Abstract
Immunoglobulin (Ig) E and IgG anti-thyroid autoantibodies (AAbs) play important roles in the immunopathogenesis of chronic spontaneous urticaria (CSU). To date, association of IgE and IgG AAbs with Chinese CSU patients has not been fully investigated. We aimed to explore prevalence rates of IgE and IgG AAbs in Chinese CSU patients and their association with clinical and laboratory parameters. Serum IgE and IgG AAbs against thyroid peroxidase (TPO) and thyroglobulin (TG), total IgE (tIgE) and specific IgEs were measured using enzyme-linked immunosorbent assay, chemiluminescence microparticle immunoassay and immunoblotting. Meta-analyses and literature review were conducted. The meta-analyses indicated that CSU cases were 4.98, 6.90 and 6.68 times more likely to have positive anti-TPO IgE, anti-TPO IgG and anti-TG IgG (all P < 0.001) compared with controls, respectively, and revealed a positive correlation between the prevalence rates of anti-TPO IgE and anti-TPO IgG (r = 0.53, P = 0.025). A total of 1,100 Chinese Han adult CSU patients and 1,100 ethnicity-, age- and sex-matched healthy controls were recruited from 15 centers. Prevalence rates of anti-TPO IgE, anti-TPO IgG, anti-TG IgE or anti-TG IgG in the patients were all significantly higher than those in the controls. Significant correlations were observed between prevalence rates of anti-TPO IgE and anti-TPO IgG (r = 0.297, P < 0.001) as well as between those of anti-TG IgE and anti-TG IgG in the patients (r = 0.137, P < 0.001). Patients with anti-TPO IgE or anti-TPO IgG had significantly lower tIgE levels (P < 0.001). Positive anti-TPO IgE, positive anti-TPO IgG and tIgE < 40 IU/mL were independent predictors of antihistamine-refractory cases. In conclusion, the prevalence rates of IgE and IgG AAbs in Chinese CSU patients are significantly elevated and reciprocally correlated. This study verifies the results of previous case-control studies of CSU patients from other populations and ethnicities.
Keywords: Chronic spontaneous urticaria, thyroid peroxidase, thyroglobulin, immunoglobulin E
INTRODUCTION
Chronic spontaneous urticaria (CSU) is an autoimmune skin disease characterized by spontaneous occurrence of wheals and/or angioedema for more than 6 weeks.1
The association of thyroid autoimmunity with chronic urticaria has been reported since 1983. Elevated prevalence rates of IgE and IgG anti-thyroid autoantibodies (AAbs) in CSU patients from different populations and ethnicities have been observed (Supplementary Table S1).2 Anti-thyroid IgG AAbs cause autoimmune thyroid inflammation, disrupts normal architecture of the thyroid gland, leads to release of sequestered autoantigens, such as thyroid peroxidase (TPO) and/or thyroglobulin (TG), and induce a low-grade autoimmune response. The resulting thyroid protein immune complexes activate the classic complement pathway to generate C3a and C5a, which cause degranulation of mast cells. IgG anti-thyroid AAbs enhance mast cell’s susceptibility to activating signals including anti-FcεRIα and C5a. Upon exposure to circulatory TPO and/or TG, mast cell- and basophil-bound IgE anti-TPO/TG AAbs cause autoallergic activation and degranulation of the cells.3
So far, prevalence rates of anti-thyroid AAbs in Chinese CSU patients and their roles in predicting antihistamine-refractory cases have not been fully investigated.
MATERIALS AND METHODS
Literature search
Literature search was performed in PubMed, Web of Science, Embase, Google Scholar and Cochrane Library by July, 2021, according to the Preferred Reporting Items for Meta-Analyses guidelines,4 using the following terms: urticaria, chronic urticaria, chronic spontaneous urticaria, chronic idiopathic urticaria, thyroid, TPO and TG.
Inclusion and exclusion criteria
For inclusion, studies should provide sufficient information to allow analysis of positivity for anti-TPO and anti-TG AAbs in both CSU and control individuals. Studies that enrolled CSU patients with or without history of thyroid disease were both considered eligible. No restrictions on the methodologies for detecting anti-TPO and anti-TG AAbs were made. The studies should be published in English.
Exclusion criteria were as follows: 1) reviews, meta-analyses, guidelines, editorials, comments, case reports on fewer than 5 cases, letters to the editor or other communications without original data; 2) conference abstracts; and 3) animal or in vitro studies.
Data extraction and quality assessment
Two authors (LZ and LQ) performed the study assessment independently, and data were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Any discrepancies were discussed with additional reviewers (TX and HC). Study quality was assessed using the modified version of Newcastle-Ottawa Scale.5 A score of up to 9 points was assigned to each study based on the quality of population selection (0–4), the comparability between groups (0–2), and assessment of exposure (0–3).
