Abstract
Mediator is the evolutionarily conserved coactivator required for the integration and recruitment of diverse regulatory signals to basal transcription machinery. To elucidate the functions of metazoan Mediator, we isolated Drosophila melanogaster Med6 mutants. dMed6 is essential for viability and/or proliferation of most cells. dMed6 mutants failed to pupate and died in the third larval instar with severe proliferation defects in imaginal discs and other larval mitotic cells. cDNA microarray, quantitative reverse transcription-PCR, and in situ expression analyses of developmentally regulated genes in dMed6 mutants showed that transcriptional activation of many, but not all, genes was affected. Among the genes found to be affected were some that play a role in cell proliferation and metabolism. Therefore, dMed6 is required in most cells for transcriptional regulation of many genes important for diverse aspects of Drosophila development.
The development of multicellular organisms, even at the level of a single cell, demands a complex array of transcriptional regulation mechanisms for proper proliferation and development. To meet the demand, eukaryotic cells utilize transcriptional machinery comprising dozens of proteins that recognize and initiate RNA synthesis from promoters and that regulate the efficiency of transcription using thousands of specialized transcription factors. In addition, a number of coactivator complexes working at diverse stages of transcription add to the depth of regulatory complexity to achieve the orchestrated developmental control of gene expression in higher eukaryotes. Although these coactivator proteins appear to be required for transcriptional regulation in general, different groups of genes show different coactivator requirements. In addition, these coactivator functions are carried out by a number of complexes. Therefore, each coactivator complex appears to have unique and specific roles in transcriptional regulation of diverse developmental processes.
Two major coactivator complexes, TFIID (20, 38) and Mediator (24, 25), integrate and relay diverse regulatory signals to the basal transcription machinery through their association with TATA-box binding protein (TBP) and RNA polymerase II (pol II), respectively. Both complexes were shown to be required for transcriptional activation in an in vitro transcription system under specific conditions (2, 3, 17, 32). Depletion or inactivation of TFIID-specific TBP-associated factors affects the transcription of a subset of the genes involved in cell cycle regulation and development, suggesting that TFIID may act as a gene-specific rather than a general coactivator (21, 33, 47).
The Mediator complex was first identified in budding Saccharomyces cerevisiae as a general intermediary complex that mediates signal transfer between transcriptional activator proteins and the basal transcription machinery (24, 25). The search for similar Mediator complexes in mammalian systems led to the identification of a number of homologous complexes (5, 15, 18, 23, 35, 39, 41, 45). These complexes contain more than one Mediator homolog and share several components, but their overall compositions are different from each other and from that of the yeast Mediator complex.
Biochemical analysis of the yeast Mediator complex revealed that it is composed of several functional modules, each of which regulates distinct groups of genes (28, 30, 34). Mutations in the Gal11 and Med10 proteins caused severe transcriptional defects specifically to the genes involved in carbon metabolism (e.g., GAL1) and amino acid synthesis (e.g., HIS4), without affecting the expression of other groups of genes (19). The distinct activator-specific binding regions of Mediator underlie the gene-specific regulatory mechanism of Mediator subunits (37). In addition, alleles of gal11, sin4, and rgr1 affect the process of transcriptional repression as well as activation (11, 42). In vitro transcriptional analysis of mammalian Mediator homologs also demonstrated the requirement for the Mediator complexes for both positive and negative regulation of transcription (18, 45). Compared to the extensive genetic analysis of the Mediator complex in yeast, the functional analysis of Mediator genes in multicellular organisms is currently limited. Analysis of evolutionarily conserved subunits of Mediator (Med6, Srb7, Med7, and Med10) with the use of an RNA interference assay revealed that Caenorhabditis elegans Mediator homologs are required for transcriptional activation of developmentally regulated genes (26). These conserved subunits of Mediator complexes appear to have similar roles in mammals as well: murine Srb7 is essential for embryonic stem cell viability and development (46). On the other hand, disruption of a metazoan-specific subunit of Mediator revealed a gene-specific function. The C. elegnas Trap230 gene was shown to regulate lineage-specific expression of transcription factors (51). Ablation of the murine Trap220 gene revealed that null mutants die during an early gestational stage with heart failure and impaired neuronal development (22). Clonal analyses of Drosophila melanogaster Trap80 and Trap240 mutants revealed their functions in the specification of adult cell and segment identity (4). Therefore, the metazoan Mediator subunits appear to contribute their gene- or activator-selective functions to diverse developmental processes.
To pinpoint the physiological functions of Mediator homologs in higher eukaryotes, we isolated mutants for a Drosophila homolog of yeast Med6 (dMed6) and examined their effects on development and transcriptional activation. Our results suggest that dMed6 is essential for cell viability and/or proliferation of diverse germ line and somatic cells and is required for transcriptional activation of a subset of genes involved in diverse aspects of development. Therefore, dMED6 appears to play an important role as a gene-specific transcriptional coactivator in Drosophila as does Med6 in yeast.
MATERIALS AND METHODS
Degenerate PCR-based cloning of Drosophila Med6 homolog.
To clone a Drosophila Med6 homolog, degenerate PCR primers were designed for the conserved regions of yeast, C. elegans, and human Med6 proteins and used to amplify Drosophila embryonic cDNA. Sequencing analysis of the fragments amplified with diverse sets of the Med6 degenerate PCR primers revealed that primers M1–1 (5′-cggaattcGTN TTR GAY TAY TTT-3′) and M5–1 (5′-cgggatccDAT DAT RTA RTA RTC-3′) amplified a fragment with a sequence homologous to those of other Med6 genes (lowercase letters indicate the restriction enzyme site incorproated at the end of each PCR primer). By using the PCR fragment as a probe, cDNA and genomic DNA clones were isolated from screens of a Drosophila adult λZAPII cDNA library (Stratagene) and a Drosophila genomic Charon 4A genomic DNA library, respectively. Three independent cDNA clones and the 4.2-kb BamHI-EcoRI genomic fragment identified by Southern analysis to contain the dMed6 gene were sequenced and analyzed for gene structure with the use of the GCG program (Wisconsin package). The sequence and structure of the dMed6 gene (CG9473) were described in the Genome Annotation Database for Drosophila (http://www.fruitfly.org).
Preparation of larval nuclear extracts.
Whole animals (0.l ml), carrying dMed626/26 and dMed626/+, at the late-third-instar larval stage were resuspended in 0.25 ml of NEB(0.3) (0.3 M sucrose, 10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM dithiothreitol, 1× protease inhibitor), homogenized with a micropestle, and filtered through synthetic cotton. Filtered supernatant was loaded on 0.25 ml of NEB(1.7) [same as NEB(0.3) except 1.7 M sucrose] and centrifuged for 15 min at 12,500 rpm and 4°C. Nuclear pellets were resuspended in NEB(0.3) for immunoblotting or in 0.1 ml of HEMG-0.4K (25 mM HEPES-KOH [pH 7.6], 400 mM potassium acetate, 5 mM magnesium acetate, 0.1 mM EDTA, 5 mM β-mercaptoethanol, 20% glycerol, and 0.5 mM phenylmethylsulfonyl fluoride). Nuclei resuspended in HEMG-0.4K were disrupted by three cycles of freezing and thawing in liquid nitrogen and a water bath. After the lysates were centrifuged for 15 min at 15,000 × g and 4°C, the supernatants were collected and used for immunoprecipitation with anti-dSOH1 antibody beads or subjected to Superose-6 chromatography.
Transcriptional and biochemical analyses.
Nuclear extract (Drosophila Oregon R embryo) preparation, in vitro transcription, immunodepletion, and gel filtration analyses were carried out as described previously (36). The antibodies against each Drosophila Mediator protein used in this study were described previously (36).
EMS mutagenesis.
Two hundred 3-day-old isogenic w1118 male flies were fed with ethane methyl sulfonate (EMS) solution (25 mM) as described by Huang and Baker (21a) and crossed with an equal number of TM3 Sb Ser virgin female flies to balance the third chromosome with TM3 Sb Ser. Each of 6,555 males carrying the EMS-mutagenized third chromosome over the balancer was crossed individually with three Df(3R)by10/TM3 Sb females. After 15 days, the lines without viable Sb+ progeny were selected, and the balanced males from each of the selected lines were crossed with Df(3R)γB104/TM3 females to identify the lines that produce viable Sb+ progeny. Thirty-six lines suffering lethality whose genes were isolated within the nonoverlapping regions of the Df(3R)by10 and Df(3R)γB104 chromosomes were crossed to each other to identify the complementation groups. A single male from each complementation group was crossed with w1118; p{w+=dMed6 (10.4 kb)}/CyO; Df(3R)by10/TM3 females, and the viability of the w1118; p{w+=dMed6 (10.4 kb)}/+; +*/Df(3R)by10 progeny was examined to identify the dMed6 mutants.
SSCP analysis.
The transcribed region of dMed6 was amplified in three fragments of 300 to 400 bp from wild-type and mutant heterozygous genomic DNAs. Each PCR product was cloned in pBluescript SK(+) vector (Stratagene). Ten independent clones were amplified for each PCR fragment and displayed along with the corresponding wild-type PCR fragment on 8% nondenaturing polyacrylamide gels (acrylamide-bisacrylamide ratio, 49:1) with 10% glycerol for 12 h at room temperature or 4°C and visualized by silver staining. The PCR products that showed abnormal migration on the single-strand conformational polymorphism (SSCP) analysis were sequenced to find the mutations.
FLP-FRT-mediated clonal analysis.
