Abstract
Background
Acute pancreatitis is a relatively common acute abdominal emergency but there is no specific therapy for it. Traditional Chinese medicinal herbs have been used widely for many years in China to treat acute pancreatitis, and several controlled trials have been carried out to investigate their efficacy.
Objectives
To assess the efficacy and safety of traditional Chinese medicinal herbs for acute pancreatitis.
Search methods
The following electronic databases were searched, in September 2002: the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library Issue 3, 2002, MEDLINE, EMBASE, AHMED (Allied and Complementary Medicine Database) and SIGLE (System for Information on Grey Literature). Four Chinese journals and conference proceedings were handsearched. No language restriction was used. The searches were updated in October 2003, October 2004 and October 2005 but nothing new was found. The updated search in July 2008 found four new included studies.
Selection criteria
All randomized controlled trials involving traditional Chinese medicinal herbs in the treatment of acute pancreatitis and published in any language, regardless of whether they were single‐blinded, double‐blinded, or not blinded.
Data collection and analysis
Data were extracted independently by two reviewers. The methodological quality of trials was evaluated using the Jadad scale plus allocation concealment.
Main results
Fifteen randomized clinical trials (including a total of 845 participants) were identified in which Chinese medicinal herbs or Chinese medicinal herbs plus routine treatment were compared with routine treatment. All of these trials were published in Chinese and all included inpatients. Only three of the articles described the method of randomisation. According to the analysis result, there appeared to be benefit from Chinese medicinal herbs over control for mortality rates, operative intervention, multiple organ failure and systemic infection. But for local septic complications, there was no difference between the treatment and control. But the trials were of low quality.
Authors' conclusions
Some Chinese medicinal herbs may work in acute pancreatitis. However, because the trials were of low quality, the evidence is too weak to recommend any single herb. Rigorously designed, randomized, double‐blind, placebo‐controlled trials are required.
Keywords: Humans; Acute Disease; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Pancreatitis; Pancreatitis/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Chinese medicinal herbs for treating acute inflammation of the pancreas
The pancreas is a gland near the stomach that helps digestion by secreting insulin into the blood and digestive enzymes into the small intestine. Acute pancreatitis is a relatively common acute abdominal emergency with no specific therapy. It is potentially fatal. In China, traditional Chinese medicinal herbs have been used for many years to treat acute pancreatitis. This review identified fifteen randomized trials but they were poorly controlled and used different herbs. It appeared that some Chinese medicinal herbs may have positive effects on mortality but there is no strong evidence and adverse effects have not been studied.
Background
Description of the condition
Acute pancreatitis is a common hypermetabolic, hyperdynamic disease process of variable severity that has multiple etiologies and which creates a catabolic stress state (Steinberg 1994). It is a relatively common disease with an incidence of about 30 per 100,000 persons per year throughout the world. It is a potentially fatal disease with an overall mortality of 5% to 10% (Lowham 1999; Mergener 1999). Death usually occurs from massive inflammatory responses causing multiple organ‐system failure, usually in association with pancreatic necrosis. The mortality risk increases to around 40% when initial pancreatic necrosis becomes super infected, which requires surgical intervention to control persisting inflammatory responses (Blamey 1984; Bradley 1993; Bassi 1994; Dervenis 1999). The in‐hospital case mortality rate of around 10% has remained fairly static for over four decades worldwide. However, there is no specific therapy for acute pancreatitis, placing reliance on intensive supportive therapy, and surgical and radiological interventions.
Description of the intervention
Traditional Chinese medicinal herbs have been used for many years to treat acute pancreatitis in China and many controlled trials have been carried out. The quality of these trials, however, has not been assessed systematically. Traditional Chinese Medicine (TCM) has its unique theories for concepts of etiology, systems of diagnosis and treatment which are vital to its practice.
How the intervention might work
TCM drug treatment consists typically of complex prescriptions of a combination of several components. The combination, based on the Chinese diagnostic patterns (i.e., inspection, listening and smelling, inquiry, and palpation), follows a completely different rationale for than many western drug treatments. The mechanism of TCM in the treatment of acute pancreatitis is various. For example, rhubarb has inhibitive effect on trypsin, lipase and amylase, is effective in purgation and in improving peristalsis. Salvia miltiorrhiza, another important TCM, acts to improve peripheral circulation, tranquillise and abate pain. Most of the Chinese Medicinal Treatments include these two herbs. In the present review, therefore, Chinese medicinal herbs were considered in combination as a specific treatment for acute pancreatitis.
Why it is important to do this review
In China, traditional Chinese medicinal herbs have been used for many years to treat acute pancreatitis. A summary of the evidence for and against the effectiveness of using TCM to treat acute pancreatitis is required.
Objectives
The primary objective was to compare the effects of Chinese medicinal herbs versus routine treatment, or a combination of TCM and Western medicine versus Western medicine, by assessing mortality and length of period of hospitalization in patients with acute pancreatitis.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomized controlled trials involving orally administered traditional Chinese medicinal herbs in the treatment of acute pancreatitis with reports published in any language.
Types of participants
Participants included patients with mild or severe acute pancreatitis (AP). Diagnosis of acute pancreatitis was defined by the Atlanta criteria/Santorini criteria (Bradley 1993; Dervenis 1999) and established by clinical presentation and elevated serum amylase.
Types of interventions
Chinese medicinal herbs, a single medicine or mixtures (compounds), compared with routine treatments, such as fasting, fluid replacement, antispasmodics, therapy according to symptoms and nutritional support, and Chinese medicinal herbs plus routine treatment versus routine treatment alone.
Types of outcome measures
We considered trials if any of the following clinical outcomes were reported.
Primary outcomes
Death
Secondary outcomes
Length of period of hospitalization.
Systemic inflammatory response syndrome (SIRS).
Multiple organ failure (MOF).
Operative intervention (need for operation).
Local septic complications(pancreatic abscess formation, infected necrosis).
Other local complications (fluid collection, pseudocyst, sterile pancreatic necrosis, fistula).
Systemic sepsis (septicemia, urinary tract infection (UTI), pneumonia).
Subgroup analysis was to be performed comparing mild to severe pancreatitis.