Study subjects
This multicenter cross-sectional study was carried out from June 2014 to May 2021. A total of 1,100 Chinese Han adult patients with active and uncontrolled CSU were recruited from 15 medical centers in China. CSU diagnosis was based on the EAACI/GA2LEN/EDF/WAO guidelines.1 CSU patients with chronic inducible urticaria, active atopic diseases, urticarial vasculitis, or recent treatment history of systemic corticosteroids or immunosuppressants were excluded. In order to investigate the real prevalence of IgE and IgG AAbs to TPO or TG in CSU patients, we did not recruit CSU patients who were associated with physician-diagnosed overt hypothyroidism, overt hyperthyroidism or other thyroid diseases in this study. In total, 1,100 ethnicity-, sex- and age-matched healthy controls were enrolled. The urticaria activity score for 1 day (UASday) was used to evaluate CSU disease activity. Ethical approval was obtained from the Institutional Ethics Committees (2014-4-2) and written consent was obtained from each participant.
Measurement of anti-TPO IgE/IgG, anti-TG IgE/IgG, thyrotropin, free thyroxine (fT4), free triiodothyronine (fT3), total IgE (tIgE) and specific IgEs (sIgEs)
Anti-TPO IgE and anti-TG IgE (Meimian, Yancheng, China) were measured using enzyme-linked immunosorbent assay (ELISA) kits. The optical density (OD) values were detected at 450 nm. The cut-off value for anti-TPO IgE or anti-TG IgE was calculated as the sum of mean OD and triple the SD of healthy controls with negative IgG anti-thyroid AAbs.6 An Abbott ARCHIITECT i2000SR chemiluminescence microparticle immunoassay analyzer was used to detect anti-TPO, anti-TG IgG AAbs, thyrotropin (or thyroid stimulating hormone, TSH), fT4 and fT3 (Abbott Park, Middletown, USA). The normal ranges of anti-TPO IgG, anti-TG IgG, TSH, fT4 and fT3 were 0.00–5.61 IU/mL, 0.00–4.11 IU/mL, 0.35–4.94 mIU/L, 9.01–19.05 pmol/L and 2.43–6.01 pmol/L, respectively. Serum tIgE was measured using ELISA kits (Euroimmun, Lübeck, Germany). The normal range of tIgE was 0.00–100.00 IU/mL.7 Serum sIgEs were determined using immunoblotting (Euroimmun). A test result of 0.7 kU/L or higher (level 2 to 6) was considered positive.7,8
Control of disease activity and designation of antihistamine-refractory cases
As we have previously described, control of disease activity was classified as being in complete control (UASday = 0), partial control (UASday ≥ 1) or uncontrolled (UASday unchanged or increased).9 The failure of disease control means uncontrolled or partial control. Before complete control was achieved, the patients were followed up weekly or biweekly, and UASday was assessed. CSU cases that were not completely controlled by 4-fold doses or 4-fold equivalent doses of second-generation H1-antihistamines for at least 4 weeks were designated as antihistamine-refractory cases.
Statistical analysis
STATA 16.0 was used for the meta-analysis. To reduce influence of different detecting methods, dichotomous outcomes were extracted as odds ratio (OR) with 95% confidence interval (CI), and continuous outcomes were extracted as weighted mean difference (WMD) with 95% CI. An estimate of the pooled correlation coefficient between IgE and IgG anti-thyroid AAbs was calculated by combining the correlation coefficients obtained in previous individual studies. Correlation coefficient (r) values were extracted from each study and 95% CI was calculated after applying Fisher’s z transformation in order to ensure an unbiased estimate. Heterogeneity among the studies included was assessed using Cochran's Q test (P < 0.05) and I2 statistic. When I2 > 50% or P < 0.05, substantial heterogeneity was considered to exist among the studies, and a random-effect model was used. When I2 ≤ 50% or P ≥ 0.05, a fixed-effect model was used for the meta-analysis. No subgroup analysis was performed owed to the limited number of studies. The possibility of publication bias was evaluated by a funnel plot, Begg’ s test and Egger’ s test.
Normally distributed data are presented as mean ± SD and nonnormally distributed data are expressed as median and interquartile range. Prevalence rates were shown as percentage values. For comparisons of proportions, Chi-square and Fisher exact tests were used. Mann-Whitney U tests and t tests were used for comparing data lack of a normal distribution and data in a normal distribution, respectively. Spearman’s rank correlation coefficients were used to assess potential correlations between prevalence rates of IgE and IgG AAbs. Multivariate logistic regression was used to calculate associations of sex, age, laboratory parameters and antihistamine-refractory cases. The association is expressed as OR with a 95% CI. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 26 and Graphpad prism 7 programs.