Clones of mutant cells were generated by the Flip recombinase-FRT site (FLP-FRT)-mediated mitotic recombination system (49). y w hsFLP/+; FRT-82B p{w+mC=ovoD1–18}3R1 p{w+mC=ovoD1–18}3R2/FRT-82B dMed626 and y w hsFLP/+; FRT-82B p{w+mC=ovoD1–18}3R1 p{w+mC=ovoD1–18}3R2/FRT-82B ry506 females (10) were generated by standard crosses. For germ line clonal analysis, 200 female flies (3 to 5 days old) were heat treated at 37°C for 2 h during the late-first-instar larval stage and mated with 50 w1118 male flies. After 2 days embryos were collected at 4-h intervals. For twin spot analysis of adult tissues, dMed6− clones were monitored using the associated marker w in eyes and the green fluorescent protein (GFP) marker expressed from the ubiquitin-63E (Ubi) promoter in imaginal discs and ovarian follicle cells. For these experiments, y w hsFLP/+; FRT-82B P{w+mC=Ubi-GFP}/FRT-82B dMed626 and y w hsFLP/+; FRT-82B P{w+mC=Ubi-GFP}/FRT-82B ry506 females were generated. Clones were induced by heat shock (2 h, 37°C) during the first or second instar. Imaginal discs were dissected from late L3 larvae. Eyes and ovaries from 2- to 5-day-old adult females that were heat shocked for 2 h at the end of the late-first-instar larval stage were analyzed. The dissected imaginal discs and ovaries were fixed with 4% formaldehyde for 20 min at 22°C. GFP expression was analyzed by confocal laser microscopy (Bio-Rad; MR1024).
GFP in larval tissues.
Larval tissues were dissected and mounted in 1× phosphate-buffered saline (PBS). Whole larvae were etherized in 20% (vol/vol) diethyl ether in ethanol for 5 min, mounted in a 70% (vol/vol) glycerol (in PBS) on a standard slide glass with a paper tape support for a standard coverslip, and viewed directly. Samples were observed with a plane fluorescence microscope (Carl Zeiss; Axioscop 2) or confocal laser scanning microscope (Carl Zeiss; LSM510) under Hg illumination with standard fluorescein isothiocyanate fluorescence filters for the observation of modified GFPS65T.
DNA chip analysis.
cDNA expression profiles of 192 genes were analyzed by microarray analysis on a polylysine-coated glass slide as described previously (48) with the following modification. Total RNA was isolated from whole flies carrying dMed626/26 and dMed626/+ at the late-third-instar larval stage. Fluorescent cDNA was produced with 5 μg of total RNA, oligo(dT) primers (Gibco-BRL), and Superscript II reverse transcriptase (Gibco-BRL) in the presence of Cy3 or Cy5 fluorescence-tagged dUTP (Amersham-Pharmacia). The labeled cDNA was dissolved in 3× saline sodium citrate buffer (SSC; 1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and hybridized to microarrays for 12 to 16 h at 65°C in humidified incubation chambers. Arrays were then washed for 5 min in 0.6× SSC–0.03% sodium dodecyl sulfate and rinsed for 10 min in 0.06× SSC, spun dry, and scanned with a confocal laser array scanner (GSI Lumonics; Scanarray Lite). The results were analyzed with Quantarray (GSI Lumonics) and Gene Spring, version 3.1 (Silicon Genetics). Results from two independent hybridization analyses were averaged.
Quantitative reverse transcription-PCR (RT-PCR).
Total RNAs (5 μg) isolated from dMed626/26 or dMed626/+ flies at the late-third-instar larval stage were incubated with oligo(dT) primers (Gibco-BRL), Superscript II reverse transcriptase (Gibco-BRL), and deoxynucleoside triphosphate (dNTP; Boehringer Mannheim) in 30-μl reaction buffer supplied by the manufacturer. The specific gene of interest was amplified with 1 μl of the cDNA synthesized in the presence of a mixture containing 5 pmol of specific primers, 1 μl of a 10× SYBR Green I solution (Roche), 2 μl of 10× PCR buffer (100 mM Tris-Cl [pH 8.8], 500 mM KCl, 25 mM MgCl2, 1% Triton X-100), 1.6 μl of 2.5 mM dNTP mixture, and 15 U of Taq DNA polymerase in a 20-μl reaction mixture. The product was amplified by 35 cycles of PCR (30 s at 94°C, 30 s at 63°C, and 1 min at 72°C). The incorporation of the dye into the amplified products was monitored by iCycler (Bio-Rad), and the concentration of a specific transcript in the sample was analyzed by the associated software based on the standard curves predetermined with known amounts of target transcripts. Quantities of rp49 gene transcripts were used as a total-cDNA input control. Results from three independent RT-PCR analyses were averaged.
LacZ activity staining in larval tissues.
Enhancer trap LacZ lines (43) (provided by the Bloomington Stock Center) were crossed with a dMed626 mutant to make p{ry+t7.2=PZ}*/+; dMed626/TM6B Tb p{w+mC=Ubi-GFP} flies, and their males were crossed with dMed626/TM6B Tb p{w+mC=Ubi-GFP}virgin females. Larval progeny were washed with water extensively and stored in 1× PBS. Tissues were dissected in cold 1× EBR (130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM HEPES-NaOH [pH 6.9]) and then fixed within 5 min with fixative (4:1:1 ratio of water–37% formaldehyde–buffer B [100 mM KH2PO4-K2HPO4 {pH 6.8}, 450 mM KCl, 150 mM NaCl, 20 mM MgCl2)]). The fixed tissues were stained with LacZ staining solution {10 mM NaH2PO4-Na2HPO4 [pH 7.2], 150 mM NaCl, 1 mM MgCl2, 3.1 mM K4[Fe2+(CN)6], 3.1 mM K3[Fe3+(CN)6], 0.3% Triton X-100, 0.05% X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside]} at 37°C for 16 h. The stained tissues were dissected on a slide and mounted in 1× PBS, and the coverslip was sealed with nail polish. The samples were analyzed by using Nomarski images (Carl Zeiss; Axioscope 2).
RESULTS
Cloning and expression of Drosophila Med6
To study the function of Mediator in Drosophila development, we cloned a Drosophila Med6 homolog by PCR. We used degenerate primers based on the conserved regions of the yeast, nematode, and human MED6 proteins (Table 1). The Drosophila Med6 homolog was isolated and termed dMed6, and it encodes a 247-amino-acid polypeptide (GadFly CG9473). This predicted polypeptide has 43 and 19% identity to human and yeast MED6, respectively. Sequence analysis of the dMed6 cDNA and genomic clones revealed that the dMed6 gene is composed of four exons and controlled by a distal promoter element-containing TATA-less promoter. In situ hybridization of the dMed6 cDNA to polytene chromosomes revealed that dMed6 is located on the third chromosome at the 85E10-13 locus, where the dMed6 sequence was identified in the Drosophila genome project.
TABLE 1.
DNA sequences of oligomers used in RT-PCR analyses
| Gene | Directiona | Sequence (5′–3′) |
|---|---|---|
| Rp49 | F | CAG TCG GAT CGA TAT GCT AAG CTG T |
| R | TAA CCG ATG TTG GGC ATC AGA TAC T | |
| Adh | F | GAG AAC TTC GTC AAG GCT ATC GAG C |
| R | TGG TTC GCA GAA CCC TAT GAA CTA A | |
| cdc2 | F | ACA TGG AGA GTG AAT TGG TCC GTA |
| R | TTT ATG AGG CCA CTC TTG TCG ATT A | |
| Dhr78 | F | GAT ATC GAT AAG ATC GAA CCG TTG AA |
| R | GTA GAG TTG GAC TCT GCG GAC GTA | |
| 18w | F | GTG CTC ATC ATT GTC TTC GTC TTC C |
| R | AAA CTC GTA GTC CTT CTC CGA GTG C | |
| brn | F | GGT ATG TTC GAT CAG AAG TCA ACG G |
| R | AAT ATT TCA ATC TCC TCC TCG CTG C | |
| dl | F | CTG GAG ATC AAC AGT GAG ACA ATG C |
| R | GAA TTC AGA TCT ATG CTC GAG GGC T | |
| exd | F | ACA GTC ACT TGA GCA ACC CAT ATC C |
| R | CCT CCT GTG CCT TAC CAA TGT TCT T | |
| Hsf | F | CCT CAC ATT ATG ACC AAG AGA GCG T |
| R | CCG CCT ACT AGA ATA TTA CCG CCA G | |
| Kr | F | GCT GCA TTA GCT GGC ATA AAA CAA G |
| R | AGC CAG AAG TTG GGT AGG TGA TAG C | |
| Lsp-1β | F | TCT ACG AGT TCA ACC AGG AGA CCA A |
| R | GAT AGT ACC AGT AGG CGT TCC AGC C | |
| Pka-Cl | F | ATT ATG CCA TGA AGA TCC TCG ACA A |
| R | GGG AAC ATA CTC CAG CAC CAT GTA A | |
| tkv | F | GAT TAC CAT TGC TGG TGC AAA GAA C |
| R | AAG AAG CCT CTT CGG TCG TAA AGA A | |
| trx | F | CCA ACC GAT GTA CTA TGG ACT GGA G |
| R | ATC GGT TCG ATT TTA CTA GCC TGG A | |
| zip | F | TCT GTA CAA GGA GCA GCT GGC TAA G |
| R | CAG ATA CGA ATA CCC TCG AGC ACA C | |
| Ap | F | TCA ACA CTG AGT ACG TGG ACT TTG G |
| R | TTG TTT TAG ATC CTT TGC ATC AGG G | |
| CycE | F | AAG TTT ACA AGC TGC ATC GGG AGA C |
| R | TGG CGG ATA AAT CTC CTC TAC CTT G | |
| dpp | F | GTG CAG ACC CTG GTC AAC AAT ATG |
| R | ACC ACG GTC ATC TCC TGG TAG TTC T | |
| EGFR | F | GTA TTC ACC AGC AAG TCC GAT GTC T |
| R | CCA GCA CGA GAG CAG TGT ACA GTA A | |
| Salm | F | TGT TGT TCG AGC AAA AGC TGA GAA T |
| R | GAT AAC CTG GGA TGA TGC ATG TAC G |
F, forward; R, reverse.