Search methods for identification of studies
Electronic searches
The following bibliographic databases were searched to identify all published and unpublished randomised controlled trials:
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library);
MEDLINE;
EMBASE;
AHMED (Allied and Complementary Medicine Database);
SIGLE (System for Information on Grey Literature).
The searches were updated in October 2003, October 2004 and October 2005 but nothing new was found. The searches were run again in July 2008 (for the period 1st January 2005 to July 2008) for this updated version of the review.
The search strategy in Appendix 1 was constructed by using a combination of MESH subject headings and text words relating to the treatment of acute pancreatitis with traditional Chinese medicine or medical and surgical interventions.
Searching other resources
Reference lists from trials selected by electronic searching were hand searched to identify further relevant trials. Published abstracts from the conference proceedings were hand searched:
International Association of Pancreatology;
European Pancreatic Club;
Pancreatic Society of Great Britain and Ireland;
American Pancreatic Association;
Pancreas Club Inc (USA);
British Society of Gastroenterology;
United European Gastroenterology Week;
American Gastroenterological Association;
World Congress of Gastroenterology.
The following journals were hand searched from their first date of publication:
Chinese Journal of Integrated Traditional and Western Medicine of Zhejiang;
Chinese Journal of Beijing University of Traditional Chinese Medicine;
Chinese Surgical Journal of Integrated Traditional and Western Medicine;
Chinese Journal of Integrated Traditional and Western Medicine.
In addition members of the Cochrane UGPD Group and experts in the field were contacted and asked to provide details of outstanding clinical trials and any relevant unpublished materials.
Data collection and analysis
Selection of studies
Two reviewers independently selected the trials according to the selection criteria. Disagreement was resolved by discussion. Trials published only in abstract form were included if full details of the protocol and results could be obtained from the authors. To avoid over‐representing duplicate studies in the review, duplicate publications were excluded.
Data extraction and management
The reviewers extracted data using a standard form and recorded data independently. Recorded data were cross checked by the reviewers. The data were entered into RevMan (version 4.2.3) for analysis. The following parameters were extracted: number of deaths, local septic complications (pancreatic abscess formation, infected necrosis), other local complications, systemic infection (septicemia, UTI, pneumonia), and number of days in hospital. If the above data were not available in the trial reports, additional information was sought by correspondence with the principal author. If the information was still lacking after the author was contacted, this was recorded.
Assessment of risk of bias in included studies
Assessment of study quality
The quality of included trials was assessed using the instrument developed by Jadad et al. (Jadad 1996). In addition, concealment of the allocation sequence was scored as A (adequate), B (unclear) or C (inadequate), following the criteria adopted from The Cochrane Handbook and Schulz et al. (Schulz 1995) as follows A ‐ Documented adequate allocation concealment (e.g. central randomisation). B ‐ Uncertain allocation concealment(e.g. authors did no report an allocation concealment approach). C ‐ Allocation definitely not adequately concealed.
Other validity criteria to assess studies included the following: baseline comparability of treatment groups (severity score); presence of inclusion and exclusion criteria; intervention described in detail; definition of outcomes; stated time for outcome assessment; stated indications for further interventions.
Measures of treatment effect
Dichotomous data were presented as relative risk (RR) and continuous outcomes as weighted mean difference (WMD), both with 95% confidence intervals (CI). Analyses were performed an on intention‐to‐treat basis where possible.
Results
Description of studies
All fifteen RCTs included adults diagnosed according to the domestic diagnosis standard. Only four articles (Fang 2007; Peng 2007; Zhao 2008) used an Acute Physiology and Chronic Health Evaluation (APACHE) II score (Knaus 1985) when patients were admitted. Three of the articles (Fang 2007; Peng 2007; Zhao 2008) described in detail the methodology of the RCT. According to the category of Chinese medicinal herbs, only one trial studied a single herb (Rheum) (Bao 2000), ten trials studied compounds of herbs (Qingyitang, Dacaihu Tang, Huanglianjiedu Tang and Dachengqi Tang, Salvia miltiorrhiza, Dan‐shen compound, Qingganlidan Tang, Danyi Tang, Chaishaochengqi Tang, Tongxiahuayu compound). The constituents and dosage of medicinal herbs varied. The large heterogeneity of the intervention prevented us from doing a meaningful subgroup analysis on herbs either single or compound. Only three article had definite exclusion criteria (Bao 2000; Fang 2007; Peng 2007). The common outcomes reported were symptoms and signs, serum amylase and complications. None of the fifteen articles reported adverse effects.
Results of the search
The search strategy generated 181 studies. After reading titles and abstracts, one hundred and forty‐eight of these articles were excluded because they were non‐clinical studies or had study objectives different from this review. A total of 33 articles published in Chinese or English were retrieved for further assessment.
Included studies
Fifteen randomized clinical trials (a total of 845 Chinese participants) comparing Chinese medicinal herbs plus routine treatment and routine treatment were included (Characteristics of included studies). All of these study reports were published in Chinese and all included inpatients.
Excluded studies
Of the 33 articles published in Chinese or English which were retrieved for further assessment., eighteen articles were excluded because they did not meet our inclusion criteria. The reasons for exclusion were listed under 'Characteristics of excluded studies'.
Risk of bias in included studies
Methodological quality
Applying the Jadad score, all fifteen articles were assessed as low quality (a Jadad score of one or two). They had poor methodological quality, providing only limited descriptions of study design, randomisation, and allocation concealment. All trials stated only that random assignment was used but did not give sufficient information to allow a judgment of whether or not it was conducted properly. None of the articles reported adequate concealment of the treatment allocation. No trials were blinded. Only three (Fang 2007; Peng 2007; Zhao 2008) of the fifteen RCTs gave information on how the allocation sequence was generated. None of the trials reported a sample size calculation or stated that they used an intention‐to‐treat analysis to evaluate their data. No multi‐centre, large scale RCTs were identified. Only one study reported on dropouts and the rate of dropouts was more than 20% (Huang 2001), but the authors didn't report how drop‐outs were treated. Three studies (Fang 2007; Peng 2007; Zhao 2008) made baseline comparisons. All studies had inclusion criteria, described the intervention in detail and defined outcomes, but only four articles described exclusion criteria (Bao 2000; Chen 2001; Fang 2007; Peng 2007). Outcome assessment was made after treatment but there were no stated indications for further interventions.
