
Tkacz 1983 {published data only}
- Tkacz B, Dworniak D. Sylimarol in the treatment of acute viral hepatitis [Sylimarol w leczeniu ostrego wirusowego zapalenia watroby]. Wiadomosci Lekarskie 1983;36:613‐6. [PMID: 6353775] [PubMed] [Google Scholar]
Vailati 1993 {published data only}
- Vailati A, Aristia L, Sozzé E, Milani F, Inglese V, Galenda P, et al. Randomized open study of the dose‐effect relationship of a short course of IdB in 1016 in patients with viral or alcoholic hepatitis. Fitoterapia 1993;64(3):219‐28. [Google Scholar]
Additional references
Akriviadis 2000
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short‐term survival in severe acute alcoholic hepatitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119(6):1987‐91. [DOI] [PubMed] [Google Scholar]
Bindoli 1977
- Bindoli A, Cavallini L, Siliprandi N. Inhibitory action of silymarin of lipid peroxide formation in rat liver mithochondria and microsomes. Biochemical Pharmacology 1977;26:2405‐9. [DOI] [PubMed] [Google Scholar]
Bird 1998
- Bird GL, Prach AT, McMahon AD, Forrest JA, Mills PR, Danesh BJ. Randomised controlled double‐blind trial of the calcium channel antagonist amlodipine in the treatment of acute alcoholic hepatitis. Journal of Hepatology 1998;28(2):194‐8. [DOI] [PubMed] [Google Scholar]
Boigk 1997
- Boigk G, Stroedter L, Herbst H, Waldschmidt J, Rieken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology 1997;26:643‐9. [DOI] [PubMed] [Google Scholar]
Brok 2005a
Brok 2005b
Campos 1989
- Campos R, Garrido A, Guerra R, Valenzuela A. Silybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetamiophen on rat liver. Planta Medica 1989;55(5):417‐9. [PMID: 2813577] [DOI] [PubMed] [Google Scholar]
Chang 1994
- Chang TT, Lin CY, Chow NH, Hsu PI, Yang CC, Lin ZZ, et al. Hepatitis B and hepatitis C virus infection among chronic alcoholic patients with liver disease in Taiwan. Journal of Formosan Medical Association 1994;93(2):128‐33. [PubMed] [Google Scholar]
Christensen 1995
- Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis. Gut 1995;37(1):113‐8. [PMID: 7672658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corrao 1998a
- Corrao G, Zambon A, Torchio P, Arico S, Vecchia C, Orio F. Attributable risk for symptomatic liver cirrhosis in Italy. Collaborative Groups for the Study of Liver Diseases in Italy. Journal of Hepatology 1998;28(4):608‐14. [DOI] [PubMed] [Google Scholar]
Corrao 1998b
- Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998;27(4):914‐9. [DOI] [PubMed] [Google Scholar]
De Franceschi 2000
- Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology 2000;31(4):997‐1004. [PMID: 10733558] [DOI] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Practical aspects in monitoring: a brief review. Statisctics in Medicine 1987;6(7):753‐60. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Di Bisceglie 2002
- Bisceglie AM, Hoofnagle JH. Optimal therapy of hepatitis C. Hepatology 2002;36(5 Suppl 1):S121‐S127. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Floersheim 1978
- Floersheim GL, Eberhard M, Tschumi P, Duckert F. Effects of penicillin and silymarin on liver enzymes and blood clotting factors in dogs given a boiled preparation of Amanita phalloides. Toxicology and Applied Pharmacology 1978;46(2):455‐62. [DOI] [PubMed] [Google Scholar]
Flora 1998
- Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. The American Journal of Gastroenterology 1998;93(2):139‐43. [DOI] [PubMed] [Google Scholar]
Geier 1990
- Geier J, Fuchs T, Wahl R. Anaphylactic shock due to an extract of Silybum marianum in a patient with immediate‐type allergy to kiwi fruit [Anaphylaktischer shock durch einen mariendistel‐extrakt bei soforttyp‐allergie auf kiwi]. Allergologie 1990;10:S387‐S388. [Google Scholar]
Gluud 1988
- Gluud C. Testosterone and alcoholic cirrhosis. Epidemiologic, pathophyphysiologic and therapeutic studies in men. Danish Medical Bullettin 1988;35(6):564‐75. [PMID: 3064977] [PubMed] [Google Scholar]
Gluud 1993
- Gluud C, Afroudakis A, Caballeria J, Laskus T, Morgan MY, Rueff B, et al. Diagnosis and treatment of alcoholic liver disease in Europe. Gastroenterology International 1993;6(4):221‐30. [Google Scholar]
Gluud 2001
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. Falk Symposium 121. Steatosis (NASH and ASH). Dordrecht/Boston/London: Kluwer Academic Publishers, 2001; Vol. 121:322‐42.