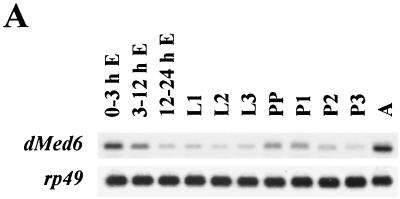
Developmental Northern analysis showed that a single 1.7-kb dMed6 transcript was maternally deposited and gradually decreased through embryogenesis. In wild-type flies, the number of dMed6 transcripts increased during the early stages of pupation and reached the highest level during adulthood (Fig. 1A). Thus, dMed6 expression appears to be correlated with those developmental stages that involve high developmental activities.
FIG. 1.
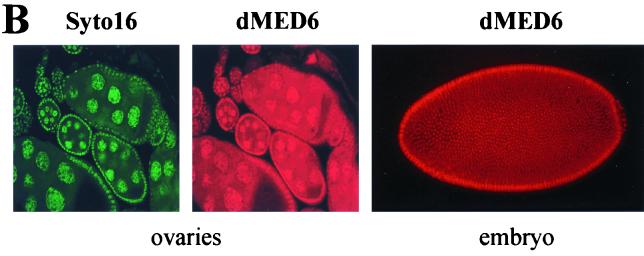
Expression of dMed6. (A) Developmental Northern analysis of dMed6. Poly(A+) RNA (3 μg) isolated from each indicated developmental stage was analyzed with a dMed6 cDNA probe. The amount of rp49 transcript is shown as a loading control. E, embryo; L, larva; PP, prepupa; P, pupa; A, adult. (B) Immunostaining of ovaries and embryos with anti-dMED6 Ab (red) or Syto16 (green; nucleic acid).
Immunostaining of Drosophila embryos and adult tissue sections with anti-dMED6 polyclonal antibodies (Abs), which were raised in rabbits, against the full-length recombinant dMED6 protein revealed that the dMED6 protein was expressed ubiquitously throughout development and was localized mainly in the nuclei (Fig. 1B and data not shown). Double staining of Drosophila ovaries with a dMED6 Ab and Syto16 (Molecular Probes; S-7875) showed a high level of dMED6 protein in the nuclei of nurse and follicle cells. In addition, the cytoplasm of nurse cells contained a significant amount of dMED6 protein for later deposition in oocytes (Fig. 1B).
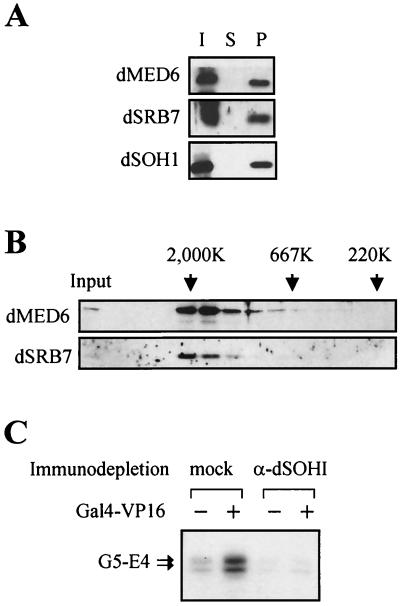
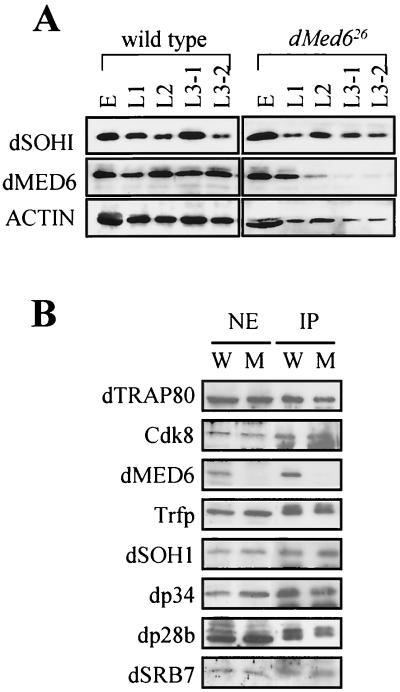
In order to examine whether dMED6 is the true functional homolog of the yeast and human Med6 proteins, the biochemical characteristics of dMED6 were determined by coimmunoprecipitation and gel filtration analysis. Affinity-purified anti-dMED6 Abs or anti-dSOH1 Abs precipitated all of the dMED6 protein together with the other Drosophila Mediator subunits, dSRB7 and dSOH1 (Fig. 2A and data not shown). When Drosophila nuclear extract was analyzed by Superose-6 gel filtration chromatography, both dMED6 and dSRB7 migrated at a molecular size of 2 MDa (Fig. 2B). In addition, the immunodepletion of the dMED6-containing complex from the Drosophila embryo soluble nuclear fraction with the anti-dSOH1 Ab abolished the transcriptional activation activity of the extract (Fig. 2C). These lines of evidence strongly indicate that dMED6 is a genuine Mediator component.
FIG. 2.
Association of dMED6 with Mediator complex. (A) Coimmunoprecipitation of dMED6 with other Mediator subunits. Drosophila embryo nuclear extract was immunoprecipitated with anti-dSOH1 Ab, and the input (I), supernatant (S), and pellet (P) were analyzed by immunoblotting with Abs against the proteins indicated at the left. (B) Gel filtration analysis of dMED6-containing complex. Embryo nuclear extract was put on a Superose-6 gel filtration column, and the filtrates were analyzed with Abs against the proteins indicated at the left. The input and the elution positions of size markers are marked. (C) Transcriptional activation of the E4 promoter constructs by Gal4-VP16 in nuclear extracts. Before the in vitro transcription assay, the nuclear extracts were immunodepleted with anti-dSOH1 (α-dSOH1) or anti-β-galactosidase (mock). Recombinant Gal4-VP16 (40 ng) was added to the reaction mixtures as indicated. Arrows, transcripts from the E4 templates containing five tandem Gal4 DNA binding sites (G5-E4).
Isolation of dMed6 mutants.
Although the requirement of the Mediator complex for transcriptional activation has been well documented in yeast, there is little information about the physiological function of Mediator in higher eukaryotes. This prompted us to examine the gene-specific requirement of dMed6 for transcriptional activation and the role of dMed6 in developmental processes in Drosophila. To address these questions, we isolated dMed6 mutant alleles using mutagenesis induced by EMS (Sigma; M-0880).
A database search for chromosomal deletions at the dMed6 locus identified two deficiency lines: Df(3R)by10 (deficient for 85D8–12 to 85E7-F1) and Df(3R)GB104 (deficient for 85D12 to 85E10) lines. Although there is an extensive overlap in the deleted area between these two deficiency chromosomes, in situ hybridization of heterozygous polytene chromosomes revealed that dMed6 was uncovered only by the Df(3R)by10 chromosome (data not shown). Therefore, we used Df(3R)by10 in an F2 screen for EMS-induced lethal mutations at the dMed6 locus.
Of 6,693 EMS-mutagenized F1 flies, 102 chromosomes caused lethality to Df(3R)by10. Among them, 34 mutant chromosomes were complemented by the Df(3R)GB104 chromosome, which contained dMed6. A complementation test of the 34 lines suffering lethality identified 11 complementation groups. Among them, the lethality (fertility as well) for one complementation group, which contained two independently screened lines suffering lethality (BE026 and BE064), was rescued by a genomic DNA fragment that encompasses the dMed6 gene.
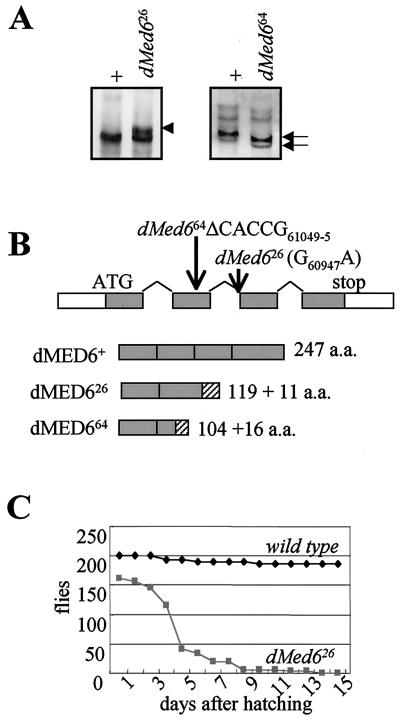
To identify the mutation sites of these dMed6 mutant alleles, we examined sequence variations between the wild-type and mutant alleles of dMed6 by the SSCP method. Both of the mutant alleles had alterations in the SSCP banding pattern due to a PCR fragment spanning the second and third exons (nucleotide positions 61159 to 60748 of the sequence with GenBank accession no. AE003648). The BE026 allele (dMed626) displayed an extra band, and the BE064 allele (dMed664) displayed a faster-migrating band (Fig. 3A). Sequence analysis of these PCR fragments identified that dMed626 had a G60947-to-A change, which disrupts the 3′ splicing acceptor site of the second intron, whereas dMed664 had a five-nucleotide deletion within the coding region of the second exon (ΔCACCG61049–61045) (Fig. 3B). Both mutations caused severe truncation of the C-terminal coding region. The dMED626 and dMED664 mutant proteins were truncated at amino acid residues 119 and 104, which were followed by 11 and 16 nonauthentic amino acids, respectively (Fig. 3B). The deleted regions of the dMed6 mutant proteins include a 40- to 50-amino-acid region with extensive sequence conservation from yeasts to humans. A small deletion in this region was lethal in yeast (S. Min and Y.-J. Kim, unpublished data). Immunoblot analysis of dMed6 mutant heterozygotes with Abs that recognize the N-terminal regions of dMed6 did not detect any sign of the truncated dMED6 mutant proteins (data not shown). Therefore, these dMed6 mutant alleles appear to be null alleles.