Effects of interventions
Mortality
We looked at mortality in individual trials. In Bao 2000, nine patients died: four in the TCM group and five in the control group (relative risk (RR) for death with TCM versus control was 0.68; 95% CI 0.22 to 2.14; Analysis 1.1). In Huang 2001 six patients died, all in the control group (RR 0.08; 95% CI 0.00 to 1.32). Ten patients died: two in the TCM group and eight in the control group in Li 2001 (RR 0.22; 95% CI 0.05 to 0.92). In Mo 2000, nine patients died: four in the TCM group and five in the control group (RR 0.71; 95% CI 0.22 to 2.37). Four patients died in Liu 2000: one in the TCM group and three in the control group (RR 0.33; 95% CI 0.04 to 2.83). Four patients died: one in the TCM group and three in the control group (Wang 2007) (0.24 (0.03 to 2.13).
1.1. Analysis.
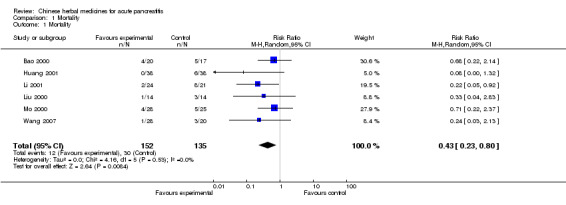
Comparison 1 Mortality, Outcome 1 Mortality.
Overall the RR was (RR 0.43; 95% CI 0.23 to 0.80; P= 0.008; Analysis 1.1). This indicates a statistically significant effect in favour of the intervention.
Length of hospital stay
There were only three trials in this category (Bao 2000; Huang 2001; Wang 2007). Bao stated that the mean duration of hospital stay was 25.90 days in the TCM group and 36.71 days in the control group. In the other study the mean number of days of hospital stay were 24 (21 to 32) in the TCM group and 55 (34 to 133) in the control group (Huang 2001). But Wang stated that the mean duration of hospital stay was 24±3.6 days in the TCM group and 32.5±2.5 days in the control group (Wang 2007).
Systemic inflammatory response syndrome (SIRS)
This was not reported as an outcome measure in any of the studies.
Multiple organ failure (MOF)
Two trial reported this as an outcome measure (Bao 2000; Wang 2007). Bao reported that there were seven patients who suffered from MOF in the TCM group and 11 patients in the control group (Bao 2000). In the other study two patients suffered from MOF in the TCM group and 4 patients in the control group (Wang 2007). For these two included studies, there was a statistically significant effect in favour of the intervention (RR 0.51; 95% CI 0.27 to 0.96; P=0.04; Analysis 2.1).
2.1. Analysis.
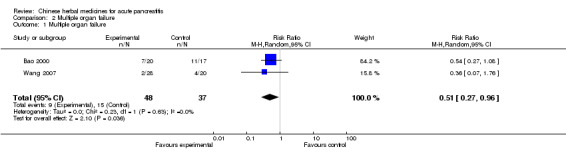
Comparison 2 Multiple organ failure, Outcome 1 Multiple organ failure.
Need for operation
There were three included trials (Huang 2001; Wu 1998; Wang 2007): ten patients underwent an operation in the TCM group and 16 patients in the control group (Wu 1998) in the second trial, 25 patients were operated on in the TCM group and 28 patients in the control group (Huang 2001). Only one patient needed to be operated in the TCM group and 3 patients in the control group (Wang 2007). There was no evidence of effect in favour of the intervention (RR 0.72; 95% CI 0.44 to 1.18; P= 0.19 Analysis 3.1).
3.1. Analysis.
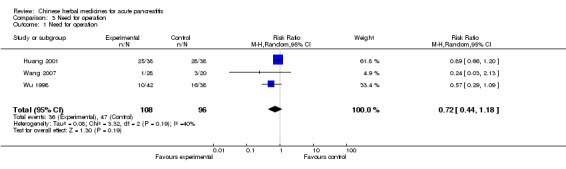
Comparison 3 Need for operation, Outcome 1 Need for operation.
Systemic infection
There were four trials in this category (Bao 2000; Huang, W‐q 2001; Li 2001; Wang 2007). In the first, two patients suffered from septicemia in the TCM group and seven patients in the control group (Bao 2000); only one patient suffered from pneumonia in the TCM group and three patients in the control group (Huang, W‐q 2001) in the second; and two patients suffered from a fungal infection in the TCM group with eight patients in the control group (Li 2001). No one suffered from septicemia in the TCM group and one patient in the control group (Wang 2007). There was evidence in favour of the intervention (RR 0.24; 95% CI 0.10 to 0.59; P=0.002; Analysis 4.1).
4.1. Analysis.
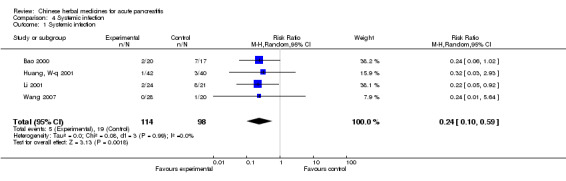
Comparison 4 Systemic infection, Outcome 1 Systemic infection.
Local septic complication
There were two trials in this category (Bao 2000; Huang, W‐q 2001): two patients suffered from pancreatic abscess in the TCM group and seven patients in the control group (Bao 2000); no patient suffered from pancreatic abscess in the TCM group but there was one patient in the control group (Huang, W‐q 2001). There was no evidence in favour of the intervention (RR 0.70; 95% CI 0.39 to 1.25; P= 0.23; Analysis 5.1)
5.1. Analysis.
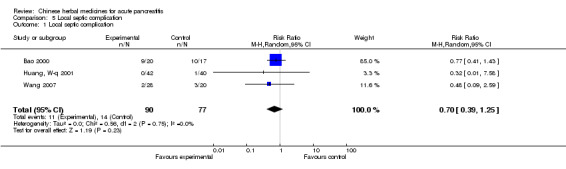
Comparison 5 Local septic complication, Outcome 1 Local septic complication.