RESULTS
Meta-analysis of anti-TPO IgE and anti-thyroid IgG in CSU cases and controls
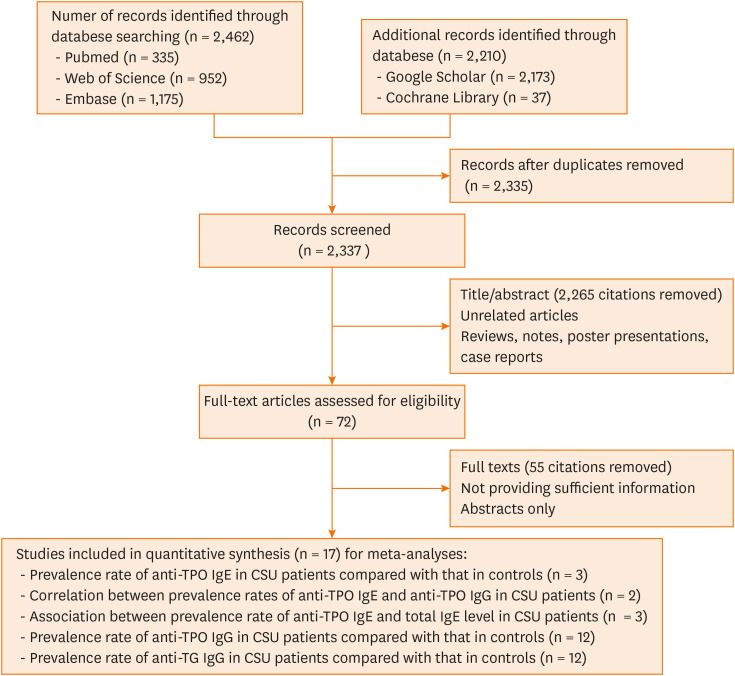
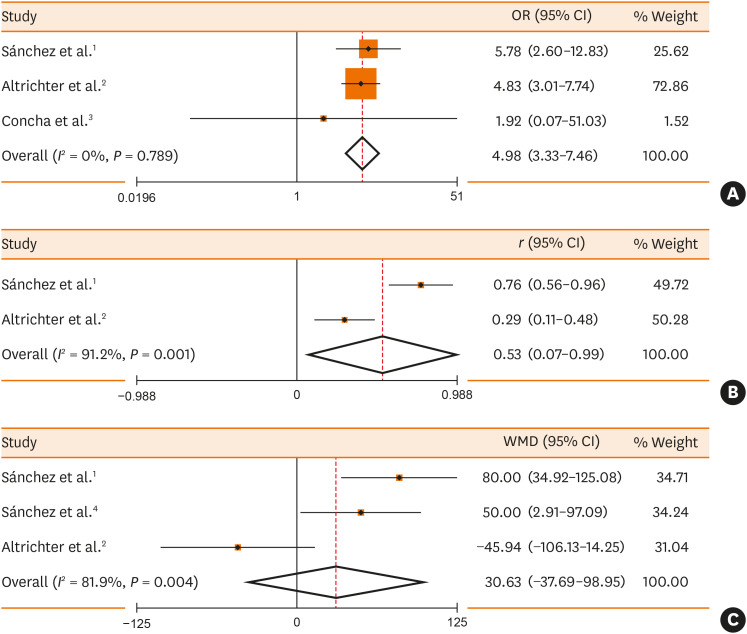
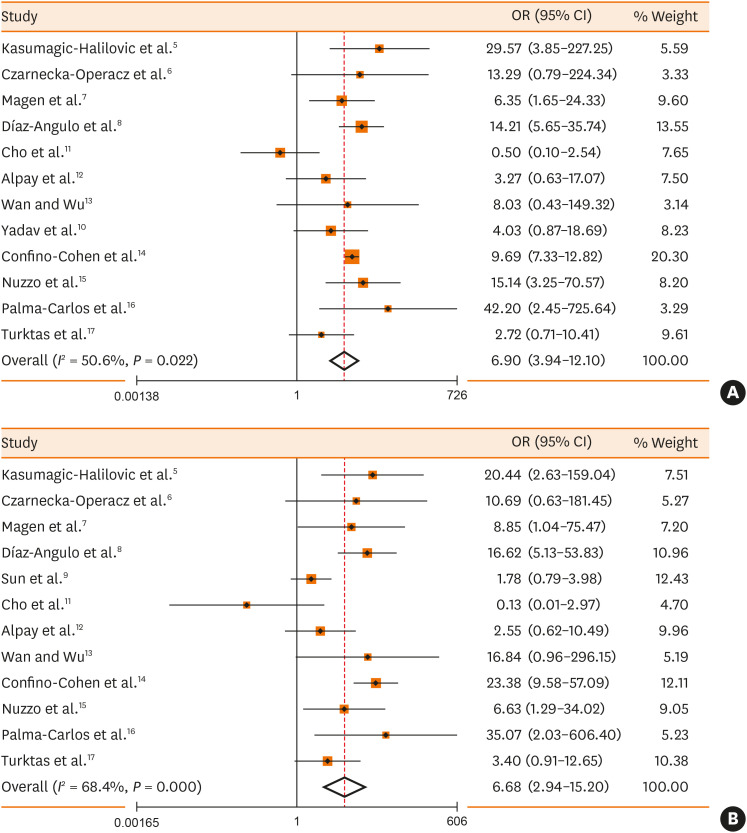
A total of 4,672 published articles were yielded based on the search strategy, and 17 articles were ultimately included in the 5 meta-analyses after screening (Fig. 1). Characteristics and methodological quality of the qualified studies are shown in Supplementary Table S1. Significantly higher prevalence rate of positive anti-TPO IgE (OR, 4.98; 95% CI, 3.33, 7.46; P < 0.001) in CSU patients than that in controls (Fig. 2A) and positive correlation (r = 0.53, P = 0.025) between prevalence rates of positive anti-TPO IgE and anti-TPO IgG were observed (Fig. 2B). In CSU patients, no significant difference in tIgE levels was observed between the positive and negative anti-TPO IgE groups (WMD, 30.63; 95% CI, −37.69, 98.95, P = 0.380; Fig. 2C). There were significantly higher prevalence rates of positive anti-TPO IgG (OR, 6.90; 95% CI, 3.94, 12.10; P < 0.001) or positive anti-TG IgG (OR, 6.68; 95% CI, 2.94, 15.20, P < 0.001) in CSU patients than in controls (Fig. 3).