Gluud 2002
- Gluud C. Acute, serious drug‐induced liver injury. Journal of Hepatology 2002;37:675‐7. [DOI] [PubMed] [Google Scholar]
Gluud 2003
- Gluud C, Als‐Nielsen B, D'Amico G, Gluud LL, Khan S, Klingenberg SL, et al. Hepato‐Biliary Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005, Issue 3. Art. No.: LIVER.
Hakova 1993
- Hakova H, Misurova E. The effect of silymarin and gamma radiation on nucleid acids in rat organs. The Journal of Pharmacy and Pharmacology 1993;45:910‐2. [DOI] [PubMed] [Google Scholar]
Halim 1997
- Halim AB, el‐Ahmady O, Hassab‐Allah S, Abdel‐Galil F, Hafez Y, Darwish A. Biochemical effect of antioxidants on lipids and liver function in experimentally‐induced liver damage. Annals of Clinical Biochemistry 1997;34(Pt 6):656‐63. [PMID: 9367004] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & sons, Ltd. [Google Scholar]
ICH‐GCP 1997
- International Committee on Harmonization. Code of Federal Regulations & Guidelines. Vol. 1, Philadelphia, US: Barnett International/PAREXEL, 1997. [Google Scholar]
Ioannidis 2001
- Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001;286:821‐30. [PMID: 11497536] [DOI] [PubMed] [Google Scholar]
Jacobs 2002
- Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta‐analysis. American Journal of Medicine 2002;113(6):506‐15. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [PMID: 11730399] [DOI] [PubMed] [Google Scholar]
Kjaergard 2003
- Kjaergard LL, Liu J, Als‐Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute‐on‐chronic liver failure: a systematic review. JAMA 2003;289(2):217‐22. [DOI] [PubMed] [Google Scholar]
Kunz 2002
- Kunz R, Vist G, Oxman AD. Randomisation to protect against selection bias in healthcare trials. The Cochrane Database of Methodology Reviews 2002, Issue 4. Art. No.: MR000012. DOI: 10.1002/14651858.MR000012. [DOI] [PMC free article] [PubMed]
Lawrence 2000
- Mulrow C, Lawrence V, Jacobs B, Dennehy C, Sapp J, Ramirez G, et al. Milk thistle: effects on liver disease and cirrhosis and clinical adverse effects. Evidence Report/Technology Assessment (Summary) 2000;21:1‐3. [PMC free article] [PubMed] [Google Scholar]
Liaw 2003
- Liaw YF, Leung N, Guan R, Lau GK, Merican I, Asian Pacific Consensus Working Parties on Hepatitis B. Asian‐Pacific consensus statement on the management of chronic hepatitis B: an update. Journal of Gastroenterology and Hepatology 2003;18:239‐45. [PMID: 12603522] [DOI] [PubMed] [Google Scholar]
Lieber 2000
- Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. Journal of Hepatology 2000;32(1 Suppl):113‐28. [PMID: 10728799] [DOI] [PubMed] [Google Scholar]
Lieber 2001
- Lieber CS. Beneficial effects of polyenylphosphatidylcholine (PPC) in alcoholic and non‐alcoholic liver injury. In: Leuschner U, James O, Dancygier H editor(s). Falk Symposium 121. Steatohepatitis (NASH and ASH). Vol. 121, Dordrecht/Boston/London: Kluwer Academic Publishers, 2001:345‐61. [Google Scholar]
Lieber 2003a
- Lieber CS, Leo MA, Cao Q, Ren C, DeCarli L. Silymarin retards the progression of alcohol‐induced hepatic fibrosis in baboons. Journal of Clinical Gastroenterology 2003;37:336‐9. [DOI] [PubMed] [Google Scholar]
Lieber 2003b
- Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S, for the Veterans Affairs Cooperative Study 391 Group. II. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease. Alcoholism, Clinical and Experimental Research 2003;27(11):1765‐72. [DOI] [PubMed] [Google Scholar]
Lok 2001
- Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology 2001;34(6):1225‐41. [DOI] [PubMed] [Google Scholar]
Luper 1998
- Luper S. A review of plants used in the treatment of liver disease: part 1. Alternative Medicine Review 1998;3(6):410‐21. [PubMed] [Google Scholar]