Other local complications
There were three trials in this category (Bao 2000; Huang, W‐q 2001;Wang 2007): nine patients suffered from a pseudocyst in the TCM group with ten patients in the control group (Bao 2000); no patient suffered from pseudocyst in the TCM group but there was one patient in the control group (Huang, W‐q 2001). Two patients suffered from a pseudocyst in the TCM group with three patients in the control group (Wang 2007).
Protection of the gut mucosal barrier
This was not reported as an outcome measure in any of the studies.
Adverse events
No trials reported the adverse events.
Sensitivity analysis
An insufficient number of trials were found to perform a meaningful sensitivity analysis by excluding studies with inadequate concealment of allocation.
Heterogeneity
I2 = 0% for the overall mortality data and for all other comparisons, except 'Need for operation' where I2 = 40%. There was no statistically significant heterogeneity found for any of the comparisons.
Discussion
Summary of main results
Although the analyses indicate that there is statistically significant evidence of effect for Chinese herbal medicine in acute pancreatitis in terms of mortality, multiple organ failure, and systemic infection, at present there is no strong evidence to recommend any of these medicinal herbs for treatment of acute pancreatitis, due to the general high risk of bias in these trials.
Overall completeness and applicability of evidence
According to the meta‐analysis results, there appeared to be benefit from Chinese medicinal herbs compared with control for mortality rates, multiple organ failure and systemic infection. But for operative intervention (need for operation), and local septic complications, there was no difference between the treatment and control. All the trials were of low quality. There is clinical heterogeneity in the form of different categories of Chinese Medicinal herbs used, age, sex, etiology and severity of pancreatitis. Inadequate concealment, no reporting on dropouts, and different assessments of outcomes point to the possibility of bias. In addition, other important outcomes such as protection of the gut mucosal barrier were not measured in any of the trials.
Quality of the evidence
The majority of the RCTs included in this review had high risk of bias. They provided only limited descriptions of study design, randomization, and allocation concealment. Almost all trials stated only that random assignment was used but did not give sufficient information to allow a judgment of whether or not it was conducted properly.
Methodologically less rigorous trials show larger differences between experimental and control groups than do those conducted with better rigor. The insufficient number of trials prohibited us from performing meaningful sensitivity analysis to illuminate how robust the results of the review are to the exclusion of the trials with inadequate methodology. No multi‐centre, large scale RCTs were identified.
Agreements and disagreements with other studies or reviews
In China, Chinese medicinal herbs have been used for treating acute pancreatitis for many years. In this review some Chinese medicinal herbs seemed to have a beneficial effect on patients with acute pancreatitis but there was no standard prescription, dosage or period of treatment. Therefore, it is necessary to explore which drug combinations or sequences of drug combinations are likely to be most useful. The combination of 'Western' conventional therapy with 'Eastern' classical herbal therapy may reveal itself as an attractive option. Any definite conclusions on adverse events associated with Chinese medicinal herbs cannot be drawn from this review due to the limited number of trials identified and inadequate recording and reporting of adverse events. In China, only about 50% of clinical trials of Chinese medicinal herbs report adverse effects. Perhaps the reason for this is that Chinese practitioners perceive Chinese medicinal herbs as free of side effects. However, there are reports of liver toxicity and other serious adverse events associated with using Chinese herbal medicines (Gottlieb 2000; Melchart 1999; Tomlinson 2000) highlighting that the safety of complementary medicines needs to be monitored. In clinical trials efficacy and safety should receive equal attention; occasional and severe adverse events identified from large scale epidemiological studies need to be investigated.
Authors' conclusions
Implications for practice.
Based on this systematic review it appears that some Chinese medicinal herbs may have positive effects on mortality in acute pancreatitis. However, due to the high risk of bias in the RCTs and the risk of publication bias, there is no strong evidence for treating acute pancreatitis with Chinese medicinal herbs.
Implications for research.
The methodological quality of clinical trials of treatment with Chinese medicinal herbs for acute pancreatitis needs to be improved. The following aspects should be attended to: (i) detailed reporting of the generation of allocation sequence and the allocation concealment; (ii) application of blinding and placebo control; (iii) clear descriptions of withdrawal/dropout during the trial. Rigorously designed, multicentre, large scale RCTs are required to evaluate Chinese medicinal herbs versus placebo, with improved follow up of patients and increased reporting of adverse events and quality of life. Herbs should be tested in the same way as any drug.
What's new
| Date | Event | Description |
|---|---|---|
| 30 March 2009 | New search has been performed | New studies sought, four new studies included. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 30 July 2008 | Amended | Converted to new review format. |
| 5 October 2005 | New search has been performed | New studies added, conclusions not changed. |
| 28 October 2004 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We thank Janet Lilleyman and Dr Cathy Bennett for help with the review. We also appreciate the helpful suggestions for search strategy design from Iris Gordon. We are grateful to Klaus Linde, Paul Moayyedi, and Dr Edzard Ernst for expert suggestions and corrections. We thank all staff of the Chinese Cochrane Center.