FIG. 3.
Identification of dMed6 mutations. (A) SSCP analysis of dMed6 mutant alleles. PCR fragments (positions 61159 to 60748) amplified from wild-type (+), dMed626, and dMed664 mutant chromosomes were analyzed by SSCP gel electrophoresis. The extra band (arrowhead) and faster-migrating bands (arrows) are marked. (B) Mutation sites of dMed626 and dMed664. The structure of the dMed6 gene is marked with the mutations in each dMed6 mutant allele identified. dMed626 has one nucleotide change (G60947A) at the splicing acceptor of the second intron, and dMed664 has a five-nucleotide deletion (ΔCACCG61049–5). Both mutations cause truncation of the dMED6 protein, as shown beneath. Gray boxes, wild-type coding regions; hatched boxes, nonauthentic amino acids added to the C-terminal ends of the mutant proteins due to the mutations. (C) Lethality of dMed6 homozygous mutants. The numbers of larvae, pupae, and flies viable after hatching from eggs were determined every day for wild-type (200 hatched larvae) and dMed626 mutant flies (168 hatched larvae) at 25°C. Most of the wild-type flies developed to adults in 10 days after hatching. However, a significant number of mutant flies died 3 to 5 days after hatching. The mutant flies showed no apparent developmental defect until the third larval instar but never developed to the prepupa stage.
Requirement of dMed6 for cell viability.
To identify the developmental defects associated with the dMed6 mutations, we first determined the lethal phase of the dMed6 homozygous mutants. Among the embryos from dMed626/TM6B(GFP) and dMed664/TM6B(GFP) heterozygous flies, we collected dMed6 homozygous mutant embryos that do not express green fluorescence in the central nervous system (CNS). Developmental progress of these embryos was scored at 24-h intervals. Both types of dMed6 mutant embryos developed normally and reached third-instar larvae 2 days after hatching, as did the wild-type embryos. After two more days, all of the wild-type larvae quadrupled their size and had entered pupation, while the mutant larvae showed a slower growth rate and most of them died without pupating. A small number of mutant larvae survived for several more days but never developed into pupae (Fig. 3C). When we examined the dMed626/dMed664, dMed626/Df(3R)by10, and dMed664/Df(3R)by10 flies, we found that they all showed the third-instar larval lethality (data not shown). Therefore, dMed6 mutants are defective in a developmental process required for the transition from third-instar larva to pupa.
To test whether maternally deposited dMED6 supplies the necessary activity during the early developmental stages, we examined the level of dMED6 and several other Mediator proteins in the mutants at each developmental stage. Western blot analyses with an anti-dMED6 Ab showed that wild-type flies contained an almost-constant level of Mediator protein during development from embryo to larva. On the other hand, the mutant embryos contained large amounts of maternally deposited wild-type dMED6 protein, but the level of dMED6 protein diminished greatly in the second-instar larva stage and became almost undetectable in the third instar. Therefore, the depletion of the maternally deposited dMED6 proteins in the mutant caused the developmental defects.
Because dMED6 is a component of a coactivator complex, the loss of dMED6 may affect the structural integrity of the Mediator complex. However, immunoblot analysis of the mutant nuclear extracts revealed that the amounts of other Drosophila Mediator homologs were maintained at a level comparable to that for the wild type in the absence of dMED6 (Fig. 4A). When the nuclear extracts were immunoprecipitated with the anti-dSOH1 Ab, all the Mediator proteins we tested were precipitated together with dSOH1 except dMED6 (Fig. 4B). In addition, the dMED6-deficient complex migrated on a Superose-6 gel filtration column at the position identical to that for the wild-type Mediator (data not shown). These results suggest that the dMED6-deficient Mediator retains all of the other Mediator subunits, as the Med6ts Mediator in yeast does (29). Therefore, the gene-specific defects described here appear to have resulted mainly from the loss of dMed6 function rather than from the inactivation of the whole Mediator complex.
FIG. 4.
dMED6-deficient Mediator complex. (A) Levels of dMED6 and dSOH1 proteins in wild-type and dMed626 mutant flies. Whole-cell extracts (20 μg) were prepared from wild-type and dMed626 embryos (E), first-instar larvae (L1), second-instar larvae (L2), early-third-instar larvae (L3–1), and late-third-instar larvae (L3–2). Proteins were immunoblotted with the antibodies indicated at the left. The actin protein was the loading control. (B) Immunoprecipitation of nuclear extracts of dMed6+/26 (W) and dMed626/26 (M) third-instar larvae with the anti-dSOH1 Ab. Equivalent amounts of the nuclear extract input (NE) and the immunoprecipitation pellet (IP) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with the Drosophila Mediator Abs against the proteins indicated at the left (36).
Clonal analysis of dMed6 mutant.
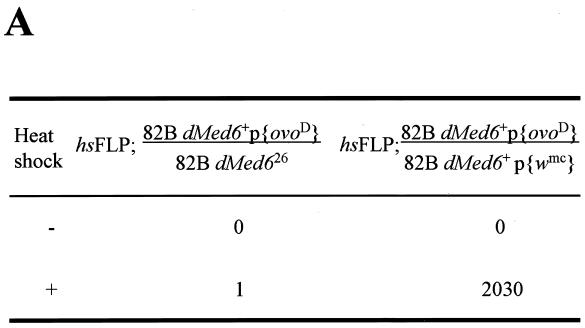
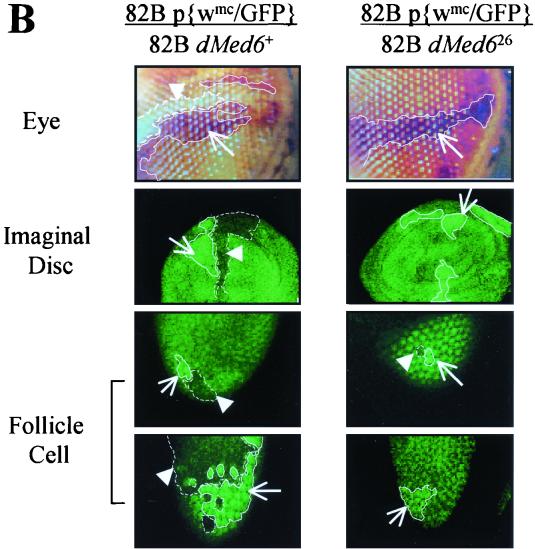
To confirm the requirement of dMed6 function for cell viability, we made dMed6 homozygous mutant cells with the FLP-FRT system (49). First, we used the FRT-dominant female sterile system (10) to examine the fate of dMed6 null germ line cells during oogenesis. Because ovoD is a dominant female sterile mutation, the parental strain containing one copy of ovoD is completely sterile. When wild-type homozygous clones without ovoD were generated by the FLP-FRT system upon heat shock, the flies generated several fertile germ cells. However, when the dMed626 mutation was placed at a position trans-heterozygous to the site of ovoD, mitotic recombination at a site proximal to dMed6 generated recombinant clones that were homozygous for dMed626 and that lacked ovoD. The flies remained sterile even after the induction of mitotic recombination, which indicates that the dMed626 mutant clones were not able to generate mature germ line cells (Fig. 5A). Thus, dMed6 is needed for proliferation and/or development of germ line cells.
FIG. 5.
Clonal analysis of dMed626 homozygous cells. (A) Numbers of egg laid in 4 h by the female flies of the indicated genotypes with (+) and without (−) the induction of mitotic recombination in germ line cells by heat shock. The two hundred female flies (3 to 5 days old) of the indicated genotypes were heat treated at 37°C for 2 h during the late-first-instar larval stage and mated with 50 w1118 male flies for 2 days before egg collection. (B) Clonal analysis of dMed626 homozygous clones in somatic cells. The genotypes of the third chromosome, where the mitotic recombination was induced, are shown at the top. The boundaries of the twin spots are marked with solid (dMed6+) and dashed (dMed626) lines. Arrows and arrowheads, dMed6+ and dMed626 homozygous clones (two copies of w+ or GFP-expressing patches in eye, imaginal disc, and follicle cells), respectively. Clones were induced by heat shock (2 h, 37°C) during the first or second instar, and discs were dissected from late L3 larvae, whereas ovaries and eyes in 2- to 5-day-old adult females were examined.
To find out whether dMed6 is also required for cell autonomous function in other tissue types, we examined the fate of dMed6 homozygous mutant clones in ovarian follicle cells, wing imaginal discs, and eye. We used P{w+mC=Ubi::GFP}83 (13) for follicle cells and imaginal discs or P{w+mC=NM}88C (49) for eye as a marker instead of ovoD in the generation of mitotic recombinant clones. When the dMed626 mutant clone was induced in the ovarian follicle cells, twin spots were detected just after recombination up to the two-cell stage (Fig. 5B). However, when we examined these twin spots several days after the recombination had taken place, only the wild-type GFP homozygous clones had proliferated, whereas the dMed626 homozygous clone had disappeared (Fig. 5B). Similarly, the dMed626 homozygous clone was not detected in the eye and imaginal discs and only the w+mC or GFP homozygous clones were detected (Fig. 5B). Therefore, dMed6 was required for cell division and/or viability in somatic cells as well.
dMed6 mutant phenotype.
Although the growth rate of the dMed6 mutant decreased in the third instar, the mutant larvae continued to grow until they reached the size of fully grown wild-type larvae. Therefore, the death of dMed6 mutant larvae appeared to result from stage-specific developmental defects rather than simple growth defects. Dissection of the fully grown mutant third-instar larvae showed that most of the larval organs were a little bit smaller than wild-type organs (80 to 90% of wild-type size) but showed no other obvious abnormality. However, we were not able to detect antenna, wing, and leg imaginal discs in the mutant comparable in size to those in wild-type animals. The lack of obvious imaginal discs was intriguing and hinted at a specific requirement of dMed6 in imaginal disc development.