Main 1998
- Main J, McCarron B, Thomas HC. Treatment of chronic viral hepatitis. Antiviral Chemistry and Chemotherapy 1998;9(6):449‐60. [PMID: 9865383] [DOI] [PubMed] [Google Scholar]
Marbet 1987
- Marbet UA, Bianchi L, Meury U, Stalder GA. Long‐term histological evaluation of the natural history and prognostic factors of alcoholic liver disease. Journal of Hepatology 1987;4(3):364‐72. [DOI] [PubMed] [Google Scholar]
Mato 1999
- Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, et al. S‐adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo‐controlled, double‐blind, multicenter clinical trial. Journal of Hepatology 1999;30:1081‐9. [DOI] [PubMed] [Google Scholar]
Mezey 1991
- Mezey E, Caballeria J, Mitchell MC, Pares A, Herlong HF, Rodes J. Effect of parenteral amino acid supplementation in severe alcoholic hepatitis: a randomised controlled trial. Hepatology 1991;14(6):1090‐6. [PMID: 1959859] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher R, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐anlyses. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Morgan 1999
- Morgan MY. Alcoholic liver disease: natural history, diagnosis, clinical features, management, prognosis and prevention. In: Bircher J, Benhamou J‐P, McIntyre N, Rizzetto M, Rodés J editor(s). Oxford Text Book of Clinical Hepatology. Second Edition. Vol. 2, Oxford: Oxford University Press, 1999:1185‐238. [Google Scholar]
Muriel 1992
- Muriel P, Garciapina T, Perez Alvarez V, Mourelle M. Silymarin protects against paracetamol‐induced lipid peroxidation and liver damage. Journal of Applied Toxicology 1992;12:439‐42. [PMID: 1360480] [DOI] [PubMed] [Google Scholar]
Pelletier 2003
- Pelletier G, Roulot D, Davion T, Masliah C, Causse X, Oberti F, et al. A randomized controlled trial of ursodeoxycholic acid in patients with alcohol‐induced cirrhosis and jaundice. Hepatology 2003;37:887‐92. [PMID: 12668982] [DOI] [PubMed] [Google Scholar]
Pianko 2000
- Pianko S, McHutchison JP. Treatment of hepatitis C with interferon and ribavirin. Journal of Gastroenterology and Hepatology 2000;15(6):581‐6. [PMID: 10921409] [DOI] [PubMed] [Google Scholar]
Powell 1968
- Powell WJ Jr, Klatskin G. Duration of survival with Laennec's cirrhosis. Influence of alcohol withdrawal, and possible effects of recent changes in general managment of the disease. American Journal of Medicine 1968;44(3):406‐20. [DOI] [PubMed] [Google Scholar]
Poynard 1994
- Poynard T, Barthelemy P, Fratte S, Boudjema K, Doffoel M, Vanlemmens C. Evaluation of efficacy of liver transplantation in alcoholic cirrhosis by a case control study and simulated controls. Lancet 1994;344(8921):502‐7. [PMID: 7914613] [DOI] [PubMed] [Google Scholar]
Rambaldi 2001a
- Rambaldi A, Gluud C. Colchicine for alcoholic and non‐alcoholic liver cirrhosis and fibrosis. The Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No.: CD002148. DOI: 10.1002/14651858.CD002148.pub2. [DOI] [PMC free article] [PubMed]
Rambaldi 2001b
Rambaldi 2002a
- Rambaldi A, Gluud C. Anabolic‐androgenic steroids for alcoholic liver disease ‐ a Cochrane Review. The American Journal of Gastroenterology 2002;97(7):1674‐81. [DOI] [PubMed] [Google Scholar]
Rambaldi 2002b
- Rambaldi A, Gluud C. Propylthiouracil for alcoholic liver disease ‐ Cochrane Review. Liver 2002;21:398‐404. [DOI] [PubMed] [Google Scholar]
Rambaldi 2003
Ramellini 1974
- Ramellini G, Meldolesi J. Stabilization of isolated rat liver plasma membranes by treatment in vitro with silymarin. Arzneimittel Forschung 1974;24(5):806‐8. [PubMed] [Google Scholar]
Rauen 1971