Appendices
Appendix 1. Search strategy
randomized controlled trial.pt. controlled clinical trial.pt. randomized controlled trials.sh. random allocation.sh. double blind method.sh. single‐blind method.sh. or/1‐6
(animal not human).sh. 7 not 8 clinical trial.pt. exp clinical trials/ (clin$ adj25 trial$).ti,ab. ((singl$ or doubl$ or trebl$ or tripl$) adj25 blind$).mp. or mask$.ti,ab. [mp=title, abstract, cas registry/ec number word,mesh subject heading] placebos.sh. placebo$.ti,ab. random$.ti,ab. research design.sh. or/10‐17 18 not 8 19 not 9 comparative study.sh. exp evaluation studies/ follow up studies.sh. prospective studies.sh. (control$ or prospectiv$).mp. or volunteer$.ti,ab. [mp=title, abstract, cas registry/ec number word, mesh subject heading] or/21‐25 26 not 8 27 not (9 or 20) 9 or 20 or 28 exp pancreas/ exp pancreatitis/ pancrea$.tw. (acute adj5 pancrea$).tw. (necro$ adj5 pancrea$).tw. (chronic adj5 pancrea$).tw. or/30‐35 dachengqitang.tw. (yue adj3 ju adj3 wan).tw. (danyi adj3 tang).tw. miltiorrhiza.tw. (yinchenhao adj3 tang).tw. (dachaihu adj3 tang).tw. (qingyi adj3 tang).tw. senna.tw. rhubarb.tw. RHODIOLA/ rhodiola.tw. EMODIN/ emodin.tw. saikosaponin.tw. saiko‐keshi‐to.tw. keshi‐to.tw. sho‐saiko‐to.tw. (qing adj2 yi adj2 tang).tw. (liyi adj2 tang).tw. (fructus adj2 gardeniae).tw. ginsenoside.tw.glycyrrhizin.tw. tetramethylpyrazine.tw. (gardenia adj2 jasminoides).tw. exp traditional chinese medicine/ Drugs, Chinese Herbal/ (trad$ adj5 chinese adj5 med$).tw. (chinese adj5 herb$).tw. or/37‐64 65 and 36
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
Comparison 2. Multiple organ failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Multiple organ failure | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.96] |
Comparison 3. Need for operation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for operation | 3 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.18] |
Comparison 4. Systemic infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic infection | 4 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.10, 0.59] |
Comparison 5. Local septic complication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Local septic complication | 3 | 167 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.39, 1.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bao 2000.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 37 patients (12 males and 8 females, average age 48 years in rheum group; 9 males and 8 females, average age 51 years in internal routine treatment group) Setting: inpatients Inclusion criteria: acute hemorrhagic necrotic pancreatitis Exclusion criteria: less than 18 years, pregnant or lactating women, coma before admission, death in 48 hours | |
| Interventions | Experimental: frying Rheum 200ml bid and routine therapy Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; period of hospitalization; death; period of high amylase; concentration of endotoxin and TNF‐¦Á | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Chen 2001.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 65 patients (35 in Dan‐shen group; 30 in routine treatment group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: in addition to routine therapy, intravenous infusion of Ranitidine 200mg and Danshen injection 20ml in 250ml of 5% glucose daily for 4‐7days. Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; normal amylase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Fang 2007.
| Methods | Randomized clinical trial. Generation of allocation sequence: computer randomization. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 53 patients (17 males and 11 females, average age 41.8 years in TCM group; 15 males and 10 females, average age 42.3 years in internal routine treatment group) Setting: inpatients Inclusion criteria: severe acute pancreatitis Exclusion criteria: acute biliary pancreatitis accompanying obstructive suppurative cholangitis or gangrenous cholecystitis | |
| Interventions | Experimental: Tongxia Huayu Decoction and routine therapy Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; concentration of amylase and C‐reactive protein; concentration of endothelin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer randomization. |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
He 1996.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 98 patients (18 males and 30 females, average age 38.2 years in Danyitang group; 19 males and 31 females, average age 37.8 years in routine treatment group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: Danyitang, a compound herbs (including rhubarb, chaihu, liquorice, et al), one dose, daily and routine therapy for five days Control: routine therapy (fasting, fluid replacement, antibiotics, et al ) | |
| Outcomes | symptoms and signs; normal amylase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Huang 2001.
| Methods | Randomized clinical trial.
Generation of allocation sequence: no information.
Allocation concealment: no information.
Blinding: no blinding. Loss to follow‐up: 7 patients in Danyitang group, 11 patients in control group , but we do not know the reason. |
|
| Participants | 76 patients (20 males and 18 females, average age 49 years in Qingganlidantang group; 22 males and 16 females, average age 37.8 years in internal routine treatment group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: Qingganlidantang (including rhubarb, Chaihu, Salvia miltiorrhiza, skullcap, et al), compound herbs, one dose, daily and routine therapy or after drainage operation Control: routine therapy (fasting, fluid replacement, antibiotics, et al) or drainage operation | |
| Outcomes | symptoms and signs; period of hospitalization; death; local complication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Huang, W‐q 2001.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 82 patients (27 males and 15 females, average age 46.1 years in Experimental group; 23 males and 17 females, average age 47.2 years in routine treatment group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: For mild acute pancreatitis: Dacaihutang (including rhubarb, Chaihu, skullcap, et al) one dose, daily and routine therapy for five days; For severe acute pancreatitis: Huanglianjiedutang and Dachengqitang (including rhubarb, Chaihu, Glauber's salt, skullcap, et al) one dose, daily and routine therapy for five days Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; normal amylase; complication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Li 2001.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 45 patients (6 males and 18 females, average age 55.9 years in Qingyitang group; 5 males and 16 females, average age 56.3 years in operation group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: frying Qingyitang (including rhubarb, Chaihu, skullcap, et al) 200ml qd and routine therapy Control: operation and routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | concentration of TNF; death; complication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Liu 2000.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 28 patients (17 males and 11females, average age 47.3 years,14 in experimental group and 14 in control group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: Dacaihutang (no information about dosage) and internal routine therapy Control: operation and routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | death; white blood cells; blood sugar; partial pressure of oxygen; death; complication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Mo 2000.
| Methods | Randomized clinical trial. Generation of allocation sequence: no details. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 53 patients (12 males and 16 females, average age 45.5 years in Danshen injection and Qingyitang group; 12 males and 13 females, average age 44.8 years in control group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: in addition to routine therapy, intravenous infusion of Danshen injection 16ml in 500ml of 5% glucose, daily, and Qingyitang, one dosage daily for 7days. Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; death; level of amylase and C reactive protein | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Pan 2001.
| Methods | Randomized clinical trial. Generation of allocation sequence: no details. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 28 patients (10 males and 6 females in Danshen injection group; 7 males and 5 females in control group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: in addition to routine therapy, intravenous infusion of Danshen injection 30‐50ml in 500ml of 5% glucose daily for 3‐10 days. Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; normal amylase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Peng 2007.