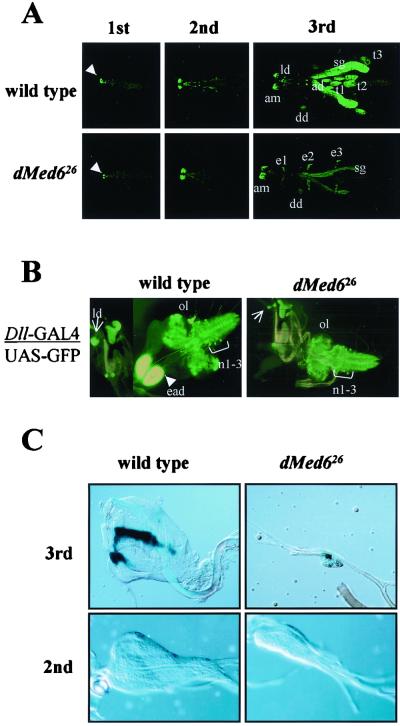
To confirm the putative developmental defect in imaginal discs, development was monitored using a GFP reporter under the control of the Distal-less (Dll) promoter (43), which is one of the most active promoters in imaginal discs (7). The whole fly was scanned under a confocal laser microscope to detect the GFP-expressing cells. In the wild type, the GFP signal began to appear at the antennomaxillary complex in the first-instar larva, and the expression was maintained throughout larval development. GFP expression in the salivary glands, imaginal discs (antenna and legs), and CNS began to appear at the second instar and reached the highest level in the third instar as the organs grew (Fig. 6A). However, the GFP expression pattern in the dMed6 mutant was quite different. Although the GFP expression in the antennomaxillary complex, T1 to T3 thoracic ectoderms, and CNS was maintained at the wild-type level, the expression in the salivary glands was reduced severalfold in the mutant (Fig. 6A and B). But above all, no GFP expression was detected at all from antenna, wing, and leg imaginal discs in the dMed6 mutant larvae (Fig. 6A and B). These results suggested that imaginal discs were not properly developed in the dMed6 mutant.
FIG. 6.
Arrest of imaginal disc development in dMed626. (A) Effect of dMed6 mutation on the expression of Dll promoters. Whole mounts of wild-type and dMed6 mutant larvae containing a GFP expression construct under the control of the Dll promoter are shown for the indicated larval stages. GFP expression at the antennomaxillary complex is visible in the first-instar larvae of both the wild-type and dMed6 mutant (arrowhead). Organs with strong GFP expression are marked. ad, antenna imaginal disc; am, antennomaxillary complex; dd, dorsal T1 disc; sg, salivary gland; t1 to -3, leg imaginal discs; e1 to -3, thoracic ectoderm. (B) Dll induced GFP expression in CNS and imaginal discs. Dll expression patterns in the CNS and imaginal discs dissected from wild-type and dMed6 mutant third-instar larvae are shown. Arrowhead and arrows, eye-antennal imaginal discs (ead) and labial imaginal discs (ld), respectively. The GFP expression in thoracic neuromere cells (n1–3) is marked. ol, optic lobe. (C) Wild-type and dMed6 mutant wing imaginal discs dissected from second- and third-instar larvae. The wing imaginal discs dissected from third-instar larva were stained with LacZ driven by the dpp promoter. The second-instar wing imaginal discs are shown at higher magnification than the third-instar wing imaginal discs.
To examine whether the loss of GFP signals from antenna, wing, and leg imaginal discs originated from the arrest of the imaginal disc development at early stage or from the inactivation of the Dll promoter in developed imaginal discs, we marked the imaginal discs with β-galactosidase (LacZ) activity driven by the decapentaplegic (dpp) promoter, which is activated at the initial stage of wing imaginal disc development (43). In the wild-type third-instar larvae, we could detect the wing imaginal disc marked by LacZ staining of the typical dpp expression pattern (8). However, in the dMed6 mutant larvae, we could also detect only a very small mass of cells (less than 1/10 the size of the wild-type wing imaginal disc) with LacZ staining of a typical dpp expression pattern; the cells were attached at the correct site on the trachea, where the wild-type wing imaginal disc attaches (Fig. 6C). When we examined the wing imaginal disc in second-instar larvae, we found that dMed6 mutant larvae had quite small wing imaginal discs with an abnormal shape (Fig. 6C). These results suggest that the wing imaginal disc cells failed to proliferate and/or died at an early stage. Because dMed6 is required for cell division and/or viability in all of the cell types we examined, the rather specific defects in imaginal disc development caused by the dMed6 mutation suggest that imaginal discs may require a higher level of transcriptional activity and more-complicated arrays of developmental regulators.
Expression profile analysis of dMed6 mutants.
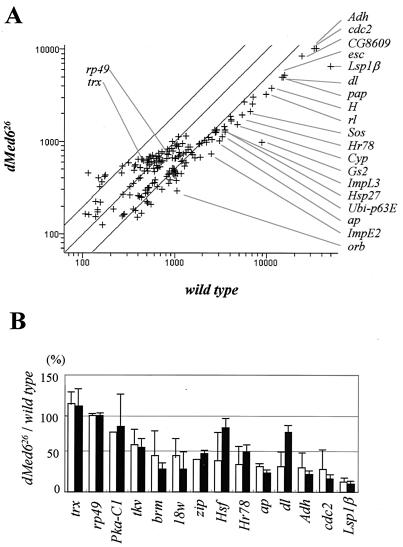
Studies with a mutant allele of yeast MED6 conferring temperature sensitivity revealed that MED6 is required for transcriptional activation of most but not all of the genes transcribed by pol II (21). To identify the genes responsible for the dMed6 mutant phenotype, the level of mRNAs in the dMed6 mutant and wild-type third-instar larvae was analyzed with microarrays. The microarray contained 192 different cDNA probes, which covered genes involved in diverse aspects of Drosophila development (Table 2). Wild-type and dMed6 mutant cDNAs labeled with Cy5 and Cy3 dyes, respectively, were hybridized simultaneously to the microarrays. The average of results from two experiment showed that 12% of the genes (22 out of 184 transcripts assayed) were down-regulated more than threefold, 27% (50 out of 184) were down-regulated two- to threefold, and the remaining 61% were changed less than twofold in the mutant compared with wild-type genes (Fig. 7A and Table 2). Genes required for metabolism (Glutamine synthetase 2 [Gs2], Larval serum protein 1β [Lsp1β], Alcohol dehydrogenase [Adh], and cytochrome P450 [Cyp]), cell cycle control (cdc2 and Cyclin E [CycE]), and differentiation (Son-of-sevenless [Sos], oo18 RNA-binding protein [orb], and rolled [rl]) were down-regulated more than threefold in the mutant. In particular, the expression of developmentally regulated transcription factors (dorsal [dl], Hairless [H], apterous [ap], extra sexcombs [esc], dTrap240, and CG8609) and genes induced by the larval hormone (Hormone-receptor-like in 78 [Hr78], Ubiquitin-63E [Ubi-p63E], Heat shock protein 27 [Hsp27], and Ecdysone-inducible gene E2 [ImpE2]) was significantly reduced in the dMed6 mutant.
TABLE 2.