Fig. 1. Flowchart of the study selection process.
TPO, thyroid peroxidase; Ig, immunoglobulin; CSU, chronic spontaneous urticaria; TG, thyroglobulin.
Fig. 2. Meta-analyses of the prevalence rate of anti-TPO IgE in CSU patients compared with the controls (A), correlation between prevalence rates of anti-TPO IgE and anti-TPO IgG in CSU patients (B), association between the prevalence rate of anti-TPO IgE and the total IgE level in CSU patients (C).
TPO, thyroid peroxidase; Ig, immunoglobulin; CSU, chronic spontaneous urticaria; OR, odds ratio; CI, confidence interval; WMD, weighted mean difference.
Fig. 3. Meta-analyses of the prevalence rates of anti-TPO IgG (A) or anti-TG IgG (B) in CSU patients compared with the controls.
TPO, thyroid peroxidase; TG, thyroglobulin; IgG, immunoglobulin G; CSU, chronic spontaneous urticaria; OR, odds ratio; CI, confidence interval.
Demographic and laboratory data
The demographic and laboratory characteristics of the 1,100 patients and the 1,100 controls are presented in Table 1. CSU occurred more frequently in females and mostly from 21 to 50 years (Supplementary Fig. S1A). The median disease duration was 12 months, with 46.1% ≤ 6 months and 17.3% > 5 years (Supplementary Fig. S1B). In the 1,100 CSU patients, 16 had elevated levels of TSH but normal levels of fT4 and fT3 and could be diagnosed as subclinical hypothyroidism; another 5 had reduced levels of TSH but normal levels of fT4 and fT3 and could be diagnosed as subclinical hyperthyroidism, and the remaining 1,079 had normal levels of TSH, fT4 and fT3. All the 1,100 controls had normal levels of TSH, fT4 and fT3.
Table 1. Characteristics of the CSU patients and the healthy controls.
| Parameter | CSU patients | Healthy controls | P value | |
|---|---|---|---|---|
| No. | 1,100 | 1,100 | ||
| Age (yr) | 38.9 ± 11.4 (18–60) | 38.6 ± 9.9 (18–60) | 0.481 | |
| Sex (female/male) | 766/334 | 748/352 | 0.434 | |
| Age at initial CSU onset | 34.4 ± 13.0 | NA | NA | |
| Disease duration (mon) | 12 (3–36; 2–240) | NA | NA | |
| UASday | 4.4 ± 1.3 | NA | NA | |
| Positive anti-TPO IgE or anti-TG IgE | 243 (22.1) | 86 (7.8) | < 0.001 | |
| Positive anti-TPO IgE | 198 (18.0) | 75 (6.8) | < 0.001 | |
| Positive anti-TG IgE | 77 (7.0) | 32 (2.9) | < 0.001 | |
| Positive anti-TPO IgG or anti-TG IgG | 423 (38.5) | 250 (22.7) | < 0.001 | |
| Positive anti-TPO IgG | 292 (26.5) | 139 (12.6) | < 0.001 | |
| Positive anti-TG IgG | 334 (30.4) | 227 (20.6) | < 0.001 | |
| Correlation between IgE and IgG anti-TPO AAbs | r = 0.297; P < 0.001 | r = 0.179; P < 0.001 | NA | |
| Correlation between IgE and IgG anti-TG AAbs | r = 0.137; P < 0.001 | r = 0.139; P < 0.001 | NA | |
| tIgE (IU/mL) | 102.9 (45.4–173.6) | 12.5 (0–82.9) | < 0.001 | |
| > 100 IU/mL | 551 (50.1) | 242 (22.0) | < 0.001 | |
| > 150 IU/mL | 357 (32.5) | 176 (16.0) | < 0.001 | |
| < 40 IU/mL | 243 (22.1) | 693 (63.0) | < 0.001 | |
| Positive sIgE | 263 (23.9) | 242 (22.0) | 0.311 | |
| Positive inhalant sIgE | 149 (13.5) | 167 (15.2) | 0.301 | |
| Positive food sIgE | 176 (16.0) | 146 (13.3) | 0.080 | |
Values are presented as mean ± SD, median (interquartile range) or number (%).
The P values in bold are statistically significant at level P < 0.05.
CSU, chronic spontaneous urticaria; UASday, urticaria activity score for 1 day; TPO, thyroid peroxidase; Ig, immunoglobulin; TG, thyroglobulin; AAbs, autoantibodies; tIgE, total IgE; sIgE, specific IgE; NA, not significant.