| Methods | Randomized clinical trial. Generation of allocation sequence: random digits table. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 36 patients (14 males and 4 females, average age 40.5 years in Salvia miltiorrhiza group; 13 males and 5 females, average age 46.4 years in routine treatment group) Setting: inpatients Inclusion criteria: severe acute pancreatitis Exclusion criteria: patients with gastrointestinal disease, diabetes mellitus, tumor and autoimmune disorder; acute biliary pancreatitis in need of emergency endoscopic treatment; chronic pancreatitis with acute exacerbation | |
| Interventions | Experimental: in addition to routine therapy, intravenous infusion of Salvia miltiorrhiza (Danshen) injection 250ml daily . Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | serum levels of the three cytokines(IL‐6, IL‐8, and TNF‐ α) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random digits table |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Wang 2000.
| Methods | Randomized clinical trial. Generation of allocation sequence: no details. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 60 patients (26 males and 34 females, average age 45.5 years; 30 in experimental group; 30 in control group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: compound herbs (including rhubarb, Chaihu, skullcap, Glauber's salt et al), one dose daily and routine therapy for five days Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; normal amylase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Wang 2007.
| Methods | Randomized clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 48 patients (30 males and 18 females, average age 45 years; 28 in experimental group; 20 in control group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: modified decoction of chengqi including bupleurum and peony into the stomach and bowels via a stomach tube and a retention enema and routine therapy Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; normal amylase; complication; death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Wu 1998.
| Methods | Randomized clinical trial. Generation of allocation sequence: no details. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 80 patients (14 males and 28 females, average age 49 years in Qingyitang group; 13 males and 25 females, average age 48.1 years in routine treatment group) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: Qingyitang one dose, daily and internal routine therapy Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; normal amylase; operation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information about sequence generation |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Zhao 2008.
| Methods | Randomized clinical trial. Generation of allocation sequence: random sampling. Allocation concealment: no information. Blinding: no blinding. Description of dropout: no. | |
| Participants | 50 patients (35 males and 15 females, average age 54 years) Setting: inpatients Inclusion criteria: unstated Exclusion criteria: unstated | |
| Interventions | Experimental: Dachengqitang, a compound herbs (including rhubarb, Sodium Sulfate, Immature orange fruit, et al), 100ml, tid and routine therapy for four weeks Control: routine therapy (fasting, fluid replacement, antibiotics, et al) | |
| Outcomes | symptoms and signs; concentration of serum amylase, urea nitrogen, blood calcium and blood glucose; APACHE II score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random sampling. |
| Allocation concealment? | Unclear risk | No information about allocation concealment |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 1997 | Participants were not really allocated randomly to treatment group and control group. |
| Deng 2000 | TCM administered by enema not orally. |
| Deng 2007 | Both of treatment and control group were treated by TCM. |
| Gao 1997 | Participants were not really allocated randomly to treatment group and control group. |
| He 2001 | Participants were not really allocated randomly to treatment group and control group. |
| Li R‐x 2001 | Participants were not really allocated randomly to treatment group and control group. |
| Li, J‐y 2001 | Participants were not really allocated randomly to treatment group and control group. |
| Liu 1997 | Participants were not really allocated randomly to treatment group and control group. |
| Sen 1999 | Participants were not really allocated randomly to treatment group and control group. |
| Wang 1996 | Participants were not really allocated randomly to treatment group and control group. |
| Wang M 2000 | Participants were not really allocated randomly to treatment group and control group. |
| Xia 2005 | Both of treatment and control group were treated by TCM. |
| Xie 1995 | Participants were not really allocated randomly to treatment group and control group. |
| Xue 2008 | Both of treatment and control group were treated by TCM. |
| Yan 2001 | The study is about TCM for complication of severe acute pancreatitis. |
| Zeng 1999 | Participants were not really allocated randomly to treatment group and control group. |
| Zhang 1996 | Participants were not really allocated randomly to treatment group and control group. |
| Zhou 2000 | Participants were not really allocated randomly to treatment group and control group. |
Contributions of authors
Qiong Wang designed, drafted and revised the protocol, performed searches, selected trials, extracted data, wrote and revised the review. Yiping Wang developed the search strategy, provided methodological perspective, and revised the protocol and the review. Jinlin Yang , Tao Gan, Zhen Guo and Pengcheng Zhao handsearched journals, retrieved papers, and extracted data.
Sources of support
Internal sources
Chinese Cochrane Center, Chinese Center of Evidence‐based Medicine, West China Hospital of Sichuan University, China.