DNA chip results from cDNA microarray analyses and functions of gene products
| Protein (abbreviation)a | WT/dMed6b cDNA ratio | Confidencec | Description |
|---|---|---|---|
| Ribosomal protein L32 (RpL32, rp49) | 1.00 | Good | Protein biosynthesis; ribosomal protein |
| cAMP-dependent protein kinase 1 (Pka-C1) | 1.27 | Poor | Protein kinase; anterior and posterior axis determination |
| Decapentaplegic (dpp) | 0.91 | Poor | Signal transduction; TGF-βd receptor signaling pathway |
| Extradenticle (exd) | 0.74 | Poor | Specific RNA pol II transcription factor |
| Trithorax (trx) | 0.86 | Poor | Transcription factor; positive regulation of homeotic gene (trithorax group) |
| Spalt major (salm) | 1.05 | Poor | Specific RNA pol II transcription factor |
| Glutamine synthetase 2 (Gs2) | 9.00 | Excellent | Glutamate-ammonia ligase |
| Larval serum protein 1 beta (Lsp1b) | 7.50 | Excellent | Storage protein; larval serum protein |
| Orb | 3.65 | Good | oo18 RNA-binding protein |
| Ecdysone-induced gene E2 (ImpE2) | 3.38 | Excellent | Component of the extracellular Elk-like repeat |
| Cdc2 | 3.32 | Excellent | Cell cycle regulator; cyclin-dependent protein kinase |
| Alcohol dehydrogenase (adh) | 3.14 | Excellent | Alcohol dehydrogenase |
| Son of sevenless (Sos) | 3.14 | Excellent | Signal transduction; RAS guanyl-nucleotide exchange factor |
| Cytochrome P450 | 3.12 | Excellent | Cytochrome P450 |
| Dorsal (dl) | 3.01 | Excellent | Transcription factor; NF-κB/Rel/dorsal domain signature |
| Rolled (rl) | 2.99 | Excellent | MAPe kinase; transmenbrane receptor protein tyrosine kinase signaling pathway |
| Apterous (ap) | 2.98 | Excellent | Transcription factor; LIM domain, homeobox domain |
| Extra sexcomb (esc) | 2.97 | Excellent | Transcription factor; beta-transducin family |
| Hairless (H) | 2.94 | Excellent | Transcription corepressor; paired-box domain |
| Ubi-p63E | 2.89 | Excellent | Protein degradation tagging; ubiquitin-like |
| Poils aux pattes (pap; dTrap240) | 2.88 | Excellent | Transcription factor complex; pap |
| Fork head (fkh) | 2.81 | Good | Transcription factor; fork head domain |
| CG8609 (dP34) | 2.80 | Excellent | Component of the cytoskeleton |
| Hormone receptor-like in 78 (Hr78) | 2.80 | Excellent | Transcription factor; ligand-dependent nuclear receptor |
| Heat shock protein 27 (Hsp27) | 2.76 | Excellent | Chaperone; heat shock response |
| Heat shock protein 22 (Hsp22) | 2.75 | Good | Chaperone; heat shock response |
| CG1057 (dSoh1) | 2.71 | Excellent | Transcription factor complex |
| Corkscrew (csw) | 2.62 | Good | Tyrosine phosphatase; receptor signaling protein tyrosine phosphatase |
| Ecdysone-induced gene L3 (ImpL3) | 2.62 | Excellent | Glycolysis: l-lactate dehydrogenase |
| Glycerol-3-phosphate dehydrogenase (Gpdh) | 2.50 | Good | Glycerophosphate shuttle; NAD+ |
| Heat shock factor (Hsf) | 2.47 | Excellent | Specific RNA pol II transcription factor; heat shock transcription factor |
| Neuroglian | 2.45 | Good | Neuronal cell adhesion |
| CG7008 (dP100) | 2.42 | Excellent | Transcription coactivator; RNA binding, etc. |
| Prospero (pros) | 2.40 | Good | Transcription factor; homeobox domain |
| Dacapo (dap) | 2.38 | Good | Cyclin-dependent protein kinase inhibitor |
| Trap100 | 2.37 | Excellent | Transcription factor complex; transcription cofactor |
| 14-3-3zeta (leonardo) | 2.36 | Excellent | Enzyme inhibitor; RAS protein signal transduction |
| Zipper (zip) | 2.36 | Good | Motor; cytoplasmic myosin II heavy chain |
| Clock (Clk) | 2.34 | Excellent | Transcription factor; circadian rhythm |
| Ras85D | 2.32 | Good | Signal transduction; RAS pathway |
| Discs lost (dlt) | 2.27 | Good | Enzyme; establishment of cell polarity |
| CG1245 (dCrsp34) | 2.25 | Excellent | Transcription factor binding; transcription coactivator |
| Pyruvate kinase (Pyk) | 2.23 | Good | Enzyme; main pathways of carbohydrate metabolism |
| Aldolase 2 (Adl2) | 2.22 | Excellent | Glycolysis; fructose-bisphosphate aldolase |
| Calmodulin (Cam) | 2.21 | Excellent | Calcium binding protein |
| Inositol 1,4,5-trisphosphate receptor (ITP-r83A) | 2.19 | Good | Calcium channel protein |
| Myocyte enhancing factor 2 (Mef2) | 2.18 | Good | RNA pol II transcription factor; mesoderm development |
| Phosphogluconate dehydogenase (Pgd) | 2.15 | Excellent | Pentose-phosphate shuttle |
| Piwi | 2.13 | Good | Expressed in the ovary |
| 18 wheeler (18w) | 2.12 | Excellent | Transmenbrane receptor; cell adhesion |
| Screw (scw) | 2.11 | Excellent | TGF-β receptor ligand-like |
| Brahma (brm) | 2.09 | Excellent | DNA binding; Swi/Snf, bromodomain signature |
| Hedgehog (Hh) | 2.09 | Good | Cysteine-type endopeptidase; smo receptor signaling pathway |
| Aldolase 1 (Ald) | 2.04 | Excellent | Glycolysis; fructose-bisphosphate aldolase |
| cAMP response element binding protein A (CrebA) | 2.03 | Good | RNA pol II transcription factor |
| Abl oncogene (Abl) | 2.03 | Good | Protein tyrosine kinase-like; CNS development, axon |
| Snail (sna) | 2.01 | Excellent | RNA pol II transcription factor |
| CG8491 (dTrap230) | 2.01 | Good | Transcription factor complex; transcription cofactor |
| Zeste (Z) | 1.98 | Good | Transcription factor |
| Ecdysone-induced protein 75B (Eip75B) | 1.97 | Good | Transcription factor; nuclear receptor NR1D3 |
| Saxophone (sax) | 1.97 | Good | Type I TGF-β receptor |
| CG12254 (dDrip97) | 1.96 | Good | Transcription factor complex; transcription cofactor |
| Achaete (ac) | 1.96 | Good | Transcription factor; Myc-type, helix-loop-helix dimerization domain |
| Lethal-scute [1(1)sc] | 1.94 | Good | Transcription factor; Myc-type, helix-loop-helix dimerization domain |
| Sprouty (sty) | 1.93 | Good | Plasma membrane protein; terminal branches form development |
| Medea (Med) | 1.93 | Good | Transcription factor; TGF-β receptor signaling pathway |
| Squid (sqd) | 1.93 | Good | RNA binding, RRM motif |
| Apontic (apt) | 1.92 | Good | Transcription factor; RNA binding, bZIP motif |
| Kekkonl (kekl) | 1.92 | Good | Cell adhesion; immunoglobulin C2-type domain |
| Arrowhead (Awh) | 1.84 | Good | Transcription factor; LIM domain |
| Vacuolar H+-ATPase 55-kDa B subunit (Vha55) | 1.82 | Good | Enzyme; hydrogen-transporting ATPase, VI, B subunit |
| Relish (Rel) | 1.74 | Good | Specific RNA pol II transcription factor; ankyrin repeat, PEST domain |
| Tolkin (tok) | 1.71 | Good | Endopeptidase; TGF-β-associated protein-like |
| Suppressor of variegation 205 [Su(var)205] | 1.71 | Good | Chromatin binding protein; chromo domain, shadow domain |
| String (stg) | 1.66 | Good | Non-membrane-spanning protein tyrosine phosphatase |
| Thickveins (tkv) | 1.62 | Good | Type I TGF-β receptor |
| Rpd3 | 1.60 | Good | Histone deacetylase |
| Amalgam (Ama) | 1.59 | Good | Cell adhesion; immunoglobulin C2-type domain |
| Held out wings (how) | 1.55 | Good | RNA binding, KH domain |
| CG8117 (dCrsp70) | 1.54 | Good | TFIIS-like; transcription factor complex, transcription cofactor like |
| Haplo-, diplolethal (Hdl) | 1.45 | Good | Females with one dose and males with two suffer almost complete lethality |
| Males absent on the first (mof) | 1.41 | Good | Histone acetyltransferase; dosage compensation zinc finger, C2HC type |
| Protein phosphatase 2A at 29B (Pp2A-29B) | 1.27 | Good | Protein dephosphorylation; serine/threonine phosphatase |
| Trithorax-like (Trl) | 1.25 | Good | RNA pol II transcription factor; GAGA factor |
| Scratch (scrt) | 1.23 | Good | Transcription factor; zinc finger C2H2 type |
| Antennapedia (antp) | 1.21 | Good | Transcription factor; homeobox domain |
| Absent, small, or homeotic disc 1 (ash1) | 1.17 | Good | Transcription factor; positive regulation of homeotic gene (trithorax group) |
| RNAonX (rox1) | 1.16 | Good | Nuclear untranslated RNA gene |
| Calpain-A (CalpA) | 1.15 | Good | Calpain; EF-hand calcium binding domain |
| Groucho (gro) | 1.14 | Good | Transcription corepressor, signal transduction; Trp-Asp (WD) repeats |
| Sugarless (sgl) | 1.12 | Good | Glycosaminoglycan biosynthesis; UDP-glucose-6-dehydrogenase |
| Cactus (cact) | 1.12 | Good | Transcription factor; cytoplasmic sequestering, cytoskeletal structural protein |
| Downstream of receptor kinase (drk) | 1.11 | Good | Signal transduction; RAS protein signal transduction |
| GTPase-activating protein 1 (Gap 1) | 1.10 | Good | Signal transduction; RAS GTPase-activating protein |
| Bithorax complex (BX-C) | 0.97 | Good | Transcription factor; homeobox domain |
| Pipsqueak (psq) | 0.93 | Good | Transcription factor; BTB/POZ-domain protein |
Gene names and abbreviations can be found at http://flybase.bio.indiana.edu/. cAMP, cyclic AMP.
WT, wild type.
Excellent, hybridization signal intensity at least sixfold above the background level; good, hybridization signal intensity three- to sixfold above the background level; poor, hybridization signal intensity less than threefold above the background level.
TGF-β, transforming growth factor β.
MAP, mitogen-activated protein.
FIG. 7.
Expression profile of a dMed6 mutant. (A) Scatter plot analysis of microarray experiment. The hybridization intensities of the wild-type (x axis) and dMed6 mutant cDNA probes (y axis) are plotted on a log scale. rp49, trx, and genes down-regulated more than three-fold in the dMed6 mutant larvae are indicated. Diagonal lines, range of a twofold difference. Results from two independent hybridization experiments were averaged. (B) Comparison of expression levels of individual genes assayed by cDNA microarray (open bar) and quantitative RT-PCR (solid bar). Percentages of transcript in dMed6 mutant are compared to those for the wild type. Results from three independent quantitative RT-PCR experiments were averaged, and the deviations are marked with error bars.
To confirm the microarray results, we examined the levels of transcripts in the wild-type and mutant larvae using quantitative RT-PCR. The levels of transcripts for Lsp1β, cdc2, Adh, ap, brm, 18 wheeler (18w), and extradenticle (exd) were reduced 3- to 10-fold in the dMed6 mutant compared to those in the wild type (Fig. 7B). However, the amounts of transcript for rp49 and trithorax (trx) were not reduced in the mutant, thus confirming the microarray results. The microarray and quantitative RT-PCR analyses for dl and Heat shock factor (Hsf) transcripts showed a twofold discrepancy, but, aside from these two cases, RT-PCR confirmed the microarray result. Therefore, the microarray and quantitative RT-PCR results indicated that the dMed6 mutant has defects in transcriptional activation of a distinct group of genes.
Requirement of dMed6 for tissue-specific transcriptional activation in vivo.