Prevalence of anti-thyroid AAbs
The prevalence rates of subclinical hypothyroidism and subclinical hyperthyroidism showed no significant difference between IgE and IgG AAbs to TPO or TG-positive patients and IgE and IgG AAbs to TPO or TG-negative patients (Tables 2 and 3). Compared with the 1,100 controls, prevalence rates of anti-TPO IgE (18.0% vs 6.8%) or anti-TG IgE (7.0% vs 2.9%) were significantly higher in the 1,100 patients (Table 1). Prevalence rates of anti-TPO IgE or anti-TG IgE were the highest in patients with positive anti-TPO IgG (Supplementary Fig. S2). In both the patients and the controls, significant correlations between prevalence rates of IgE anti-thyroid AAbs and IgG anti-thyroid AAbs were observed (Table 1). Compared with patients with negative anti-TPO IgE, patients with positive anti-TPO IgE had significantly higher percentage of females, significantly higher percentage of positive anti-TPO IgG or significantly lower tIgE level (Table 2). Patients with positive anti-TG IgE had significantly higher percentages of positive anti-TG IgG or positive sIgE compared with patients with negative anti-TG IgE (Table 2).
Table 2. Characteristics of CSU cases with positive anti-TPO IgE or positive anti-TG IgE.
| Parameter | Anti-TPO IgE | Anti-TG IgE | |||||
|---|---|---|---|---|---|---|---|
| Positive (n = 198) | Negative (n = 902) | P value | Positive (n = 77) | Negative (n = 1,023) | P value | ||
| Age (yr) | 38.2 ± 11.3 | 39.1 ± 11.4 | 0.326 | 36.8 ± 11.4 | 39.1 ± 11.4 | 0.105 | |
| Female | 152 (76.8) | 614 (68.1) | 0.017 | 59 (76.6) | 707 (69.1) | 0.199 | |
| Disease duration (mon) | 7.5 (3–36) | 12 (3–36) | 0.158 | 12 (2.5–60) | 10.5 (3–36) | 0.790 | |
| UASday | 4.4 ± 1.3 | 4.4 ± 1.3 | 0.989 | 4.7 ± 1.1 | 4.4 ± 1.3 | 0.055 | |
| Positive anti-TPO IgG or anti-TG IgG | 130 (65.7) | 293 (32.5) | < 0.001 | 58 (75.3) | 365 (35.7) | < 0.001 | |
| Positive anti-TPO IgG | 108 (54.5) | 184 (20.4) | < 0.001 | 41 (53.2) | 251 (24.5) | < 0.001 | |
| Positive anti-TG IgG | 96 (48.5) | 238 (26.4) | 0.016 | 41 (53.2) | 293 (28.6) | < 0.001 | |
| Reduced TSH | 1 (0.51) | 4 (0.44) | 0.999 | 0 | 5 (0.49) | 0.999 | |
| Elevated TSH | 3 (1.52) | 13 (1.44) | 0.999 | 2 (2.60) | 14 (1.37) | 0.310 | |
| tIgE (IU/mL) | 63.0 (25.2–157.2) | 109.8 (54.0–180.4) | < 0.001 | 76.5 (36.0–143.7) | 105.2 (46.3–177.9) | 0.103 | |
| > 100 IU/mL | 76 (38.4) | 475 (52.7) | < 0.001 | 35 (45.5) | 516 (50.4) | 0.411 | |
| > 150 IU/mL | 53 (26.8) | 304 (33.7) | 0.065 | 19 (24.7) | 338 (33.0) | 0.164 | |
| < 40 IU/mL | 78 (39.4) | 165 (18.3) | < 0.001 | 21 (27.3) | 222 (21.7) | 0.256 | |
| Positive sIgE | 61 (30.8) | 202 (22.4) | 0.016 | 38 (49.4) | 225 (22.0) | < 0.001 | |
| Positive inhalant sIgE | 31 (15.7) | 118 (13.1) | 0.359 | 17 (22.1) | 132 (12.9) | 0.036 | |
| Positive food sIgE | 40 (20.2) | 136 (15.1) | 0.086 | 23 (29.9) | 153 (15.0) | 0.002 | |
| Antihistamine-refractory cases | 78 (42.6) | 104 (14.4) | < 0.001 | 17 (23.9) | 165 (19.7) | 0.440 | |
Values are presented as mean ± SD, median (interquartile range) or number (%).
The P values in bold are statistically significant at level P < 0.05.
CSU, chronic spontaneous urticaria; TPO, thyroid peroxidase; TG, thyroglobulin; Ig, immunoglobulin; UASday, urticaria activity score for 1 day; TSH, thyroid stimulating hormone, or thyrotropin; tIgE, total IgE; sIgE, specific IgE.