External sources
China Medical Board of New York (Grant number 98‐680), China.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bao 2000 {published data only}
- Bao Shi‐ning, Zhang Shu‐wen, Wang Bao‐en. The therapeutic effect of Rheum on the patients with acute necrotic pancreatitis [Zhongyao Dahuang dui jixing chuxue huaishixing yixianyan zhiliao zuoyong de yanjiu]. Chinese Journal of Combination of TCM and Western Medicine Emergency 2000;7(6):362‐4. [Google Scholar]
Chen 2001 {published data only}
- Chen Honghua, Xia Shujiao. Therapeutic analysis of 35 patients with acute pancreatitis with ranitidine and Dan‐shen injection [Leinitiding yu fufangdanshen lianhe zhiliao jixing yixianyan 35 li liaoxiao fenxi]. Chinese Journal of Integrated Traditional and Western Medicine of Zhejiang 2001;11(7):406‐7. [Google Scholar]
Fang 2007 {published data only}
- Fang B‐J. Gao P‐Y. He S‐H. Chen H. Shen P. Zhang Y‐Y. Zhang J‐Z. Clinical early intervention of Tongxia Huayu Decoction on pancreatic microcirculatory disturbance in severe acute pancreatitis. Journal of Chinese Integrative Medicine 15 Mar 2007;5(2):134‐136. [DOI] [PubMed] [Google Scholar]
He 1996 {published data only}
- He Guanhua, Shen Jiaxing. Combination of TCM and western medicine in treatment acute gallstone pancreatitis [Zhongxiyijiehe zhiliao jixing danyuanxing yixianyan]. Practical Clinical Emergency of Combination of TCM and Western Medicine 1996;3(7):300‐1. [Google Scholar]
Huang 2001 {published data only}
- Huang Peile, Li Xinyu, Liang Jun, Wei Xiaoyi, Gao Pengcheng. The effects of Qingganlidantang on severe acute pancreatitis [Zhongyao Qingganlidantang zai zhongzheng jixing yixianyan zhiliao zhong de zuoyong]. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(6):461‐2. [Google Scholar]
Huang, W‐q 2001 {published data only}
- Huang Wenqiang, Zhong Miaowen, Wu Yiqiong, Zhang Guopei, Luo Zhihua. Treatment of acute pancreatitis by TCM combined with western medicine: a clinical observation of 42 cases [Zhongxiyijiehe zhiliao jixing yixianyan 42 li liaoxiao guancha]. New Traditional Chinese Medicine 2001;33(7):27‐8. [Google Scholar]
Li 2001 {published data only}
- Li Songlin. Combination of TCM and western medicine in therapy of 24 patients with acute pancreatitis [Zhongxiyijiehe zhiliao zhongzheng jixing yixianyan 24 li]. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(10):781. [Google Scholar]
Liu 2000 {published data only}
- Liu Shuli. The method of Huoxuegongxia in treatment of severe acute pancreatitis [Huoxuegongxiafa zhiliao zhongzheng jixing yixianyan]. Chinese Journal of Integrated Traditional and Western Medicine 2000;6(4):261‐2. [Google Scholar]
Mo 2000 {published data only}
- Mo Shaoxiong, Wu Songhe. The combination of TCM and western medicine in treatment of 28 patients with severe acute pancreatitis [Zhongxiyijiehe zhiliao jixing zhongzheng yixianyan 28 li]. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(10):789. [Google Scholar]
Pan 2001 {published data only}
- Pan Xiaofeng. Clinical observation of Dan‐shen compound in treatment of acute pancreatitis [Fufangdanshen zhiliao jixing yixianyan linchuang guancha]. Chinese Journal of Integrated Traditional and Western Medicine of Zhejiang 2001;11(8):495‐6. [Google Scholar]
Peng 2007 {published data only}
- Guolin PENG, Xiaoyun ZHANG. Effects of Salvia miltiorrhiza on serum levels of inflammatory cytokines in patients with severe acute pancreatitis. Journal of Chinese Integrative Medicine January 2007;5(1):28‐31. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang Feng, Li Yingqun. Combination of TCM and western medicine in therapy of 30 cases with acute edematous pancreatitis [Zhonxiyijiehe zhiliao jixing shuizhongxing yixianyan 30 li]. Chinese Surgical Journal of Integrated Traditional and Western Medicine 2000;6(1):45. [Google Scholar]
Wang 2007 {published data only}
- Wang L.‐M. Luo H. Wang Y.‐N. Zeng Y. Su H.‐F. Integrated traditional Chinese and Western medicine in treatment of severe acute pancreatitis: An analysis of 28 cases. World Chinese Journal of Digestology Sep 2007;15(25):2747‐2749. [Google Scholar]
Wu 1998 {published data only}
- Wu Gude, Wu Chengwu, Xu Hongbing. Qingyitang decoction in treatment of 42 patients with severe acute pancreatitis [Qingyitang zhiliao zhongzheng jixing yixianyan 42 li]. Digistive Journal of Chinese 1998;6(7):619‐21. [Google Scholar]
Zhao 2008 {published data only}
- Hong‐Zhi Zhao. Curative effect of Dachengqi decoction as an adjutant therapy on recurrent acute pancreatitis: an analysis of 25 cases. World Chinese Journal of Digestology May 2008;16(16):1825‐1827. [Google Scholar]
References to studies excluded from this review
Chen 1997 {published data only}
- Chen Qiduo, Chen Wuqing, Li Juncai. Effect of the combination of TCM and western medicine on hemorrhagic necrotic gallstone pancreatitis [Zhongxiyijiehe zhiliao danyuanxing chuxue huaisixing yixianyan liaoxiao guancha]. Chinese Journal of Integrated Traditional and Western Medicine of Zhejiang 1997;7(1):9‐10. [Google Scholar]
Deng 2000 {published data only}
- Deng Yi, Chen Hang, Cui Chaoyang, Lin Xinchao. Retention enema with Tongfutang in treatment of 45 patients with acute pancreatitis [Tongfutang baoliu guanchang zhiliao jixing yixianyan 45 li]. Chinese Journal of Beijing University of Traditional Chinese Medicine 2000;23(3):59‐61. [Google Scholar]
Deng 2007 {published data only}
- Bin Deng, Yan‐Bing Ding, Zhi‐Gang Yan, Yuan‐ZhiWang, Jian Wu, Wei‐Ming Xiao. Treatment of severe acute pancreatitis by different administration route: a comparative analysis. World Chinese Journal of Digestology May 2007;15(14):1673‐1675. [Google Scholar]
Gao 1997 {published data only}
- Gao Zhenyu. Effect of the combination of TCM and western medicine on 42 patients with acute edematous pancreatitis [Zhongxiyijiehe zhiliao jixing shuizhongxing yixianyan 42 li liaoxiao guancha]. Chinese Journal of Integrated Traditional and Western Medicine of Zhejiang 1997;7(1):48‐9. [Google Scholar]
He 2001 {published data only}
- He Guixiang, Wang Suqing. Effect of combination of TCM and western medicine on acute pancreatitis [Zhongxiyijiehe zhiliao jixing yixianyan liaoxiao guancha]. Sichuan Medical Journal 2001;22(8):748‐9. [Google Scholar]