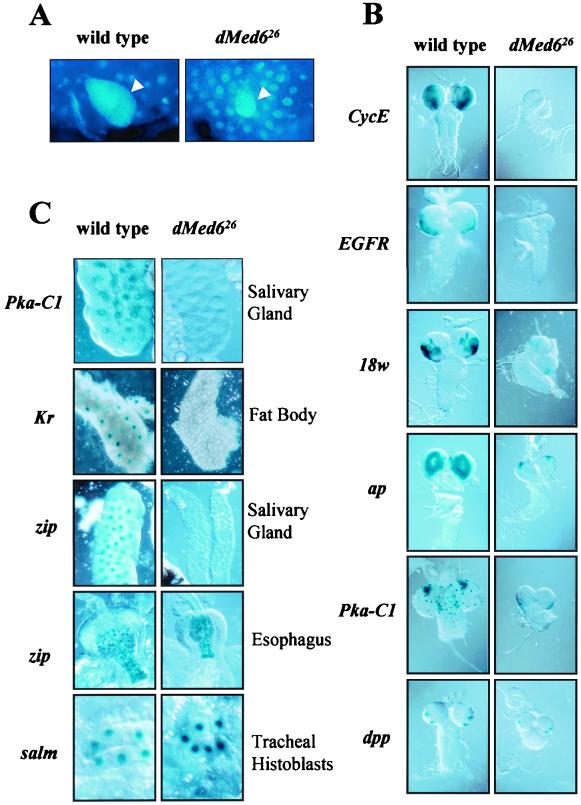
Down-regulation of some genes involved in cell proliferation in the dMed6 mutant partly explains the mutant phenotype and suggested that other mitotically active larval tissues (gonad and neuroblasts) might also be affected by the dMed6 mutation. 4′-6-Diamidino-2-phenylindole (DAPI) staining of the larval ovary showed that the mutant ovary was significantly smaller and contained fewer cells than the wild-type ovary (Fig. 8A).
FIG. 8.
Requirement of dMed6 for the activation of developmental promoters. (A) DAPI staining of larval ovary. Arrowheads, ovaries. (B) Requirement for dMed6 for transcriptional activation in larval CNS. The expression of lacZ was driven by the promoters of the genes indicated at the left. (C) Effect of dMed6 mutation on the promoters of the genes listed at the left in the larval tissues indicated at the right.
The mutant larvae also showed a smaller brain size, indicating mutational effects on the proliferation of neuroblasts. Therefore, we examined the transcriptional activities in the neuroblasts with the use of lacZ reporters controlled by the promoters of several important regulatory genes. The LacZ staining pattern showed that genes involved in cell cycle (CycE) and signal transduction (epidermal growth factor receptor [EFGR], 18w, ap, and cAMP-dependent protein kinase 1 [Pka-C1]) were expressed at high levels in the wild-type, but much less in the mutant, optic lobes (Fig. 8B).
Along with the defects in cell proliferation, the down-regulation of genes involved in metabolism suggested that other larval tissues, such as salivary glands, were also affected by the dMed6 mutation. Examination of the transcriptional activities revealed that the Pka-C1 promoter was down-regulated not only in the optic lobes but also in salivary gland, gastric cecum, and muscle cells (Fig. 8C). We also detected a severe reduction in the tissue-specific activation of Krüppel (Kr) and Calmodulin (Cam) promoters in the fat body and ring glands, respectively (Fig. 8C and data not shown). Even though dMed6 is not absolutely required for the growth of these larva-specific tissues, their specific metabolic and developmental activities require functional dMed6.
Despite the universal requirement for dMed6 in many cell types, dpp expression at the optic lobe and spalt major (salm) expression in the trachea were not affected by the dMed6 mutation (Fig. 8C). This result, along with the results of microarray analysis, demonstrates that mutations in dMed6 affect the transcriptional activation of a group of genes. Even more interesting were the differential effects of the dMed6 mutation on the zipper (zip) promoter in different tissues (Fig. 8C). The activation of the zip promoter in the mutant salivary glands was severely compromised, while its esophagus-specific activation was not affected by the dMed6 mutation. Therefore the defects of dMed6 mutations appear to be specific not only to a group of genes but also to some specific tissue types in which these genes are expressed.
DISCUSSION
The Mediator complex is generally required for most gene expression and functions in the recruitment of transcriptional machinery to promoters by activator-specific interaction of Mediator subunits (19, 21, 29). Therefore, the Mediator complex has an essential role in most developmental processes as a whole but still shows gene specificity in the requirement for each Mediator subunit. The distinct dMed6 mutant phenotype in most Drosophila cells reflects both the fundamental and the specific aspects of the complex in developmental regulation.
Although yeast Med6 plays a central role in the Mediator complex, only a distinct group of genes transcribed by pol II (15% of the yeast genome) requires Med6 activity (21). Consistent with this result, the microarray analysis of dMed6 mutants for transcriptional defects revealed that dMed6 is required for transcriptional regulation of a subset of genes in Drosophila as well. In particular, defects in cell proliferation and metabolism were most easily detected due to the down-regulation of several key regulators by dMed6 mutation. It is intriguing that Drosophila cdc2 and Cyclin-dependent kinase 7 (Cdk7), the essential regulators of cell proliferation, cause similar mutant phenotypes; larvae with mutations in these genes were also restricted in the mitotic proliferation of imaginal cells, while nonimaginal larval cells continued to grow and replicate their DNA (27, 44). In addition, the reduced level of CycE and brahma (brm) transcription may be partly responsible for the proliferation defects of the dMed6 mutant (14, 31). In addition to these, the transcriptional activation of Gs2 (16), Cyp (9), Adh (12), and Ecdysone-inducible gene L3 (ImpL3) (1), which are involved in the biogenesis of cellular components or removal of toxic metabolites, was severely reduced in the dMed6 mutants. Therefore, most of the genes expressed at high levels during the developmental transition from larva to pupa appear to require the function of dMed6 directly or indirectly for transcriptional activation.
Because the expression of these genes is highly stimulated by 20-hydroxyecdysone, dMed6 activity appears to be required for the mediation of regulatory signals from ligand-bound nuclear receptors to basal transcription machinery. In mammals, ligand-bound nuclear receptors (e.g., the vitamin D receptor and thyroid receptor) bind tightly to a mammalian Mediator homolog, the vitamin D receptor-interacting protein (DRIP)-thyroid receptor-associated protein (TRAP) complex (39, 50). The DRIP-TRAP complex has been suggested to activate transcription by recruitment of the Mediator complex to the promoter along with other transcription factors. Homologs for dMED6 and its associated Drosophila Mediator subunits (dSOH1 and dSRB7) are components of the DRIP-TRAP complex, suggesting that the mammalian MED6 homolog may function in transcriptional activation by nuclear receptors. However, the nuclear receptors interact with the Mediator complex via different subunits (e.g., DRIP205-TRAP220) (6, 40, 50). Therefore, dMED6 may function at the post-activator (nuclear receptor) binding stage in the relay of activation signals from ecdysone-induced nuclear receptors to basal transcription machinery, as does yeast Med6 in transcriptional activation by Gal4, which binds to the Gal11 subunit of the Mediator complex (30).
Although the microarray and quantitative RT-PCR analyses of whole mutant flies identified genes whose expression was affected by the dMed6 mutation, the examination of the tissue-specific expression of developmentally regulated promoters with LacZ and GFP reporters revealed several interesting details. First, dMed6 is required for activation of most but not all of the developmentally regulated promoters. Neither dpp expression at the optic lobe of the third-instar larval brain and imaginal discs nor esophagus-specific expression of zipper was defective, whereas a number of developmentally regulated promoters were inactive in the dMed6 mutants. Second, the transcriptional activation of a specific promoter was sometimes affected differently depending on where the gene was expressed. For example, the level of the Pka-C1 transcript measured by the microarray and quantitative RT-PCR decreased about 2-fold in the mutant but the Pka-C1::lacZ expression analysis for various tissues revealed transcriptional defects from 2- to 3-fold in muscles to more than 20- to 30-fold in salivary glands and brain. Transcriptional activation of the zip promoter was defective in the mutant salivary glands and was without abnormality in the esophagus. Similarly, transcriptional activation of the Dll promoter was completely lost in mutant imaginal discs, whereas a comparable level of Dll promoter activity was detected in the mutant CNS. Therefore, we conclude that dMed6 is required for gene-specific transcriptional activation of a group of genes required for diverse aspects of cell metabolism. However, whether all of these genes require dMED6 directly for transcriptional activation remains to be addressed.
ACKNOWLEDGMENTS
We thank Jeongsil Kim-Ha for expert assistance. We thank Juri Kim for assistance to isolate dMed6 mutants. We thank Thomas Kaufman, Michael Levine, Ulrich Nauber, Todd Laverty, Kei Ito, Carl Hashimoto, Marcelo Jacobs-Lorena, Judith Lengyel, Michael Weir, Carl Thummel, and the Bloomington and Umea stock centers for kindly providing plasmids and fly stocks. We are grateful to John T. Lis and Bruce Baker for critical reading and comments.
This work was supported by a Creative Research Initiatives (CRI) grant from the Ministry of Science and Technology, Korea, to Y.-J.K. and the Brain Korea 21 Project to C.K.