Table 3. Characteristics of CSU cases with positive anti-TPO IgG or positive anti-TG IgG.
| Parameter | Anti-TPO IgG | Anti-TG IgG | |||||
|---|---|---|---|---|---|---|---|
| Positive (n = 292) | Negative (n = 808) | P value | Positive (n = 334) | Negative (n = 766) | P value | ||
| Age (yr) | 38.4 ± 11.6 | 39.1 ± 11.4 | 0.392 | 39.2 ± 11.7 | 38.8 ± 11.3 | 0.575 | |
| Female | 218 (74.7) | 548 (67.8) | 0.031 | 250 (74.9) | 516 (67.4) | 0.015 | |
| Disease duration (mon) | 10.5 (3–36) | 12 (3–36) | 0.710 | 12 (3–36) | 10 (3–36) | 0.568 | |
| UASday | 4.4 ± 1.3 | 4.4 ± 1.3 | 0.416 | 4.4 ± 1.3 | 4.4 ± 1.3 | 0.733 | |
| Reduced TSH | 2 (0.68) | 3 (0.37) | 0.613 | 1 (0.30) | 4 (0.52) | 0.999 | |
| Elevated TSH | 7 (2.40) | 9 (1.11) | 0.153 | 5 (1.50) | 11 (1.44) | 0.999 | |
| tIgE (IU/mL) | 77.6 (35.5–153.8) | 109.8 (53.1–186.2) | < 0.001 | 99.4 (43.0–170.2) | 105.2 (47.3–179.2) | 0.229 | |
| > 100 IU/mL | 128 (43.8) | 423 (52.4) | 0.014 | 165 (49.4) | 386 (50.4) | 0.793 | |
| > 150 IU/mL | 77 (26.4) | 280 (34.7) | 0.011 | 103 (30.8) | 254 (33.2) | 0.484 | |
| < 40 IU/mL | 87 (29.8) | 156 (19.3) | < 0.001 | 81 (24.3) | 162 (21.1) | 0.269 | |
| Positive sIgE | 83 (28.4) | 180 (22.3) | 0.038 | 81 (24.3) | 182 (23.8) | 0.878 | |
| Positive inhalant sIgE | 43 (14.7) | 106 (13.1) | 0.486 | 43 (12.9) | 106 (13.8) | 0.702 | |
| Positive food sIgE | 57 (19.5) | 119 (14.7) | 0.062 | 55 (16.5) | 121 (15.8) | 0.789 | |
| Antihistamine-refractory cases | 95 (34.1) | 87 (13.9) | < 0.001 | 76 (25.1) | 106 (17.5) | 0.008 | |
Values are presented as mean ± SD, median (interquartile range) or number (%).
The P values in bold are statistically significant at level P < 0.05.
CSU, chronic spontaneous urticaria; TPO, thyroid peroxidase; TG, thyroglobulin; Ig, immunoglobulin; UASday, urticaria activity score for 1 day; TSH, thyroid stimulating hormone, or thyrotropin; tIgE, total IgE; sIgE, specific IgE.
Compared with the 1,100 controls, prevalence rates of anti-TPO IgG (26.5% vs 12.6%) or anti-TG IgG (30.4% vs 20.6%) were significantly higher in the 1,100 patients (Table 1), especially for the female patients (Table 3).
The tIgE levels in patients with positive anti-TPO IgG were significantly lower compared with those in patients with negative anti-TPO IgG. The percentage of tIgE < 40 IU/mL was significantly higher in patients with positive anti-TPO IgG than in patients with negative anti-TPO IgG, while the percentages of tIgE > 100 IU/mL or tIgE > 150 IU/mL were significantly lower in patients with positive anti-TPO IgG than in patients with negative anti-TPO IgG, respectively (Table 3).
Risk factors for antihistamine-refractory CSU cases
Among the 1,100 cases, 193 were not evaluated for the efficacy of antihistamines because they were lost to follow-up. A total of 182 cases were antihistamine-refractory. Compared with the 725 non-refractory cases, percentages of females, tIgE < 40 IU/mL, positive anti-TPO IgE, positive anti-TPO IgG or positive anti-TG IgG were significantly higher, while percentages of tIgE > 100 IU/mL, tIgE > 150 IU/mL or positive inhalant sIgE were significantly lower in the 182 antihistamine-refractory cases (Table 4). In addition, mean UASday was significantly higher in the 182 refractory cases than in the non-refractory cases (Table 4). Multivariate logistic regression analysis was performed with statistically significant variables (Table 4) in one model and demonstrated significant associations between tIgE < 40 IU/mL, positive anti-TPO IgE, positive anti-TPO IgG and increased risk of antihistamine-refractory cases (Table 5).