Li R‐x 2001 {published data only}
- Li Rongxiang, Li Jin, Zhang Tianying. Yi‐antang in treatment of 80 cases with acute pancreatitis [Yi‐antang zhiliao jixing yixianyan 80 li]. Chinese Surgical Journal of Integrated Traditional and Western Medicine 2001;7(2):112. [Google Scholar]
Li, J‐y 2001 {published data only}
- Li Jingyu, Zheng Yuchu. Effect of combination of TCM and Western medicine on acute pancreatitis [Zhongxiyaojiehe zhiliao jixing yixianyan liaoxiao guancha]. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(5):395. [Google Scholar]
Liu 1997 {published data only}
- Liu Yumao, He Dongchu. Effect of Senna leaf in treatment of acute edematous pancreatitis [Fanxieye wei zhufang zhiliao jixing shuizhongxing yixianyan]. Emergency of Traditional Chinese Medicine 1997;6(4):151‐152. [Google Scholar]
Sen 1999 {published data only}
- Jiang Sen. Dahuangzhizitang in treatment of 28 cases with acute edematous pancreatitis [Dahuangzhizitang zhiliao jixing shuizhongxing yixianyan]. Chinese Journal of Integrated Traditional and Western Medicine 1999;19(3):183. [Google Scholar]
Wang 1996 {published data only}
- Wang Changyong, Li Zengcan, Hou Peng, Du Min, Han Jinjiang. Clinical observation of combination of TCM and western medicine on acute edematous pancreatitis [Zhongxiyijiehe zhiliao jixing shuizhongxing yixianyan linchuang guancha]. Chinese Journal of Integrated Traditional and Western Medicine 1996;16(3):170‐171. [Google Scholar]
Wang M 2000 {published data only}
- Wang Min, Lu Yongming, Zhang Ruili. Effect of combination of TCM and western medicine on severe acute pancreatitis [Zhongxiyijiehe zhiliao zhongzheng jixing yixianyan liaoxiao fenxi]. Chinese Surgical Journal of Integrated Traditional and Western Medicine 2000;6(3):151‐152. [Google Scholar]
Xia 2005 {published data only}
- Qing Xia, Lin Yuan, Xiao‐Nan Yang, WenFu Tang, ]unMing]ian. Comparison of integrated Chinese and Western medicine with and without somatostatin supplement in the treatment of severe acute pancreatitis. World Journal of Gastroenterolog February 2005;11(7):1073‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 1995 {published data only}
- Xie Min, Jin Zunyu, Ye Genghui, Zhou Jianxin, Zhang Kai, Qiu Yudong. Clinical investigation of Dan‐shen injection in treatment severe acute pancreatitis [Fufangdanshen zhusheye zhiliao zhongzheng yixianyan de linchuang yanjiu]. Chinese Journal of Integrated Traditional and Western Medicine 1995;15(5):269‐270. [PubMed] [Google Scholar]
Xue 2008 {published data only}
- Xue Ping, Deng Lihui, Zhang Zhaoda, Xia Qing, Huang Zongwen, Yang Xiaonan, Jiang Junming. Clinical study of Yihuo Qingxia method in treating hyperlipoidemia‐related severe acute pancreatitis in early stage. Journal of Chinese Integrative Medicine March 2008;6(3):262‐265. [DOI] [PubMed] [Google Scholar]
Yan 2001 {published data only}
- Yan Ming, Yang Xingyi, Chen Dechang, Xu Yonghua, Guo Changxing. Effect of Rhubarb on severe acute pancreatitis complicated with acute respiratory distress syndrome [Dahuang dui zhongzheng jixing yixianyan bingfa jixing huxi jiongpo zonghezheng de zhiliao zuoyong]. Chinese Journal of Gastroenterology 2001;6(2):94‐96. [Google Scholar]
Zeng 1999 {published data only}
- Zeng Yaqing. Jiedusanyutang in treatment of 50 patients with acute pancreatitis [Jiedusanyutang zhiliao jixing yixianyan 50 li]. Chinese Journal of Integrated Traditional and Western Medicine 1999;19(7):437‐438. [Google Scholar]
Zhang 1996 {published data only}
- Zhang Zhihua. Yuejuwan in treatment acute gallstone pancreatitis [Yuejuwan jibenfang weizhu zhiliao jixing danyuanxing yixianyan]. Practical Clinical Emergency of Integrated Traditional and Western Medicine 1996;3(3):97‐98. [Google Scholar]
Zhou 2000 {published data only}
- Zhou Ying, Li Rongxiang, Pan Wanneng, Li Jinlong, Li Jin, Pu Qingfan. Effect of combination of TCM and western medicine on acute pancreatitis [Zhongxiyijiehe zhiliao jixing yixianyan de liaoxiao guancha]. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(6):461‐462. [Google Scholar]
Additional references
Bassi 1994
- Bassi C, Pederzoli P, Vesentini S, Falconi M, Bonora A, Abbas H, Benini A, Bertazzoni EM. Behaviour of antibiotics during human necrotising pancreatitis. Antimicrobial Agents and Chemotherapy 1994;38(4):830‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blamey 1984
- Blamely SL, Imrie CW, O'Neill J, et al. Prognostic factors in acute pancreatitis. Gut 1984;25:1304‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL. A clinically based classification system for acute pancreatitis: Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11‐13, 1992. Archives of Surgery 1993;128(5):586‐90. [DOI] [PubMed] [Google Scholar]
Dervenis 1999
- Dervenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity and management of acute pancreatitis. Santorini Consensus Conference. International Journal of Pancreatology 1999;25(3):195‐210. [DOI] [PubMed] [Google Scholar]
Gottlieb 2000
- Gottlieb S. Chinese herb may cause cancer (news). BMJ 2000;320:1623. [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Knaus 1985
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE‐II: a severity of disease classification. Critical Care Medicine 1985;13:818‐29. [PubMed] [Google Scholar]
Lowham 1999
- Lowham A, Lavelle J, Leese T. Mortality from acute pancreatitis. International Journal of Pancreatology 1999;25(2):103‐6. [DOI] [PubMed] [Google Scholar]
Melchart 1999
- Melchart D, Linde K, Weidenhammer W, Hager S, Shaw D, Bayer R. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA 1999;282(1):28‐9. [DOI] [PubMed] [Google Scholar]
Mergener 1999
- Mergener K, Baillie J. Endoscopic treatment for acute biliary pancreatitis. When and in whom?. Gastroenterology Clinics of North America 1999;28(3):601‐613. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Steinberg 1994
- Steinberg W, Tenner S. Acute pancreatitis. New England Journal of Medicine 1994;330:1198‐210. [DOI] [PubMed] [Google Scholar]
Tomlinson 2000
- Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an Eastern perspective. Journal of Clinical Pharmacology 2000;40(5):451‐6. [DOI] [PubMed] [Google Scholar]