REFERENCES
- 1.Abu-Shumays R L, Fristrom J W. IMP-L3, a 20-hydroxyecdysone-responsive gene, encodes Drosophila lactate dehydrogenase: structural characterization and developmental studies. Dev Genet. 1997;20:11–22. doi: 10.1002/(SICI)1520-6408(1997)20:1<11::AID-DVG2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Berk A J. Activation of RNA polymerase II transcription. Curr Opin Cell Biol. 1999;11:330–335. doi: 10.1016/S0955-0674(99)80045-3. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund S, Almouzni G, Davidson I, Nightingale K P, Weiss K. Global transcription regulators of eukaryotes. Cell. 1999;96:759–767. doi: 10.1016/s0092-8674(00)80586-3. [DOI] [PubMed] [Google Scholar]
- 4.Boube M, Faucher C, Joulia L, Cribbs D L, Bourbon H M. Drosophila homologs of transcriptional mediator complex subunits are required for adult cell and segment identity specification. Genes Dev. 2000;14:2906–2917. doi: 10.1101/gad.17900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer T G, Martin M E, Lees E, Ricciardi R P, Berk A J. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature. 1999;399:276–279. doi: 10.1038/20466. [DOI] [PubMed] [Google Scholar]
- 6.Burakov D, Wong C W, Rachez C, Cheskis B J, Freedman L P. Functional interactions between the estrogen receptor and DRIP205, a subunit of the heteromeric DRIP coactivator complex. J Biol Chem. 2000;275:20928–20934. doi: 10.1074/jbc.M002013200. [DOI] [PubMed] [Google Scholar]
- 7.Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila J, Estrada M P, Sanchez-Herrero E, Guerrero I. The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J. 1994;13:71–82. doi: 10.1002/j.1460-2075.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez V M, Marques G, Delbecque J P, Kobayashi K, Hollingsworth M, Burr J, Natzle J E, O'Connor M B. The drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development. 2000;127:4115–4126. doi: 10.1242/dev.127.19.4115. [DOI] [PubMed] [Google Scholar]
- 10.Chou T B, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covitz P A, Song W, Mitchell A P. Requirement for RGR1 and SIN4 in RME1-dependent repression in Saccharomyces cerevisiae. Genetics. 1994;138:577–586. doi: 10.1093/genetics/138.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David J R, Bocquet C, Arens M F, Fouillet P. Biological role of alcohol dehydrogenase in the tolerance of Drosophila melanogaster to aliphatic alcohols: utilization of an ADH-null mutant. Biochem Genet. 1976;14:989–997. doi: 10.1007/BF00485131. [DOI] [PubMed] [Google Scholar]
- 13.Davis I, Girdham C H, O'Farrell P H. A nuclear GFP that marks nuclei in living Drosophila embryos; maternal supply overcomes a delay in the appearance of zygotic fluorescence. Dev Biol. 1995;170:726–729. doi: 10.1006/dbio.1995.1251. [DOI] [PubMed] [Google Scholar]
- 14.Elfring L K, Daniel C, Papoulas O, Deuring R, Sarte M, Moseley S, Beek S J, Waldrip W R, Daubresse G, DePace A, Kennison J A, Tamkun J W. Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics. 1998;148:251–265. doi: 10.1093/genetics/148.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fondell J D, Guermah M, Malik S, Roeder R G. Thyroid hormone receptor-associated proteins and general positive cofactors mediate thyroid hormone receptor function in the absence of the TATA box-binding protein-associated factors of TFIID. Proc Natl Acad Sci USA. 1999;96:1959–1964. doi: 10.1073/pnas.96.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frenz L M, Glover D M. A maternal requirement for glutamine synthetase I for the mitotic cycles of syncytial Drosophila embryos. J Cell Sci. 1996;109:2649–2660. doi: 10.1242/jcs.109.11.2649. [DOI] [PubMed] [Google Scholar]
- 17.Green M R. TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes. Trends Biochem Sci. 2000;25:59–63. doi: 10.1016/s0968-0004(99)01527-3. [DOI] [PubMed] [Google Scholar]
- 18.Gu W, Malik S, Ito M, Yuan C X, Fondell J D, Zhang X, Martinez E, Qin J, Roeder R G. A novel human SRB/MED-containing cofactor complex, SMCC, involved in transcription regulation. Mol Cell. 1999;3:97–108. doi: 10.1016/s1097-2765(00)80178-1. [DOI] [PubMed] [Google Scholar]
- 19.Han S J, Lee Y C, Gim B S, Ryu G H, Park S J, Lane W S, Kim Y J. Activator-specific requirement of yeast mediator proteins for RNA polymerase II transcriptional activation. Mol Cell Biol. 1999;19:979–988. doi: 10.1128/mcb.19.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann A, Horikoshi M, Wang C K, Schroeder S, Weil P A, Roeder R G. Cloning of the Schizosaccharomyces pombe TFIID gene reveals a strong conservation of functional domains present in Saccharomyces cerevisiae TFIID. Genes Dev. 1990;4:1141–1148. doi: 10.1101/gad.4.7.1141. [DOI] [PubMed] [Google Scholar]
- 21.Holstege F C, Jennings E G, Wyrick J J, Lee T I, Hengartner C J, Green M R, Golub T R, Lander E S, Young R A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 21a.Huang S L, Baker B S. The mutability of the minute loci of Drosophila melanogastr with ethyl methanesulfonate. Mutat Res. 1976;34:407–414. doi: 10.1016/0027-5107(76)90218-9. [DOI] [PubMed] [Google Scholar]
- 22.Ito M, Yuan C X, Okano H J, Darnell R B, Roeder R G. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol Cell. 2000;5:683–693. doi: 10.1016/s1097-2765(00)80247-6. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y W, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway J W, Conaway R C, Kornberg R D. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc Natl Acad Sci USA. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 25.Koleske A J, Young R A. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 26.Kwon J Y, Park J M, Gim B S, Han S J, Lee J, Kim Y J. Caenorhabditis elegans mediator complexes are required for developmental-specific transcriptional activation. Proc Natl Acad Sci USA. 1999;96:14990–14995. doi: 10.1073/pnas.96.26.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larochelle S, Pandur J, Fisher R P, Salz H K, Suter B. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev. 1998;12:370–381. doi: 10.1101/gad.12.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y C, Kim Y J. Requirement for a functional interaction between mediator components Med6 and Srb4 in RNA polymerase II transcription. Mol Cell Biol. 1998;18:5364–5370. doi: 10.1128/mcb.18.9.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y C, Min S, Gim B S, Kim Y J. A transcriptional mediator protein that is required for activation of many RNA polymerase II promoters and is conserved from yeast to humans. Mol Cell Biol. 1997;17:4622–4632. doi: 10.1128/mcb.17.8.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y C, Park J M, Min S, Han S J, Kim Y J. An activator binding module of yeast RNA polymerase II holoenzyme. Mol Cell Biol. 1999;19:2967–2976. doi: 10.1128/mcb.19.4.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilly M A, Spradling A C. The Drosophila endocycle is controlled by cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996;10:2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- 32.Malik S, Roeder R G. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem Sci. 2000;25:277–283. doi: 10.1016/s0968-0004(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 33.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 34.Myers L C, Gustafsson C M, Hayashibara K C, Brown P O, Kornberg R D. Mediator protein mutations that selectively abolish activated transcription. Proc Natl Acad Sci USA. 1999;96:67–72. doi: 10.1073/pnas.96.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naar A M, Beaurang P A, Zhou S, Abraham S, Solomon W, Tjian R. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999;398:828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 36.Park J M, Gim B S, Kim J M, Yoon J H, Kim H S, Kang J G, Kim Y J. Drosophila Mediator complex is broadly utilized by diverse gene-specific transcription factors at different types of core promoters. Mol Cell Biol. 2001;21:2312–2323. doi: 10.1128/MCB.21.7.2312-2323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J M, Kim H S, Han S J, Hwang M S, Lee Y C, Kim Y J. In vivo requirement of activator-specific binding targets of Mediator. Mol Cell Biol. 2000;20:8709–8719. doi: 10.1128/mcb.20.23.8709-8719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pugh B F, Tjian R. Diverse transcriptional functions of the multisubunit eukaryotic TFIID complex. J Biol Chem. 1992;267:679–682. [PubMed] [Google Scholar]
- 39.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 40.Ren Y, Behre E, Ren Z, Zhang J, Wang Q, Fondell J D. Specific structural motifs determine TRAP220 interactions with nuclear hormone receptors. Mol Cell Biol. 2000;20:5433–5446. doi: 10.1128/mcb.20.15.5433-5446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryu S, Zhou S, Ladurner A G, Tjian R. The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature. 1999;397:446–450. doi: 10.1038/17141. [DOI] [PubMed] [Google Scholar]
- 42.Sakai A, Shimizu Y, Kondou S, Chibazakura T, Hishinuma F. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spradling A C, Stern D, Beaton A, Rhem E J, Laverty T, Mozden N, Misra S, Rubin G M. The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern B, Ried G, Clegg N J, Grigliatti T A, Lehner C F. Genetic analysis of the Drosophila cdc2 homolog. Development. 1993;117:219–232. doi: 10.1242/dev.117.1.219. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Zhang Y, Cho H, Rickert P, Lees E, Lane W, Reinberg D. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol Cell. 1998;2:213–222. doi: 10.1016/s1097-2765(00)80131-8. [DOI] [PubMed] [Google Scholar]
- 46.Tudor M, Murray P J, Onufryk C, Jaenisch R, Young R A. Ubiquitous expression and embryonic requirement for RNA polymerase II coactivator subunit Srb7 in mice. Genes Dev. 1999;13:2365–2368. doi: 10.1101/gad.13.18.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIS. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 48.White K P, Rifkin S A, Hurban P, Hogness D S. Microarray analysis of Drosophila development during metamorphosis. Science. 1999;286:2179–2184. doi: 10.1126/science.286.5447.2179. [DOI] [PubMed] [Google Scholar]
- 49.Xu T, Rubin G M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 50.Yuan C X, Ito M, Fondell J D, Fu Z Y, Roeder R G. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA. 1998;95:7939–7944. doi: 10.1073/pnas.95.14.7939. . (Erratum, 95:14584.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Emmons S W. A C. elegans mediator protein confers regulatory selectivity on lineage-specific expression of a transcription factor gene. Genes Dev. 2000;14:2161–2172. doi: 10.1101/gad.814700. [DOI] [PMC free article] [PubMed] [Google Scholar]