- Rauen HM, Schriewer H. The antihepatotoxic effect of silymarin on liver damage induced by carbon tetrachloride, d‐galactosamine, and allyl alcohol [Die antihepatotoxische wirkung von silymarin bei experimentellen leberschädigunug der ratte durch tetrachlorkohlenstoff, D‐galaktosamin und allylalkohol]. Arzneimittel Forschung 1971;21:1194‐212. [PMID: 5110018] [PubMed] [Google Scholar]
Rauen 1973
- Rauen HM, Schriewer H. The antihepatotoxic effect of parenteral silymarin liver injury caused by CCL4 in the rat [Die antihepatotoxische wirkung von parenteral verabreichten silymarin bei der leberbeschädigung der ratte durch carbonium tetrachloride (CCL4)]. Arzneimittel Forschung 1973;23:148‐9. [PubMed] [Google Scholar]
Rubin 1968
- Rubin E, Lieber CS. Alcohol‐induced hepatic injury in non‐alcoholic volunteers. New England Journal of Medicine 1968;278(16):869‐76. [DOI] [PubMed] [Google Scholar]
Russo 2000
- Russo F, Bacosi, Miglioresi L, Ricci GL. Leucopenia is a side effect of combination therapy for hepatitis C infection. The American Journal of Gastroenterology 2000;95(4):1100‐1. [PMID: 10763981] [DOI] [PubMed] [Google Scholar]
Saller 2001
- Saller R, Meier R, Brignoli R. The use of sillymarin in the treatment of liver diseases. Drugs 2001;61:2035‐63. [PMID: 11735632] [DOI] [PubMed] [Google Scholar]
Schriewer 1973
- Schriewer H, Badde R, Roth G, Rauen HM. The anti‐hepatoxic effect of silymarin on thioacetamide‐induced liver‐damage. Arzneimittelforschung 1973;23:160. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methological quality associated with estimates of treatment effects in controlled trials. Journal of American Medical Association 1995;273(5):408‐12. [PMID: 7823387] [DOI] [PubMed] [Google Scholar]
Szilard 1988
- Szilard S, Szentgyorgyi D, Demeter I. Protective effect of Legalon in workers exposed to organic solvent. Acta Medica Hungarica 1988;45(2):249‐56. [MEDLINE: ] [PubMed] [Google Scholar]
Sørensen 1984
- Sørensen TIA, Orholm M, Bentsen KD, Høybye G, Eghøje K, Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in as predictors of developement of cirrhosis. Lancet 1984;2(8397):241‐4. [DOI] [PubMed] [Google Scholar]
Tanasescu 1988
- Tanasescu C, Petrea S, Baldescu R, Macarie E, Chiriloiu C, Purice S. Use of the Romanian product Silimarina® in the treatment of chronic liver diseases. Medecine Interne 1988;26(4):311‐22. [PMID: 3072661] [PubMed] [Google Scholar]
Trinchet 1992
- Trinchet JC, Balkau B, Poupon RE, Heintzmann F, Callard P, Gotheil C, et al. Treatment of severe alcoholic hepatitis by infusion of insulin/glucagon: a multicenter sequential trial. Hepatology 1992;15(1):76‐81. [PMID: 1727803] [DOI] [PubMed] [Google Scholar]
Tuchweber 1979
- Tuchweber B, Sieck R, Trost W. Prevention of silybin of phalloidin‐induced acute hepatotoxicity. Toxicology and Applied Pharmacology 1979;51:265‐75. [DOI] [PubMed] [Google Scholar]
Valenzuela 1985
- Valenzuela A, Barria T, Guerra R, Garrido A. Inhibitory effect of the flavonoid silymarin on the erythrocyte hemolysis induced by phenylhydrazine. Biochemical and Biophysical Research Communications 1985;126:712‐5. [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19(2):159‐66. [DOI] [PubMed] [Google Scholar]
Zavaglia 2000
- Zavaglia C, Airoldi A, Pinzello G. Antiviral therapy of HBV ‐ and HCV induced liver cirrhosis. Journal of Clinical Gastroenterology 2000;30(3):234‐41. [PMID: 10777179] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rambaldi 2005
- Rambaldi A, Jacobs BP, Iaquinto G, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C liver diseases‐‐a systematic cochrane hepato‐biliary group review with meta‐analyses of randomized clinical trials. American Journal of Gastroenterology 2005;100(11):2583‐91. [DOI] [PubMed] [Google Scholar]