Table 4. Comparisons between the characteristics of the antihistamine-refractory and non-refractory cases.
| Parameter | Refractory cases | Non-refractory cases | P value | |
|---|---|---|---|---|
| No. | 182 | 725 | ||
| Age (yr) | 39.5 ± 11.0 | 38.5 ± 11.6 | 0.289 | |
| Female | 142 (78.0) | 496 (68.4) | 0.011 | |
| Disease duration (mon) | 6 (3–36) | 10 (3–36) | 0.359 | |
| UASday | 4.6 ± 1.2 | 4.3 ± 1.3 | 0.017 | |
| Positive anti-TPO IgE | 78 (42.9) | 61 (8.4) | < 0.001 | |
| Positive anti-TG IgE | 17 (9.3) | 54 (7.4) | 0.440 | |
| Positive anti-TPO IgG | 95 (52.2) | 184 (25.4) | < 0.001 | |
| Positive anti-TG IgG | 76 (41.8) | 227 (31.3) | 0.008 | |
| tIgE (IU/mL) | 36.0 (18.3–107.9) | 117.2 (63.2–195.8) | < 0.001 | |
| > 100 IU/mL | 48 (26.4) | 409 (56.4) | < 0.001 | |
| > 150 IU/mL | 28 (15.4) | 277 (38.2) | < 0.001 | |
| < 40 IU/mL | 119 (65.4) | 97 (13.4) | < 0.001 | |
| Positive sIgE | 42 (23.1) | 204 (28.1) | 0.192 | |
| Positive inhalant sIgE | 18 (9.9) | 122 (16.8) | 0.021 | |
| Positive food sIgE | 29 (15.9) | 135 (18.6) | 0.451 | |
Values are presented as mean ± SD, median (interquartile range) or number (%).
The P values in bold are statistically significant at level P < 0.05.
UASday, urticaria activity score for 1 day; TPO, thyroid peroxidase; TG, thyroglobulin; Ig, immunoglobulin; tIgE, total IgE; sIgE, specific IgE.
Table 5. Risk factors of antihistamine-refractory cases.
| Parameter | P value | OR (95% CI) |
|---|---|---|
| Female | 0.258 | 1.30 (0.82–2.06) |
| UASday > 4 | 0.103 | 1.39 (0.94–2.06) |
| Positive anti-TPO IgE | < 0.001 | 2.89 (1.73–4.84) |
| Positive anti-TPO IgG | < 0.001 | 3.10 (1.95–4.95) |
| Positive anti-TG IgG | 0.796 | 0.94 (0.59–1.50) |
| tIgE < 40 IU/mL | < 0.001 | 11.58 (7.77–17.25) |
| Positive inhalant sIgE | 0.030 | 0.49 (0.26–0.94) |
The P values in bold are statistically significant at level P < 0.05.
OR, odds ratio; CI, confidence interval; UASday, urticaria activity score for 1 day; TPO, thyroid peroxidase; TG, thyroglobulin; Ig, immunoglobulin; tIgE, total IgE; sIgE, specific IgE.
DISCUSSION
Since the first report on IgE anti-thyroid microsomal antibodies in patients with chronic urticaria,10 to date the largest study on the prevalence rate of anti-TPO IgE in CSU patients recruited 478 patients (54.2%) and 127 healthy controls (19.7%).11 Another study has demonstrated that anti-TPO IgE is present at higher frequency (34.0% vs 8.1%) in CSU patients as well as a significant correlation between prevalence rates of anti-TPO IgE and anti-TPO IgG is also present.6 The authors also found up-regulation of basophil activation markers in CSU subjects upon exposure to TPO and positive skin reactions upon passive transfer of anti-TPO IgE from a CSU patient to the skin of a healthy subject. In this large-sample-size study, we have confirmed higher prevalence rates of anti-TPO IgE and anti-TG IgE in a Chinese Han CSU population and significant correlation between the prevalence rates of anti-TPO IgE and anti-TPO IgG. In line with Sauer et al’s study,12 we found that the elevated prevalence rates of anti-TPO IgE were associated with lower tIgE levels. The elevated levels of anti-TG IgE in patients with severe CSU decrease after omalizumab treatment.13
Based on the literature and our real-world experience on patients with atopic dermatitis and CSU, systemic corticosteroids or immunosuppressants (such as cyclosporine) reduce serum levels of tIgE and possibly reduce serum levels of anti-TPO IgE or anti-TG IgE. In fact, only a few CSU patients were excluded because they were first treated by intermittent systemic corticosteroids at local clinics or hospitals before presentation.
In this study, 26.5% of Chinese CSU patients were positive for anti-TPO IgG and 30.4% positive for anti-TG IgG. These prevalence rates are similar to those of previous reports from other populations and ethnicities (Supplementary Table S1).
Up to 41% of autoimmune CSU patients exhibit significantly lower tIgE levels (< 40 IU/mL) and higher anti-TPO IgG levels.14 Elevated anti-TPO IgG and low tIgE have been shown as a predictor for low response to antihistamine and a useful diagnostic marker for autoimmune CSU.15 In this study, we confirmed that 29.8% of CSU patients with positive anti-TPO IgG had tIgE < 40 IU/mL versus 19.3% in those with negative anti-TPO IgG.
In our study, positive anti-TPO IgE, positive anti-TPO IgG and tIgE < 40 IU/mL are independent predictors of antihistamine-refractory CSU cases. Our findings are consistent with the results of a previous study which has shown that lower tIgE is associated with higher prevalence rates of anti-TPO IgE.12 The antihistamine-refractory CSU patients with positive anti-TPO IgE can be successfully treated with omalizumab.16
This study has some limitations. First, as the basic medical system in China was very different from that in Europe and the US, Chinese patients could see several dermatologists in the same hospital on the same day and their overall compliance was poor. In addition, because this study was not a sponsor-initiated drug clinical trial, it was difficult to persuade the CSU patients to discontinue use of antihistamines for 7 days. In this study, because most patients had taken antihistamines in the week before presentation and they could not remember the exact daily number of wheals in the past week, we used the UASday before the day of blood sample collection. Secondly, the IgE AAbs against other autoantigens, such as interleukin 24 and tissue factor, were not detected.
In conclusion, our study confirms the elevated prevalence rates of IgE and IgG anti-thyroid AAbs in Chinese CSU patients and highlights the correlation between IgE and IgG anti-thyroid AAbs.
ACKNOWLEDGMENTS
This study was supported by the National Key Clinical Specialist Subject Construction Project on Urticaria from National Health Commission, China ([2012]649).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Studies on the prevalence rates of IgE or IgG anti-thyroid autoantibodies in cases with CSU qualified for meta-analyses
(A) Number of patients with CSU in different age-based subgroups according to age at initial onset. (B) Number of CSU patients with different disease duration.
Prevalence rates of anti-TPO IgE and anti-TG IgE in CSU patients with positive anti-TPO IgG, positive anti-TG IgG or negative anti-thyroid IgG AAbs, and in healthy controls with positive anti-TPO IgG, positive anti-TG IgG or negative anti-thyroid IgG AAbs.
References
- 1.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 2.Ulambayar B, Park HS. Anti-TPO IgE autoantibody in chronic urticaria: Is it clinically relevant? Allergy Asthma Immunol Res. 2019;11:1–3. doi: 10.4168/aair.2019.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolkhir P, Metz M, Altrichter S, Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy. 2017;72:1440–1460. doi: 10.1111/all.13182. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] Ottawa: Ottawa Hospital Research Institute; 2019. [cited 2019 August 24, 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 6.Sánchez J, Sánchez A, Cardona R. Causal relationship between anti-TPO IgE and chronic urticaria by in vitro and in vivo tests. Allergy Asthma Immunol Res. 2019;11:29–42. doi: 10.4168/aair.2019.11.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KW, Myers RA, Lee JH, Igartua C, Lee KE, Kim YH, et al. Genome-wide association study of recalcitrant atopic dermatitis in Korean children. J Allergy Clin Immunol. 2015;136:678–684.e4. doi: 10.1016/j.jaci.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang KL, Yang YH, Yu HH, Lee JH, Wang LC, Chiang BL. Analysis of serum total IgE, specific IgE and eosinophils in children with acute and chronic urticaria. J Microbiol Immunol Infect. 2013;46:53–58. doi: 10.1016/j.jmii.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Wu J, Qi Y, Zhu H, Yao X, Li M, et al. Long-term combinations and updosing of second-generation H1-antihistamines show efficacy and safety in the treatment of chronic spontaneous urticaria: a multicenter real-life pilot study. J Allergy Clin Immunol Pract. 2020;8:1733–1736.e11. doi: 10.1016/j.jaip.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Bar-Sela S, Reshef T, Mekori YA. IgE antithyroid microsomal antibodies in a patient with chronic urticaria. J Allergy Clin Immunol. 1999;103:1216–1217. doi: 10.1016/s0091-6749(99)70204-6. [DOI] [PubMed] [Google Scholar]
- 11.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase--a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794. doi: 10.1371/journal.pone.0014794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer M, Scheffel J, Frischbutter S, Kolkhir P, Xiang YK, Siebenhaar F, et al. Lower IgA levels in chronic spontaneous urticaria are associated with lower IgE levels and autoimmunity. Front Immunol. 2021;12:657211. doi: 10.3389/fimmu.2021.657211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cugno M, Asero R, Ferrucci S, Lorini M, Carbonelli V, Tedeschi A, et al. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy. 2018;73:2408–2411. doi: 10.1111/all.13587. [DOI] [PubMed] [Google Scholar]
- 14.Schoepke N, Asero R, Ellrich A, Ferrer M, Gimenez-Arnau A, E H Grattan C, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST study. Allergy. 2019;74:2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 15.Kolkhir P, Kovalkova E, Chernov A, Danilycheva I, Krause K, Sauer M, et al. Autoimmune chronic spontaneous urticaria detection with IgG anti-TPO and total IgE. J Allergy Clin Immunol Pract. 2021;9:4138–4146.e8. doi: 10.1016/j.jaip.2021.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Maurer M, Altrichter S, Bieber T, Biedermann T, Bräutigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202–209.e5. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Studies on the prevalence rates of IgE or IgG anti-thyroid autoantibodies in cases with CSU qualified for meta-analyses
(A) Number of patients with CSU in different age-based subgroups according to age at initial onset. (B) Number of CSU patients with different disease duration.
Prevalence rates of anti-TPO IgE and anti-TG IgE in CSU patients with positive anti-TPO IgG, positive anti-TG IgG or negative anti-thyroid IgG AAbs, and in healthy controls with positive anti-TPO IgG, positive anti-TG IgG or negative anti-thyroid IgG AAbs.